Highlights
-
•
Osseous metastases (OMs) occur in only 4% of all thyroid cancer patients but are associated with greatly increased morbidity and mortality.
-
•
OMs are about twice as frequent in follicular, hurthle cell, and medullary thyroid cancers as compared to papillary thyroid cancers.
-
•
OMs are often lytic, triggered via activation of osteoclasts by tumor cells in a “vicious cycle”.
-
•
OMs are often initially asymptomatic, but associated with eventual skeletal related events in >75%.
-
•
Early identification of OMs, preemptive treatment with antiresorptive agents, and aggressive treatment of focal lesions before crisis are key.
Keywords: Thyroid cancer, Bone metastasis, Bisphosphonate, RANK ligand
Abstract
Whereas preemptive screening for the presence of lymph node and lung metastases is standard-of-care in thyroid cancer patients, bone metastases are less well studied and are often neglected in thyroid cancer patient surveillance. Bone metastases in thyroid cancer are, however, independently associated with poor/worse prognosis with a median overall survival from detection of only 4 years despite an otherwise excellent prognosis for the vast majority of thyroid cancer patients. In this review we summarize the state of current knowledge as pertinent to bony metastatic disease in thyroid cancer, including clinical implications, impacts on patient function and quality of life, pathogenesis, and therapeutic opportunities, proposing approaches to patient care accordingly. In particular, bone metastasis pathogenesis appears to reflect cooperatively between cancer and the bone microenvironment creating a “vicious cycle” of bone destruction rather than due exclusively to tumor invasion into bone. Additionally, bone metastases are more frequent in follicular and medullary thyroid cancers, requiring closer bone surveillance in patients with these histologies. Emerging data also suggest that treatments such as multikinase inhibitors (MKIs) can be less effective in controlling bone, as opposed to other (e.g. lung), metastases in thyroid cancers, making special attention to bone critical even in the setting of active MKI therapy. Although locoregional therapies including surgery, radiotherapy and ablation play important roles in palliation, antiresorptive agents including bisphosphonates and denosumab appear individually to delay and/or lessen skeletal morbidity and complications, with dosing frequency of every 3 months appearing optimal; their early application should therefore be strongly considered.
1. Introduction
1.1. Overview
Osseous metastases (OMs) occur in only ~4% of all thyroid cancer (TC) patients and are consequently poorly studied but nevertheless associated with considerably heightened morbidity and mortality [1], [2], [3]. Although 10-year overall survival (OS) in metastatic TC is ~40% [1], soberingly, 70% of TC patients with OMs have historically died within 4 years of discovery [4]. Recent data remain limited, with 5 and 10-year OS from initial OM diagnosis of 61% and 27%, respectively, in patients with differentiated thyroid cancer (DTC) [5], with poorly differentiated thyroid cancer (PDTC), having worse 10-year OS of only 15% [6]. OMs represent thereby a major clinical problem and the focus of this review.
1.2. Prevalence/Incidence
OMs are observed in all TC histologies, occurring in 2–15% of pooled differentiated TC (DTC) patient series [2,3,[7], [8], [9], [10], [11], [12], [13]; this literature is summarized in Table 1. Importantly, OMs are at least twice more frequent in follicular (FTC, 7–28%) than in papillary DTC patients (PTC, 1.4–7%) [3,11], and occur also at similarly higher (16–19%) frequency in medullary thyroid cancer (MTC) patients [3,14], stressing the importance of looking for OMs especially diligently in FTC and MTC patients.
Table 1.
Osseous metastases (OMs) in thyroid cancers (TCs) – major published studies.
| Study | OMs/Sample Size/% | Histology | Number of OMs reported/% of all TC OMs | Prevalence by histology | Number of SREs (%) | Clinical data | Mortality/survival data | Notes |
|---|---|---|---|---|---|---|---|---|
| [4] | 44/1142 (3.9%) | PTC | 15 (34%) | 2% of PTCs | Not described | Solitary OMs in 13 (30%) Multiple OMs in 31 (70%) |
70% of TC patients with OMs, died <4 years of detection. At last FU, 42/44 (95%) with OMs had died from TC | |
| Consecutive Referral Mayo Clinic USA | FTC | 14 (32%) | 12% of FTCs | |||||
| HCC | 5 (11%) | 17% of HCCs | ||||||
| MTC | 6 (14%) | 10% of MTCs | ||||||
| ATC | 4 (9%) | 5% of ATCs | ||||||
| [10] | 30/780 (3.8%) | PTC | 4 (13%) | 0.7% of PTCs | Fracture 23%, Surgery 47% | Bone pain initial symptom in 36.7%, Multiple OMs in 11 (37%) | Mean survival 7.1 years; survival at 5 and 10 years - 65% and 18% | |
| Consecutive referral Univ. of Pisa, Italy | ||||||||
| FTC | 26 (87%) | 15% of FTCs | ||||||
| [12] | 28/600 (4%) | PTC | 2 (7%) | 0.9% of PTCs | EBRT, 64%; Surgery in 25% | OMs at Diagnosis, 54%; Multiple OMs, 75% | 75% died of TC | RAI cures only 7% of pts with OMs. |
| consecutive surgeries CHUP, Lille, France | ||||||||
| FTC | 26 (93%) | 7% of FTCs | ||||||
| [13] | 180/2200 (8%) | DTC | Not reported by histology | Not reported | EBRT+ RAI, 64%; EBRT only, 12%; Surgery, 39% |
33% with single OM | 21% 10-yr survival | |
| Consecutive referral, Institut Gustave-Roussy, France | ||||||||
| [8] | 39/907 (4.3%) | PTC | 11 (28%) | Not described | Multiple OMs, 80% | 65% 5 year survival | ||
| Taipei, Taiwan | FTC | 28 (72%) | ||||||
| [9] | 62/1197 (5%) | PTC | 20 (32%) | 2% of PTCs | Not evaluated | Surgery and EBRT to spine OMs associated with better prognosis | At 6.6 years, 24% of PTC patients with OMs had died. At 7 years, 36% of FTCs with OMs had died |
|
| CGMH Linkou, Taiwan | FTC | 42 (68%) | 21% of FTCs | |||||
| [2] | 146/1636 (9%) Data at right for subset of 83: |
PTC | 19 (23%) | Not reported | EBRT, 75%; surgery, 26%; Fracture 27%; Cord comp., 14% |
Multiple OMs in 53%; OMs at diagnosis in 47% | 10yr-survival from the time of diagnosis of TC was 35% and from diagnosis of OM was 13% | |
| MSKCC New York 1960–1998 USA |
FTC | 17 (21%) | ||||||
| HCC | 9 (11%) | |||||||
| MTC | 6 (7%) | |||||||
| ATC | 10 (12%) | |||||||
| MTC | 6 (7%) | |||||||
| Lymphoma | 3 (4%) | |||||||
| Other | 13 (16%) | |||||||
| [7] | 109/1977 (5.5%) | PTC | 19 (17%) | Not reported | Surgery, 77%; EBRT, 36%; Fracture, 13%; cord comp., 34% | Multiple OM in 52% The detection of OM as revealing symptom of TC, was associated with improved survival |
84% died; alive at 5, 10, and 20 years: 41, 15, and 7%; median survival 3.9 years (mean, 5.6 years) | Only: bone metastases, total RAI dosage, OM resection correlated with improved survival in patients <45 years old. |
| Hopital Pitié- Salpetrière, Paris, France | FTC | 77 (71%) | ||||||
| [16] | 245 DTCs with OMs [145 from Pittas et al., 143 new] |
PTC | 46 (19%) | Not reported | 78% had ≥1 SREs, median 5 months from OM Diagnosis | OM symptomatic in 67%; EBRT, 46%; 65% developed 2nd SRE, median 10.7 months after 1st | 67% died; mortality higher in patients with OMs who developed SREs vs. those who did not (P < 0.0001). | |
| Retrospective, MSKCC, New York USA | FVPTC | 25 (10%) | ||||||
| FTC | 84 (34%) | |||||||
| HCC | 31 (13%) | |||||||
| PDTC | 59 (24%) | |||||||
| [15] | 202 TCs with vertebral OMs (VOMs, 37 single site, 165 literature) | PTC | 54 (28%) | Not reported | Cord 45%, radicular 12%, pain 28%; surg. 67%; RT 25% | Multiple 51%, PTC vertebral OMs asymptomatic 38% |
Not reported | RAI avid: -FTC 66%, -PTC 43% |
| Retrospective study Medstar, Washington DC, USA |
FTC | 120 (63%) | ||||||
| HCC | 6 (3%) | |||||||
| MTC | 9 (5%) | |||||||
| ATC | 2 (1%) | |||||||
| [3] | 2457/30,063 (8.2%) (either OMs or SREs) 1173/30,063 (3.9%) OMs |
