Abstract
Hand, foot and mouth disease (HFMD) has high prevalence around the world, with serious consequences for children. Due to the long survival period of HFMD virus in ambient air, air pollutants may play a critical role in HFMD epidemics. We collected data on daily cases of HFMD among children aged 0–14 years in Ningbo City between 2014 and 2016. Distributed lag nonlinear models were used to assess the effects of particulate matter (PM2.5), sulphur dioxide (SO2), nitrogen dioxide (NO2) and ozone (O3) on the daily incidence of HFMD among children, with analyses stratified by gender and age. Compared with moderate levels of air pollution, high SO2 levels had a relative risk (RR) of 2.32 (95% CI 1.42–3.79) and high NO2 levels had a RR of 2.01 (95% CI 1.22–3.31). The RR of O3 was 2.12 (95% CI 1.47–3.05) and that of PM2.5 was 0.77 (95% CI 0.64–0.92) at moderate levels of air pollution. Specifically, high levels of SO2 and NO2 had RRs of 2.39 (95% CI 1.44–3.96) and 2.02 (95% CI 1.21–3.39), respectively, among 0–4-year-old children, while high O3 had an RR of 2.31 (95% CI 1.09–4.89) among 5–14-year-old children. Our findings suggest significant associations of high SO2 and NO2 levels and moderate O3 levels in HFMD epidemics, and also indicate that air pollution causes lagged effects on HFMD epidemics. Our study provides practical and useful data for targeted prevention and control of HMFD based on environmental evidence.
Key words: Air pollutants, HFMD
Introduction
Hand, foot and mouth disease (HFMD) is a common illness caused by a variety of enteroviruses, such as enterovirus 71 or Coxsackie, and usually affects children [1]. The first outbreak of HFMD was reported in Canada in 1957 by Robinson et al. [2]. After that, a series of reports on individual cases of HFMD and larger outbreaks have been published across the world. In the United States, 63 cases were identified between November 2011 and February 2012, according to the U.S. Centers for Disease Control and Prevention (https://www.cdc.gov/). Asia was reported to have the most outbreaks of HFMD across the world. In China, a national survey from 2008 to 2012 estimated that ~15 million cases had been reported, with an incidence of 1.2 per 1000 person-years, and the disease is responsible for 350–900 reported deaths annually, predominantly among young children [3]. HFMD has also occurred in Japan during the past decade. Between April and September 2012, 2900 cases have been reported in children under 15 years of age [4]. Other Asian countries, including India, Singapore and South Korea, have also reported cases of HFMD [5–7]. HFMD is transmitted by faecal–oral, oral–oral and respiratory droplet contact [8]. Outbreaks can occur in the spring to fall and most cases occur in patients younger than 10 years [8]. Experimental studies have indicated that although this disease is self-limiting and the clinical symptoms are mild, such as rashes or mucosal herpes, severe complications, such as meningitis or encephalitis, occasionally occur. These can result in death, particularly among young children under 5 years of age [9]. The incubation period of HFMD is typically about 3 to 10 days [8, 10, 11]. To date, no effective vaccines have been developed against HFMD-related viruses. Hence, determining the risk factors so that we can establish an early warning system remains a crucial part of the measures to prevent and control HFMD outbreaks, and thus reduce the burden of the disease for children [12].
Air pollution has been reported to be a leading cause of global mortality. The World Health Organization estimates that ~7 million premature deaths were partly caused by air pollution in 2012. This corresponds to almost one in eight deaths globally (http://www.who.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2014.pdf?ua=1). Epidemiological and clinical studies have demonstrated that exposure to air pollution, and particulate matter (PM10 and PM2.5) in particular, is associated with childhood diseases such as allergy, asthma and congenital hypothyroidism and increases the risks once a disease has been contracted [13–15]. These findings suggest that essential air pollution sources, such as nitrogen dioxide (NO2), ozone (O3) and sulphur dioxide (SO2) could also contribute to HDMF outbreaks. Susan et al. estimated that 9–23 million asthma cases may be attributable to air pollution, specifically O3 and PM2.5 [16]. Guoqi et al. analysed the effect of air pollution on HFMD, and found that low O3 levels increased the risk, whereas low PM2.5 levels protected against HFMD [17]. However, the results of our previous study did not suggest any relationship between exposure to PM10 and the incidence of HFMD [18]. The conclusions drawn from these data are inconsistent with respect to air pollution and the incidence of HFMD. Nonetheless, to date, only limited studies have been carried out on the relationship between air pollution and HFMD incidence. For example, SO2 has not yet been included in assessments of pollutants associated with HFMD, but it is considered a significant pollutant in both cities and rural areas [19]. We hypothesised that the incidence of HFMD may be attributable to exposure to four essential air pollutants (SO2, NO2, O3 and PM2.5). We used a distributed lag nonlinear model (DLNM) to analyse the associations between air pollution and the incidence of HFMD in Ningbo City between 2014 and 2016. This is the first analysis of the relationship between the incidence of HFMD and combinations of multiple air pollutants. We expect that the results of our study will be helpful for clarifying potential relevant factors and providing a reference for establishing an early warning system to prevent children from contracting HFMD.
