Abstract
Starting in 2014, large phase III clinical trials began to disclose the study results of using programmed death (PD)-1 immune checkpoint inhibitors (ICIs) (pembrolizumab, nivolumab) and PD-ligand (L)1 (atezolizumab, durvalumab, avelumab) ICIs immunotherapy in patients with advanced head and neck squamous cell carcinoma (HNSCC). In the recurrent and metastatic (R/M), cisplatin-refractory setting, nivolumab achieved a 2.2-fold increase of the median 1-year overall survival as compared with investigators' choice of salvage chemotherapy (36.0 vs. 16.6%). A paradigm shift to the winning regimen, pembrolizumab combined with platinum and infusional fluorouracil, has outperformed the past gold standard of cetuximab-based platinum and fluorouracil combination in terms of overall survival (median, 13.6 vs. 10.1 mo) when administered as the first-line treatment for R/M HNSCC. Nevertheless, many patients still did not respond to the PD-1/PD-L1 checkpoint inhibitor treatment, indicating innate, adapted, or quickly acquired resistance to the immunotherapy. The mechanisms of resistance to ICIs targeting the PD-1/PD-L1 signaling pathway in the context of HNSCC are the focus of this review. The past 5 years have seen improved understanding of the mechanisms underlying checkpoint inhibition resistance in tumor cells, such as: tumor cell adaption with malfunction of the antigen-presenting machinery via class I human leukocyte antigen (HLA), reintroduction of cyclin D–cyclin-dependent kinase (CDK) 4 complex to cell cycles, enrichment of CD44+ cancer stem-like cells, or development of inactivating mutation in IKZF1 gene; impairment of T-cell functions and proliferation through mutations in the interferon-γ-regulating genes, suppression of the stimulator of interferon genes (STING) pathway, or resulted from constitutional nutritional iron deficiency state; metabolic reprogramming by cancer cells with changes in metabolites such as GTP cyclohydrolase 1, tetrahydrobiopterin, kynurenine, indoleamine 2,3-dioxygenase, and arginase 1; defective dendritic cells, CD-69 sufficient state; and the upregulation or activation of the alternative immune checkpoints, including lymphocyte activation gene-3 (LAG3), T-cell immunoglobulin and ITIM domain (TIGIT)/CD155 pathway, T-cell immunoglobulin mucin-3 (TIM-3), and V domain-containing Ig suppressor of T-cell activation (VISTA). Several potential biomarkers or biosignatures, which could predict the response or resistance to the PD-1/PD-L1 checkpoint immunotherapy, are also discussed.
Keywords: PD-1/PD-L1 signaling pathway, HNSCC, immune checkpoint blockade, cancer immunotherapy, innate resistance, adapted resistance, immune evasion, head and neck cancer
Introduction
Scope of Problems
Head and neck cancers encompass a group of malignancies arising from several anatomical mucosal sites, including the nasal cavity, paranasal sinuses, nasopharynx, oropharynx, hypopharynx, larynx and lips, and oral cavity. According to GLOBOCAN epidemiological estimates of incidence and mortality of cancer worldwide, in 2018, there were ~835,000 new cases of cancer arising from the lips, oral cavity, naso-, oro-, and hypo-pharynx and larynx; with the number of deaths in the same year being ~431,000 (1). The majority (95%) of histopathological types of head and neck cancer is squamous cell carcinoma (HNSCC) (2). Virus-related nasopharyngeal carcinoma will be included in this review because of its immunogenicity and encouraging trial outcomes (3, 4). The tumorigenesis can separate head and neck cancers into virus-related (Epstein-Barr virus-related nasopharyngeal carcinoma (NPC) and human papillomavirus (HPV)-positive oropharyngeal HNSCC) cancer and non-viral (HPV-negative) HNSCC, the latter being related to smoking, alcohol, and betel quid consumption in etiology.
Early-stage and locally advanced stage HNSCC should be treated with curative intent incorporating radical surgical resection or radical radiotherapy combined with chemotherapy. More than 65% of previously treated HNSCC will develop local recurrence or distant metastasis (5). It is very challenging for the head and neck cancer team to manage patients with unresectable locally advanced stage, relapsed or metastatic (R/M) HNSCC, mainly because of the high propensity for intrinsic, spatial, and acquired resistance to chemotherapeutic agents, radiotherapy, and anti-epidermal growth factor receptor monoclonal antibodies. The current first-line regimen for R/M HNSCC adopted a new paradigm in 2019, when the results of the randomized controlled trial, comparing pembrolizumab combined with platinum and infusional 5-fluorouracil to the past gold-standard regimen of cetuximab plus the same chemotherapy combination, confirmed an overall survival benefit (hazard ratio, HR, for death at 0.65, 95% confidence interval, CI, 0.53–0.80) favoring the pembrolizumab-based treatment arm (Table 1). Pembrolizumab is an anti-programmed cell death (PD)-1 monoclonal antibody, previously known as MK-3475, and subsequently, lambrolizumab (8).
Table 1.
Summary of data demonstrating the evolving new paradigms of systemic treatment for R/M HNSCC over 12 years.
| Outcomes | Platinum + 5-FU (6) | Cetuximab + Platinum + 5-FU (6) | Pembrolizumab + Platinum + 5-FU (7)* |
|---|---|---|---|
| No. of patients | n = 200 | n = 222 | n = 281 |
| Overall Response Rate (95% CI) | 20% (15–25%) | 36% (29–42%) | 36.4% (not given) |
| Progression-Free Survival (mo.) | 3.3 (2.9–4.3) | 5.6 (5.0–6.0) | HR = 0.84 (95% CI, 0.69–1.02)* |
| Overall Survival in mo. (95% CI) | 7.4 (6.4–8.3) | 10.1 (8.6–11.2) | 13.6 (not given) |
| Hazard ratio for OS (95% CI) | 0.80 (0.64–0.99) | ||
| 0.65 (0.53–0.80) | |||
Results shown here represent the subgroup of patients whose Combined Positive Score (CPS) for PD-L1 was ≥1. The exact figure for progression-free survival was not given in the Abstract.
The Rationale of Anti-PD-1/PD-Ligand (L)1 Immunotherapy for R/M HNSCC
One of the hallmarks of cancer is the ability of cancer cells to evade immune destruction (9). PD-1 is a co-inhibitory receptor on the cell surface of cytotoxic T lymphocytes. The ligation of PD-1 and PD-L1 or PD-L2 on tumor cells or antigen-presenting cells (APCs) elicits an immunosuppressive response, which implements subsequent metabolic reprogramming in T cells, decreases effector T cells and memory T cells, and increases Treg and exhausted T cell abundance (10). For the past few years, the abundance of PD-L1 protein in the HNSCC tumor, with its microenvironment sphere, has been the focus of numerous studies (2, 11–17). Observe the oral cavity squamous cell carcinoma (OCSCC) as an example, the prevalence of PD-L1 positivity has been reported in 45-87% of cases, depending on the cut-off value for positivity, whether cytoplasmic staining was counted as positive, and inclusion of the proportion of HPV+ cancer cases (2, 11). The PD-1/PD-L1 axis applies immunosuppressive signals, inducing anergy of cytotoxic T-cells; thus, the blockade of this ligation (analogous to releasing the brake) becomes a strong rationale for anti-PD-1 immunotherapy for R/M HNSCC.
The Recent Development of Anti-PD-1/PD-L1 Monoclonal Antibody-Based Treatment for R/M HNSCC
Table 2 attempts to summarize the recent relevant clinical trials investigating PD-1 or PD-L1 blockade in R/M HNSCC or NPC. Usually, NPC does not belong to the classical HNSCC membership due to unique tumor pathogenesis and different treatment protocols. However, anti-PD-1 therapy for NPC will be shown in this review to give our readers a broader picture of the immunotherapy comparing classical HNSCCs to NPC. The same would apply to HPV-positive oropharyngeal cancer. Overall response rate (ORR), progression-free survival (PFS), duration of response (DoR), and overall survival (OS) are shown if these results are available in the published paper. To date, only the following two anti-PD-1 monoclonal antibodies, pembrolizumab and nivolumab, and two anti-PD-L1 monoclonal antibodies, durvalumab, and atezolizumab (anti-PD-L1), have been tested in R/M HNSCC (Table 2). As yet, there is only one published phase I study regarding avelumab, a PD-L1 inhibitor, in R/M HNSCC (27–29).