PTC | 1703 (69%) | 6.9% of PTCs | 5.5% SREs, Pathologic Fracture 4%; myelopathy 0.9%, Surgery or EBRT 1% | Patients with FTC and MTC were more likely to develop OM (OR, 2.25; 95% CI, 1.85–2.74 and OR, 2.16; 95% CI, 1.60–2.86) | OMs associated with greater risk of overall and disease-specific mortality [HR, 2.14; 95% CI, 1.94–2.36 and HR, 1.59; 95% CI, 1.48 - 1.71; P <0.001] | Data appear impaired–as more patients were reported to have SREs than OMs, and fractures appear under-reported as treated. |
| Retrospective longitudinal population-based SEER-Medicare, USA | ||||||||
| FTC | 370 (15%) | 15% of FTCs | ||||||
| HCC | 148 (6%) | 11% of HCC | ||||||
| MTC | 142 (6%) | 16.4% of MTC | ||||||
| ATC | 94 (4%) = Bone event (either OMs or SREs) |
13.4% of ATC = Bone event (either OMs or SREs) |
||||||
| [74] | 143 DTCs with OMs | PTC FVPTC FTC HCC PDTC* |
35 (24.5%) 32 (22.4%) 48 (33.6%) 13 (9.1%) 15 (10.5%) |
Not reported | 37.1% SREs (excluding surgery and EBRT); 24.5% fracture, 7% hypercalcemia, 11.9% cord compression | Multiple OMs in 66.4% | 27.3% of patients died during the study period; overall mortality was significantly associated with development of SREs and localization of OM to the hip. Overall mortality was lower in patients who received RAI. | SRE-free survival was significantly shorter with metachronous OMs, those involving cervical spine, and those without RAI uptake. |
| Retrospective multicenter study (11 centers) Italy | ||||||||
| [6] | 86 DTC with OMs | PTC FTC PDTC |
41 (47.7%) 34 (39.5%) 11 (12.8%) |
Not reported | 76.7% at least 1 SRE, of these 53% >1 SREs; 66.3% EBRT, 26.7% surgery, 25.7% cord compression, 24.2%. | Multiple OMs in 59.3% | Five and 10 year OS were, respectively, 58 and 55% for PTC, 63 and 50% for FTC, and 61 and 15% for PDTC (P = 0.415 and P = 0.336, respectively). | Not consistent with other studies as the presence of SREs was not associated with significant increase in mortality at 5 and 10 years. |
| Portugal | ||||||||
| [33] | 64 DTC with OMs | PTC FVPTC FTC PDTC⁎⁎ |
13 (20.3%) 13 (20.3%) (32.8%) (26.6%) |
Not reported, but of 2268 patients with DTC in registry, 64 (2.8%) met inclusion criteria | Not described | OMs were synchronous in 50%. Distant metastases at DTC diagnosis in 43 (67.2%): 18 bone-only, 10 lung-only and 14 both. | Overall, 54.7% of patients died, 71.4% of DTC. The mean time to death after DTC detection was 9.6 years ±7.4 years. | Younger age and non-spinal OMs were independent predictors of improved survival. |
| Israel | ||||||||
| [5] | 77 DTC with OMs and at least one dose of RAI | PTC FVPTC FTC |
18 (23.3%) 17 (22%) 42 (54.5%) |
Not reported | 31% surgery, 47% EBRT | OMs synchronous with DTC 29 (56%), signs/symptoms of OMs 32 (42%), single OM at dx of OM 26 (45%) | The 5 and 10-year OS after OM diagnosis was 61% and 27%, respectively. The median OS for RAI alone and RAI plus combination therapy was 3.9 years and 7.7 years, respectively, |
Age at initial diagnosis of DTC, and RAI within 6 months of thyroidectomy, were independent prognostic factors for survival. |
| Retrospective study Medstar, Washington DC, USA |
Abbreviations: SRE, skeletal related events; DTC, differentiated thyroid cancer; PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; PDTC, poorly differentiated thyroid cancer; HCC, Hürthle cell carcinoma; FVPTC: follicular variant papillary thyroid cancer; EBRT, external beam radiotherapy; RAI, radioactive iodine; FTC WD, follicular thyroid cancer well-differentiated, FTC LD, follicular thyroid cancer less-differentiated; SEER, Surveillance Epidemiology and End Results; HR, hazard ratio; Comp; compression; FU, follow up; SREs, skeletal related events.
Aggressive histology DTC (e.g., presence of tall cell, columnar cell, or hobnail variants of PTC) or PDTC.
PDTC/intermediately differentiated carcinoma.
Reports of OM frequencies in the literature, however, vary widely (Table 1), likely attributable to biases among many reports that enrich or alter OM frequency in the reports. For example, among OMs in 202 TC patients pooling 37 from a single center and 165 from the literature [15], vertebral OM prevalence by histotype was overall reported very high: 63% FTC, 28% PTC, 5% MTC, 3% HCC (Hürthle cell carcinoma) and 1% for ATC (anaplastic thyroid cancer). Alternatively, a recent population-based study [3] differed; bone metastases and/or skeletal related events developed in 7–15% of DTC (15% FTC, 11% HCC, 7% PTC), 16.4% of MTC and in 13.4% of ATC patients. In consecutive TC patients receiving primary treatment during a 25-year period (1946–1970) at the Mayo Clinic, 9% developed pulmonary, and 3.9% skeletal, metastases [4], with risk of OMs highest (12–17%) among Hürthle cell (HCC) and FTC DTC histologies. Among 44 patients with OMs, the majority, 31 (70%), were multicentric. Mean time from the diagnosis to first OM was shortest in ATC (mean, 0.5 years), and longest in HCC (mean, 8 years); 70% died within 4 years of OM discovery, indicating correlation with poor prognosis.
In a later Memorial Sloan Kettering Cancer Center (MSKCC) series of 146 patients [2], OMs were present upon TC diagnosis in 47%, multiple at diagnosis of first OM in half, likely reflecting enrichment due to referral of poor prognosis patients with extensive disease. In this MSKCC cohort, 27% of patients with OMs suffered pathological fracture, and 14% developed spinal cord compression. 10-year OS from time of TC diagnosis in this group was 35%, and from initial OM was 13%. By univariate analysis, radioiodine uptake by OMs, absence of non-osseous metastases, and treatment with radioiodine were factors associated with favorable OS; the former two factors remaining significant upon multivariate analysis. A review of published data clearly indicates risk of over reporting of OMs due to referral bias in non-population-based studies.
1.3. Clinic impacts/skeletal-related events (SREs)
In a study of 245 patients with DTC and OMs [16], 78% (192/245) presented with, or developed, ≥1 skeletal-related event (SRE) after initial detection of OMs. Median time from identification of OMs to first SRE was ~5 months, excluding 97 with first SRE at the time of initial OM detection. After initial SRE, 65% (125/192) sustained a second SRE, a median of 10.7 months later. SREs were often multiple; 39% (74/192) sustained ≥3 discrete SREs.
Alternatively, a population based study [3] reported OMs and/or SREs among 7.9% of DTC patients per our analysis of their presented data. This lower prevalence appears to reflect absence of referral bias otherwise enriching OM prevalence at referral cancer centers [16]. This population study also reported increased SREs in tall/cell variant PTC (11.9% vs. 6.8% classic PTC, 6.9% follicular variant PTC and 6.3% diffuse sclerosing PTC) and in insular/trabecular FTC (22% vs. 6.8% minimally invasive FTC, 16% classic FTC). Patients >65 years of age were also found more often to develop OMs and SREs than those ≤50 years of age [OR 1.87 with 95% CI 1.45–2.45 and OR 2.4 CI 1.96–2.97]. Older age and male gender were also associated with greater mortality. Patients with FTC and MTC were more likely to develop bone metastases [OR 2.25 95% CI 1.85–2.74 and OR 2.16 CI 1.60–2.86, respectively] or SREs [OR 1.40 95% CI 1.15–1.68 and OR 1.62 CI 1.23–2.11, respectively]. Moreover, OMs were independently correlated with higher risk of overall mortality [HR 2.14 95% CI 1.94–2.36] and with disease-specific mortality [HR 1.59 95% CI 1.48–1.71]. Clearly, analysis of the presented outcome data indicate that OMs represent a major threat to patients with advanced TCs with need for improved and early detection, therapeutics, and preventative approaches.
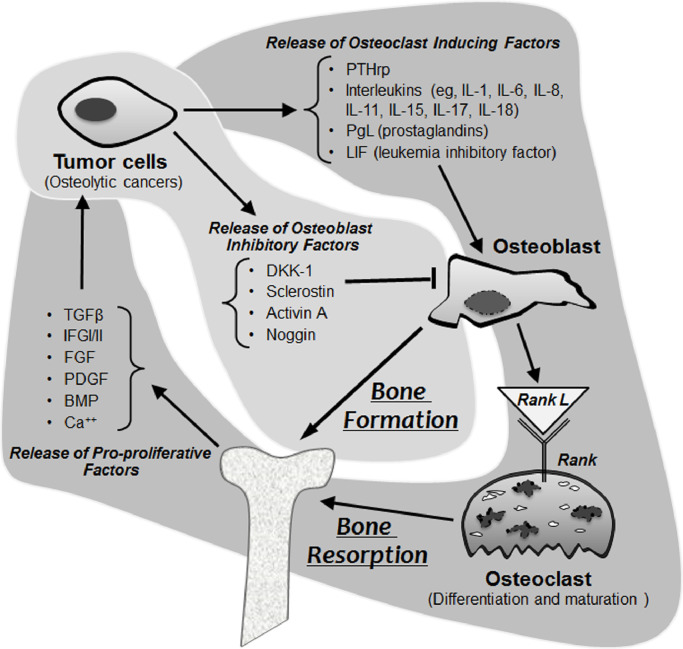
2. Biology and pathogenesis of bone metastases
Metastases, including OMs, arise from a multistep process involving loss of cell-to-cell adhesion, invasion, and dissemination through the blood and/or lymphatics, and deposition in receptive end organs/tissues (depicted in Fig. 1). Numerous processes have been implicated in fostering metastasis, including epithelial mesenchymal plasticity, cancer stem cells, noncoding RNAs, cytokines, and receptor tyrosine kinase (RTK) pathways [17].