Methods
Data collection
Ningbo City is a well-known economic centre and tourist destination in eastern China. Ningbo has an area of 9816 km2 and a population of 7.80 million (in 2016). The city is located near the coast, with an average annual temperature of 22 °C and an average relative humidity of 70%.
We obtained information regarding HFMD cases among children aged 0–14 years between January 2014 and December 2016 from the National Notifiable Disease Reporting System of the Ningbo Municipal Centre for Disease Control and Prevention. The standards for the diagnosis of HFMD were based on recommendations from the Chinese Ministry of Health [20]. We tried to ensure that our analysis was based on a stable population by only including the records of patients living in five central urban districts in our data set. To identify vulnerable populations, we reclassified the data by sex (male, female) and age (0–4, 5–14 years old).
We collected data on SO2, nitrogen dioxide (NO2), daily maximum 8 h average ambient O3 concentration (O3-8 h) and ambient fine particulate matter (⩽2.5 µm in aerodynamic diameter, PM2.5) measured at eight monitoring stations in the central urban area. These data were provided by the Ningbo Environment Monitoring Center. We averaged daily measurements from the eight monitoring stations to create a daily estimated exposure. The method has been reported previously [21, 22]. The daily meteorological data used in this study were published by the Ningbo Meteorological Bureau. They included daily average temperature, relative humidity and sunshine duration. These data were measured at the Yinzhou station, which is the only national weather monitoring station in the central urban area of Ningbo city.
Data analysis
We used a DLNM to ensure that our model had sufficient flexibility to describe the lag time dimension of the exposure–response relationship. These are usually applied to the analysis of the relationship between air quality and disease incidence due to their ability to capture predictors at both the usual pace and with the additional dimension of temporal lag [23]. For example, this model has been applied previously to assess the effect of heat waves on cardiovascular daily mortality and temperature changes on the numbers of HFMD-related emergency room visits and morbidity [24, 25]. It has been suggested that this model can be used effectively to investigate associations between HFMD incidence and air pollution.
The model is defined by the following formula:
where E(Yt) is the expected number of HFMD cases on calendar day t (1,2,…1096) and α is the model intercept; we controlled for long-term and seasonality trends using a natural cubic spline (ns) with 7 degrees of freedom (df) per year for time (1,2,…1096), and adjusted for other environmental confounding variables using a natural cubic spline with 3 df for relative humidity (RHt) and sunshine duration (Sunt) [26, 27]. Although there is no consensus on how many knots are optimal, 7 per year has been justified as a balance between providing adequate control for seasonality and other confounding by trends in time, while leaving sufficient information from which to estimate exposure effects [27]. We controlled for the potential effects of temperature using a cross-basis function (Tempt,l), natural cubic splines with 4 df for the exposure–response relationship and natural cubic splines with 4 df for the lag–response relationship. We included dummy variables for day of week (DOWt) and holidays (Holidayt), which included vacation days for elementary schools and national public holidays. APt,l represented the bi-dimensional natural cubic spline of the exposure–response association between air pollutants and daily HFMD cases, and we used a non-linear model to determine the effects of air pollutants. We used a maximum lag of 14 days to capture any lagged effects of air pollution, considering that HFMD has an incubation period that is typically less than 14 days. The Akaike's information criterion for the quasi-Poisson model (Q-AIC) was applied to verify the optimal df of the models [28]. To account for autocorrelations in the residuals, we included autoregressive terms of the HFMD daily counts at lag 1 and 2 into the model based on an autocorrelation plot of the residuals.
To assess the effects of each air pollutant, we used the 1st percentile of each air pollutant as a reference value, and the effects of moderate and high levels of air pollutants were estimated by calculating the risk of HFMD cases at the 50th and 99th percentiles of each air pollutant relative to the reference value, with lags of 0–14 days. We performed all statistical analyses using the ‘dlnm' package in the R programming language (ver. 3.1.1; R Development Core Team, Vienna, Austria) and defined statistical significance as a two-tailed P < 0.05.
Results
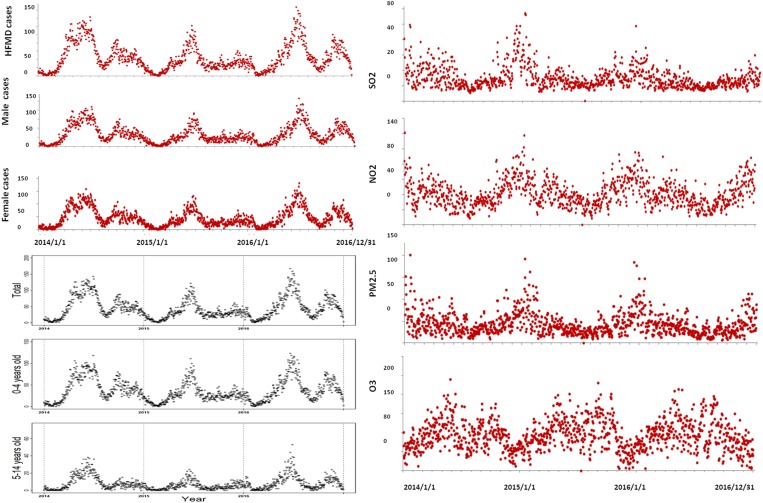
During the period from 2014 to 2016, a total of 48 209 HFMD cases occurred in Ningbo city, 28 606 (59.34%) of which were male and 19 603 (40.66%) female; about 82.88% of the cases were children less than 5 years old. Figure 1 shows the spatial distribution of environmental monitoring stations while Figure 2 illustrates daily trends in the distribution of HFMD cases and levels of air pollutants. We observed two peaks of HFMD cases in Ningbo city in April/July and September/November, and the seasonal distribution of all population groups was similar. The summary statistics of HFMD, meteorological factors and air pollution can be found in Table 1.
