Abstract
The article reports data on chemical profiling by gas chromatography-mass spectrometry (GC-MS) of aqueous and methanolic leaf extracts of Madagascar periwinkle (Catharanthus roseus) and drumstick tree (Moringa oleifera) and on their antioxidant and antibacterial effects against three clinical human pathogens. In total 105 compounds were tentatively identified; in which 65 in Catharanthus roseus and 40 in Moringa oleifera compounds. A large number of peaks with good area percentage was found in methanolic extract of Catharanthus roseus with core chemical constituents such as trans-squalene, n-hexadecanoic acid, Eicosyl acetate, stearin, 1H-Benz(G)indole-3-carboxylic acid. The corresponding constituents from Moringa oleifera include 9-Octadecenoic acid (z)-, Heptadecanoic acid and phytol acetate. The highest scavenging activity (87.7% at 200 μg/mL) was shown by DPPH aqueous leaf extract of C. roseus. Moreover, the methanolic scavenging of both plant extracts was in the order of FRAP>DPPH>NO> H2O2 with lowest antioxidant activity (51.4% at 200 μg/mL) exposed by Catharanthus roseus in comparison of all cases. Good antibacterial action was examined against three different organisms (E.coli, B. subtilis and S. aureus) of aqueous infusion of Catharanthus roseus.
Keywords: GC-MS, Catharanthus roseus, Moringa oleifera, Anti-oxidant, Antibacterial
Specifications Table
| Subject | Biology |
| Specific subject area | Medicinal plants and pharmacology |
| Type of data | Chromatogram figures, Tables, Figures, Text files |
| How data were acquired | Crude aqueous and methanolic leaf extracts of C. roseus and M. oleifera were isolated and analyzed by GC-MS, which was carried out on GC-MS QP2010 plus (Shimadzu, Japan) equipped with a flame ionization detector and GC 6890 model series. Several antioxidant activity in-vitro assays were performed with specified protocols. Antibacterial activities were determined using agar well diffusion method. |
