Abstract
Adolescence is characterized by rapid brain development in white matter (WM) that is attributed in part to surges in gonadal hormones. To date, however, there have been few longitudinal investigations relating changes in gonadal hormones and WM development in adolescents. We acquired diffusion-weighted MRI to estimate mean fractional anisotropy (FA) from 10 WM tracts and salivary testosterone from 51 females and 29 males (ages 9–14 years) who were matched on pubertal stage and followed, on average, for 2 years. We tested whether interactions between sex and changes in testosterone levels significantly explained changes in FA. We found positive associations between changes in testosterone and changes in FA within the corpus callosum, cingulum cingulate, and corticospinal tract in females (all ps<0.05, corrected) and non-significant associations in males. We also collected salivary estradiol from females and found that increases in estradiol were associated with increases in FA in the left uncinate fasciculus (p = 0.04, uncorrected); however, this effect was no longer significant after accounting for changes in testosterone. Our findings indicate there are sex differences in how changes in testosterone relate to changes in WM microstructure of tracts that support impulse control and emotion regulation across the pubertal transition.
Keywords: Adolescence, Puberty, Diffusion MRI, Testosterone, Estradiol, Cingulum cingulate
1. Introduction
Adolescence is a developmental period between childhood and adulthood that is marked by significant changes in cognition, emotion, and social functioning (Lerner and Steinberg, 2004). This period of transition is defined by the onset of puberty, which involves widespread endocrine shifts that signal a cascade of physical changes, including growth spurts, the appearance of secondary sex characteristics, and adipose tissue redistribution (Sisk and Foster, 2004; Dorn et al., 2006). Coinciding with these physical changes are significant behavioral changes, particularly those centered around social cues and peer relationships (Blakemore, 2008; Blakemore et al., 2010; Kilford et al., 2016). Relative to childhood, adolescence is also characterized by an increase in risk-taking behaviors, including higher rates of vehicular accidents, drug abuse, and unsafe sex practices (Eaton et al., 2008; Steinberg, 2007), and by an increase in rates of mental health disorders such as depression, anxiety, and suicidal thoughts and behaviors (Maslow et al., 2015; Nock et al., 2013). Consequently, adolescence has garnered widespread attention as a time of vulnerability to difficulties and disorder; however, it is also a period of opportunity for intervention to prevent longer-term adverse outcomes (Dahl et al., 2018; Quas, 2014).
It is now widely recognized that these behavioral difficulties that emerge in adolescence are supported by neurobiological changes during this developmental period (Ladouceur and Peper, 2012; Lenroot and Gogtay, 2007; Tamnes et al., 2018). Indeed, one of the most consistent findings reported in research examining developmental changes in the adolescent brain is that there is a linear increase in global white matter (WM) volume from childhood to adolescence (Giedd et al., 1999; Tamnes et al., 2013). These changes in WM volumes are posited to be driven by increased axonal caliber and/or by increased myelination (Paus, 2010); although cellular-level mechanisms cannot be examined directly in studies of brain volume, they are thought to underlie the increased cortical organization and maturation of relevant cognitive faculties that are developing throughout adolescence (e.g., cognitive control, response inhibition, emotion regulation). Recently, researchers have used diffusion-tensor imaging (DTI) to estimate age-related changes in WM microstructure across childhood (Krogsrud et al., 2016), adolescence (see Peters et al., 2012, for a review) and over the lifespan (see Tamnes et al., 2018, for a review). Fractional anisotropy (FA) is the most common diffusion MRI-based measure of WM microstructure that reflects the directionality and magnitude of diffusion, with higher FA values signifying stronger structural integrity and improved WM organization (Beaulieu, 2002; Song et al., 2002). Overall, these studies have found consistently that older adolescents exhibit higher FA in most WM regions than do younger adolescents (Asato et al., 2010; Lebel and Beaulieu, 2011; Lebel et al., 2012).
Researchers have also identified sex differences in normative adolescent WM development, even when controlling for total brain volume; males have significantly steeper increases in global and regional WM volume (primarily in prefrontal cortex and in subcortical structures) than do females (Lenroot and Gogtay, 2007). Sex differences are also commonly reported in diffusivity metrics such as FA (Herting et al., 2012; 2017; Schmithorst et al., 2008; Seunarine et al., 2016; Simmonds et al., 2014; Wang et al., 2012); however, there is little consensus about the directionality and specificity (i.e., the precise tracts) that contribute to these findings. For instance, Herting and colleagues demonstrated in a sample of adolescents ages 10–16 years that males had higher FA than did females throughout the entire brain (Herting et al., 2012). In contrast, Bava and colleagues reported in a sample of adolescents ages 12–14 years that females had higher FA than did age-matched males in corticospinal tracts and in the superior corona radiata (Bava et al., 2011). Similarly, Seunarine and colleagues examined adolescents ages 8–16 years and found that females had higher FA than did males in nearly every WM region, although these differences were found primarily at earlier ages and were largely absent by ages 10–14 years (Seunarine et al., 2016).
Despite the importance of these studies in identifying key developmental patterns of WM in adolescents and providing evidence of sexual dimorphism in several WM tracts, the majority of these investigations focused on changes as a function of age, thereby overlooking the impact of critical physiological events like puberty (although see Genc et al., 2017, who demonstrated that compared to prepubertal youth, postpubertal youth had greater fiber density in the corpus callosum), during which important endocrine and physiological changes are posited to affect the organization and activation of brain circuits during adolescence (Ladouceur and Peper, 2012; Juraska et al., 2013; Romeo, 2003; Schulz et al., 2009; Sisk and Zehr, 2005). Furthermore, given that females typically enter puberty earlier than males (Ellis, 2004,Negriff et al., 2010), examining sex differences even after accounting for the effects of chronological age does not preclude the possibility that sex differences in WM are explained by differences in pubertal maturation. Indeed, in a recent longitudinal study using fixel-based analyses to examine WM micro- and macrostructure in female and male adolescents ages 9–13 years, Genc and colleagues reported increases in WM fiber density, particularly in the corpus callosum and superior longitudinal fasciculus, over 16 months; however, sex, age, and self-reported pubertal stage did not explain these increases in this sample, raising the possibility that pubertal hormones are the primary drivers of WM changes during early adolescence (Genc et al., 2018). Similarly, in a separate longitudinal study using DTI in adolescent females and males ages 10–18 years, Herting et al. (2012) found that changes in self-reported pubertal stage over two years predicted changes in FA of the thalamus, precentral gyrus, superior corona radiata, corpus callosum, superior corona radiata, and superior frontal gyrus. Specifically, increases in both gonadal (e.g., testes) and adrenal (e.g., pubic hair) development were found to be related to increases in FA in the superior frontal gyrus and precentral gyrus in boys, whereas increases in gonadal (e.g., breast) development were related to decreases in FA in the anterior corona radiata in girls (Herting et al., 2012). While neither of these two longitudinal studies explicitly measured hormones, their findings point to the likely critical role that pubertal hormones—particularly gonadal hormones—play in WM development in adolescence.
The few studies that have explicitly investigated these issues to date have yielded conflicting findings regarding sex differences in FA and the effects of the two primary sex steroids that drive pubertal changes in WM microstructure: testosterone and estradiol. In a cross-sectional study of adolescents ages 8–25 years, Peper and colleagues found that higher levels of testosterone were associated with lower FA in females only and higher mean diffusivity (MD, a DTI metric that is inversely related to FA) in both females and males in tracts connecting frontal, temporal, and subcortical regions, including the uncinate fasciculus (although several of these associations did not survive correction for multiple comparisons; Peper et al., 2015). In a separate cross-sectional study of adolescents ages 10–16 years, Herting and colleagues examined brain regions exhibiting sex differences in FA and found that in males only, testosterone was positively associated with FA in primarily subcortical tracts, the inferior fronto-occipital fasciculus, and WM in the superior temporal gyrus; in contrast, estradiol was positively associated with FA in WM regions in lateral/middle occipital cortex (Herting et al., 2012). Among these same sexually dimorphic regions, Herting et al. found a significant negative association between estradiol and FA in subcortical tracts only in females (Herting et al., 2012). Finally, using a voxel-wise approach, Herting et al. also found that in males, testosterone was positively associated with FA in several of these same sexually dimorphic WM regions and, further, that estradiol was positively associated with FA in the cingulum cingulate; in contrast, in females, estradiol was negatively associated with FA in the superior longitudinal fasciculus and angular gyrus, whereas testosterone was positively associated with FA only in a small WM region of the precentral gyrus (Herting et al., 2012). In another cross-sectional study of adolescent males ages 12–16 years, self-reported pubertal stage was not associated with FA in any WM tract, although more mature pubertal stage was significantly correlated with weaker MD in the cingulum cingulate, the inferior fronto-occipital fasciculus, and the uncinate fasciculus; further, higher levels of testosterone, but not estradiol, were associated with lower MD in these regions that were correlated significantly with self-reported pubertal stage (Menzies et al., 2015).
It is evident from the extant literature that we do not yet fully understand the neurobiological effects of puberty-related changes in gonadal hormones on changes in WM during adolescence, or the potential roles of sex differences in these processes. We sought to address this gap by recruiting a sample of young male and female adolescents who were matched on pubertal stage at the time of recruitment and examining associations between longitudinal changes in gonadal hormones and corresponding changes in several key WM tracts. Because adolescence is a time of significant maturation in corticolimbic systems and because previous studies have reported gonadal effects in major cortical tracts (Barendse et al., 2018; Herting et al., 2012; 2017; Menzies et al., 2015; Peper et al., 2015), we focused our investigation on 10 major WM tracts: left and right cingulum cingulate (CGC), corpus callosum forceps minor or genu (CC Minor), corpus callosum forceps major or splenium (CC Major), left and right inferior fronto-occipital fasciculus (IFOF), left and right uncinate fasciculus (UF), and left and right CST. Further, because previous studies have estimated FA using voxel-wise approaches or tract-based spatial statistics rather than examining tracts at the level of an individual, we conducted tractography for each participant using Automated Fiber Quantification (AFQ) to segment individual-level tracts, a robust method that has been validated against manual tracing in the developmental age range of our study sample (Yeatman et al., 2012). Based on prior findings from human neuroimaging studies (Herting et al., 2012; Menzies et al., 2015), we hypothesized that males would exhibit positive associations between changes in testosterone and changes in FA, whereas females would exhibit negative associations between changes in estradiol and changes in FA.
2. Methods and materials
2.1. Participants and inclusion criteria
Participants were English speakers from the San Francisco Bay Area who were recruited by media and online advertisements for a longitudinal study of adolescent brain development. Males and females were matched on self-reported pubertal stage (see “Pubertal Stage,” below). Female participants were excluded from the study if they had experienced the onset of menses, which occurs relatively late in gonadarche (Dorn et al., 2006), and all participants were excluded from the study if they had any contraindications for MRI (e.g., had metal implants, braces), a history of neurological disorder or major medical illness, cognitive or physical challenges that limited their ability to understand or complete the study procedures, or were not fluent in English.
Of the 140 participants who attempted a T1-weighted and diffusion-weighted MRI scan at Time 1, 122 successfully completed both scans with usable data (see “Diffusion MRI Preprocessing” below for more details on thresholds for motion outliers). Of these 122 participants, 42 were excluded from our primary investigation: 1 for having identified as being transgender; 1 for entering menarche between study inclusion and the time of scan; 8 for not providing usable hormone samples at Time 1 (see “Gonadal Hormones” below for more details on usability); 23 for not returning for a scan at Time 2; 1 for not provide usable T1-weighted and diffusion-weighted data at Time 2; and 8 for not providing usable hormone samples at Time 2. Thus, a total of 80 participants provided usable neuroimaging and hormone data at both timepoints: 51 females (11.14 ± 1.05 years at Time 1; 12.93 ± 1.32 years at Time 2) and 29 males (ages 11.90 ± 1.02 years at Time 1; 13.88 ± 1.12 years at Time 2). See Table 1 for a summary of the demographic data. There were no differences in age (t(138) = 1.26; p = 0.21), Tanner stage (t(138) = 0.78, p = 0.44), gender distribution (χ2(1) = 1.09, p = 0.30), or race (χ2(1) = 3.17; p = 0.07) at study enrollment between participants who provided usable hormone and neuroimaging data at both timepoints and participants who did not provide usable hormone and neuroimaging data at both timepoints. The study was approved by the Stanford University Institutional Review Board. In accordance with the Declaration of Helsinki, all participants provided informed assent and their parent/legal guardian provided informed consent.
