Abstract
The association of plant with microorganisms, such as dark septate endophytic fungi, has mitigated the harmful effects of chemical, physical, and biological agents on the host. The objective of this work was to evaluate the interaction of the dark septate endophytic fungi with cowpea plants under salt stress. Endophytic fungi were isolated from Vochysia divergens root system, and molecular identification of fungi was performed by sequencing the ITS region. We selected and identified Sordariomycetes sp1-B’2 and Melanconiella elegans-21W2 for their ability to infect V. divergens root in vitro with development of typical dark septate fungi structures. Cowpea plants—inoculated or not inoculated with Sordariomycetes sp1-B’2 and M. elegans 21W2—were cultivated in 5-L pots under greenhouse conditions and submitted to four different electrical conductivities of irrigation water (1.2, 2.2, 3.6, and 5.0 dS m−1). The salinity caused decrease in leaf concentration of K and increased leaf concentration of calcium, sodium, and chlorine; and no influence of dark septate endophytic fungi was observed in these responses. On the other hand, root colonization with Sordariomycetes sp1-B’2 and M. elegans 21W2 resulted in improved nutrition with N and P in cowpea under salt stress, favoring the growth and rate of liquid photosynthesis. However, such positive responses were evident only at moderate levels of salinity.
Keywords: Vigna unguiculata L., Salinity, Plant-fungi interaction, Photosynthesis, Mineral nutrition
Introduction
The water crises observed in different regions of the planet, and particularly in semi-arid regions, have led to the increasing interest in the use of lower-quality water, such as brackish water. However, indiscriminate use of these water sources can reduce crop yield and cause soil salinization. Nonetheless, there are several management strategies for the soil-water-plant system that can minimize the impacts of using water with moderate salinity, which can favor the sustainability of agricultural systems in the semi-arid environments [1]. In addition to these techniques, the use of microorganisms, such as mycorrhizal and endophytic fungi, as salt stress-mitigating agents, has been proposed [2–5].
Endophytes are microorganisms capable of colonizing internal tissues of plant organs, at least in some period of their life cycle, without causing visible symptoms of disease or morphological alterations [6]. The diversity of the endophytic community varies with plant species [7], host genotype [8], type of tissue analyzed [9], host age [10], climatic factors [11], and geographic distribution [8, 9].
Dark septate endophytic fungi (DSE) are important root endophytes involved with host resistance to environmental stress. These fungi are characterized by the presence of brown to black mycelium with septate and melanized hyphae, capable of infecting the intercellular and intracellular spaces of root tissues where it differentiates structures of resistance called microsclerotia inside the cells within a wide range of plant species [12–14].
DSE can occur from the tropics to the Arctic and are particularly common in stressful environments, such as cold, nutritionally poor soils, alpine or subalpine ecosystems, environments with high salinity, and polar regions [15–21]. The abundance and disseminated occurrence of DSE in different biomes, colonizing more than 600 species of hosts distributed in 320 genera and 114 families, suggests lack of specificity of the host and an important role of these microorganisms in natural ecosystems [15].
In addition to natural systems, DSE are present in agroforestry systems associated with plant species of economic interest such as soybean [20, 22], rice [23], tomato [24], orchids [25], and forest species [26]. To the best of our knowledge, we found no reports of the DSE-cowpea plants association.
Cowpea is an important crop cultivated in different regions of the world, being an important source of vegetal protein for human consumption in semi-arid regions, such as the population of Brazilian Northeast [27]. This species is widely cultivated in intercropping systems and crop rotation in this region, contributing to the improvement of soil properties due to symbiotic interaction with nitrogen-fixing bacteria [28] and also with mycorrhizal fungi [29].
Dark septate fungi act as symbiont and promote host growth, facilitating the absorption of nutrients, such as nitrogen, phosphorus, and micronutrients [21, 30–35]. In plants under abiotic stress, the association of roots with DSE fungi also promotes changes in physiological and biochemical responses, resulting in improved host performance [36, 37]. For example, the presence of DSE increase tolerance of rice to water stress, reducing the oxidative stress, indicating that the beneficial effects of DSE is not due only to nutrient uptake [23]. In maize under heavy metal stress, the improvement of efficiency of photosynthesis and the decrease of translocation factor of Pb, caused by DSE fungi colonization, were efficient strategies to improve Pb tolerance of host plants [36].
The above results show that DSE fungi acts as a mitigating agent in plants under abiotic stress, and this benefic effect can be related to changes in physiological, biochemical, and nutritional responses. Since salinity is a mineral stress, our hypothesis is that the beneficial effect of DSE fungi on cowpea plants under salt stress can be related to the control on the acquisition of essential nutrients and potentially toxic ions, especially Na+ and Cl−. In this context, the objective was to evaluate the responses of cowpea plants associated with DSE fungi, when irrigated with water of different salinity levels. In this study, we selected DSE from Vochysia divergens roots, an invasive species in the Brazilian Pantanal, to evaluate the functional role of these fungi in plant of agricultural interest under salt stress conditions.
Material and methods
Isolation of root endophytes from Vochysia divergens Pohl
The fungi were obtained from the roots of Vochysia divergens Pohl (Vochysiaceae). This tree is native to the Brazilian Cerrado, popularly known in Brazil as ‘Cambará’, and is considered an invasive species in the Pantanal, preferentially occurring in open areas and alluvial floodplains forming monospecific stands due to its rapid and extensive propagation [38, 39]. This species was chosen to obtain root endophytes because of its resistance to the long drought and flooding periods of the Pantanal [39]. V. divergens roots are associated with dark septate endophytic fungi, reaching root colonization rates close to 50% [40], and the functional roles of these fungi associated with V. divergens are not known. The association between V. divergens and root endophytes interferes in the metabolic profile of the host, contributing to qualitative changes in its phytochemical profile depending on the fungal species associated [41].
Plant material was collected in the area (16° 35′ 22.9″ S; 56° 47′ 83.4″ W) in the period of low soil moisture (September–dry period). Endophytes were isolated from the roots of V. divergens using sodium hypochlorite for superficial disinfestation of the roots [42]. Root fragments were incubated in potato dextrose agar medium. The isolates were purified and stored in flasks containing PDA medium under refrigeration.
Part of the roots collected was previously fixed in FAA (formaldehyde–acetic acid–alcohol) for observation of fungal structures after the bleaching of plant tissues [43]. The segments were diaphonized with 2.5% KOH heated in a water bath for approximately 30 min in boiling water. Subsequently, KOH was drained and the roots were rinsed with tap water. The pH was acidified with 1% HCl for 5 min in water bath at 65 °C. The acid was drained from the fragments and then they were stained with 0.05% trypan blue in lactoglycerol (1:1:1, lactic acid:glycerol:water) for 15 min. The dye solution was drained and the fragments were preserved in lactoglycerol. Fungal structures were classified according to specialized literature [44]. The frequency of colonization of these structures was calculated according to the gridline intersection method [45], observing 100 squares under × 400 magnification.
