Abstract
Bovine mastitis is a common inflammatory disease caused by various factors. The main factor of mastitis is pathogenic microorganism infection, such as Staphylococcus aureus, Escherichia coli, and Streptococcus. Cronobacter sakazakii (C. sakazakii) is a newly discovered pathogenic bacteria in milk products, which seriously threat human health in recent years. At present, it has not been reported that the pathogenesis of mastitis is caused by C. sakazakii. This study investigated the inflammation of mammary gland epithelium, which was induced by C. sakazakii for the first time. We focused on bacterial isolation, histological observation, AIM2 inflammasome pathways, endoplasmic reticulum stress, and apoptosis. The results showed that C. sakazakii–induced inflammation caused damage of tissue, significantly increased the production of pro-inflammatory cytokines (including TNF-α, IL-1β, and IL-6), activated the AIM2 inflammasome pathway (increased the expression of AIM2 and cleaved IL-1β), and induced endoplasmic reticulum stress (increased the expression of ERdj4, Chop, Grp78) and apoptosis (increased the ratio of Bax/Bcl-2, a marker of apoptosis). In conclusion, it is suggested that it maybe inhibite AIM2 inflammasome pathways and alleviate endoplasmic reticulum stress (ER stress) against the C. sakazakii–induced inflammation.
Keywords: Cronobacter sakazakii, AIM2 inflammasome, ER stress, Apoptosis
Introduction
Mastitis is a common disease, which causes a decline in milk yield and milk quality and seriously hinders the development of dairy industry, even harms to human health (Zheng et al. 2016). The etiologies of bovine mastitis are complicated, including bacteria, fungi, mycoplasma, and viruses (Filioussis et al. 2019). There were abundant studies of the mechanism of mastitis, mainly focused on Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Streptococcus agalactiae (S. agalactiae), and Streptococcus (Haapala et al. 2018). Yet C. sakazakii, newly discovered in recent years and a kind of pathogenic bacteria in milk products, is an important emerging neonatal pathogen. It is associated with outbreaks of meningitis, sepsis, and necrotizing enterocolitis (Mullane et al. 2007), with a high mortality rate of 50–80% (Healy et al. 2010). It has not been reported whether C. sakazakii can cause caw mastitis. So far, the mechanism of C. sakazakii–induced inflammation remains poorly understand. Hence, it is urgent to understand its pathogenesis. In this report, we used C. sakazakii to construct inflammatory models in vivo and in vitro.
Inflammasome is a multiprotein complex composed of a sensor-receptor protein, a specular protein (ASC) associated with apoptosis, and a cysteine protease caspase-1, which activation promotes the release of mature IL-1β and IL-18 which play an indispensable role in defense factor in the innate immune response against invading microorganisms (Kovacs and Miao 2017). The NLR family consists of several receptors (Costa Franco et al. 2019). AIM2 is identified as the receptor involved in inflammasome activation, which responds to the recognition of cytoplasmic DNA during bacterial infections (Song et al. 2019). AIM2 can be activated by pathogens; its activation, Caspase-1 precursors, promotes releases of mature caspase1 and pro-inflammatory cytokine IL-1β, which result in inflammation (Schroder and Tschopp 2010; Wu et al. 2019).
Endoplasmic reticulum (ER) is an organelle with many vital cellular functions (Schwarz and Blower 2016). ER stress maybe induced by physiological and pathological stimuli, for instance inflammation, oxidative stress, hypoxia, disturbance of calcium homeostasis, increase of protein expression, and subsequent accumulation of unfolded protein (Minamino and Komuro 2010), which plays a crucial role in many diseases, such as inflammatory bowel disease (Cai et al. 2019), diabetes (Sato et al. 2015), cardiovascular diseases (Lebeaupin et al. 2018), non-alcoholic fatty liver disease (Zhang et al. 2019), viral infection (Clarke et al. 2014), and immune-related disorders (Eggleton et al. 2017). Cellular response relies on the level of ER stress. The cells can recover and adapt to the ER stress when ER stress is milder. On the contrary, ER stress is prolonged or too severe; these mechanisms will imbalance protein homeostasis, leading to autophagy. If the stress is not alleviated, apoptosis may occur (Lee et al. 2019). Numerous studies have shown that apoptosis is essential for immune defense, which is associated with many diseases (Peterson et al. 2017). In addition, certain pathogens are capable of increasing epithelial apoptosis, thereby leading to onset of diseases (Wu et al. 2017). However, an imbalance between normal rates of apoptosis and restitution may result in destroying the cell barrier allowing for bacterial invasion and ultimately leading to the development of systemic sepsis (Hunter et al. 2008).
Materials and methods
Milk sample collection
A total of 20 samples of milk, including ten normal milk samples and ten mastitis milk samples, were collected from Yonghao Animal Husbandry Company, Jiangsu, China.
Bacterial strains
C. sakazakii is a newly discovered pathogenic bacterium in milk products. C. sakazakii, a mastitis pathogen, was isolated from the fresh milk with high somatic cell count and used for the intramammary inoculum to establish mice models of mastitis, which was used in this study.
Bacterial culture was performed in C. sakazakii chromogenic medium and incubated at 37 °C for 24 h. Overnight cultures were used for the intramammary injection in mice. C. sakazakii were properly diluted in PBS to achieve the desired concentration (2 × 108 CFU/mL). The bacterial inoculum size was determined by serially diluting the culture and pour plating the dilutions on common nutrition agar.