Table 1.
Descriptive statistics of demographic and hormone data by sex. All change values reported were scaled by interval. Numbers in brackets indicate number of participants missing values for a specific measurement.
| Males (M ± SD, range) [# missing] | Females (M ± SD, range) [# missing] | |
|---|---|---|
| Age at Time 1 (years) | 11.90 ± 1.02 (10.08–14.17) | 11.14 ± 1.05 (9.25–13.75) |
| Age at Time 2 (years) | 13.88 ± 1.12 (11.92–16.50) | 12.93 ± 1.32 (9.67–15.75) |
| Interval (years) | 1.98 ± 0.33 (1.42–2.84) | 1.80 ± 0.62 (0.42–3.41) |
| Ethnicity (% Caucasian) | 62.1 % | 56.9 % |
| Menarche (% by Time 2) |
N/A | 63.3 % [2] |
| Days Since Last Period | N/A | 11.62 ± 12.7 (1–47) [5] |
| Corticosteroid Usage | 4 | 4 |
| Tanner Average (Time 1) | 2.05 ± 0.91 (1.0–5.0) | 2.09 ± 0.79 (1.0–3.5) |
| Tanner Average (Time 2) | 3.55 ± 0.89 (2.0–5.0) [1] | 3.28 ± 0.95 (1.0–5) [1] |
| Tanner Change | 0.85 ± 0.40 (0–1.52) [1] | 0.64 ± 0.50 (−1.33 – 1.47) [1] |
| Time of Saliva Collection at Time 1 (hours after midnight) |
8.12 ± 1.73 (5–14.42) | 7.36 ± 1.06 (5.5–9.75) |
| Time of Saliva Collection at Time 2 (hours after midnight) |
8.00 ± 1.86 (3.58–14.42) | 8.2 ± 1.14 (5.12–10.67) |
| Estradiol at Time 1 (pg/mL) |
N/A | 1.01 ± 0.48 (0.26–2.06) [3] |
| Estradiol at Time 2 (pg/mL) |
N/A | 1.11 ± 0.51 (0.24–2.22) [3] |
| Change in Estradiol (pg/mL) |
N/A | 0.14 ± 0.64 (−1.36 – 1.80) [6] |
| Transformed Estradiol at Time 1 (ln) | N/A | −0.12 ± 0.53 (−1.35 – 0.72) [3] |
| Transformed Estradiol at Time 2 (ln) | N/A | −0.02 ± 0.54 (−1.43 – 0.8) [3] |
| Change in Transformed Estradiol (ln/year) | N/A | 0.01 ± 0.45 (−1.56 – 0.9) [6] |
| Testosterone at Time 1 (pg/mL) | 70.44 ± 55.33 (18.29–300.16) | 51.92 ± 19.67 (16.27–112.56) |
| Testosterone at Time 2 (pg/mL) | 130.02 ± 71.15 (18.29–300.16) | 57.68 ± 28.25 (20.92–147.09) |
| Change in Testosterone (pg/mL/year) |
59.58 ± 71.54 (−103.71–299.51) | 5.76 ± 27.4 (−66.47–92.49) |
| Log Transformed Testosterone at Time 1 (ln) | 4.07 ± 0.58 (2.91–5.7) | 3.88 ± 0.38 (2.79–4.72) |
| Log Transformed Testosterone at Time 2 (ln) | 4.72 ± 0.58 (3.04–5.84) | 3.95 ± 0.44 (3.04–4.99) |
| Change in Transformed Testosterone (ln/year) | 0.66 ± 0.54 (−0.42 – 2.08) | 0.07 ± 0.44 (0.96–1.12) |
2.2. Pubertal stage
In order to match males and females based on pubertal stage at Time 1, we measured pubertal development using self-report Tanner staging (Marshall and Tanner, 1968, 1970; Marshall and Tanner, 1969,Morris and Udry, 1980). Participants reported their developmental stage by selecting how closely their pubic hair and breast/testes resembled an array of schematic drawings on a scale of 1 (prepubertal) to 5 (postpubertal). Self-report Tanner staging scores have been found to be correlated with physicians’ physical examinations of pubertal development (Coleman and Coleman, 2002; Shirtcliff et al., 2009). For the purposes of this study, we used the average of the pubic hair and breast/testes Tanner scores to index overall pubertal development (Colich et al., 2017; King et al., 2017a).
2.3. Gonadal hormones
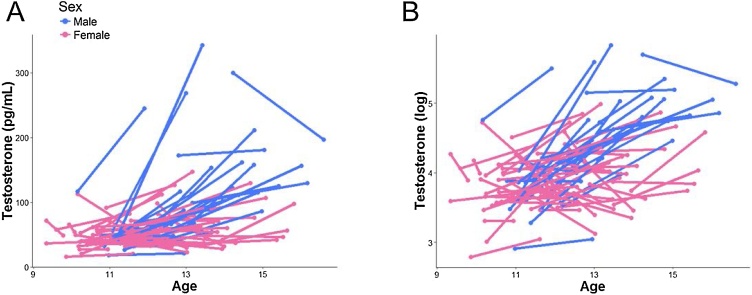
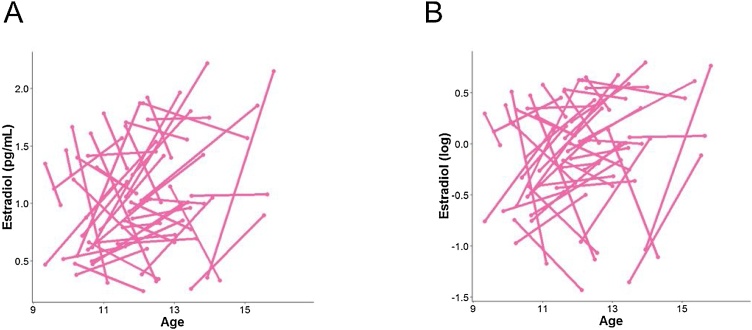
Participants were asked to provide a saliva sample through passive drool at home on the morning of their scan date (prior to eating breakfast or brushing teeth). Salivary hormonal assays were conducted for estradiol (for females only) and testosterone (for all participants). Participants recorded collection time and placed the saliva samples in their home freezer after collection. After participants returned the samples to the laboratory, samples were transferred to a −20 °C freezer in the Psychology Department at Stanford University. The samples were then shipped on dry ice to Salimetrics, LLC (State College, PA), where they were assayed for salivary testosterone using a high sensitivity enzyme immunoassay (Cat. No. 1-2312). The assay for testosterone used 25 μl of saliva per determination and had a lower limit sensitivity of 1 pg/mL and a standard curve range from 6.1 pg/mL to 600 pg/mL. Intra-assay coefficients of variation for testosterone were 2.5 % and 6.7 % at 188.83 and 18.12 pg/mL, respectively, and inter-assay coefficients of variance were 5.6 % and 14.1 % at 199.08 and 19.60 pg/mL. Samples from females were also assayed for salivary estradiol using a high sensitivity enzyme immunoassay (Cat. No. 1-3702). The assay for estradiol used 100 μl of saliva per determination and had a lower limit sensitivity of 0.1 pg/mL and a standard curve range from 1 pg/mL to 32 pg/mL. Intra-assay coefficients of variation were 7.0 %, 6.3 % and 8.1 % at 20.26 pg/mL, 7.24 pg/mL and 3.81 pg/mL, respectively, and interassay coefficients of variance were 6.0 % and 8.9 % at 24.62 pg/mL and 4.76 pg/mL. Acceptance criteria (per Salimetrics) for duplicate hormone results was a coefficient of variation <15 % between samples 1 and 2; data not meeting these quality control thresholds were excluded from final analysis. Further, any data exceeding 3 SD from the sample mean were winsorized (King et al., 2017a). Because neither testosterone nor estradiol levels were normally distributed, all values were log-transformed (natural log) for all analyses. No females reported using any form of hormonal contraceptives at Time 1 or Time 2. See Fig. 2, Fig. 3 for a visualization of the hormonal data (raw and log transformed) across all participants.
Fig. 2.
Distribution of raw testosterone levels (A) and log transformed (B) by sex across timepoints.
Fig. 3.
Distribution of raw estradiol levels (A) and log transformed (B) in females across timepoints.
2.4. MRI scanning acquisition
All participants were MRI scanned by a 3 T Discovery MR750 (GE Medical Systems, Milwaukee, WI) with a 32-channel head coil (Nova Medical) at the Stanford Center for Cognitive and Neurobiological Imaging. A high-resolution T1-weighted anatomical scan was acquired using an SPGR sequence (TR/TE/TI = 6.24/2.34/450 ms; flip angle = 12°; 186 sagittal slices; 0.9 mm isotropic voxels). Diffusion-weighted imaging was acquired using an EPI sequence (TR/TE = 8500/93.5 ms; 64 axial slices; 2 mm isotropic voxels; 60 b = 2000 diffusion-weighted directions, and 6 b = 0 acquisitions at the beginning of the scan; A/P phase encoding direction).
2.5. Diffusion MRI preprocessing
As described previously (Ho et al., 2017), diffusion-weighted MRI data were processed using the open-source mrVista software distribution developed by the VISTA lab (Stanford Vision and Imaging Science and Technology): http://vistalab.stanford.edu/software. Briefly, after the T1-weighted images were manually aligned to the anterior commissure-posterior commissure (AC-PC) line, the mean of the non-diffusion-weighted (b = 0) images was aligned to the T1 image using a rigid body 3D motion correction (6 parameters) with a constrained non-linear warping (8 parameters, which consisted of a spherical harmonics series expansion in Cartesian coordinates, up to quadratic terms) based on a model of the expected eddy-current distortions. Each registration consisted of estimating the 14 eddy-current/motion correction parameters simultaneously and optimization was performed using a gradient-ascent-type technique within a multi-resolution framework. Initial estimates of the registration parameters were obtained using low-resolution approximations of the images and these estimates were then used to initialize the optimization using higher-resolution representations of the data. The eddy-current and motion corrected diffusion-weighted data were then resampled to 2 mm isotropic voxels using a trilinear interpolation algorithm based on SPM5 (Friston and Ashburner, 2004). Since these data were rotated from their original orientation by these alignment and motion correction steps, the diffusion-weighted directions (bvecs matrix) were adjusted appropriately by combining the rotation matrix from the alignment step with the rotation matrix from the rigid-body component of the Rohde transform and applying it to the bvecs matrix before computing the tensors (Leemans and Jones, 2009). Excessive motion was censored by excluding data that were translated greater than 3 mm or rotated over 1.5° from one diffusion direction acquisition to the next (Ho et al., 2017; Leong et al., 2016, 2018). Participants with excessive movement in more than 15 directions (25% of all directions) were excluded from final analysis. Diffusion tensors were fitted to the resampled data using a least squares fit.