Selection of dark septate strains
Potentially dark septate strains were selected based on morphological characteristics of the mycelium and capacity to infect seedlings of the host. Strains showing brown or dark mycelium with septate and melanized hyphae were activated in PDA medium at 28 °C for 7 days. Mycelium fragments were inoculated close to the radicles of V. divergens seedlings obtained according to [40]. The plates were kept under natural illumination at 28 °C. After 2 weeks, the radicles were processed according to [43] and observed under the microscope. The presence of microsclerotia and brown septate hyphae were considered indicative of dark septate.
Identification of dark septate strains
Molecular identification was carried out only for strains capable of infecting V. divergens radicles in vitro with microsclerotial differentiation. The total DNA of the strains 21W2 and B’2 was obtained with the extraction kit (Norgen Biotek Corp., Canada) and the ITS region was amplified with the primers ITS1 and ITS4 [46]. The amplicons were purified using polyethylene glycol 6000 (PEG) and sequenced by the Sanger method (BigDye Terminator Cycle Sequencing). The sequences were aligned (MEGA 7) and compared with sequences obtained from the GenBank database through the nBLAST tool (http://www.ncbi.nlm.nih.gov/) and deposited in GenBank (Access Number): MK680501 (Melanconiella elegans-21W2) and MK680499 (Sordariomycetes sp-B’2).
Inoculation of cowpea roots under salt stress
The experiment was carried out in a greenhouse at the Agricultural Engineering Department, located in the experimental area of the Agrometeorological Station of the Federal University of Ceará (UFC), Brazil, with coordinates 3° 45′ S, 38° 33′W and average altitude of 20 m.
A completely randomized 4 × 3 factorial design was used, represented by four saline concentrations of irrigation water (1.2; 2.2; 3.6; and 5.0 dS m−1) and three treatments of inoculation of plants with dark septate endophytic fungi (without inoculation and inoculation with two different strains). The experiment was conducted with 10 replicates, totaling 120 experimental plots, each plot represented by a pot with one plant.
The experiment used seeds of cowpea (Vigna unguiculata (L.) Walp.), cultivar ‘Setentão’, obtained from the seed bank of the Plant Science Department–UFC. A pre-germination was performed on a tray with sterilized river sand to select uniform plants for the experiment. Five days after germination, the seedlings were transferred to pots containing 5.0 L of substrate, composed of river sand sterilized by sodium hypochlorite, mixed with vermiculite at 1:1 proportion, with the following characteristics: 763 g kg−1 of coarse sand, 201 g kg−1 of fine sand, 20 g kg−1 of silt, 16 g kg−1 of clay, 14 g kg−1 of natural clay, sand textural class, degree of flocculation of 8.0 g kg−1, bulk density 1.54 g cm−3, particle density of 2.59 g cm−3, pH in water of 7.5, EC of 0.30 dS m−1, Na+ of 0.11 cmolc kg−1, C/N ratio of 11 g kg−1, and OM of 1.34 g kg−1.
The strains Sordariomycetes sp1-B’2 and M. elegans 21W2 were used for inoculation, with the following treatments: without inoculation (NI), plants inoculated with 21 W2 strain, and plants inoculated with B’2 strain. Endophytic fungi were activated on PDA plates (infusion of 200 g potato, 20 g dextrose, 15 g agar, and water-q.s. 1000 mL) for 7 days at room temperature.
Fungal inoculation was performed 5 days after germination, at the moment of transferring cowpea seedlings to the 5.0-L pots. The inoculation was performed by adding in the planting hole two fragments of culture medium (4 × 2 cm) containing mycelium of the strains used. In the control treatment, the same procedure was performed using fragments of sterile culture medium.
Fertilization began at 7 days after germination and consisted of weekly application of 250 mL per pot of Hoagland nutrient solution. Irrigation with water of different salinity levels began at 20 days after planting and continued until the end of the experiment. The highest levels of irrigation water salinity were obtained by adding in well water (1.2 dS m−1) the salts: sodium chloride (NaCl), calcium chloride (CaCl2.2H2O), and magnesium chloride (MgCl2.6H2O), dissolved at 7:2:1 ratio (proportion commonly observed in waters of Northeast Brazil), assuming the relationship between the irrigation water electrical conductivity and their concentrations (mmolc L−1 = EC × 10). Irrigation was performed daily, in a localized manner, with a leaching fraction of 0.15.
Plant measurements
At 57 days after planting, the rates of net carbon assimilation (A), transpiration (E), and stomatal conductance (gs) were measured in the first completely mature leaf from the apex, using an infrared gas analyzer (IRGA, LI-6400XT, Licor, USA). Readings were taken between 8 and 10 h, under saturating light (1500 μmol m−2 s−1) and under natural conditions of temperature and CO2 concentration.
Plants were then collected and their leaf area was determined (LI–3100, Area Meter, Li-Cor., In. Lincoln, NE, USA). Shoot and root materials were dried in a forced air circulation oven at 60 °C, to obtain the dry biomass. Samples of leaves, previously dried in the oven, were ground in a Wiley-type mill and used for extraction and determination of Na+, Ca2+, K+, N, P, and Cl− contents.
Root samples were separately collected to evaluate the colonization by dark septate endophytic fungi. Roots that were thinner and at least 1 cm long were selected for this purpose. Then, these roots were washed in tap water using a sieve and subjected to clearing [47]. In order to determine the frequency of root system colonization, 40 root fragments were separated and fixed on slides for observation under light microscope with × 40 magnification. The percentage of colonization by the endophytic dark septate fungus was determined using the method of [48]. On the slides, all the root/grid intersection points were visualized and considered positive when the structures typical of the dark septate endophytic fungi were observed. Colonization percentage was calculated based on three replicates, considering the number of typical structures observed in the 40 root fragments in each slide.
Statistical analyses
Initially, the Shapiro-Wilk test was used to verify the normality of the data. As the data followed normal distribution, the analysis of variance was then performed by the F test. Regression analysis was performed to show the effects of salinity and the interaction between salinity and DSE. Tukey’s test was used to compare the means of inoculated treatments within the same salinity level. For all evaluations, significance levels of 0.05 and 0.01 were used. The statistical procedures were conducted using the ASSISTAT 7.5 BETA program [49].