Table 2.
Summary of clinical trial results of PD-1 or PD-L1 blockade in R/M HNSCC and NPC showing overall response rate (ORR), duration of response (DoR), progression-free survival (PFS), and overall survival (OS).
| 1st Author/ published year/ (References)/EudraCT No. | Phase of study/Study Name/No. pts | Key immunotherapy drug | Biomarker | Failed treatment previously | Treatment outcomes |
|---|---|---|---|---|---|
| Chow/2016/(18)/2012-005771-14 | Phase Ib/KEYNOTE-012 Expansion Cohort/n = 132 |
Pembrolizumab at a fixed dose, 200 mg Q3W | Irrespective of biomarker status | 57% failed two or more lines of chemo | 6-mo. PFS = 23%; 6-mo. OS = 59%. ORR = 22% in PD-L1+ tumors. Duration of response = not reached (range, ≥ 2 to ≥ 11 mo.) |
| Bauml/2017/(19)/2014-002447-18 | Phase II/KEYNOTE-055/n = 171 |
Pembrolizumab | 82% PD-L1 positive (CPS ≥ 1%) | 75% failed platinum and cetuximab or more | ORR = 16% (95% CI, 11% to 23%). Duration of response = 8 mo. (2+ to 12+ mo.); Median PFS = 2.1 mo., and median OS = 8 mo. |
| Hsu/2017/(13)/ 2013-004507-39 | Phase Ib/KEYNOTE-028/n = 27 NPC |
Pembrolizumab | Dako 22C3 positive ≥ 1% | 70.4% failed three or more lines | ORR = 25.9% (95% CI, 11.1 to 46.3) |
| Cohen/2019/(20)/2014-001749-26 | Phase III/KEYNOTE-040/n = 247 (pembro. arm) |
Pembrolizumab at a fixed-dose, 200 mg Q3W | PD-L1 tumor proportion score (≥ 50% vs. < 50%) | Failed platinum-containing chemo | ORR = 14·6% (95% CI, 10.4–19.6); Duration of response = 18.4 mo (95% CI 5.8–18.4); Median PFS = 2·1 mo (95% CI 2.1–2.3); Median OS = 8.4 mo. (95% CI 6.4–9.4). |
| Rischin/2019/(7)/2014-003698-41 | Phase III/KEYNOTE-048/n = 882 (entire) |
Pembrolizumab vs. pembrolizumab + PF vs. cetuximab + PF | CPS for PD-L1 protein expression. | First-line for R/M HNSCC | In the CPS ≥ 1 group, ORR = 36.4% and median OS = 13.6 mo. (in pembrolizumab + PF) vs. ORR, 35.7%; OS, 10.4 mo. (in cetuximab + PF); HR = 0.65, 95% CI, 0.53–0.80). |
| Ferris/2016/(21);Ferris/2018/(22)/ 2013-003622-86 | Phase III/CHECKMATE-141/n = 240 (nivo. arm) |
Nivolumab 3 mg/kg Q2W | Dako positive ≥ 1%, ≥ 5%, vs. ≥ 10%. | Failed within 6 mo. of platinum therapy | ORR = 13.3% (9.3–18.3); OS = 7.7 mo. (5.7–8.8). 24-mo. OS = 16.9%. |
| Colevas/2018/(23)/2011-001422-23 | Phase Ia/PCD4989g/n = 32 |
Atezolizumab | Responses observed irrespective of HPV or PD-L1 status. | Heavily pretreated | ORR = 22% (95% CI, 9–40%); PFS = 2.6 mo. (0.5–48.4 mo.); Median OS = 6.0 mo (range 0.5–51.6+ mo). |
| Segal/2019/(24)/not available | Phase I/II expansion/n = 62 |
Durvalumab 10 mg/kg Q2W for 12 mo | 32.3% had tumor cell PD-L1 expression ≥ 25% | Failed median of 2 prior systemic treatments (range, 1-13) | ORR = 6.5% (15.0% for PD-L1 ≥ 25%, 2.6% for < 25%); TTP = 2.7 months (range, 1.2-5.5); PFS = 1.4 mo; OS = 8.4 mo. OS rate = 62% at 6 mo and 38% at 12 mo (42% for PD-L1 ≥ 25%, 36% for < 25%). |
| Siu/2018/(25)/not available | Phase II randomized, open-label/CONDOR/n = 67 |
Durvalumab (10 mg/kg Q2W) monotherapy | PD-L1–low/negative | Failed 1 platinum-containing regimen | ORR = 9.2% (3.46-19.02) |
| Siu/2018/(25)/not available | Phase II randomized, open-label/ CONDOR/n = 133 |
Durvalumab + tremelimumab (anti-CTLA-4) | PD-L1–low/negative | Failed 1 platinum-containing regimen | ORR = 7.8% (3.78-13.79%) |
| Bahig/2019/(26)/not available | Phase I-II/n = 35 (non-NPC) |
Durvalumab (1500 mg Q4W) + tremelimumab (75 mg Q4W × 4 doses) + SBRT to metastases at cycles 2 and 3 of immunotherapy | Biomarker-unselected | Patients with ≥ 2 extracranial metastatic lesions. | Ongoing study |
| Elbers/2019(27)/not available | Phase I/n = 9 (cisplatin-unfit) |
Cetuximab-radiotherapy + avelumab (concurrent 10 mg/kg Q2W + 4 months maintenance) | None | Unfit for cisplatin but with an indication for concurrent bioradiotherapy | At 12 (median, 95% CI, 8–26) months follow-up, recurrence occurred in 4/8 patients (50%). |
| Merlano/2018/(28)/2017-000353-39 | Phase Ib-II/CONFRONT/n = |
Avelumab 10 mg/kg Q2W + Cyclophosphamide 50 mg daily + 8 Gy radiotherapy day 8. | None | Failed at least therapy with platinum, fluorouracil, and cetuximab | Ongoing study. |
EudraCT, European Union Drug Regulating Authorities Clinical Trials; NPC, nasopharyngeal carcinoma; SBRT, stereotactic body radiotherapy; CPS, Combined Positive Score.
The efficacy of anti-PD-1 and anti-PD-L1 for R/M HNSCC, regardless of its use at as salvage therapy, in the second-line, or even in the first-line while combined with platinum plus infusional 5-fluorouracil, the ORRs were fairly poor across the board. When used in the first-line combined with PF chemotherapy for R/M HNSCC, the response rate was reported as 36.4% (7). Nearly 64% of the patients' tumors demonstrated either primary or adaptive resistance [cancer cells salvaged themselves by resorting to immunoediting and thus, further created an immunosuppressive tumor microenvironment (TME)] (30) to the combination immunochemotherapy.
This review serves to present the recent research findings with implications on the mechanisms of immune evasion from the anti-PD1 or anti-PD-L1 immune checkpoint blockade.
Methods
Methodology for the Literature Search
A dynamic PubMed literature search until September 14, 2019, using the Medical Subject Headings and the Boolean search terms, was used to retrieve articles indexed under keywords, such as “head and neck cancer,” “head and neck squamous cell carcinoma,” “HNSCC,” “oral cavity squamous cell carcinoma,” “OCSCC,” “immunotherapy,” “pembrolizumab,” “nivolumab,” “atezolizumab,” “durvalumab,” “avelumab,” “immune escape,” “immune evasion,” “resistance,” “relapsed or metastatic,” “unresected locally advanced,” “mechanism,” “PD-L1,” “PD-1,” “immune checkpoint inhibitor,” “immunoediting,” “tumor microenvironment,” “immunosuppressive,” and “adapted resistance.” The American Society of Clinical Oncology Meeting Abstract database was also searched for the relevant trials. Also, a hand search from the “Similar Articles” inside the PubMed panel was performed to retrieve related articles.
