Abstract
Proteasome inhibitor bortezomib is one of the most effective drugs currently available for the treatment of multiple myeloma (MM). However, the intrinsic and acquired resistance to bortezomib can limit its effectiveness. The activation of heat shock response has been characterized as a potential resistance mechanism protecting MM cells from bortezomib-induced cell death. In this study, in response to bortezomib therapy, we discovered that HSP70 is one of the most substantially upregulated heat shock proteins. In order to further explore approaches to sensitizing bortezomib-based treatment for MM, we investigated whether targeting HSP70 using a specific inhibitor VER-155008 combined with bortezomib could overcome the acquired resistance in MM. We found that HSP70 inhibitor VER-155008 alone significantly decreased MM cell viability. Moreover, the combination of VER-155008 and bortezomib synergistically induced MM cell apoptosis markedly in vitro. Notably, the combined treatment was found to increase the cleavage of PARP, an early marker of chemotherapy-induced apoptosis. Importantly, the reduction of anti-apoptotic Bcl-2 family member Bcl-2, Bcl-xL, and Mcl-1 and the induction of pro-apoptotic Bcl-2 family member BH3-only protein NOXA and Bim were confirmed to be tightly associated with the synergism. Finally, the ER stress marker CHOP (CCAAT-enhancer binding protein homologous protein), which can cause transcriptional activation of genes involved in cell apoptosis, was markedly induced by both VER-155008 and bortezomib. Taken together, our finding of a strong synergistic interaction between VER-155008 and bortezomib may support for combination therapy in MM patients in the future.
Electronic supplementary material
The online version of this article (10.1007/s12192-020-01078-0) contains supplementary material, which is available to authorized users.
Keywords: Bortezomib, Multiple myeloma, HSP70, VER-155008, Synergism, Apoptosis
Introduction
Multiple myeloma (MM) is a progressive malignant plasma cell disorder that accounts for 1% of all cancers and 10% to 15% of all hematologic malignancies (Kyle and Rajkumar 2008). In the past decades, despite the emergence of novel therapeutics such as proteasome inhibitors (bortezomib, carfilzomib, ixazomib), immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide), and autologous stem cell transplant which have partially improved the outcomes, MM remains an incurable malignancy with most patients experiencing relapse and refractory (Cavo 2006; Morgan et al. 2012; Palumbo et al. 2008). The major reason for this unsatisfactory therapeutic effect may be related to the resistance of chemotherapy drugs, in addition to tumor cells escape of immune surveillance. Currently, combinational therapy remains fundamental to an effective treatment against MM with minimal side effects in patients.
Bortezomib (PS-341) is the first therapeutic proteasome inhibitor recommended for the first-line treatment in refractory, relapsed, and newly diagnosed MM (Mulligan et al. 2007). Treatment of multiple myeloma with bortezomib has dramatically improved the survival of MM patients. However, some MM patients do not show beneficial improvement from this treatment and those who respond eventually acquire resistance to the drug (Oerlemans et al. 2008). In this regard, the activation of the unfolded protein response (UPR) has been correlated with the development of resistance to apoptosis initiated by proteasome inhibition (Vincenz et al. 2013). Heat shock proteins (HSPs) are molecular chaperones with a central role in protein folding and cellular protein homeostasis. The bortezomib-induced UPR leads to the induction of HSPs, which are a group of ubiquitously expressed chaperone proteins that ensure correct folding and prevent aggregation of specific target proteins. These chaperone proteins coordinate as well as rescue endoplasmic reticulum (ER) stress and are thought to contribute toward bortezomib resistance and myeloma growth and survival (Roue et al. 2011; Vincenz et al. 2013; Yoo et al. 2014; Yuan et al. 2009).
In this study, we found that HSP70 is one of the most significantly upregulated HSPs by bortezomib in MM cells. Given the potential protective role of HSP70 in MM, we further investigated whether the combination of bortezomib and HSP70 inhibitor VER-155008 could synergistically induce MM cell apoptosis and the underlying molecular mechanisms.
Materials and methods
Cell lines and reagents
MM cell lines RPMI 8226, OPM2, and MM.1S were purchased from American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium (Basal Media, Shanghai, China) supplemented with 10% fetal bovine serum (Biological Industries, Israel), 2-mM L-glutamine (Beyotime, China) and 1% penicillin and streptomycin (Genview, USA). MM cells were maintained in 37 °C humidified air with 5% CO2.
VER-155008 was purchased from Selleck Chemicals Company and dissolved in dimethyl sulfoxide (DMSO) at 20-μmol/L stock solution for in vitro studies. Bortezomib bought from LC Laboratories was reconstituted in DMSO at a stock concentration of 10 mmol/L and stored at − 20 °C until use. The concentration of DMSO never exceeded 0.1%. The two reagents working dilutions were always freshly attenuation in the medium.
Cell viability assay
Cell viability assay was determined in 96-well plate with Cell Counting Kit-8 (7Sea Pharmatech Co., Ltd., Shanghai, China). Briefly, MM cells were seeded in 96-well plates (1 × 104 cells/well) and treated for indicated times with different doses of drugs 3 h before the end of treatment. Ten microliter (1:10 dilution) of CCK-8 solution was added to each well. The absorbance of each sample was then measured in a 96-well plate using an enzyme-linked immunosorbent assay reader (Bio-Tek, China) at 450 nm as the reference filters to determine the cell viability. The cell viability rate, compared to the control (untreated cells), was calculated using the following equation (Bae et al. 2007; Yuan et al. 2009).