| Data format | Raw, analyzed and expressed as mean ± SEM, One-way ANOVA analysis of variance. |
| Experimental factors | Aqueous and methanolic extracts of Catharanthus roseus and Moringa oleifera prepared to isolate secondary metabolites through GC-MS and tested in different in-vitro antioxidant assays and for antimicrobial activity. |
| Experimental features | Extraction and isolation of both plant crude extracts (Soxhlet, column chromatography, GC-MS analysis); In-vitro antioxidant assays (DPPH, FRAP, NO, H2O2.); Test microorganism (One Gram negative Escherichia coli (MTCC 443) and two Gram positive Bacillus subtilis (MTCC 441), Staphylococcus aureus (ATCC 259323). |
| Data source location | The fresh leaves of the two species of Catharanthus roseus and Moringa oleifera were gathered from campus of Yogi Vemana University and near Raychotighat, Kadapa, India, Department of Biotechnology and Bioinformatics, Yogi Vemana University, Kadapa, India. |
| Data accessibility | Provided data in this article |
| Related research article | Mehdi Soltani Howyzeh, Seyed Ahmad Sadat Noori, Vahid Shariati J (2018).Essential oil profiling of Ajowan (Trachyspermum ammi) industrial medicinal plant. Industrial Crops and Products 119, 255–259. |
Value of the Data
|
1. Data
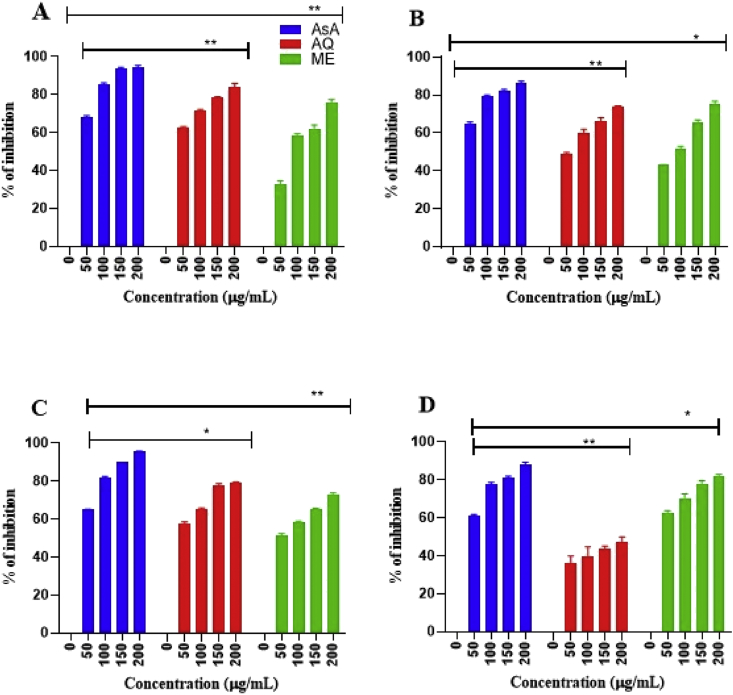
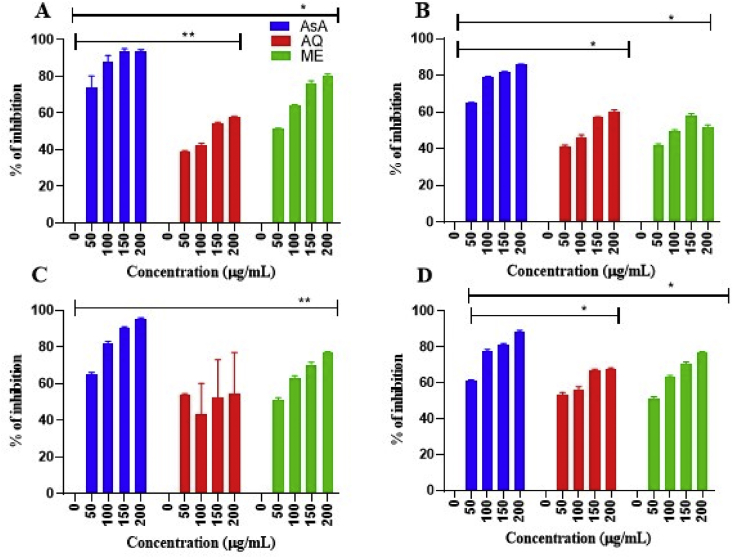
The current data pertains to GC–MS chromatogram of the methanolic and aqueous leaf extract of C. roseus (Fig. 1, Fig. 2) and M. oleifera (Fig. 3, Fig. 4) with their corresponding secondary metabolites as depicted in Table 1, Table 2 respectively. In-vitro antioxidant assays with percentage of inhibition as a parameter are presented in Fig. 5, Fig. 6. Antimicrobial activity (in terms of inhibition zones) of C. roseus and M. oleifera leaves against selected bacterial strains was shown in Table 3.
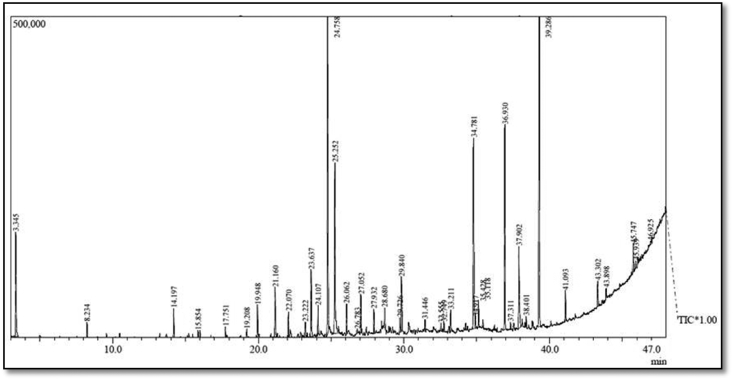
Fig. 1.
GC-MS Chromatogram of methanolic Catharanthus roseus leaf extract.
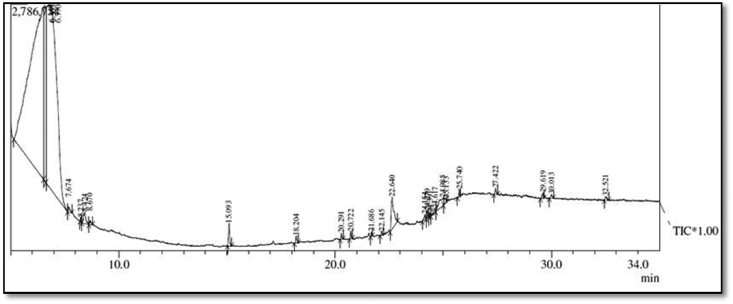
Fig. 2.