Fig. 1.
Cellular compartments and their effects on bone on osseus metastases.
2.1. Epithelial to mesenchymal transition (EMT)
EMT serves essential roles in embryology and in the differentiation of multiple tissues and organs [18] and also contributes to tissue repair. Alternatively, EMT can be disruptive and coopted to cause organ fibrosis and promote cancer progression, convey migratory and invasive properties, induce stem cell-like properties, attenuate apoptosis and senescence, and contribute to immunosuppression to allow tumor evasion of the immune system [19,20].
2.2. Tumor microenvironment
The tumor microenvironment (TM), and not exclusively cancer cells per se, is also intimately involved in metastasis formation via genetic and epigenetic alterations that affect the ability of cancer cells to form viable metastases. Cancers “activate” tumor stroma to release factors, including hepatocyte growth factor (HGF), tumor necrosis factor (TNF)-alfa, Notch, Hedgehog, transforming growth factor-beta (TGF-β), Wingless/int (Wnts), and platelet derived growth factor (PDGF) [18] promoting tumor sustenance, progression and EMT. Hypoxia and inflammation in the TM also induce EMT and metastasis [17,21].
Focal adhesion kinase (FAK), a non-receptor protein kinase implicated in integrin-mediated signaling, oncogenic transformation, and invasion [22,23] is moreover highly expressed in aggressive TCs, functioning as a scaffold for protein-protein interactions and also as a kinase capable of phosphorylating multiple substrates. However, FAK expression also plays a dominant role in regulating TC independent of its kinase functions [24]. cDNA expression in primary, compared to metastatic, FTC has also shown differential gene alterations associated with cell cycle regulation, apoptosis, DNA damage response, angiogenesis, cell adhesion and mobility, invasion, and immune response - emphasizing the importance of TM in metastasis [25].
2.3. Osteoclasts, osteoblasts and bone metastases
Bone itself is also sometimes specifically permissive of metastasis, but effects of tumor on bone are variable. Bone microenvironment contains stroma as well as osteoclasts and osteoblasts that can be coopted or “reprogrammed” by metastatic tumor cells to promote OMs. OMs are broadly defined as lytic, sclerotic, or mixed; many DTC metastases are predominantly osteolytic.
Integrin αvβ3 elaboration by tumor accelerates development of osteolytic lesions, presumably through increased adhesion and bone invasion [26]. Solid tumor cells and multiple myeloma coopt osteoclast and osteoblast functions, triggering cytokine release from bone matrix to disrupt physiological bone remodeling. Marrow-resident tumor cells also secrete factors including parathyroid hormone-related peptide [PTHrp], interleukins [IL-6, IL-8, IL-11, IL-15, IL-18], prostaglandins, and leukemia inhibitory factor (LIF) to activate osteoblast RANKL release to promote lytic lesions. Many cancers also secrete activin A, dickkopf-1, sclerostin, and/or noggin, that alternately inhibit osteoblast differentiation and activity, impairing repair of lytic lesions [27], [28], [29].
2.4. The “vicious cycle” of osteolytic bone metastases (Fig. 1)
Bone is unique, in that it is a large repository of immobilized growth factors [TGFβ, IGF-I/II, FGF, PDGF, bone morphogenetic protein (BMP)], and calcium. Perhaps unexpectedly, osteolysis is triggered principally by tumor-stimulated osteoclast differentiation and activation - rather than by replacement of bone by tumor per se [30]. Tumor-associated macrophages and metastatic cancer cells often overexpress osteoclast-inducing factors to prompt bone resorption/osteolysis, this osteolysis in turn, leads to the release of active matrix-embedded cytokines/growth factors, like TGFβ and IGFs [31,32], thereby promoting tumor growth in bone in a “vicious cycle” (Fig. 1).
Primary and metastatic tumors are frequently heterogeneous [17], with metastases often clonally distinct from their primary tumors and sometimes better suited to preferential adhesion and metastasis formation at specific anatomical sites. The ability of tumor cells to recruit blood supply is critical to the development of macrometastases; >80% of DTC OMs are located in the axial skeleton red marrow - where blood flow is highest, with vertebrae (29–47%%), pelvis (22–38%), ribs (17–22%), and femur (11%) representing the most common sites of metastases [2,33]. Bone also contains niches wherein vascular sinusoids lacking basement membranes are permissive of invasion [23,34]. Moreover, cancer subclonal cells that exchange biological information with bone are best able to establish OMs [11,35] via disrupting a normally tightly regulated process called “coupling” linking bone resorption to bone formation [30].
3. Genomic and signaling milieu in advanced thyroid cancer involving bone
3.1. Discovery of pathogenic and “driver” mutations in thyroid cancer
The first evidence to conclusively link genomic alterations to TC came with the appreciation that MTC occurs in a heritable form linked to gain-of-function (activating) germ line mutations in RET (REarranged during Transfection). RET is a transmembrane receptor protein kinase, signaling through downstream pathways including the mitogen activated protein kinase (MAPK) cascade involving RAS/RAF/MEK/ERK. Activated/mutated RET thus drives pro-proliferative signaling and is thought to represent the dominant MTC driver in the majority of patients, realizing that mutated HRAS alternatively drives a minority of MTCs. Because RET requires ATP as a substrate to phosphorylate and thus activate downstream proteins, the hypothesis arose that RET activity might be blocked by small molecule ATP decoys that block downstream signal transduction. Two multi- and RET-kinase inhibitors (vandetanib and cabozantinib) are now approved for treatment of advanced MTC, two additional ATP mimics are approved in DTC (sorafenib and lenvatinib) and a combination therapy (dabrafenib plus trametinib) is presently approved for BRAFV600E mutated ATC based on limited data [36,37], with additional and more selective RET inhibitors now in clinical trial in MTC.
Several key somatic mutations associated with the development and progression of DTC have now been identified, termed “driver mutations” leading to constitutive activation of either of two seminal signaling pathways in TC: the mitogen activated protein kinase (MAPK: also known as RAS/RAF/MEK/ERK signaling transduction cascade) or/and the phosphatidylinositol 3-kinase/v-AKT murine thymoma viral oncogene homolog 1/mammalian target of rapamycin (PI3K/AKT/mTOR) [38,39] pathway. Tumor initiation is believed to be the consequence of activation of various growth factors or proto-oncogenes like RET or RAS [39]. Angiogenesis, in part due to increased expression of VEGF in TC, is typical in advanced disease [40], [41], [42].
The best characterized mutation in DTC occurs in PTC and involves BRAF, in particular BRAF V600E, that occurs in 45–59% of PTCs [43], especially in classic and tall cell variant PTC. However, only 10–15% of these tumors evolve into a more aggressive phenotype. Cancer Genome Atlas (TCGA) Project [43] results demonstrated a significant heterogeneity in gene expression pattern among tumors with BRAF V600E mutations, identifying at least 4 distinct molecular subgroups among BRAF-mutant tumors, indicating that they are heterogeneous despite a common driver mutation. The second most frequently identified DTC driver was RAS, which leads to constitutive activation of both MAPK and PI3K/AKT pathways, found mutated in 40–50% of FTC [38].
Of consideration is that the relatively low overall frequency of somatic mutations in PTC compared to most other cancers may contribute to its generally more indolent clinical behavior [43]. TCGA data identified candidate driver mutations in 96% of assessed PTCs, reporting new driver mutations in PTC (e.g. EIF1AX) or novel alterations of known drivers (e.g. of RET, BRAF and ALK). TCGA defined individual genes (CHEK2, ATM, and TERT) - and sets of functionally related genes - with alterations or expression patterns defining clinically relevant PTC subclasses. TERT promoter mutations (the gene encoding telomerase reverse transcriptase: TERT 228C>T), in particular, defined a subset of more aggressive, less differentiated PTCs.
As expected, among TCGA-assessed PTCs, the two most-common drivers were BRAF V600E and RAS; these seemed largely mutually exclusive, allowing the development of a “BRAF V600E-RAS score” (BRS). Likewise, a measure of thyroid cell differentiation, termed the “Thyroid Differentiation Score” (TDS), was developed through analysis of clustered thyroid-related gene expression profiles [44]. TGCA further described meta-clusters of BRAFV600E-like PTCs (BVL-PTCs) and RAS-like PTCs (RL-PTCs). The BVL-PTCs signal preferentially through MAPK, while RL-PTCs signal through both MAPK and PI3K. The RL-PTCs were highly differentiated follicular tumors, occurring in younger patients with lower risk of recurrence. However, BVL-PTCs were less-differentiated tall cell and classical type histological variants with greater ERK pathway activation than RL-PTCs. One of the pathological subgroups of BVL-PTCS comprised >70% of the tall cell variant tumors, which were typically BRAFV600E-mutated, had lower BRS and TDS values, and were associated with more advanced stage, high-risk disease.