Cell culture and treatment
Bovine mammary epithelial cells (BMECs) were donated by professor Yuping Sun. Cells were cultured in DMEM containing 10% FBS at 37 °C in a humidified incubator under 5% CO2. The medium was changed every 2 days. Cells were treated for 24 h by 50 μL (108 CFU/mL) inactivated bacteria which were diluted in PBS.
Animals
A total of 30 female and 10 male C57BL/6J mice (6–8 weeks old, weighing20–25 g) were kept in our own SPF mice house. The female and male mice cohabited at a 3 female to 1 male ratio for conception. They were housed separately after pregnancy until 5 to 7 days after calving. Lactating female mice were raised in a room maintained at 24 °C ± 1 °C with 40–80% humidity and were allowed access to food and water ad libitum. Feeding and management of experimental animals shall be executed in accordance with the relevant regulations of the national institutes of health on the use of experimental animals.
The procedures involving animals were approved by the Animal Welfare Committee of Nanjing Agricultural University, Nanjing, China, approval No. 20190205. And all animal experiments were carried out in strict accordance with the guidelines and rules.
Mouse model of mastitis
Female mice were separated into two groups at random: C. sakazakii group, treatment with C. sakazakii; PBS group, treatment with sterile PBS after postpartum 7 days. The pups were removed 2 h before the experiment. The female mice were anesthetized with ether and disinfected. The fourth pair of milk areas with 75% alcohol fully exposes the breast. The nipple was fixed in the left, and a 100 μL microinjector needle was slowly inserted into the milk ducts with the right hand, and C. sakazakii group was injected 50 μL of a certain concentration of bacterium into the mammary gland, while PBS group was injected with 50 μL PBS.
All mice were sacrificed by cervical dislocation after 24-h infection. The L4 (the left fourth mammary gland) and R4 (the right fourth mammary gland) mammary glands of each animal were sterile collected for follow-up analysis.
Histopathological analysis
The mammary gland samples of C. sakazakii group and PBS group were collected and fixed in 10% neutral buffered formalin for about 48–72 h, then embedded in paraffin, sectioned, and stained by hematoxylin and eosin (H&E), and the pathological changes were observed by microscopy.
Total RNA isolation and qRT-PCR analysis
The tissue of appropriate size was ground into a powder, and RNA was extracted by using Trizol (Invitrogen) following the manufacturer’s instructions. The RNA was reverse-transcribed into cDNA in accordance with the Reverse Transcription System’s instructions (Takara) according to the manufacturer’s instructions. Real-time quantitative PCR was performed on an ABI 7500 RT-PCR instrument by using 2X SYBR Green (Vazyme, Q111-02) and the appropriate primers. According to the GenBank sequence, the primer sequence of the target genes and the β-actin gene were designed using the software PrimerPremier5.0 (Table 1).
Table 1.
The primer used for qRT-PCR analysis
| Gene | Primer sequence |
|---|---|
| TNF-α | ATGAGCACAGAAAGCATGATC |
| TACAGGCTTGTCACTCGAATT | |
| IL-1β | ATGGCAACTGTTCCTGAACTCAACT |
| CAGGACAGGTATAGATTCTTTCCTTT | |
| IL-6 | CTCCCAACAGACCTGTCTATAC |
| CCATTGCACAACTCTTTTCTCA | |
| Grp78 | ACTTGGGGACCACCTATTCCT |
| ATCGCCAATCAGACGCTCC | |
| Chop | GCATGAAGGAGAAGGAGCAG |
| ATGGTGCTGGGTACACTTCC | |
| ERdj4 | TAAAAGCCCTGATGCTGAAGC |
| TCCGACTATTGGCATCCGA | |
| Bax | TGCAGAGGATGATTGCTGAC |
| GATCAGCTCGGGCACTTTAG | |
| Bcl-2 | ACTGTGTTAACTCCTGCCCG |
| GCAGCAAGCTACTCAGACGA | |
| Actin | GACCTCTATGCCAACACAGT |
| AGTACTTGCGCTCAGGAGGA |
Western blot analysis
The tissue (50-mg tissue) was ground into a powder in lysis buffer (mixture protein lysate and proteinase inhibitor as 1:9). The lysate was centrifuged at 13,000g for 20 min at 4 °C, and the supernatant was collected into a new centrifuge tube, and the supernatant was quantified using a BCA Protein Assay Kit (Beyotime Biotechnology Co.). Equal amounts of protein from each sample were mixed with SDS-PAGE loading buffer, then placed into metal incubator for 95 °C 10 min and preserved at 4 °C. Proteins were separated by SDS-PAGE and transferred to the PVDF membrane. After protein transfer, membranes were blocked for 1 h with 5% no-fat dried milk in TBST. The membranes were incubated overnight at 4 °C with rabbit monoclonal. The antibodies used in this study include the following: GAPDH (CST, Boston, MA), Tublin (CST, Boston, MA), Bax (Proteintech Co LTD, Wuhan, Hubei, China), Bcl-2 (Proteintech Co LTD, Wuhan, Hubei, China), AIM2 (CST, Boston, MA), and Cleaved IL-1β (CST, Boston, MA). Finally, membranes were processed using an enhanced chemiluminescence solution detection system (Amersham, Piscataway, NJ). Densitometry was performed by using the ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Statistical analyses were performed by t test with GraphPad Prism version 7.0 (GraphPad Sofware, San Diego, CA, USA). Data were presented as mean ± the standard deviation (SD). P < 0.05 were considered statistically significant.