In the EndNote software, “Find Duplicates” function was activated to remove duplicated papers. “Find Full Text” was then activated to download those articles available for download. The Harvard Medical School digital library, “BrowZine,” was then used to complete all full-text downloads for the EndNote library. Review articles represented most retrieved articles in the library, and only a few of them would be cited in this review. Whereas, articles with primary research data from innovative experiments and clinical trials would be selected.
Results and Discussion
HNSCC Cancer Cells Remodel and Shape an Immunosuppressive TME
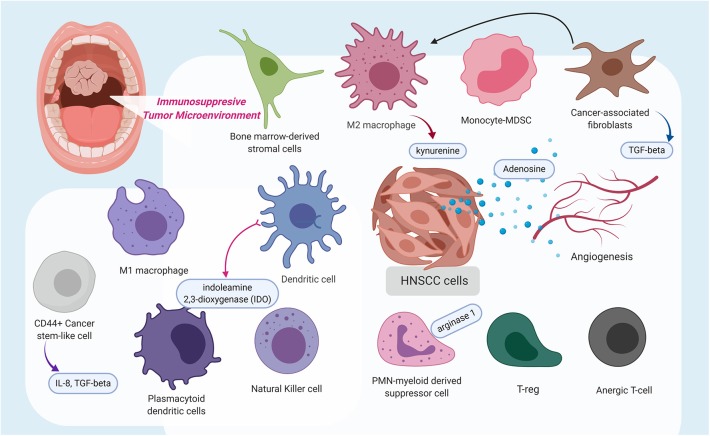
Within the tumor microenvironment, there exists a plethora of cytokines, and various infiltrating immune cells, such as CD8+ T cells and APCs, including dendritic cells, anti-tumoral M1 macrophages, pro-tumoral M2 macrophages [tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), cancer-associated fibroblasts (CAFs), and regulatory T cells (Tregs) (Figure 1)]. Like the immunosuppressive lymphocytic Tregs, MDSC functions as myeloid regulatory cells (MRC) and is further separated into monocytic-MDSC and polymorphonuclear-MDSC (31). A study by Takahashi and colleagues identified that CAFs stimulate and polarize the increase of CD68+ and CD163+ pro-tumoral macrophages in the TME (32).
Figure 1.
This schematic diagram highlights the immunosuppressive tumor microenvironment (TME) in which a variety of immune cells are polarized to possess pro-tumoral features, stimulated cancer-associated fibroblasts, which release transforming growth factor-β, and even angiogenesis contribute to the immunosuppressive state. Additionally, several molecules, such as kynurenine, adenosine, indoleamine 2,3-dioxygenase, arginase 1, interleukin (IL)-8, and IL-10, were identified to contribute to a pro-tumoral immunosuppressive TME or at extreme, an immune-desert. The figure was created with BioRender.com and was exported under a paid subscription.
Primary Resistance to ICIs in HNSCC
A Birmingham research group developed a 54-gene hypoxia-immune classifier to prognosticate patients with HNSCC, and uncovered that tumors that are high-hypoxia/low-immune are associated with “immune-desert” microenvironmental profiles (33). Hypoxia within a tumor microenvironment will increase the immune-inhibitory PD-L1 generation through the hypoxia-inducible factor (HIF)-1α signaling (33, 34). An immune desert tumor microenvironment promotes immune evasion for cancer cells. These cold tumors respond poorly to single-agent PD-1/PD-L1 immune checkpoint (ICP) immunotherapy. The JAK family of kinases (JAK1/2/3, TYK2) is involved in PD-L1 induction in tumor, stromal, and immune cells (35). A group of investigators from Foundation Medicine Inc., Cambridge, Boston, showed that in a preclinical study focusing on endometrial carcinoma and stomach adenocarcinoma, somatic mutation of JAK1 with loss of function (meaning loss of JAK1-mediated interferon response) results in immune evasion, particularly in microsatellite instability-high (MSI-H) tumors with high total mutational burden (TMB) (35).
Soft tissue infections or deep neck infection in the head and neck region can occur in patients with locally advanced HNSCC. Antibiotic usage around the time of ICI treatment could dampen the effects of PD-1/PD-L1 blockade (36–39), as antibiotics usage for head and neck soft tissue infection will disrupt the integrity of the gut microbiome. A recent study demonstrates that disruptions in the gut microbiome composition, also known as dysbiosis, is one of the mechanisms of primary resistance to ICI therapy (36). Experiments using fecal microbiota transplantation in avatar mice demonstrate that a combination of A. muciniphila and E. hirae would increase the CCR9- or CXCR3-expressing central memory T cells in the mesenteric lymph nodes and induce dendritic cells to secrete IL-12, a Th1 cytokine essential to elicit immunogenicity during PD-1/PD-L1 blockade (36). However, the actual mechanisms by which the intestinal flora modulate ICP immunotherapy is still unclear.
An immunosuppressive tumor microenvironment can also result from immune modulatory effects of CAF (32), TAM (40), and MDSC (41) (Figure 1). In addition, extracellular signals from angiogenesis can also drive immune suppression by directly suppressing APCs and immune effector cells, or by augmenting the effect of Tregs, MDSC, and TAM. Reciprocally, the above-mentioned immune suppressive cells can also drive angiogenesis (Figure 1), rendering a vicious cycle of disrupted immune activation (42).
What Does HNSCC Possess to Avoid Immunosurveillance or Destruction via the PD-1/PD-L1 Immune Checkpoint (ICP) Blockade?
To understand the tumor microenvironment of the oral cavity SCC using the infiltrating lymphocyte repertoires as an example, several studies identified considerable antigen-experienced CD4+ and CD8+ cells, Tregs, and PD-1-expressing and Tim-3-expressing T cells, as well as lymphoid follicles with germinal center-like structures (11, 43–48). These immunosuppressive characteristics are indicative of T-cell exhaustion. Table 2 shows that ~60% of patients with R/M HNSCC would not respond to the PD-1/PD-L1 ICP therapy. This means that even the “release of the brakes” through blocking the ligation of PD-1/PD-L1, cannot adequately allow the cytotoxic T cells to mount an attack of significant proportions on cancer cells, which would prevent tumor shrinkage or induce mixed responses amongst measurable tumor sites. The potential mechanisms being recognized for causing acquired immune escape from the PD-1/PD-L1 ICP in the setting of head and neck cancer are summarized in Table 3.
Table 3.
Mechanisms of immune escape that are implicated in HNSCC.