2.6. Tractography and white matter segmentation using automated Fiber quantification
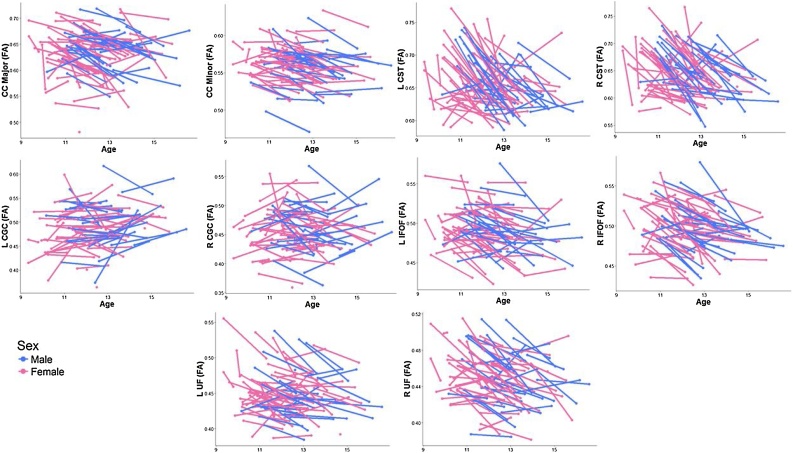
As described in previous work (Ho et al., 2017), whole-brain fiber tracking was performed on AC-PC aligned tensor maps using Automated Fiber Quantification (AFQ), which is an automated tractography tool that has been validated in adolescents (Yeatman et al., 2012; https://github.com/jyeatman/AFQ). Whole-brain fiber tracts were mapped onto the ACPC-aligned T1-weighted image using an established deterministic algorithm with a fourth-order Rungeñ-Kutta path integration method and 1 mm fixed-step size (Yeatman et al., 2012; Ho et al., 2017). A continuous tensor field was estimated with trilinear interpolation of the tensor elements. The seeds for tractography were selected from a uniform 1 mm 3D grid spanning the whole brain mask for voxels with FA > 0.3. Path tracing proceeded until FA fell below 0.15 or until the minimum angle between the current and previous path segments was greater than 30°. Streamlines for each of the five (four primary and one control) bilateral tracts of interest were automatically generated using a two planar waypoint region of interest (ROI) approach (see Yeatman et al., 2012 for more details). Each respective tract was built from two ROIs; the tracts were then traced along these seeds according to probabilistic fiber groupings based on an established white matter atlas; these candidate fibers were then assessed based on their similarity to the standard probability map (Wakana et al., 2007; Mori et al., 2002). Outliers were defined along a Gaussian curve as anything more than 4 standard deviations away from the spatial core of the tract and excluded until there were no remaining outlier volumes (Ho et al., 2017). All tracts at each time point were visually assessed and checked for consistency. Errant streamline fibers whose tract profiles differed significantly from established white matter anatomy were manually corrected. Tracts that were too thin or misshapen beyond the scope of this manual correction were excluded. FA, as well as other diffusivity metrics including mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), was calculated along each fiber group by assessing the value at each of the 100 nodes along the tract. In the present study, a single diffusivity metric per white matter tract was computed by averaging across the 100 nodes. Diffusivity metrics that exceeded 3 SD from the group mean (computed from all participants who provided usable DTI data for a given tract) were further omitted. See Fig. 4 for boxplots of FA from each tract by timepoint for the present sample and Figure S1 for visualizations of each tract from a representative participant.
Fig. 4.
Distribution of FA for each tract of interest by sex across timepoints. See Figure S3 for visualization of the group effect.
2.7. Statistical analyses
All statistical analyses were conducted using R version 3.3 (https://www.r-project.org/) using RStudio (RStudio Team, 2015). Repeated measures ANOVA, t-tests, and χ2 tests, where appropriate, were conducted to examine sex differences in demographic and diffusivity variables.
Fixed effect linear regression models were used to test for significant interaction effects between sex and (log transformed) testosterone levels between Time 1 and Time 2 on changes in FA of each of the 10 WM tracts (in separate models). We chose fixed effects modeling over mixed effects modeling because our study goal was to examine how changes in hormones relate to changes in FA, which, within a mixed effects framework, would necessitate a single model that allowed for random intercepts/slopes and, critically, for the correlation between these two change processes. While predictors of change can be time-varying within a linear mixed effects framework and, in principle, such a modeling approach is possible with our data given that we have individual variation in age and intervals between timepoints (Singer and Willett, 2003), because we only have two timepoints of data, the identifiability of such models relies more strongly on assumptions about the change across age than it does on the actual data. In addition, we would have to impose several model restrictions, such as fixing residual variances to be equal across timepoints and limiting the number of parameters—and thus, variables—in the model. For instance, simply using a mixed effects model to test whether changes in testosterone are associated with changes in FA already yields six free parameters (global mean, testosterone, age, random intercept, random slope, and residual error) and this is without including the moderating effect of sex—which is the primary goal of our study—as well as other important covariates (e.g., motion during scan, time of saliva collection).
The covariates in our fixed effects models included age at Time 1, testosterone levels at Time 1, FA of the tract at Time 1, motion (mean of Time 1 and Time 2), time of saliva collection (mean of Time 1 and Time 2), and tract length as covariates. Because individuals had different intervals between timepoints, all longitudinal changes in FA (i.e., values at Time 2 – values at Time 1) and (log transformed) hormones (i.e., ln(values at Time 2) – ln(values at Time1)) were scaled by the time interval between assessments (i.e., number of years between Time 1 and Time 2). Significance was determined using false discovery rate (FDR) correction across the 10 tracts of interest for all models testing the interactive effect of sex and changes in testosterone and for the post-hoc follow-up analyses examining the associations between changes in testosterone and changes in FA in females.
In females only, we also explored whether changes in estradiol between Time 1 and Time 2 were significantly associated with changes in FA for each of the 10 WM tracts, using the same covariates as in our models (except including estradiol levels at Time 1 instead of testosterone levels at Time 1). For tracts that did exhibit significant interactive effects with changes in estradiol, we also ran additional analyses where we included changes in both testosterone and estradiol (and their levels at Time 1) as predictors of changes in FA in the same model.
Finally, although FA is the most commonly examined DTI metric, given that other studies have examined other diffusivity metrics in the context of adolescent neurodevelopment and puberty (Asato et al., 2010; Herting et al., 2012; 2017; Peper et al., 2015; Menzies et al., 2015; Wang et al., 2012), we explored whether changes in testosterone (and in females, changes in estradiol) was associated with changes in MD, RD, and AD in each tract.
2.8. Sensitivity analyses
To demonstrate that our results are specific to the biological effects of gonadal hormones and not to broad changes in pubertal development and/or to self-perceived puberty-related changes, we also reran all statistical models that yielded a significant interaction effect using changes in Tanner stage as a covariate. We also reran all our models covarying for corticosteroid usage, removing the two participants with intervals between Time 1 and Time 2 that were shorter than 6 months, and excluding any individuals with negative (raw) hormone change scores. Running these analyses did not change the significance of the majority of our findings. See “Sensitivity Analyses” in the Supplement for more details.
2.9. Supplemental analyses
To replicate prior studies (Peper et al., 2015; Herting et al., 2012; Menzies et al., 2015), we tested whether testosterone levels were associated with FA at Time 1 in both males and females (separately), whether sex moderated any of these associations, and whether there was a main effect of testosterone on FA at Time 1. We also tested whether estradiol levels were associated with FA at Time 1 in females. Please see “Cross-Sectional Associations between Gonadal Hormones and FA” in the Supplement for more details.
3. Results
3.1. Sex differences in demographic, hormonal, and diffusivity variables
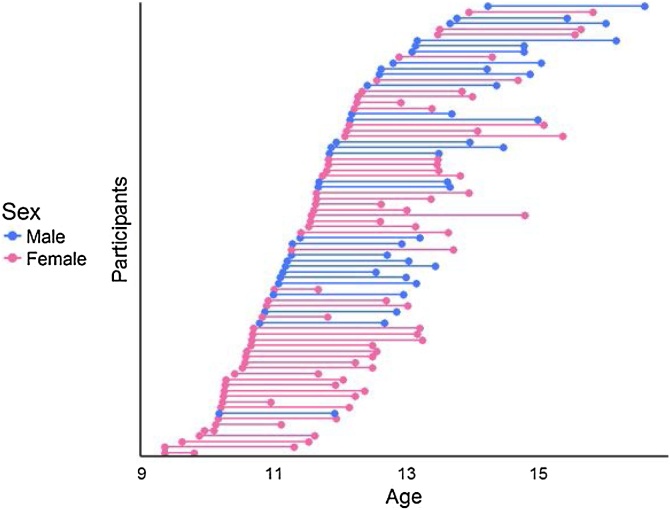
As we noted above, 80 participants (29 males) provided usable hormone data and DTI data for both Times 1 and 2. By design, males and females did not differ in self-reported pubertal staging at Times 1 or 2, but did differ significantly in age (see Fig. 1). Males and females did not differ in testosterone levels at Time 1 but, as expected, differed significantly in testosterone levels at Time 2 and in the change in testosterone levels between timepoints (see Fig. 2). Males and females also did not differ in mean FA for the majority of the tracts at either Time 1 or Time 2, with the exception of the left CGC and right CST at Time 1 only, for which females exhibited a trend for smaller FA and significantly larger FA, respectively (see Fig. 4). Males and females did not differ in other covariates of interest including time of saliva collection (p = 0.38) and motion (p = 0.29). Males and females did not differ in tract length for all tracts (all ps>0.1) except R IFOF at Time 2, where males exhibited longer tracts (p = 0.02). See Table 1, Table 2 for more details. See Figure S2 in the Supplement for an overview of the zero-order correlations among age, hormones, diffusivity metrics, time of saliva collection, and motion during the scan at each and between timepoints.
Fig. 1.
Age distribution by sex. The interval between Time 1 and Time 2 for each participant is represented by a connected line.
Table 2.
Descriptive statistics of DTI data by sex. All change values reported were scaled by interval. Motion is the average of translation across x, y, z dimensions (in mm) and pitch, roll, and yaw (in degrees) across all non-outlier directions; negative values refer to displacement in the leftward direction for x, the posterior direction for y, the inferior direction for z, leftward tilt for pitch, counterclockwise rotation for roll, and downward tilt for yaw. ICC values were computed based off of all participants who provided usable DTI data. Numbers in brackets indicate the number of participants who did not have tracts adequately resolve or were outliers.