3.2. Chromosomal rearrangements
The 2 most common chromosomal rearrangements described in TC are RET/PTC (papillary TC) and PAX8/PPARγ (paired box 8-peroxisome proliferator activated receptor). RET/PTC rearrangement results in constitutive activation of the MAPK and PI3K/AKT pathways. Clonal RET/PTC rearrangements occur in 10–20% of PTCs and are more commonly found in patients with a history of neck radiation treatment. The PAX8/PPARγ rearrangement inhibits the tumor suppressor PPARγ and activates genes responsive to PAX8, occurring in 30–35% of FTCs.
4. Clinical evaluation and diagnosis of OMS
OMs are often initially occult. Pain, resulting from mechanical damage from mass effect, or due to release of cytokines triggering periosteal irritation and stimulating pain receptors, is the most common OM presentation [45,46]. After pain, fracture is responsible for presentation, with the least common clinical presentations now hypercalcemia or spinal cord compression [46]. Skeletal-related events (SRE) constitute a standardized composite endpoint encompassing: (1) spinal cord compression, (2) pathological fracture, (3) requirement for external beam irradiation or surgery to control pain or prevent impending fracture, and/or (4) hypercalcemia of malignancy (rare).
4.1. Laboratory evaluation
Complete blood count; serum calcium/phosphorus, albumin, total alkaline phosphatase, parathyroid hormone (PTH), and markers of bone turnover (serum bone alkaline phosphatase and beta-CTx-telopeptide) constitute key laboratory studies that have potential to provide insights related to the extent of compromise of baseline bone health and related to the extent of active bone destruction ongoing in response to metastatic bony disease. Biomarkers of bone remodeling can also be assessed, but seldom are [30]. In particular, peptides released by osteoblasts/bone formation (including osteocalcin [OC], bone alkaline phosphatase [BAP], or procollagen type 1 aminoterminal propeptide [P1NP]), or by osteoclasts/bone resorption (including urinary hydroxyproline, or β-isomerized carboxyterminal crosslinking telopeptide of type I collagen[β-CTX]) are the primary surrogate means of biochemical assessment of bone remodeling. In clinical practice, however, wherein the primary focus of care is usually on palliation of OMs, these marker are not typically drivers of care or actionable in-and-of themselves and are thus not often assessed.
4.2. Imaging
Imaging is critical to assessing the extent of OMs and to defining optimal treatment, both locoregional and systemic (Table 2). Plain films are most useful in assessing structural bone integrity, risk of fracture, and need for surgery; >50% trabecular bone destruction may be required for lesions to be evident on X-ray, however [46]. More sensitive, are CT (71–100%) [11], and MR (94%) - with 91% accuracy in screening for OMs at 2-mm lesion size [47] and also in defining soft tissue extent and neural compromise [48]. MR T1 images are particularly useful (metastatic lesions typically produce decreased T1 signal due to replacement of fat with water-containing tumor, and correspondingly increased T2 marrow signal) in locations including spine and pelvis, where both insufficiency fractures and metastatic disease commonly occur [49,50]. Corresponding gadolinium uptake and T1-weighted images with fat presaturation render OMs more obvious [51].
Table 2.
Approaches to imaging osseous metastases (OMs).
| Imaging modality | Strengths | Shortcomings | OM detection sensitivity | References |
|---|---|---|---|---|
| Plane Films (X-rays) | Assessment of structural integrity/surgical necessity | Low sensitivity | 44–50% | [46] |
| CT (Computerized Tomography) | Readily available, good at detecting structural bone disease | Intermediate sensitivity | 71–100% | [11] |
| MR (Magnetic Resonance) | Assessment of neurological invasion and soft tissue extent | Higher cost, can affect implantable electronics, longer residence time in scanner, requires general anesthesia in severely claustrophobic patients | 94% | [47] |
| Bone Scan planar scintigraphy | Assesses metabolism, supplements anatomical imaging | Relatively Insensitive | 78% | [52] |
| Bone SPECT (single-photon emission CT) | Improves detection rate when added to bone scan | Higher cost than planar scintigraphy bone scan | 90.5% | [53] |
| 123/131I RAI (Radioactive Iodine) Whole Body Scan (WBS) | Useful in RAI-avid disease | Useless in RAI non-avid disease | 75% (83% lesion detectability) |
[55] [57] |
| 124I PET/CT | Useful in RAI-avid disease, improved sensitivity over RAI WBS | Useless in RAI non-avid disease; not commonly available | 87% lesion detectability | [57] |
| 18FDG-PET (Positron Emission Tomography)/CT | Most useful in RAI-insensitive disease, in which case sensitivity is very high | Lower quality structural assessment than dedicated CT or MR | 73–92% NSCLC, 95% breast cancer |
[115,116] [117] |
Abbreviations: OM, osseous metastases; CT, computed tomography; MR, magnetic resonance; SPECT single-photon emission computed tomography; RAI, radioactive iodine; PET, positron emission tomography; NSCLC, non-small cell lung cancer.
Bone scan (with 99mtechnetium-labeled diphosphonates such as methylene diphosphonate [MDP]) detects osteoblastic reaction to bone damage; since TC OMs are primarily osteolytic, bone scans are relatively insensitive [52]. SPECT (single-photon tomography) can improve OM detection sensitivity and specificity [53,54]. 131I or 123I whole-body scans (WBS) can alternatively be helpful among RAI-avid thyroid tumors [11,55,56], with 124I-PET more sensitive than 131I-WBS [11]. A small study showed 56, 87 and 100% OM detection for CT, 124I-PET, and combined 124I-PET/CT imaging, respectively [57]. Alternatively, 18FDG-PET is readily available and especially helpful in defining bone involvement in RAI non-avid disease. Although rhTSH stimulation might improve OM detection [58], it remains of uncertain incremental clinical benefit and is not favored.
An important question that is unfortunately not meaningfully addressed in the literature is that of how to most productively integrate the use of various imaging modalities in the clinical evaluations of patients with OMs. It has been our experience that, once OMs are suspected clinically, metabolic imaging using FDG-PET is a very useful screening approach to identify thyroid cancer OMs in the cases of differentiated and anaplastic thyroid cancers; alternatively, gallium dotatate PET imaging may be more helpful in screening for OMs in medullary thyroid cancer. Lesions identified via these screening approaches should trigger further targeted imaging. In the case of identified long bone metastases, plain films are required to best assess structural integrity of bone. Alternatively, in the case of spine metastases, MR imaging is required to in parallel assess the extent of neural compromise so as to adequately inform surgical decision making.
4.3. Selective proactive surveillance and early detection of OMS
Because OMs represent a major problem, contributor to morbidity, and a poor prognostic correlate in thyroid cancers, it is our view that there is a strong rationale to undertake proactive surveillance for OMs in high risk and metastatic thyroid cancers – especially in follicular, Hurthle cell, medullary, and also in all anaplastic thyroid cancers. This said, there is little motivation alternatively to screen for OMs in most PTCs that involve only the thyroid gland or/and neck lymph nodes and in most patient with follicular, Hurthle cell, and medullary thyroid cancers that are resected with expectation of curative intention. We thus propose selective, not universal, screening for OMs based upon thyroid cancer histology, disease extent, risk factors and also cancer behavior.
Firstly, it is critical to aggressively assess symptoms that might reflect bony metastatic disease—this is a given, and does not reflect screening but simply good clinical care. Secondly, patients with prior OMs are at greater risk of additional OMs, and they constitute a particular high risk group that should be proactively and regularly screened for OMs. Thirdly, patients with metastatic and/or high risk follicular, Hurthle cell, and medullary thyroid cancers represent also higher risk patients wherein unexpected changes in tumor markers and/or unpredictable cancer behavior should trigger institution of proactive OM screening.
There are nuisances in terms of how best to screen for OMs that vary depending upon clinical situation. Metabolic/functional imaging can be a very helpful as a rule, as it can image the entire skeleton at once, and has high sensitivity. In follicular cell derived thyroid cancers deemed worthy of proactive OM screening, the first question is whether they might have iodine avid disease; if so, diagnostic RAI imaging can be very helpful; if not, FDG PET is superior for Om screening. In medullary thyroid cancer, gallium dotatate PET is sometimes (or even often) more sensitive that FDG PET in detection OMs and should be alternatively considered if available. In anaplastic thyroid cancer, FDG PET is a suitable screening approach. The key is to be aware of, and prominently consider, OMs in metastatic high risk patient contexts.
5. Treatment of OMS
Treatments can be broadly classified as: symptomatically palliative, disease-modifying, or structurally preservative/restorative – with considerable potential for overlap. Therapeutic options include: pain management/symptom control (symptomatically palliative); systemic therapy (e.g. radioactive iodine, kinase inhibitors, antiresorptive agents; disease-modifying); and regional approaches including: radiotherapy, directed (thermal) ablation, and/or surgery (structurally preservative/restorative), best applied and coordinated by experienced multispecialty teams.
