Abstract
Primary familial brain calcification (PFBC) is a rare neurodegenerative disorder characterized by a combination of neurological, psychiatric, and cognitive decline associated with calcium deposition on brain imaging. To date, mutations in five genes have been linked to PFBC. However, more than 50% of individuals affected by PFBC have no molecular diagnosis. We report four unrelated families presenting with initial learning difficulties and seizures and later psychiatric symptoms, cerebellar ataxia, extrapyramidal signs, and extensive calcifications on brain imaging. Through a combination of homozygosity mapping and exome sequencing, we mapped this phenotype to chromosome 21q21.3 and identified bi-allelic variants in JAM2. JAM2 encodes for the junctional-adhesion-molecule-2, a key tight-junction protein in blood-brain-barrier permeability. We show that JAM2 variants lead to reduction of JAM2 mRNA expression and absence of JAM2 protein in patient’s fibroblasts, consistent with a loss-of-function mechanism. We show that the human phenotype is replicated in the jam2 complete knockout mouse (jam2 KO). Furthermore, neuropathology of jam2 KO mouse showed prominent vacuolation in the cerebral cortex, thalamus, and cerebellum and particularly widespread vacuolation in the midbrain with reactive astrogliosis and neuronal density reduction. The regions of the human brain affected on neuroimaging are similar to the affected brain areas in the myorg PFBC null mouse. Along with JAM3 and OCLN, JAM2 is the third tight-junction gene in which bi-allelic variants are associated with brain calcification, suggesting that defective cell-to-cell adhesion and dysfunction of the movement of solutes through the paracellular spaces in the neurovascular unit is a key mechanism in CNS calcification.
Keywords: primary familial brain calcification, Fahr disease, JAM2, recessive brain calcification, knock out mouse model, familial idiopathic basal ganglia calcification, MYORG, SLC20A2, JAM3, OCLN
Main Text
Primary familial brain calcification (PFBC [MIM: 213600]), often referred as Fahr disease, constitutes a heterogeneous neurodegenerative disorder that presents with mineral calcium deposits in the brain. The clinical manifestations can include, but are not restricted to, movement disorders such as parkinsonism and ataxia, seizures, migraine, and neuropsychiatric symptoms. Both autosomal-dominant and -recessive inheritance patterns have been reported.1 The clinical picture and severity are often variable between and within families, with some family members being clinically asymptomatic. The advent of widespread brain imaging for individuals presenting with parkinsonism, who were previously clinically diagnosed, has significantly increased the number of PFBC-affected families identified.2
There are two main pathogenic mechanisms described so far in PFBC. On the one hand, the calcium and phosphate homeostasis dysfunction via dominant mutations in SLC20A2 (MIM: 158378) and XPR1 (MIM: 605237).1 The inhibition of phosphate uptake by mutations in SLC20A2 encoding for sodium-dependent phosphate transporter 2 (PiT-2) leads to deposition of calcium in the vascular extracellular matrix, and inhibition of phosphate export associated with XPR1 mutations is expected to increase intracellular phosphate concentration and provoke calcium phosphate precipitation.3
On the other hand, the second mechanism causes PFBC through disruption of the neurovascular unit (NVU). Endothelial integrity and function affecting the blood-brain barrier (BBB) is altered via dominant mutations in PDGFB (MIM: 190040) and PDGFRB (MIM: 173410) encoding for the platelet-derived growth factor B and its receptor, that lead to the impairment of pericytes recruitment and BBB integrity, causing vascular and perivascular calcium accumulation.2 The recessive brain calcification phenotype due to MYORG4 (MIM: 618255) mutations, has been shown to present specific myorg mRNA expression in mouse astrocytes and disturb the normal function of the NVU. Mutations in JAM35 (MIM: 613730) and OCLN6 (MIM: 602876) encoding for tight junction proteins lead to excess solutes crossing the BBB causing CNS calcification and hemorrhage.
Interestingly, a study on the Slc20a2 null mouse suggested that the calcified nodules present in the brain initiated in pericytes and astrocytes and found endogenous IgG around nodules proposing that there was increased BBB permeability7 and identifying a possible link between different PFBC causative genes.
Pathologically, human brains exhibit calcium salt deposits predominantly distributed around small blood vessels,2 and a reported subject with a SLC20A2 mutation presented calcification in the tunica media of small arteries, arterioles, and capillaries, but not in veins distributed in the basal ganglia, thalamus, cerebellar white matter, and deeper layers of the cerebral cortex.8
Despite important progress in discovering the genetic architecture of PFBC, more than half of the case subjects remain genetically unsolved.2
In this study we report four unrelated families with seven individuals affected by autosomal-recessive primary familial brain calcification. In the families described here, we used a combination of homozygosity mapping, exome sequencing (ES), functional studies, and mouse model to identify and characterize the causal variants in JAM2 (MIM: 606870) encoding for the junctional-adhesion-molecule-2, a tight-junction protein as a cause of PFBC.