| Males (M ± SD, range) [# missing] | Females (M ± SD, range) [# missing] | |
|---|---|---|
| Motion at Time 1 | −0.25 ± 0.56 (−1.87 – 0.41) | −0.30 ± 0.57 (−2.14 – 0.66) |
| Motion at Time 2 | −0.05 ± 0.41 (−1.18 – 0.56) | −0.18 ± 0.47 (−2.32 – 0.49) |
| CC Major Tract Length at Time 1 (mm) |
7260.59 ± 3934.30 (1503.00–20847.00) | 6862.99 ± 3110.91(1503–14564.00) |
| CC Major Tract Length at Time 2 (mm) |
8133.73 ± 5380.28 (3064.40–21775.00) |
7544.03 ± 4798.33(2686.7–29362.00) [1] |
| CC Major FA at Time 1 (mm) |
0.65 ± 0.03 (0.59 – 0.72) | 0.64 ± 0.05 (0.48 – 0.72) |
| CC Major FA at Time 2 (mm) |
0.63 ± 0.04 (0.55 – 0.70) | 0.62 ± 0.04 (0.53 – 0.70) [1] |
| CC Major FA Change (mm/year) |
−0.01 ± 0.01 (−0.03 – 0.02) | −0.01 ± 0.03 (−0.13 – 0.1) [1] |
| CC Major FA ICC | 0.766 | 0.709 |
| CC Minor Tract Length at Time 1 (mm) | 5559.82 ± 3242.67 (1737.60–14832.00) |
4979.91 ± 2847.41(1233.00–14596.00) |
| CC Minor Tract Length at Time 2 (mm) | 4380.64 ± 4113.12 (995.89–21482.00) |
4088.80 ± 2365.96(1227.60–11338.00) |
| CC Minor FA at Time 1 | 0.56 ± 0.03 (0.50 – 0.62) | 0.57 ± 0.02 (0.52 – 0.63) |
| CC Minor FA at Time 2 | 0.55 ± 0.03 (0.47 – 0.59) | 0.56 ± 0.02 (0.51 – 0.61) |
| CC Minor FA Change (FA/year) | −0.01 ± 0.01 (−0.03 – 0.01) | −0.01 ± 0.02 (−0.08 – 0.01) |
| CC Minor FA ICC | 0.878 | 0.832 |
| L CGC Tract Length at Time 1 (mm) | 4928.53 ± 3688.88 (1493.80–15169.00) |
4363.01 ± 2614.94(1393.50–17185.00) [3] |
| L CGC Tract Length at Time 2 (mm) | 3673.77 ± 1799.68 (1404.70–9363.20) |
3952.86 ± 2517.83(1484.10–14946.00) |
| L CGC FA at Time 1 | 0.49 ± 0.05 (0.37 – 0.62) | 0.49 ± 0.04 (0.38 – 0.60) [3] |
| L CGC FA at Time 2 | 0.50 ± 0.04 (0.41 – 0.59) | 0.48 ± 0.04 (0.36 – 0.58) |
| L CGC FA Change (FA/year) | 0 ± 0.02 (−0.05 – 0.08) | 0 ± 0.02 (−0.09 – 0.06) [3] |
| L CGC FA ICC | 0.794 | 0.808 |
| R CGC Tract Length at Time 1 (mm) | 3846.29 ± 2801.03(1190.8 –15899.00) [1] |
3236.92 ± 1937.37(1376.20 –12995.00) [2] |
| R CGC Tract Length at Time 2 (mm) | 3622.68 ± 2941.97 (792.76–15076.00) |
3185.05 ± 1881.30(896.94–9721.00) [1] |
| R CGC FA at Time 1 | 0.47 ± 0.05 (0.38 – 0.57) [1] | 0.46 ± 0.04 (0.38 – 0.55) [2] |
| R CGC FA at Time 2 | 0.46 ± 0.04 (0.36 – 0.55) | 0.45 ± 0.04 (0.36 – 0.54) [1] |
| R CGC FA Change (FA/year) | 0 ± 0.02 (−0.04 – 0.02) [1] | −0.01 ± 0.03 (−0.15 – 0.07) [3] |
| R CGC FA ICC | 0.791 | 0.753 |
| L CST Tract Length at Time 1 (mm) | 3525.02 ± 2180.66 (1602.80–11567.00) |
2944.4 ± 2181.38 (1467.00–16473.00) [1] |
| L CST Tract Length at Time 2 (mm) | 2693.47 ± 1103.89 (1150.20–5449.40) |
2611.05 ± 1853.27 (1140.00–13198.00) [1] |
| L CST FA at Time 1 | 0.68 ± 0.04 (0.61 – 0.74) | 0.67 ± 0.04 (0.59 – 0.77) [1] |
| L CST FA at Time 2 | 0.63 ± 0.02 (0.59 – 0.70) | 0.64 ± 0.03 (0.59 – 0.73) [1] |
| L CST FA Change (FA/year) | −0.02 ± 0.02 (−0.06 – 0.05) | −0.02 ± 0.04 (−0.17 – 0.06) [2] |
| L CST FA ICC | 0.281 | 0.262 |
| R CST Tract Length at Time 1 (mm) | 1684.67 ± 949.80 (755.31–4178.30) |
1700.05 ± 674.31 (592.17–3258.60) [1] |
| R CST Tract Length at Time 2 (mm) | 2702.29 ± 5089.14 (664.57–27899.00) |
1748.62 ± 788.43 (682.88–4116.3) [1] |
| R CST FA at Time 1 | 0.67 ± 0.04 (0.60 – 0.73) | 0.66 ± 0.04 (0.58 – 0.77) [1] |
| R CST FA at Time 2 | 0.62 ± 0.03 (0.55 – 0.70) | 0.63 ± 0.04 (0.57 – 0.74) [1] |
| R CST FA Change (FA/year) | −0.03 ± 0.02 (−0.07– −0.05) | −0.03 ± 0.06 (−0.24 – 0.11) [2] |
| L CST FA ICC | 0.313 | 0.218 |
| L IFOF Tract Length at Time 1 (mm) | 7281.90 ± 3271.67 (2295.4–13068.00) |
6195.69 ± 2729.11 (2597.50–15686.00) |
| L IFOF Tract Length at Time 2 (mm) | 6149.32 ± 3611.85 (2515.70–15931.00) |
6936.38 ± 6086.30 (2013.30–36239.00) |
| L IFOF FA at Time 1 | 0.50 ± 0.03 (0.45 – 0.58) | 0.50 ± 0.03 (0.43 – 0.56) [1] |
| L IFOF FA at Time 2 | 0.48 ± 0.02 (0.44 – 0.53) | 0.49 ± 0.03 (0.42 – 0.55) |
| L IFOF FA Change (FA/year) | −0.01 ± 0.01 (−0.03 – 0.02) | −0.01 ± 0.02 (−0.09 – 0.02) [1] |
| L IFOF FA CC | 0.742 | 0.787 |
| R IFOF Tract Length at Time 1 (mm) | 4188.4 ± 2118.76 (1949.60–11720.00) |
4499.06 ± 2559.66(1750.20–12205.00) [1] |
| R IFOF Tract Length at Time 2 (mm) | 4757.27 ± 5329.21 (1833.33–26274.00) |
2886.18 ± 933.55(1562.90–5659.4) |
| R IFOF FA at Time 1 | 0.51 ± 0.03 (0.47 – 0.58) | 0.51 ± 0.03 (0.44 – 0.57) [1] |
| R IFOF FA at Time 2 | 0.49 ± 0.03 (0.43 – 0.53) | 0.49 ± 0.03 (0.43 – 0.55) |
| R IFOF FA Change (FA/year) | −0.01 ± 0.01 (−0.04 – 0.01) | −0.01 ± 0.02 (−0.12 – 0.04) [1] |
| R IFOF FA ICC | 0.802 | 0.817 |
| L UF Tract Length at Time 1 (mm) | 14346.56 ± 55495.79 (1403.70–302810.00) |
3914.91 ± 1984.77(780.98–9607.00) [1] |
| L UF Tract Length at Time 2 (mm) | 3663.3 ± 2556.06 (1370.50–10637.00) |
3593.40 ± 1872.59(925.19–9508.6) |
| L UF FA at Time 1 | 0.45 ± 0.03 (0.41 – 0.54) | 0.46 ± 0.03 (0.39 – 0.56) [1] |
| L UF FA at Time 2 | 0.44 ± 0.03 (0.38 – 0.48) | 0.44 ± 0.03 (0.39 – 0.51) |
| L UF FA Change (FA/year) | −0.01 ± 0.03 (−0.17 – 0.02) | −0.01 ± 0.02 (−0.1 – 0.02) [1] |
| L UF FA ICC | 0.525 | 0.816 |
| R UF Tract Length at Time 1 | 2915.81 ± 1889.54 (1267.90–10555.00) |
2569.18 ± 1887.93(789.63–10393.00) |
| R UF Tract Length at Time 2 | 2537.53 ± 1656.42 (556.03–7101.70) |
2198.94 ± 1696.88(789.63–10393.00) |
| R UF FA at Time 1 | 0.46 ± 0.03 (0.39 – 0.51) | 0.46 ± 0.03 (0.39 – 0.51) [1] |
| R UF FA at Time 2 | 0.44 ± 0.03 (0.38 – 0.49) | 0.44 ± 0.03 (0.38 – 0.50) |
| R UF FA Change (FA/year) | −0.01 ± 0.01 (−0.03 – 0.03) | −0.01 ± 0.02 (−0.08 – 0.02) [1] |
| R UF FA ICC | 0.832 | 0.868 |
3.2. Increases in gonadal hormones are associated with increases in FA in females only
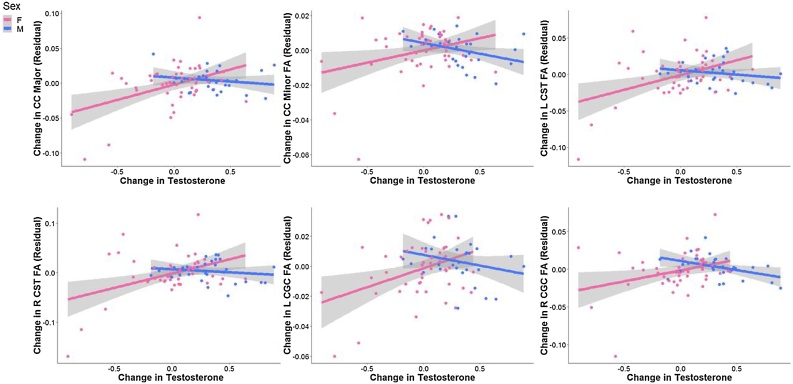
We tested whether sex moderated associations between rates of change in testosterone levels with rates of change in FA. The interaction effect was significant for CC Major, CC Minor, L CGC, R CGC, L CST, R CST (all ps<0.033, FDR-corrected). Simple slopes analyses revealed that in females only, increases in testosterone were significantly associated with increases in FA (all ps<0.05, FDR-corrected); in contrast, in males, changes in testosterone were not associated significantly with changes in FA in any of these tracts (all ps>0.27, uncorrected). See Fig. 5 and Table 3 for more details. Almost all effects that were significant remained significant after including changes in Tanner as a covariate. See “Sensitivity Analyses” in the Supplement for more details.
Fig. 5.
Tracts exhibiting significant interaction effects of sex and changes in testosterone in predicting changes in FA. Data and trends plotted include adjustment of covariates (Time 1 levels of testosterone, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length) on FA (i.e., regressing out the effects of these covariates). Change in testosterone refers to the difference in log (ln) transformed testosterone values scaled by the interval between timepoints. See Table 3 for more details.
Table 3.
Summary of estimates from modeling the interactive effects of sex and change in testosterone on change in FA. All models include Time 1 levels of testosterone, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length as covariates. We also report the simple slopes for males and females for any tracts exhibiting significant interaction effects (at uncorrected significance values), as well as partial values for any significant interaction effects and effects of testosterone in females, and the adjusted R2 for the full models. See Fig. 5 for more details. *indicates significance at p < 0.05; **indicates significance at p < 0.01.
