Abstract
Lower growth rate of the açaí palm seedlings limits the crops’ commercial expansion. The goal was evaluating the biometry, biomass accumulation, nutrient contents, chlorophyll-a fluorescence, and gas exchange in açaí seedlings inoculated with rhizobacteria. The treatments were individual inoculations of the seven rhizobacteria isolates and one control (without inoculation) on the roots. Biometry and biomass data were submitted to cluster analysis to separate the isolates into groups according to the similarity degree, and groups’ means were compared through the SNK test. Three groups were formed; group 1 was composed of the control; group 2 of the UFRA-35, UFRA-38, UFRA-58, UFRA-61, UFRA-92, and BRM-32111 isolates; and group 3 was composed of the BRM-32113 isolate. Group 2 and 3 isolates promoted an increase in growth, biomass accumulation, higher levels of nutrients and chlorophyll, and improvements in the gas exchange and chlorophyll-a fluorescence in comparison with the control. The results evidenced that the rhizobacteria accelerate the growth, increase the photosynthetic efficiency, and induce the leaf nutrient accumulation in açaí palm seedlings. The rhizobacteria inoculation can contribute to the sustainable management of the açaí palm seedling production in nurseries.
Keywords: Euterpe oleracea, Biostimulants, Biofertilizer, Photosynthesis
Introduction
Açaí (Euterpe oleracea Mart.) is a palm native to the Amazon region and distributed along the banks of the Amazon River in northern Brazil [1]. The domestic and international demand for fruit in the last decade, due to its nutritional benefits [2], stimulated the extractive production system change for commercial crops in land areas [3]. The seedling production has increased, and it faces some obstacles such as a slow initial growth and low availability of differentiated genetic material [4].
Several studies report the use of rhizobacteria to promote the growth of palm seedlings such as oil palm [5] and coconut [6]. Rhizobacteria are found in free-living in the soil and promote benefits to the plant through colonization of the root system [7]. The benefits include increased recycling, solubilization, and absorption of mineral nutrients, synthesis of vitamins, amino acids, siderophores, and plant growth, promoting hormones such as gibberellin, auxin, and cytokinin [8, 9].
The auxins are responsible for the induction of primary and secondary root growths [10]. Rhizobacteria can make auxins available through root exudates or induce their biosynthesis in plants [11]. However, root growth induction occurs when higher auxin levels are balanced with ethylene lower levels. Aslantaş et al. [12] report that root growth increase may be attributed to the ability of the rhizobacteria in synthesizing the ACC deaminase enzyme, which decreases ethylene to non-inhibitory levels.
The more extensive root system increases the root contact area with the soil and enhances water and nutrient uptakes [13]. In the oil palm seedlings inoculated with rhizobacteria, the expanded root system improves the efficiency of nitrogen, phosphorus, and potassium uptakes besides the influence on the increase in height and biomass of the aerial part [14]. Rhizobacteria induce the gibberellin synthesis to the direct nutrient utilization in the aerial part biomass, as observed in tomato seedlings inoculated with them [10, 15].
The most significant growth in the aerial part includes in more leaves and a greater expansion in the leaf area, which contributes to a higher light absorption and CO2 assimilation [16]. The best photosynthetic performance, resulting from the inoculation of rhizobacteria, can be measured through the evaluation of the gas exchange and chlorophyll-a fluorescence. Gas exchanges indicate the efficiency of stomatal opening regulation that directly influences CO2 assimilation [17], while parameters of chlorophyll-a fluorescence indicate the efficiency of light utilization in the photochemical processes of the photosynthesis [18].
Açaí seedlings inoculated with rhizobacteria may accelerate the growth and decrease the time needed to obtain the seedlings in nurseries, contributing to a decrease in the mortality rate of the plants in the field. The objective of this study was to evaluate the biometry, biomass accumulation, nutrient contents, chlorophyll-a fluorescence, and gas exchange in leaves of açaí seedlings inoculated with rhizobacteria.
Material and methods
Plant growth
Açaí seeds (cultivar BRS-Pará) were sown in plastic trays containing 2.5 L of substrate composed of crushed coconut fiber (Golden mix). On day 32 after germination, seedlings with two expanded leaves and a height of about 13 cm were transplanted into plastic bags (15 × 25 cm, length × height) containing a substrate composed of 60% of Oxisol and 40% of decomposed poultry litter. Cultivation was carried out in nursery of the Federal Rural University of the Amazon in Belém, PA, which presents AMI climatic type, hot and rainy, according to the Köppen-Geiger classification. During the experimental period (from August to December 2016), the environmental conditions were 32 ± 2 °C air temperature, 75 ± 5% relative humidity, 2 ± 0.2 kPa of air vapor pressure deficit, and 800 ± 100 μmol m−2 s−1 of incident radiation. The pH of the substrate and concentrations of macro- and micronutrients were adjusted as recommended for açaí palms [19]. The seedlings were irrigated daily between 8:00 and 9:00 a.m. sing self-compensating drippers (PCJ, Netafim, São Paulo, Brazil) with a flow rate of 1.92 L water h−1 per dripper. The operating time of drippers was estimated to maintain field capacity soil [20].
Rhizobacteria characterization
Rhizobacteria from açaí palms (UFRA-35, UFRA-38, UFRA-58, UFRA-61, and UFRA-92) and rice plants (BRM-32113 and BRM-32111) are stored and preserved in the collection of microorganisms owned by Plant Protection Laboratory (PPL) of the Federal Rural University of Amazonia, Belém, state of Pará, Brazil. Rhizobacteria from açaí palms were isolated and characterized as described by Filippi et al. [21]. BRM-32113 and BRM-32111 were identified by Martins [22] and tested for plant growth promotion by Filippi et al. [21].
Rhizobacteria identification from açaí
Rhizobacteria isolates from açaí palm were identified through 16S rRNA gene sequencing. Rhizobacteria cells were grown in solid medium 523 [23] at 28 °C for 48 h until they reached stationary phase. One millimeter of culture was transferred to a 2-mL microcentrifuge tube and centrifuged at 13000g for 5 min using the Eppendorf centrifuge 5810R. Genomic DNA was isolated as described by Xiang and Boer [24]. The universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) were used to amplify the 16S rRNA coding region. The 50-μL PCR reaction mixture was composed of 2 μL of bacterial DNA, 25 μM of each primer, 10× PCR reaction buffer (Fermentas, USA), 10 mM of dNTP mix, 2.5 U of Taq polymerase (Fermentas, USA), 50 mM of MgCl2 and tops up with sterile distilled water. The thermal cycling condition was as follows: one cycle at 94 °C for 4 min, 23 cycles at 94 °C for 1 min, 23 cycles at 48 °C for 30 s, and 23 cycles at 72 °C for 2 min. The amplified 16S gene fragments were purified using QIAquick Gel Extraction Kit (QIAGEN Inc., USA) and sent for sequencing. The obtained sequences were analyzed through comparison with sequences in GenBank database by the BLAST program, and the percentage similarity was determined. A phylogenetic tree was constructed by using the MEGA X version software following the neighbor-joining method.
