Abstract
Vitiligo is a chronic, autoimmune destruction of melanocytes, resulting in progressively expanding depigmented skin patches. Severity of the disorder, which affects approximately 1% of humans, may be mitigated using topical corticosteroids combined with phototherapy; along with other clinical strategies; however, no definitive cures are currently available. Here, the capacity of apigenin, a plant-derived aglycone, to inhibit oxidative stress–mediated melanocyte depletion in vitro using a PIG3V vitiligo perilesional melanocyte cell model is evaluated. PIG3V cells, treated with selected doses of apigenin, were challenged with H2O2, then assessed for viability and the oxidative stress–related parameters: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) by enzyme-linked immunoabsorbent assay (ELISA). Additionally, expression of nuclear factor erythroid 2p45 (NF-E2)–related factor 2 (Nrf2) and downstream targets was detected using Western blotting. Outcomes demonstrated that compared with negative control cultures, apigenin-treated cells exhibited enhanced viability. Likewise, apigenin enhanced expression of the cellular anti-oxidants SOD, CAT, and GSH-Px, but inhibited production of MDA, an oxidative stress biomarker. Interestingly, the expression and nuclear localization of the Nrf2 transcription factor, an important regulator oxidative stress and its downstream target genes, was significantly increased by apigenin treatment. Apigenin influence on Nrf2 was further validated by experiments demonstrating that Nrf2 knockdown cells failed to exhibit significant apigenin-mediated effects on cell viability and oxidative stress. Apigenin’s non-toxicity and ability to affect multiple oxidative stress–related parameters through its effects on Nrf2 signaling in melanocytes suggests that it may prove to be a valuable therapeutic tool in long-term management of vitiligo.
Keywords: Vitiligo, Apigenin, Oxidative stress, Nuclear factor erythroid 2p45 (NF-E2)–related factor 2 (Nrf2), Melanocyte
Introduction
Approximately 1% of the world’s population is afflicted with vitiligo (Whitton et al. 2015), characterized as the appearance of irregularly shaped depigmented skin patches of various sizes. Destruction of melanocytes is the cause leading to pigment loss in the skin and mucosal surfaces of patients with vitiligo. This notwithstanding, current treatments such as corticosteroids and phototherapies have demonstrated only limited ability to attenuate the major symptoms of vitiligo. Moreover, at the time of this writing, no definitive cures have been identified for the disease. Therefore, it becomes important to explore the pathogenesis of vitiligo, which could help the identification of novel therapies. To date, investigations have revealed multiple causative factors involved in the initiation and progression of vitiligo, such as dysregulation of immune cells, genetic susceptibility, trauma, parasitic infection, chemical toxicity, and oxidative stress (Halder and Chappell 2009; Mahmoud et al. 1998, 2002; Mahmoud et al. 2006; Qiu et al. 2014).
Previous studies demonstrated that patients with vitiligo exhibited high level of reactive oxygen species (ROS) in their skin (Boissy and Manga 2004; Schallreuter et al. 2012). In melanocytes, melanin synthesis produces superoxide anion (O2−) and hydrogen peroxide (H2O2), rendering melanocytes vulnerable to oxidative stress (Simon et al. 2009). Unbalanced oxidants and anti-oxidants result in loss of homeostasis in melanocytes, which may cause apoptosis or malignant transformation (Chang et al. 2017; Fried and Arbiser 2008; Gavalas et al. 2006). Accumulations of H2O2 in the epidermis of patients with vitiligo suggest an essential role for oxidative stress in the initial pathogenic event of vitiligo (Maresca et al. 1997). Further evidence has shown the importance of oxidative stress in vitiligo, where superoxide dismutase (SOD) level was demonstrated to be elevated in the skin and erythrocytes (Agrawal et al. 2004; Yildirim et al. 2004).
The transcription factor nuclear factor erythroid 2p45 (NF-E2)–related factor 2 (Nrf2) is a crucial regulator for the maintenance of oxidative stress, which controls the expression levels of several phase II detoxifying/anti-oxidant enzymes (Tanaka et al. 2008). Abnormal expression and an allelic variant of Nrf2 contribute to the vitiligo pathogenesis (Guan et al. 2008; Natarajan et al. 2010). Apigenin is a flavonoid found in numerous fruits and vegetables (Shukla and Gupta 2010) and possesses anti-oxidative and anti-inflammation properties (Wang et al. 2014). Apigenin exerts its anti-oxidation function by upregulating and activating Nrf2 signaling (Paredes-Gonzalez et al. 2014; Xu et al. 2016). In addition, apigenin increases melanin content in human melanoma cells (Takekoshi et al. 2014) and attenuates melanocyte apoptosis induced by dopamine (Lin et al. 2011). In light of the reported protective role of apigenin in melanocytes, we proposed that apigenin could regulate the oxidative activities in melanocytes through its anti-oxidative property by regulating Nrf2 signaling to enhance melanocyte survival. Therefore, in this study, we tested the effects of apigenin on melanocyte survival under H2O2 treatment. Several markers of oxidative stress were measured with or without apigenin treatment. The mechanism underlying the anti-oxidation function of apigenin in melanocytes was investigated.