| Tract | β±SE | Statistic | Significance (uncorrected) | Significance (FDR-corrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|---|
| CC Major | Interaction: -0.46 ± 0.02Males: -0.29 ± 0.01Females: 0.42 ± 0.02 |
Interaction: t(69)=-2.62 Males: t(21)=-0.13 Females: t(42) = 3.00 |
Interaction: p = 0.01*Males: p = 0.75Females: p = 0.005** |
Interaction: p = 0.026*Females: p = 0.029* |
Interaction: R2 = 0.09Females: R2 = 0.12 |
All: R2 = 0.25Males: R2 = 0.08Females: R2 = 0.20 |
| CC Minor | Interaction: -0.50 ± 0.01Males: -0.18 ± 0.01Females: 0.30 ± 0.01 |
Interaction: t(70)=-2.56 Males: t(21)=-0.86 Females: t(43) = 2.07 |
Interaction: p = 0.02*Males: p = 0.40Females: p = 0.04* |
Interaction: p = 0.033*Females: p = 0.049* |
Interaction: R2 = 0.09Females: R2 = 0.02 |
All: R2 = 0.18Males: R2 = 0.07Females: R2 = 0.12 |
| L CGC | Interaction: -0.42 ± 0.01Males: -0.12 ± 0.01Females: 0.32 ± 0.01 |
Interaction: t(67)=-2.65 Males: t(21)=-0.77 Females: t(40) = 2.36 |
Interaction: p = 0.01* Males: p=0.45 Females: p = 0.02* |
Interaction: p = 0.025*Females: p = 0.043* |
Interaction: R2 = 0.09Females: R2 = 0.06 |
All: R2 = 0.42 Males: R2 = 0.49Females: R2 = 0.38 |
| R CGC | Interaction: -0.52 ± 0.02Males: -0.21 ± 0.01Females: 0.28 ± 0.02 |
Interaction: t(66)=-2.78 Males: t(20)=-1.03 Females: t(40) = 2.03 |
Interaction: p = 0.007**Males: p = 0.31Females: p = 0.049* |
Interaction: p = 0.02*Females: p = 0.05 |
Interaction: R2 = 0.10Females: R2 = 0.09 |
All: R2 = 0.26Males: R2 = 0.23Females: R2 = 0.27 |
| L CST | Interaction: -0.33 ± 0.03Males: -0.34 ± 0.01Females: 0.31 ± 0.02 |
Interaction: t(68)=-2.42 Males: t(21)=-0.25 Females: t(41) = 2.72 |
Interaction: p = 0.02*Males: p = 0.80Females: p = 0.009** |
Interaction: p = 0.03*Females: p = 0.03* |
Interaction: R2 = 0.08Females: R2 = 0.15 |
All: R2 = 0.56Males: R2 = 0.61Females: R2 = 0.55 |
| R CST | Interaction: -0.35 ± 0.03Males: -0.05 ± 0.02Females: 0.36 ± 0.02 |
Interaction: t(68)=-2.33 Males: t(21)=-0.29 Females: t(41) = 2.73 |
Interaction: p = 0.02*Males: p = 0.77Females: p = 0.009** |
Interaction: p = 0.03*Females: p = 0.03* |
Interaction: R2 = 0.08Females: R2 = 0.10 |
All: R2 = 0.45Males: R2 = 0.36Females: R2 = 0.41 |
| L IFOF | Interaction: -0.23 ± 0.01 | Interaction: t(69)=-1.39 | Interaction: p = 0.17 | |||
| R IFOF | Interaction: -0.26 ± 0.02 | Interaction: t(69)=-1.42 | Interaction: p = 0.15 | |||
| L UF | Interaction: -0.19 ± 0.02 | Interaction: t(69)=-0.98 | Interaction: p = 0.32 | |||
| R UF | Interaction: -0.25 ± 0.01 | Interaction: t(69)=-1.47 | Interaction: p = 0.15 |
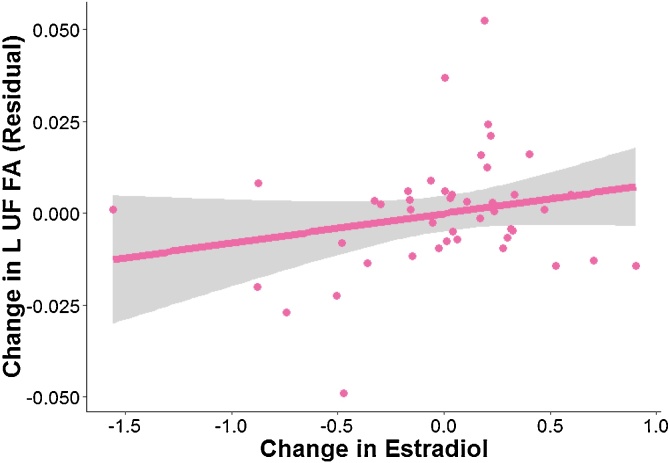
In females only, we found that changes in estradiol were positively associated with changes in FA in L UF (p = 0.04, uncorrected). See Fig. 6 for more details and Table 4A for more details. This association was no longer significant when we included changes in Tanner as a covariate or when we included changes in testosterone as a covariate. See Table 4B, Table 4C for more details.
Fig. 6.
Tract exhibiting significant associations between changes in estradiol and changes in FA in females only. Data and trends plotted include adjustment of covariates (Time 1 levels of testosterone, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length) on FA (i.e., regressing out the effects of these covariates). Change in estradiol refers to the difference in log (ln) transformed estradiol values scaled by the interval between timepoints. See Tables 4A, 4B, 4C for more details.
Table 4A.
Summary of estimates from modeling the effects of change in estradiol on change in FA in females only. All models include Time 1 levels of estradiol, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length as covariates. We report partial and adjusted R2 values for models yielding significant effects of estradiol. See Fig. 6 for more details. *indicates significance at p < 0.05.
| Tract | β±SE | Statistic | Significance (uncorrected) | Significance (FDR-corrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|---|
| CC Major | 0.21 ± 0.02 | t(36) = 0.99 | p = 0.33 | p = 0.74 | ||
| CC Minor | 0.09 ± 0.01 | t(37) = 0.50 | p = 0.62 | p = 0.78 | ||
| L CGC | 0.12 ± 0.01 | t(36) = 0.65 | p = 0.52 | p = 0.74 | ||
| R CGC | 0.04 ± 0.03 | t(36) = 0.23 | p = 0.82 | p = 0.91 | ||
| L CST | 0.18 ± 0.02 | t(37) = 1.05 | p = 0.30 | p = 0.74 | ||
| R CST | 0.23 ± 0.02 | t(37) = 1.24 | p = 0.22 | p = 0.74 | ||
| L IFOF | 0.02 ± 0.01 | t(37) = 0.93 | p = 0.93 | p = 0.93 | ||
| R IFOF | 0.14 ± 0.01 | t(37) = 0.67 | p = 0.50 | p = 0.74 | ||
| L UF | 0.38 ± 0.01 | t(37) = 2.10 | p = 0.04* | p = 0.40 | R2 = 0.10 | R2 = 0.06 |
| R UF | 0.15 ± 0.01 | t(37) = 0.73 | p = 0.47 | p = 0.74 |
Table 4B.
Summary of estimates when modeling the effects of change in estradiol and change in Tanner on change in FA in L UF in females only. All models include Time 1 levels of estradiol, Time 1 Tanner score, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length as covariates. *indicates significance at p < 0.05.
| Variable | β±SE | Statistic | Significance (uncorrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|
| Change in Estradiol | 0.04 ± 0.01 | t(36) = 1.87 | p = 0.07 | R2 = 0.04 | R2 = 0.03 |
| Change in Tanner | 0.01 ± 0.01 | t(36)=−0.64 | p = 0.53 | R2 = 0.04 | R2 = 0.03 |
Table 4C.
Summary of estimates when modeling the effects of change in estradiol and change in testosterone on change in FA in L UF in females only. All models include Time 1 levels of estradiol, Time 1 levels of testosterone, Time 1 age, FA of tract at Time 1, time of saliva collection, motion during scan, and tract length as covariates. *indicates significance at p < 0.05.
| Variable | β±SE | Statistic | Significance (uncorrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|
| Change in Estradiol | 0.27 ± 0.01 | t(36) = 1.15 | p = 0.26 | R2 = 0.04 | R2 = 0.05 |
| Change in Testosterone | 0.28 ± 0.01 | t(36) = 1.26 | p = 0.22 | R2 = 0.04 | R2 = 0.05 |
3.3. Increases in testosterone are associated with changes in MD, RD, and AD in females only
We explored whether sex moderated associations between changes in testosterone and changes in MD. The interaction effect was significant for L CST (p = 0.023, uncorrected) and R CST (p = 0.039, uncorrected). Simple slopes analyses revealed that in females only, increases in testosterone were significantly associated with decreases in MD in L CST (p = 0.005, uncorrected) and in R CST (p = 0.013, uncorrected); in contrast, in males, changes in testosterone were not associated significantly with changes in MD (all ps>0.84, uncorrected).
We explored whether sex moderated associations between changes in testosterone and changes in RD. The interaction effect was significant for L CST (p = 0.02, uncorrected) and R CST (p = 0.03, uncorrected). Simple slopes analyses revealed that in females only, increases in testosterone were significantly associated with decreases in RD in L CST (p = 0.006, uncorrected) and in R CST (p = 0.01, uncorrected); in contrast, in males, change in testosterone were not associated significantly with change in RD (all ps>0.79).
We explored whether sex moderated associations between changes in testosterone and changes in AD. The interaction effect was significant for CC Minor (p = 0.02, uncorrected), L CGC (p = 0.009, uncorrected), and R CGC (p = 0.04, uncorrected). Simple slopes analyses revealed that in females only, increases in testosterone were significantly associated with increases in AD in CC Minor (p = 0.02, uncorrected), L CGC (p = 0.004, uncorrected), and R CFC (p = 0.008, uncorrected); in contrast, in males, change in testosterone were not associated significantly with change in AD (all ps>0.31). See Table 5 for a summary of significant (uncorrected) effects of changes in testosterone on changes in MD, RD, and AD.
Table 5.
Summary of estimates from modeling the effects of changes in testosterone on changes in MD, RD, and AD. All models include Time 1 levels of testosterone, Time 1 age, Time 1 diffusivity metric of tract, time of saliva collection, motion during scan, and tract length as covariates. We also report the simple slopes for males and females for any tracts exhibiting significant interaction effects (at uncorrected significance values), as well as partial values for any significant interaction effects and effects of testosterone in females, and the adjusted R2 for the full models. *indicates significance at p < 0.05; **indicates significance at p < 0.01.
| Tract | Diffusivity Metric | β±SE | Statistic | Significance (uncorrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|---|
| L CST | MD | Interaction: 0.29 ± 0.03Males: 0.15 ± 0.01Females: -0.33 ± 0.02 |
Interaction: t(68) = 2.32Males: t(21) = 0.14 Females: t(41)=-3.01 |
Interaction: p = 0.023*Males: p = 0.89Females: p = 0.005** |
Interaction: R2 = 0.07Females: R2 = 0.18 |
All: R2 = 0.62 Males: 0.75 Females: R2 = 0.60 |
| R CST | MD | Interaction: 0.28 ± 0.03Males: 0.22 ± 0.01Females: -0.30 ± 0.02 |
Interaction: t(68) = 2.10Males: t(21) = 0.21 Females: t(41)=-2.60 |
Interaction: p = 0.039*Males: p = 0.84Females: p = 0.013* |
Interaction: R2 = 0.06Females: R2 = 0.14 |
All: R2 = 0.59 Males: 0.76 Females: R2 = 0.56 |
| L CST | RD | Interaction: 0.31 ± 0.03Males: 0.03 ± 0.02Females: -0.32. ± 0.02 |
Interaction: t(68) = 2.45Males: t(21) = 0.25 Females: t(41)=-2.91 |
Interaction: p = 0.016*Males: p = 0.80Females: p = 0.006** |
Interaction: R2 = 0.08Females: R2 = 0.17 |
All: R2 = 0.61 Males: 0.74 Females: R2 = 0.59 |
| R CST | RD | Interaction: 0.44 ± 0.02Males: 0.03 ± 0.01Females: -0.34. ± 0.02 |
Interaction: t(68) = 2.21Males: t(21) = 0.26 Females: t(41)=-2.67 |
Interaction: p = 0.031*Males: p = 0.79Females: p = 0.01* |
Interaction: R2 = 0.07Females: R2 = 0.15 |
All: R2 = 0.52 Males: 0.65 Females: R2 = 0.49 |
| CC Minor | AD | Interaction: -0.38 ± 0.05Males: -0.19 ± 0.02Females: 0.38 ± 0.03 |
Interaction: t(70)=-2.10 Males: t(21)=-1.04 Females: t(43) = 2.99 |
Interaction: p = 0.018*Males: p = 0.31Females: p = 0.005** |
Interaction: R2 = 0.07Females: R2 = 0.15 |
All: R2 = 0.39Males: R2 = 0.39Females: R2 = 0.40 |
| L CGC | AD | Interaction: -0.41 ± 0.04Males: -0.33 ± 0.02Females: 0.47 ± 0.03 |
Interaction: t(67)=-2.68 Males: t(21)=-0.02 Females: t(40) = 3.85 |
Interaction: p = 0.009**Males: p = 0.98Females: p = 0.004** |
Interaction: R2 = 0.10Females: R2 = 0.27 |
All: R2 = 0.46Males: R2 = 0.53Females: R2 = 0.48 |
| R CGC | AD | Interaction: -0.34 ± 0.04Males: -0.62 ± 0.03Females: 0.35 ± 0.03 |
Interaction: t(66)=-2.15 Males: t(20)=-0.32 Females: t(40) = 2.80 |
Interaction: p = 0.035* Males: p = 0.75Females: p = 0.008** |
Interaction: R2 = 0.10Females: R2 = 0.16 |
All: R2 = 0.42Males: R2 = 0.36Females: R2 = 0.46 |
3.4. Increases in estradiol are associated with changes in AD in females
Finally, we explored whether changes in estradiol were associated with changes in MD, RD, and AD in all tracts of interest in females. We found that increases in estradiol were associated with increases in AD in L CGC (p = 0.042, uncorrected). When including changes in testosterone as a covariate, however, changes in estradiol were no longer significantly associated with changes in AD in L CGC (p = 0.30, uncorrected) whereas increases in testosterone remained significantly associated with increases in AD in L CGC (p = 0.005, uncorrected). There were no significant effects of change in estradiol on change in MD or change in RD for any of the tracts of interest in females. See Table 6A, Table 6Bfor details.