Results
Isolation and identification of C. sakazakii
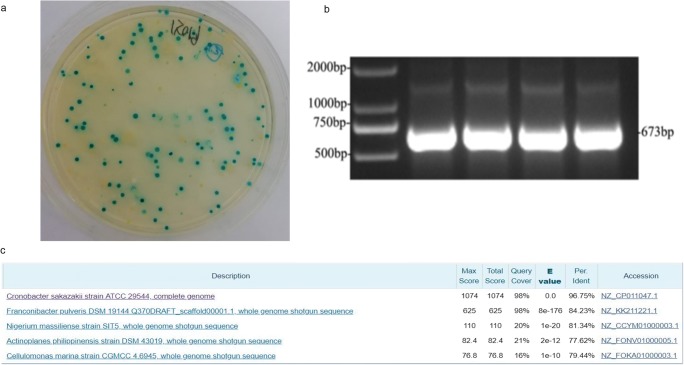
C. sakazakii is a pathogenic bacterium frequently detected in dairy products in recent years. We isolated five strains from 54 colonies by 16sRNA sequencing, including C. sakazakii, Pantoea rwandensis, Escherichia coli, Staphylococcus aureus, and Aerococcus vitidans. In order to further confirm, we used the characteristic of C. sakazakii showing blue colonies in C. sakazakii chromogenic medium (Fig. 1a). PCR assay was developed based on α-1, 4-glusidase gene. The ban size was 673 bp (Fig. 1b), and PCR products were recovered and purified, which were sent to sequencing (Sangon Biotech, Shanghai, China). All the obtained sequences were submitted to the GenBank. The results showed that the homology of the target fragment of α-1, 4-glucosidase gene (673 bp) was 96% (Fig. 1c).
Fig. 1.
Isolation and identification of C. sakazakii. Identification of C. sakazakii: aC. sakazakii chromogenic medium is a selective medium. C. sakazakii showed green colonies. b PCR assay was developed based on α-1, 4-glusidase genes, which were positive (673 bp). c Sequencing: the homology of the target fragment of α-1, 4-glucosidase gene (673 bp) was 96%
C. sakazakii–induced histopathological impairment and increased pro-inflammatory cytokine levels of the mammary gland in mice
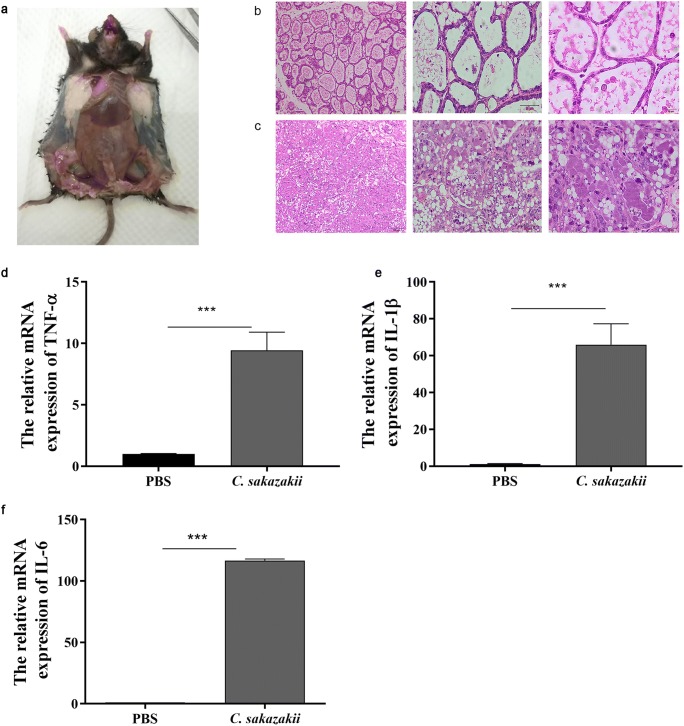
Mice were sacrificed 24 h later. After dissection, we found that the tissues of the fourth breast area, injected with bacterium, were thickened and congested, while the first and second breast areas, injected with PBS, were full of milk (Fig. 2a). To test whether the mouse mastitis model was successfully constructed, the mammary gland was collected, sectioned, and stained with H&E. The results showed that there were no pathological injuries of mammary gland from the PBS group mice (Fig. 2b). Compared with the PBS group, mammary gland from the C. sakazakii group presented severe pathological injuries, such as tissue congestion and edema, interstitial thickening, and a large number of inflammatory cells infiltration. Most of the epithelial cells were necrosis, exfoliation, disintegration, or even disappearance. Neutrophils were scattered in mammary gland acinus, which were necrosis and disintegration (Fig. 2c). Pro-inflammatory cytokines play important roles in the development of inflammation to further examine the degree of inflammation in mice with mastitis induced by C. sakazakii. The production of the pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, was measured by qRT-PCR. It showed that C. sakazakii group markedly increased the mRNA levels of TNF-α (Fig. 2d), IL-1β (Fig. 2e), and IL-6 (Fig. 2f) compared with the PBS group.
Fig. 2.