Methods
Cell culture
PIG3V cells, a perilesional melanocyte cell line from vitiligo, were cultured in Medium 254 (Cascade Biologics, USA) supplemented with 5% fetal bovine serum (FBS, Gibco, USA) and human melanocyte growth supplement (Cascade Biologics), and maintained in a humidified incubator at 37 °C with 5% CO2. For H2O2 (Sigma-Aldrich, USA) treatment, cells were treated with 1 mM H2O2 for 24 h after adding indicated concentrations of apigenin for 1 h. Knockdown of Nrf2 was achieved by transfecting cells with si-RNA (si-Nrf2). Si-NC was transfected as control. The sequences for si-Nrf2 and si-NC are shown below: si-Nrf2: GGGAGGAGCTATTATCCATTC and si-NC: TTCTCCGAACGTGTCACGT.
Cell viability
Cell viability was tested by the MTT assay. Briefly, cells (1 × 104/well) were seeded in 96-well plates and cultured until the confluence reached about 80%. Then cells were treated by apigenin with indicated concentrations for 1 h and then 1 mM H2O2 for 24 h. The control group was treated by 0.1% DMSO. MTT assay was performed using an MTT Assay Kit (#ab211091, Abcam, USA) according to manufacturer’s instruction. Cell medium was replaced by MTT solution diluted in same volume of serum-free medium. After 3 h incubation, MTT solvent was added into each well and the absorbance was detected at OD 590 nm after 15 min.
Measurements of the activities of SOD, CAT, and GSH-Px and MDA level
Cells were lysed by ultrasound (10% efficiency, 5 s/10s for 10 cycle) in PBS. The activities of SOD, catalase (CAT), and glutathione peroxidase (GSH-Px) in cells were measured using corresponding kits (#S0101, SOD assay kits, and # S0051 Total Catalase Analysis Kit, Beyotime Biotechnology, China) (#A005 GSH-Px assay kit, Nanjing Jian Cheng Institute of Bioengineering, China) according to manufacturer’s instructions. The malondialdehyde (MDA) level was measured MDA assay kit (#A003-1, Nanjing Jiancheng Bioengineering Institute, China).
Quantitative real-time PCR
Cells were harvested and RNA was isolated using RNeasy Mini Kit (QIAGEN, China). For cDNA synthesis, 1 μg RNA was reverse transcribed by a PrimeScript RT reagent Kit (TaKarRa, Ohtsu, Japan) according to manufacturer’s instructions. For quantitative real-time PCR, SYBR™ Green PCR Master Mix (Takara Bio, Kusatsu, Japan) was employed. The primers used are shown below: Nrf2 forward, 5′-CTTGGCCTCAGTGATTCTGAAGTG-3′ and reverse, 5′-CCTGAGATGGTGACAAGGGTTGTA-3′); HO-1 forward, 5′-CAGGAGCTGCTGACCCATGA-3′ and reverse, 5′-AGCAACTGTCGCCACCAGAA-3′; NQO1 forward, 5′-GGATTGGACCGAGCTGGAA-3′ and reverse, 5′-GGATTGGACCGAGCTGGAA-3′. Actin was used as an internal control: Actin forward, 5′-AGAAAATCTGGCACCACACC-3′ and reverse, 5′-AGAGGCGTACAGGGATAGCA-3′.
Western blotting
Cells were collected in RIPA buffer (Beyotime) and protein samples were prepared by adding loading buffer (Beyotime). The proteins were separated by a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto nitrocellulose membranes (Millipore, USA). The membranes were blotted by 10% non-fat milk and incubated with primary antibodies purchased from Sigma-Aldrich (USA) including anti-Nrf2, anti-HO-1, anti-NQO1, anti-Lamin A/C, and anti-tubulin at 4 °C overnight. Then, after washed by Tris-buffered saline buffer with Tween 20, second antibodies, anti-mouse IgG or anti-rabbit IgG (Santa Cruz, USA) was applied for 1 h at 37 °C. The signal was detected by ECL Western blotting detection system (Millipore).
Statistical analysis
All data was represented as mean ± standard deviation (SD). The analysis of data was performed by GraphPad Prism (GraphPad Software 5.0, USA). The comparisons of differences were achieved by using one-way ANOVA analysis followed with a Tukey’s post hoc test. A p value less than 0.05 was considered as statistically significant.