Results
Isolation, selection, and identification of root endophytes of Vochysia divergens Pohl
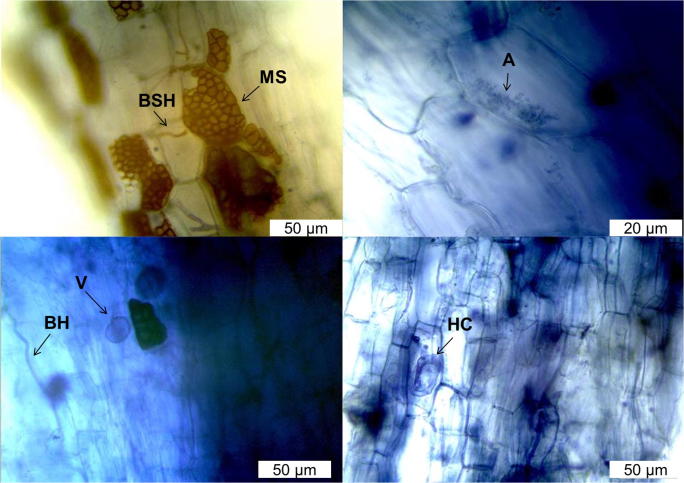
The preparations showed that V. divergens internally have a set of structures characteristic of dark septate endophytic and arbuscular mycorrhizal fungi (Fig. 1; Table 1). Dark septate fungi were characterized by brown septate hyphae (BSH) growing inter- and intracellularly in the root cortex. Another characteristic typical of this endophytic group was the differentiation of structures called microsclerotium (MS) intracellularly. These structures were characterized by intracellular masses of hyphae in the plant cell. Besides the colonization by dark septate fungi, V. divergens roots showed interaction with arbuscular endomycorrhizal fungi. Different structures, differentiated by this group of fungus, were observed, such as hyphal coil (HC), vesicles (V), and arbuscules (A) located intracellularly in the host tissue (Fig. 1; Table 1).
Fig. 1.
Morphological structural differences observed in V. divergens roots: microsclerotium (MS), vesicle (V), blue hypha (BH), brown septate hypha (BSH), arbuscule (A), and hyphal coil (HC)
Table 1.
Frequency of colonization (%) of microsclerotia (MS), brown septate hyphae (BSH), blue hypha (BH), vesicle (V), arbuscule (A), and hyphal coil (HC)
| MS | BSH | BH | V | A | HC |
|---|---|---|---|---|---|
| 67.7* ± 5.7 | 72.7 ± 5.6 | 84.7 ± 2.3 | 47.7 ± 5.2 | 81.7 ± 2.6 | 27.7 ± 1.7 |
*Values represent the mean ± standard error of the mean. n = 3
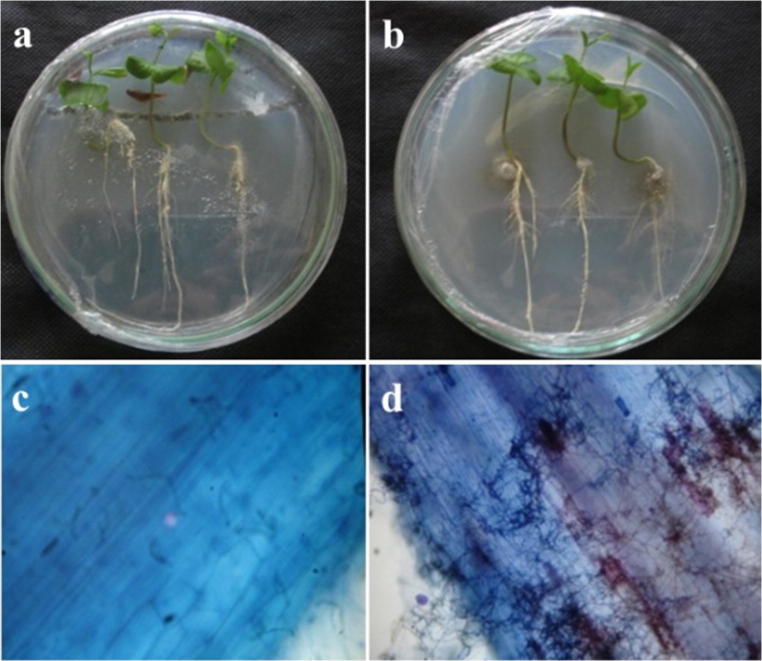
The observations indicate the existence of 10 strains with morphological characteristics of dark septate (brown to black and septate hyphae), but only two strains (21W2 and B’2) were able to infect V. divergens roots kept in vitro (Fig. 2). These two strains colonized the inter- and intracellular spaces of the root tissue, with dark septate hyphae and differentiation of microsclerotium.
Fig. 2.
V. divergens associated with the strain B’2 in in-vitro conditions. Seedlings were cultivated without (a) and with inoculation (b) on Petri dishes containing mineral medium. Radicles of seedlings not inoculated (c) and associated with the fungus B’2 (d) were observed under the optical microscope with × 100 magnification
Molecular identification of the strain 21W2 indicated high identity (> 98% and 100% coverage) with the ITS sequence of the fungus Melanconiella elegans (KJ173701.1) (hereinafter referred to as 21W2). Differently, the ITS sequence of the strain B’2 does not have sufficient identity capable of identifying at species level (> 97%), allowing only its identification as a representative of the class Sordariomycetes (96% of identity and 100% of coverage with ITS sequence of the fungus Sordariomycetes sp. V19-540–KJ439202.1) (hereinafter referred to as B’2).
Colonization rate and cowpea growth
Colonization rate, growth variables (leaf area, shoot dry biomass, and root dry biomass), leaf gas exchanges (photosynthesis and stomatal conductance rates), and N and P contents were influenced by the interaction between salinity and inoculation with endophytic fungi (p < 0.01 or p < 0.05) (Table 2). On the other hand, transpiration rate and Na, Cl, Ca, and K contents were influenced only by the salinity factor (p < 0.01 or p < 0.05).
Table 2.