C. sakazakii induced histopathological impairment and increased pro-inflammatory cytokines levels of mammary gland in mice. a A mouse model of mastitis: the fourth pair of mammary glands were injected with C. sakazakii and the first and second pairs of mammary glands were injected with PBS and dissected. Mouse was dissected 24 h after infection. b HE staining of mammary glands histology: each nipple was injected with 100-μL C. sakazakii fluid. c HE staining of mammary glands histology: each nipple was injected with 100-μL PBS. The mRNA level of TNF-α (d), IL-1β (e), IL-6 (f), and relative mRNA level of β-actin were determined by qRT-PCR. Values are presented as means ± the standard deviation (SD) (n ≥ 3) (***P<0.001)
C. sakazakii increased the expression of AIM2 and cleaved IL-1β in mice and in BMECs
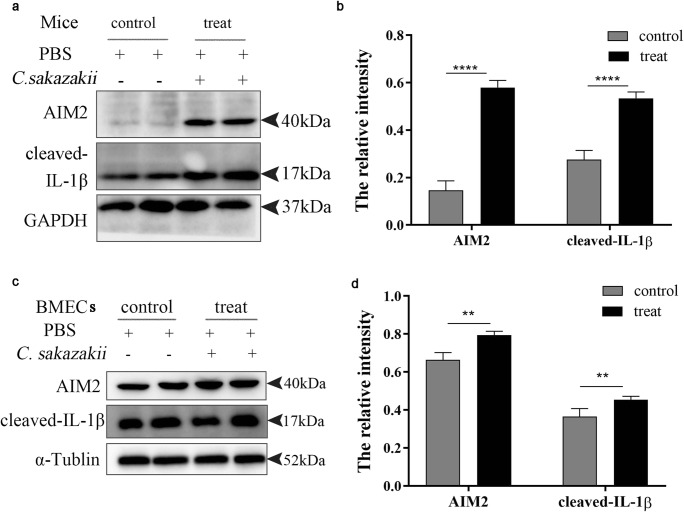
Studies have shown that AIM2 is required for the release of IL-1β in the macrophage infected with virulent B. abortus (Gomes et al. 2013). AIM2 can be activated by pathogens; its activation promotes the cleavage of caspase-1 and releases the mature pro-inflammatory cytokine IL-1β, which induce inflammation (Gong et al. 2019). To study the role of AIM2 in the C. sakazakii–induced inflammation in mice and BMECs, the expression of AIM2 and cleaved IL-1β were detected by western blot. The result showed that the C. sakazakii group significantly increased the expression of AIM2 and cleaved IL-1β protein in mice and BMECs (Fig. 3 a and c). The quantitative analysis of protein further confirmed that the C. sakazakii group significantly increased in the expression of AIM2 and cleaved IL-1β comparing with the PBS group (P < 0.0001, Fig. 3 b and d).
Fig. 3.
C. sakazakii increased the expression of AIM2 and cleaved IL-1β in vivo and vitro. The mammary gland tissues of each group (n ≥ 3) were obtained at 24 h after C. sakazakii and PBS infection. a, c AIM2 protein and cleaved IL-1β protein were detected by western blot in mice and in BMECs. b, d The relative AIM2 protein and cleaved IL-1β protein levels were quantified by ImageJ and normalized to α-Tublin. Data are presented as means ± the standard deviation (SD) (n ≥ 3) (**P<0.01, ****P<0.0001)
C. sakazakii–induced ER stress in mice and in BMECs
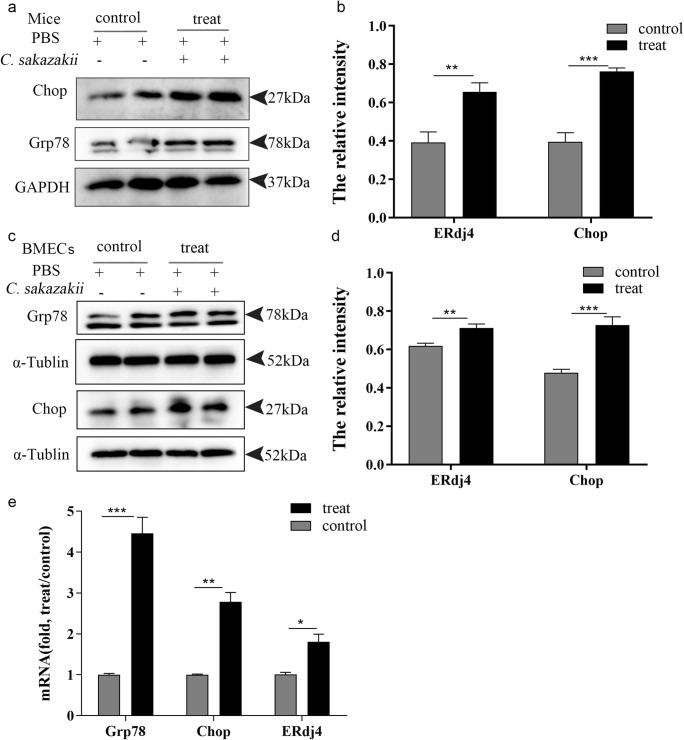
Recently, substantial evidences have proved that inflammatory response is related to ER stress, and pro-inflammatory cytokines could induce excessive ER stress response (Brenjian et al. 2019) to assess whether C. sakazakii stimulated ER stress in mice and in BMECs. The expression of ER stress marker was detected by western blot. The result showed that C. sakazakii–injected significantly increased the Grp78, a member of the HSP protein family, which was found in the ER that was upregulated after ER stress (Zheng et al. 2019) and C/EBP homologous protein (Chop) (Pejman et al. 2019), a pro-apoptotic transcription factor that was overexpressed following the disruption of ER homeostasis, compared with the PBS group in mice (Fig. 4 a and c). Further confirmed C. sakazakii group resulted in a significant increase in the expression of AIM2 and cleaved IL-1β compared with the PBS group (P < 0.0001, Fig. 4 b and d). We also detected the mRNA expression of ERdj4, a BiP cochaperone that was upregulated after ER stress (Fritz et al. 2014), Chop, and glucose-regulated protein 78 (Grp78) by qRT-PCR. The result showed that C. sakazakii–injected significantly increased the mRNA expression of ERdj4, Chop, and Grp78 (Fig. 4e) compared with the PBS group in mice. Moreover, BMECs were treated with bacterium and the result showed that the expression of ERdj4, Chop, and Grp78 increased compared with the control group (Fig. 4).