GC-MS Chromatogram of aqueous Catharanthus roseus leaf extract.
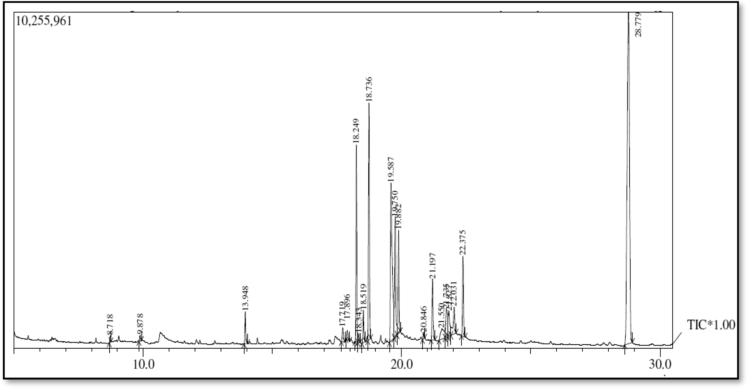
Fig. 3.
GC-MS Chromatogram of methanolic Moringa oleifera leaf extract.
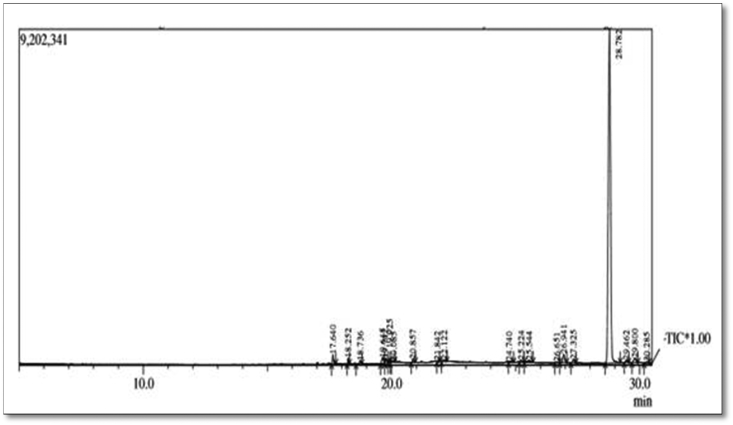
Fig. 4.
GC-MS Chromatogram of aqueous Moringa oleifera leaf extract.
Table 1.
Phytochemicals tentatively identified based on retention time (RT) matching in the methanolic (Left) and aqueous (Right) extracts of Catharanthus roseus leaf extract by GC-MS.
| Sl. No | RT (min.) | NIST DATABASE/Wiley 2007/FAME ID/(Methanolic) | RT (min.) | NIST DATABASE/Wiley 2007/FAME ID/(Aqueous) |
|---|---|---|---|---|
| 1. | 3.34 | 2-Hydroxy-2-methyl-4-pentanone (diacetone) | 6.46 | R (-)-2-Amino-1-butanol |
| 2. | 8.23 | 4-Penten-2-Ol, 3-methyl- | 6.55 | Phenethylamine, alpha-ethyl- |
| 3. | 14.17 | Quinoline, 1,2-dihydro-2,2,4-trimethyl- | 6.77 | 1-Butanol, 2-amino- |
| 4. | 15.85 | Hexathiane | 7.67 | 2,4(1H,3H)-Pyrimidinedione |
| 5. | 17.75 | Pentadecane | 8.23 | Naphthalene |
| 6. | 19.20 | 2(3H)-Benzothiazolone | 8.42 | 2,2,5,5-Tetramethylhex-3-ene, 3,4-dideutero |
| 7. | 19.94 | Octadecane | 8.67 | 4-Pyrimidinamine, 2,6-dimethyl |
| 8. | 21.16 | Tetradecanoic Acid | 15.09 | Phenol, 2,4-Bis(1,1-dimethylethyl |
| 9. | 22.07 | Tetracosane | 18.20 | Cyclooctasiloxane, hexadecamethyl |
| 10. | 23.22 | Octadecanoic Acid | 20.29 | 1,3-Diphenyl-1,3,5,5-tetramethyl- |
| 11. | 23.63 | 3-(2-Chloroethyl)-1,3-benzothiazol-2(3H)-one | 20.72 | 1,1,3,3,5,5,7,7,9,9,11,11,13,13-Tetradeca |
| 12. | 24.10 | Tetracosane | 21.68 | Phosphine Oxide, bis(Pentamethylphenyl)- |
| 13. | 24.75 | 2-(1,3-Benzothiazol-2-ylsulfanyl)ethanol | 22.14 | Hexadecanoic Acid, methyl ester |
| 14. | 25.25 | n-Hexadecanoic acid | 22.64 | n-Hexadecanoic Acid |
| 15. | 26.06 | Tetracosane | 24.15 | 9-Octadecenoic acid, Methyl ester, (E)- |
| 16. | 26.78 | Dodecane, 1,1′-oxybis- | 24.27 | Cyclododecasiloxane, tetracosamethyl |
| 17. | 27.05 | Octathiocane | 24.39 | Hexacosanoic acid, Methyl Ester |