Rhizobacteria inoculation
The rhizobacteria were grown in 523 solid medium [23] for 48 h at 28 °C. Bacterial suspensions were prepared with distilled and sterilized water, and the concentration was adjusted in a spectrophotometer to 540 nm optical density = 0.5 ABS corresponding to 108 colony-forming unit (CFU). The seedlings had their roots sectioned for root length standardization at 7 cm, and before transplantation to the plastic bags filled with the substrate, they were immersed in 500 mL of each bacterial suspension for 20 min. The control seedlings were immersed in distilled and sterilized water. Then, irrigation was performed every week for 1 month using 50 mL/seedling of each bacterial suspension for treated plants, and 50 mL/seedling of sterile water was applied to the control plants. After this period, irrigation was maintained every month until the third age month of the açaí seedlings.
Leaf gas exchange
The gas exchange parameters were estimated on the second physiologically mature and fully expanded leaf, from apex to base, 5 months after inoculation of rhizobacteria on açaí palm seedlings. The net assimilation of CO2 (A), stomatal conductance to water vapor (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) were estimated between 08:00 and 10:00 using a portable open-flow gas-exchange system (LI-6400XT, LI-COR, Lincoln, NE) under an external CO2 concentration of 400 μmol mol−1 of air and artificial photosynthetically active radiation (PAR) of 900 μmol of photons m−2 s−1. This measurement interval (08:00–10:00) was adjusted according to the results obtained with the daytime curve of gas exchange for the species [25]. The A/E ratio estimated the water use instantaneous efficiency. All measurements were performed under the air temperature of 31 ± 2 °C, relative air humidity of 63 ± 2%, incident radiation of 600 ± 100 μmol m−2 s−1, and air vapor pressure deficit of 1.9 ± 0.2 kPa. The amount of blue light was adjusted to 10% of the photosynthetically active radiation to optimize the stomatal opening.
Leaf chlorophyll-a fluorescence
The chlorophyll-a fluorescence was determined simultaneously with the gas exchanges using a fluorescence chamber (IG 6400-40; LI-COR Inc.) integrated into the portable open-flow gas-exchange system. The leaves were adapted to the dark for 20 min and after that illuminated with a weak and modulated light pulse (0.03 μmol m−2 s−1) to obtain the initial fluorescence (Fo). A saturating white light pulse of 6000 μmol m−2 s−1 was applied for 0.8 s to ensure maximum fluorescence emission (Fm). The sampled leaves were then illuminated for 300 s with an actinic light (250 μmol m−2 s−1) to achieve the steady-state fluorescence yield (Fs). Subsequently, white light saturating pulses were applied to achieve maximum fluorescence (Fm′). The actinic light was then switched off, and a far-red illumination (2 μmol m−2 s−1) was applied to estimate the initial fluorescence adapted to light (Fo′). From these measurements, the following parameters were calculated: potential PSII activity (Fv/Fo = (Fm − Fo)/Fo), effective photochemical efficiency of PSII (Fv′/Fm′ = (Fm′ − Fo′)/Fm′) [26], coefficients of photochemical (qp = (Fm′ − Fs)/(Fm′ − Fo′)) and non-photochemical dissipation (qN = 1 – qP), and the rate of electron transport (ETR = φPSII.PPFD.0,42) [18].
Chlorophyll content
The chlorophyll relative content was initially estimated by the chlorophyll meter (SPAD-502) in those leaves used for measurements of gas exchange and chlorophyll-a fluorescence. Afterwards, these values were used to estimate the chlorophyll-a absolute contents (Chla), chlorophyll-b (Chlb), Chla + b sum, and Chla/Chlb ratio from the equations of the adjustment curves obtained by the relationship between the relative values of chlorophylls obtained by SPAD-502 and chlorophyll absolute content (Supplementary Figure), described in the item below.
SPAD-502 and chlorophyll content
The relationship between the chlorophyll relative content obtained by the SPAD and the chlorophyll absolute content was determined as described by Jesus et al. [27], with some modifications. Açaí palm seedling leaves with different green shades were selected to obtain a gradient of chlorophyll content. Leaves at different ages were used: from the early stages, with low contents of chlorophylls, up to stages of complete photosynthetic activity (mature) and fully expanded leaves, with high levels of chlorophylls. The SPAD-502 readings were performed at three points on either side of the central leaf vein, on the adaxial side of the leaf. After the readings, the leaves were detached and stored in aluminum foil bags inside a Styrofoam box with ice and taken to the laboratory to determine the absolute chlorophyll content. Seven 5-mm diameter discs were removed from each leaf blade site where SPAD-502 measurements were performed. Absolute chlorophyll content was extracted by macerating the leaf tissue in 250 μL of ethanol 98% (v/v). The macerated tissue was incubated for 20 min at 80 °C and subsequently centrifuged at 12,000×g for 5 min at 4 °C. The supernatant was collected, and the residue was washed twice in 200 μL of ethanol. The first wash used 80% ethanol, and the second, 50% ethanol. The volume was adjusted to 600 μL with the same extractor, and a 20-μL aliquot of the ethylic extract from each sample was added to a reaction medium containing 120 μL of 98% ethanol and 40 μL of the ethyl mix (final volume of 180 μL per well/sample). The absorbance of the samples was recorded at 645 and 665 nm, and the concentrations of Chla, Chlb, Chla/Chlb, and Chla + b were estimated according to formulas (a) and (b) described by Porra et al. [28], later normalized to the leaf disc area of each sample and total leaf area of each plant.
Leaf nutrient content
The leaf samples were dried in a forced air circulation oven (72 h at 65 °C), milled (< 5 mm) in a Wiley type mill, weighed, and taken to the soil analysis laboratory owned by Embrapa Amazônia Oriental, Belém, state of Pará, for the nutrient content determination, as described by Silva et al. [29]. Phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) were extracted through perchloric nitric digestion, and the measurements were done through flame photometry for P and K, and microwave plasma-atomic emission spectrometer (AES) for Ca and Mg. Nitrogen (N) was extracted by sulfuric digestion and determined by the Kjeldahl method.