Summary values of variance analysis for colonization rate (CR), photosynthetic rate (A), stomatal conductance (gs), leaf area, shoot dry mass (SDM), roots dry mass (RDM), and leaf concentration of N, P, Ca, K, Na, and Cl in cowpea plants grown under different salinity levels and different inoculation treatments with DSE fungi
| Mean square | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source of variation | DF | CR | A | gs | DF | Leaf area | SDM | RDM |
| Salinity (A) | 3 | 80.52** | 25.838** | 0.140** | 3 | 2849611.87** | 4858541.42** | 1189254.78** |
| Fungi (B) | 2 | 16593.36** | 13.364** | 0.021** | 2 | 148290.158** | 72432.70* | 353454.15** |
| A × B | 6 | 142.87** | 10.813** | 0.011** | 6 | 112424.358** | 58591.38* | 40449.86* |
| Error | 24 | 8.58 | 1.519 | 0.003 | 108 | 23101.869 | 22511.45 | 19468.09 |
| CV% | - | 6.80 | 10.00 | 20.94 | - | 14.52 | 13.48 | 18.23 |
| Source of variation | DF | N | P | Ca | K | Na | Cl | |
| Salinity (A) | 3 | 251.22** | 2.26** | 30.42** | 77.48** | 3.50** | 26721.18** | |
| Fungi (B) | 2 | 40.90** | 7.81** | 0.40ns | 1.55ns | 0.01ns | 24.77ns | |
| A × B | 6 | 4.33** | 1.01** | 0.20ns | 5.07ns | 0.01ns | 6.74ns | |
| Error | 48 | 0.87 | 0..20 | 0.50 | 3.87 | 0.04 | 70.12 | |
| CV% | - | 5.13 | 9.26 | 6.77 | 9.17 | 17.87 | 7.92 | |
**,*, and nsSignificant at 0.01 and 0.05 probability, and not significant by the F test, respectively. DF degrees of freedom, CV coefficient of variation
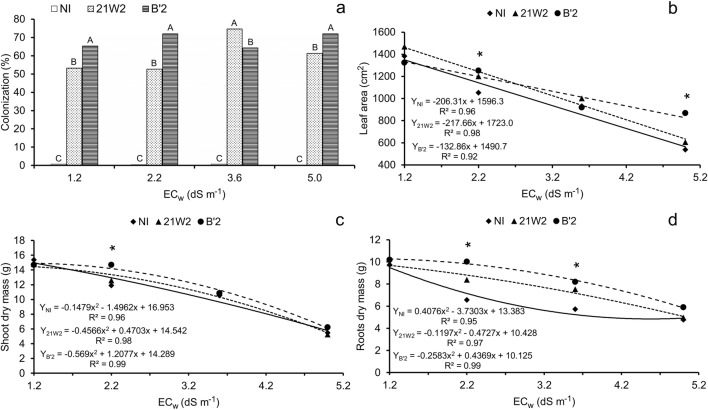
The rates of root colonization by dark septate fungi, measured at the end of the experiment, were significant for both 21W2 and B’2 (Fig. 3a). On average, the isolate B’2 had higher colonization rate, being inferior to 21W2 only in the treatment with 3.6 dS m−1. Although it was not possible to fit a mathematical model, it is clear that salinity did not negatively influence the rate of root colonization.
Fig. 3.
Root colonization (a), leaf area (b), shoot dry mass (c), and root dry mass (d) of cowpea plants irrigated with saline water and inoculated with dark septate endophytic fungi. NI = without inoculation; 21W2 = roots inoculated with strain 21W2; and B’2 = roots inoculated with strain B’2. Fig. 3a: bars with the same letter do not differ by Tukey’s test (p < 0.01), n = 3. Fig. 3 b, c, and d: *significant differences by Tukey’s test (p < 0.05), n = 10
Leaf area (Fig. 3b), shoot dry mass (Fig. 3c), and root dry mass (Fig. 3d) data decreased as the concentration of salts in the irrigation water increased, following linear or quadratic models. In general, treatments that were inoculated showed higher means compared with the control for the same level of irrigation water salinity, with significant differences for the three variables studied.
The stress linearly reduced cowpea leaf area in the three treatments of inoculation, and the differences between them were significant at the highest salinity level. The increase in electrical conductivity from 1.2 to 5.0 dS m−1 caused reductions of 58.2%, 56.6%, and 38.0% in leaf area for the treatments NI, 21W2, and B’2, respectively, and this last-mentioned isolate stood out at this level of severe stress (Fig. 3b).
In terms of root biomass production, the differences between treatments with and without inoculation were higher at the intermediate levels of salinity (2.2 and 3.6 dS m−1). For these salinity levels, the degree of reduction in the treatment without inoculation was 2.4 and 4.0 times higher than that observed in the treatments 21W2 and B’2, respectively. The reductions observed in the treatment of 5.0 dS m−1, compared with the lowest salinity level (1.2 dS m−1), were similar between treatments with and without inoculation, reaching values of 48.2, 45.4, and 43.0%, respectively, in the treatments NI, 21W2, and B’2 (Fig. 3d). Similar results were found for shoot biomass production (Fig. 3c), but the differences between treatments with and without inoculants were much lower than those observed for root biomass (Fig. 3d), even at the intermediate levels of salinity.
Leaf gas exchanges
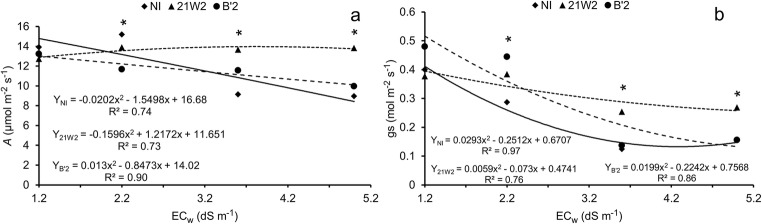
Increasing irrigation water salinity from 1.2 dS m−1 to 5.0 dS m−1 caused reductions in the photosynthetic rate of 40.0, 0.6, and 22.4%, respectively, in the treatments NI, 21W2, and B’2 (Fig. 4a). In the treatment with the fungus 21W2, a quadratic response was observed, with reduction in the photosynthetic rate from the salinity level of 3.8 dS m−1. Significant differences between the treatments with inoculation were observed from the salinity level of 3.6 dS m−1, when the NI treatment showed lower means compared with the inoculated treatments, demonstrating that the plant-dark septate fungus association improved the photosynthetic rate of cowpea plants. Improvement in photosynthetic rate seems to be related to the maintenance of higher values of stomatal conductance (Fig. 4b), and higher values were found in inoculated plants, especially with the strain 21W2.
Fig. 4.
Net photosynthetic rate-A (a) and stomatal conductance-gs (b) of cowpea plants irrigated with saline water and inoculated with dark septate endophytic fungi. NI = without inoculation; 21W2 = roots inoculated with strain 21 W2; and B’2 = roots inoculated with strain B’2. *significant differences by Tukey’s test (p < 0.05). n = 3
Mineral contents
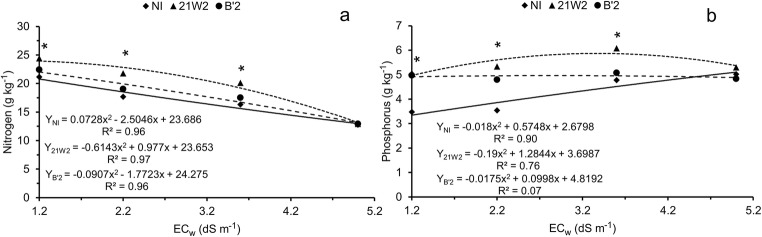
Leaf contents of nitrogen decreased with increasing levels of irrigation water salinity (Fig. 5a). However, plants inoculated with dark septate endophytic fungi showed higher values, except at the highest level of salinity. Inoculated plants also had higher leaf contents of P (Fig. 5b), especially at low and intermediate levels of salinity.