Two unrelated consanguineous families from traveller communities in England (family 1) and Northern Ireland (family 2), one non-consanguineous family from the United States (family 3) identified using GeneMatcher, and one Turkish consanguineous family (family 4) were included in this study (Figure 1A). Clinical features of affected individuals are presented in Table 1. The proband in family 1 (F1-II:2) had a normal birth and early milestones. He presented with childhood-onset cerebellar ataxia and learning difficulties. His symptoms progressed and were associated with additional behavioral problems and worsening cognitive impairment. He was examined by a neurologist at the age of 23 years old. At that stage, he already had difficulties following commands and had alternate exotropia with left eye preference for fixation, slow and jerky pursuit, and ophthalmoplegia. He had reduced ability to control tongue movements and was unable to protrude his tongue. He was dysarthric with dysphagia and a percutaneous endoscopic gastrostomy (PEG) insertion at the age of 22 years. He had increased tone in the upper and lower limbs with ankle contractures, brisk reflexes throughout, and upgoing plantars. There was upper and lower limb ataxia, bradykinesia, and generalized dystonia, worse in the upper limbs (Video S1). Occasional seizures were seen later in the disease course. The interictal electroencephalography (EEG) showed moderate generalized slowing of cortical rhythms.
Figure 1.
Clinical and Neuroimaging Features of JAM2-Related Disease
(A) Pedigrees of the four families with bi-allelic JAM2 mutations.
(B) Brain images of JAM2-related disease. B1 to B3 are CT scans acquired from case subject F1-II:2 from family 1 and B4 to B6 are CT acquired from case subject F2-III:3 from family 2. In both individuals there is extensive, symmetrical, bilateral calcification involving the basal ganglia, deep cortical gray matter and cerebellum. B7, 8, and 9 are CT from case subject F3-II:1 demonstrating calcification in the basal ganglia and cortical gyri but not in the cerebellum. B10, 11, and 12 are axial MRI scans from individual F3-II:1 from family 3 showing basal ganglia and frontal calcification (B10 is T1 MRI, B11 is T2 MRI, and B12 is Axial Ven Bold reconstruction MRI that is sensitive to calcium shown as hypointense regions). Asterisk (∗) areas of calcification on MRI.
Table 1.
Clinical Features of Affected Individuals with JAM2 Bi-allelic Variants
| Individual F1-II:2 | Individual F2-III:2 | Individual F2-III:3 | Individual F2-III:4 | Individual F2-III:5 | Individual F3-II:1 | Individual F4-II:3 | |
|---|---|---|---|---|---|---|---|
| cDNA sequence | c.685C>T | c.685C>T | c.685C>T | c.685C>T | c.685C>T | c.395−1dupG, c.323G>A | c.177_180delCAGA |
| Amino acid change | p.Arg229Ter | p.Arg229Ter | p.Arg229Ter | p.Arg229Ter | p.Arg229Ter | IVS4-1dupG, p.Arg108His | p.Arg60Ter |
| Zygosity | homozygous | homozygous | homozygous | homozygous | homozygous | compound heterozygous | homozygous |
| Gender (male/female) | male | male | female | male | male | male | female |
| Birth and early milestones | normal | normal | normal | normal | normal | normal | normal |
| Onset of symptoms | childhood | late 20s | late 30s | teenage | teenage | childhood | early childhood |
| Symptom at onset | cerebellar ataxia and cognitive decline | cognitive decline, depression | difficulty walking | depression, dysarthria | depression, dysarthria | autism spectrum disorder | seizures |
| Age at examination (in years) | 24 | 41 | 39 | 40 | 49 | 15 | 7 |
| Phenotype at Last Examination | |||||||
| Pyramidal syndrome | yes; increased tone, brisk reflexes, upgoing plantars | yes; increased tone, brisk reflexes, upgoing plantars | yes; increased tone, brisk reflexes, upgoing plantars | yes; increased tone, brisk reflexes, upgoing plantars | yes; increased tone, brisk reflexes, upgoing plantars | no | no |
| Cerebellar syndrome | yes; upper and lower limb ataxia, dysarthria, nystagmus | yes; upper and lower limb ataxia | yes; upper and lower limb ataxia | yes; upper and lower limb ataxia, dysarthria | yes; upper and lower limb ataxia, dysarthria | yes; upper and lower limb mild ataxia, nystagmus | no |
| Parkinsonism | yes; rigidity, bradykinesia. | yes; hypophonia, hypomimia, bradykinesia | yes; hypophonia, hypomimia, bradykinesia | yes; rigidity, bradykinesia | yes; rigidity, bradykinesia | no | no |
| Dystonia | yes; generalized | yes; limb dystonia and orofacial dyskinesias | yes; limb dystonia and orofacial dyskinesias | no | no | no | no |
| Other | seizures, ophthalmoplegia, PEG inserted in advance stage | PEG inserted in advance stage | became anarthric in advanced stage | – | – | autism spectrum disorder | – |
| Cognitive function | severe cognitive decline | memory decline with severe impaired recall | unable to comment on cognition due to anarthria. | severe cognitive decline | severe cognitive decline | decline in academic performance | normal for her age |