Fig. 1.
The spatial distribution of environmental monitoring stations in Ningbo city.
Fig. 2.
Raw plots showing the temporal trends of HFMD cases in Ningbo city, 2014–2016.
Table 1.
Summary statistics of HFMD, meteorological factors and air pollution in Ningbo city, during 2014–2016
| Groups | Mean | s.d. | Percentile | ||
|---|---|---|---|---|---|
| P1 | P50 | P99 | |||
| All age | 44.0 | 32.4 | 2.0 | 35.0 | 134.0 |
| Sex | |||||
| Male | 26.1 | 19.5 | 0.0 | 21.0 | 80.0 |
| Female | 17.9 | 13.6 | 0.0 | 14.5 | 55.0 |
| Age | |||||
| 0–4 years | 36.5 | 25.9 | 1.0 | 30.0 | 106.0 |
| 5–14 years | 7.5 | 7.4 | 0.0 | 5.0 | 33.1 |
| Environment factors | |||||
| PM2.5 (μg/m3) | 43.2 | 26.4 | 9.7 | 37.0 | 133.4 |
| SO2 (μg/m3) | 41.1 | 17.5 | 12.4 | 38.1 | 89.9 |
| NO2 (μg/m3) | 15.2 | 7.8 | 6.6 | 13.1 | 44.6 |
| O3-8 h (μg/m3) | 94.0 | 40.1 | 13.1 | 90.0 | 197.7 |
| Average temperature (°C) | 17.9 | 8.2 | 2.0 | 19.1 | 31.3 |
| Relative humidity (%) | 76.8 | 11.5 | 46.0 | 78.0 | 96.0 |
| Sunshine duration (h) | 4.1 | 3.9 | 0.0 | 3.4 | 11.6 |
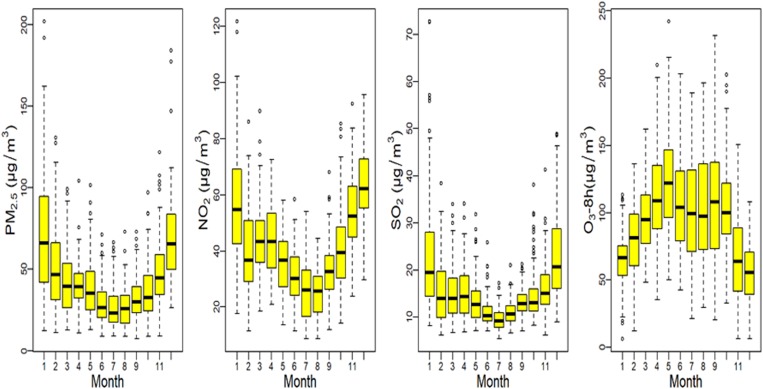
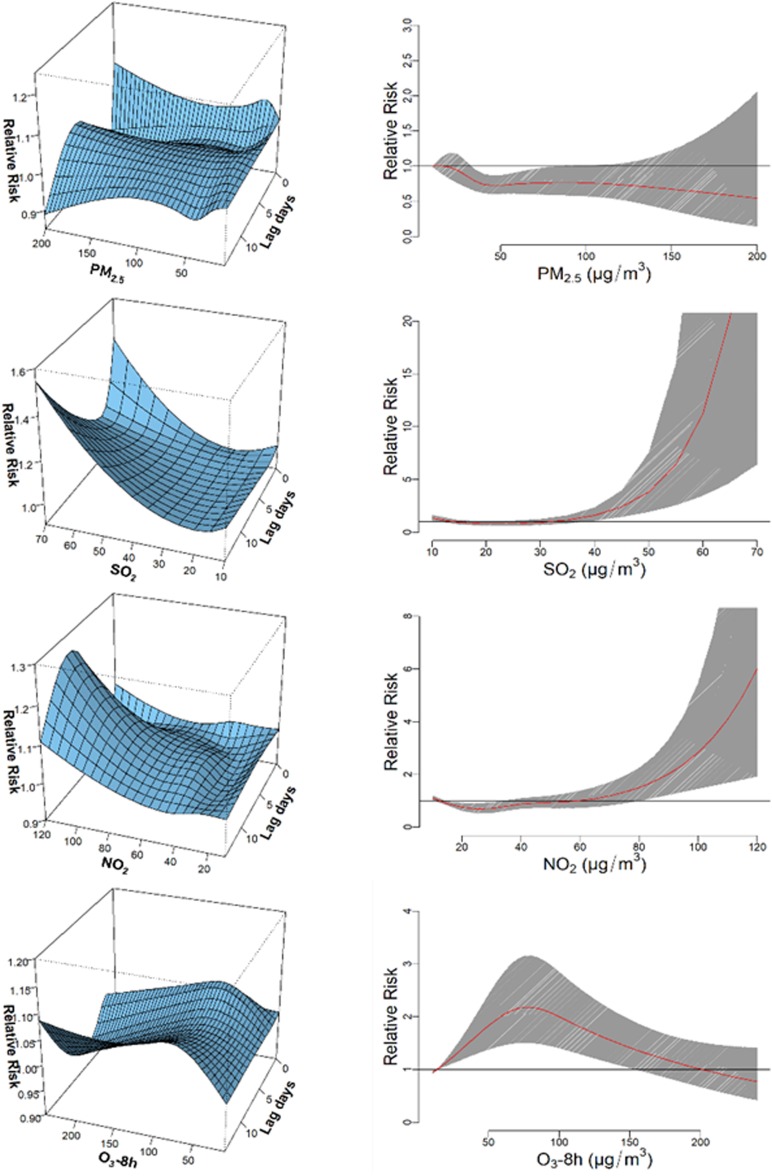
Figure 3 shows boxplots of monthly ambient air pollutant levels. The concentrations of PM2.5, SO2 and NO2 peaked in winter, with low values in summer, while the distribution of O3-8 h showed the opposite pattern. Table 2 shows the Spearman correlation coefficients for comparisons between different environment factors. Coefficients for relationships among PM2.5, SO2 and NO2 were high, while those among PM2.5, SO2 and O3-8 h were not statistically significant. Figure 4 shows the association between air pollution and HFMD cases. In general, we found positive associations between NO2, SO2, O3-8 h and the number of daily HFMD cases, but none for PM2.5. The exposure–response associations of NO2 and SO2 with HFMD cases were approximately linear, but the effect of O3-8 h decreased about 80 µg/m3. The overall estimated relative risk (RR) values for moderate levels of PM2.5, SO2, NO2 and O3-8 h with a lag of 14 days were 0.77 (0.64–0.92), 1.06 (0.87–1.29), 0.85 (0.68–1.07) and 2.12 (1.47–3.05), respectively. The overall RR estimates for high levels of PM2.5, SO2, NO2 and O3-8 h with a lag of 14 days were 0.71 (0.46–1.09), 2.32 (1.42–3.79), 2.01 (1.22–3.31) and 1.02 (0.69–1.53), respectively (Table 3).