Cell Viability (%) = 100 × Absorbance of treated group/Absorbance of untreated group
Analysis of apoptosis by flow cytometry
MM.1S cells (2 × 1025/mL) were cultured for 24 h in the presence of the indicated doses of bortezomib and/or VER-155008. The cell apoptosis was then measured with annexin V-FITC/7-AAD detection kit (BioLegend, USA), according to the manufacturer’s instructions. All treated MM cells were washed twice with cold PBS before stained with 5 μL of 7-AAD and 5-μL annexin V in 100 μL of binding buffer. Then the cells were incubated at 4 °C for 15 min before detection. Finally, cells were diluted in 400 μL of binding buffer and immediately analyzed using FACS Beckman Coulter CytoFLEX flow cytometer (Beckman Coulter, American), and the results were analyzed using FlowJo software version 7.6.2.
Western blotting
Western blotting analysis was conducted as previously described (Hu et al. 2010). Briefly, cells were lysed using buffer solution consisting of 50-mM Tris (pH 7.4), 150-mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS and with additional sodium orthovanadate, sodium fluoride, EDTA, leupeptin, and other inhibitors (Beyotime, Shanghai, China). Protein extracts were loaded onto an 8–15% SDS-PAGE and transferred to 0.45-μm polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Billerica, USA). The blots were incubated with antibodies against BIK, Bim, Mcl-1, and PARP (Cell Signaling Technology, Beverly, MA, USA); Bcl-2, Bcl-xL, NOXA, and GRP-78 (Proteintech, Wuhan, China); and CHOP (Santa Cruz Biotech, Santa Cruz, CA, USA). Following incubation with horseradish peroxidase (HRP)-linked secondary antibody (Proteintech, Wuhan, China), the signal was detected by the chemiluminescence phototope-HRP Substrate (Millipore Corporation, Billerica, USA) on the instrument of MiniChemi 610 (Beijing Sage, China). The relative densities of proteins were quantified with ImageJ software (version 2x) and normalized against β-actin (Proteintech, Wuhan, China) as a loading control.
Quantitative real-time PCR (qRT-PCR)
RNA extraction was conducted using an Ultrapure RNA Kit (CWBio, Beijing, China). Total RNA was reverse transcribed using a HiFiScript cDNA Synthesis Kit (CWBio, Beijing, China) according to the manufacturer’s instructions. Quantitative real-time PCR was performed on a Bio-Rad CFX Connect Real-Time System instrument using gene-specific primers, which were synthesized by Invitrogen (Shanghai, China). The PCR cycling program consisted of incubation for enzyme activation at 95 °C for 20 min, followed by melting at 95 °C for 5 s and annealing at 60 °C for 30 s, for 40 cycles. The expression levels of HSP70, HSP90, GRP-78, and HSP27 were normalized to the internal control β-actin reference to obtain the relative threshold cycle (ΔCT). The relative expression levels were calculated by the comparative Ct (ΔΔCt) method, and relative expression folds (2−△△Ct) were calculated. The sequences of primers are shown in Table 1.
Table 1.
Primer sequences used for quantitative real-time PCR
| Gene | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| β-actin | ATCGTGCGTGACATTAAGGAGAAG | AGGAAGGAAGGCTGGAAGAGTG |
| GRP-78 | AGGAGGAGGACAAGAAGGAGGAC | CAGGAGTGAAGGCGACATAGGAC |
| HSP70 | 5′-AGGGTCAGATCCACGACATC-3 | 5′-CGTCTGGGTTGATGGATAGG-3 |
| HSP27 | CACGAGGAGCGGCAGGAC | GGACAGGGAGGAGGAAACTTGG |
| HSP90 | CCCCCGGATCCATGTCCACCGAGACCTTTGG | CCCCCCGAATTCTTAGTCAACCTCCTCCATGGAG |
| CHOP | TGCTTCTCTGGCTTGGCTGAC | CCGTTTCCTGGTTCTCCCTTGG |
Statistical analysis
All statistical analyses were determined using GraphPad Prism version 7.0 software (GraphPad Software Inc., USA). The differences between groups were tested by using the two-tailed log-rank test. Differences between two groups were determined by Mann-Whitney t test. The results are expressed as the mean ± SD. The criterion for statistical significance was taken as p < 0.05. To analyze synergism, the combination index (CI) for each treatment was determined using the CompuSyn software (ComboSyn Inc.) developed by Chou (Chou 2010).