| 18 | 27.93 | Tetracosane | 24.61 | 1,5,9,9-Tetramethyl-2-oxatricyclo [6.4.0.0 (4,8) |
| 19. | 28.68 | Urea | 24.98 | 2-Furanpentanoic acid, tetrahydro-5-nonyl-, methyl |
| 20. | 29.72 | Spiro [Cyclopentane-1,2′ (1′h)-quinoxaline], 3′-(4-morpholinyl)-6′,8′-dinitro- | 25.13 | (2-Methyl-1-phenyl-2-propenyl)Be |
| 21. | 29.63 | Eicosyl Acetate | 25.74 | Cyclononasiloxane, Octadecamethyl- |
| 22. | 31.44 | Tetracosane | 27.42 | 1H-Purin-6-Amine, [(2-fluorophenyl |
| 23. | 32.55 | Stannane, Tributyl (2,5-dimethyl-1-phenyl-4-hexenyl)-, (R∗,R∗)-(.+-.)- | 29.61 | Heptasiloxane, Hexadecamethyl- |
| 24. | 32.74 | Methyl 6,7-dideoxy-6-C-methyl-2,3-di-o-methyl-.alpha.-D-gluco-oct-6-eno-1,5-pyranosid)Urono-8,4-lactone | 30.01 | 1,2-Benzenedicarboxylic acid |
| 25. | 33.21 | Tricosyl acetate | 32.52 | Cyclononasiloxane, octadecamethyl- |
| 26. | 34.78 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | ||
| 27. | 34.91 | 1,4-Cyclooctanedione | ||
| 28. | 35.42 | 1,2-Benzenedicarboxylic acid | ||
| 29. | 35.11 | Pyrrolo [3,4-C]pyrrole-1-carboxylic Acid, 3-cyclopropyloctahydro-4,6-dioxo-1,5-diphenyl-, methyl ester | ||
| 30. | 36.93 | 4,4′((phenylene)diisopropylidene)diphenol | ||
| 31. | 37.31 | 1H-indole-3-ethanamine | ||
| 32. | 37.90 | Octadecanoic acid, 2,3-dihydroxypropyl ester | ||
| 33. | 38.40 | Heptacyclo [6.6.0.0(2,6).0(3,13).0(4,11).0(5,9).0(10,14)]Tetradecanone | ||
| 34. | 39.28 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-Hexamethyl- | ||
| 35. | 41.09 | 1H-Benz [G]indole-3-carboxylic acid, 1-(2,2-dimethoxyethyl)-5-methoxy-2-methyl-, ethyl Ester | ||
| 36. | 43.30 | Cholest-5-en-3-ol (3.Beta.)- | ||
| 37. | 43.89 | 6-Methoxy-2,8-dimethyl-(4′,8′-dimethyl-3′,7′-nonadienyl)-3,4-dihydro-2H-1-Benzopyran | ||
| 38. | 45.74 | Beta.-Sitosterol | ||
| 39. | 45.93 | Ethanone, 1,1′-[3,3′-biisoxazole]-5,5′-diylbis- | ||
| 40. | 46.92 | 3-Butoxy-1,1,1,5,5,5-hexamethyl-3-(Trimethylsiloxy)trisiloxane |
Table 2.