Biomass and growth evaluation
Growth promotion and biomass accumulation were evaluated for 5 months after seed germination. Plant height and root length were measured using a graduated metallic ruler, while the collar diameter was measured with a digital caliper (0.02 mm accuracy). The robustness index was calculated by the ratio between plant height and collar diameter. The leaf number was evaluated by direct counting of the emitted leaves, and the total leaf area was estimated by the dry mass of the leaf discs at 7 mm in diameter (6 discs per leaf). The seedlings were sectioned into the root, leaf blade, and aerial part and dried in a forced ventilation oven at 65 °C, until constant weight, for weighing and dry biomass determination [30].
Statistical analysis
The experiment was conducted in a completely randomized design with eight treatments (seven rhizobacteria isolates and one control without rhizobacteria) and five replicates. Growth and biomass data were submitted to cluster analysis using the mean values of each, and the similarity degree was obtained by the standard Euclidean distance. The groups were submitted to variance analysis (ANOVA), and the means were compared through the Student-Newman-Keuls (SNK) test, P < 0.05, using R programming language [31].
Results
Rhizobacteria isolate selection for growth promotion
All the evaluated isolates promoted the growth of açaí palm seedlings compared with the absolute control (Figs. 1 and 2). The cluster analysis showed the formation of the three groups, where group 1 which is composed of the control treatment was lower for all growth promotion variables than groups 2 and 3, which are composed of the isolates UFRA-35, UFRA-38, UFRA-58, UFRA-61, UFRA-92, and BRM-32111; and BRM-32113, respectively. For the growth variables, the increase ranged from 31 to 38% for plant height, from 14 to 21% for the collar diameter, and up to 33% for the number of leaves, from 53 to 112% for the leaf area, from 17 to 23% for the robustness index, and up to 36% for the root length in the isolates of groups 2 and 3, respectively. In relation to biomass, root dry mass, leaf dry mass, and shoot dry mass and total dry mass in group 2 and 3 isolates, the increase ranged from 69 to 67%, from 53 to 113%, from 64 to 110%, and from 65 to 95%, respectively. The root/shoot ratio was lower in 22% for the group 3 isolates in relation to the control, with no difference between the group 1 and 2 isolates (Table 1).
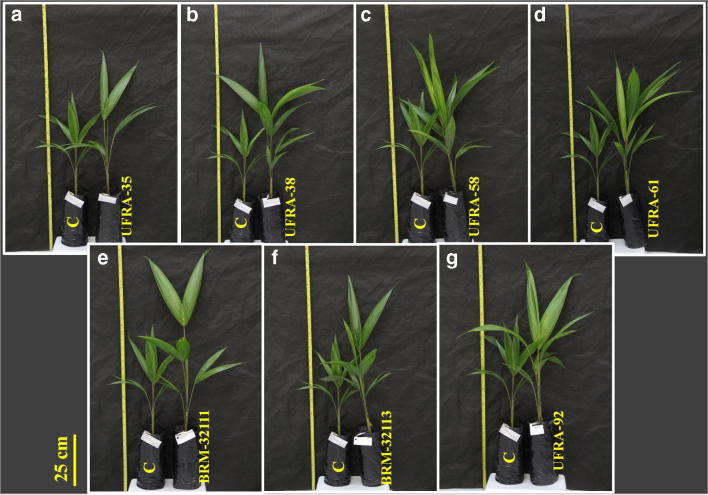
Fig. 1.
Açaí palm seedlings shoot (5 months old) inoculated with rhizobacteria: UFRA-35 (a), UFRA-38 (b), UFRA-58 (c), UFRA-61 (d), BRM-32111 (e), BRM-32113 (f), UFRA-92 (g), and control (C). The figure was elaborated by author
Fig. 2.
Açaí palm seedlings roots (5 months old) inoculated with rhizobacteria: UFRA-35, UFRA-38, UFRA-58, UFRA-61, UFRA-92, BRM-32111, BRM-32113, and control (C). The figure was elaborated by author
Table 1.
Biometry and biomass in açaí palm seedlings (5 months old) inoculated with rhizobacteria: UFRA-35, UFRA-38, UFRA-58, UFRA-61, UFRA-92, BRM-32111, and BRM-32113
| Variables/isolatesa | Groups | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Control | UFRA-35; UFRA-38; UFRA-58; UFRA-61; UFRA-92; BRM-32111 | BRM-32113 | |
| Biometry | |||
| Plant height (cm) | 34.26 B* | 44.88 A | 47.34 A |
| Collar diameter (mm) | 6.36 C | 7.25 B | 7.67 A |
| Leaf number | 3.0 B | 3.0 B | 4.0 A |
| Leaf area (cm2) | 183.46 C | 281.15 B | 388.88 A |
| Root length (cm) | 22.70 B | 30.76 A | 30.08 A |
| Robustness index | 5.16 B | 6.07 A | 6.37 A |
| Biomass | |||
| Root dry mass (g) | 0.72 B* | 1.22 A | 1.20 A |
| Leaf dry mass (g) | 0.78 C | 1.19 B | 1.66 A |
| Shoot dry mass (g) | 1.42 C | 2.33 B | 2.98 A |
| Total dry mass (g) | 2.14 C | 3.54 B | 4.18 A |
| Root/shoot ratio | 0.51 A | 0.52 A | 0.40 B |
Isolates were clustered into class according to Euclidian similarity matrix. *Means with different letters are significantly different at P ≤ 0.05 according to the Student-Newman-Keuls test, using the R software
Rhizobacteria identification from the açaí plant
Rhizobacteria isolates UFRA-35, UFRA-38, UFRA-58, UFRA-61, and UFRA-92 were identified at the molecular level. The 16S rDNA gene sequences of rhizobacteria isolates were PCR amplified and approximately 1200 bp were sequenced. The rhizobacteria isolate identification based on 16S rDNA gene partial sequences revealed that the UFRA-35 (GenBank MN173354) and UFRA-58 (GenBank MN173368) isolates match with Burkholderia sp. with 98 and 96% similarity, respectively. The UFRA-38 (GenBank MN173367) isolate matches with Bacillus safensis with 99% similarity. The UFRA-61 (GenBank MN173370) isolate matches with the Pseudomonas fluorescens with 96% similarity, while the UFRA-92 (GenBank MN175193) isolate matches with Bacillus subtilis with 99% similarity. The phylogenetic analysis based on the neighborhood-joining tree method (100 bootstrap sampling) was also done to validate the identification of rhizobacteria.