Results
Bortezomib markedly induces HSP70 expression in MM cells
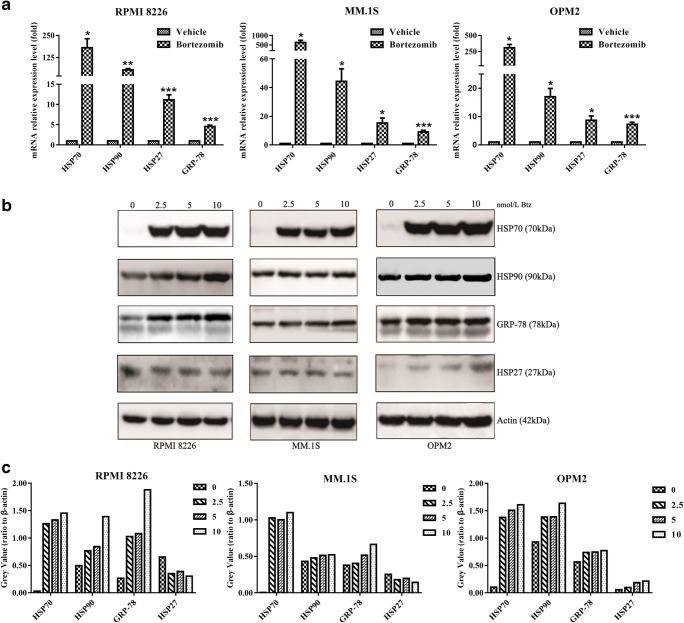
To evaluate the expression of HSPs in bortezomib-treated MM cells, we treated RPMI 8226, OPM2, and MM.1S cells with 5-nM bortezomib for 24 h, and then qRT-PCR was used to detect the expression of HSP70, HSP90, GRP-78, and HSP27 on the mRNA level. As shown in Fig. 1a, the four molecules, as mentioned, were all increased in the above cell lines. Clearly, HSP70 was the most one upregulated by bortezomib. Moreover, we confirmed bortezomib-induced HSP70, HSP90, GRP-78, and HSP27 expression on the protein level by western blotting. As shown in Fig. 1b and c, the expression of HSP70 was dose-dependently induced by bortezomib in all tested cell lines. In addition, HSP90, GRP-78, and HSP27 were also upregulated, but not as obviously as HSP70. These results showed that HSP70 was one of the major chaperones induced by bortezomib treatment.
Fig. 1.
Bortezomib markedly induces HSP70 expression in multiple myeloma. a qRT-PCR analysis of the expression HSP70, HSP90, GRP-78, and HSP27 in bortezomib-treated RPMI 8226, MM.1S, and OPM2 cell lines. Cells were cultured with vehicle or 5-nmol/L bortezomib for 24 h and then harvested for RNA extraction, cDNA synthesis, and PCR analysis. The vertical axis indicates mRNA expression levels (*p < 0.05, **p < 0.01, ***p < 0.001). b Bortezomib activates HSPS, markedly induces HSP70 expression in MM cell lines. Cells were cultured with vehicle or 2.5-, 5-, and 10-nmol/L bortezomib for 24 h and then harvested and probed with the indicated antibodies by western blotting analysis. c Grayscale of the detected proteins on the above were analyzed by ImageJ software. These experiments were repeated in triplicate
Targeting HSP70 with VER-155008 inhibits MM cell viability
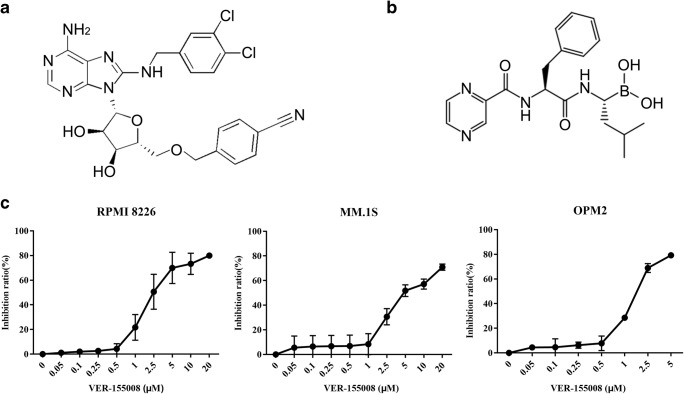
Considering that HSP70 is a master player in protein homeostasis and has an important role in the acquired drug resistance (Mitsiades et al. 2002; Nimmanapalli et al. 2008), we investigated whether targeting HSP70 could exhibit cytotoxicity against multiple myeloma cells. We treated RPMI 8226, MM.1S, and OPM2 cells with different doses of VER-155008 in 96-well plates for 48 h; then cell viability was analyzed by CCK-8 assay. As represented in Fig. 2c, the corresponding IC50 values were 3.04 ± 1.55, 6.48 ± 1.65, and 1.74 ± 0.14 μM, respectively, for RPMI 8226, MM.1S, and OPM2 cells. The results indicated that inhibition of HSP70 with VER-155008 can decrease cell viability in MM cell lines in a dose-dependent manner.
Fig. 2.
HSP70 inhibitor VER-155008 can inhibit cell viability in multiple myeloma cell lines. a The chemical structure of VER-155008. b The chemical structure of bortezomib. c RPMI 8226 and MM.1S cells were treated with different doses of VER-155008 between 0 and 20 μM and OPM2 0 and 5 μM. Cell viability was evaluated at 48-h post-treatments by the CCK-8 kit. The half-maximum inhibitory concentration 50 values (IC50 values) were calculated by nonlinear regression in GraphPad Prism version 7.0 software. These experiments were repeated in triplicate
Bortezomib and VER-155008 synergistically induce MM cell death
Given that bortezomib can significantly induce HSP70 expression in MM cells, we further evaluated whether combination of bortezomib with HSP70 inhibitor could show synergistic antitumor activity. To achieve this aim, MM cells were seeded at 2 × 104 cells/well in 96-well plates and treated with a combination of VER-155008 (0, 1, 2.5, 5 μM) and bortezomib (0, 2.5, 5 nM) at a constant ratio for 24 h followed by CCK-8 analysis to determine cell viability. The combination index (CI) was calculated using CompuSyn software (Chou 2010). As shown in Table 2, the CIs for the two drugs were in the range varying between 0.60749 and 1.08376. Almost all the combinations show synergistic effect, except the group of 5-nmol/L bortezomib and 5-μmol/L VER-155008. Similar results were also observed for 48-h combination treatment (Supplementary Table 1).
Table 2.