| Potential mechanisms | HPV+ or HPV- | Component in the immunity against cancer | References |
|---|---|---|---|
| TUMOR CELLS ADAPTION | |||
| Antigen presenting machinery (APM) via class I HLA | No data | Activated CD8+ immunologic pressure could induce transcriptional loss of HLA class one loci; deleterious alterations in JAK1/2 and β2-microglobulin. | (49–52) |
| Downregulation of the transporter associated with antigen processing (TAP)-1/2 heterodimer | APM component downregulated by the IFN-γ-phosphorylated STAT1-mediated signaling pathway; results in escaping recognition by tumor antigen-specific cytotoxic T lymphocytes. | (53, 54) | |
| JAK mutation | Both | Leads to loss of sensitivity to IFN-γ signals. | (35) |
| Cyclin D–CDK4 kinase re-introduction | No data | Destabilizes PD-L1 and controls the PD-L1 abundance in tumor cells. | (55) |
| Enrichment of CD44+ cancer stem-like cells | No data | Activates immunosuppressive network through cytokine release. | (54) |
| IKZF1-inactivating mutations | No data | Genomic alterations of the master regulator IKZF1 correlates with low immune recruitment. | (56) |
| IMPAIRED T-CELL FUNCTIONS AND PROLIFERATION | |||
| Nutritional iron deficiency state | Both | Affects T-cell proliferation | (57–59) |
| Mutations in interferon-γ-regulating genes | Both | Exhausted “Immune Class” enriched with M2 macrophages, WNT/TGF-β activation | (60, 61) |
| Suppression of stimulator of interferon genes (STING) pathway | HPV+ | Dampens the antitumor immune response. | (62) |
| Inhibition of STAT1 phosphorylation | Both | Enhanced T-cell exhaustion and accumulation of MDSCs | (63) |
| CHANGES IN METABOLITE- AND CYTOKINE-RICH TUMOR MICROENVIRONMENTS | |||
| Defective dendritic cells (DC) | Both | Defective cytokine- and STAT-mediated regulation of DC. | (64–66) |
| CD69-sufficient state | Both | Leads to effector T-cell exhaustion. | (67) |
| Genetic inactivation of GTP cyclohydrolase 1 (GCP1) | No data | Drastically impairs T-cell maturation. | (58) |
| Metabolite tetrahydrobiopterin (BH4) inhibited by kynurenine | No data | Will impair T cell function. | (58) |
| Indoleamine 2,3-dioxygenase-1 (IDO1); Tryptophan metabolite, kynurenine (Kyn) level | Both | IDO1 inhibits T cell proliferation, restricts tumor immune infiltration, and retards antitumor immune responses. Kyn released from ϕ, and myeloid cells activate T-reg cells. | (12, 68, 69) |
| Cancer-associated fibroblasts secrete TGF-β | Both | Results in restraining CD8+ T effector cells infiltrating into microenvironment. TGF-β1 also decreases the number of dendritic cells in the draining lymph nodes. |
(32, 70–73) |
| Arginase 1 expression on microenvironment myeloid cells | Both | Arg1 leads to L-arginine depletion depriving T cells and NK cells of essential nutrients required for proliferation. | (74) |
| CD38-upregulation | Both | CD38 inhibits CD8+ T-cell function via adenosine receptor signaling. | (75, 76) |
| Ectonucleotidases CD39/CD73 axis | Both | CD39 is considered a tumor-specific dysfunction marker. Tregs use the axis to diminish anti-cancer killing. | (76–79) |
| Polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) activation | Both | Through the nitric oxide pathway, PMN-MDSCs impair proliferation and expression molecules in activated T cells. | (41) |
| Nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3) inflammasome activation | Both | Leads to downstream interleukin (IL)-1β release. NLRP3 inflammasome/IL-1β axis increases MDSCs, Tregs and TAMs creating an immunosuppressive microenvironment. | (80) |
| ACTIVATION OF AND DEPENDENCE ON ALTERNATIVE IMMUNE CHECKPOINTS | |||
| Lymphocyte activation gene-3 (LAG3) (=CD223) upregulation | More in HPV+ | Induces a state of functional exhaustion in effector T-cells. | (81, 82) |
| T-cell immunoglobulin and ITIM domain (TIGIT)/CD155 pathway activation | Both | Augments TIGIT+ T-regs, a unique T-reg subset, leading to active suppression of anti-tumor immune response and T-cell exhaustion. | (82–85) |
| T-cell immunoglobulin mucin-3 (TIM-3) upregulation | Both | TIM-3 is considered a tumor-specific dysfunction marker. It dampens effector T-cell functions in the microenvironment. | (44, 48, 77, 86) |
| V domain-containing Ig suppressor of T-cell activation (VISTA) | Both | Leads to T-cell exhaustion and T-reg recruitment in the microenvironment. | (87, 88) |
Investigated mechanisms of acquired immune escape from PD-1/PD-L1 checkpoint blockade relevant to head and neck cancer.
There are currently four areas of research on the molecular resistance mechanisms underlying the selective pressure from PD-1/PD-L1 ICB (Table 3). In this review, they are categorized as tumor cell adaption, impairment of T cell functions and proliferation, changes in metabolite- and cytokine-rich tumor microenvironments, and activation of and dependence on alternative immune checkpoints.
Tumor Cell Adaption
Tumor cell adaption occurs through various processes, namely with modification of molecules causing immune escape via malfunction of the antigen-presenting machinery via class I HLA, reintroduction of cyclin D–CDK4 kinase heterodimers to cell cycles (55), enrichment of CD44+ cancer stem-like cells (54), JAK mutation (35), or development of inactivating mutation in IKZF1 gene, the master regulator of immune infiltrates recruitment (56).
Speckle-type POZ protein (SPOP) is an E3 ubiquitin ligase adaptor protein mediating poly-ubiquitination and proteasome-mediated degradation of several proteins, such as PD-L1 (55, 89). SPOP mutations or copy number variation can act as a tumor suppressor or progressor depending on the different context in different cancer types. In head and neck cancer, TCGA dataset, <2% of patient tumors exhibited mutated or altered SPOP, which was associated with non-significant reduction of the relative risk of relapse or disease progression (relative risk = 0.4, 95% confidence interval, 0.06–2.61) (Supplementary Table 1). Recent research identified that cyclin D-CDK4 kinase reintroduction in CAF would destabilize PD-L1 molecules via the cullin 3-SPOP pathway (55). Whereas, loss of function mutations of SPOP will increase the level of PD-L1 and tumor-infiltrating lymphocytes, as observed in mouse models. CDK4/6 inhibitor treatment will theoretically increase PD-L1 levels thus potentiating the ICB treatment (55).
In the milieu of the HNSCC immune microenvironment (Figure 1), there exists a kind of CD44+ stem-like cell, whose immunosuppressive capabilities have been demonstrated to be more effective than CD44-negative stem cells to inhibit the effector T-cell population while simultaneously inducing immunosuppressive Tregs and MDSC (54).
Genes function as a master regulator to control the expression of downstream genes by controlling transcription factors. IKZF1 is such a master regulator that could lead to enhanced recruitment of immune infiltrate and tumor sensitivity to ICP inhibitors in several cancer types. Genomic alterations of this gene could negatively affect immunogenicity and tumor response to ICB (56).
Results from genomic studies of HNSCC demonstrate that the immune evasion genetic pathways are different between HPV-negative and HPV-positive HNSCCs (24). The HLA mutations are uncommonly found with <10% prevalence rate in HPV-negative HNSCCs, whereas, the HPV-positive tumors will have more common HLA and Beta2-microglobulin (B2M) mutations and TRAF3 loss (90). B2M is a light chain incorporated with the MHC Class I heavy chain to form a capable antigen-presenting machinery complex. B2M deficiency in tumor cells, which results in defective cell surface HLA Class I complex or subsequent acquired loss of B2M while under immunotherapy, has been recognized as a crucial immune escape mechanism as demonstrated in various solid tumor models (91).
Impairment in T Cell Functions and Proliferation
Potential mechanisms of intrinsic or adaptive resistance rest upon T-cell functions and proliferation, which is the most essential weapon the human body utilizes to destroy cancer cells. T-cell functions and proliferation can be impaired through mutations in interferon-γ-regulating genes, suppression of the Stimulator of Interferon genes (STING) pathway or result from constitutional nutritional iron deficiency states (57–59, 62). It is noted that HPV-antigen expression levels in the tumor microenvironment would enhance cytotoxic T lymphocyte dysregulation (77).
Changes in Metabolite- and Cytokine-Rich Tumor Microenvironments
The other well-known hallmark of cancer is metabolic reprogramming by cancer cells. The tumor extracellular microenvironment contains a vibrant display of metabolites and cytokines released from different cell types aiming to create an immunosuppressive environment for the proliferation of tumor cells. Table 3 highlights several notable changes in metabolite-rich and cytokine-rich tumor stroma with consequences of dampening anti-cancer immunity. Defective cytokine- and STAT-mediated regulation of dendritic cells (DCs) leads to defective DCs. CD-69 sufficient state will cause effector T-cell exhaustion. The actual mechanism is still elusive; however, it is apparent that CD-69 expressed on leucocytes is responsible for cell retention in the tumor microenvironment and the CD-69 expression on T-cells is associated with the expression of PD-1 and Tim-3 in T-cells (67).