Leaf gas exchanges
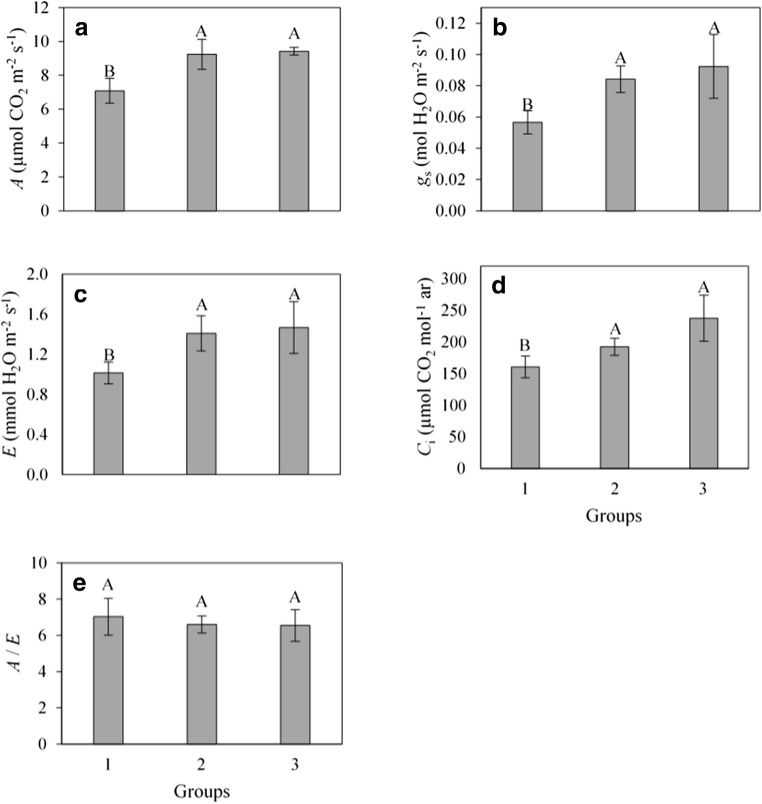
All isolates from groups 2 and 3 induced an increase in gas exchange compared with those from group 1 (control). Increases ranged from 31 to 33% for A, from 50 to 64% for gs, and from 39 to 46% for E, in groups 2 and 3, respectively. The Ci increased by 19 and 32% for groups 2 and 3, respectively (Fig. 3).
Fig. 3.
a CO2 net assimilation rate (A), b stomatal conductance to water vapor (gs), c transpiration rate (E), d CO2 intercellular concentration (Ci), and e water use instantaneous efficiency (A/E) in açaí palm seedlings inoculated with rhizobacteria: group 1 (control—without rhizobacteria), group 2 (UFRA-35, UFRA-38, UFRA-58, UFRA-61, BRM-32111, and UFRA-92), and group 3 (BRM-32113). The means were compared by the Student-Newman-Keuls test (P ≤ 0.05)
Chlorophyll content
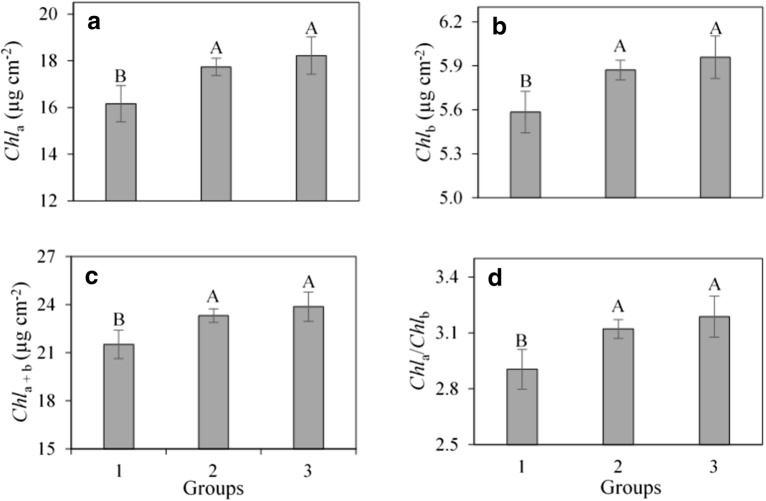
All rhizobacteria induced an increase in the chlorophyll content. In comparison with group 1, the increment ranged from 10 to 13%, from 7 to 9%, from 8 to 11%, and from 7 to 10% in Chla, Chlb, Chla + b, and Chla/Chlb in groups 2 and 3, respectively (Fig. 4).
Fig. 4.
a Chlorophyll-a (Chla), b chlorophyll-b (Chlb), c total chlorophyll (Chla + b), and d ratio between Chla and Chlb (Chla/Chlb) in açaí palm seedlings’ leaves inoculated with rhizobacteria: group 1 (control—without rhizobacteria), group 2 (UFRA-35, UFRA-38, UFRA-58, UFRA-61, BRM-32111, and UFRA-92), and group 3 (BRM-32113). The means were compared by the Student-Newman-Keuls test (P ≤ 0.05)
Chlorophyll-a fluorescence
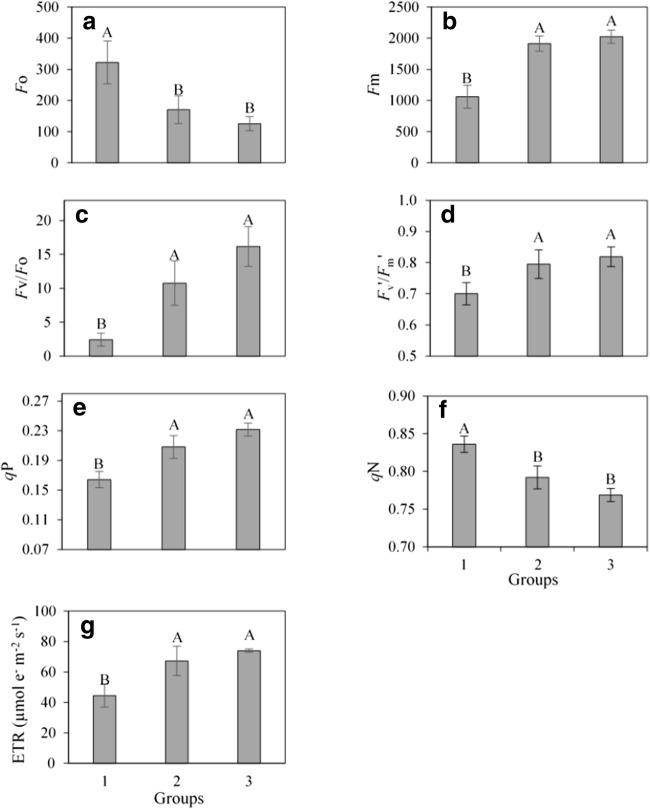
All rhizobacteria isolates induced average reductions of 54% for Fo and 6% for qN in relation to control plants. However, all other variables increased in groups 2 and 3 in comparison with those in group 1. The increment was greater than 81% for Fm, between 343 and 565% for Fv/Fo, between 13 and 17% for Fv′/Fm′, between 31 and 44% for qP, and between 51 and 66% for ETR in groups 2 and 3, respectively (Fig. 5).