5.1. Pain management - systemic and regional analgesic therapies
The four key medicinal therapeutic approaches to the management of structural pain are: pure systemic analgesics (e.g. acetaminophen, opioids), systemic anti-inflammatory agents (e.g. nonsteroidal anti-inflammatory agents like ibuprofen, glucocorticoids like dexamethasone), agents targeting neuropathic pain (e.g. gabapentin, pregabilin), and regional analgesic approaches (e.g. regional injections or blocks, pain infusion pumps). Broadly construed, palliation of symptoms arising from OMs is now commonly within the purview of the specialty of Palliative Care, yet all providers need familiarity with available approaches as expeditious symptom palliation is central to the care of any patient.
Pure systemic analgesics include non-narcotic agents such as acetaminophen and oral narcotics (tramadol, codeine derivatives, and morphine derivatives – all of which have long and shorter acting preparations). Our initial approach is to optimize use of non-opioid approaches and to rely upon shorter acting agents up to the point wherein sleep is compromised by pain—in which case longer active preparations become a requirement. Importantly, when other approach to treating pain effectively exist (e.g. focal radiation therapy, ablation, surgery), these should be pursued aggressively in parallel with pharmacological approaches. The goal is to attain robust pain control and to minimize chronic narcotic usage to the extent possible and humane.
There are important reasons to consider shorter acting opioids in preference to sustained release preparations if pain is generally absent at rest and is not disrupting sleep. In particular, if there is no pain to be treated at rest and no disruption of sleep, during those times opioids are potentially adding side effects (e.g. constipation, neurocognitive effects, nausea) withoutproviding counterbalancing benefits, making the rationale for use of sustained release preparations unclear. Thus we favor titrating long acting opioid preparations to allow for fitful sleep, relying upon fast acting/immediate release preparations to treat and preemptively treat activity related pain.
Systemic anti-inflammatory agents include non-steroidal anti-inflammatory agents (e.g. ibuprofen, naproxen) which can relieve pain and also inflammation - and corticosteroids (e.g. dexamethasone, prednisone); these agents can sometimes relieve pain more effectively and/or in a complementary fashion when used in conjunction with opioids. When acute structural compromise such as spinal cord compression is to be palliated, corticosteroids are imperative to minimize neural compromise from inflammation itself, until such time as more definitive structural palliation can be undertaken.
Systemic agents targeting neuropathic pain include newer agents including pregabalin and gabapentin, as well as historical agents such as amitriptyline, which may also assist with sedation and sleep.
Regional analgesia can be accomplished either by local infusion of analgesics, for example by intrathecal infusion of narcotics, or using ablative “nerve block” procedures, such as celiac plexus block.
5.2. Adaptive approaches – physical and occupational medicine
Debility even in the absence of pain from OMs also requires palliation, often best addressed via physical and occupational medicine specialists. Restoration of function through rehabilitation should also be sought whenever possible, else adaptive approaches used, such as braces (in foot drop, for example) or adaptive equipment used to best advantage.
5.3. Structure preservation/restoration
Locoregional/focal bone metastasis treatment strategies targeting preservation or remediation of structural damage from bone metastases include surgery, radiotherapy, embolization, and thermal ablation (e.g., radiofrequency ablation, RFA; or cryoablation); these approaches are best applied in the setting of focally painful or threatening bone metastatic lesions and ineffective systemic therapies (Table 3). Antiresorptive therapies (e.g. zoledronic acid, denosumab), however, should be strongly considered in parallel.
Table 3.
Locoregional therapeutic approaches to osseous metastases (OMs).
| Modalities | Technique | Strengths | Weaknesses | References |
|---|---|---|---|---|
| Surgery | Prophylactic fixation | For impending pathologic fracture | • Curative OM resection is rare • Usually requires postoperative radiotherapy |
[59] [60] [61] |
| En bloc resection (usually for solitary OM) | Radical removal and improved survival | |||
| De-compression surgery | Rapid treatment of Spinal Cord compression syndrome | |||
| External Beam Radiation Therapy (EBRT) | • Single fraction: 8 Gy • Multiple fraction: 30 Gy in 10 treatments |
• Complete or partial pain relief • Single and multiple fraction regimens equal pain relief • Can at times be done in 1 fraction |
• Risks of adjacent tissue and neurological damage upon re-irradiation |
[70] [71] [118,119] |
|
Stereotactic radiosurgery: Stereotactic Body Radiation Therapy (SBRT), CyberKnife |
• Non-invasive and painless • No anesthesia needed • High energy photon radiation produced by linear accelerator: o Single fraction 8 Gy o Multiple fraction to 30 Gy in (2–5) fractions |
• >80% tumor control rate • Can at times be done in 1 fraction. • Minimizes damage to adjacent unaffected tissues via better sculpting of field compared to EBRT • Can be used in selected cases of prior EBRT |
• Side effects such as myelopathy or vertebral fractures can occur |
[120] [121] |
| Stereotactic radiosurgery: Gamma Knife | • Requires a collimator “helmet” surrounding patient´s head • Targets only brain or cervical spine Single treatment of high dose radiation. Fixed-array of Cobalt-60 sources |
• Gamma units have a superior mechanical precision compared to linear accelerator units • Can use for skull, as well as for brain, metastases. |
• Gamma Knife require rigid fixation of the head to a reference frame, and this can be uncomfortable • Usually used for brain metastases; cannot treat OMs outside of cranium and upper cervical spine |
[122] |
| Stereotactic radiation: Proton Beam Radiotherapy “Charged particle radiosurgery” |
• Uses heavy charged particles such as protons | • Newest type of stereotactic radiosurgery • Higher radiation doses while sparing surrounding healthy tissue • Best control of penetration depth compared to photon or gamma radiation • Can deliver high-quality Planning Target Volume (PTV) that surround the spinal cord |
• Not available in most centers • Cost |
[123] |
| Thermal Ablation | • RFA exposure to >50–60 °C • Cryoablation exposure to −40 °C |
• Less invasive than surgery • Can be highly effective |
• Under general anesthesia • Pain, transient neurologic deficits or vertebral fracture can result |
[65], [66], [67], [68] |
| Arterial Embolization | • Vertebral body embolization usually performed using right common femoral artery approach • Embolization should be performed only if a secure catheter position is attainable and spinal arteries are not visualized during spinal angiography • Conscious sedation • Particle embolization • Common choice 150 to 250 µm diameter particles of polyvinyl alcohol |
• Alternative approach • Palliation of unresectable lesions • Pain and growth control • Can be used preoperatively to embolize hypervascular bone metastases to reduce intraoperative bleeding |
• Rapid but transient relief of symptoms • Cervical metastases obtain blood supply form the vertebral arteries and embolization carries risk of ischemic stroke in the posterior circulation of the brain |
[62], [63], [64],124] |
Abbreviations: Gy, Gray.
Surgery: Primary surgical indications include structural instability, fracture and/or pain refractory to other therapeutic approaches. Prophylactic fixation for impending pathologic fracture is usually considered if there is >50% cortical loss in long bones [59]. En bloc resection may optimize local tumor control, and radical surgical removal of OMs in DTC has also been associated with improved survival [60]. Curative OM resection is rare; therefore, radiotherapy is best also administered postoperatively. In spinal cord compression due to cancer, de-compressive surgery plus postoperative radiotherapy appears superior to treatment with radiotherapy alone [61].
In patients with pain but without neurological damage or spinal instability, less invasive techniques such as percutaneous vertebroplasty and kyphoplasty may be considered. Percutaneous vertebral augmentation with or without polymethylmethacrylate (PMMA) has also been used after vertebral compression fracture.
5.4. Arterial embolization
Arterial embolization may palliate pain and growth of OMs, particularly in large unresectable lesions [62,63], but is uncommonly applied due to the high success of alternative approaches. Selective embolization of 41 skeletal lesions among 16 DTC patients with symptomatic OMs [64] was deemed successful in 24 lesions (59%); tumor control lasted 6.5 vs. 15 months for embolization alone compared to RAI or irradiation (P = 0.0146). Arterial embolization can also be used as a prodrome to surgery, wherein tumor vascularity is reduced with potential to lessen bleeding complications.
5.5. Ablative techniques
Imaging-guided ablative techniques are less invasive than surgery and can be highly effective [65,66], accomplished via exposure to increased (>50–60 °C, radiofrequency ablation, RFA) or decreased (−40 °C, cryoablation) temperatures using thermal probes inserted under general anesthesia. Bone defects developing consequent to thermal ablation can be reinforced by percutaneous PMMA cement (“cementoplasty”); however, such approaches are palliative, mainly used to alleviate pain [65].
RFA and cryoablation require highly specialized teams and technologies [11], but has “salvage” potential even after progression despite prior radiotherapy, and among patients not candidates for surgery. RFA is more commonly used in treating liver metastases; cryoablation is preferable in ablating OMs, as it is associated with lessened immediate post-procedural pain compared with RFA and avoids electrical conduction, thus allowing treatments despite presence of metallic surgical or electrical cardiac devices such as pacemakers or defibrillators; ablation of large tumor volumes using multiple cryoprobes is feasible [67].