Fig. 4.
C. sakazakii induced endoplasmic reticulum (ER) stress in mouse models and in BMECs. The mammary gland tissues of each group (n ≥ 3) were obtained at 24 h after C. sakazakii and PBS infection. a, c Grp78 protein and Chop protein were detected by western blot in mice and in BMECs. b, d The relative Grp78 protein and Chop protein levels were quantified by ImageJ and normalized to α-Tublin and GAPDH in mice and BMECs. e The mRNA level of Grp78, Chop, ERdj4, and relative mRNA level of β-actin were determined by qRT-PCR in mice. Data are presented as means ± the standard deviation (SD) (n ≥ 3) (*P<0.05, **P<0.01, ***P<0.001)
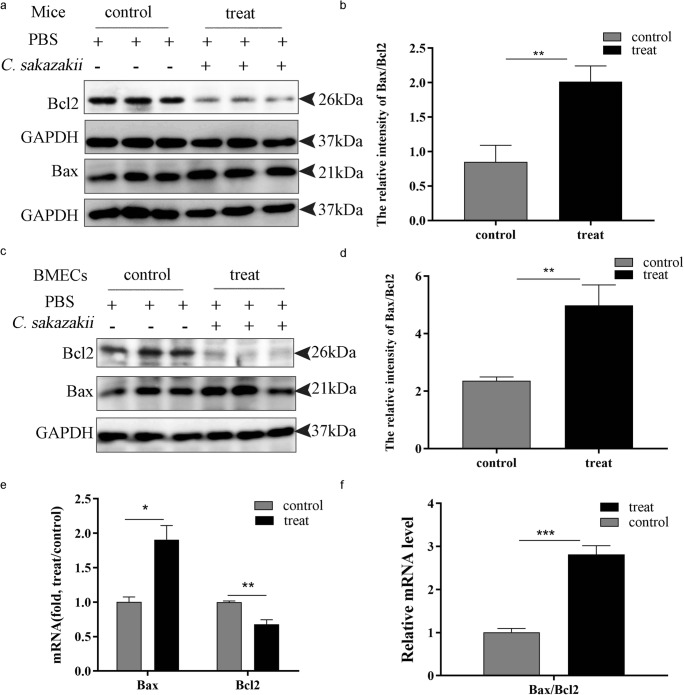
C. sakazakii–induced apoptosis in mice and BMECs
Apoptosis interacted with many vital processes such as inflammation (Rathinam et al. 2010). Therefore, we examined the apoptosis process by western blot to investigate whether the inflammation caused by C. sakazakii activated apoptosis. The expression of Bax, a pro-apoptotic protein (Bialuk et al. 2019), and Bcl-2, antiapoptotic protein (Bialuk et al. 2019), was detected by western blot in mice and BMECs. As shown in Fig. 5, the C. sakazakii group significantly increased the level of Bax and decreased the expression of Bcl-2 in mice and BMECs (Fig. 5 a and c ). The further confirmed C. sakazakii group resulted in a significant increase in the ratio of Bax/Bcl-2 (P < 0.001) compared with the PBS group (Fig. 5 b and d). Moreover, we detected the mRNA expression of Bax and Bcl-2 by qRT-PCR. It showed that the C. sakazakii treatment group significantly increased the mRNA level of Bax (P < 0.05) and decreased the expression of Bcl-2 (P < 0.01) (Fig. 5e), and the ratio of Bax/Bcl-2 significantly increased in mice (Fig. 5f).
Fig. 5.