Results and discussion
Improved cell viability by apigenin under H2O2-induced oxidative damage
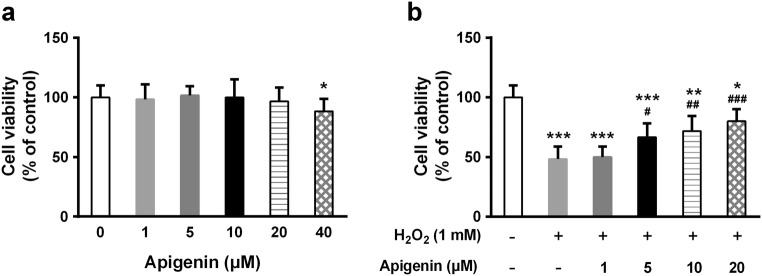
We first tested the effects of different doses of apigenin on cell viability and found that high concentration (40 μM) of apigenin was toxic to PIG3V human melanocytes (Fig. 1a). Therefore, we next examined cell viability after treatment with 1, 5, 10, and 20 μM apigenin for 1 h and then 1 mM H2O2 for another 24 h. Interestingly, although H2O2 significantly reduced cell viability, apigenin was able to attenuate this effect in a dose-dependent manner (Fig. 1b).
Fig. 1.
Apigenin promotes viability of melanocytes under H2O2-induced oxidative damage. a Melanocytes were treated with different concentrations of apigenin alone for 24 h and cell viability was determined by MTT assay. b Melanocytes were exposed to indicated concentrations of apigenin for 1 h and were further treated with 1 mM H2O2 for another 24 h; cell viability was determined by MTT assay. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with H2O2-treated group
Enhanced anti-oxidant capacity of melanocytes by apigenin
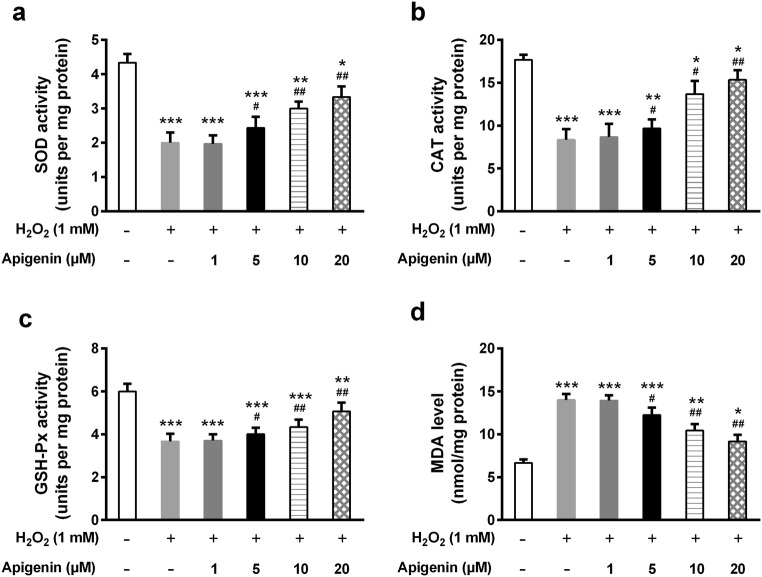
Since H2O2 induces oxidative damage, we speculated that apigenin may suppress oxidation reactions. We next tested several markers of oxidative stress including SOD, CAT, GSH-Px, and MDA. As expected, under H2O2 treatment, apigenin dramatically elevated the activities of SOD, CAT, and GSH-Px, three main anti-oxidant enzymes, in a dose-dependent manner (Fig. 2a–c). Lipid peroxidation was evaluated by measuring MDA, a product of lipoperoxidation. H2O2 induced the level of MDA whereas apigenin significantly repressed it (Fig. 2d).
Fig. 2.
Apigenin enhances the antioxidant capacity of H2O2-treated human melanocytes. SOD activity (a), CAT activity (b), GSH-Px activity (c), and MDA level (d) were detected by ELISA. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated group; #p < 0.05, ##p < 0.01 compared with H2O2-treated group