Specific experience with ablative therapies in TC is limited, so most data are from treating OMs from other solid tumors. In a study of cryoablation of sternal metastases that [68] included one patient with TC; 12 patients underwent 15 sternal cryoablation procedures for pain palliation and local tumor control. The TC patient enjoyed complete resolution of pain with short follow up (1.9 months). Another single institution retrospective DTC study among 25 patients undergoing 49 procedures [cementoplasty (77.5%), cryoablation (14.3%) or radiofrequency ablation (8.2%)] [67] with 4.6 year median follow-up reported complete local remission rates of 71.6%, 66.8% and 60.1% at 1, 2 and 3 years, respectively. Another study of which 16% of patients had TC [69] showed favorable prognostic factors by multivariate analyses to be: oligometastatic (P = 0.02), metachronous (P = 0.004) or small-size (P = 0.001) disease; absence of cortical destruction (P = 0.01); or absence of neurological invasion (P = 0.002). Toxicity from thermal ablation is low, but local pain or transient neurological deficits or vertebral fracture arise in 5–6%.
5.6. External beam radiation therapy (EBRT) and stereotactic body radiotherapy (SBRT)
EBRT to bone is indicated when pain, risk for fracture, and/or neurological complications are present or predicted and is standard of care when surgery is not indicated [70]. Non-RAI-avid DTC OMs often respond to EBRT with complete or partial pain relief (~80%, lasting >6 months in 50% of cases) [11,71].
SBRT, or similar proprietary technologies such as “CyberknifeR”, allow the delivery of more focused/concentrated radiotherapy compared to EBRT, potentially lessening collateral damage to adjacent structures. SBRT is especially useful in re-irradiating previously EBRT-treated lesions and in treating liver and lung metastases, wherein preservation of adjacent normal tissue is critical. SBRT delivers a maximum of 30 Gy administered in 1–5 fractions, but a lower single dose of 12.5–15 Gy may achieve similar results. Prominent SBRT side effects, especially with treatment of large-volume OMs, however, can include myelopathy or late vertebral fractures. Newer approaches using alternatives to photons - such as protons or other “heavy particles” - can also be very helpful in retreating previously irradiated areas and those close to critical adjacent structures, but is not widely available and is less well studied.
5.7. Systemic therapies - radioactive iodine (DTC only)
RAI targets only RAI-avid disease, and is thus ineffective in RAI-insensitive DTC; RAI is moreover ineffective in MTC and ATC. Factors causing low RAI efficacy (29–35%) [10,11,72] in DTC include: large skeletal burden and high dose requirements for effective treatment of OMs. One study of 394 individuals with lung and/or OMs showed that, although two-thirds of patients had 131I uptake in their metastases, only 46% achieved a complete response to therapy [13]. Prognostic factors for complete response in OMs to RAI were: younger age, 131I uptake in metastases, and smaller extent of skeletal disease. Long-term survival in this cohort was 33% at 15 years; multivariate analysis demonstrated younger age, early metastasis detection, well-differentiated TC, RAI-avid metastases, small skeletal extent of disease, and year of discovery of metastases to be favorable factors with regard to OS. Among the 46% of patients who achieved complete RAI response, 15-year survival was 89% vs. 8% among those who did not. Better prognosis in patients with solitary OMs, or alternatively among those who underwent bone surgery before RAI, has also been observed [73], suggesting benefit of RAI in a subset of DTC patients with OMs. In an Italian multicenter study of 143 patients with DTC-related OMs, overall mortality was found lower in association with RAI therapy (HR 0.10; P = 0.02) [74]. However, the time frame of RAI administration seems to have importance, with patients receiving RAI within 6 months of thyroidectomy having better outcomes. It is also important to aim for a multimodal approach; median OS for OM DTC patients is of 3.9 years vs 7.7 years for those treated with RAI alone vs those treated with RAI plus combination therapies, respectively [5].
5.8. Re-differentiation agents in DTC: RAI “resensitization” therapy (DTC only)
Re-differentiation of TCs may allow for reconstituted effective RAI therapy. Selumetinib, a MAPK kinase [MEK1 and MEK2] inhibitor, increased uptake of iodine-124 in 12 of 20 patients (4 of 9 patients with BRAF mutations, and 5 of 5 patients with NRAS mutations) [75]. Eight of the 12 reached the dosimetry threshold for RAI, including all 5 with NRAS mutations. Of 8 treated with radioiodine, 5 had confirmed partial responses and 3 had stable disease, while all patients enjoyed decreased thyroglobulin levels (mean reduction, 89%). Striking increases in selumetinib-induced ioidine-124 uptake were also observed in OMs, suggesting potential benefit even in this context.
Another candidate therapeutic strategy involves mutation -guided “personalized” targeted therapy. Because aggressive TC develops when multiple signaling pathways are involved and new mutations are acquired, characterizing which genetic alterations and pathways are involved in a specific patient may reveal additional patient-specific therapeutic options that may be in flux [38].
5.9. Systemic approaches other than RAI (cytotoxics)
The majority of therapeutic emphasis in the context of bone metastases in thyroid cancer has been placed on treating established and threatening lesions, as opposed to prevention and preemptive approaches. Moreover, emphasis has been on focal therapies, as opposed to systemic therapies in this setting. However, emerging data support efficacy also of systemic approaches to treating OMs in thyroid cancer with systemic approaches; we review available data below and place it into clinical context.
For many years doxorubicin was the only FDA-approved chemotherapy for advanced TCs; low efficacy [11] associated with significant toxicities [76,77] limit use. Durable responses are uncommon [39]. Among 49 non-anaplastic patients treated over 10-years with 5 successive chemotherapeutic protocols containing doxorubicin [78] only 2 objective responses were noted (3%). Nonetheless, cytotoxic chemotherapy might occasionally have a role in selected patients unresponsive to RAI and to the newer kinase inhibitors therapies [66,79], but its roles in treating OMs in thyroid cancer remain unknown.
5.10. Radiopharmaceuticals other than RAI for bone metastatic disease
Radioisotopes emitting beta particles [e.g. strontium-89 (89SR), samarium-153 (153Sa)] that home to bone may also palliate bone pain from OMs in a variety of cancers, typically requiring 1–4 weeks for symptom palliation, and yet are uncommonly utilized. These agents target healthy as well as diseased bone, and can damage hematopoietic reserve, and have therefore traditionally been applied primarily for pain palliation in the setting of end-stage disease, especially in prostate cancer. Duration of pain palliation can be >18 months, reducing analgesic use 40–95% [80], but when used alone may not convey OS benefit [46], perhaps because it is used so late in disease course.
Radionuclides with high linear energy transfer α-particles like Radium-223 (223Ra) have enhanced radiobiological potency compared to β-emitting radionuclides [81]. Advantages of α-emitters include delivery of high-energy radiation over a much shorter distance than β-emitting 153Sm or 89Sr. 223Ra is FDA-approved for the treatment of castration-resistant prostate cancer with symptomatic OMs, but is being investigated in a phase II clinical trial in TC (NCT02390934) at the Gustave-Roussy Institute in France. These agents are presently best otherwise reserved for persistent or recurrent multifocal bone pain after EBRT and/or other forms of local therapy.
5.11. Bone microenvironment-targeted treatments
The concept that locally increased bone resorption and decreased bone formation are critical for OM progression (Fig. 1) suggests that, if osteolysis might be disrupted, bone lesions might be stabilize even without direct cancer targeting. The clinical rationale for the development of specific inhibitors of bone resorption has arisen from an understanding that the bone microenvironment itself helps facilitate OM growth (Fig. 1).
At present, two classes of antiresorptive agents are available for use in thyroid cancer OMs, realizing that most data relate to other solid tumor OMs. Bisphosphonates represent one of the two classes, and bind preferentially to hydroxyapatite crystals in bone at sites of active metabolism, reaching very high local concentrations. Bisphosphonates are slowly released from bone matrix during bone resorption, and are internalized by osteoclasts within bone resorption lacunae, leading to osteoclast apoptosis, in turn reducing osteoclast-mediated bone resorption including that stimulated by OMs.
Nitrogen-containing bisphosphonates (zoledronic acid, pamidronate, ibandronate) also inhibit farnesyl diphosphate synthase, an enzyme in the mevalonate pathway, thereby attenuating prenylation of small GTPase signaling proteins in osteoclasts as required for normal cellular function. Inhibition of farnesyl diphosphate synthase seems to account for in vitro antitumor effects, and for activation of T-cells resulting in release of tumor necrosis factor-α, a feature of the acute-phase response sometimes seen after bisphosphonate treatment in humans. Non-nitrogen-containing bisphosphonates such as clodronate do not inhibit farnesyl diphosphate synthase or protein prenylation, but instead trigger formation of cytotoxic metabolites in osteoclasts that can lead to osteoclast dysfunction [82].