CI analysis of VER-155008 combined with bortezomib at a nonconstant ratio in MM.1S cells
| Bortezomib (nmol/L) | VER-155008 (μmol/L) | Effect | CI | Description |
|---|---|---|---|---|
| 2.5 | 1 | 0.224869 | 0.82237 | Synergism |
| 5 | 1 | 0.404416 | 0.60749 | Synergism |
| 2.5 | 2.5 | 0.439279 | 0.88300 | Synergism |
| 5 | 2.5 | 0.553167 | 0.75474 | Synergism |
| 2.5 | 5 | 0.673446 | 0.94774 | Synergism |
| 5 | 5 | 0.642069 | 1.08376 | Antagonism |
Analysis was conducted using the CompuSyn software (ComboSyn, Inc.). Descriptions are based on CI values and the recommendations of CompuSyn: < 1.0, synergism; > 1.0, antagonism. The enzyme-labeling instrument 450 measured the numbers in column effect after 24 h in MM.1S cells with each indicated treatment
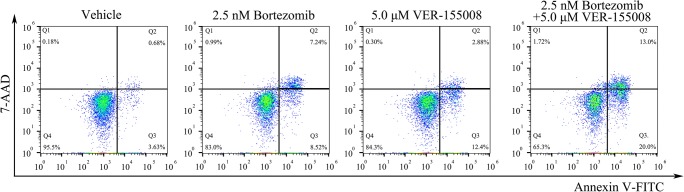
To further confirm the synergism of the combination of bortezomib with VER-155008, we analyzed the effects on cell apoptosis. MM.1S cells were respectively exposed to a lower dose of bortezomib (2.5 nM) and VER-155008 (2.5 μM) for 48 h; then cell apoptosis was measured by flow cytometry. As shown in Fig. 3, the combination of bortezomib with VER-155008 significantly increased the number of apoptotic cells, compared with the single-compound treated group in MM.1S cells. Collectively, these results demonstrated that targeting HSP70 with VER-155008 could synergistically enhance bortezomib-induced MM cell death.
Fig. 3.
Bortezomib in combination with VER-155008 induces apoptosis in MM.1S cells. MM.1S cells were treated with vehicle, bortezomib, VER-155008, and the combination at the indicated concentrations for 48 h, followed by annexin V-FITC/7-AAD staining and flow cytometry analysis. Percentages of cells were shown within each quadrant
The combination of bortezomib and VER-155008 increases the cleavage of PARP, an apoptotic marker
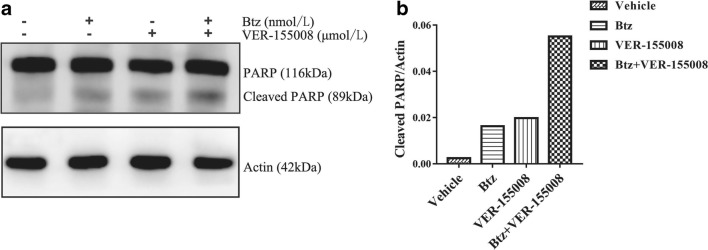
To characterize the mechanisms involved in bortezomib- and VER-155008-mediated antitumor synergism in MM, we detected PARP proteins by western blotting in MM.1S cells. As a final event leading to cell death, 2.5-nM bortezomib and 2.5-μM VER-155008 interacted for 48 h can induce apoptosis, as demonstrated by an increase of the cleaved PARP, compared with monotherapy or vehicle (Fig. 4). This indicated that the extrinsic and intrinsic pathways of apoptosis might participate in the synergy effect.
Fig. 4.
Bortezomib in combination with VER-155008 induces and the cleavage of PARP in MM.1S cells. MM.1S cells were treated with vehicle, bortezomib (2.5 nmol/L), VER-155008 (2.5 μmol/L), and the combination therapy for 48 h. Whole cell lysates were immunoblotted with the indicated antibodies. a Western blotting analysis of the formation of the cleaved PARP by bortezomib and VER-155008 treatment. b The grayscale analysis of the cleaved PARP blots by ImageJ software. These experiments were repeated in triplicate
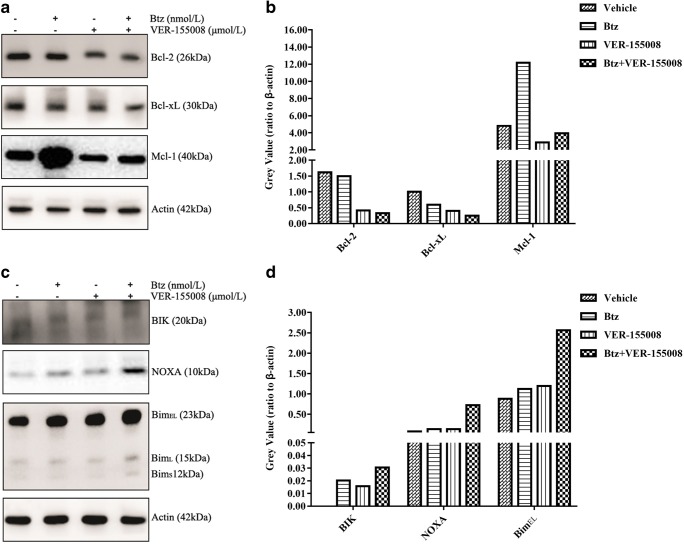
Combinational treatment with bortezomib and VER-155008 alters the balance of Bcl-2 family proteins
To further clarify the mechanisms underlying the combinational treatment, we investigated the changes of Bcl-2 family. Bcl-2 family proteins are the key mediators of the apoptotic response to targeted anticancer therapeutics, which regulate apoptosis via selective interactions between its pro- and anti-apoptotic members (Hata et al. 2015). As shown in Fig. 5a and b, the combination of VER-155008 and bortezomib significantly decreased the expression of Bcl-2 and Bcl-xL, in contrast to VER-155008 or bortezomib treatment alone. However, focusing on the changes of the anti-apoptotic Mcl-1, we found that the combination counteracted the increase induced by bortezomib. On the other hand, we confirmed that the combination induced a higher level of pro-apoptotic Bcl-2 proteins NOXA, BIK, and Bim than the single compound alone (Fig. 5c and d).