Upregulation of Nrf2 and its downstream genes by apigenin in H2O2-treated melanocytes
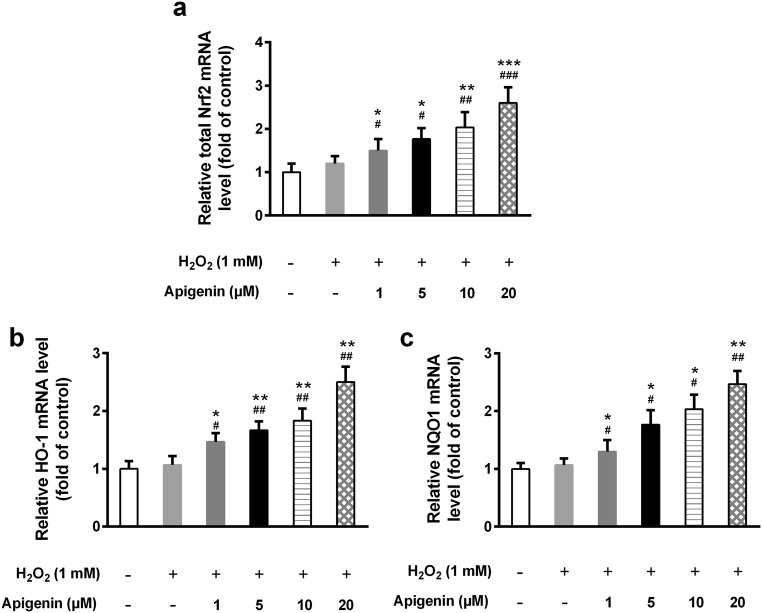
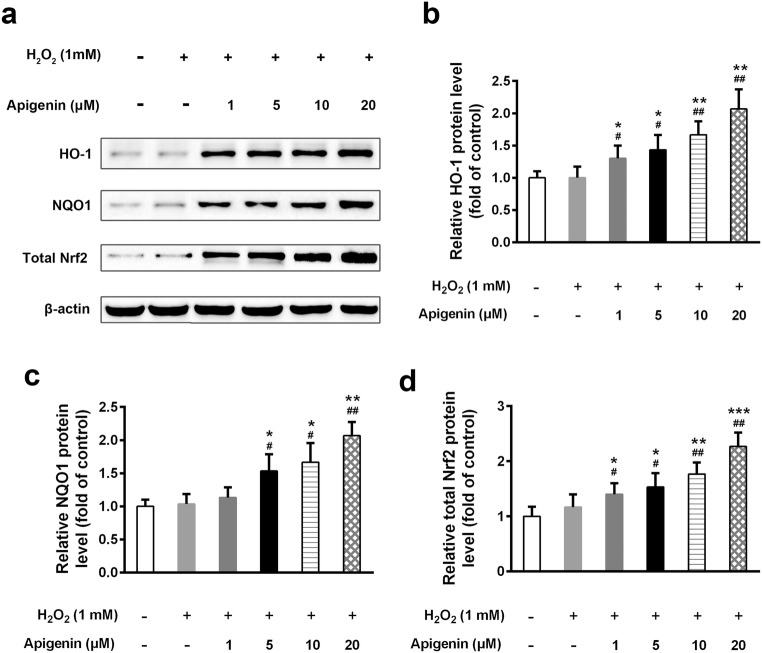
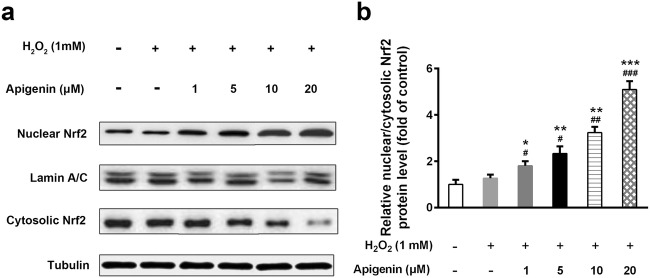
The transcription factor Nrf2 is an important regulator of cellular anti-oxidation and plays essential roles in redox balance. We found that the mRNA level of Nrf2 was markedly increased in apigenin-treated cells (Fig. 3a). Furthermore, apigenin also upregulated the mRNA levels of HO-1 and NQO1, both of which are downstream targets of Nrf2, under oxidative stress (Fig. 3b, c). We therefore assessed their protein levels and found that apigenin significantly increased the protein expression levels of Nrf2, as well as HO-1 and NQO1, in a dose-dependent manner (Fig. 4a–d). Interestingly, when we tested the nuclear and cytosolic Nrf2 expression, we observed that nuclear Nrf2 expression was significantly elevated by apigenin compared with untreated cells under H2O2 treatment (Fig. 5a, b). In contrast, cytosolic Nrf2 expression declined with increasing doses of apigenin. The ratio of nuclear to cytosolic Nrf2 levels was significantly elevated upon apigenin treatment. These results suggest that apigenin not only affects the transcriptional and translational expression of Nrf2 but also impacts its nuclear translocation.
Fig. 3.
Apigenin increases the mRNA expression of Nrf2 and its target genes in H2O2-treated human melanocytes. The relative mRNA levels of Nrf2 (a), HO-1 (b), and NQO1(c) were detected by qRT-PCR. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with H2O2-treated group
Fig. 4.
Apigenin elevated the protein levels of Nrf2 and its target genes in H2O2-treated human melanocytes. The protein expressions of Nrf2, HO-1, and NQO1 were determined by Western blot (a) and relative levels (b, c, and d) were analyzed. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated group; #p < 0.05, ##p < 0.01 compared with H2O2-treated group
Fig. 5.