C. sakazakii induced apoptosis in mouse model and in bovine mammary epithelial cells. The mammary gland tissues of each group (n ≥ 3) were obtained at 24 h after C. sakazakii and PBS infection. a, c Bcl-2 protein and Bax protein were detected by western blot in mice and in BMECs. b, d The ratio of Bax/Bcl-2 protein was analyzed in mice and BMECs. e The mRNA level of Bcl-2, Bax, and relative mRNA level of β-actin were determined by qRT-PCR in mice. f The ratio mRNA level of Bcl-2/Bax and relative mRNA level of β-actin were determined by qRT-PCR in mice. Data are presented as means ± the standard deviation (SD) (n ≥ 3) (*P<0.05, **P<0.01, ***P<0.001)
Discussion
Mastitis seriously affects the milk yield and quality of dairy cows and seriously hinders the development of dairy industry, even threats to human health. In recent years, C. sakazakii was frequently detected in certain infant formulas made with milk (Chen et al. 2019). With the increased with the number of using infant formula, C. sakazakii–related infections have substantially increased. It has been reported that necrotizing enterocolitis may be associated with C. sakazakii. At present, the mechanism of C. sakazakii–induced mastitis inflammation has not been reported. We were the first to used C. sakazakii to construct inflammatory models to study the mastitis in vivo and in vitro. Our results were consistent with the previous studies which LPS induced mastitis in BMECs that the production of TNF-α increased significantly, which was a crucial immunoregulatory cytokine that could trigger a series of immune responses and subsequently accelerate the production of other cytokines (Li et al. 2019). It has not been reported the C. sakazakii cause caw mastitis. In this study, we used the desired concentration (2 × 108 CFU/mL) to construct the mouse mastitis model. We found that mammary gland from the C. sakazakii group presented severe pathological injuries compared with the PBS group, and the production of IL-6 and IL-1β were significantly increased in the mammary gland tissues treated by C. sakazakii than treated by PBS.
Studies have shown that AIM2 is required for the release of IL-1β in the macrophage infected with virulent B. abortus (Gomes et al. 2013). Moreover, B. abortus infection eliminated IL-1β in NLRP3-deficient macrophages, but did not affect IL-1β production in AIM2-deficient macrophages (Bronner et al. 2015). It has been shown that nigericin and ATP-stimulated total bone marrow cells lead to the NLRP3 inflammasome activation, and Salmonella-stimulated bone marrow neutrophil leads to the NLRC4 inflammasome activation, which promotes the maturation of IL-1β (Chen et al. 2014; Mankan et al. 2012). Our results were consistent with the previous studies that AIM2 is necessary for the release of IL-1β during macrophage infection; we revealed that AIM2 and cleaved IL-1β increased in the C. sakazakii treatment group compared with the PBS group. We then reasoned that C. sakazakii activated AIM2 inflammasome pathway. Thus, it is possible to inhibit AIM2 inflammasome pathways to treat C. sakazakii–induced inflammation.
ER stress is associated with the activation of inflammation (Bronner et al. 2015). ER is a large membranous organelle with a series of functions, such as folding newly synthesized proteins, maintenance of calcium homeostasis and phospholipid synthesis, and regulating intracellular signaling pathways (Kleizen and Braakman 2004). Subversion of ER function is a feature shared by multiple intracellular bacteria and viruses, and this disruption of cellular function activates pathways of the unfolded protein response (UPR) (English et al. 2017). Substantial evidences have proved that inflammatory response is related to ER stress, and pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and interleukin 8 (IL-8), are closely related to the unfolded protein response (UPR) (Li et al. 2005). Our results showed that the C. sakazakii treatment group increased the expression of ERdj4, Chop, and Grp78 compared with the PBS group. Chop is a transcription factor, which is induced in response to physiological changes in the cell, including ER stress, DNA damage, nutrient deprivation, hypoxia, and viral and fungal infection (Kleizen and Braakman 2004; Li et al. 2014). Our research indicated that C. sakazakii induces excessive ER stress response.
Excessive ER stress results in apoptosis. Therefore, we further investigated the cell apoptosis. Numerous studies have shown that apoptosis is crucial for immune defense and associated with many diseases (Namgaladze et al. 2019). Moreover, certain pathogens are capable of increasing epithelial apoptosis, thereby contributing to disease pathogenesis (Ruan et al. 2016). However, when there is an imbalance between normal rates of apoptosis and restitution, this may result in decreased barrier integrity allowing for bacterial translocation and the development of systemic sepsis (Hunter et al. 2008). In agreement with this concept, our studies showed that the C. sakazakii treatment group significantly increased the expression of the Bax/Bcl2 than PBS group, which demonstrated that the C. sakazakii treatment group was significant apoptosis in BMECs and in mice.
Conclusion
We separated C. sakazakii from the fresh milk, which was used to construct the model of inflammation. C. sakazakii–induced inflammation significantly increased the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, activated AIM2 inflammasome, and induced excessive ER stress response and apoptosis. However, there has been no evidence to support the relationship between AIM2 inflammasome activation and ER stress (Fig. 6). These data emphasized that C. sakazakii induced mammary epithelial cell apoptosis and stimulated the expression of inflammatory cytokines via AIM2 inflammatory activation and ER stress in vivo and in vitro, leading to tissue damage. Further understanding the role of C. sakazakii in the pathogenesis of mastitis will provide clues for developing new therapeutic strategies to prevent this disease.
Fig. 6.