Fig. 5.
Changes of Bcl-2 family members induced by bortezomib and VER-155008 treatment. MM.1S cells were treated with vehicle, bortezomib (2.5 nmol/L), VER-155008 (2.5 μmol/L), and the combination therapy for 48 h. Whole cell lysates were immunoblotted with the indicated antibodies. a and c separately showed changes of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 and pro-apoptotic BIK, NOXA, and Bim, induced by bortezomib and VER-155008 treatment by western blotting analysis. b and d represented the grayscale analysis of protein levels by ImageJ software. The gray values were displayed on the vertical axis. These experiments were repeated in triplicate
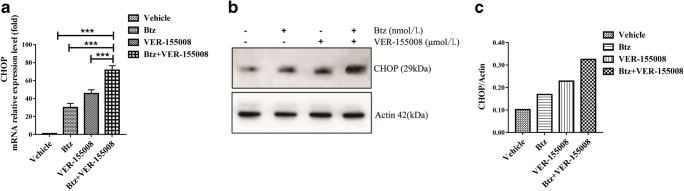
ER stress marker and transcription factor CHOP were induced by both bortezomib and VER-155008
The action of bortezomib was suggested to be related to the activation of ER stress signaling or UPR ((Obeng et al. 2006; Vincenz et al. 2013); Christianson MG and Lo DC. 2011). To answer why both compounds and the combination induced BH3-only pro-apoptotic proteins, we further investigated the changes of ER stress regulator CHOP in this process. As shown in Fig. 6, when cells were exposed to bortezomib and VER-155008 alone or the combination, the expressions of the transcription factor CHOP were significantly upregulated.
Fig. 6.
Changes of CHOP by bortezomib and VER-155008 combination treatment. a. qRT-PCR analysis of CHOP expression in MM.1S cells. Cells were cultured with vehicle, bortezomib (2.5 nmol/L), VER-155008 (2.5 μmol/L), and the combination therapy for 48 h and then harvested for RNA extraction, cDNA synthesis, and PCR analysis. The vertical axis indicates mRNA expression levels (***p < 0.001). b After cells were cultured with different treated groups mentioned above, CHOP expression was detected by western blotting. c Grayscale of the CHOP protein was analyzed by ImageJ software. These experiments were repeated in triplicate
Discussion
HSPs are chaperone proteins induced by a variety of different stresses. As a member of this family, HSP70 is highly expressed in a variety of cancers and correlated with tumor grade, metastasis, poor prognosis, and chemo-resistance (Behnsawy et al. 2013; Daugaard et al. 2007; Kumar et al. 2016; Mori et al. 2017; Yang et al. 2012; Yoshidomi et al. 2014). In the current study, we found that HSP70 was one of the most important HSPs induced by bortezomib and demonstrated that targeting HSP70 in combination with bortezomib could synergistically induce cytotoxicity, therefore improving the efficacy of MM treatment.
The proteasome inhibitor bortezomib is a promising novel drug for MM therapy. However, drug resistance still represents a major treatment obstacle as bortezomib can activate ER stress signaling or unfolded protein response to protect the cell from bortezomib-induced apoptosis (Leung-Hagesteijn et al. 2013; Rodvold et al. 2017). It is well known that proteasome acts to degrade and eliminate ubiquitinated proteins, whereas proteasome inhibition abrogates the degradation and induces cytoplasmic accumulation of the proteins. On the other hand, proteasome inhibition can also induce the formation of aggresome to degradate the accumulated misfolded/unfolded proteins in a histone deacetylase 6 (HDAC6)-dependent way. In this process, HDAC6 was also found to induce the expression of major cellular chaperones by triggering the dissociation of a repressive HDAC6/HSF1 (heat shock factor 1)/HSP90 complex (Boyault et al. 2007; Imai et al. 2019). Therefore, the intense activity of HSPs is required to balance the proteotoxic stress and cell death (Cavenagh et al. 2017; Eugênio et al. 2017; Khong and Spencer 2011; Shah et al. 2015). In this regard, the induction of HSPs is an adaptive response to further recruit the other proteostasis pathways (such as the aggresome or macroautophagy pathways) to either maintain proteostasis or induce apoptosis if ER stress stays persistent (Guang et al. 2019). Among these bortezomib-induced HSPs, HSP90 and HSP27 have been demonstrated to confer resistance to bortezomib (Cavenagh et al. 2017; Chauhan et al. 2003; Khong and Spencer 2011; Richardson et al. 2011; Roue et al. 2011). However, there is still no substantial evidence that clarify the role of HSP70 in this process. Therefore, we investigated the changes of HSP70 in bortezomib-treated MM cells, and HSP70 was discovered to be the most substantially upregulated of the HSPs by bortezomib, strongly suggesting a pivotal role of HSP70 in bortezomib-mediated MM cell death (Fig. 1). Given the essential role in protein folding, disaggregation, and degradation, targeting HSP70 has been suggested to be an attractive strategy for solid cancer therapy (Galluzzi et al. 2009; Kumar et al. 2016; Qi et al. 2013; Yerlikaya et al. 2010). More recently, a study in MM also showed that targeting HSP70 can inhibit tumor growth in a mouse xenograft model (Eugênio et al. 2017). Of note, we further demonstrated that the combination of bortezomib and HSP70 inhibitor VER-155008 synergistically induce MM cell death in vitro (Figs. 2 and 3).