Fig. 5.
a Initial (Fo) and b maximum (Fm) fluorescence, c PSII potential activity (Fv/Fo), d PSII effective photochemical efficiency (Fv′/Fm′), e photochemical (qP) and f non-photochemical (qN) extinction coefficients, and g electron transfer rate (ETR) in açaí palm seedlings inoculated with rhizobacteria: group 1 (control—without rhizobacteria), group 2 (UFRA-35, UFRA-38, UFRA-58, UFRA-61, BRM-32111, and UFRA-92), and group 3 (BRM-32113). The means were compared by the Student-Newman-Keuls test (P ≤ 0.05)
Leaf nutrient content
All rhizobacteria induced an increase in nutrient content in leaves of açaí seedlings, except for calcium (Ca) and magnesium (Mg). Compared with the control, the seedlings inoculated with rhizobacteria increased by 12 and 18% for nitrogen (N), 24 and 73% for phosphorus (P), and 11 and 14% for potassium (K) for groups 2 and 3, respectively (Table 2).
Table 2.
Nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) contents in açaí palm seedlings’ leaves without (group 1) and with (groups 2 and 3) rhizobacteria inoculation
| Group | Isolates | Nutrient content (g kg−1 shoot) | ||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | ||
| 1 | Control | 13.31* b | 2.13 c | 52.95 b | 6.71 a | 2.22 a |
| 2 | UFRA-35, UFRA-38, UFRA-58, BRM-32111, UFRA-61 e UFRA-92 | 14.84 a | 2.64 b | 58.55 a | 7.33 a | 2.43 a |
| 3 | BRM-32113 | 15.74 a | 3.69 a | 60.47 a | 6.95 a | 2.35 a |
| CV(%) | 4.90 | 12.64 | 4.57 | 7.10 | 8.52 | |
*Values are the means of five replicates. Equal lowercase letters in the column indicate non-significant differences by the SNK test (P < 0.05)
Discussion
The present study reports for the first time the growth promotion, biomass and nutrient accumulation, and photosynthetic performance improvement in açaí palm seedlings inoculated with the rhizobacteria UFRA-35, UFRA-38, UFRA-58, UFRA-61, UFRA-92, BRM-32111 (group 2), and BRM-32113 (group 3). The benefits of the rhizobacteria inoculation to growth promotion have been reported in monocotyledons such as oil palm [5], coconut [6], and rice [21, 32], which resulted in increased root system growth and efficiency in water and nutrient absorption.
Burkholderia sp. (UFRA-35 and UFRA-58), Burkholderia pyrrocinia (BRM-32113), Pseudomonas fluorescens (UFRA-61 and BRM-32111), Bacillus safensis (UFRA-38), and Bacillus subtilis (UFRA-92) are widely used as growth promoters of plants such as tomato [33], peanut [34], and rice [35]. Other studies report the use of these rhizobacteria such as in biofertilization, phytostimulation, and biocontrol [36, 37].
The roots attract rhizobacteria by releasing exudates such as carbohydrates, amino acids, organic acids, proteins, lipids, vitamins, and phenolic compounds, and this interaction constitutes a mechanism known as the rhizosphere priming effect, which favors the bacterium nutrition and growth [9, 38], and starts the process of signal exchange and root colonization by rhizobacteria [7]. The result of this plant-rhizobacteria interaction has been named as biostimulants and biofertilizers.
In the present study, all rhizobacteria induced an increase in the growth of primary, secondary, and tertiary roots, which can be attributed to the direct mechanisms when the rhizobacteria act in the direct solubilization of insoluble P sources, N fixation, and/or regulation of the concentration of plant growth regulators, such as indole-acetic acid (IAA); and indirect mechanisms when providing precursors such as tryptophan amino acid to IAA biosynthesis in the plant [17]. Another indirect form may occur when the plant provides tryptophan, through root exudates, to be converted into IAA by the rhizobacteria [11] and used in the induction of root growth [39]. In oil palm seedlings inoculated with rhizobacteria, an increase in the growth of primary and secondary roots was observed, and it was attributed to a higher IAA synthesis [10].
Root system development induced by the rhizobacteria (groups 2 and 3) was directly associated with the aerial part growth, which can be attributed to the higher water absorption and nutrient translocation resulting from the greater root contact area with the soil [14]. The BRM-32113 induced the largest collar diameter. However, all rhizobacteria induced an increase in the robustness index, a parameter that is related to the greater seedling vigor, as observed in coconut seedlings inoculated with rhizobacteria [6].
The aerial part growth, as well as the leaf number and leaf area, can be attributed to the ability of the rhizobacteria to induce the gibberellin and cytokinin syntheses that regulate leaf expansion and chlorophyll synthesis [40, 41]. In the present study, the more significant leaf number was obtained only with BRM-32113. However, the other group 2 rhizobacteria induced a greater leaf area that resulted in more significance in the aerial part growth and the leaf biomass accumulation. In tomato seedlings inoculated with rhizobacteria, similar results were obtained, which is attributed to the rhizobacteria ability to signal the route gibberellin and cytokinin biosyntheses [42].
The largest leaf area directly influences the higher light absorption and CO2 assimilation [16]. In the present study, all rhizobacteria induced an increase in the CO2 net assimilation (A) concerning the control plants. The highest A value can be attributed to the greater stomatal opening degree (gs), which allows higher CO2 entrance in the leaves and favors the increase in net photosynthesis. In rice plants inoculated with rhizobacteria, an increase in the photosynthetic net was observed, which was attributed to the positive influence on the opening and closing of the stomata, directly affecting the gas exchange and contributing to the plant growth [17].
Besides promoting the entry of CO2 into the leaves, the higher stomatal opening directly influences the more significant loss of water by transpiration [43]. In the present study, the higher transpiration rate can be attributed to the more significant stomatal opening induced by all rhizobacteria. When water is abundant and the solar radiation incident on the leaves favors the high photosynthetic activity, there is a high demand for CO2 within the leaf, and the stomatal pores open widely, reducing the stomatal resistance to CO2 diffusion [44]. In these conditions, a high water loss occurs due to transpiration; however, since the water supply is abundant, it is advantageous for the plant to exchange water for photosynthesis products, resulting in growth gain [45].
The changes in the CO2 intercellular concentration (Ci) evidence that the rhizobacteria isolates modulate the stomatal opening and Rubisco carboxylation activity to increase the CO2 fixation in mesophile. Similar results were found for other growth biopromotors, such as Trichoderma spp., inoculated in rice plants [46]. These benefits may result in increased photoassimilate production, such as sucrose and raffinose, which can be allocated to plant heterotrophic organs to support growth or be converted into reverse products [47] or be used by rhizobacteria by the root exudates [38].