Effect of apigenin treatment on Nrf2 nuclear translocation in H2O2-treated human melanocytes. Western blots of nuclear and cytoplasmic Nrf2 (a) were detected and the ratio of nuclear/cytosolic of Nrf2 (b) was analyzed. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated group; #p < 0.05, ##p < 0.01 compared with H2O2-treated group
Nrf2 knockdown blocks the functions of apigenin in H2O2 treated cells
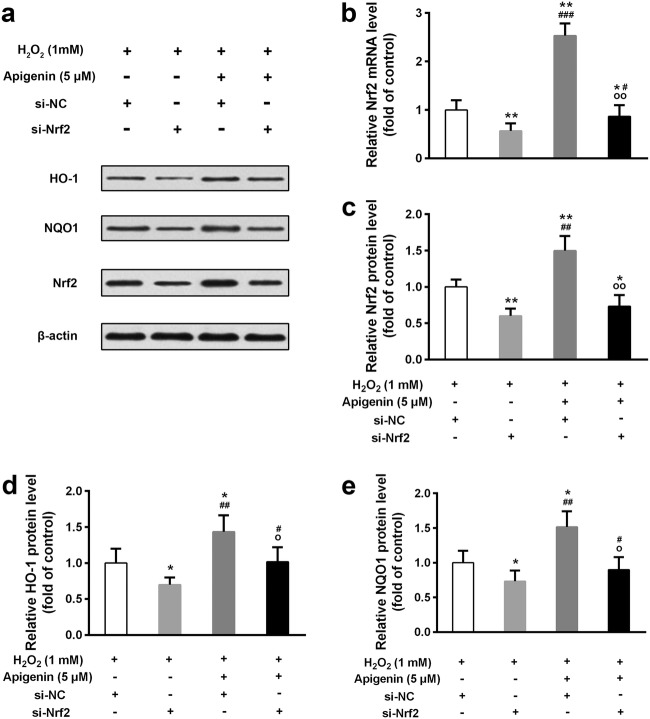
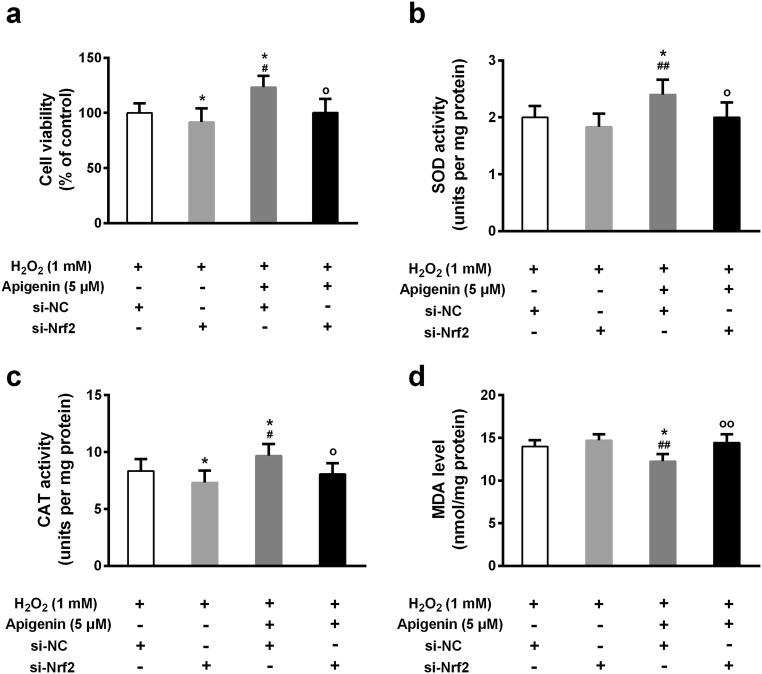
To confirm whether apigenin exerts its functions through regulating Nrf2, we knocked down Nrf2 and treated cells with or without apigenin. The efficiency of Nrf2 knockdown was confirmed by both quantitative real-time PCR and Western blotting (Fig. 6a–c). Although apigenin improved the mRNA and protein levels of Nrf2, the knockdown efficiency was dramatically high. The HO-1 and NQO1 protein levels were also downregulated in Nrf2 knockdown cells compared with control cells (Fig. 6d, e). We further tested the effects of apigenin on viability of Nrf2 knockdown cells. Intriguingly, in Nrf2 knockdown cells, apigenin (5 μM)-treated cells showed no significant change in cell viability compared with untreated cells (Fig. 7a). However, apigenin (5 μM) was still able to improve cell viability in control cells. Moreover, the same concentration of apigenin elevated SOD and CAT activities and repressed MDA levels in control cells, but not in Nrf2 knockdown cells (Fig. 7b–d). These results indicate that apigenin may affect anti-oxidation in cells mainly through regulating Nrf2 and its downstream target genes.
Fig. 6.
The upregulation effect of apigenin on Nrf2 and its target genes expressions in H2O2-treated human melanocytes was reduced by transfection of Nrf2 si-RNA. a Western blot was used to determine protein levels. b The relative mRNA levels of Nrf2 was detected by qRT-PCR. The relative protein levels of Nrf2 (c), HO-1 (d), and NQO1 (e) from Western blot were analyzed. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 compared with si-NC group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with si-Nrf2 group; op < 0.05, oop < 0.01 compared with Apigenin-si-NC group
Fig. 7.