The function of metabolites GTP cyclohydrolase 1 (GCH1) and tetrahydrobiopterin (BH4) has been identified to be able to increase T-cell proliferation and promote their maturation. GCH1 is the first rate-limiting enzyme in the de novo BH4-synthesis pathway. Whereas, metabolite kynurenine has been found to activate Treg cells, and the enzyme indoleamine 2,3-dioxygenase (IDO) inhibits T cell proliferation and restricts tumor infiltration. Arginase 1 (Arg1) depletes L-arginine, depriving the essential nutrients that T cells and NK cells need to proliferate. The alterations of these metabolites will adversely impact anti-cancer immunity through various molecular mechanisms.
Activation of and Dependence on Alternative Immune Checkpoints
The fourth group of mechanisms underlying the resistance to PD-1-PD-L1 blockade includes activation of and dependence on alternative immune checkpoints. These alternative immune checkpoints other than PD-L1 include lymphocyte activation gene-3 (LAG3) (=CD223) upregulation, T-cell immunoglobulin and ITIM domain (TIGIT)/CD155 pathway activation, T-cell immunoglobulin mucin-3 (TIM-3) upregulation, and V domain-containing Ig suppressor of T-cell activation (VISTA). The past two decades have seen research identify the G-protein-coupled adenosine receptors mediating downregulation of the inflammatory tumor microenvironment creating an immunosuppressive milieu. Adenosine and adenosine triphosphate (ATP) are the most abundant metabolites within a cell and in the extracellular space, which acts as an autocrine and paracrine messenger. While ATP acts as an accelerator to promote proinflammatory activities, adenosine, via the Gs-coupled A2a and A2b receptors, suppresses various immune cells, including T lymphocytes, NK cells, neutrophils, dendritic cells, and macrophages (75, 76, 78, 92–95).
There are several vital molecules interplaying within the adenosine receptor signaling. The molecule, CD73, is an ecto-5-nucleotidase, which will dephosphorylate extracellular AMP to immunosuppressive adenosine (78, 79, 94). As a ubiquitous membranous ectozyme, CD38, cleaves NAD(+) and NADP(+), generating cyclic ADP ribose (cADPR), NAADP, and ADPR, which are directly involved in the calcium signaling essential for a cell (96). Current evidence suggests that one of the significant mechanisms in acquired immune escape from PD-1/PD-L1 inhibition is CD38 upregulation. CD38 promotes adenosine production through the CD38-CD203a-CD73 axis. Studies discovered that the tumor cells hijacked and leveraged the adenosine receptor signaling pathway by upregulating the activities of CD38, to develop resistance to PD-1/PD-L1 immunotherapy through inhibition of CD8+ T-cell function. Upregulation of CD38 is induced by tumor-derived soluble mediators, all-trans retinoic acid (ATRA) and IFNβ, in the tumor microenvironment.
Prediction of Response to Anti-PD-1 Immunotherapy in HNSCC
The development of predictive precision oncology aiming to discover and validate biomarkers that can predict the response from PD-1/PD-L1 immunotherapy has evolved relentlessly in the past years. Table 4 highlights the current discovery of biomarker predictors for immunotherapy response in patients who received PD-1/PD-L1 ICB. The eagerly awaited issues to be solved in predictive biomarker development are to achieve adequate clinical evidence about the discriminative capacity (power) of each biomarker. While the evidence is being investigated through clinical studies, Table 4 aims to demonstrate some potentially investigated or helpful biomarkers related to the field of HNSCC. These include the combined positive score (CPS) for PD-L1 protein expression, mismatch repair (MMR)-deficient status, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC)-driven mutations status, the molecular exhausted immune class, molecular active immune class, the interferon-γ signature (6-genes), the expanded immune signature (18-genes), the condition of somatic frameshift events in tumor suppressor genes, the total mutational burden (TMB), and the microenvironment infiltrating arginase 1 (Arg1)+/CD68+ macrophage-mediated immune evasion state.
Table 4.
Response prediction to anti-PD-1 immunotherapy in HNSCC.
| Gene alterations or signature | Testing platform | Response to anti-PD-1 checkpoint blockade | References |
|---|---|---|---|
| Combined Positive Score (CPS) for PD-L1 protein Expression | Immunohistochemistry on formalin-fixed paraffin-embedded tissue samples | CPS = number of PD-L1+ tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells, and multiplying by 100. In various trials, CPS ≥ 1 predicts response. | (7, 19, 97, 98) |
| MMR-deficient | Quantification of genomic MSI level (MSI intensity) | Higher insertion-deletion (Indel) load predicts response. | (99) |
| Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC)-driven mutations | APOBEC enrichment scores. | Upregulated as an innate immune response particularly in HPV+ tumors. APOBEC3 mutation leads to driver mutation in PI3KCA. | (100–102) |
| Molecular exhausted immune class | Gene expression pattern analyzed by non-negative matrix factorization algorithm | Portends a worse prognosis than active immune class in overall survival. | (61) |
| Molecular active immune class | Better prognosis (overall survival) than exhausted class. May predict immune responses. | (61) | |
| Interferon-γ signature (6-genes) | NanoString nCounter mRNA | Low signature score did not respond to pembrolizumab. | (103) |
| Expanded immune signature (18-genes) | NanoString nCounter mRNA | Low signature score did not respond to pembrolizumab. | (103) |
| Somatic frameshift events in tumor suppressor genes | Targeted massively parallel sequencing | More frequently seen in HPV- responders. | (104, 105) |
| Total mutational burden (TMB) | Targeted massively parallel sequencing | Predicts response in HPV- HNSCC. | (104, 105) |
| Microenvironment infiltrating arginase 1 (Arg1)+/CD68+ macrophage-mediated immune evasion | Enzyme-Linked Immunosorbent Assay (ELISA) | Plasma Arg1 level (ng/mL) to predict immune evasion (cutoff to be determined) | (74) |
The immunohistochemistry combined positive score (CPS) for quantifying the PD-L1 expression in a tumor sample has been adopted to predict tumor response to ICB treatment. This score is calculated as the number of PD-L1+ tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells, and multiplying by 100. In various trials, CPS ≥ 1% predicts immunotherapy response. The caveat of using CPS at the 1% cutoff to select HNSCC patients for PD-1 ICB is that intratumor heterogeineity should be considered. Rasmussen et al. have prospectively investigated the intratumor heterogeneity in PD-L1 expression in HNSCC in 28 patients with a total of 33 whole surgical specimens (98). With 1% cut off, 52% of the six random core biopsies from each surgical specimen was concordant with CPS. Defining a tumor as positive if just a single-one of the core biopsies was positive using CPS at 1% cut-off, the negative predictive value of a single negative core biopsy was 0% (98).
DNA mismatch repair (MMR) genes, hMLH1 and hMSH2, once inactivated, can accumulate thousands of mutations in simple repeats in genomic DNA and develop microsatellite instability (MSI). A defective MMR system results in both MSI and high tumor mutation burden (TMB-high), which further generates neoantigens to be identified by APCs to mount an effective cytotoxic killing of tumor cells. It has been estimated that MSI is present in ~40% of patients with HNSCC (106). The frequency of MSI in an endemic betel-quid chewing region is about the same as the otherwise non-endemic regions, having a rate of 37.9% (107). Despite the fact that these TMB-high cancers are more immunogenic, unlike those immunogenic-desert tumors, half of the TMB-high patients still do not respond to anti-PD-1 immunotherapy (108). Subsequently, a study looking at the mutational landscape of MSI-high discovered that the extent of immunotherapy response is specifically associated with a mutational load accumulating the insertion-deletion (indel) mutations (99). The authors emphasize that there is a greater impact of frameshifting indel mutations over the more general missense mutations, in eliciting anti-PD-1 immunotherapy response.