Table 6A.
Summary of estimates from modeling the effects of change in estradiol on change in AD in females only. All models include Time 1 levels of estradiol, Time 1 age, Time 1 diffusivity metric of tract, time of saliva collection, motion during scan, and tract length as covariates. *indicates significance at p < 0.05.
| Tract | Diffusivity Metric | β±SE | Statistic | Significance (uncorrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|---|
| L CGC | AD | 0.36 ± 0.03 | t(35) = 2.11 | p = 0.042* | R2 = 0.11 | R2 = 0.33 |
Table 6B.
Summary of estimates from modeling the effects of change in estradiol while accounting for change in testosterone on change in L CGC AD in females only. All models include Time 1 levels of estradiol, Time 1 levels of testosterone, Time 1 age, Time 1 diffusivity metric of tract, time of saliva collection, motion during scan, and tract length as covariates. **indicates significance at p < 0.01.
| Variable | β±SE | Statistic | Significance (uncorrected) | Partial R2 | Adjusted R2 |
|---|---|---|---|---|---|
| Change in estradiol | 0.13 ± 0.04 | t(33) = 0.70 | p = 0.49 | R2 = 0.01 | R2 = 0.45 |
| Change in testosterone | 0.46 ± 0.04 | t(33) = 2.84 | p = 0.008** | R2 = 0.20 | R2 = 0.45 |
4. Discussion
Despite the fact that adolescence is a period of notable WM development, due in part to puberty-related changes (e.g., increases in gonadal hormones), few studies have examined the effects of puberty on WM microstructure longitudinally. To address this gap, we examined associations between longitudinal changes in gonadal hormones and longitudinal changes in WM for several major tracts in a sample of adolescent males and females who were matched on pubertal stage. We found that sex moderates the associations between changes in testosterone levels and changes in WM microstructure, such that in females, increases in testosterone from early to mid-puberty were associated with increases in FA in the splenium and genu of the corpus callosum (CC Major and CC Minor, respectively), bilateral cingulum cingulate (CGC), and bilateral corticospinal tract (CST), whereas in males there were no significant associations between changes in testosterone and changes in FA in these tracts. Importantly, changes in testosterone explained changes in FA even after accounting for changes in Tanner staging. Additionally, changes in estradiol were associated with increases in FA in left UF; however, these effects of estradiol were no longer significant when controlling for changes in testosterone. Overall, our findings demonstrate that pubertal maturation, indexed by changes in levels of gonadal hormones, explains important variation in WM microstructure changes occurring during the pubertal transition in females. Secondly, we show that changes in testosterone specifically explain changes in WM microstructure above and beyond the effects of self-reported pubertal staging and chronological age.
Gonadal hormones—and in particular, testosterone—have been consistently reported to influence the characteristics of WM microstructure in the brain by acting on both neurons and supporting glial cells to support myelination (Garcia-Segura and Melcangi, 2006; Jordan and Williams, 2001; Leonelli and Ballabio, 2006). With respect to human neuroimaging data, Herting et al. showed in a cross-sectional study that testosterone was positively associated with FA in IFOF (as well as in subcortical tracts and in the superior temporal gyrus) in males, and positively associated with FA in a small WM region of the precentral gyrus in females (Herting et al., 2012). These findings stand in contrast to the results of the present study: we found that changes in testosterone significantly explained changes in WM microstructure in females, but did not in males. It is possible that our relatively small sample of 29 males is not sufficiently powered to detect these associations (Herting et al., 2012, studied 38 males); it is important to note, however, that in our supplemental cross-sectional analysis of 43 males at Time 1 we also did not detect any significant associations between testosterone and FA. Another possibility is that our sample, while ideally suited for examining the potential early emergence of sex differences during the pubertal transition, is too early in the pubertal process to adequately capture how greater increases in testosterone may affect development of WM microstructure in males. However, consistent with our findings, Menzies et al. (2015) also recently reported in a recent cross-sectional study of 61 adolescent males, over half of whom were in the later stages of puberty (Tanner stage > 4), that testosterone was not associated with FA in any regions.
Thus, a significant contribution of the present study is that we identified, in females, that increases in gonadal hormones were associated with increases in FA in the corpus callosum (CC Major and CC Minor), the cingulate (CGC), the cortical spinal tract (CST), and the uncinate fasciculus (UF). The CC Major, also known as the splenium, connects portions of the temporal and parietal cortex with occipital cortices, while the CC Minor, also known as the genu, connects the medial and frontal portions of frontal cortex. The CGC connects cingulate fibers along the dorsal portion of the corpus callosum, while the UF connects the amygdala and hippocampus with lateral and medial portions of frontal cortex. Finally, the CST connects motor and sensory areas with the brainstem. All of these WM tracts are involved in cognitive control, response inhibition, and emotion regulation (Keedwell et al., 2012; Silk et al., 2009; Yurgelun-Todd, 2007) and have been found to have altered FA in individuals with a variety of mental health conditions, including substance abuse and depression (Bava et al., 2009, 2010; Jacobus et al., 2013; LeWinn et al., 2014; Sacchet et al., 2014; Albaugh et al., 2020). Our sex-specific findings in adolescent females have important implications for understanding not only why adolescence is a period of increased risk for developing disorders characterized by disturbances in impulse control and emotion regulation, but also why there are robust sex differences in the rates of these, and other related, behaviors (Cyranowski et al., 2000; Steinberg, 2007). For instance, risk for depression in female adolescents has been found to be associated with higher levels of testosterone (Copeland et al., 2019) and with altered microstructure in several of the WM tracts in which we found positive associations between changes in testosterone and changes in FA in females, including the CC Major, CC Minor, and CGC (Aghajani et al., 2014; Bracht et al., 2015; Connolly et al., 2017 Heilbronner and Haber, 2014; Keedwell et al., 2012; LeWinn et al., 2014; Tromp et al., 2019). However, whereas we found that increases in testosterone were associated with increases in FA, these cross-sectional studies have reported associations between lower FA and higher levels of anxiety and depression, thus highlighting the need for researchers to examine longitudinal associations of cognitive and affective symptoms with testosterone and WM in order to understand the nuanced differences in these findings. Interestingly, we did not find main effects of testosterone (or interaction effects with sex) in our cross-sectional analyses of the data at Time 1 (i.e., earlier in puberty). One possibility is that testosterone is not strongly associated with WM microstructure during early puberty, particularly in a sample that was recruited so that males and females were matched on pubertal stage (and, presumably, testosterone levels) at this point in time. Alternatively, levels of testosterone (and estradiol) may not be reliably measured at a single point in time, in contrast to other puberty-related measures like Tanner staging. Despite fluctuations in measurement at a single point in time, changes in testosterone (and estradiol) from early to later puberty may be a more reliable and sensitive index in relation to accompanying WM changes. Clearly, it will be important for investigators to examine in larger samples whether testosterone has different sex-related effects on WM at certain stages of development, or only when considered longitudinally across development. Future research is also critically needed to examine the processes that contribute to any sex differences, and whether these sex differences also explain the development of behaviors that are supported by the brain circuits that are most sensitive to puberty-related changes.
We also explored whether changes in testosterone (and in estradiol for females) were associated with changes in other diffusivity metrics. We found that sex moderated the associations between changes in testosterone and changes in MD and RD in bilateral CST: whereas females exhibited significant negative associations, males exhibited no significant associations. Similarly, sex also moderated the associations between changes in testosterone and changes in AD in CC Minor and bilateral CGC: whereas females exhibited significant positive associations, males demonstrated no significant associations. Although increases in estradiol were associated with increases in AD in L CGC, these associations were no longer significant when accounting for changes in testosterone.
Because previous cross-sectional studies examining associations between hormones and diffusivity metrics focused on regions that first yielded significant age-, sex-, and/or puberty-related effects in FA (or MD), it is difficult to directly compare their results with ours. Compounding this difficulty, these studies have not yielded consistent results. For instance, Herting et al. (2012) reported that white matter regions in which testosterone was positively associated with FA were also related to lower RD and higher AD in males; further, the authors reported that higher levels of testosterone were associated with greater MD in males but not in females. Although Peper et al. (2015) also reported that higher levels of testosterone were associated with greater MD in males, they found this positive association in females as well; moreover, Peper et al. reported that in the regions in which there were positive associations between testosterone and MD, there were also positive associations between testosterone and RD in females, but not in males. In contrast to both of these studies, Menzies et al. (2015) reported that higher levels of testosterone were associated with lower MD in both males and females. With respect to associations between estradiol and other diffusivity metrics in females, Herting et al. (2012) found that regions in which estradiol levels were associated with lower FA also showed positive associations with RD but negative associations with AD. In contrast, Peper et al. (2015) did not find any associations between estradiol and RD and AD in females or males.
Although our longitudinal study is the first to investigate the association between changes in gonadal hormones and changes in WM microstructure in adolescents, several studies have examined the relation between self-reported pubertal stage and WM microstructure (Asato et al., 2010; Chahal et al., 2019; Herting et al., 2012). Consistent with our findings, in a recent longitudinal study of 107 young adolescent females, Chahal and colleagues reported that females with more advanced self-reported pubertal status specific to gonadal-related physical changes (e.g., breast development) at age 9 had higher FA at age 19 in tracts related to cognitive control, responsive inhibition, and emotion regulation, including the UF (Chahal et al., 2019). In the only other longitudinal study in this area with measurements of puberty (via self-report) and FA at two timepoints, Herting et al. found in 18 adolescent males and 15 adolescent females that self-reported pubertal stage was associated with FA of the thalamus, precentral gyrus, superior corona radiata, CC Major, superior corona radiata, and superior frontal gyrus (Herting et al., 2017). Specifically, Herting and colleagues found that more advanced gonadal (e.g., testes) and adrenal (e.g., pubic hair) development were related to higher FA in the superior frontal gyrus and precentral gyrus in boys, whereas more advanced gonadal (e.g., breast) development was related to lower FA in the anterior corona radiata in girls (Herting et al., 2017). One explanation for the apparent inconsistency between the results of that study and our current findings involves the use of different measures of puberty (subjective measures of pubertal stage versus objective measurements of gonadal hormones). It is worth noting, however, that the statistical models used in the study by Herting et al. did not explicitly examine whether individual change in pubertal status predicted individual change in FA. Rather, the interpretation of their model is that pubertal stage, averaged over the two timepoints, is associated with changes in FA. Our study builds significantly on these prior studies by measuring gonadal hormones in addition to self-reported pubertal stage, by demonstrating that changes in testosterone and estradiol are positively associated with changes in FA in females, and by reporting that changes in these hormones are stronger predictors of changes in FA than are changes in self-reported pubertal stage. A strength of our study is that we used two different measurements of puberty—gonadal hormones and self-report Tanner staging. Nevertheless, it is important to recognize that puberty is a multifaceted developmental stage that is a difficult construct to assess (Bordini and Rosenfield, 2011). Despite this concern, our results suggest that gonadal hormones, compared with self-reported pubertal measures, may represent a more sensitive measure when seeking to understand puberty-related changes in WM microstructure. Moreover, our study illustrates the benefits of including multiple measurements of the construct of puberty and highlights the need for consensus concerning robust measurements of pubertal development.