However, the more significant carboxylation activity for CO2 fixation depends on a constant supply of chemical energy in the form of ATP and NADPH generated from the light absorption by chlorophylls [16]. In rice plants inoculated with rhizobacteria, the increase in the net photosynthesis was associated with the increase in the chlorophyll content [48]. Similar results were observed in this study, where all rhizobacteria induced an increase in Chla, Chlb, Chla/Chlb ratio, and Chltotal. However, the estimation of total content of Chla, Chlb, and Chltotal per plant was higher for BRM-32113 due to the higher total leaf aeration induced by these rhizobacteria, suggesting that rhizobacteria increase net photosynthesis by inducing chlorophyll content increments, which in turn increase the efficiency of the light conversion into chemical energy to support the energy expenditure of the higher carboxylation activity for the CO2 fixation in the leaves.
The higher chlorophyll content (Chla and Chlb) induced by all rhizobacteria may have contributed to greater efficiency in light absorption, energy transfer, electron transfer, and greater control in the dissipation of thermal energy surplus. This can be demonstrated by chlorophyll-a fluorescence parameters, such as the increases in the productive photochemical efficiency of PSII (Fv′/Fm′) and an increase in potency activity of PSII (Fv/Fo), as observed in pepper plants inoculated with Bacillus spp. [49]. In this study, the increments in electron transfer rate (ETR) and photochemical dissipation coefficients (qP) indicate a greater light utilization capacity and higher efficiency of photosyntheses I and II, which may have contributed to lower generation and dissipation of non-photochemical quenching (qN).
Lucas et al. [50] report that the increment in Fv′/Fm′ is associated to the D1 protein integrity, which is responsible for the electron transfer from the water to the Chla associated with PSII. The increase in ETR may indicate a more exceptional ability of plastoquinones to perform oxidation-reduction reactions [51], while lower values of qN point to the best control of photosynthetic pigment photoprotection mechanisms [52]. This work shows that açaí palm seedlings inoculated with rhizobacteria increased qP and ETR, which influenced the decrease in qN.
The benefits induced by all rhizobacteria in promoting growth, gas exchange, and chlorophyll-a fluorescence are likely to be associated with the best nutritional state of açaí palm seedlings, as demonstrated by the accumulation of nitrogen (N), phosphorus (P), and potassium (K) in the leaves. Similar results were observed in rice plants inoculated with rhizobacteria, where the higher accumulation of N, P, and K positively influenced the gas exchange and increased the photosynthetic performance, contributing to the higher growth and biomass accumulation [17].
Nitrogen is one of the most important elements in plant nutrition since it is used in the synthesis of the cellular compounds, such as chlorophylls [53]. In the present study, N accumulation influenced positively the higher chlorophyll content in the leaves of the seedlings inoculated with the rhizobacteria, which may be attributed to the N-fixing capacity of UFRA-35, UFRA-38, and UFRA-58 rhizobacteria, or other mechanisms involved in the higher N absorption efficiency by other rhizobacteria. Similar results were observed in oil palm seedlings inoculated with rhizobacteria [54].
Phosphorus (P) is the primary nutrient that influences plant growth as it is the essential element in the protein composition, nucleic acids, membranes, and energy molecules, such as ATP, GTP, and NADPH [55]. One of the rhizobacteria action mechanisms to promote growth is phosphate solubilization [56]. In this experiment, the higher phosphorus content in the leaves contributed to the growth and biomass accumulation in the seedlings inoculated with rhizobacteria. The UFRA-35 and BRM-32111 were positive for phosphate solubilization, while phosphate solubilization may have occurred in the other rhizobacteria through mechanisms involving the secretion of organic and inorganic acids, as observed in oil palm inoculated with rhizobacteria [14].
Potassium (K) is correlated with enzymatic activation, carbohydrate transport, and stomatal opening regulation [57]. The PGPRs may benefit plants through their ability of solubilizing potassium and make it available to the plant, therefore contributing to a higher growth [8, 58], as observed in the present study, where inoculation of rhizobacteria induced K accumulation in leaves and positively influenced the growth of açaí palm seedlings. In rice plants inoculated with rhizobacteria, the higher potassium absorption efficiency contributed to higher roots and the aerial part growth [59].
Rhizobacteria accelerate growth, increase photosynthetic efficiency, and induce leaf nutrient accumulation in açaí seedlings, contributing to obtaining quality seedlings for the field in less time (5 months). The BRM-32113 isolate presents a top highlight in all parameters evaluated. Rhizobacteria inoculation can contribute to the sustainable management of the production of açaí palm seedlings in nurseries.
Acknowledgments
The authors thank the Coordination for Higher Education Staff Development (CAPES) for granting fellowships, the Plant Protection Laboratory (PPL) for its logistical support, and Dr. Alessandra Jackeline G. Moraes and Msc. Gleiciane Rodrigues dos Santos of the Universidade Federal Rural da Amazônia for the rhizobacteria molecular identification.
Abbreviations
- UFRA-35
Burkholderia sp.
- UFRA-38
Bacillus safensis
- UFRA-58
Burkholderia sp.