In vitro and in vivo studies indicate that nitrogen-containing bisphosphonates induce apoptosis of osteoclasts and tumor cells alike [83]. In TC cell lines, clodronate inhibits cell growth of endocytic macrophages, osteoclasts, and cancer cells in a dosage-dependent manner [84]. Concentrations required for these effects in vitro, however, are much higher than those used in clinical practice, so relevance to observed clinical effects remains uncertain [11]. In postmenopausal breast cancer, early zoledronic acid use seems to produce improved cancer-specific and all-cause mortality [85]. A recent meta-analysis showed that adjuvant bisphosphonate use reduces the rate of breast cancer recurrence in the bone and improve breast cancer survival in women who were postmenopausal when treatment was started, indicating that decrease in bone recurrence was the likely driver of disease free survival [86].
Alternatively, denosumab is a humanized monoclonal antibody [directed against receptor-activator of nuclear factor-kappa B (RANKL)] that has potent activity blocking bone resorption when administered subcutaneously, with good results in treating OMs in a variety of solid tumors. Unlike bisphosphonates, wherein dosage-reduction is required in patients with renal dysfunction, denosumab has no renal liability – but comes at greater cost than bisphosphonates. Zoledronic acid and denosumab are approved for treatment of OMs based upon delayed time to first SRE and decrease SRE incidence across multiple cancers, but is not specifically approved for TC OMs [87], [88], [89], [90], [91], [92], [93] (Supplemental Table 1). Randomized controlled trials have been performed to evaluate bone-directed therapies for treatment of bone metastasis (Supplemental Table 1), but not yet in TC. On this basis, antiresorptive agents are the standard of care for treatment and prevention of OMs in solid tumors and myeloma.
Very few studies specifically evaluate antiresorptive therapy in advanced TC (Table 4). In an early study [94], 10 TC patients with painful osteolytic bone metastases receiving pamidronate monthly experienced decreased bone pain, improved quality of life, and partial radiographic responses. Another study [95] evaluated 50 patients with DTC OMs; 22 received zoledronic acid (ZA) and 28 did not; SREs occurred at lower frequency in ZA-treated patients (3/22, 14% vs. 14/28, 50%; P = 0.007) also associated with delayed onset of SREs (P = 0.04) - but two ZA-treated patients (9%) developed jaw osteonecrosis. The same group [96] later studied 19 ZA-treated patients compared to 16 historical controls. Eight patients (42%) experienced one SRE during an observation period [EBRT, n = 4 (21%); surgery, n = 3(16%); metastatic spinal cord compression, n = 1 (5%)], but none receiving ZA experienced bone fracture or hypercalcemia, with fewer (P = 0.017) and later onset (P = 0.042) of spinal cord compression also noted, suggesting benefit specifically in TC OMs. In a recent retrospective multicenter study in a “real life setting”, of 143 DTC—OMs patients, 32 (22.4%) received antiresorptive therapies (31 zoledronic acid and 1 denosumab) and in most cases these drugs were started after the development of a SRE. The limited number of patients treated with ZA precluded the evaluation of the potential effect of ZA in preventing SRE in clinical practice [74].
Table 4.
Antiresorptive therapy in thyroid cancer (TC) patients with osseous metastases (OMs) – published studies.
| Study | Sample size/% | Histology | Number of OMs reported/% of all TC OMs | Therapy administered | Number of SREs (%) | Clinical data | Mortality/survival data | Notes |
|---|---|---|---|---|---|---|---|---|
| [94] | 10 TC patients with painful OMs | PTC | 2 (20%) | Pamidronate (90 mg IV monthly, 12 months) | Not Assessed | Decrease bone pain (p<0.005); performance status improved (p<0.05) | >50% decrease in OM lesion in 2/10; 5/10 achieved OM stabilization. | Small study population |
| Prospective trial Napoli, Italy |
FTC | 6 (60%) | ||||||
| MTC | 2 (20%) | |||||||
| [95] | 50 patients with OM from DTC |
PTC | 26 (52%) [GrpA 17; GrpB 9] |
Group A: sham (n = 28); Group B: zoledronic acid (n = 22) 4 mg IV/month |
SREs: 50% Gp A; 14% Gp B (P = 0.007) | Multiple OMs in 28 (56%); Two Group B patients developed ONJ | 27 (54%) died of the disease. | Zoledronic acid associated with delayed SREs (P = 0.04). |
| Retrospective study Cancer Institute Hospital, Tokyo, Japan | ||||||||
| FTC | 24 (48%) [GrpA 11; GrpB 13] |
|||||||
| PTC | 46 (19%) | |||||||
| [96] | 19 patients with OM from DTC | PTC | 14 (74%) | Zoledronic acid, 4 mg IV monthly | SREs 42%; EBRT 21%; Surgery, 15% |
Multiple OMs 47% No responses; stable disease, 53% |
32% died of TC. | No correlations between changes in bone metabolic markers and SRE development. |
| Prospective, single arm; Cancer Institute Hospital, Tokyo, Japan | FTC | 5 (26%) | ||||||
| [14] | 188 MTCs with OM of 1008 MTC (19%) | MTC | Not reported | 84/177 (47%) received: Zoledronic acid IV (n = 64), or Pamidronate IV (n = 16) or Denosumab IV (n = 12) |
≥ 1 SRE in 48%; EBRT 74%, Fracture 23%, Surgery 13%, HCM 7%, Cord comp 3% | Only 6% had single OM lesion 168 (89%) had non-OM distant metastases. |
At 1.6 year (0–23.2) Median follow-up 59% dead No difference in mortality was seen between patients with and without antiresoptive agents. |
Out of 120 patients with germline mutation RET testing, 25% were positive. |
|
Re Retrospective study M MDACC, Houston, Texas USA | ||||||||
| Mazziotti et al. 2018 Retrospective multicenter study (11 centers) Italy |
143 DTCs with OMs | PTC FVPTC FTC HCC PDTC* |
35 (24.5%) 32 (22.4%) 48 (33.6%) 13 (9.1%) 15 (10.5%) |
32 (24%) received anti-resorptives: 31 zoledronic acid, 1 denosumab | At the start of treatment, 24 of 32 (75%) receiving zoledronic acid or denosumab had ≥1 SRE. | Patients treated with ARs had less frequently PTC and >frequently aggressive histotypes, >frequent metachronous OMs, and > frequent pathological fractures. | 27.3% of patients died during the study period; antiresorptives had no significant effect over OS (P = 0.36). | Use of ARs was not included in the SRE-free survival analysis, since in most cases the drugs were started after SRE development. |
| [6] | 86 DTC with OMs | PTC FTC PDTC |
41 (47.7%) 34 (39.5%) 11 (12.8%) |
17 (19.8%) received bisphosphonates: pamidronic acid in 9, zoledronic acid in 5 and clodronic acid in 3 | 76.7% at least 1 SRE | The presence of SRE was not significantly associated with therapy with bisphosphonates (P = 0.454). | At the end of the study, 50 (58.1%) had died. | Only a small number of patients received bisphosphonates. |
| Portugal | ||||||||
| [5] | 77 DTC with OMs and at least one dose of RAI | PTC FVPTC FTC |
18 (23.3%) 17 (22%) 42 (54.5%) |
Bisphosphonate 30 (39%), denosumab 22 (29%) | 31% surgery, 47% EBRT | OMs synchronous with DTC 29 (56%), signs/symptoms of OMs 32 (42%), single OM at dx of OM 26 (45%). | In the total cohort, patients who received denosumab had longer median OS than those who did not (7.7 vs 5.2 years; P = 0.03). Those who received bisphosphonates seemed to have slightly improved survival (7.8 vs. 6.0 years; P = 0.31). | In the RAI plus combination group, individual analysis showed no significant improvement in median OS with antiresorptives. |
| Retrospective study Medstar, Washington DC, USA |
Abbreviations: SRE, skeletal related events; OM, osseous metastases; DTC, differentiated thyroid cancer; PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; MTC, medullary thyroid cancer; Grp, group; Mo, month, EBRT, external beam radiotherapy; HCM, hypercalcemia of malignancy; Comp, compression.
Aggressive histology DTC (e.g., presence of tall cell, columnar cell, or hobnail variants of PTC) or PDTC.; ARs, antiresportives.
The situation in MTC has also been preliminarily examined. In a study [14] of OMs among 188 MTC patients, 45/180 (25%) were found to have OMs within 3 months of MTC diagnosis. The numbers of OMs were as follows: >10 (65%), 6–10 (12%), 2–5 (16%), and 1 (6%). Spine was most commonly affected (92%), followed by pelvis (69%) and ribs (53%); 48% experienced ≥1 SREs (60% had 1 and 40% had ≥ 2 SREs), most commonly requiring radiotherapy (67/90; 74%) or developing pathological fracture (21/90; 23%). The first SRE most often affected spine (58%), followed by pelvis (17%) and extremities (11%). Patients with >10 OMs were more likely to experience later SREs (odds ratio [OR] = 2.4; P = 0.007), but no difference in 5-year OS after MTC diagnosis was observed between patients with (31%) or without (23%) SREs (P = 0.11). In all, 84 of 177 (47%) received antiresorptive agents, 13 receiving multiple agents; median duration of antiresorptive treatment was 3.9 months (0–117) with median total doses of 2 (1–64). ARs were given to patients with more lesions (P = 0.006) and more involved sites (P = 0.026). In a published abstract [97], fewer patients receiving antiresorptive therapy developed SREs compared with controls (25% vs 42%, P = 0.026), suggesting benefit, with the effect remaining significant after adjusting for age, gender, and distant non-bone metastases (P = 0.047). In addition, fewer antiresorptive therapy patients developed subsequent OMs (59% vs 84%, P = 0.005), suggesting also a chemopreventative role.