Phytochemicals tentatively identified based on retention time (RT) matching in the methanolic (Left) and aqueous (Right) extracts of Moringa oleifera leaf extract by GC-MS.
| Sl. No | RT (min.) | NIST DATABASE/Wiley 2007/FAME ID/(Methanolic) | RT (min.) | NIST DATABASE/Wiley 2007/FAME ID/(Aqueous) |
|---|---|---|---|---|
| 1. | 8.718 | 1,1-Diethoxy-2-ethylhexane | 17.640 | Benzene, 1,1′-(1,2-cyclobutanediyl |
| 2. | 9.878 | Azulene | 18.252 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 3. | 13.948 | 2,6-Di-butyl-2,5-cyclohexadiene-1 | 18.736 | pentadecanal- |
| 4. | 17.719 | 9-Octadecenoic acid, ethyl ester | 19.645 | 9-Octadecenoic acid (z)- |
| 5. | 17.896 | 2(4H)-Benzofuranone, 5,6,7,7a tetrahydro | 19.742 | 1,2-Benzenedicarboxylic acid, diheptyl ester |
| 6. | 18.249 | 2,6,10-Trimethyl,14-ethylene-14-pe | 19.925 | 2,5-Pyrrolidinedione, 1-hydroxy- |
| 7. | 18.343 | 2-Pentadecanone, 6,10,14-trimethyl- | 20.085 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl |
| 8. | 18.519 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 20.857 | Phosphonic acid, dioctadecyl ester |
| 9. | 18.736 | Phthalic acid, isobutyl undec-2-en-1-yl ester | 21.842 | Dimethylaminato [4-methyl-2-(e) |
| 10. | 19.587 | l-(+)-Ascorbicacid2,6-dihexadecanoate | 22.122 | Tetradecanamide |
| 11. | 19.750 | Dibutyl phthalate | 24.740 | Heptadecanoic acid, ethyl ester |
| 12. | 19.882 | Hexadecanoic acid, ethyl ester | 25.224 | Hexanedioic acid, mono (2-ethylhexyl)ester |
| 13. | 20.846 | Behenic alcohol | 25.544 | Tetracosyl acetate |
| 14. | 21.197 | 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, | 26.651 | borneol, Pentamethyldisilanyl ether |
| 15. | 21.550 | 9,12-Octadecadienoic acid (z,z)- | 26.941 | 7-Propyl-1,3,5-cycloheptatriene |
| 16. | 21.735 | (r)-(-)-14-Methyl-8-hexadecyn-1-ol | 27.325 | Octane, 1,1′-oxybis- |
| 17. | 21.827 | 9,12,15-Octadecatrienoic acid, (z,z,z)- | 28.782 | Bis(2-ethylhexyl) phthalate |
| 18. | 22.031 | Heptadecanoic acid, ethyl ester | 29.462 | (2,3-Diphenylcyclopropyl)methyl phenyl sulfoxide, |
| 19. | 22.375 | Phytol, acetate | 29.800 | 1,2-Diphenyl-1-isocyanoethane |
| 20. | 28.779 | Bis(2-ethylhexyl) phthalate | 30.285 | 7-(Isobut-1-yl)cyclohepta-1,3,5-tr |
Fig. 5.
In-vitro antioxidant activity of aqueous and methanolic leaf extracts of Catharanthus roseus. (A) DPPH scavenging activity (B) H2O2 scavenging activity (C) Nitric oxide scavenging activity (D) FRAP assay. Values are expressed as Mean ± SEM (n = 3). One-way ANOVA followed by Dunnett's test was employed to compare each concentration with positive control. ∗Statistical significance at p < 0.05; ∗∗ statistical significance at p < 0.01. AsA-Ascorbic acid (Positive control); AQ - Aqueous; ME-MeOH.
Fig. 6.
In-vitro antioxidant activity of aqueous and methanolic leaf extracts of Moringa oleifera. (A) DPPH scavenging activity (B) H2O2 scavenging activity (C) Nitric oxide scavenging activity (D) FRAP assay. Values are expressed as Mean ± SEM (n = 3). One-way ANOVA followed by Dunnett's test was employed to compare each concentration with positive control. ∗Statistical significance at p < 0.05; ∗∗ statistical significance at p < 0.01. AsA-Ascorbic acid (Positive control); AQ-Aqueous; ME-MeOH.