As a master player in protein homeostasis, HSP70 is essential for normal cell functional by balancing protein synthesis, folding, trafficking, assembly, and degradation (Fernandez-Fernandez and Valpuesta 2018). HSP70 protects cancer cells from death by directly or indirectly interacting with the apoptosis signaling (Lanneau et al. 2008). High level of HSP70 has been shown to inhibit apoptosis and to prevent caspase activation in many different cellular models upon a variety of cellular stresses (Creagh et al. 2000; Lanneau et al. 2008; Sabirzhanov et al. 2012). Therefore, it is reasonable that selective inhibition of HSP70 or depletion of HSP70 under stress could trigger apoptosis. Accordingly, we observed that targeting HSP70 by VER-155008 in the presence of bortezomib significantly induced the formation of the cleaved PARP, which is an early apoptosis marker and the early initiator involved in promoting apoptosis by further cleaving diverse cellular protein substrates (Fig. 4). Although the cleavage of PARP plays a key role in mediating apoptosis, it is well accepted that apoptosis is governed by Bcl-2 family proteins, which include anti-apoptotic and pro-apoptotic members that interact with one another (Elkholi et al. 2011; Hata et al. 2015). The anti-apoptotic Bcl-2 family proteins, which include Bcl-2, Bcl-XL, Bcl-W, Mcl-1, and BFL-1/A1, share structural homology in the Bcl-2 homology (BH) 1, 2, 3, and 4 domains, whereas pro-apoptotic Bcl-2 members, Bim, PUMA, BAD, BID, BIK, BMF, HRK, and NOXA, only contain the BH3 domain. The dynamic balance that occurs between anti-apoptotic members and pro-apoptotic members determines whether the cell initiates apoptosis. In this regard, bortezomib has been shown to affect the expressions of both anti-apoptotic and pro-apoptotic Bcl-2 proteins. For instance, unwanted Mcl-1 was shown to be accumulated by bortezomib (Hu et al. 2012); pro-apoptotic BH3-only members Bim, BIK, and NOXA were induced by bortezomib (Gomez-Bougie et al. 2007; Herrant et al. 2004; Nikrad et al. 2005; Rizzatti et al. 2008). In the current study, we demonstrated that bortezomib alone can increase Mcl-1 expression. However, the combination with HSP70 inhibitor VER-155008 significantly attenuated the level of Mcl-1 (Fig. 5a and b). In this regard, Mcl-1 has been demonstrated to be the customer protein of HSP70 (Stankiewicz et al. 2009). Therefore, inhibition HSP70 could destabilize Mcl-1 and enhance caspase-mediated cleavage (Clohessy et al. 2004; Herrant et al. 2004). On the contrary, we observed that HSP70 inhibition by VER-155008 and the combination with bortezomib can induce a higher level of Bim, BIK, and NOXA in MM cells (Fig. 5c and d). Collectively, these results support that the enhanced apoptosis by combinational targeting HSP70 is tightly associated with the changes of Bcl-2 family proteins.
CHOP (CCAAT-enhancer binding protein homologous protein), also known as growth arrest and DNA damage-inducible protein 153 (GADD153), is a transcription factor specific for ER stress and an important regulator for the apoptotic pathway (Hu et al. 2018; Nishitoh 2012). Focusing on the mechanism underlying the upregulation of Bim, which is one of the highest expressed pro-apoptotic BH3-only proteins in MM cells, we further investigated the role of CHOP in the synergism and confirmed that bortezomib, VER-155008, and the combination could induce CHOP expression (Fig. 6). Importantly, previous studies have validated the transcriptional role of CHOP in upregulating the expression of Bim (Wali et al. 2014). Therefore, it is reasonable that CHOP-mediated upregulation of Bim plays an important role in the synergistic apoptosis by bortezomib and HSP70 inhibition.
There are limitations to this study. First, to find the essential client proteins of HSP70 and their functions during proteasome inhibition, it is important for a deeper understanding of the mechanisms underlying the combinational treatment with proteasome inhibitor and HSP70 inhibitor VER-155008. Second, in this study, the synergistical anti-MM effects of the combinational were only tested in vitro, and the efficacy of the combination still needs to be evaluated in vivo at preclinical stage in the near future.
In conclusion, we demonstrated in the current study that combinational targeting HSP70 by VER-155008 and bortezomib shows synergism in inducing MM cell death. The combination may offer a novel approach to overcome bortezomib resistance in MM.
Electronic supplementary material
(DOC 32 kb)
Authors’ contributions
JH and AH designed the experiments, analyzed and interpreted the experimental results, and wrote the manuscript. LH and YW contributed to conception and design; acquired, analyzed, and interpreted data; and wrote the manuscript. LL, NL, JB, FW, YY, YF, and RZ carried out western blotting and real-time PCR analysis. FL and PZ made substantial contributions to the conception and design of the study and revised the manuscript. All authors read and approved the manuscript.
Funding information
This research was supported by grants from the National Natural Science Foundation of China (No. 81570192 and 81372534), Shaanxi Provincial Key Research and Development Project (2018ZDXM-SF-039), Basic Research Program of Natural Science of Shaanxi Province (2017JM8160), and Science and Technology Action Plan Medical Research Project of Xi’an Science and Technology Bureau in 2018 (201805095YX3SF29).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingjuan Huang and Yanmeng Wang contributed equally to this work.