Fig. 3.
Boxplots of monthly air pollution in Ningbo city, during 2014–2016.
Table 2.
The spearman correlations between different environment factors
| Factors | PM2.5 (μg/m3) | SO2 (μg/m3) | NO2 (μg/m3) | O3-8 h (μg/m3) | Temperature (°C) | Humidity (%) | Sunshine (h) |
|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 1.000 | ||||||
| SO2 (μg/m3) | 0.773 | 1.000 | |||||
| NO2 (μg/m3) | 0.728 | 0.720 | 1.000 | ||||
| O3-8 h (μg/m3) | 0.026 | 0.046 | −0.242 | 1.000 | |||
| Temperature (°C) | −0.464 | −0.483 | −0.579 | 0.376 | 1.000 | ||
| Humidity (%) | −0.230 | −0.437 | −0.032 | −0.394 | 0.183 | 1.000 | |
| Sunshine (h) | 0.049 | 0.165 | −0.149 | 0.491 | 0.166 | −0.687 | 1.000 |
Note. Statistically significant (P < 0.05) were labelled in bold font.
Fig. 4.
Association between air pollution and HFMD cases. Left panels: 3-D graphs of the exposure-lag-response risk surface. Right panels: overall cumulative exposure–response associations.
Table 3.
The overall estimated RR values for air pollutants at different levels with a lag of 14 days
| Moderate level | High level | |
|---|---|---|
| PM2.5 | 0.77 (0.64–0.92) | 0.71 (0.46–1.09) |
| SO2, | 1.06 (0.87–1.29) | 2.32 (1.42–3.79) |
| NO2 | 0.85 (0.68–1.07) | 2.01 (1.22–3.31) |
| O3-8 h | 2.12 (1.47–3.05) | 1.02 (0.69–1.53) |
Tables 4 and 5 show the effects of moderate and high levels of air pollution on HFMD cases in various population groups. We found that the effects of SO2 and NO2 were stronger in children less than 5 years old, with overall RR values for high levels thereof 2.39 (1.44–3.96) and 2.02 (1.21–3.39), respectively. On the other hand, the effects of O3-8 h were stronger in children aged 5–14 years, and the overall RR for high levels of O3-8 h was 2.31 (1.09–4.89).
Table 4.