Fig. 5.
Leaf concentrations of nitrogen (a) and phosphorus (b) of cowpea plants irrigated with saline water and inoculated with dark septate endophytic fungi. NI = without inoculation; 21W2 = roots inoculated with strain 21W2; and B’2 = roots inoculated with strain B’2. *significant differences by Tukey’s test (p < 0.05). n = 5
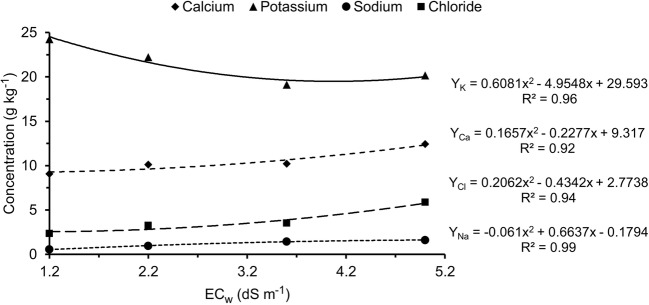
Salinity caused reduction in potassium contents and increase in calcium, sodium, and chloride contents (Fig. 6), and these responses were not affected by the inoculation of dark septate endophytic fungi. Increase in irrigation water salinity from 1.2 dS m−1 to 5.0 dS m−1 caused increment of more than 200% in the sodium content of cowpea leaves. It is worth emphasizing that, for the chloride content, the increment in salinity from 1.2 dS m−1 to 2.2 dS m−1 was already significant and the increment from 1.2 dS m−1 to 5.0 dS m−1 led to an increase of more than 140%.
Fig. 6.
Leaf concentrations of calcium, potassium, sodium, and chloride of cowpea plants as a function of levels of irrigation water salinity. n = 5
Discussion
The strains Melanconiella elegans-21W2 and Sordariomycetes sp.-B’2 infected the root system and showed characteristics of dark septate in the roots of both V. divergens and cowpea plants. Sordariomycetes is a class commonly reported in the communities of root endophytic fungi of different hosts in Brazilian biomes [8, 42, 50] and is one of the representative classes of dark septate fungi [51, 52].
The colonization rates of DSE in cowpea roots were high under control conditions and remained high even at high levels of salinity (Fig. 3a), demonstrating the capacity of resistance of endophytic fungi under salt stress conditions [17–20, 23].
Such ease of plant root colonization by DSE has been observed under different natural conditions, from regions of Antarctica to areas of temperate, tropical, and arid and semi-arid climates [8, 15, 53, 54]. For instance, the characteristic structures of dark septate fungi (MS and BSH) in V. divergens roots (Fig. 1; Table 1) have also been reported in other plant species of the Brazilian Pantanal, including representatives of the families Combretaceae [42], Lamiaceae [50], Vochysiaceae, and Poaceae [40], and in various hosts of other humid areas, such as Serratula tinctoria L, Betonica officinalis, Lycopodiella inundata (L.) Holub, and Carex sp [55]. On the other hand, [56] observed colonization of endomycorrhizal and dark septate endophytic fungi in different grass species in southern India. According to [57], myelination of the hypha causes DSE to be more resistant and may be related to the defense mechanism of the fungus under more severe environmental conditions.
Dark septate endophytes fungi were shown to increase the tolerance of rice plants to stress caused by water deficiency [23]. Cowpea growth data (Fig. 3) demonstrate that plants inoculated with the fungal isolates were significantly more tolerant to the negative effects of salinity than non-inoculated plants, and the responses were more evident at the intermediate levels of salinity, notably for the biomass data. These results are similar to those observed in other crops, such as cucumber [58] and soybean [2, 59], where plants inoculated with endophytic fungi also had higher values of biometric variables than non-inoculated plants under salt stress conditions. Such behavior can be explained by the capacity of endophytic fungi to produce growth-inducing phytohormones, especially indoleacetic acid and gibberellins [2, 59, 60].
Inoculated plants were also superior in terms of photosynthesis and stomatal conductance (Fig. 4), showing better physiological performance at the end of the experiment. However, the instantaneous measurements of photosynthesis were not similar to those of the growth data (Fig. 3), which reflect the whole response of the plant along the entire experimental period. In relation to that, it was observed that the inoculation of B’2 caused the best response in terms of growth whereas the inoculation of 21W2 led to the best physiological response of leaves at the end of the experiment. It becomes clear, however, that root colonization with dark septate endophytic fungi results in benefits to plants, compared with not inoculated ones. Such beneficial effect of endophytic fungi on C assimilation rate has also been observed in maize plants under conditions of stress by heavy metals [36, 61].
DSE-rice plants association increasing the tillering and nutrients uptake, especially N (due to an enhanced affinity for N uptake) and P [33]. Plants associated with the fungus 21W2 showed higher contents of N and P than the plants of the other treatments (Fig. 5). This result is consistent with the evaluations of photosynthetic rate, which was higher in plants inoculated with this strain (Fig. 4), indicating that their leaves showed better nutritional status at the end of the experiment, resulting in higher capacity for C assimilation.
Lower contents of nutrients in plants under salt stress are mainly due to the osmotic effects and predominance of some ionic species in saline media, which act by competing with essential nutrients for absorption sites in the root cell membranes [62]. In the case of N, the reduction caused by salt stress has been attributed to the excess of chloride, which causes decrease in nitrate absorption due to the direct competition between these ions for the same transporter, or to reduced water flow caused by the excess of salts in the root zone [1, 63].
Dark septate endophytic fungi facilitate the absorption of nutrients such as N and P [21, 30, 32, 33, and]. Therefore, the higher contents of P and N in the leaves of cowpea inoculated with dark septate endophytic fungus (Fig. 5) can be justified by the ability of the fungus to produce extraradical hyphae which explore soil regions that roots cannot reach, allowing higher absorption of nutrients necessary for plant growth. It should be pointed out that dark septate endophytic fungi are able to solubilize P that is unavailable to plants, favoring their nutrition and growth in stressful environments [64–66].
Inoculation of cowpea roots with dark septate endophytic fungi did not influence the acquisition of K, Ca, Na, and Cl by cowpea plants, under conditions of high and low salinity (Fig. 6). The increase in irrigation water salinity from 1.2 dS m−1 to 5.0 dS m−1 caused an increase of 37%, on average, in leaf Ca content. Such increment in Ca content in the leaves can be explained by the high concentration of Ca in the irrigation water, notably in the treatments of highest salinity, since CaCl2 was used as source of water salinization.
The contents of sodium and chloride, ions considered potentially toxic [67], increased as water salinity increased, regardless of the inoculation treatments (Fig. 6). The accumulation of sodium in the root environment, due to salinity, causes reduction in the leaf contents of K+, as observed in the present study (Fig. 6). According to [62], the lower K+ absorption is caused by the competition with Na+ ions for the same sites of the absorption system in the plasma membrane of root cells.