- UFRA-61
Pseudomonas fluorescens
- UFRA-92
Bacillus subtilis
- BRM-32111
Pseudomonas fluorescens
- BRM-32113
Burkholderia pyrrocinia
- N
nitrogen
- P
phosphorus
- K
potassium
- Ca
calcium
- Mg
magnesium
- A
CO2 net assimilation rate
- gs
stomatal conductance to water vapor
- E
transpiration rate
- Ci
CO2 intercellular concentration
- A/E
water use instantaneous efficiency
- Chla
chlorophyll-a
- Chlb
chlorophyll-b
- Chla + b
total chlorophyll
- Chla/Chlb
ratio between Chla and Chlb
- Fo
initial fluorescence
- Fm
maximum fluorescence
- Fv/Fo
PSII potential activity
- Fv′/Fm′
PSII effective photochemical efficiency
- qP
photochemical extinction coefficients
- qN
non-photochemical extinction coefficients
- ETR
electron transfer rate
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamaguchi KKDL, Pereira LFR, Lamarão CV, Lima ES, da Veiga-Junior VF. Amazon acai: chemistry and biological activities: a review. Food Chem. 2015;179:137–151. doi: 10.1016/j.foodchem.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Cantu-Jungles TM, Iacomini M, Cipriani TR, Cordeiro LMC. Extraction and characterization of pectins from primary cell walls of edible açaí (Euterpe oleraceae) berries, fruits of a monocotyledon palm. Carbohydr Polym. 2017;158:37–43. doi: 10.1016/j.carbpol.2016.11.090. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira MDSP, Neto JTDF. Cultivar BRS-Pará: Açaizeiro para Produção de Frutos em Terra Firme. Embrapa Comun Técnico. 2004;114(1):1–3. [Google Scholar]
- 4.Costa MR, De Oliveira MDSP, Ohaze MMM. Divergência Genética No Açaizeiro Com Base Em Marcadores Rapd. Rev Ciênc Agrár. 2004;41:89–95. [Google Scholar]
- 5.Om AC, Ghazali AHA, Keng CL, Ishak Z. Microbial inoculation improves growth of oil palm plants (Elaeis guineensis Jacq.) Trop Life Sci Res. 2009;20(2):71–77. [PMC free article] [PubMed] [Google Scholar]
- 6.George P, Gupta A, Gopal M, Thomas L, Thomas GV. Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.) World J Microbiol Biotechnol. 2013;29(1):109–117. doi: 10.1007/s11274-012-1163-6. [DOI] [PubMed] [Google Scholar]
- 7.Haldar S, Sengupta S. Plant-microbe cross-talk in the rhizosphere: insight and biotechnological potential. Open Microbiol J. 2015;9(iii):1–7. doi: 10.2174/1874285801509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary SR, Sindhu SS. Growth stimulation of clusterbean (Cyamopsis tetragonoloba) by coinoculation with rhizosphere bacteria and Rhizobium. Legum Res - An Int J. 2016;39(OF):1003–1012. doi: 10.18805/lr.v0iOF.8605. [DOI] [Google Scholar]
- 9.Van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol. 2007;119(3):243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- 10.Astriani M, Mubarik NR, Tjahjoleksono A. Selection of bacteria producing indole-3-acetic acid and its application on oil palm seedlings (Elaeis guineensis Jacq.) Malays J Microbiol. 2016;12(2):147–154. doi: 10.21161/mjm.74615. [DOI] [Google Scholar]
- 11.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo) 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslantaş R, Çakmakçi R, Şahin F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic (Amsterdam) 2007;111(4):371–377. doi: 10.1016/j.scienta.2006.12.016. [DOI] [Google Scholar]
- 13.Asari Shashidar, Tarkowská Danuše, Rolčík Jakub, Novák Ondřej, Palmero David Velázquez, Bejai Sarosh, Meijer Johan. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta. 2016;245(1):15–30. doi: 10.1007/s00425-016-2580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir HG, Shamsuddin ZH, Halimi MS, Marziah M, Ramlan MF. Enhancement in nutrient accumulation and growth of oil palm seedlings caused by PGPR under field nursery conditions. Commun Soil Sci Plant Anal. 2005;36(15–16):2059–2066. doi: 10.1080/00103620500194270. [DOI] [Google Scholar]
- 15.Noor Ai’shah O, Tharek M, Keyeo F, et al. Influence of indole-3-acetic acid (IAA) produced by diazotrophic bacteria on root development and growth of in vitro oil palm shoots (Elaeis guineensis Jacq.) J Oil Palm Res. 2013;25(APR):100–107. [Google Scholar]
- 16.Zhang K, Liu Z, Shan X, et al. Physiological properties and chlorophyll biosynthesis in a Pak-choi (Brassica rapa L. ssp. chinensis) yellow leaf mutant, pylm. Acta Physiol Plant. 2017;39(1):22. doi: 10.1007/s11738-016-2321-5. [DOI] [Google Scholar]
- 17.Nascente AS, de Filippi MCC, Lanna AC, de Souza ACA, da Silva Lobo VL, da Silva GB. Biomass, gas exchange, and nutrient contents in upland rice plants affected by application forms of microorganism growth promoters. Environ Sci Pollut Res. 2016;24(3):2956–2965. doi: 10.1007/s11356-016-8013-2. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell K, Johnson GN. Chlorophyll fluorescence--a practical guide. J Exp Bot. 2000;51(345):659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 19.Silva Cravo M, Viégas IJM, Brasil EC. Recomendações de Adubação e Calagem Para o Estado Do Pará. Bélem: EMBRAPA Amazonia Oriental; 2007. [Google Scholar]
- 20.Klar AE, Villa Nova NA, Marcos ZZ, Cervéllini A. Determinação da umidade do solo pelo método das pesagens. An da Esc Super Agric Luiz Queiroz. 1966;23:15–30. doi: 10.1590/S0071-12761966000100003. [DOI] [Google Scholar]
- 21.Filippi MCC, da Silva GB, Silva-Lobo VL, Côrtes MVCB, Moraes AJG, Prabhu AS. Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biol Control. 2011;58(2):160–166. doi: 10.1016/j.biocontrol.2011.04.016. [DOI] [Google Scholar]
- 22.Martins BEM (2015) Caracterização morfológica, bioquímica e molecular de isolados bacterianos antagonistas a Magnaporthe oryzae. 80 f (Doctoral dissertation, Dissertação, Universidade Federal de Goiás).