| Brain imaging calcification pattern | basal ganglia, thalamus, cerebellum, deep gray matter | basal ganglia, thalamus, cerebellum, deep gray matter | basal ganglia, thalamus, cerebellum, deep gray matter | basal ganglia, thalamus, cerebellum, deep gray matter | basal ganglia, thalamus, cerebellum, deep gray matter | basal ganglia, and frontal cortex | basal ganglia, dentate nucleus and cerebellar hemispheres |
There were four affected individuals in the second family. Two siblings (F2-III:2 and F2-III:3) presented with borderline low IQ in childhood but had no definite physical limitations in early life. In their twenties they had social withdrawal and severe depression requiring treatment. The disease progressed and examination at 41 and 39 years revealed severe speech hypophonia, dysphagia, hypomimia, reduced vertical up gaze, orofacial dyskinesias, slow and reduced tongue movements with bradykinesia, and dystonic limb posturing in both affected individuals. Tone was increased in an extrapyramidal pattern with lower limb hyperreflexia and extensor plantar responses, grasp reflexes, positive glabellar tap, and brisk jaw jerk. They both had memory decline with impaired recall. Treatment with ropinirole did not lead to any significant improvement in symptoms. The disease progressed, they became bedridden and case F2-III:2 needed a PEG insertion 10 years after the onset of movement problems due to recurrent aspiration pneumonia, and the proband died in the late 40s. Case subjects F2-III:4 and F2-III:5 are maternal first cousins of the index case subject in family 2. They presented an almost identical phenotype. On a background of depression, both brothers noted progressive “slurring” of speech, slowing of all movements, difficulty with walking, recurrent falls, and poor memory. Examination demonstrated a similar phenotype with dysarthria, abnormal pursuit with frequent saccadic intrusions, pronounced bradykinesia, extrapyramidal rigidity, and bilaterally extensor plantar responses.
The proband in family 3 (F3-II:1) had normal early development but later developed mild delay in fine motor and language milestones that were progressive. He also developed mild coordination problems and autism spectrum disorder (ASD) and received special education services at school age. At age 11 years, repeat neuropsychologic evaluation showed a continuous decline in academic performance. Parents were asymptomatic, an older brother had some anxiety and hyperactivity, and a younger brother aged 13 years had mild autistic features. At the last examination of the proband aged 15 years, he had autism spectrum features, hyperactivity, developmental delay, and coordination problems. The coordination difficulties were mild but affected both fine motor skills (buttons, zippers, hooks) and complex gross motor tasks. His learning difficulties were progressive.
The affected member of family 4 (F4-II:3) was born to Turkish consanguineous parents and presented with seizures when she was 18 months of age; current age is 7.5 years and she has had a total of 3 seizures. Her development so far has been in keeping with her peers and her last examination did not reveal any neurological signs. Brain calcification was identified on MRI imaging with bilateral symmetric calcification of the basal ganglia, dentate nucleus, and subcortical white matter of cerebellar hemispheres. Prior to exome sequencing, the known Fahr’s genes was sequenced and negative.
All seven case subjects reported here had brain calcification identified on brain CT and/or MRI imaging. They had a consistent pattern of bilateral symmetric calcification of the basal ganglia and deep cortical gray matter. In addition, the older individuals from families 1 and 2 had severe calcification in the cerebellum folia and the thalamus (Figure 1B). All families had extensive genetic, metabolic, and mitochondrial investigations carried out that excluded acquired and other inherited causes of brain calcification.
In order to localize the chromosomal location of the pathogenic variant, we genotyped three affected and two unaffected individuals from extended family 2 genome-wide by using Illumina HumanCytoSNP-12v2-1 Beadchip array incorporating ∼200,000 genetic markers. Three regions of homozygosity were detected on chr10:37,414,883–43,132,376, chr13:88,327,643–93,518,692, and chr21:22,370,881–28,338,710. Next, we performed exome sequencing on probands of families 1 and 2 to identify the causative variant(s). On the assumption that the disease follows an autosomal-recessive pattern of inheritance in the families as well as presence of consanguinity in two families, we prioritized the bi-allelic potentially functional variants residing within the runs of homozygosity. These variants were screened through all publicly available population databases and our in-house database. We excluded synonymous variants, intronic variants (>7 bp from exon boundaries) and common variants (minor allele frequency > 0.001%). The selected variants were validated, and segregation analysis was performed using Sanger sequencing.