The differences of growth in plants under salt stress can be explained, at least in part, by the lower accumulation of potentially toxic ions or by the maintenance of K nutrition [67]. In the present study, however, the differences observed in the growth (Fig. 3) and photosynthetic rate (Fig. 4) of plants under salt stress, comparing plants inoculated and not inoculated, seem to be associated with the beneficial effect of the fungus on the acquisition of N and P (Fig. 5) and not with the control over Na, Cl, and K absorption (Fig. 6). The capacity of dark septate endophytic fungi to reduce the absorption and translocation of potentially toxic ions has been reported for conditions of stress by heavy metals [36, 68, 69]. Nonetheless, heavy metals are normally found at concentrations that are much lower than those observed for cations and anions in saline environments, especially Na+ and Cl−. Therefore, our results indicate that endophytic fungi were not able to limit the absorption and transport of these potentially toxic ions present at high concentrations in the irrigation water.
Conclusions
Cowpea plants associated with DSE Sordariomycetes sp1-B'2 and Melanconiella elegans-21W2 fungi, isolated from the V. divergens root system, and this association improved nutrition with N and P in cowpea under salt stress, favoring growth, and net photosynthetic rate. Therefore, endophytic fungi were not able to limit the absorption and transport of potentially toxic ions (Na+ and Cl−) present at high concentrations in the irrigation water.
Acknowledgments
We would like to thank the following Brazilian institutions: ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)’, ‘Instituto Nacional de Ciência e Tecnologia em Salinidade (INCTSal)’, ‘Instituto Nacional de Ciência e Tecnologia de Áreas Úmidas (INAU)’, and ‘Fundação de Amparo à Pesquisa do Mato Grosso (FAPEMAT)’.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lacerda CF, Ferreira JFS, Liu X, Suarez DL. Evapotranspiration as a criterion to estimate nitrogen requirement of maize under salt stress. J Agron Crop Sci. 2016;202:192–202. doi: 10.1111/jac.12145. [DOI] [Google Scholar]
- 2.Khan AL, Hamayun M, Kim YH, Kang SM, Lee JH, Lee IJ. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011;46(2):440–447. doi: 10.1016/j.procbio.2010.09.013. [DOI] [Google Scholar]
- 3.Lúcio WS, Lacerda CF, Mendes Filho PF, Hernandez FFF, Neves ALR, Gomes Filho E. Crescimento e respostas fisiológicas do meloeiro inoculado com fungos micorrízicos arbusculares sob estresse salino. Semina: Ciências Agrárias. 2013;34(4):1587–1602. doi: 10.5433/1679-0359.2013v34n4p1587. [DOI] [Google Scholar]
- 4.Mahmoud FM, Krimi Z, Maciá-Vicenteh JG, Errahmani MB, Lopez-Llorca LV. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Rev Iberoam Micol. 2017;34(2):116–120. doi: 10.1016/j.riam.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Moreira SD, França AC, Rocha WW, Tibães ESR, Neiva Júnior E. Inoculation with mycorrhizal fungi on the growth and tolerance to water deficit of coffee plants. Rev Bras Eng Agríc Ambient. 2018;22(11):747–752. doi: 10.1590/1807-1929/agriambi.v22n11p747-752. [DOI] [Google Scholar]
- 6.Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Sasse F, Jansen R, Murali TS. Fungal endophytes and bioprospecting. Fungal Biol Rev. 2009;23:9–19. doi: 10.1016/j.fbr.2009.07.001. [DOI] [Google Scholar]
- 7.Souza WP, Mello IS, Vendruscullo SJ, Silva GF, Cunha CN, White JF, Soares MA. Endophytic fungal communities of Polygonum acuminatum and Aeschynomene fluminensis are influenced by soil mercury contamination. PLoS One. 2017;12(7):e0182017. doi: 10.1371/journal.pone.0182017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva FA, Liotti RG, Boleti APA, Reis EM, Passos MBS, Santos EL, Sampaio OM, Januario AH, Branco CLB, Silva GF, Mendonca EAF, Soares MA. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS One. 2018;13(4):e0195874. doi: 10.1371/journal.pone.0195874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotti RG, Figueiredo MIS, Silva GF, Mendonca EAF, Soares MA. Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol Res. 2017;207:8–18. doi: 10.1016/j.micres.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 10.JianQui S, LiangDong G, Weiz Z, WenXiang P, DeFu C. Diversity and ecological distribution of endophytic fungi associated with medicinal plants. Sci China Ser C-Life Sci. 2008;51(8):751–759. doi: 10.1007/s11427-008-0091-z. [DOI] [PubMed] [Google Scholar]
- 11.Naik BS, Shashikala J, Krishnamurthy YL. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiol Res. 2009;164(3):290–296. doi: 10.1016/j.micres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Piercey MM, Graham SW, Currah RS. Patterns of genetic variation in Phialocephala fortinii across a broad latitudinal transect in Canada. Mycol Res. 2004;108(8):955–964. doi: 10.1017/S0953756204000528. [DOI] [PubMed] [Google Scholar]
- 13.Knapp DG, Kovács GM, Zajta E, Groenewald JZ, Crous PW. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia. 2015;35:87–100. doi: 10.3767/003158515X687669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan ZL, Su ZZ, Zhang CL. Understanding the biodiversity and functions of root fungal endophytes: the ascomycete Harpophora oryzae as a model case. In: Druzhinina IS, Kubicek CP, editors. Environmental and Microbial Relationships. Berlin: Springer; 2016. pp. 205–214. [Google Scholar]
- 15.Jumpponen A, Trappe JM. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. 1998;140:295–310. doi: 10.1046/j.1469-8137.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 16.Jumpponen A. Dark septate endophytes–are they mycorrhizal? Mycorrhiza. 2001;11(4):207–211. doi: 10.1007/s005720100112. [DOI] [Google Scholar]
- 17.Newsham KK, Upson R, Read DJ. Mycorrhizas and dark septate root endophytes in polar regions. Fungal Ecol. 2009;2(1):10–20. doi: 10.1016/j.funeco.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Fracchia S, Krapovickas L, Aranda-Rickert A, Valentinuzzi VS. Dispersal of arbuscular mycorrhizal fungi and dark septate endophytes by Ctenomys cf. knighti (Rodentia) in the northern Monte Desert of Argentina. J Arid Environ. 2011;75(11):1016–1023. doi: 10.1016/j.jaridenv.2011.04.034. [DOI] [Google Scholar]