- 23.Kado CI, Heskett MG. Selective Media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 1970;60(6):969. doi: 10.1094/Phyto-60-969. [DOI] [PubMed] [Google Scholar]
- 24.Xiang L, de Boer SH. Selection of polymerase chain reaction primers from an RNA intergenic spacer region for specific detection of <i xmlns= Phytopathology. 1995;85:837–842. doi: 10.1094/Phyto-85-837. [DOI] [Google Scholar]
- 25.Silvestre Walter V. D., Pinheiro Hugo A., Souza Rodrigo O. R. de M., Palheta Lenilson F. Morphological and physiological responses of açaí seedlings subjected to different watering regimes. Revista Brasileira de Engenharia Agrícola e Ambiental. 2016;20(4):364–371. doi: 10.1590/1807-1929/agriambi.v20n4p364-371. [DOI] [Google Scholar]
- 26.Oxborough K, Baker NR. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components - calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth Res. 1997;54(2):135–142. doi: 10.1023/A:1005936823310. [DOI] [Google Scholar]
- 27.De Jesus SV, Marenco RA. O SPAD-502 como alternativa para a determinação dos teores de clorofila em espécies frutíferas. Acta Amaz. 2008;38(4):815–818. doi: 10.1590/S0044-59672008000400029. [DOI] [Google Scholar]
- 28.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenergetics. 1989;975(3):384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- 29.Silva F. Manual de Análises Químicas de Solos, Plantas e Fertilizantes. Embrapa Informação Tecnológica: Brasília; 2009. [Google Scholar]
- 30.Benincasa MMP. Análise de Crescimento de Plantas: Noções Básicas. FUNEP: Jaboticabal; 1988. [Google Scholar]
- 31.Team RC (2017) R: A language and environment for statistical computing. R Found Stat Comput Vienna, Austria https://www.R-project.org. Accessed 3 Dec 2018
- 32.Rais A, Shakeel M, Hafeez FY, Hassan MN. Plant growth promoting rhizobacteria suppress blast disease caused by Pyricularia oryzae and increase grain yield of rice. BioControl. 2016;61(6):769–780. doi: 10.1007/s10526-016-9763-y. [DOI] [Google Scholar]
- 33.Gu A, Ozaktan H, Yolageldi L (2017) Rhizobacteria promoted growth and yield of tomato plants and control of Fusarium oxysporum f. sp. Acta Hortic:345–352. 10.17660/ActaHortic.2017.1164.44
- 34.Prasad AA, Babu S. In growth promotion of groundnut ( Arachis hypogea L.) An Acad Bras Cienc. 2017;89(2):1027–1040. doi: 10.1590/0001-3765201720160617. [DOI] [PubMed] [Google Scholar]
- 35.De Sousa TP, Carlos A, De Souza A, et al. Bioagents and silicon promoting fast early upland rice growth. Environ Sci Pollut Res. 2018;25:3657–3668. doi: 10.1007/s11356-017-0753-0. [DOI] [PubMed] [Google Scholar]
- 36.Bloemberg GV, Lugtenberg BJJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol. 2001;4:343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 37.Shameer S, Prasad TNVKV. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018;84(3):603–615. doi: 10.1007/s10725-017-0365-1. [DOI] [Google Scholar]
- 38.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57(1):233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad F, Ahmad I, Khan MS. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol. 2005;29:29–34. [Google Scholar]
- 40.Dodd IC, Zinovkina NY, Safronova VI, Belimov AA. Rhizobacterial mediation of plant hormone status. Ann Appl Biol. 2010;157(3):361–379. doi: 10.1111/j.1744-7348.2010.00439.x. [DOI] [Google Scholar]
- 41.Kang SM, Khan AL, You YH, Kim JG, Kamran M, Lee IJ. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J Microbiol Biotechnol. 2014;24(1):106–112. doi: 10.4014/jmb.1304.04015. [DOI] [PubMed] [Google Scholar]
- 42.Kang SM, Khan AL, Hamayun M, et al. Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones. J Microbiol. 2012;50(6):902–909. doi: 10.1007/s12275-012-2273-4. [DOI] [PubMed] [Google Scholar]
- 43.Silva PA, Cosme VS, Rodrigues KCB, et al. Drought tolerance in two oil palm hybrids as related to adjustments in carbon metabolism and vegetative growth. Acta Physiol Plant. 2017;39(2):58. doi: 10.1007/s11738-017-2354-4. [DOI] [Google Scholar]
- 44.Flexas J, Barbour MM, Brendel O, et al. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci. 2012;193–194:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Fan X, Hu H, Huang G, Huang F, Li Y, Palta J. Soil inoculation with Burkholderia sp. LD-11 has positive effect on water-use efficiency in inbred lines of maize. Plant Soil. 2015;390(1–2):337–349. doi: 10.1007/s11104-015-2410-z. [DOI] [Google Scholar]
- 46.Doni F, Isahak A, Che Mohd Zain CR, Wan Yusoff WM. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express. 2014;4(1):45. doi: 10.1186/s13568-014-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Y, Lou K, Li C. Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth Res. 2010;105(1):5–13. doi: 10.1007/s11120-010-9547-7. [DOI] [PubMed] [Google Scholar]
- 48.Alam S, Cui Z-J, Yamagishi T, Ishii R. Grain yield and related physiological characteristics of rice plants (Oryza sativa L.) inoculated with free-living rhizobacteria. Plant Prod Sci. 2001;4(2):126–130. doi: 10.1626/pps.4.126. [DOI] [Google Scholar]
- 49.Samaniego-Gámez BY, Garruña R, Tun-Suárez JM, Kantun-Can J, Reyes-Ramírez A, Cervantes-Díaz L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil J Agric Res. 2016;76(4):409–416. doi: 10.4067/S0718-58392016000400003. [DOI] [Google Scholar]
- 50.Lucas JA, García-Cristobal J, Bonilla A, Ramos B, Gutierrez-Mañero J. Beneficial rhizobacteria from rice rhizosphere confers high protection against biotic and abiotic stress inducing systemic resistance in rice seedlings. Plant Physiol Biochem. 2014;82:44–53. doi: 10.1016/j.plaphy.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Suresh K, Nagamani C, Kantha DL, Kumar MK. Changes in photosynthetic activity in five common hybrids of oil palm (Elaeis guineensis Jacq.) seedlings under water deficit. Photosynthetica. 2012;50(4):549–556. doi: 10.1007/s11099-012-0062-2. [DOI] [Google Scholar]
- 52.Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42(1):313–349. doi: 10.1146/annurev.pp.42.060191.001525. [DOI] [Google Scholar]
- 53.Wolff WM, Floss EL. Correlação entre teores de nitrogênio e de clorofila na folha com o rendimento de grãos de aveia branca. Ciência Rural. 2008;38(6):1510–1515. doi: 10.1590/S0103-84782008000600003. [DOI] [Google Scholar]
- 54.Amir HG, Shamsuddin ZH, Halimi MS, Ramlan MF, Marziah M. N2 fixation, nutrient accumulation and plant growth promotion by rhizobacteria in association with oil palm seedlings. Pak J Biol Sci. 2003;6(14):1269–1272. doi: 10.3923/pjbs.2003.1269.1272. [DOI] [Google Scholar]
- 55.Acevedo E, Galindo-Castañeda T, Prada F, Navia M, Romero HM. Phosphate-solubilizing microorganisms associated with the rhizosphere of oil palm (Elaeis guineensis Jacq.) in Colombia. Appl Soil Ecol. 2014;80(August):26–33. doi: 10.1016/j.apsoil.2014.03.011. [DOI] [Google Scholar]
- 56.Velásquez E, Rodríguez-Barrueco C. In: First International Meeting on Microbial Phosphate Solubilization. Velázquez E, Rodríguez-Barrueco C, editors. Dordrecht: Springer Netherlands; 2007. [Google Scholar]
- 57.Pan Y, Lu Z, Lu J, Li X, Cong R, Ren T. Effects of low sink demand on leaf photosynthesis under potassium deficiency. Plant Physiol Biochem. 2017;113:110–121. doi: 10.1016/j.plaphy.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Hu X, Chen J, Guo J. Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol. 2006;22(9):983–990. doi: 10.1007/s11274-006-9144-2. [DOI] [Google Scholar]
- 59.Duarah I, Deka M, Saikia N, Deka Boruah HP. Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech. 2011;1(4):227–238. doi: 10.1007/s13205-011-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]