Generally speaking, TMB was defined as the total number of somatic, coding, base substitution, and indel mutations counted per megabase (Mb) of genome interrogated (109). In terms of the mutational burden as assessed by real-time gene sequencing, HNSCC exhibits median mutations/Mb of 5.0 with 10.1% (95% CI, 8.5%−11.9%) having > 20 mutations/Mb (109). In a group of 81 patients with HNSCC, higher TMB, as demonstrated by the targeted massively parallel tumor sequencing, predicted PD-1/PD-L1 ICB response (P < 0.01) (105).
T Cell-Inflamed Gene Signature
IFN-γ signaling (expanded immune) signature consists of the following 18 genes: CD3D, CCL5, CD3D, CD3E, IL2RG, CIITA, GZMK, CXCL9, CXCR6, TAGAP, CD2, HLA-E, IDO1, LAG3, NKG7, GZMB, STAT1, and CXCL10 (60). Typically, the core IFN-γ gene signature comprises six genes, i.e., IDO1, CXCL9, CXCL10, HLA-DRA, STAT1, and IFNG (60). In a retrospective analysis of the correlation of the 18-gene signature score (as a continuous variable) with the best of response (BOR) and PFS for 43 patients with HNSCC of the KEYNOTE-012 cohort (18, 110), strong statistical significance (P = 0.015 and P < 0.001, respectively) was obtained. The 18-gene IFN-γ signature profile derived from the NanoString platform is under development as a clinical-grade companion diagnostic incorporated into the future or ongoing pembrolizumab trials (111).
An enriched proinflammatory M1 macrophage signature, enhanced cytolytic activity, abundant tumor-infiltrating lymphocytes, high human papillomavirus (HPV) infection, and favorable prognosis were associated with active immune class (all, P < 0.05).
A bioinformatics study using a non-negative matrix factorization algorithm of the RNA sequencing profiles of 522 patients with HNSCC collected in the TCGA identified an immune class (61). This immune class was determined based on the enriched inflammatory response, enhanced cytolytic activity, and active interferon-γ signaling. There are two subclasses within the immune class, namely, the exhausted immune class, with a poor prognosis, and the active immune class, with a favorable prognosis. The active immune class was highlighted to have enriched anti-tumoral M1 macrophage polarization, stronger cytolytic activity, abundant tumor-infiltrating lymphocytes, and higher HPV infection rates (61). The research findings are relevant to the clinical application on selecting patients with active immune signatures for immunotherapy.
Finally, IFN-gamma signaling depends on the integrity of the JAK/STAT pathway. An orthotopic head and neck squamous cell carcinoma model experiment using Stat1 deficient [Stat1(–/–)] mice observed enhanced T-cell exhaustion and accumulation of MDSCs, creating a tumor-permissive microenvironment in Stat1 deficient mice (63).
Based on current research findings, if tumor samples are discovered possessing any of the aforementioned genetic aberrations or signatures indicating a hot immune tumor, a treatment recommendation incorporating the blockade of the PD-1/PD-L1 axis is justified. The status of recommendation on selecting a predictive biomarker will be refined when new evidence or data is available to suggest otherwise.
Conclusions
Hnsccs have more immunosuppressive tumor microenvironments although the initial research indicated that HNSCCs are amongst the top malignancies ranked from high to low by the neoantigen loads and total tumor burdens. Although a new treatment paradigm has been established placing pembrolizumab + platinum and infusional fluorouracil as the new standard as the first line for R/M HNSCC, ~64% of patients will not benefit from the significant tumor regression criteria, indicating innate, adaptive resistance to the blockade of PD-1/PD-L1 ligation. This review assimilated the research findings after an extensive literature review and presents the potential mechanisms of immune escape from the PD-1/PD-L1 checkpoint inhibition into four aspects as follows: tumor cell adaption, impairment of T-cell functions and proliferation, changes in metabolite- and cytokine-rich tumor microenvironments, and activation of the alternative immune checkpoints. There are no easy methods for immunotherapy response prediction in HNSCC. Even the widely accepted criteria of the combined positivity score for PD-L1 protein expression, commonly adopted in several clinical trials to stratify patients with HNSCC by the degree of immunogenicity, may have a negative predictive value of zero percent if just one core-biopsy was examined, questioning the usefulness of this immunohistochemistry test in unresectable patients. Targeted massively parallel sequencing and NanoString nCounter mRNA analysis showing the results of total mutational burden or certain immune signatures present promising tests with the potential to discriminate between immune responsive or unresponsive patients with HNSCC, thus requiring further research to confirm their utility in predictive precision immuno-oncology. Every patient with HNSCC contemplating ICI therapy, theoretically, should be considered for several tests, namely TMB, MSI, PD-L1/L2 genome amplification, viral antigens, and next-generation sequencing, to identify actionable targets to combine with ICI therapy.
Author Contributions
The sole author of this work performed all aspects related to a review article, such as research idea conception, research strategy planning, literature search, retrieval and selection, extensive analysis of data, drafting the manuscript, drawing figures, creating tables, and finally approved this manuscript for publishing.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author is grateful that part of this review has been critically reviewed as a Capstone Project Topic Review by an anonymous expert in the field of Harvard Medical School, Boston, in the High-Impact Cancer Research: Cancer Biology and Therapeutics program. A significant amount of time was dedicated to the Capstone Project during the 1 year program. The author of this review took part in the program as a student and this review is a result of these activities.
Glossary
Abbreviations
- APOBEC
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like
- BDSC
Bone marrow-derived stromal cell
- BMSC
Bone marrow-derived suppressor cell
- CPS
Combined positive score
- HNSCC
Head and neck squamous cell carcinoma
- ICB
Immune checkpoint blockade
- ICP
Immune checkpoint
- IDO1
Indoleamine 2,3-dioxygenase-1
- IKZF1
IKAROS family zinc finger 1
- JAK
Janus kinase
- MDSC
Myeloid-derived suppressor cell
- MMR
Mismatched Repair
- ORR
Overall Response Rate
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- R/M
Relapsed/metastatic
- STAT
signal transducer and activator of transcription
- STING
Stimulator of Interferon Genes
- TCGA
The Cancer Genome Atlas (program)
- TIGIT
T-cell immunoglobulin and ITIM domain
- Tim-3
T cell immunoglobulin and mucin-domain containing-3
- TMB
Tumor mutation burden
- TME
Tumor microenvironment
- VISTA
V domain-containing Ig suppressor of T-cell activation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00268/full#supplementary-material
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Straub M, Drecoll E, Pfarr N, Weichert W, Langer R, Hapfelmeier A, et al. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. (2016) 7:12024–34. 10.18632/oncotarget.7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martorelli D, Houali K, Caggiari L, Vaccher E, Barzan L, Franchin G, et al. Spontaneous T cell responses to Epstein-Barr virus-encoded BARF1 protein and derived peptides in patients with nasopharyngeal carcinoma: bases for improved immunotherapy. Int J Cancer. (2008) 123:1100–7. 10.1002/ijc.23621 [DOI] [PubMed] [Google Scholar]
- 4.Wu TS, Wang LC, Liu SC, Hsu TY, Lin CY, Feng GJ, et al. EBV oncogene N-LMP1 induces CD4 T cell-mediated angiogenic blockade in the murine tumor model. J Immunol. (2015) 194:4577–87. 10.4049/jimmunol.1400794 [DOI] [PubMed] [Google Scholar]
- 5.Chow LQM. Head and neck cancer. N Engl J Med. (2020) 382:60–72. 10.1056/NEJMra1715715 [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 7.Rischin D, Harrington K, Greil R, Soulieres D, Tahara M, de Castro G, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. (2019) 37:6000. [Google Scholar]
- 8.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. (2013) 369:134–44. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 10.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. (2016) 7:550. 10.3389/fimmu.2016.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. (2013) 73:128–38. 10.1158/0008-5472.CAN-12-2606 [DOI] [PubMed] [Google Scholar]