- 19.Silvani V, Rothen C, Rodriguez MA, Cisneiros G, Godeas A, Aranda-Rickert A, Sebastián F. Fungal root colonization of Puccinellia frigida (Phil.) Johnston, a dominant grass species inhabiting the margins of high-altitude hypersaline Andean wetlands. Aquatic Bot. 2013;108:26–32. doi: 10.1016/j.aquabot.2013.03.001. [DOI] [Google Scholar]
- 20.Rothen C, Miranda V, Aranda-Rickert A, Fracchia S, Rodríguez MA. Characterization of dark septate endophyte fungi associated with cultivated soybean at two growth stages. Appl Soil Ecol. 2017;120:62–69. doi: 10.1016/j.apsoil.2017.07.033. [DOI] [Google Scholar]
- 21.Mesquita CPB, Sartwell SA, Ordemann EV, Porazinska DL, Farrer EC, King AJ, Spasojevic MJ, Smith JG, Suding KN, Schmidt SK. Patterns of root colonization by arbuscular mycorrhizal fungi and dark septate endophytes across a mostly-unvegetated, high-elevation landscape. Fungal Ecol. 2018;36:63–74. doi: 10.1016/j.funeco.2018.07.009. [DOI] [Google Scholar]
- 22.Russo ML, Pelizza SA, Cabello MN, Stenglein SA, Vianna MF, Scorsetti AC. Endophytic fungi from selected varieties of soybean (Glycine max L. Merr.) and corn (Zea mays L.) grown in an agricultural area of Argentina. Rev Argent Microbiol. 2016;48(2):154–160. doi: 10.1016/j.ram.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Santos SG, Silva PRA, Garcia AC, Zilli JE, Berbara RLL. Dark septate endophyte decreases stress on rice plants. Braz J Microbiol. 2017;48(2):333–341. doi: 10.1016/j.bjm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergara C, Araujo KEC, Urguiaga S, Schultz N, Balieiri FC, Medeiros PS, Santos LA, Xavier GR, Zilli JE. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front Microbiol. 2017;2437:1–12. doi: 10.3389/fmicb.2017.02437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick MK, Whigham DF, Sloan D, O’Malley K, Hodkinson B. Orchidfungus fidelity: a marriage meant to last? Ecology. 2006;87:903–911. doi: 10.1890/0012-9658(2006)87[903:OFAMMT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Peay KG, Kennedy PG, Bruns TD. Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation? Fungal Ecol. 2011;4(3):233–240. doi: 10.1016/j.funeco.2010.09.010. [DOI] [Google Scholar]
- 27.Cardoso JM, Melo FB, Lima MG. Ecofisiologia e manejo de plantio. In: Freire Filho FR, Lima JAA, Ribeiro VQ, editors. Feijão-caupi: avanços tecnológicos. Brasilia: Embrapa; 2005. pp. 213–225. [Google Scholar]
- 28.Guimarães AA, Jaramillo PMD, Nóbrega RSA, Florentino LA, Silva KB, Moreira FMS. Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Appl Environ Microbiol. 2012;78(18):6726–6733. doi: 10.1128/AEM.01303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezerra MEJ, Lacerda CF, Sousa GG, Gomes VFF, Mendes Filho PF. Biomassa, atividade microbiana e FMA em rotação cultural milho/feijão-de-corda utilizando-se águas salinas. Rev Ciênc Agron. 2010;41(4):562–570. doi: 10.1590/S1806-66902010000400008. [DOI] [Google Scholar]
- 30.Scervino JM, Gottlieb A, Silvani VA, Pérgola M, Fernández L, Godeas AM. Exudates of dark septate endophyte (DSE) modulate the development of the arbuscular mycorrizal fungus (AMF) Gigaspora rosea. Soil Biol Biocherm. 2009;41(8):1753–1756. doi: 10.1016/j.soilbio.2009.04.021. [DOI] [Google Scholar]
- 31.Upson R, Newsham KK, Bridge PD, Pearce DA, Read DJ. Taxonomic affinities of dark septate root endophytes of Colobanthus quitensis and Deschampsia antarctica, the two native Antarctic vascular plant species. Fungal Ecol. 2009;2(4):184–196. doi: 10.1016/j.funeco.2009.02.004. [DOI] [Google Scholar]
- 32.Chen XM, Dong HL, Hu KX, Sun ZR, Chen J, Guo SX. Diversity and antimicrobial and plant-growth-promoting activities of endophytic fungi in Dendrobium loddigesii rolfe. J Plant Growth Regul. 2010;29(3):328–337. doi: 10.1007/s00344-010-9139-y. [DOI] [Google Scholar]
- 33.Vergara C, Araujo KEC, Alves LS, Souza SR, Santos LA, Santa-Catarina C, Silva K, Pereira GMD, Xavier GR, Zilli JE. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol. 2018;49(1):67–78. doi: 10.1016/j.bjm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vergara C, Araujo KEC, Souza SR, Schultz N, Saggin Junior OJ, Sperandio MVL, Zilli JE. Plant-mycorrhizal fungi interaction and response to inoculation with different growth-promoting fungi. Pesq Agropec Bras. 2019;54(e25140):1–24. doi: 10.1590/s1678-3921.pab2019.v54.25140. [DOI] [Google Scholar]
- 35.Vergara C, Araujo KEC, Sperandio MVL, Santos LA, Urguiaga S, Zilli JE. Dark septate endophytic fungi increase the activity of proton pumps, efficiency of 15 N recovery from ammonium sulphate, N content, and micronutrient levels in rice plants. Braz J Microbiol. 2019;50(3):825–838. doi: 10.1007/s42770-019-00092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ban Y, Xu Z, Yang Y, Zhang H, Chen H, Tang M. Effect of dark septate endophytic fungus Gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.) Pedosphere. 2017;27(2):283–292. doi: 10.1016/S1002-0160(17)60316-3. [DOI] [Google Scholar]
- 37.Zhang Q, Gong M, Yuan J, Hou Y, Zhang H, Wang Y, Hou X. Dark septate endophyte improves drought tolerance in sorghum. Int J Agri Biol. 2017;19:53–60. doi: 10.17957/IJAB/15.024. [DOI] [Google Scholar]
- 38.Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil, 2nd edn (2) São Paulo: Instituto Plantarum; 2002. [Google Scholar]
- 39.Cunha CN, Junk WJ. Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Appl Veg Sci. 2004;7:103–110. [Google Scholar]
- 40.Biz AR, Mendonca EAF, Almeida EG, Soares MA. Endophytic fungal diversity associated with the roots of cohabiting plants in the Pantanal wetland. In: Soares MA, Jardim MAG, editors. Natural resources in wetlands: from Pantanal to Amazonia. 1. Belém: Museu Paraense Emílio Goeldi; 2017. pp. 37–70. [Google Scholar]