Perhaps the most significant limitation of our investigation was that estradiol and testosterone were not assayed in both boys and girls. Given low reliability of salivary measures of estradiol in males in peripuberty period, most likely due to limited assay sensitivity (Shirtcliff et al., 2000), we did not assay this hormone in males. Consequently, we cannot determine whether the positive associations between changes in estradiol and changes in FA in females are also present in males, or if sex moderates associations between estradiol and WM microstructure. Thus, it is possible that higher levels of estradiol may are associated with higher FA in males. Previous studies in humans, however, have not consistently documented associations between estradiol and FA in males (Herting et al., 2012; Menzies et al., 2015). To our knowledge, no work in non-human animals has explicitly examined associations between estradiol and WM microstructure in males; however, estrogen receptors are expressed in oligodendrocytes and oligodendrocyte precursor cells and have been posited to regulate oligodendrocyte progenitor proliferation (Marin-Husstege et al., 2004; Zhang et al., 2004). Further, several studies have identified sex-specific effects in the molecular and biochemical processes related to myelination (e.g., turnover rate) that are likely mediated by estradiol (Cerghet, 2006; 2009). It is important to note, however, that the methods used in murine studies to isolate the effects of hormones on the brain (e.g., castration) do not translate well to human studies.
It is possible that differences in samples (e.g., saliva versus serum) and/or immunoassay kits used to assay gonadal hormones may contribute to differences in our results with those from prior studies (e.g., Herting et al., 2012). The immunoassay kits we used show high correlations between serum and saliva levels of testosterone and estradiol—ranging from 0.61–0.96 (https://salimetrics.com/assay-kit/salivary-testosterone-elisa-kit/), suggesting that our results would be unchanged had we assayed these hormones from blood samples. Moreover, the Salimetric kits we used have a lower threshold of detection and, therefore, are more sensitive than those used in 2012 by Herting et al. (100 pg/mL versus 1 pg/mL for testosterone and 2.2 pg/mL versus 0.1 pg/mL for estradiol). That said, the correlation between salivary testosterone and serum testosterone assayed from the Salimetrics kits in our study is lowest in females (r = 0.61), which is an important caveat to consider when interpreting our results in the context of the broader literature. Other limitations of this study include the fact that we did not collect hormones in a manner that accounted for diurnal or even monthly fluctuations (Dorn, 2006; Janfaza et al., 2006), and that we focused solely on testosterone and estradiol. In order to gain a more comprehensive understanding of the neuroendocrine processes involved in both pubertal maturation and WM development, future investigations should acquire repeated (e.g., weekly) samples, and examine other hormones, including dehydroepiandrosterone (DHEA; see Byrne et al., 2017, for a review, Klauser et al., 2015 for evidence that DHEA is negatively correlated with frontal white matter volume, and Barendse et al., 2018, who showed that the effect of testosterone on white matter microstructure in adolescents is dependent on levels of DHEA), which is a metabolic intermediate in the biosynthesis of sex steroids, and progesterone, that modulates the luteal and follicular phases of the menstrual cycle (Reed and Carr, 2015). Future work should also consider explicitly testing whether objectively assessed puberty measures related to adrenal processes (e.g., pubic hair) versus gonadal processes (e.g., breast/testes development) are more strongly associated with DHEA than with testosterone (and/or estradiol). Another important point to consider is that while testosterone is synthesized mainly in the gonads and ovaries, it can also be synthesized in the adrenal glands and, ultimately, converted to estradiol through activation of the aromatase enzyme (Dorn, 2006; Janfaza et al., 2006); thus, future work is needed to comprehensively characterize the dynamic synthesis of testosterone and other related hormones in order to understand their changes across puberty and their effects on adolescent brain development.
Most studies to date examining adolescent WM development have acquired diffusion MRI data on a 1.5 T scanner (although see Herting et al., 2012; 2017) and sampled a maximum of 32 directions; a major strength of our investigation is that all MRI data were collected on a 3 T scanner and our diffusion-weighted MRI sequence sampled 60 directions. Further, we used AFQ to estimate FA in specific WM tracts within each individual brain instead of using voxel-based methods which estimate diffusivity properties on an aggregate level. Future work is needed to overcome inherent limitations of DTI in order to better characterize WM development in the adolescent brain. Although we acquired 60 diffusion-weighted directions in diffusion MRI scans and focused on larger tracts, the issue of crossing fibers remains problematic (Auriat et al., 2015). Higher-order tractography methods such as constrained spherical deconvolution (Tournier et al., 2004, 2007), combined with multi-shell diffusion MRI sequences for data acquisition, will increase our ability to distinguish among crossing fibers. Other analytical methods such as neurite orientation dispersion and density imaging (NODDI) also have the potential to assess specific markers of brain tissue microstructure that are better indicators of myelination than are FA and other DTI-based metrics (Jelescu et al., 2015; Zhang et al., 2012). Further, for the purposes of consistency, we maintained the same tracking parameters across all participants and conservatively excluded diffusivity values from tracts that did not adequately resolve and/or were outliers; nevertheless, it is likely that there are individual differences in terms of optimal tracking parameters for certain tracts (Colby et al., 2012). Additional research is needed to identify the set of optimal parameters for tractography on the basis of such factors, including sex, developmental stage, and age.
The novelty and a strength of our study design is that males and females were matched on puberty at Time 1. A challenge in recruiting such a sample, however, was that we were unable to acquire self-reported Tanner information for the potential participants through their parents/legal guardians, whom we had permission to contact before they came to our lab. Thus, in addition to recruiting on the basis of age, we excluded female participants if their parent/guardian reported they had experienced the onset of menarche; we did this as a means of ensuring that the female participants who we recruited, and who we intended to follow-up with approximately 2 years later, were not at the later stages of pubertal development at entry into the study (menarche occurs relatively late in gonadarche, Dorn et al., 2006). A limitation of this recruitment strategy, however, is that we did not apply an exclusionary criterion equivalent to the onset of menses for males who were potential participants in the study; indeed, spermarche is an ambiguous equivalent that is also not as reliably assessed through self- or parent-report (Rasmussen et al., 2015), and tends to occur earlier in pubertal development in males than menarche does in females (Dorn et al., 2006).
Finally, while the longitudinal design of this study and our repeated assessments of puberty and WM microstructure are notable strengths of this investigation, two timepoints of data does not allow us to examine nonlinear changes (King et al., 2017b; Vijayakumar et al., 2018). It is important that future studies examining normative adolescent brain development not exclude female participants on the basis of menarche status and also acquire multiple timepoints of data for each individual to characterize nonlinear changes more comprehensively (Tamnes et al., 2018). For example, the Adolescent Brain Cognitive Development (ABCD) study, in which diffusion-weighted MRI and salivary hormones will be obtained in over 11,500 adolescents starting at ages 9–10 years for the next decade (Uban et al., 2017; Casey et al., 2018), represents an excellent opportunity for researchers to replicate the findings we report in the present study.
5. Conclusions
This study was motivated in part by the lack of current studies in which puberty— measured by gonadal hormones—is examined explicitly as a contributing factor to sex differences in WM development—measured by FA—in adolescents. To address this gap, we recruited young adolescent males and females matched on pubertal stage and examined the association between longitudinal changes in gonadal hormones and in WM microstructure. We found that increases in testosterone during puberty are associated with increases in WM organization in several tracts associated with cognitive control, response inhibition, and emotion regulation in adolescent females, but not in adolescent males. Our results should be replicated in larger samples in which puberty-related hormones and WM metrics are obtained at more than two timepoints. Nevertheless, our findings may explain previously reported sex differences in the developmental trajectories of several WM tracts that have been shown to support several key cognitive and socioemotional processes that develop during adolescence.
Conflicts of Interest and Acknowledgments
This research was supported by National Institutes of Health (R37MH101495 to IHG, K01MH117442 to TCH, F32MH114317 to NLC, and K01MH106805 to SO), the Stanford University Precision Health and Integrated Diagnostics (IHG and TCH), the Klingenstein Third Generation Foundation (Child and Adolescent Depression Award to TCH), the National Science Foundation (Graduate Research Fellowship Program to NLC), and Stanford Bio-X (Undergraduate Summer Research Program to KO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors report no biomedical conflicts of interest. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We thank Josiah Leong for assistance with setting up VISTA software and AFQ on our servers, Sarah Ordaz for providing funding, and Maria “Cat” Camacho, Anna Cichocki, Monica Ellwood-Lowe, Meghan Goyer, Amar Ojha, Holly Pham, Morgan Popolizio, Alexandra Price, and Sophie Schouboe for assistance with data collection and organization. We also thank David Piekarski for helpful comments on the manuscript. Finally, we wish to thank the participants and their families for contributing to this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100773.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aghajani M., Veer I.M., van Lang N.D.J., Meens P.H.F., van den Bulk B.G., Rombouts S.A.R.B., Vermeiren R.R.J.M., van der Wee N.J. Altered white-matter architecture in treatment naïve adolescents with clinical depression. Psychol. Med. 2014;44(11):2287–2298. doi: 10.1017/S0033291713003000. [DOI] [PubMed] [Google Scholar]
- Albaugh, M.D., Ducharme, S., Karama, S., Watts, R., Lewis, J.D., Orr, C., Brain Development Cooperative Group, et al. (2016). Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Developmental Psychopathology, 1-8. [DOI] [PubMed]
- Auriat A.M., Borich M.R., Snow N.J., Wadden K.P., Boyd L.A. Comparing a diffusion tensor and non-tensor approach to white matter fiber tractography in chronic stroke. NeuroImage: Clinical. 2015;7:771–781. doi: 10.1016/j.nicl.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse M.E.A., Simmons J.G., Byrne M.L., Seal M.L., Patton G., Mundy L.…Whittle S. Brain structural connectivity during adrenarche: Associations between hormone levels and white matter microstructure. Psychoneuroendocrinology. 2018;88:70–77. doi: 10.1016/j.psyneuen.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Bava S., Frank L.R., McQueeny T., Schweinsburg B.C., Schweinsburg A.D., Tapert S.F. Altered white matter microstructure in adolescent substance users. Psychiatry Res. Neuroimaging. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Jacobus J., Mahmood O., Yang T.T., Tapert S.F. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72(3):347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Boucquey V., Goldenberg D., Thayer R.E., Ward M., Jacobus J., Tapert S.F. Sex differences in adolescent white matter architecture sunita. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system- a technical review. NMR Biomedical. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini B., Rosenfield R.L. Normal pubertal development: part II: clinical aspects of puberty. Pediatr. Rev. 2011;32:281–292. doi: 10.1542/pir.32-7-281. [DOI] [PubMed] [Google Scholar]
- Bracht T., Linden D., Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J. Affect. Disord. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Byrne M.L., Whittle S., Vijayakumar N., Dennison M., Simmons J.G., Allen N.B. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev. Cogn. Neurosci. 2017;25:12–28. doi: 10.1016/j.dcn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M.…Orr C.A. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerghet M. Proliferation and Death of Oligodendrocytes and Myelin Proteins Are Differentially Regulated in Male and Female Rodents. J. Neurosci. 2006;26(5):1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerghet M., Skoff R.P., Swamydas M., Bessert D. Sexual dimorphism in the white matter of rodents. J. Neurol. Sci. 2009;286(1–2):76–80. doi: 10.1016/j.jns.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R., Weissman D.G., Marek S., Rhoads S.A., Hipwell A.E., Forbes E.E., Keenan K., Guyer A.E. Girls’ brain structural connectivity in late adolescence relates to history of depression symptoms. Clin Child Psychol P. 2019 doi: 10.1111/jcpp.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J.B., Soderberg L., Lebel C., Dinov I.D., Thompson P.M., Sowell E.R. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59:3227–3242. doi: 10.1016/j.neuroimage.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L., Coleman J. The measurement of puberty: a review. J. Adolesc. 2002;25(5):535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Colich N.L., Williams E.S., Ho T.C., King L.S., Humphreys K.L., Price A.N.…Gotlib I.H. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Development and psychopathology. Psychopathol. 2017;29(5):1851–1864. doi: 10.1017/S0954579417001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., Ho T.C., Henje Blom E., LeWinn K.Z., Sacchet M.D., Tymofiyeva O., Simmons A.N., Yang T.T. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J. Affect. Disord. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Worthman C., Shanahan L., Costello E.J., Angold A. Early pubertal timing and testosterone associated with higher levels of adolescent depression in girls. J Am Acad Child Psy. 2019;58(12):1197–1206. doi: 10.1016/j.jaac.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski J.M., Frank E., Young E., Shear M.K. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch. Gen. Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Allen N.B., Wilbrecht L., Suleiman A.B. Importance of investing in adolescence from a developmental science perspective. Nature. 2018;554(7693):441–450. doi: 10.1038/nature25770. [DOI] [PubMed] [Google Scholar]