- 12.Foy JP, Bertolus C, Michallet MC, Deneuve S, Incitti R, Bendriss-Vermare N, et al. The immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann Oncol. (2017) 28:1934–41. 10.1093/annonc/mdx210 [DOI] [PubMed] [Google Scholar]
- 13.Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. (2017) 35:4050–6. 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Lee JY, Lim SH, Park K, Sun JM, Ko YH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat. (2016) 48:527–36. 10.4143/crt.2015.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mletzko S, Pinato DJ, Robey RC, Dalla Pria A, Benson P, Imami N, et al. Programmed death ligand 1 (PD-L1) expression influences the immune-tolerogenic microenvironment in antiretroviral therapy-refractory Kaposi's sarcoma: a pilot study. Oncoimmunology. (2017) 6:e1304337. 10.1080/2162402x.2017.1304337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. (2015) 51:221–8. 10.1016/j.oraloncology.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 17.Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. (2016) 22:704–13. 10.1158/1078-0432.CCR-15-1543 [DOI] [PubMed] [Google Scholar]
- 18.Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. (2016) 34:3838–45. 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. (2017) 35:1542–9. 10.1200/JCO.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. (2019) 393:156–67. 10.1016/s0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 21.Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. (2018) 81:45-51. 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colevas AD, Bahleda R, Braiteh F, Balmanoukian A, Brana I, Chau NG, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. (2018) 29:2247–53. 10.1093/annonc/mdy411 [DOI] [PubMed] [Google Scholar]
- 24.Segal NH, Ou SI, Balmanoukian A, Fury MG, Massarelli E, Brahmer JR, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer. (2019) 109:154-61. 10.1016/j.ejca.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 25.Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. (2018) 5:195-203. 10.1001/jamaoncol.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahig H, Aubin F, Stagg J, Gologan O, Ballivy O, Bissada E, et al. Phase I/II trial of durvalumab plus tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma. BMC Cancer. (2019) 19:68. 10.1186/s12885-019-5266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbers JBW, Al-Mamgani A, Tesseslaar MET, van den Brekel MWM, Lange CAH, van der Wal JE, et al. Immuno-radiotherapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: Results from a phase-I trial. Radiother Oncol. (2019) 142:79-84. 10.1016/j.radonc.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 28.Merlano MC, Merlotti AM, Licitra L, Denaro N, Fea E, Galizia D, et al. Activation of immune responses in patients with relapsed-metastatic head and neck cancer (CONFRONT phase I-II trial): multimodality immunotherapy with avelumab, short-course radiotherapy, and cyclophosphamide. Clin Transl Radiat Oncol. (2018) 12:47-52. 10.1016/j.ctro.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Lee NY. JAVELIN head and neck 100: a phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. (2019) 15:687–94. 10.2217/fon-2018-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassetta L, Baekkevold ES, Brandau S, Bujko A, Cassatella MA, Dorhoi A, et al. Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates. Cancer Immunol Immunother. (2019) 68:687–97. 10.1007/s00262-019-02302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. (2017) 8:8633–47. 10.18632/oncotarget.14374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks JM, Menezes AN, Ibrahim M, Archer L, Lal N, Bagnall CJ, et al. Development and validation of a combined hypoxia and immune prognostic classifier for head and neck cancer. Clin Cancer Res. (2019) 25:5315–28. 10.1158/1078-0432.CCR-18-3314 [DOI] [PubMed] [Google Scholar]
- 34.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. (2014) 74:665–74. 10.1158/0008-5472.CAN-13-0992 [DOI] [PubMed] [Google Scholar]
- 35.Albacker LA, Wu J, Smith P, Warmuth M, Stephens PJ, Zhu P, et al. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS ONE. (2017) 12:e0176181. 10.1371/journal.pone.0176181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 37.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. (2019) 8:e1568812. 10.1080/2162402x.2019.1568812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalani AA, Xie W, Braun DA, Kaymakcalan M, Bosse D, Steinharter JA, et al. Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. Eur Urol Oncol. (2019). 10.1016/j.euo.2019.09.001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. (2019) 130:10-7. 10.1016/j.lungcan.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 40.Sakakura K, Takahashi H, Kaira K, Toyoda M, Murata T, Ohnishi H, et al. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab Invest. (2016) 96:994–1003. 10.1038/labinvest.2016.70 [DOI] [PubMed] [Google Scholar]
- 41.Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. (2014) 134:2853–64. 10.1002/ijc.28622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. 10.1158/1078-0432.Ccr-18-1543 [DOI] [PubMed] [Google Scholar]
- 43.Kato R, Yamasaki M, Urakawa S, Nishida K, Makino T, Morimoto-Okazawa A, et al. Increased Tim-3(+) T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother. (2018) 67:1673–83. 10.1007/s00262-018-2225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, McMichael EL, Shayan G, Li J, Chen K, Srivastava R, et al. Novel effector phenotype of Tim-3(+) regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin Cancer Res. (2018) 24:4529–38. 10.1158/1078-0432.CCR-17-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan H, Fang L, Pan H, Deng Z, Gao S, Liu O, et al. An adaptive immune response driven by mature, antigen-experienced T and B cells within the microenvironment of oral squamous cell carcinoma. Int J Cancer. (2016) 138:2952–62. 10.1002/ijc.30019 [DOI] [PubMed] [Google Scholar]
- 46.Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. (2016) 6:e1261779. 10.1080/2162402X.2016.1261779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linedale R, Schmidt C, King BT, Ganko AG, Simpson F, Panizza BJ, et al. Elevated frequencies of CD8 T cells expressing PD-1, CTLA-4 and Tim-3 within tumour from perineural squamous cell carcinoma patients. PLoS ONE. (2017) 12:e0175755. 10.1371/journal.pone.0175755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. (2018) 24:5368–80. 10.1158/1078-0432.CCR-18-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Concha-Benavente F, Srivastava R, Ferrone S, Ferris RL. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. (2016) 58:52-8. 10.1016/j.oraloncology.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA class i antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. (2017) 7:1420–35. 10.1158/2159-8290.Cd-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. (2006) 66:9281–9. 10.1158/0008-5472.CAN-06-0488 [DOI] [PubMed] [Google Scholar]
- 52.Paulson KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, Perdicchio M, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nature Commun. (2018) 9:3868. 10.1038/s41467-018-06300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. (2011) 60:525–35. 10.1007/s00262-010-0961-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck. (2011) 33:208–15. 10.1002/hed.21420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. (2018) 553:91–5. 10.1038/nature25015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen JC, Perez-Lorenzo R, Saenger YM, Drake CG, Christiano AM. IKZF1 enhances immune infiltrate recruitment in solid tumors and susceptibility to immunotherapy. Cell Syst. (2018) 7:92–103.e4. 10.1016/j.cels.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 57.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. (2013) 13:509–19. 10.1016/j.chom.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. (2018) 563:564–8. 10.1038/s41586-018-0701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung N, Shen CC, Hu YW, Hu LY, Yeh CM, Teng CJ, et al. Risk of cancer in patients with iron deficiency anemia: a nationwide population-based study. PLoS ONE. (2015) 10:e0119647. 10.1371/journal.pone.0119647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. (2017) 127:2930–40. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YP, Wang YQ, Lv JW, Li YQ, Chua MLK, Le QT, et al. Identification and validation of novel microenvironment-based immune molecular subgroups of head and neck squamous cell carcinoma: implications for immunotherapy. Ann Oncol. (2019) 30:68–75. 10.1093/annonc/mdy470 [DOI] [PubMed] [Google Scholar]