Contributor Information
Jinsong Hu, Email: jinsong.hu@xjtu.edu.cn.
Aili He, Email: heaili@xjtu.edu.cnc.
References
- Bae SN, Kim J, Lee YS, Kim JD, Kim MY, Park LO. Cytotoxic Effect of Zinc–Citrate Compound on Choriocarcinoma Cell Lines. Placenta. 2007;28(1):22–30. doi: 10.1016/j.placenta.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Behnsawy HM, Miyake H, Kusuda Y, Fujisawa M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urologic Oncology: Seminars and Original Investigations. 2013;31(6):843–848. doi: 10.1016/j.urolonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc’h C, Matthias P, Khochbin S. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21(17):2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh J, Oakervee H, Baetiong-Caguioa P, Davies F, Gharibo M, Rabin N, Kurman M, Novak B, Shiraishi N, Nakashima D, Akinaga S, Yong K. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br J Cancer. 2017;117(9):1295–1302. doi: 10.1038/bjc.2017.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavo M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia. 2006;20(8):1341–1352. doi: 10.1038/sj.leu.2404278. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Shringarpure R, Podar K, Ohtake Y, Hideshima T, Anderson KC. Blockade of Hsp27 overcomes Bortezomib/proteasome inhibitor PS-341resistance in lymphoma cells. Cancer Res. 2003;63(19):6174–6177. [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Clohessy JG, Zhuang J, Brady HJ. Characterisation of Mcl-1 cleavage during apoptosis of haematopoietic cells. Br J Haematol. 2004;125(5):655–665. doi: 10.1111/j.1365-2141.2004.04949.x. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Carmody RJ, Cotter TG. Heat Shock Protein 70 Inhibits Caspase-Dependent and -Independent Apoptosis in Jurkat T Cells. Exp Cell Res. 2000;257(1):58–66. doi: 10.1006/excr.2000.4856. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Elkholi R, Floros KV, Chipuk JE. The Role of BH3-Only Proteins in Tumor Cell Development, Signaling, and Treatment. Genes Cancer. 2011;2(5):523–537. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugênio AIP, Fook-Alves VL, de Oliveira MB, Fernando RC, Zanatta DB, Strauss BE, Silva MRR, Porcionatto MA, Colleoni GWB. Proteasome and heat shock protein 70 (HSP70) inhibitors as therapeutic alternative in multiple myeloma. Oncotarget. 2017;8(70):114698–114709. doi: 10.18632/oncotarget.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Fernández María Rosario, Valpuesta José María. Hsp70 chaperone: a master player in protein homeostasis. F1000Research. 2018;7:1497. doi: 10.12688/f1000research.15528.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Giordanetto F, Kroemer G. Targeting HSP70 for cancer therapy. Mol Cell. 2009;36(2):176–177. doi: 10.1016/j.molcel.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, Trichet V, Robillard N, Philippe M, Bataille R, Amiot M. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67(11):5418–5424. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- Guang Matthew Ho Zhi, Kavanagh Emma L., Dunne Luke Paul, Dowling Paul, Zhang Li, Lindsay Sinéad, Bazou Despina, Goh Chia Yin, Hanley Cathal, Bianchi Giada, Anderson Kenneth C., O’Gorman Peter, McCann Amanda. Targeting Proteotoxic Stress in Cancer: A Review of the Role that Protein Quality Control Pathways Play in Oncogenesis. Cancers. 2019;11(1):66. doi: 10.3390/cancers11010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015;5(5):475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F, Auberger P. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene. 2004;23(47):7863–7873. doi: 10.1038/sj.onc.1208069. [DOI] [PubMed] [Google Scholar]
- Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, Liu Q, Sun JD, Van Camp B, Hart CP, Vanderkerken K. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116(9):1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- Hu J, Dang N, Menu E, De Bruyne E, Xu D, Van Camp B, Van Valckenborgh E, Vanderkerken K. Activation of ATF4 mediates unwanted Mcl-1 accumulation by proteasome inhibition. Blood. 2012;119(3):826–837. doi: 10.1182/blood-2011-07-366492. [DOI] [PubMed] [Google Scholar]
- Hu H, Tian M, Ding C, Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Yoichi, Hirano Mitsuhito, Kobayashi Masayuki, Futami Muneyoshi, Tojo Arinobu. HDAC Inhibitors Exert Anti-Myeloma Effects through Multiple Modes of Action. Cancers. 2019;11(4):475. doi: 10.3390/cancers11040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong T, Spencer A. Targeting HSP 90 induces apoptosis and inhibits critical survival and proliferation pathways in multiple myeloma. Mol Cancer Ther. 2011;10(10):1909–1917. doi: 10.1158/1535-7163.MCT-11-0174. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stokes J, 3rd, Singh UP, Scissum Gunn K, Acharya A, Manne U, Mishra M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016;374(1):156–166. doi: 10.1016/j.canlet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12(3):743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, Chung KC, Tiedemann RE. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24(3):289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GJ, Gregory WM, Davies FE, Bell SE, Szubert AJ, Brown JM, Coy NN, Cook G, Russell NH, Rudin C, Roddie H, Drayson MT, Owen RG, Ross FM, Jackson GH, Child JA, National Cancer Research Institute Haematological Oncology Clinical Studies G The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- Mori Y, Terauchi R, Shirai T, Tsuchida S, Mizoshiri N, Arai Y, Kishida T, Fujiwara H, Mazda O, Kubo T. Suppression of heat shock protein 70 by siRNA enhances the antitumor effects of cisplatin in cultured human osteosarcoma cells. Cell Stress Chaperones. 