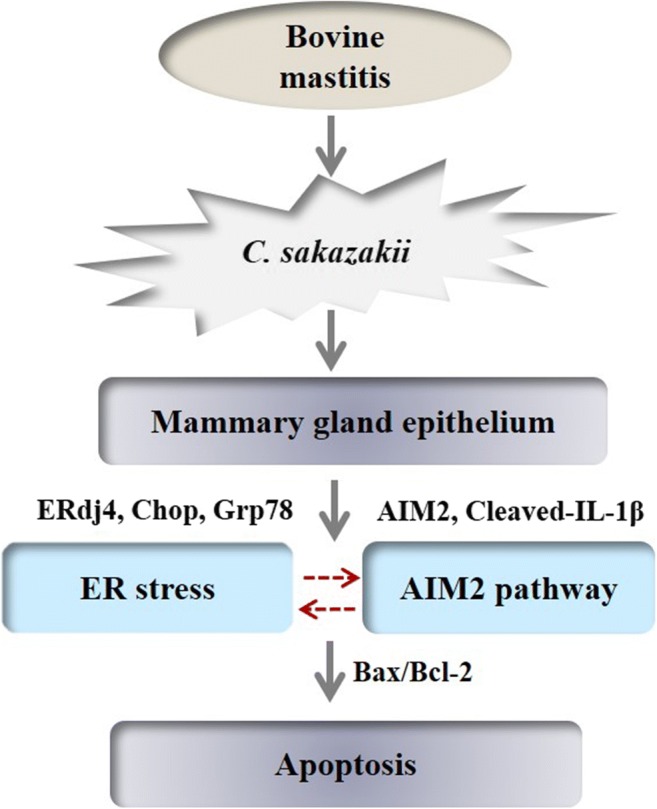
C. sakazakii–stimulated mammalian mammary gland epithelium induced apoptosis via activating AIM2 pathway and excessive ER stress response. E. sakazakii was isolated from bovine mastitis milk sample, which was used to infected mice and BMECs. E. sakazakii–infected mammary gland epithelium activated AIM2 inflammasome and induced excessive endoplasmic reticulum stress response. However, there has been no evidence to support the relationship between AIM2 inflammasome activation and endoplasmic reticulum stress. Finally, apoptosis occurred in mammary epithelial cells
Funding information
This study was supported by The National Natural Science Foundation of China (Grant No. 31970413), National Key R&D Program of China (Grant No. 2018YFC1200201), and Start-up grant from Nanjing Agricultural University (Grant No. 804090).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bialuk Izabela, Cieślińska Magdalena, Kowalczuk Oksana, Bonda Tomasz A., Nikliński Jacek, Winnicka Maria M. IL-6 deficiency attenuates p53 protein accumulation in aged male mouse hippocampus. Biogerontology. 2019;21(1):29–43. doi: 10.1007/s10522-019-09841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenjian S et al (2019) Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol:e13186. 10.1111/aji.13186 [DOI] [PubMed]
- Bronner DN, et al. Endoplasmic reticulum stress activates the Inflammasome via NLRP3- and Caspase-2-driven mitochondrial damage. Immunity. 2015;43:451–462. doi: 10.1016/j.immuni.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, et al. Indispensable role of the ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov. 2019;5:7. doi: 10.1038/s41421-018-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Gro CJ, Flor Vásquez S, Stacey KJ, Jurg T, Sweet MJ, Kate S. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Reports. 2014;70:68. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Chen D, et al. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 2019;290:270–276. doi: 10.1016/j.foodchem.2019.03.149. [DOI] [PubMed] [Google Scholar]
- Clarke H, Chambers J, Liniker E, Marciniak S. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25:563–573. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Costa Franco MMS, Marim FM, Alves-Silva J, Cerqueira D, Rungue M, Tavares IP, Oliveira SC. AIM2 senses Brucella abortus DNA in dendritic cells to induce IL-1beta secretion, pyroptosis and resistance to bacterial infection in mice. Microbes Infect. 2019;21:85–93. doi: 10.1016/j.micinf.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleton P, Smerdon GR, Holley JE, Gutowski NJ (2017) Manipulation of oxygen and endoplasmic reticulum stress factors as possible interventions for treatment of multiple sclerosis: evidence for and against [DOI] [PubMed]
- English BC, Prooyen NV, Örd T, Örd T, Sil A. The transcription factor CHOP, an effector of the integrated stress response, is required for host sensitivity to the fungal intracellular pathogenHistoplasma capsulatum. Plos Pathogens. 2017;13:e1006589. doi: 10.1371/journal.ppat.1006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filioussis George, Kachrimanidou Melina, Christodoulopoulos Georgios, Kyritsi Maria, Hadjichristodoulou Christos, Adamopoulou Maria, Tzivara Athanasia, Kritas Spyridon K., Grinberg Alex. Short communication: Bovine mastitis caused by a multidrug-resistant, mcr-1-positive (colistin-resistant), extended-spectrum β-lactamase–producing Escherichia coli clone on a Greek dairy farm. Journal of Dairy Science. 2020;103(1):852–857. doi: 10.3168/jds.2019-17320. [DOI] [PubMed] [Google Scholar]
- Fritz JM, Dong M, Apsley KS, Martin EP, Na CL, Sitaraman S, Weaver TE. Deficiency of the BiP cochaperone ERdj4 causes constitutive endoplasmic reticulum stress and metabolic defects. Mol Biol Cell. 2014;25:431–440. doi: 10.1091/mbc.E13-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MT, et al. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol (Baltimore, Md : 1950) 2013;190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- Gong Zhenwei, Zhang Xinyi, Su Kai, Jiang Ruihua, Sun Zhe, Chen Wei, Forno Erick, Goetzman Eric S., Wang Jieru, Dong H. Henry, Dutta Partha, Muzumdar Radhika. Deficiency in AIM2 induces inflammation and adipogenesis in white adipose tissue leading to obesity and insulin resistance. Diabetologia. 2019;62(12):2325–2339. doi: 10.1007/s00125-019-04983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala V, et al. Semen as a source of mycoplasma bovis mastitis in dairy herds. Vet Microbiol. 2018;216:60–66. doi: 10.1016/j.vetmic.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Healy B, et al. Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathogens Dis. 2010;7:339–350. doi: 10.1089/fpd.2009.0379. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Singamsetty VK, Chokshi NK, Boyle P, Camerini V, Grishin AV, Upperman JS, Ford HR, Prasadarao NV. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198:586–593. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis trends. Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B (2018) Endoplasmic reticulum stress signaling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol S0168827818321615- [DOI] [PubMed]