Table 3.
Antimicrobial activity of aqueous and methanolic leaf extracts of Catharanthus roseus and Moringa oleifera against selected bacterial strains.
| Leaf Extracts (50 μg/mL) | Zone of inhibition (mm) |
||
|---|---|---|---|
| B. subtilis | S. aureus | E. coli | |
| C. roseus | |||
| Aqueous | 22 ± 0.33 | 23 ± 0.18∗∗ | 25 ± 0.25 |
| Methanolic | 23 ± 0.23 | 23 ± 0.44∗∗ | 20 ± 0.41 |
| M. oleifera | |||
| Aqueous | 23 ± 0.23∗ | 20 ± 0.25 | 18 ± 0.41 |
| Methanolic | 23 ± 0.65∗ | 24 ± 0.44 | 16 ± 0.23 |
| Tetracycline (1 μg/mL) | 29 ± 0.25 | 36 ± 0.33 | 28 ± 0.46 |
∗Statistical significance at p < 0.05; ∗∗ statistical significance at p < 0.01.
2. Experimental design, materials and methods
2.1. Collection of plant material and preparation
The fresh leaves of Catharanthusroseus (Apocynaceae) and Moringa oleifera (Moringaceae) were gathered from campus of Yogi Vemana University, Kadapa and near Raychotighat, India respectively. The plant specimens were recognized and authenticated by Department of Botany at Yogi Vemana University, Kadapa, India. The leaves of both the plants were harvested at the vegetative phase.
2.2. Plant sample extraction and column chromatography
Dried powdered leaf samples were successively extracted by soxhlet apparatus, as described by Sadasivam and Manickam [1] and extracts were subjected to column chromatography over silica gel (60–120 mesh) and eluted with n-hexane, chloroform and methanol respectively. n-hexane and chloroform did not elute much of the compounds. Both aqueous and methanolic fractions of Catharanthus roseus and Moringa oleifera were kept under vacuum desiccators until used for gas chromatography/mass spectrometry (GC–MS) analysis.
2.3. Gas chromatography/mass spectroscopy (GC/MS) analysis
The GC-MS analysis was conducted on GC-MS QP2010 Plus (Shimadzu, Japan) equipped with a flame ionization detector and GC 6890 model series. The GC was equipped with a fused silica (30 m × 0.25 mm ID × 0.25 μm) capillary column. Injection temperature was maintained at 250 °C by employing helium (99.995%) as a carrier gas at a constant flow rate of 1.5 ml/min. 1 mg/1 ml absolute alcohol at a split ratio of 1: 10 was injected. The instrument was set to an initial temperature of 50 °C for 2 min. At the end of this period the oven temperature was arisen up to 300 °C, at the rate of 12 hold/40 min. The mass spectra of compounds in samples were obtained by electron ionization (EI) at 70 eV, and the data was evaluated using total ion count (TIC) for compound identification and quantification. The MS start and end time (3 and 32 min.) was performed at a scan speed of 2000. The spectrum of the unknown components were compared with spectrum of known components stored both in the “NIST-MS Library 05”, “Wiley GC-MS Library 2007” as well as FAME with more patterns.
2.4. In-vitro anti-oxidant assays
2.4.1. DPPH free radical scavenging assay
The DPPH radical-scavenging activity of the test extracts was examined using the modified method by Brand-Williams et al. [2]. Leaf extracts of different concentrations (50–200 μg/mL) were mixed with an equal volume of methanolic solution of DPPH (Sigma Aldrich). The mixture was allowed to react at room temperature in dark for 30 min. Ascorbic acid (1 mg/mL (50–200 μg/mL)) was used as positive control. After 30 min the absorbance was measured at 517 nm and converted into percentage of antioxidant activity using the following equation.
| % of inhibition = [A0-A1/A0] ∗100 |
where A0 = Absorbance of control.
A1 = Absorbance of test.