The protective effect of apigenin against oxidative stress in H2O2-treated human melanocytes was abolished by transfection of Nrf2 si-RNA. a Cell viability was determined by MTT assay. SOD activity (b), CAT activity (c), and MDA level (d) were tested. Data are presented as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 compared with si-NC group; #p < 0.05, ##p < 0.01 compared with si-Nrf2 group; op < 0.05 compared with Apigenin-si-NC group
Apigenin has been considered an active flavonoid that protects the skin from carcinogenesis due to its multifunction in prevention of DNA damage and immunomodulatory ability (Patel et al. 2007). Besides, with low chemical toxicity, apigenin was found to possess the ability to penetrate the skin, expanding its potential in clinical applications (Li and Birt 1996; Merfort et al. 1994). For patients with vitiligo, easy application of apigenin on the skin could dramatically improve their quality of life. Therefore, it is important to investigate whether apigenin could attenuate vitiligo. Of note, apigenin is an anti-oxidant (Andueza et al. 2015), and oxidative stress is believed to be a major and early event occurring during the development of vitiligo. In fact, apigenin alleviated melanocyte apoptosis induced by dopamine and reduced ROS accumulation through various mechanisms, inhibiting the activation of p38, JNK, and Akt signalings (Lin et al. 2011). These signaling activations may induce the expression of various transcription factors. Thus, more specific and detailed mechanisms underlying the impact of apigenin on vitiligo lesions need to be revealed. In our present study, we treated melanocytes directly by H2O2, a reaction product during dopamine oxidation, and measured several markers of oxidative stress. A higher dose of apigenin was required to achieve the protective effect in our study, that treated melanocytes with H2O2 compared with the previous study (Lin et al. 2011), that treated with dopamine. However, further studies are necessary to confirm the efficiency of apigenin in vitiligo treatment by providing more convincing in vivo evidences in animal models.
Melanocytes from patients with vitiligo were more sensitive to ultra violet B, leading to increased cell apoptosis in comparison with normal melanocytes (Jimbow et al. 2001). This is mainly due to their compromised capacity of balancing oxidative stress (Jian et al. 2011; Jimbow et al. 2001), leading to the accumulation of ROS and H2O2 (Kang et al. 2018; Xie et al. 2016). We therefore proposed that apigenin could exert its anti-oxidant function in vitiligo melanocytes to improve cell viability. As expected, PIG3V cell viability was dramatically reduced when treated with H2O2. However, apigenin treatment significantly promoted cell viability under H2O2-induced oxidative damage. Apigenin was less toxic to a murine skin epidermal cell line JB6 P+ when its concentration was lower than 6.25 μM (Paredes-Gonzalez et al. 2014). However, we found that high concentration of apigenin was toxic, as 40 μM apigenin reduced viability of PIG3V cells. This difference may be caused by less sensitivity of melanocytes to apigenin compared with JB6 P+ epidermal cells. It is likely because the oxidative stress in melanocytes was reduced by apigenin, which on the other hand alleviated the cytotoxicity of apigenin. Nevertheless, the detailed mechanism of this phenomenon needs to be revealed, which may involve different modes of cell signaling activations in the two cell lines. In further clinical investigation and application, the dose of apigenin needs to be carefully considered.
Given the sensitivity of melanocytes to ROS and the ability of excessive ROS to induce cell death, the beneficial effect of apigenin on cell viability under H2O2 treatment may be attributed to its anti-oxidant property. Our results indicated that the activities of several important enzymes participating in anti-oxidant defense, including SOD, CAT, and GFH-Px, were significantly enhanced by apigenin under H2O2 treatment. While on the other hand, the MDA level was inhibited by apigenin. These results are consistent with the beneficial effects of apigenin on levels of SOD, CAT, GFH-Px, and MDA (Feng et al. 2017; Xu et al. 2016). In this context, our study served as the first demonstration of these beneficial functions of apigenin in melanocytes, providing evidences supporting the use of apigenin in vitiligo treatment.
Nrf2 is a transcription factor regulating a set of genes encoding phase II detoxification enzymes to protect cells against oxidative stress. Nrf2 normally localizes in the cytoplasm where it binds to kelch-like ECH associating protein 1 (Keap1) mediating its own ubiquitination (Itoh et al. 1999). Once cells are confronted with oxidative stress, Nrf2 is activated and released from Keap1, and subsequently translocates into the nucleus, where it stimulates the transcription of phase II enzymes such as HO-1 and NQO1. Nrf2 signaling was impaired under H2O2-induced oxidative damage (He et al. 2017; Jian et al. 2014). Of note, polyphenols, especially flavonoids, activate Nrf2 and stimulate its nuclear translocation (Kansanen et al. 2013; Köhle and Bock 2006). Apigenin is one type of flavonoids that has been shown to possess the Nrf2 activating function (Andueza et al. 2015; Feng et al. 2017; Paredes-Gonzalez et al. 2014). In the present study, we further confirmed that apigenin promoted the mRNA and protein expression of Nrf2 as well as its nuclear localization. Knockdown of Nrf2 expression significantly blocked the impacts of apigenin on regulation of oxidative stress and cell viability. These results suggest that apigenin may exert its anti-oxidant function via modulating Nrf2 expression and activation in melanocytes under H2O2-induced oxidative stress. In addition to apigenin, other phytochemicals such as curcumin were also documented to elevate Nrf2 expression (He et al. 2012; Khor et al. 2011; Yang et al. 2009). In contrast, nuclear factor k-light-chain enhancer of activated B cells (NF-κB) level was downregulated by curcumin (Li et al. 2016; Zeng et al. 2015). NF-κB is a crucial transcription factor modulating cell activities such as immune reactions. There may be a crosstalk between ROS and cell immune responses regulating the balance between Nrf2 and NF-κB. Interestingly, a recent study has demonstrated that mycoplasmal membrane lipoproteins are capable of regulating pro- and anti-inflammatory host cell responses, substantially through their effects on Nrf2 (Chernov et al. 2018), further demonstrating the role of Nrf2 in immune responses. Elevated ROS stimulated inflammation via activation of p65 subunit of NF-κB (Wenzel et al. 2017). It is possible that anti-oxidation by apigenin elevates Nrf2, which reduces ROS and represses NF-κB. However, it remains to be explored whether apigenin could also affect NF-κB and immune reactions in melanocytes.