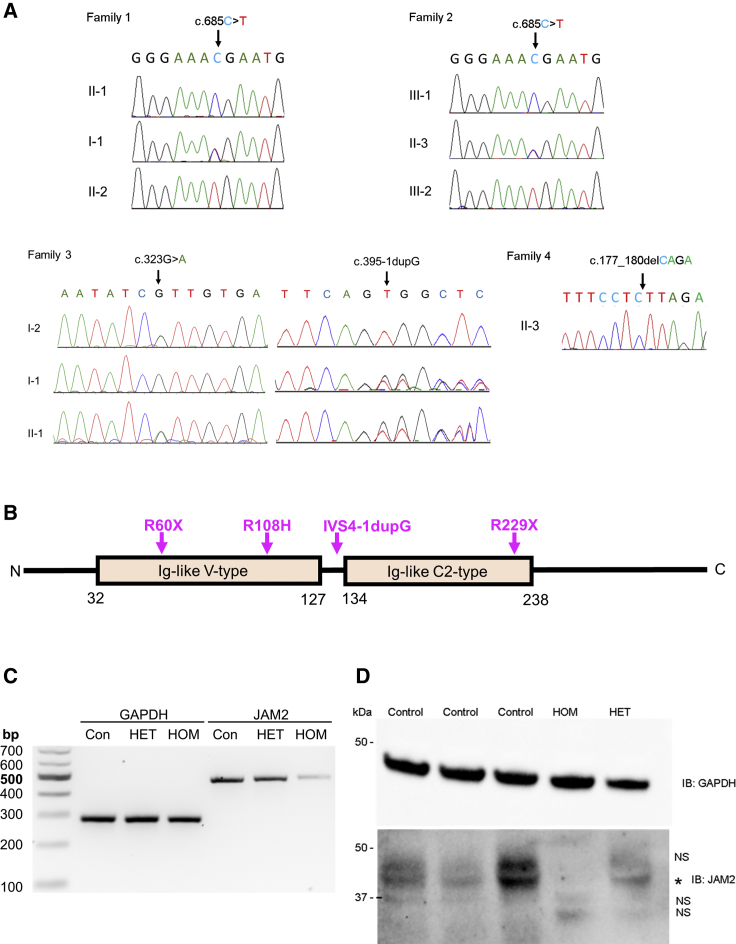
In families 1 and 2, filtered exome-sequencing data narrowed down the variants to the same homozygous nonsense variant (GenBank: NM_021219; c.685C>T [p.Arg229Ter]) in JAM2 residing within a 5 Mb region of homozygosity on chr21q21.3 (Figure S1). Sanger sequencing verified the correct segregation of the variant on available samples of both families (Figures 1A, 2A, and 2B). This variant was absent in our in-house exome database of more than 10,000 exomes, absent in homozygous state in all publicly available databases, and present in heterozygous state with a minor allele frequency (MAF) of 0.00002 in gnomAD (6/280662). The early stop codon introduced by c.685C>T is predicted to result in production of a truncated protein lacking the transmembrane and cytoplasmic regions of JAM2 and cause reduction in total JAM2 as a consequence of nonsense-mediated decay (NMD) of the mutant transcript. Indeed, RT-PCR analysis confirmed a reduction of JAM2 mRNA expression levels in the proband in family 1 compared to the heterozygous carrier and unrelated control subject (Figure 2C). Furthermore, western blot analysis confirmed the absence of JAM2 protein in the homozygous proband (Figure 2D). The reduction of RNA expression and absent JAM2 protein in the fibroblast cell lines of the proband support the loss-of-function role of this variant. As JAM2, JAM3, and TJP1 proteins are all junctional components and associated with brain calcification phenotype, we investigated whether JAM2 c.685C>T variant affected the localization of the other two proteins. We show that there was no difference in the localization of JAM3 and TJP1 in primary dermal fibroblasts from JAM2 homozygous affected individuals (Figure S2).
Figure 2.
Validation of JAM2 Variants Identified in This Study
(A) Validation by Sanger sequencing of the stop variant c.685C>T (p.Arg229Ter) in families 1 and 2, the bi-allelic variants in family 3 (IVS4-1dupG and p.Arg108His), and the homozygous change in family 4 c.177_180delCAGA (p.Arg60Ter).
(B) Protein structure of JAM2 showing the 2 main domains (Ig-like V-type and Ig-like C2-type) as well as the variants identified in the 4 families.
(C) Total RNA and protein extracts were prepared from fibroblast cell lines isolated from the proband of family 1 (HOM), his unaffected mother (HET), and unrelated control subjects (Con) to assess the c.685C>T (p.Arg229Ter) JAM2 variant. Agarose gel separation of RT-PCR products shows a reduction in JAM2 mRNA expression in the homozygous proband (HOM) compared to the proband’s mother (HET) and unrelated control subject (Con). GAPDH RT-PCR served as a loading control.
(D) Western blot analysis of total protein lysates using anti-JAM2 and GAPDH (loading control) antibodies revealed presence of JAM2 protein in control (Control) and unaffected carrier (HET) cells but complete loss of JAM2 protein in the homozygous proband of family 1 (HOM). NS, non-specific band; asterisk, JAM2.
In family 3, Trio-ES revealed compound heterozygous variants in JAM2 (GenBank: NM_021219; c.395−1dupG [IVS4-1dupG] and c.323G>A [p.Arg108His]). The c.395−1dupG was inherited from the father and c.323G>A was inherited from the mother. The c.323G>A was reported once in gnomAD in heterozygous state (MAF 0.00003, 1/31408), three times in GeneDx in-house database (MAF 0.00002, 3/167854), and was absent in all databases as homozygous. It disrupts a highly conserved residue (CADD:34) and is predicted to be damaging to the protein function by in silico prediction tools (SIFT, PROVEAN, Mutation Taster, Mutation Assessor, and PolyPhen). The c.395−1dupG is predicted to cause the retention of the canonical splice acceptor site of intron 4 with insertion of the G nucleotide as the first base of exon 5 causing a frameshift and a truncated protein (p.Val132GlyfsX9). This variant was absent from all public databases and present five times in heterozygous state in GeneDx in-house database (MAF 0.00003, 5/171284). The variants segregated fully within the family (Figures 1A, 2A, and 2B).