Cumulative relative risk (RR) and 95% confidence intervals (95% CI) for medium levels of air pollution on HFMD over a lag of 14 days
| Groups | PM2.5 | SO2 | NO2 | O3-8 h |
|---|---|---|---|---|
| All age | 0.77 (0.64–0.92) | 1.06 (0.87–1.29) | 0.85 (0.68–1.07) | 2.12 (1.47–3.05) |
| Sex | ||||
| Male | 0.78 (0.62–0.97) | 1.05 (0.83–1.33) | 0.84 (0.65–1.10) | 2.01 (1.30–3.11) |
| Female | 0.76 (0.60–0.97) | 1.08 (0.83–1.40) | 0.91 (0.68–1.23) | 2.31 (1.44–3.71) |
| Age, years | ||||
| 0–4 | 0.74 (0.61–0.90) | 1.02 (0.83–1.26) | 0.85 (0.67–1.08) | 1.82 (1.24–2.67) |
| 5–14 | 0.89 (0.62–1.27) | 1.23 (0.83–1.80) | 0.93 (0.60–1.43) | 4.20 (2.12–8.33) |
Note. Statistically significant (P < 0.05) were labelled in bold font. The reference values were the 1st percentiles of each air pollutant. The effects of medium levels of air pollution were estimated by calculating the risk of HFMD at the 50th percentiles of each air pollutants relative to the reference.
Table 5.
Cumulative relative risk (RR) and 95% confidence intervals (95% CI) for high levels of air pollution on HFMD over a lag of 14 days
| Groups | PM2.5 | SO2 | NO2 | O3-8 h |
|---|---|---|---|---|
| All age | 0.71 (0.46–1.09) | 2.32 (1.42–3.79) | 2.01 (1.22–3.31) | 1.02 (0.69–1.53) |
| Sex | ||||
| Male | 0.79 (0.47–1.31) | 2.38 (1.33–4.25) | 2.23 (1.24–4.01) | 1.06 (0.66–1.70) |
| Female | 0.62 (0.36–1.10) | 2.20 (1.15–4.18) | 1.82 (0.83–1.40) | 1.02 (0.60–1.71) |
| Age, years | ||||
| 0–4 | 0.73 (0.47–1.15) | 2.39 (1.44–3.96) | 2.02 (1.21–3.39) | 0.88 (0.58–1.33) |
| 5–14 | 0.59 (0.26–1.32) | 1.86 (0.71–4.89) | 2.21 (0.85–5.76) | 2.31 (1.09–4.89) |
Note. Statistically significant (P < 0.05) were labelled in bold font. The reference values were the 1st percentiles of each air pollutant. The effects of high levels of air pollution were estimated by calculating the risk of HFMD at the 99th percentiles of each air pollutants relative to the reference.
Discussion
HFMD affects children, in particular children less than 5 years old, resulting in serious complications including pneumonia and even death. As currently no effective vaccine is available, seeking risk factors and improving prevention strategies are crucial for children health. Although previous studies have indicated that temperature and some air pollutants including PM10 are associated with risk of HFMD [18, 29, 30], few focus on analysis of the effects of PM2.5 accompanied by the other air pollutants such as SO2, NO2 and O3. The present study included 48 209 HFMD cases that occurred from 2014 to 2016 in Ningbo city. The DLNM study indicated associations between the daily incidence of HFMD and air pollutants, including SO2, NO2, O3 and PM2.5. The data also suggested that high SO2 levels had an elevated RR of 2.38 (95% CI 1.33–4.25) among male children, compared with 2.20 (95% CI 1.15–4.18) among female children. Specifically, high levels of SO2 and NO2 had RRs of 2.39 (95% CI 1.44–3.96) and 2.02 (95% CI 1.21–3.39), respectively, among 0–4-year-old children, while O3 had an RR of 2.31 (95% CI 1.09–4.89) among 5–14-year-old children. Our findings suggest significant associations of high SO2 and NO2 levels and moderate O3 levels with HFMD epidemics, and indicate that air pollution has lagged effects on HFMD epidemics.
We found that the overall RR for moderate levels of SO2 with a lag of 14 days was 1.06 (95% CI 0.87–1.29) while that for high levels was 2.32 (95% CI 1.42–3.79), which is consistent with previous research [31]. SO2 is a toxic gas that is released naturally from volcanic activity and produced from the burning of fossil fuels, heavy metal extraction and chemical industries [32]. SO2 is a major air pollutant and has been reported to impact children's health. A study by Kathuria et al. indicated that the prevalence and severity of eczema in children in the United States is associated with higher mean annual SO2 levels [33]. Le et al. demonstrated that in Ho Chi Minh City, Vietnam, hospital admissions of young children for acute lower respiratory infection were generally positively correlated with ambient levels of SO2 during the dry season (November–April), but not the rainy season (May–October) [34]. In China, Song et al. explored the acute effects of SO2 on children's outpatient visits for respiratory diseases and found that a 10 µg/m3 increase in the 2-day average concentration (lag01) of SO2 corresponded to an increase of 0.33% (95% CI 0.10–0.56%) in daily hospital visits [35].