- Dorn L.D. Measuring puberty. J. Adolesc. Health. 2006;39(5):625–626. doi: 10.1016/j.jadohealth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Dahl R.E., Woodword, Rojahn, Hermi, Biro F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl. Dev. Sci. 2006;10(1):30–56. [Google Scholar]
- Eaton L.K., Kann L., Kinchen S., Shanklin S., Ross J., Hawkins J. Youth risk behavior surveillance—United States, 2007, surveillance summaries. Morbid. Mortal. Wkly Rep. 2008;57:1–131. [PubMed] [Google Scholar]
- Ellis B.J. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23:21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L.M., Melcangi R.C. Steroids and glial cell function. Glia. 2006;54(6):485–498. doi: 10.1002/glia.20404. [DOI] [PubMed] [Google Scholar]
- Genc S., Seal M.L., Dhollander T., Malpas C.B., Hazell P., Silk T.J. White matter alterations at pubertal onset. Aug. Neuroimage. 2017;156:286–292. doi: 10.1016/j.neuroimage.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Genc S., Malpas C.B., Ball G., Silk T.J., Seal M.L. Age, sex, and puberty related development of the corpus callosum: a multi-technique diffusion MRI study. Brain Struct Funct. 2018;223(6):2753–2765. doi: 10.1007/s00429-018-1658-5. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Heilbronner S.R., Haber S.N. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. J. Neurosci. 2014;34:10041–10054. doi: 10.1523/JNEUROSCI.5459-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Kim R., Uban K.A., Kan E., Binley A., Sowell E.R. Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology. 2017;81:70–79. doi: 10.1016/j.psyneuen.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., King L.S., Leong J.K., Colich N.L., Humphreys K.L., Ordaz S.J., Gotlib I.H. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc. Cogn. Affect. Neurosci. 2017;12:1460. doi: 10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Thayer R.E., Trim R.S., Bava S., Frank L.R., Tapert S.F. White matter integrity, substance use, and risk taking in adolescence. Psychol. Addict. Behav. 2013;27(2):431. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janfaza M., Sherman T.I., Larmore K.A., Brown-Dawson J., Klein K. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: a 10 year experience. J. Pediatr. Endocrinol. Metab. 2006;19(7):901–909. doi: 10.1515/jpem.2006.19.7.901. [DOI] [PubMed] [Google Scholar]
- Jelescu I.O., Veraart J., Adisetiyo V., Milla S.S., Novikov D.S., Fieremans E. One diffusion acquisition and different white matter models: How does microstructure change in human early development based on WMTI and NODDI? NeuroImage. 2015;107:242–256. doi: 10.1016/j.neuroimage.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C.L., Williams T.J. Testosterone regulates terminal Schwann cell number and junctional size during developmental synapse elimination. Dev. Neurosci. 2001;23(6):441–451. doi: 10.1159/000048731. [DOI] [PubMed] [Google Scholar]
- Juraska J.M., Sisk C.L., DonCarlos L.L. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm. Behav. 2013;64(2):203–210. doi: 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Chapman R., Christiansen K., Richardson H., Evans J., Jones D.K. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol. Psychiatry. 2012;72(4):296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kilford E.J., Garrett E., Blakemore S.J. The development of social cognition in adolescence: an integrated perspective. Neurosci. Biobehav. Rev. 2016;70:106–120. doi: 10.1016/j.neubiorev.2016.08.016. [DOI] [PubMed] [Google Scholar]
- King K.M., Littlefield A., McCabe C., Mills K.L., Flournoy J., Chassin L. Longitudinal modeling in developmental neuroimaging research: common challenges and solutions from developmental psychology. Dev. Cogn. Neurosci. 2017;33:54–72. doi: 10.1016/j.dcn.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.S., Colich N.L., LeMoult J., Humphreys K.L., Ordaz S.J., Price A.N., Gotlib I.H. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 2017;77:68–74. doi: 10.1016/j.psyneuen.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser P., Whittle S., Simmons J.G., Byrne M.L., Mundy L.K., Patton G.C., Fornito A., Allen N.B. Reduced frontal white matter volume in children with early onset of adrenarche. Psychoneuroendocrinology. 2015;52:111–118. doi: 10.1016/j.psyneuen.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Krogsrud S.K., Fjell A.M., Tamnes C.K., Grydeland H., Mork I., Due-Tonnessen P., Walhovd K.B. Changes in white matter microstructure in the developing brain- a longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage. 2016;124:473–486. doi: 10.1016/j.neuroimage.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Peper J.S. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev. Cogn. Neurosci. 2012;2(1):36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicolli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonelli E., Ballabio M. Neuroactive steroids: a therapeutic approach to maintain peripheral nerve integrity during neurodegenerative events. J. Mol. Neurosci. 2006;28(1):65–76. doi: 10.1385/jmn:28:1:65. [DOI] [PubMed] [Google Scholar]
- Lerner R.M., Steinberg L., editors. Handbook of Adolescent Psychology. 2nd ed. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- LeWinn K.Z., Connolly C.G., Wu J., Drahos M., Hoeft F., Ho T.C., Simmons A.N., Yang T.T. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J. Am. Acad. Adolesc. Psychiatry. 2014;53(8):899–909. doi: 10.1016/j.jaac.2014.04.021. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J.K., Pestilli F., Wu C.C., Samanez-Larkin G.R., Knutson B. White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron. 2016;89(1):63–69. doi: 10.1016/j.neuron.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J.K., MacNiven K.H., Samanez-Larkin G.R., Knutson B. Distinct neural circuits support incentivized inhibition. Neuroimage. 2018;178:435–444. doi: 10.1016/j.neuroimage.2018.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M., Muggironi M., Raban D., Skoff R.P., Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev. Neurosci. 2004:10. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Growth and physiological development during adolescence. Ann. Rev. Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44(235):291. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow G.R., Dunlap K., Chung R.J. Depression and suicide in children and adolescents. Prog. Pediat. Study. 2015;36(7) doi: 10.1542/pir.36-7-299. [DOI] [PubMed] [Google Scholar]
- Menzies L., Goddings A.L., Whitaker K.J., Blakemore S.J., Viner R.M. The effects of puberty on white matter development in boys. Dev. Cogn. Neurosci. 2015;11:116–128. doi: 10.1016/j.dcn.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Kaufmann W.E., Davatzikos C., Stieltjes B., Amodei L., Fredericksen K., Pearlson G.D., Melhem E.R., Solaiyappan M., Raymond G.V., Moser H.W., van Zijl P.C. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn. Reson. Med. 2002;47(2):215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Negriff S., Susman E.J., Trickett P.K. The developmental pathway from pubertal timing to delinquency and sexual activity from early to late adolescence. J. Youth Adolesc. 2010;40(10):1343–1356. doi: 10.1007/s10964-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M.K., Green J.G., Hwang I., McLaughlin K.A., Sampson N.A., Zaslavsky A.M., Kessler R.C. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70(3):300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Peters B.D., Szeszko P.R., Radua J., Ikuta T., Gruner P., DeRosse P., Zhang J.P., Giorgio A., Qiu D., Tapert S.F., Brauer J. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophrenia bulletin. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., De Reus M.A., Van Den Heuvel M.P., Schutter D.J. Short fused? associations between white matter connections, sex steroids, and aggression across adolescence. Hum. Brain Mapp. 2015;36(3):1043–1052. doi: 10.1002/hbm.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas J.A. American Psychological Association Child, Youth, and Families News; 2014. Adolescence: A Unique Period of Challenge and Opportunity for Positive Development.http://www.apa.org/pi/families/resources/newsletter/2014/12/adolescence-development.aspx [Google Scholar]
- Rasmussen A.R., Wohlfahrt-Veje C., Tefre de Renzy-Martin K., Hagen C.P., Tinggard J., Mouritsen A., Mieritz M.G., Main K.M. Validity of self-assessment of pubertal maturation. Pediatrics. 2015;135(1):86–93. doi: 10.1542/peds.2014-0793. [DOI] [PubMed] [Google Scholar]
- Reed B.G., Carr B.R. MDText.com, Inc; South Dartmouth (MA): 2015. The Normal Menstrual Cycle and the Control of Ovulation. Endotext [Internet] [Google Scholar]
- Romeo R.D. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J. Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- RStudio Team . RStudio, Inc.; Boston, MA: 2015. RStudio: Integrated Development for R.http://www.rstudio.com/ URL. [Google Scholar]
- Sacchet M.D., Prasad G., Foland-Ross L.C., Joshi S.H., Hamilton J.P., Thompson P.M., Gotlib I.H. Structural abnormality of the corticospinal tract in major depressive disorder. Biol Mood Anxiety Disord. 2014;4(1):8. doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Dardzinski B.J. Developmental differences in white matter architecture between boys and girls. Hum. Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K.M., Molenda-Figueira H.A., Sisk C.L. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seunarine K.K., Clayden J.D., Jentschke S., Munoz M., Cooper J.M., Chadwick M.J., Banks T., Vargha-Khaden F., Clark C.A. Sexual dimorphism in white matter developmental trajectories using tract-based spatial statistics. Brain Connection. 2016;6(1):37–47. doi: 10.1089/brain.2015.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Granger D.A., Schwartz E.B., Curran M.J., Booth A., Overman W.H. Assessing estradiol in biobehavioral studies using saliva and blood spots: simple radioimmunoassay protocols, reliability, and comparative validity. Horm. Behav. 2000;38:137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- Singer J.D., Willett J.B. Oxford university press; 2003. Applied longitudinal data analysis: Modeling change and event occurrence. [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk T.J., Vance A., Rinehart N., Bradshaw J.L., Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum. Brain Mapp. 2009;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D.J., Hallquist M.N., Asato M., Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C.L., Foster D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk C.L., Zehr J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr. Dir. Psychol. Sci. 2007;16(2):55–59. [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Østby Y., Grydeland H., Richardson G., Westlye L.T., Roddey J.C., Hagler D.J.J., Due-Tønnessen P., Holland D., Fjell A.M. Brain development and aging: overlapping and unique patterns of change. Neuroimage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Roalf D.R., Goddings A.L., Lebel C. Diffusion MRI of white matter microstructure development in childhood and adolescence: methods, challenges, and progress. Dev. Cogn. Neurosci. 2018;(33):161–175. doi: 10.1016/j.dcn.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Gadian D.G., Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;23(3):1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negtivity constrained super-resolved spherical deconvolution. NeuroImage. 2007;35(4):1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tromp D.P., Williams L.E., Fox A.S., Oler J.A., Roseboom P.H., Rogers G.M.…Kalin N.H. Altered Uncinate Fasciculus Microstructure in Childhood Anxiety Disorders in Boys But Not GirlsAmerican Journal ofuncinate fasciculus microstructure in childhood anxiety disorders in boys but not girls. Psychiatry. 2019 doi: 10.1176/appi.ajp.2018.18040425. appi-ajp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban K.A., Herting M.M., Wozniak J.R., Sowell E.R., CIFASD Sex differences in associations between white matter microstructure and gonadal hormones in children and adolescents with prenatal alcohol exposure. . Sep. Psychoneuroendocrinology. 2017;1(83):111–121. doi: 10.1016/j.psyneuen.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Mills K.L., Alexander-Bloch A., Tamnes C.K., Whittle S. Structural brain development: a review of methodological approaches and best practices. Dev. Cogn. Neurosci. 2018;33:129–148. doi: 10.1016/j.dcn.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Pansenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Adamson C., Yuan W., Altaye M., Rajagopal A., Byars A.W., Holland S.K. Sex differences in white matter development during adolescence: a DTI study. Brain Resolution. 2012;1478:1–15. doi: 10.1016/j.brainres.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr. Opin. Neurobiol. 2007;17(2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cerghet M., Mullins C., Williamson M., Bessert D., Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and Beta in oligodendrocytes. J. Neurochem. 2004;89(3):674–684. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.