In family 4, clinical ES uncovered a homozygous 4 bp deletion in JAM2 (GenBank: NM_021219; c.177_180delCAGA [p.Arg60Ter]) (Figures 1A, 2A, and 2B) that causes a frameshift and an early termination of the protein affecting the extracellular, transmembrane, and cytoplasmic domains of JAM2. The variant was not reported in any public databases and was predicted pathogenic by MutationTaster causing loss of function by NMD.
In order to characterize the link between JAM2 variants and the human neurological phenotype, we developed jam2 knockout (jam2 KO) mice. Behavioral tests in the jam2 KO mice showed significant difficulties in beam walking test and gait abnormalities when compared to wild-type mice (Figure 3). There was a significant reduction in stride length (wild-type: 8.14 ± 0.9, jam2 KO: 6.3 ± 1; ∗∗∗p < 0.0001) and increase in sway length (wild-type: 0.13 ± 0.14, jam2 KO: 0.9 ± 0.4; ∗∗p = 0.002) when comparing jam2 KO to wild-type littermates’ controls (Figure 3A). Additionally, the number of missed steps (wild-type: 1.2 ± 1.3, jam2 KO: 6.5 ± 3.6; ∗p = 0.017) in the beam-walking test was higher in jam2 KO compared to controls (Figure 3B and 3C, Video S2).
Figure 3.
Behavioral Study on jam2 KO Mice and Wild-Type (WT)
(A) Representative images of wild-type (left) and jam2 KO strides (right) in the gait test. Forepaw (blue) and hindpaw (red). Altered gait of the jam2 KO mice was analyzed in stance length, stride, and sway compared to wild-type (∗∗∗p < 0.0001, ∗∗p = 0.002; means ± SEM; n = 6 mice per genotype).
(B and C) Walking beam performance on test day, showing elevated latency to cross the beam in jam2 KO (∗p = 0.017; means ± SEM; n = 5 wild-type, n = 6 jam2 KO). See Video S2 for example beam walking test of a jam2 KO mouse and a control (wild-type).
Brains of two jam2 KO and two wild-type (C57BL/6) mice were examined at a young age (6 months old) and four jam2 KO and four wild-type mice were examined at an old age (18 months old). In addition, spinal cord sections from one of the young jam2 KO mice and from all old jam2 KO and control mice were examined. We observed prominent widespread vacuolation in the midbrain and some in the thalamus and cerebral and cerebellar cortex of young jam2 KO mice. In the midbrain, the vacuolar change was accompanied by prominent reactive astrogliosis, mild microglial activation, and mild reduction in the neuronal density compared to controls (Figure 4). Brains of aged jam2 KO mice showed similar changes, with prominent widespread neuropil vacuolation in the midbrain accompanied by marked astrogliosis, mild microglial activation, and moderately reduced neuronal density. In contrast to young jam2 KO mice, there was more prominent vacuolation in the cerebral cortex, thalamus, and cerebellar cortex and particularly widespread vacuolation in the cerebellar white matter. To a lesser extent, neuropil vacuolation in the same regions was also seen in the age-matched control wild-type mice, suggesting that jam2 KO mice develop age-related changes at a much younger age, which in some areas, such as cerebellar white matter, midbrain, thalamus, and cerebellar cortex, increase in severity with age. In addition, we performed automated quantification of the neuropil vacuolation on H&E-stained sections, GFAP immunoreactive gliosis and Iba1-positive microglial activation in young and aged wild-type and jam2 KO mice (Figure S3). Automated quantification of the percentage of vacuolation, gliosis, and microglial activation in the cortex, midbrain, and cerebellum was performed on digitalized slides, using open source software QuPath. There was a significant increase in the degree of neuropil vacuolation (p < 0.00007) and astrogliosis (p < 0.0138) in the midbrain of old jam2 KO mice when compared with age-matched wild-type mice, whereas Iba1-positive microglial activation in old jam2 KO mice was less pronounced than in age-matched wild-type mice (p < 0.035). No mineralization or calcification was observed in the brains of young or old jam2 KO mice or controls at the time of examination.
Figure 4.
Brain Pathology in Control and jam2 KO Mice of Young (6 Months Old) and Old Age (18 Months Old)
Littermate controls (wild-type) (a, a1–a6) show no significant pathology on (H&E) stained sections (a, cortex; a1, cerebellum; a2, midbrain) and on immunohistochemistry for astrocytes (GFAP) (a3, midbrain), microglia (Iba1) (a4, midbrain), neuropil (APP) (a5, midbrain), and neurones (NeuN) (a6, midbrain). In age-matched jam2 KO mice, occasional cortical vacuolation is evident in the cerebral (b) and cerebellar (b1) cortex and widespread prominent vacuolation is seen in the midbrain (b2). In the midbrain region there is also marked reactive astrocytosis (b3) and mild microglial activation (b4). APP immunostaining highlights vacuolar change in the neuropil (b5) and NeuN shows mild reduction in the neuronal density (b6). Aged wild-type mice show occasional vacuolation in the cerebral cortex (c), cerebellar cortex (c1), and midbrain (c2) (H&E). There is mild patchy astrogliosis in the midbrain (c3) and mild microglial activation (c4). No significant disruption of the cytoarchitecture is seen on APP immunostaining (c5), but NeuN highlights some degree of neuronal loss (c6). In age-matched old jam2 KO mice, there is increasingly prominent vacuolation in the cerebral cortex (d) and in the cerebellar white matter (d1), midbrain (d2), and thalamus (not shown). Similar to young jam2 KO mice, there is widespread astrogliosis in the midbrain (d3), but microglial activation remains mildly increased (d4). Frequent vacuolation is highlighted in the neuropil with APP immunostaining (d5). Similar to age-matched littermate controls, there is reduction of the neuronal density in the midbrain of jam2 KO mouse (d6). Scale bar: 100 μm in a–d, a2–a6, b2–b6, c2–c6, d2–d6; 200 μm in a1–d1.