In addition to SO2 pollution, we also explored the effect of NO2 on the HFMD incidence. Compared with moderate levels of air pollution, the high NO2 level had an RR of 2.01 (95% CI 1.22–3.31). We also found that high levels of NO2 had an RR of 2.02 (95% CI 1.21–3.39) among 0–4-year-old children. These results indicate that NO2 is associated with an increased risk of HFMD, particularly in children under 5 years old. NO2 is a key component of air pollution, is predominantly caused by traffic exhaust. A number of studies have reported that NO2 is associated with childhood respiratory diseases. Lai et al. found that NO2 exposure was associated with an increased number of thunderstorm asthma related visits to health services. It is hypothesised that NO2 acutely exacerbates asthma, resulting in respiratory system sensitivity [36]. A study by Finke et al. indicated that NO2 exposure is related to lower pulmonary function among school children [37]. Kravitz-Wirtz et al. showed that early life NO2 exposure is associated with subsequent cases of childhood asthma [38]. However, some studies found the opposite results regarding this pollutant. A study published in 2009 found no significant cross-sectional association between NO2 concentrations and respiratory and allergic disorders in adults [39]. Lan et al. investigated the effects of NO2 on children's respiratory health and found that children's lung function indicators, such as forced vital capacity, were inversely correlated with annual NO2 levels (−0.0023 l/μg per m3; −0.0044 to −0.0002; P = 0.033) [40]. Although the results of our study demonstrated that NO2 is positively associated with childhood HFMD incidence in Ningbo City, there have been few studies regarding the association between NO2 and HFMD in children to date. Considering that Ningbo City is an industrialised city with a large number of factories and vehicles, and the annual NO2 concentration is 41.06, which is above the national environmental air quality standard limit of 40 µg/m3, large-scale actions to reduce emissions of NO2 and guidelines to protect 0–5 year-old children from its effects are urgently needed.
In addition to NO2 and SO2, we included O3 in the DLNM so that we could explore its potential effects on HFMD incidence. O3 is a potent airway irritant as well as a factor affecting respiratory morbidity, especially in young people. According to a study conducted in three cities in the U.S., O3 is significantly related to pediatric respiratory morbidity (OR 1.08, 95% CI 1.06–1.11) [41]. High O3 levels were reported to have a protective effect against HFMD in Guilin City, China [17]. In contrast, some previous studies reported no association between O3 exposure and respiratory diseases in children. For example, Li et al. reported no association between O3 exposure and upper respiratory tract infection in hospital outpatients aged 0–14 years in Hefei Province, China [42]. Our study supports previous findings that O3 was associated with an increased risk of HFMD in children. In our results, The RR of O3 was 2.12 (95% CI 1.47–3.05) at moderate levels of air pollution; specifically, high levels of O3 had an RR of 2.31 (95% CI 1.09–4.89) among 5–14-year-old children. Possible explanations for this discrepancy include that the O3 concentration in Ningbo City may be different from that in other regions, or that other air pollutants may interact with O3. Liang L et al. analysed the association between dairy SO2, NO2, O3, CO, PM10 and PM2.5 and hospitalisations for acute exacerbation of COPD in Beijing from 2013 to 2017 and the results indicated that exposure–response association of NO2 with COPD cases was linear, while the SO2, CO, PM10 and PM2.5 were non-linear, no significance was found in O3 with COPD incidence [43]. Liu C et al. investigated the air pollution and daily mortality in 652 cities, the results demonstrated that exposure–response association of PM2.5 with mortality was non-linear [26]. However, till now, the exposure–response of associations of air pollutants and some certain diseases are not well consistent. The possible reasons may be due to the different study regions, different air pollutants' concentrations or other confounder factors such as differential culture background. Therefore, in the future, the studies should be conducted at multiple-cities levels.
PM2.5, another potent airway irritant, is reported to be closely related to HFMD incidence in Guilin City, China. Low PM2.5 levels decrease the risk of HFMD, whereas high levels increase the incidence of HFMD [17]. Our previous studies on the effects of PM10 on HFMD in Ningbo City found no significant correlation between PM10 and HFMD incidence, except in females [18, 44]. Yu et al. indicated that low PM2.5 levels had a protective effect on HFMD incidence, as the corresponding RR value was 0.85 (95% CI 0.74–0.98), which is similar to our results. In this study, we found that PM2.5 was a protective factor against HFMD in children, with an RR of 0.77 (95% CI 0.64–0.92) at moderate levels of air pollution. The discrepancy among these studies may be explained as follows. The PM2.5 level varies among different regions; for instance, the mean PM2.5 concentration in Guilin City is 52.8 µg/m3, whereas in Ningbo City it is 43.15 µg/m3. The former is located in western China, whereas the latter is adjacent to the sea, where the east sea wind may decrease the concentration of PM2.5. Another reason may be that air pollutants form a complex mixture of compounds, thus confounding each other's effects. Third, low levels of PM2.5 exposure may stimulate the immune system, while high levels may compromise immune function, as environmental genotoxicants have been shown to exert different effects (i.e. harmful or beneficial) depending on molecular and ecological factors [45–47]. This study shows that the impacts of these air pollutants on HFMD are complex and require further exploration, particularly of the underlying mechanisms. Meanwhile, policy makers should pay more attention to children aged less than 5 years, as they are more sensitive to air pollutants than older children.
We should mention the limitations of this study. First, most HFMD cases were diagnosed clinically rather than by laboratory tests, which may have biased the data. Second, only 3 years of data were analysed. Third and the major limitation of the time-series study is that we only use the concentration of air pollution at the city level, not at the individual level, which may cause ecological bias. In addition, individuals have different activity levels, which might lead to significant biases in exposure assessments. Despite these limitations, this is the first investigation of the effect of NO2 on HFMD incidence. Our results will further our understanding of the effects of air pollutants on vulnerable populations.