- 62.Cadwell K, Wu L, Cao J, Cai WL, Lang SM, Horton JR, et al. KDM5 histone demethylases repress immune response via suppression of STING. PLoS Biology. (2018) 16:e2006134 10.1371/journal.pbio.2006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan N, Anderson K, Volpedo G, Hamza O, Varikuti S, Satoskar AR, et al. STAT1 inhibits T cell exhaustion and myeloid derived suppressor cell accumulation to promote anti-tumor immune responses in head and neck squamous cell carcinoma. Int J Cancer. (2019) 146:1717–1729. 10.1002/ijc.32781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. (2018) 24:1178–91. 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chrisikos TT, Zhou Y, Slone N, Babcock R, Watowich SS, Li HS. Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol Immunol. (2019) 110:24-39. 10.1016/j.molimm.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Versteven M, Van den Bergh JMJ, Marcq E, Smits ELJ, Van Tendeloo VFI, Hobo W, et al. Dendritic cells and programmed death-1 blockade: a joint venture to combat cancer. Front Immunol. (2018) 9:394. 10.3389/fimmu.2018.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mita Y, Kimura MY, Hayashizaki K, Koyama-Nasu R, Ito T, Motohashi S, et al. Crucial role of CD69 in anti-tumor immunity through regulating the exhaustion of tumor-infiltrating T cells. Int Immunol. (2018) 30:559–67. 10.1093/intimm/dxy050 [DOI] [PubMed] [Google Scholar]
- 68.Laimer K, Troester B, Kloss F, Schafer G, Obrist P, Perathoner A, et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol. (2011) 47:352–7. 10.1016/j.oraloncology.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 69.Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. (2019) 18:379–401. 10.1038/s41573-019-0016-5 [DOI] [PubMed] [Google Scholar]
- 70.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. (2018) 6:47. 10.1186/s40425-018-0356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. (2018) 554:544–8. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. (2018) 554:538–43. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- 73.Weber F, Byrne SN, Le S, Brown DA, Breit SN, Scolyer RA, et al. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother. (2005) 54:898–906. 10.1007/s00262-004-0652-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. (2017) 5:101. 10.1186/s40425-017-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. (2018) 8:1156–75. 10.1158/2159-8290.CD-17-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mittal D, Vijayan D, Smyth MJ. Overcoming acquired PD-1/PD-L1 resistance with CD38 blockade. Cancer Discov. (2018) 8:1066–8. 10.1158/2159-8290.CD-18-0798 [DOI] [PubMed] [Google Scholar]
- 77.Krishna S, Ulrich P, Wilson E, Parikh F, Narang P, Yang S, et al. Human papilloma virus specific immunogenicity and dysfunction of CD8(+) T cells in head and neck cancer. Cancer Res. (2018) 78:6159–70. 10.1158/0008-5472.Can-18-0163 [DOI] [PubMed] [Google Scholar]
- 78.Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. (2018) 67:1271–84. 10.1007/s00262-018-2186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng W-W, Li Y-C, Ma S-R, Mao L, Yu G-T, Bu L-L, et al. Specific blockade CD73 alters the “exhausted” phenotype of T cells in head and neck squamous cell carcinoma. Int J Cancer. (2018) 143:1494–504. 10.1002/ijc.31534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Huang C-F, Li Y-C, Deng W-W, Mao L, Wu L, et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol. Life Sci. (2018) 75:2045–58. 10.1007/s00018-017-2720-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. (2017) 276:80–96. 10.1111/imr.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gameiro SF, Ghasemi F, Barrett JW, Koropatnick J, Nichols AC, Mymryk JS, et al. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology. (2018) 7:e1498439. 10.1080/2162402x.2018.1498439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. (2016) 1:e89829. 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shayan G, Kansy BA, Gibson SP, Srivastava RM, Bryan JK, Bauman JE, et al. Phase Ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin Cancer Res. (2018) 24:62–72. 10.1158/1078-0432.Ccr-17-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, et al. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. (2019) 7:1700–13. 10.1158/2326-6066.Cir-18-0725 [DOI] [PubMed] [Google Scholar]
- 86.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. (2016) 7:10501. 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. (2016) 57:54-60. 10.1016/j.oraloncology.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. (2017) 66:627–36. 10.1007/s00262-017-1968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang XJ, Jin Y, Song JL, Deng F. MiR-373 promotes proliferation and metastasis of oral squamous cell carcinoma by targeting SPOP. Eur Rev Med Pharmacol Sci. (2019) 23:5270–6. 10.26355/eurrev_201906_18193 [DOI] [PubMed] [Google Scholar]
- 90.Hammerman PS, Hayes DN, Grandis JR. Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discov. (2015) 5:239–44. 10.1158/2159-8290.CD-14-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. (2012) 61:1359–71. 10.1007/s00262-012-1321-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mediavilla-Varela M, Castro J, Chiappori A, Noyes D, Hernandez DC, Allard B, et al. A Novel antagonist of the immune checkpoint protein adenosine A2a receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia. (2017) 19:530–6. 10.1016/j.neo.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. (2014) 4:879–88. 10.1158/2159-8290.CD-14-0341 [DOI] [PubMed] [Google Scholar]
- 94.Hausler SF, Montalban del Barrio I, Strohschein J, Chandran PA, Engel JB, Honig A, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. (2011) 60:1405–18. 10.1007/s00262-011-1040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. (2006) 103:13132–7. 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. (2008) 88:841–86. 10.1152/physrev.00035.2007 [DOI] [PubMed] [Google Scholar]
- 97.Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. (2019) 7:184. 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasmussen JH, Lelkaitis G, Hakansson K, Vogelius IR, Johannesen HH, Fischer BM, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer. (2019) 120:1003–6. 10.1038/s41416-019-0449-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandal R, Samstein RM, Lee KW, Havel JJ, Wang H, Krishna C, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. (2019) 364:485–91. 10.1126/science.aau0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faden DL, Ding F, Lin Y, Zhai S, Kuo F, Chan TA, et al. APOBEC mutagenesis is tightly linked to the immune landscape and immunotherapy biomarkers in head and neck squamous cell carcinoma. Oral Oncol. (2019) 96:140-7. 10.1016/j.oraloncology.2019.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faden DL, Thomas S, Cantalupo PG, Agrawal N, Myers J, DeRisi J. Multi-modality analysis supports APOBEC as a major source of mutations in head and neck squamous cell carcinoma. Oral Oncol. (2017) 74:8-14. 10.1016/j.oraloncology.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 102.Gillison ML, Akagi K, Xiao W, Jiang B, Pickard RKL, Li J, et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. (2019) 29:1–17. 10.1101/gr.241141.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Albright A, et al. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. J Immunother Cancer. (2015) 3(Suppl. 2):P80 10.1186/2051-1426-3-s2-p80 [DOI] [Google Scholar]
- 104.Georgiadis A, Durham JN, Keefer LA, Bartlett BR, Zielonka M, Murphy D, et al. Noninvasive detection of microsatellite instability and high tumor mutation burden[[Inline Image]] in cancer patients treated with PD-1 blockade. Clin Cancer Res. (2019) 25:7024–34. 10.1158/1078-0432.CCR-19-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. (2018) 3:98811. 10.1172/jci.insight.98811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demokan S, Suoglu Y, Demir D, Gozeler M, Dalay N. Microsatellite instability and methylation of the DNA mismatch repair genes in head and neck cancer. Ann Oncol. (2006) 17:995–9. 10.1093/annonc/mdl048 [DOI] [PubMed] [Google Scholar]
- 107.Lin JC, Wang CC, Jiang RS, Wang WY, Liu SA. Microsatellite alteration in head and neck squamous cell carcinoma patients from a betel quid-prevalent region. Sci Rep. (2016) 6:22614. 10.1038/srep22614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. (2016) 17:956–65. 10.1016/s1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 111.Wallden B, Pekker I, Popa S, Dowidar N, Sullivan A, Hood T, et al. Development and analytical performance of a molecular diagnostic for anti-PD1 response on the nCounter Dx Analysis System. J Clin Oncol. (2016) 34(15_suppl):3034 10.1200/JCO.2016.34.15_suppl.3034 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.