The spinal cord morphology was similar in young and old jam2 KO-deficient mice but remarkably differed from that of control mice. The main findings in jam2 KO spinal cords were widespread neuronal, perivascular, and neuropil mineralization as well as widespread vacuolation in the gray matter. The mineralized deposits were negative for PAS and showed weak reactivity for Alcian blue, excluding calcification, cartilagination, or ossification stage. The mineralization and vacuolar change were evident across the gray matter in both anterior and posterior horns bilaterally and at all levels of the spinal cord. Although mineralization and calcification are known to occur with age in wild-type mice, in the four spinal cords from our control group (age-matched to old jam2 KO mice), no mineralization or neuropil vacuolation at any of the spinal cord levels was observed (Figure S4). JAM3 and TJP1 tight-junction proteins were investigated in the KO mice, and there was no difference in localization between affected and wild-type mice, in different areas of the brain (Figures S5 and S6).
Mouse models of other brain calcification genes have shown brain calcification in similar areas to those found in humans. Such is the case of Slc20a2-null mice exhibiting progressive calcification of the thalamus, basal ganglia, and cortex beginning at 8 weeks and present in close proximity or within blood vessels and affecting astrocytes and perycites7,9,10 and for myorg-null mice developing calcifications at 9 months.4 A study on mice carrying hypomorphic Pdgfb alleles interestingly showed that the calcifications depend on the loss of endothelial PDGF-B and correlate with the degree of pericyte and BBB deficiency.11 Ocln-null mice present with similar calcification surrounding blood vessels as seen in an autopsied case,6 but there were no abnormalities reported in the brain of jam3 KO mice, and there was absence of Jam3 in the vasculature of the adult mouse brain.5 In our study, we were not able to detect calcification in the brain of our jam2 mouse model even though we studied these mice until the age of 18 months, an age at which other PFBC models already present calcification and thus it does not seem to be age related. However, our jam2 KO mice did show prominent vacuolation, inflammatory response, and neuronal density reduction in the same areas affected by calcification in humans. Further studies are needed to understand better the disease mechanisms and the differences between humans and mice for JAM2 and also JAM3.
Together, our results confirm that JAM2 variants underlie the phenotype in the four families reported here. This implicates JAM2 as a cause of human Mendelian disease. JAM2 encodes for the junctional-adhesion-molecule-2 (JAM2), member of the junctional adhesion molecules family, localized in the tight junctions of endothelial cells and the NVU.12,13 JAM2 is an adhesive ligand interacting with various immune cell types and regulating vascular function and was recently identified as an inhibitor of somatodendritic myelination in spinal cord neurons.14
Junctional adhesion molecules are a family of proteins that play an important role in the regulation of cell polarity, endothelium permeability, and leukocyte migration and the blood-brain-barier (BBB) function. In addition to JAM2, recessive variants in JAM3 and OCLN were linked to complex neurological disorders presenting with calcification in the brain,5,6 suggesting that deregulation of the central NVU is important in pathogenesis of PFBC. Even though increased permeability of the BBB hasn’t been confirmed in jam2 KO mice so far,13 including this study, mutations have now been identified in three genes encoding tight junction proteins in humans (JAM2, JAM3, and OCLN), suggesting that loss of cell-to-cell adhesion with subsequent dysfunction of solute passage is an important cause of brain calcification. Furthermore, a multicenter collaboration from China recently described four patients from three families that presented with PFBC and bi-allelic mutations in JAM2.15 They interestingly show failure of JAM2 to translocate to the plasma membrane in JAM2 transfected mutants of hamster ovary cells, and they propose a cell-to-cell adhesion impairment as the mechanism causing failure of the NVU and consequent brain calcification phenotype. This work further supports our hypothesis and together with PDGFB, PDGFRB, and MYORG the tight junction genes solidify dysregulation of BBB integrity as a key PFBC pathomechanism (Figure S7).