2017;22(5):699–706. doi: 10.1007/s12192-017-0793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S, Koenig E, Fergus A, Huang Y, Richardson P, Trepicchio WL, Broyl A, Sonneveld P, Shaughnessy JD, Jr, Bergsagel PL, Schenkein D, Esseltine DL, Boral A, Anderson KC. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109(8):3177–3188. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4(3):443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- Nimmanapalli R, Gerbino E, Dalton WS, Gandhi V, Alsina M. HSP70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br J Haematol. 2008;142(4):551–561. doi: 10.1111/j.1365-2141.2008.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151(3):217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, van der Heijden JW, Ylstra B, Peters GJ, Kaspers GL, Dijkmans BA, Scheper RJ, Jansen G. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112(6):2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, Moreau P, Waage A, Spencer A, Ludwig H, Boccadoro M, Harousseau JL. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111(8):3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- Qi W, White MC, Choi W, Guo C, Dinney C, McConkey DJ, Siefker-Radtke A. Inhibition of inducible heat shock protein-70 (hsp72) enhances bortezomib-induced cell death in human bladder cancer cells. PLoS One. 2013;8(7):e69509. doi: 10.1371/journal.pone.0069509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Mitsiades CS, Laubach JP, Lonial S, Chanan-Khan AA, Anderson KC. Inhibition of heat shock protein 90 (HSP90) as a therapeutic strategy for the treatment of myeloma and other cancers. Br J Haematol. 2011;152(4):367–379. doi: 10.1111/j.1365-2141.2010.08360.x. [DOI] [PubMed] [Google Scholar]
- Rizzatti EG, Mora-Jensen H, Weniger MA, Gibellini F, Lee E, Daibata M, Lai R, Wiestner A. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma. 2008;49(4):798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- Rodvold Jeffrey J., Chiu Kevin T., Hiramatsu Nobuhiko, Nussbacher Julia K., Galimberti Valentina, Mahadevan Navin R., Willert Karl, Lin Jonathan H., Zanetti Maurizio. Intercellular transmission of the unfolded protein response promotes survival and drug resistance in cancer cells. Science Signaling. 2017;10(482):eaah7177. doi: 10.1126/scisignal.aah7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roue G, Perez-Galan P, Mozos A, Lopez-Guerra M, Xargay-Torrent S, Rosich L, Saborit-Villarroya I, Normant E, Campo E, Colomer D. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2011;117(4):1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- Sabirzhanov B, Stoica BA, Hanscom M, Piao CS, Faden AI. Over-expression of HSP70 attenuates caspase-dependent and caspase-independent pathways and inhibits neuronal apoptosis. J Neurochem. 2012;123(4):542–554. doi: 10.1111/j.1471-4159.2012.07927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Lonial S, Boise LH. When Cancer Fights Back: Multiple Myeloma, Proteasome Inhibition, and the Heat-Shock Response. Mol Cancer Res. 2015;13(8):1163–1173. doi: 10.1158/1541-7786.MCR-15-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AR, Livingstone AM, Mohseni N, Mosser DD. Regulation of heat-induced apoptosis by Mcl-1 degradation and its inhibition by Hsp70. Cell Death Differ. 2009;16(4):638–647. doi: 10.1038/cdd.2008.189. [DOI] [PubMed] [Google Scholar]
- Vincenz L, Jager R, O’Dwyer M, Samali A. Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol Cancer Ther. 2013;12(6):831–843. doi: 10.1158/1535-7163.MCT-12-0782. [DOI] [PubMed] [Google Scholar]
- Wali JA, Rondas D, McKenzie MD, Zhao Y, Elkerbout L, Fynch S, Gurzov EN, Akira S, Mathieu C, Kay TW, Overbergh L, Strasser A, Thomas HE. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell Death Dis. 2014;5:e1124. doi: 10.1038/cddis.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang J, Zhou Y, Wang Y, Wang S, Zhang W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012;321(2):137–143. doi: 10.1016/j.canlet.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Yerlikaya A, Okur E, Eker S, Erin N. Combined effects of the proteasome inhibitor bortezomib and Hsp70 inhibitors on the B16F10 melanoma cell line. Mol Med Rep. 2010;3(2):333–339. doi: 10.3892/mmr_00000262. [DOI] [PubMed] [Google Scholar]
- Yoo JY, Hurwitz BS, Bolyard C, Yu JG, Zhang J, Selvendiran K, Rath KS, He S, Bailey Z, Eaves D, Cripe TP, Parris DS, Caligiuri MA, Yu J, Old M, Kaur B. Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clin Cancer Res. 2014;20(14):3787–3798. doi: 10.1158/1078-0432.CCR-14-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshidomi K, Murakami A, Yakabe K, Sueoka K, Nawata S, Sugino N. Heat shock protein 70 is involved in malignant behaviors and chemosensitivities to cisplatin in cervical squamous cell carcinoma cells. J Obstet Gynaecol Res. 2014;40(5):1188–1196. doi: 10.1111/jog.12325. [DOI] [PubMed] [Google Scholar]
- Yuan P, Salvadore G, Li X, Zhang L, Du J, Chen G, Manji HK. Valproate activates the Notch3/c-FLIP signaling cascade: a strategy to attenuate white matter hyperintensities in bipolar disorder in late life? Bipolar Disord. 2009;11(3):256–269. doi: 10.1111/j.1399-5618.2009.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 32 kb)