- Lee Kuan-I, Lin Jhe-Wei, Su Chin-Chuan, Fang Kai-Min, Yang Ching-Yao, Kuo Chun-Ying, Wu Chin-Ching, Wu Cheng-Tien, Chen Ya-Wen. Silica nanoparticles induce caspase-dependent apoptosis through reactive oxygen species-activated endoplasmic reticulum stress pathway in neuronal cells. Toxicology in Vitro. 2020;63:104739. doi: 10.1016/j.tiv.2019.104739. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and Interleukin-6 Model Of NF-κB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- Li BX, Li WY, Tian YB, Guo SX, Huang YM, Xu DN, Cao N. Polysaccharide of Atractylodes macrocephala Koidz enhances cytokine secretion by stimulating the TLR4-MyD88-NF-kappaB signaling pathway in the mouse spleen. J Med Food. 2019;22:937–943. doi: 10.1089/jmf.2018.4393. [DOI] [PubMed] [Google Scholar]
- Mankan AK, Therese D, Dieter J, Veit H. The NLRP3/ASC/Caspase-1 axis regulates IL-1β processing in neutrophils. Eur J Immunol. 2012;42:710–715. doi: 10.1002/eji.201141921. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro IM. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circulation Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- Mullane NR, Iversen C, Healy B, Walsh C, Whyte P, Wall PG, Quinn T, Fanning S. Enterobacter sakazakii an emerging bacterial pathogen with implications for infant health. Minerva Pediatr. 2007;59:137–148. [PubMed] [Google Scholar]
- Namgaladze Dmitry, Khodzhaeva Vera, Brüne Bernhard. ER-Mitochondria Communication in Cells of the Innate Immune System. Cells. 2019;8(9):1088. doi: 10.3390/cells8091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejman Sina, Kamarehei Maryam, Riazi Gholamhossein, Pooyan Shahriar, Balalaie Saeed. Ac-SDKP ameliorates the progression of experimental autoimmune encephalomyelitis via inhibition of ER stress and oxidative stress in the hippocampus of C57BL/6 mice. Brain Research Bulletin. 2020;154:21–31. doi: 10.1016/j.brainresbull.2019.09.014. [DOI] [PubMed] [Google Scholar]
- Peterson LW, Philip NH, DeLaney A, Wynosky-Dolfi MA, Asklof K, Gray F, Choa R, Bjanes E, Buza EL, Hu B, Dillon CP, Green DR, Berger SB, Gough PJ, Bertin J, Brodsky IE. RIPK1-dependent apoptosis bypasses pathogen blockade of innate signaling to promote immune defense. J Exp Med. 2017;214:3171–3182. doi: 10.1084/jem.20170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Zhang Z, Tian L, Wang S, Hu S, Qiao JJ. The salmonella effector SopB prevents ROS-induced apoptosis of epithelial cells by retarding TRAF6 recruitment to mitochondria. Biochem Biophys Res Commun. 2016;478:618–623. doi: 10.1016/j.bbrc.2016.07.116. [DOI] [PubMed] [Google Scholar]
- Sato AY, Tu X, Mcandrews KA, Plotkin LI, Bellido T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice ☆. Bone. 2015;73:60–68. doi: 10.1016/j.bone.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Ma F, Herrup K. Accumulation of cytoplasmic DNA due to ATM Deficiency activates the microglial viral response system with neurotoxic consequences. J Neurosci. 2019;39:6378–6394. doi: 10.1523/JNEUROSCI.0774-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, et al. PRDM5 promotes the apoptosis of epithelial cells induced by IFN-gamma during Crohn’s disease Pathol. Res Pract. 2017;213:666–673. doi: 10.1016/j.prp.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Wu T, Liu W, Fan T, Zhong H, Zhou H, Guo W, Zhu X. 5-Androstenediol prevents radiation injury in mice by promoting NF-kappaB signaling and inhibiting AIM2 inflammasome activation. Biomed Pharmacother. 2019;121:109597. doi: 10.1016/j.biopha.2019.109597. [DOI] [PubMed] [Google Scholar]
- Zhang B, et al. NFkappaB/Orai1 facilitates endoplasmic reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Front Cell Dev Biol. 2019;7:202. doi: 10.3389/fcell.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Xu Y, Lu J, Liu M, Dai B, Miao J, Yin Y. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free Radical Biol Med. 2016;96:166–180. doi: 10.1016/j.freeradbiomed.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Zheng Y, et al. Betulinic acid suppresses breast cancer metastasis by targeting GRP78-mediated glycolysis and ER stress apoptotic pathway. Oxid Med Cell Longev. 2019;2019:8781690. doi: 10.1155/2019/8781690. [DOI] [PMC free article] [PubMed] [Google Scholar]