Importantly, we show that the human JAM2-related neurological phenotype seen is replicated in the jam2 KO mouse. It is noteworthy that the older human individuals carrying JAM2 variants, who had longer disease duration, presented impaired gait of variable severity including ataxic and/or parkinsonian gait illustrated in the mouse model. The main walking and behavioral findings in the affected mice were progressive gait abnormalities, similar to ataxic mouse, clearly seen on standard mouse phenotype assessment. Furthermore, neuropathology of jam2 KO mouse model (prominent vacuolation in the cerebral cortex, thalamus, and cerebellar cortex and particularly widespread vacuolation in the midbrain) affected similar brain areas to those observed on brain imaging of the human phenotype. The pattern of calcification seen on the brain scans of our subjects was largely the same as that seen in PFBC-affected subjects where the brain is structurally normal and the calcification is almost exclusively in the gray matter affecting basal ganglia, thalamus, deep cortex, dental, and cerebellar folia.16 It was indistinguishable from SLC20A2, PDGFB, PDGFRB, and XPR1 case subjects and there was no calcification in the pons as seen in MYORG subjects.1,17 In contrast, the calcification pattern observed in JAM2 subjects differs to that of other tight junction genes, as OCLN subjects have band-like calcification with simplified gyration6 and JAM3 subjects present multifocal intraparenchymal hemorrhage, massive cysts, and subependymal calcification.5
In summary, we show that JAM2 is recurrently mutated in families with recessive PFBC presenting with a combination of movement disorder and/or cognitive and psychiatric manifestations. The human phenotype was replicated in a jam2 KO mouse model. The presence of mutations in several genes involved in the central NVU presenting clinically with brain calcification suggests that the NVU is likely to represent a potential therapeutic target in this group of disorders.
Declaration of Interests
The authors declare that A. Begtrup and E.T. are employees of GeneDx, Inc., USA, and S.K. and C.B. are employees of CENTOGENE AG, Rostock, Germany. The other authors declare no competing interests.
Acknowledgments
We are grateful to the patients for their essential help. We are grateful for the funding support from The Medical Research Council (MRC) (MR/S01165X/1, MR/S005021/1), The Wellcome Trust (Synaptopathies Strategic Award, WT093205MA, WT104033AIA), The Rosetree Trust, Ataxia UK, The MSA Trust, Brain Research UK, Sparks GOSH Charity, Muscular Dystrophy UK (MDUK), and Muscular Dystrophy Association (MDA USA). We also thank James Polke and Conceição Bettencourt for assistance with the text and interpretation. S.B. and H.H. are partly funded by the National Institute of Health Research (NIHR) UCLH/UCL Biomedical Research Centre and Dementia Biomedical Research Unit. J.R.M.deO. receives funding from CNP, CAPES, and DECIT-MS. We thank Joanne Lau for technical support. This research was also conducted as part of the Queen Square Genomics group at UCL, supported by the National Institute for Health Research (NIHR) award to UCLH NHS Trust/UCL Biomedical Research Centre (BRC).
Published: March 5, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.02.007.
Contributor Information
Henry Houlden, Email: h.houlden@ucl.ac.uk.
SYNAPS Study Group:
Stanislav Groppa, Blagovesta Marinova Karashova, Wolfgang Nachbauer, Sylvia Boesch, Larissa Arning, Dagmar Timmann, Bru Cormand, Belen Pérez-Dueñas, Gabriella Di Rosa, Jatinder S. Goraya, Tipu Sultan, Jun Mine, Daniela Avdjieva, Hadil Kathom, Radka Tincheva, Selina Banu, Mercedes Pineda-Marfa, Pierangelo Veggiotti, Michel D. Ferrari, Alberto Verrotti, Giangluigi Marseglia, Salvatore Savasta, Mayte García-Silva, Alfons Macaya Ruiz, Barbara Garavaglia, Eugenia Borgione, Simona Portaro, Benigno Monteagudo Sanchez, Richard Boles, Savvas Papacostas, Michail Vikelis, Eleni Zamba Papanicolaou, Efthymios Dardiotis, Shazia Maqbool, Shahnaz Ibrahim, Salman Kirmani, Nuzhat Noureen Rana, Osama Atawneh, George Koutsis, Marianthi Breza, Salvatore Mangano, Carmela Scuderi, Eugenia Borgione, Giovanna Morello, Tanya Stojkovic, Massimi Zollo, Gali Heimer, Yves A. Dauvilliers, Pasquale Striano, Issam Al-Khawaja, Fuad Al-Mutairi, and Hamed Sherifa
Accession Numbers
The variants reported in this paper have been submitted to the Leiden Open Variation Database and the accession numbers are 643210, 643209, 643163, 643162.
Web Resources
Ensembl Genome Browser, http://www.ensembl.org/index.html
ExAC Browser, http://exac.broadinstitute.org/
GeneMatcher, https://genematcher.org/
GERP, http://mendel.stanford.edu/sidowlab/downloads/gerp/index.html
gnomAD Browser, https://gnomad.broadinstitute.org/
HomozygosityMapper software, http://www.homozygositymapper.org/
Leiden Open Variation Database, https://www.lovd.nl/
Mutation Assessor, http://mutationassessor.org/
MutationTaster, http://www.mutationtaster.org/
OMIM, https://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
PROVEAN, http://provean.jcvi.org
QuPath, https://qupath.github.io/
Accession numbers
The variants reported in this paper have been submitted to the Leiden Open Variation Database and the accession numbers are 00286201, 00286202, 00286204, 00286250.