Conclusions
Our findings suggest significant associations of high SO2 and NO2 levels and moderate O3 levels with HFMD epidemics, and indicate that air pollution has a lagged effect on HFMD epidemics. Furthermore, compared with females and 5–14-year-old children, males and 0–4-year-old children are at higher risk of contracting HFMD. Our study provides useful information for targeted prevention and control measures based on environmental evidence.
Data
Raw data of HFMD incidence and daily air pollution levels were requested and obtained from Ningbo Municipal Center for Disease Control and Prevention and the Environment Monitoring Center of Ningbo, respectively. The raw meteorological data were obtained from the Ningbo Meteorological Bureau. Raw data will not be shared because the authors are not authorised for distribution of data.
Acknowledgements
Not applicable.
Conflict of interest
The authors declare no competing interests.
Author contributions
RH conceived and designed the study; RH, SG, BL and DC performed eligibility screening and data extraction; GX analysed the data and performed the statistical analysis. RH drafted the initial manuscript. GX, RH, SG, BL, DC revised the manuscript. All authors read and approved the final manuscript.
Financial support
This study is supported by grant from the National Natural Science Foundations of China (Grant No. U1803124), the Natural Science Foundations of Hunan Province (No. 2019JJ40396), the Medical Science and Technology Planning Project of Ningbo (No. 2017A40), the Natural Science Foundations of Ningbo (2019A610379) and Ningbo Health Branding Subject Fund (PPXK2018-10).
Consent for publication
Not applicable.
Ethical standards
The study was approved by the Institutional Review Board of Ningbo Municipal Center for Disease Control and Prevention (IRB 201601). Informed consent was not required because the data used in the study were deaths registration records and the data were anonymous.
References
- 1.Du Z et al. (2018) Bayesian spatiotemporal analysis for association of environmental factors with hand, foot, and mouth disease in Guangdong, China. Scientific Reports 8, 15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson CR, Doane FW and Rhodes AJ (1958) Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A Coxsackie virus. Canadian Medical Association Journal 79, 615–621. [PMC free article] [PubMed] [Google Scholar]
- 3.Xing W et al. (2014) Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. The Lancet Infectious Diseases 14, 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takechi M et al. (2019) Nationwide survey of pediatric inpatients with hand, foot, and mouth disease, herpangina, and associated complications during an epidemic period in Japan: estimated number of hospitalized patients and factors associated with severe cases. Journal of Epidemiology 29, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palani S et al. (2018) Hand, foot and mouth disease in the Andaman Islands, India. Indian pediatrics 55, 408–410. [PubMed] [Google Scholar]
- 6.Chen Y et al. (2018) The effect of school closure on hand, foot, and mouth disease transmission in Singapore: a modeling approach. The American Journal of Tropical Medicine and Hygiene 99, 1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim B et al. (2018) Factors associated with severe neurologic complications in patients with either hand-foot-mouth disease or herpangina: a nationwide observational study in South Korea, 2009–2014. PloS One 13, e0201726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saguil A et al. (2019) Hand-foot-and-mouth disease: rapid evidence review. American Family Physician 100, 408–414. [PubMed] [Google Scholar]
- 9.Aswathyraj S, Arunkumar G and Alidjinou EK (2016) Hober D: hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Medical Microbiology and Immunology 205, 397–407. [DOI] [PubMed] [Google Scholar]
- 10.Stock I (2014) [Hand, foot and mouth disease – more than a harmless “childhood disease”]. Medizinische Monatsschrift fur Pharmazeuten 37, 4–10, quiz 11–12. [PubMed] [Google Scholar]
- 11.Yang Z et al. (2017) Estimating the incubation period of hand, foot and mouth disease for children in different age groups. Scientific Reports 7, 16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen GP et al. (2016) Epidemiological characteristics and influential factors of hand, foot and mouth disease (HFMD) reinfection in children in Anhui province. Epidemiology and Infection 144, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldacci S et al. (2015) Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respiratory Medicine 109, 1089–1104. [DOI] [PubMed] [Google Scholar]
- 14.Guarnieri M and Balmes JR (2014) Outdoor air pollution and asthma. Lancet 383, 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang L et al. (2019) Maternal exposure to PM2.5 may increase the risk of congenital hypothyroidism in the offspring: a national database based study in China. BMC Public Health 19, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuertes E et al. (2013) A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ 1, e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu G et al. (2019) Short-term effects of meteorological factors and air pollution on childhood hand-foot-mouth disease in Guilin, China. The Science of the total environment 646, 460–470. [DOI] [PubMed] [Google Scholar]
- 18.Huang R et al. (2016) Effects of meteorological parameters and PM10 on the incidence of hand, foot, and mouth disease in children in China. International journal of environmental research and public health 13, pii: E481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinmayr G et al. (2010) Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environmental Health Perspectives 118, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y et al. (2015) Enterovirus 71 infection in children with hand, foot, and mouth disease in Shanghai, China: epidemiology, clinical feature and diagnosis. Virology Journal 12, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T et al. (2018) Mortality risks from a spectrum of causes associated with wide-ranging exposure to fine particulate matter: a case-crossover study in Beijing, China. Environment International 111, 52–59. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y et al. (2015) Association between ambient air pollution and hospital emergency admissions for respiratory and cardiovascular diseases in Beijing: a time series study. Biomedical and Environmental Sciences : BES 28, 352–363. [DOI] [PubMed] [Google Scholar]