Conclusion
In conclusion, this study explored the functions of apigenin in melanocytes under H2O2-induced oxidative stress. Apigenin significantly enhanced cell viability under H2O2 treatment compared with control. Apigenin increased SOD, CAT, and GSH-Px activities and inhibited MDA levels. Knockdown of Nrf2 by si-RNA significantly blocked apigenin functions in H2O2-treated melanocytes, suggesting that the anti-oxidant property of apigenin may be attributed to its regulation on Nrf2 and downstream target genes.
Funding information
The study was supported by the Scientific Research Projects of Weifang Health Care Commission (wfwsjk_2019_215).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baoxiang Zhang and Jing Wang contributed equally to this work.
References
- Agrawal D, Shajil E, Marfatia Y, Begum R. Study on the antioxidant status of vitiligo patients of different age groups in Baroda. Pigment Cell Res. 2004;17:289–294. doi: 10.1111/j.1600-0749.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- Andueza A, García-Garzón A, de Galarreta MR, Ansorena E, Iraburu MJ, López-Zabalza MJ, Martínez-Irujo JJ. Oxidation pathways underlying the pro-oxidant effects of apigenin. Free Radic Biol Med. 2015;87:169–180. doi: 10.1016/j.freeradbiomed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Chang Y, Li S, Guo W, Yang Y, Zhang W, Zhang Q, He Y, Yi X, Cui T, An Y, Song P, Jian Z, Liu L, Li K, Wang G, Gao T, Wang L, Li C. Simvastatin protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J Investig Dermatol. 2017;137:1286–1296. doi: 10.1016/j.jid.2017.01.020. [DOI] [PubMed] [Google Scholar]
- Chernov VM, Chernova OA, Mouzykantov AA, Lopukhov LV, Trushin MV. Mycoplasmas and novel HO-1 inducers: recent advances. Curr Pharm Des. 2018;24:2236–2240. doi: 10.2174/1381612824666180716170128. [DOI] [PubMed] [Google Scholar]
- Feng X, Yu W, Li X, Zhou F, Zhang W, Shen Q, Li J, Zhang C, Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–122. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- Gavalas NG, Akhtar S, Gawkrodger DJ, Watson PF, Weetman AP, Kemp EH. Analysis of allelic variants in the catalase gene in patients with the skin depigmenting disorder vitiligo. Biochem Biophys Res Commun. 2006;345:1586–1591. doi: 10.1016/j.bbrc.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Guan CP, Zhou MN, Xu AE, Kang KF, Liu JF, Wei XD, Li YW, Zhao DK, Hong WS. The susceptibility to vitiligo is associated with NF-E2-related factor2 (Nrf2) gene polymorphisms: a study on Chinese Han population. Exp Dermatol. 2008;17:1059–1062. doi: 10.1111/j.1600-0625.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- Halder Rebat M., Chappell Johnathan L. Vitiligo Update. Seminars in Cutaneous Medicine and Surgery. 2009;28(2):86–92. doi: 10.1016/j.sder.2009.04.008. [DOI] [PubMed] [Google Scholar]
- He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. 2012;3:94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. Dysregulated autophagy increased melanocyte sensitivity to H 2 O 2-induced oxidative stress in vitiligo. Sci Rep. 2017;7:42394. doi: 10.1038/srep42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li C, Gao T. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J Investig Dermatol. 2011;131:1420–1427. doi: 10.1038/jid.2011.56. [DOI] [PubMed] [Google Scholar]
- Jian Z, et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J Investig Dermatol. 2014;134:2221–2230. doi: 10.1038/jid.2014.152. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Chen H, Park JS, Thomas P. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144:55–65. doi: 10.1046/j.1365-2133.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- Kang P, Zhang W, Chen X, Yi X, Song P, Chang Y, Zhang S, Gao T, Li C, Li S. TRPM2 mediates mitochondria-dependent apoptosis of melanocytes under oxidative stress. Free Radic Biol Med. 2018;126:259–268. doi: 10.1016/j.freeradbiomed.2018.08.022. [DOI] [PubMed] [Google Scholar]
- Kansanen E, Kuosmanen SM, Leinonen H, Levonen A-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- Köhle C, Bock KW. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem Pharmacol. 2006;72:795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Li B, Birt DF. In vivo and in vitro percutaneous absorption of cancer preventive flavonoid apigenin in different vehicles in mouse skin. Pharm Res. 1996;13:1710–1715. doi: 10.1023/A:1016453009818. [DOI] [PubMed] [Google Scholar]
- Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Lin M, Lu SS, Wang AX, Qi XY, Zhao D, Wang ZH, Man MQ, Tu CX. Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J Dermatol Sci. 2011;63:10–16. doi: 10.1016/j.jdermsci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Abul H, Al-Saleh Q, Haines D, Burleson J, Morgan G. Peripheral T-cell activation in non-segmental vitiligo. J Dermatol. 1998;25:637–640. doi: 10.1111/j.1346-8138.1998.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Abul H, Haines D, Al-Saleh C, Khajeji M, Whaley K. Decreased total numbers of peripheral blood lymphocytes with elevated percentages of CD4+ CD45RO+ and CD4+ CD25+ of T-helper cells in non-segmental vitiligo. J Dermatol. 2002;29:68–73. doi: 10.1111/j.1346-8138.2002.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Haines D, Abul H, Abu-Donia M. Neuroimmune processes contributing to pathogenesis of vitiligo. Rev Int Serv Sante Forces Armees. 2006;79:113–119. [Google Scholar]
- Maresca V, Roccella M, Roccella F, Camera E, del Porto G, Passi S, Grammatico P, Picardo M. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Investig Dermatol. 1997;109:310–313. doi: 10.1111/1523-1747.ep12335801. [DOI] [PubMed] [Google Scholar]
- Merfort I, Heilmann J, Hagedorn-Leweke U, Lippold B. In vivo skin penetration studies of camomile flavones. Pharmazie. 1994;49:509–511. [PubMed] [Google Scholar]
- Natarajan VT, Singh A, Kumar AA, Sharma P, Kar HK, Marrot L, Meunier JR, Natarajan K, Rani R, Gokhale RS. Transcriptional upregulation of Nrf2-dependent phase II detoxification genes in the involved epidermis of vitiligo vulgaris. J Investig Dermatol. 2010;130:2781–2789. doi: 10.1038/jid.2010.201. [DOI] [PubMed] [Google Scholar]
- Paredes-Gonzalez X, Fuentes F, Su Z-Y, Kong A-NT. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P+ cells through epigenetics modifications. AAPS J. 2014;16:727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- Qiu L, Song Z, Setaluri V. Oxidative stress and vitiligo: the Nrf2–ARE signaling connection. J Investig Dermatol. 2014;134:2074–2076. doi: 10.1038/jid.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter KU, Salem MA, Gibbons NC, Maitland DJ, Marsch E, Elwary SM, Healey AR. Blunted epidermal l-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: epidermal H2O2/ONOO−-mediated stress in vitiligo hampers indoleamine 2, 3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling. FASEB J. 2012;26:2471–2485. doi: 10.1096/fj.11-201897. [DOI] [PubMed] [Google Scholar]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment cell & melanoma research. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- Takekoshi S, Nagata H, Kitatani K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J Exp Clin Med. 2014;39:116–121. [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Wang Q-Q, Cheng N, Yi W-B, Peng S-M, Zou X-Q. Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorg Med Chem. 2014;22:1515–1521. doi: 10.1016/j.bmc.2014.01.038. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Kossmann S, Munzel T, Daiber A. Redox regulation of cardiovascular inflammation - Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol Med. 2017;109:48–60. doi: 10.1016/j.freeradbiomed.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Whitton ME et al (2015) Interventions for vitiligo. Cochrane Database Syst Rev [DOI] [PMC free article] [PubMed]
- Xie H, Zhou F, Liu L, Zhu G, Li Q, Li C, Gao T. Vitiligo: how do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci. 2016;81:3–9. doi: 10.1016/j.jdermsci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Xu X, Li M, Chen W, Yu H, Yang Y, Hang L (2016) Apigenin attenuates oxidative injury in ARPE-19 cells thorough activation of Nrf2 pathway. Oxidative Med Cell Longev 2016 [DOI] [PMC free article] [PubMed]
- Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Baysal V, Inaloz H, Can M. The role of oxidants and antioxidants in generalized vitiligo at tissue level. J Eur Acad Dermatol Venereol. 2004;18:683–686. doi: 10.1111/j.1468-3083.2004.01080.x. [DOI] [PubMed] [Google Scholar]
- Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, Chakrabarti S, Wu L, Wang J, Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kappaB both in vitro and in vivo. J Mol Cell Cardiol. 2015;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]