Supplemental Data
References
- 1.Batla A., Tai X.Y., Schottlaender L., Erro R., Balint B., Bhatia K.P. Deconstructing Fahr’s disease/syndrome of brain calcification in the era of new genes. Parkinsonism Relat. Disord. 2017;37:1–10. doi: 10.1016/j.parkreldis.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Taglia I., Bonifati V., Mignarri A., Dotti M.T., Federico A. Primary familial brain calcification: update on molecular genetics. Neurol. Sci. 2015;36:787–794. doi: 10.1007/s10072-015-2110-8. [DOI] [PubMed] [Google Scholar]
- 3.Legati A., Giovannini D., Nicolas G., López-Sánchez U., Quintáns B., Oliveira J.R.M., Sears R.L., Ramos E.M., Spiteri E., Sobrido M.-J. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015;47:579–581. doi: 10.1038/ng.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X.-P., Cheng X., Wang C., Zhao M., Guo X.-X., Su H.-Z., Lai L.-L., Zou X.-H., Chen X.-J., Zhao Y. Biallelic Mutations in MYORG Cause Autosomal Recessive Primary Familial Brain Calcification. Neuron. 2018;98:1116–1123.e5. doi: 10.1016/j.neuron.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Mochida G.H., Ganesh V.S., Felie J.M., Gleason D., Hill R.S., Clapham K.R., Rakiec D., Tan W.-H., Akawi N., Al-Saffar M. A homozygous mutation in the tight-junction protein JAM3 causes hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Am. J. Hum. Genet. 2010;87:882–889. doi: 10.1016/j.ajhg.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Driscoll M.C., Daly S.B., Urquhart J.E., Black G.C.M., Pilz D.T., Brockmann K., McEntagart M., Abdel-Salam G., Zaki M., Wolf N.I. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am. J. Hum. Genet. 2010;87:354–364. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen N., Schrøder H.D., Hejbøl E.K., Thomsen J.S., Brüel A., Larsen F.T., Vinding M.C., Orlowski D., Füchtbauer E.-M., Oliveira J.R.M., Pedersen L. Mice Knocked Out for the Primary Brain Calcification-Associated Gene Slc20a2 Show Unimpaired Prenatal Survival but Retarded Growth and Nodules in the Brain that Grow and Calcify Over Time. Am. J. Pathol. 2018;188:1865–1881. doi: 10.1016/j.ajpath.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T., Miura T., Aoki K., Saito S., Hondo H., Konno T., Uchiyama A., Ikeuchi T., Takahashi H., Kakita A. Familial idiopathic basal ganglia calcification: Histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology. 2016;36:365–371. doi: 10.1111/neup.12280. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S., Yamadori I., Miki H., Ohmori M. Idiopathic nonarteriosclerotic cerebral calcification (Fahr’s disease): an electron microscopic study. Acta Neuropathol. 1987;73:62–66. doi: 10.1007/BF00695503. [DOI] [PubMed] [Google Scholar]
- 10.Miklossy J., Mackenzie I.R., Dorovini-Zis K., Calne D.B., Wszolek Z.K., Klegeris A., McGeer P.L. Severe vascular disturbance in a case of familial brain calcinosis. Acta Neuropathol. 2005;109:643–653. doi: 10.1007/s00401-005-1007-7. [DOI] [PubMed] [Google Scholar]
- 11.Keller A., Westenberger A., Sobrido M.J., García-Murias M., Domingo A., Sears R.L., Lemos R.R., Ordoñez-Ugalde A., Nicolas G., da Cunha J.E.G. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 2013;45:1077–1082. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]
- 12.Aurrand-Lions M., Johnson-Leger C., Wong C., Du Pasquier L., Imhof B.A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 13.Tietz S., Périnat T., Greene G., Enzmann G., Deutsch U., Adams R., Imhof B., Aurrand-Lions M., Engelhardt B. Lack of junctional adhesion molecule (JAM)-B ameliorates experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2018;73:3–20. doi: 10.1016/j.bbi.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Redmond S.A., Mei F., Eshed-Eisenbach Y., Osso L.A., Leshkowitz D., Shen Y.-A.A., Kay J.N., Aurrand-Lions M., Lyons D.A., Peles E., Chan J.R. Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron. 2016;91:824–836. doi: 10.1016/j.neuron.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cen Z., Chen Y., Chen S., Wang H., Yang D., Zhang H., Wu H., Wang L., Tang S., Ye J. Biallelic loss-of-function mutations in JAM2 cause primary familial brain calcification. Brain. 2020;143:491–502. doi: 10.1093/brain/awz392. [DOI] [PubMed] [Google Scholar]
- 16.Livingston J.H., Stivaros S., Warren D., Crow Y.J. Intracranial calcification in childhood: a review of aetiologies and recognizable phenotypes. Dev. Med. Child Neurol. 2014;56:612–626. doi: 10.1111/dmcn.12359. [DOI] [PubMed] [Google Scholar]
- 17.Grangeon L., Wallon D., Charbonnier C., Quenez O., Richard A.-C., Rousseau S., Budowski C., Lebouvier T., Corbille A.-G., Vidailhet M., French PFBC study group Biallelic MYORG mutation carriers exhibit primary brain calcification with a distinct phenotype. Brain. 2019;142:1573–1586. doi: 10.1093/brain/awz095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.