- 23.Gasparrini A (2014) Modeling exposure-lag-response associations with distributed lag non-linear models. Statistics in Medicine 33, 881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L et al. (2015) The impact of ambient temperature on childhood HFMD incidence in inland and coastal area: a two-city study in Shandong Province, China. International Journal of Environmental Research and Public Health 12, 8691–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W et al. (2016) Quantifying the adverse effect of excessive heat on children: an elevated risk of hand, foot and mouth disease in hot days. The Science of the Total Environment 541, 194–199. [DOI] [PubMed] [Google Scholar]
- 26.Liu C et al. (2019) Ambient particulate air pollution and daily mortality in 652 cities. The New England Journal of Medicine 381, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhaskaran K et al. (2013) Time series regression studies in environmental epidemiology. International Journal of Epidemiology 42, 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparrini A, Armstrong B and Kenward MG (2010) Distributed lag non-linear models. Statistics in Medicine 29, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Z et al. (2019) Interactions between climate factors and air pollution on daily HFMD cases: a time series study in Guangdong, China. The Science of the Total Environment 656, 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J et al. (2019) A method for hand-foot-mouth disease prediction using GeoDetector and LSTM model in Guangxi, China. Scientific Reports 9, 17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Q et al. (2019) Short-term exposure to sulfur dioxide and the risk of childhood hand, foot, and mouth disease during different seasons in Hefei, China. The Science of the Total Environment 658, 116–121. [DOI] [PubMed] [Google Scholar]
- 32.Lou T et al. (2017) Monitoring, exposure and risk assessment of sulfur dioxide residues in fresh or dried fruits and vegetables in China. Food Additives & Contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 34, 918–927. [DOI] [PubMed] [Google Scholar]
- 33.Kathuria P and Silverberg JI (2016) Association of pollution and climate with atopic eczema in US children. Pediatric Allergy and Immunology : Official Publication of the European Society of Pediatric Allergy and Immunology 27, 478–485. [DOI] [PubMed] [Google Scholar]
- 34.Hei Collaborative Working Group on Air Pollution P, Health in Ho Chi Minh C, Le TG et al. (2012) Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam. Research Report 169, 5–72. discussion 73–83. [PubMed] [Google Scholar]
- 35.Song J et al. (2018) Acute effects of ambient air pollution on outpatient children with respiratory diseases in Shijiazhuang, China. BMC Pulmonary Medicine 18, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai VWY et al. (2018) Residential NO2 exposure is associated with urgent healthcare use in a thunderstorm asthma cohort. Asia Pacific Allergy 8, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finke I et al. (2018) Air pollution and airway resistance at age 8 years – the PIAMA birth cohort study. Environmental Health: a Global Access Science Source 17, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kravitz-Wirtz N et al. (2018) Early-life air pollution exposure, neighborhood poverty, and childhood asthma in the United States, 1990(-)2014. International Journal of Environmental Research and Public Health 15, pii: E1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pujades-Rodriguez M et al. (2009) Effect of traffic pollution on respiratory and allergic disease in adults: cross-sectional and longitudinal analyses. BMC Pulmonary Medicine 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mudway IS et al. (2018) Impact of London's low emission zone on air quality and children's respiratory health: a sequential annual cross-sectional study. The Lancet Public Health 4, e28–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CR OL et al. (2017) Ozone and childhood respiratory disease in three US cities: evaluation of effect measure modification by neighborhood socioeconomic status using a Bayesian hierarchical approach. Environmental Health: a Global Access Science Source 16, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li YR et al. (2018) Association between air pollution and upper respiratory tract infection in hospital outpatients aged 0–14 years in Hefei, China: a time series study. Public Health 156, 92–100. [DOI] [PubMed] [Google Scholar]
- 43.Liang L et al. (2019) Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013-17: an ecological analysis. The Lancet Planetary Health 3, e270–e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang R et al. (2018) Impact of PM10 and meteorological factors on the incidence of hand, foot, and mouth disease in female children in Ningbo, China: a spatiotemporal and time-series study. Environmental Science and Pollution Research International 13, pii: E481. [DOI] [PubMed] [Google Scholar]
- 45.Huang R and Zhou P (2019) Double-edged effects of noncoding RNAs in responses to environmental genotoxic insults: perspectives with regards to molecule-ecology network. Environmental Pollution 247, 64–71. [DOI] [PubMed] [Google Scholar]
- 46.Zhou PK and Huang RX (2018) Targeting of the respiratory chain by toxicants: beyond the toxicities to mitochondrial morphology. Toxicology Research 7, 1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang R et al. (2018) Upregulated has-miR-4516 as a potential biomarker for early diagnosis of dust-induced pulmonary fibrosis in patients with pneumoconiosis. Toxicology Research 7, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]