- 41.Parpinelli BAS, Siqueira KA, Kellner Filho LC, Pimenta LP, Costa RM, Parreira RLT, Veneziani RCS, Silva MLA, Cunha WR, Pauletti PM, Soares MA, Januario AH. Effect of endophytic fungal associations on the chemical profile of in vitro Vochysia divergens seedlings. J Braz Chem Soc. 2017;28(12):2375–2381. doi: 10.21577/0103-5053.20170091. [DOI] [Google Scholar]
- 42.Siqueira KA, Brissow ER, Santos JL, White JF, Santos FR, Ameida EG, Soares MA. Endophytism and bioactivity of endophytic fungi isolated from Combretum lanceolatum Pohl ex Eichler. Symbiosis. 2017;71(3):211–222. doi: 10.1007/s13199-016-0427-6. [DOI] [Google Scholar]
- 43.Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55(1):158–161. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- 44.Peterson RL, Massicotte HB, Lewis H, Melville LH. Mycorrhizas: anatomy and cell biology. Ottawa: National Research Council; 2004. [Google Scholar]
- 45.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 46.White TJ, Bruns T, Lee SJWT, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. London: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 47.Koske RE, Gemma JN. A modified procedure for staning roots to detect VA mycorrhizas. Mycol Res. 1989;92(4):486–488. doi: 10.1016/S0953-7562(89)80195-9. [DOI] [Google Scholar]
- 48.Giovannetti M, Mosse B. An evaluation of technique for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84(3):489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x. [DOI] [Google Scholar]
- 49.Silva FAZ, Azevedo CAV. Versão do programa computacional Assistat para o sistema operacional Windows. Revista Brasileira de Produtos Agroindustriais. 2002;4:71–78. doi: 10.15871/1517-8595/rbpa.v4n1p71-78. [DOI] [Google Scholar]
- 50.Senabio JA, Silva IP, Santos JL, Soares MA. Antimicrobial and antioxidant activity of endophytic fungi isolated from Hyptis suaveolens roots. In: Soares MA, Jardim MAG, editors. Natural resources in wetlands: from Pantanal to Amazonia. 1. Belém: Museu Paraense Emílio Goeldi; 2017. pp. 115–136. [Google Scholar]
- 51.Su ZZ, Mao LJ, Li N, Feng XX, Yuan ZL, Wang LW, Lin FC, Zhang CL. Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS One. 2013;8(4):e61332. doi: 10.1371/journal.pone.0061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.David AS, Haridas S, LaButti K, Lim J, Lipzen A, Wang M, Barry K, Grigoriev IV, Spatafora JW, May G. Draft genome sequence of microdochium bolleyi, a dark septate fungal endophyte of beach grass. Genome Announc. 2016;4(2):e00270–e00216. doi: 10.1128/genomeA.00270-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kytoviita MM, Ruotsalainen AL. Mycorrhizal benefit in two low arctic herbs increases with increasing temperature. Am J Bot. 2007;94(8):1309–1315. doi: 10.3732/ajb.94.8.1309. [DOI] [PubMed] [Google Scholar]
- 54.Sieber TN, Grüning CR. Fungal roots endophytes. In: Eshel A, Beeckman T, editors. Plant roots: The hidden half. New York: CRC; 2013. p. 38/43. [Google Scholar]
- 55.Fuchs B, Haselwandter K. Red list plants: colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza. 2004;14(4):277–281. doi: 10.1007/s00572-004-0314-5. [DOI] [PubMed] [Google Scholar]
- 56.Sathiyadash K, Thangavelu M, Eswaranpillai U. Arbuscular mycorrhizal and dark septate endophyte fungal associations in South Indian grasses. Symbiosis. 2010;52(1):21–32. doi: 10.1007/s13199-010-0096-9. [DOI] [Google Scholar]
- 57.Zhang ZB, Zeng QG, Yan RM, Wang Y, Zou ZR, Zhu D. Endophytic fungus Cladosporium cladosporioides LF70 from Huperzia serrata produces Huperzine A. World J Microbiol Biotechnol. 2011;27(3):479–486. doi: 10.1007/s11274-010-0476-6. [DOI] [Google Scholar]
- 58.Khan AL, Hamayun M, Kang SM, Kim YH, Jung HY, Lee JH, Lee IJ. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol. 2012;12:3. doi: 10.1186/1471-2180-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamayun M, Hussain A, Khan SA, Kim HY, Khan AL, Waqas M, Irshad M, Iqbal A, Rehman G, Jan S, Lee IJ. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Redman RS, Kim YO, Woodward CJDA, Greer C, Espino L, Doty SL, Rodriguez RJ. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS One. 2011;6(7):e14823. doi: 10.1371/journal.pone.0014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bibi S, Hussain A, Hamayun M, Rahman H, Iqbal A, Shah M, Irshad M, Qasim M, Islam B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere. 2018;211:653–663. doi: 10.1016/j.chemosphere.2018.07.197. [DOI] [PubMed] [Google Scholar]
- 62.Marschner H. Marschner's mineral nutrition of higher plants. 3. Cambridge: Academic Press; 2012. [Google Scholar]
- 63.Aragão RM, Silveira JAG, Silva EM, Lobo AKM, Dutra ATB. Absorção, fluxo no xilema e assimilação do nitrato no feijão-caupi submetido à salinidade. Rev Ciênc Agron. 2010;41(1):100–106. doi: 10.5935/1806-6690.20100014. [DOI] [Google Scholar]
- 64.Barrow JR, Osuna P. Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J Arid Environ. 2002;51(3):449–459. doi: 10.1006/jare.2001.0925. [DOI] [Google Scholar]
- 65.Plenchette C, Duponnois R. Growth response of the saltbush Atriplex mummularia L. to inoculation with the arbuscular mycorrhizal fungus Glomus intraradices. J Arid Environ. 2005;61(4):535–540. doi: 10.1016/j.jaridenv.2004.10.003. [DOI] [Google Scholar]
- 66.Spagnoletti FN, Tobar NE, Pardo AFD, Chiocchio VM, Lavado RS. Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl Soil Ecol. 2017;111:25–32. doi: 10.1016/j.apsoil.2016.11.010. [DOI] [Google Scholar]
- 67.Lacerda CF, Cambraia J, Oliva MA, Ruiz HA, Prisco JT. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ Exp Bot. 2003;49(2):107–120. doi: 10.1016/S0098-8472(02)00064-3. [DOI] [Google Scholar]
- 68.Li T, Liu MJ, Zhang XT, Zhang HB, Sha T, Zhao ZW. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci Total Environ. 2011;409(6):1069–1074. doi: 10.1016/j.scitotenv.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Khan AR, Ullah I, Waqas M, Park GS, Khan AL, Hong SJ, Ullah R, Jung BK, Park CE, Rehman SU, Lee IJ, Shin JH. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf. 2017;136:180–188. doi: 10.1016/j.ecoenv.2016.03.014. [DOI] [PubMed] [Google Scholar]