2.4.2. Hydrogen peroxide scavenging assay
The H2O2 scavenging activities for both the leaf extracts were assayed by the modified method [3]. Different concentrations of plant leaf extracts (50–200 μg/mL) and ascorbic acid at different concentrations (50–200 μg/mL) of (1 mg/mL) were added to 40 mM H2O2 solution prepared in phosphate buffer. The absorbance of H2O2 at 230 nm was determined after 10 min. The percentage of H2O2 scavenging by the extracts and standard (H2O2) was calculated as follows.
| % of scavenged [H2O2] = [A0-A1/A0] ∗100 |
where A0 = Absorbance of control.
A1 = Absorbance of test.
2.4.3. Nitric oxide radical scavenging assay
The nitric oxide (NO) scavenging activity was determined using the method described by Parul et al. [4].10 mM sodium nitroprusside was incubated with 100 μL leaf extract for 60 min at 30 °C. After incubation, 100 μL of griess reagent was added. The absorbance of the chromatophore formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with naphthylehylendiamine was measured at 562 nm. Ascorbic acid (1 mg/mL) was at the same concentration was taken as standard.
| % NO scavenged = [A0-A1/A0] ∗100 |
where A0 = Absorbance of control.
A1 = Absorbance of test.
2.4.4. Ferric reducing power (FRAP) assay
The reducing power was determined by Benzie and Strain [5] with slight modifications. Various concentrations of plant leaf extracts (50–200 μg/mL) were mixed with phosphate buffer and 2 mM potassium ferricyanide. The mixture was incubated at 50 °C for 20 min. TCA was added to the mixture which was then centrifuged at 3000 rpm for 10 min. The upper layer of solution was mixed with distilled water and freshly prepared Fecl3 solution (0.5 mL) and the absorbance was recorded at 700 nm using UV-Visible spectrophotometer (Thermo scientific evolution -201 series). Ascorbic acid (50–200 μg/mL) was used as positive control. Reducing capacity was calculated as follows:
| % increase in reducing power = [Atest/Ablank-1]∗100 |
Where Atest = Absorbance of test solution.
Ablank = Absorbance of blank.
2.5. Antimicrobial assay
2.5.1. Test microorganism
One Gram negative Escherichia coli (MTCC 443) and two Gram positive Bacillus subtilis (MTCC 441), Staphylococcus aureus (ATCC 259323) were used as bacterial test organism. The bacterial strains were cultured overnight at 37 °C in Luria-Bertani (LB) medium.
2.5.2. Agar well diffusion method
Antibacterial activities of two plants extract (Catharanthus roseus and Moringa oleifera) was determined using Agar well diffusion method [6]. The bacterial suspensions containing 7 × 105 cells/mL were incubated overnight and used for inoculation. 20 ml of molten nutrient agar was poured into the Petri dishes and cooled. All the bacterial suspension was swapped over the medium and 3 wells of 0.5 cm deep were made by using a sterile tip. Each 50 μL of aqueous, methanolic leaf extracts were added to respective wells one with tetracycline (1 μg/mL, Sigma) was added as positive control and other with distilled water as negative control. Tetracycline (antibiotic) was used as positive control. The antimicrobial behavior was determined by measuring Zone of inhibition around the holes in diameter (mm) after incubation.
2.6. Statistical analysis
All assays were performed in triplicate. Mean and standard deviation (SD) was examined for all assays. The results were expressed as mean ± SEM of three experiments. One way ANOVA with Dunnett's test was followed to compare each concentration with positive control to analyze level of statistical significance. P < 0.05 were considered statistically significant using Graph pad PRISM v.8.0.
Acknowledgments
The first author AMS is grateful to, University Grants Commission Govt. of India for providing Maulana Azad National Fellowship [F1-17.1/2017-18/MANF-2017-18-AND-73354/(SA-III/Website)].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2020.105258.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sadasivam S., Manickam A. Tamil Nadu Agricultural University; 1996. Biochemical Methods, Second Ed. New Age International (P) Limited. ISBN: 81: 224-0976-8. [Google Scholar]
- 2.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Food Sci. Technol. LWT. 1995;28:25–30. [Google Scholar]
- 3.Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Parul R., Kundu S.K., Saha P. In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. Pharma Innov. 2013;1(12):83–88. [Google Scholar]
- 5.Benzie I.F., Strain J.J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 6.Vidya vani M., Anjum mobeen S., Riazunnisa K. Phytochemical screening and antioxidant activities and antibacterial potentials of leaf extracts of Buchanania axillaris L. J. Pharma. Res. Int. 2018;21(3):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.