The ATA 2015 DTC guidelines [66] recommend that bisphosphonate or denosumab therapy be considered in patients with diffuse and/or symptomatic OMs from RAI-refractory DTC, either alone or concomitantly with other systemic therapies. Adequate renal function is recommended before bisphosphonate therapy, with serum calcium and 25-hydroxyvitamin D levels assessed before bisphosphonante or denosumab therapy. Dental evaluation was also recommended before use of either agent. The ATA MTC guidelines [98], due in part to limited data regarding OMs in MTC, provided only one recommendation regarding use of antiresorptive agents (densoumab or bisphospanate), suggesting limiting their use to patients with painful OMs.
Importantly, care must be taken when using anti-resorptives in the setting of patients with hypocalcemia such as that due to post-surgical hypoparathyroidism; if administered, antiresorptive agents should be used cautiously and only after correction of hypocalcemia and close monitoring after administration. Of note also is that patients should be monitored for the development of additional antiresorptive related adverse events. The prevalence of atypical subtrochanteric femoral fractures in the setting of OMs treated with IV bisphosphantes appears to be low, but noteworthy [99]. Osteonecrosis of the jaw (ONJ) can occur in the setting of OM treatment with anti-resorptives, and dentoalveolar trauma seems key in its development, with denosumab likely associated with an earlier occurrence of ONJ when compared to zoledronic acid and pamidronate [100]. Preemptive dental evaluation plays an important role in the prevention of ONJ, and reduced frequency of administration of antiresorptive agents to every 3 months may also be helpful—with available evidence indicating similar efficacy and lessened toxicities in response to every three month, as opposed to monthly, dosing in several types of solid tumors.
5.12. Other non-bisphosphonate non-RANK ligand-directed candidate treatments for bone metastases
Investigation of the biology of OMs has identified additional molecular targets of potential relevance to OM pathogenesis, including TGF-β and PTHrp [27]. Moreover, cathepsin K inhibitors (e.g., odanacatib) decrease bone resorption while simultaneously maintaining bone formation, but odanacatib development has been recently stopped due to increased risk of stroke. Src inhibitors (e.g., dasatinib, saracatinib, bosutinib) may also palliate OMs, as Src is activated in response to RANKL/RANK interactions in osteoclasts. mTOR acts both upstream and downstream of AKT, at a key junction in the PI3Kinase pathway. Drugs targeting mTORC1, such as the rapamycin analogues (rapalogs) everolimus and temsirolimus, are under current investigation. Interestingly, RANKL promotes osteoclast survival by signaling through mTORC1, whereas rapamycin induces osteoclast apoptosis and suppresses in vitro bone resorption.
Other attractive emerging therapeutic targets include Endothelin-1 (ET-1), as ET-1 seems to play a role in the formation of osteosclerotic lesions; Activin–A, which in OMs is produced by tumor cells, and stimulates bone degradation, inhibits osteoblast differentiation, and stimulates osteoclast differentiation; Wnt, which drives osteoblastogenesis (bone formation), is normally inhibited by specific antagonists such as dickkopf-1 (DKK-1), sclerostin, and frizzled-related proteins, resulting in reduction of new bone formation; tumor cells (breast, prostate, lung, myeloma) that metastasize to bone are capable of producing these antagonists of bone formation, consequently blocking these antagonists represent a potential therapeutic target [27,101].
Although the imbalance between bone formation and bone resorption in osteolytic cancers is mainly due to increased bone resorption mediated by osteoclasts, decrease in bone formation may also be occurring in patients with OMs. Hence, agents that counteract inhibition of osteoblast activity may also be of therapeutic relevance in treating OMs, and is an area for future investigation.
5.13. Kinase inhibitor (MKI) therapy
Many MKIs have been specifically investigated as therapeutics in advanced DTC and MTC, including sorafenib, lenvatinib, sunitinib, axitinib, pazopanib, motesanib, vandetanib and cabozantinib—but not specifically in the context of OMs. Vandetanib and cabozantinib were approved for treatment of MTC (medullary TC) in 2011 and 2012 respectively [102], [103], [104]. More recently, sorafenib and lenvatinib were approved in advanced iodine-refractory DTC (November 2013 and February 2015, respectively) [105,106], and dabrafeneb combined with trametinib was approved in ATC. With the exception of a small, recently completed, International Thyroid Oncology Group (ITOG) trial of the effects of cabozantinib on OMs in DTC [107], MKIs, however, have not been specifically studied with respect to effect on OMs, albeit some studies have examined OM outcomes.
In the DECISION trial [105], at baseline 57 in the sorafenib group and 56 in the placebo group had OMs. Sorafenib was associated with a 5-month improvement in median progression-free survival (PFS); in the SELECT trial, lenvatinib alternatively showed a median PFS 14.7 months longer than placebo [106]. Both drugs were associated with frequent side effects, most managed by dose reduction or interruption, plus standard clinical interventions. In both trials, if progression was seen in the placebo group, patients were allowed to switch to the active treatment arm, which may be one of the reasons neither trial achieved a statistically significant prolongation of OS. Lenvatinib was associated with frequent adverse events, with 75% of treated patients suffering ≥ grade 3 toxicities; frequent adverse events remain a challenging limitation of all MKIs. Patient selection is thus critically important, with providers astute in patient selection to assure favorable risk/benefit profiles.
Emerging data, however, appear to indicate that MKIs have constrained efficacy in treating OMs [102]. A single center study [108] indicated that different responses of metastases involving different tissues may occur during MKI treatment within a single patient, with OMs more MKI refractory. In the SELECT trial [106], progression of existing OMs occurred in 9 of 38 in the lenvatinib group (23.7%), and in 23 of 39 in the placebo group (59%), however, indicating that lenvatinib likely incompletely restrains OMs, but yet has some benefit.
Given the oncogenic roles of mutations in the serine kinase BRAF, tyrosine kinase (TK) RET and RAS, selective BRAF inhibitors (vemurafenib, dabrafenib) and mTOR inhibitors (everolimus, temsirolimus) are under investigation in DTC, as are inhibitors of ALK, EGFR, MET and MEK. No trial of these agents, however, is examining bone-specific disease-modifying activity; more specific study of effect on TC OMs is clearly nonetheless needed.
6. Assessing response to therapy in OMS
A variety of imaging approaches are available to assess bone lesions, with the utilities of these approaches varying depending upon intended purpose(s). On plain films, a positive response to treatment may be visible in the form of lesional sclerosis in the absence of expanding lytic component. If a bone scan is used, a “flare phenomenon” can occur [11], with increased uptake seen initially due to healing bone that can instead be confused with disease progression. Therefore, increased bone metabolic activity on bone scan or PET imaging should be interpreted with caution. Importantly, RECIST [109] (Response Evaluation Criteria in Solid Tumors) does not include criteria specific to the evaluation of OMs. However, the WHO (World Health Organization) and the International Union Against Cancer (UICC) have suggested criteria for response in bone [110], [111], [112]. UICC criteria are based on anatomical assessment of bone lesions on plain radiography, in some ways analogous to the RECIST approach at other disease sites. A “Revised Criteria Proposed for Assessment of Bone Response” [110] added to the UICC and WHO criteria CT and MRI findings, and incorporated changes in bone sclerosis and/or metabolic activity. These revised criteria of Hamaoka et al. [110] regarding bone imaging in metastatic breast cancer, may also be relevant to the TC population.
In a practical sense, serial assessment of bone lesions is primarily intended to assure non-progression, as healing can be very slow, and difficult to assess anatomically. Metabolic responses, however, can be assessed using FDG-PET, gallium DOTATATE-PET/CT or OctreoScan, but may be confounded by the occurrence of “flare phenomenon”, also sometimes called “pseudo-progression” [113], prompting need for care in using metabolic imaging, albeit often useful.
7. Conclusions
The presence of OMs in advanced TC conveys worse prognosis [3,114], high morbidity, therapeutic challenges, and difficulties in assessing OM response to applied therapies. As the risk of OM development is increased in MTC, FTC and HCC, proactive surveillance of patients with these TC histologies in especially important. In this regard, RAI imaging (iodine avid follicular cell derived thyroid cancers only), FDG-PET, or gallium dotatate-PET, are preferable imaging approaches with regard to detecting OMs. Although kinase inhibitors have proven clinical activity in DTC and MTC, MKI effects in controlling OMs appear attenuated relative to effects at other metastatic sites. Early use of antiresorptive palliative therapy (e.g. zoledronic acid, denosumab) is thus favored based upon analogy to data from other cancers and limited data specific to TCs – and also given that advanced DTC patients are usually treated with TSH-suppressive doses of levothyroxine with consequently heightened risk for bone loss. Providers should therefore have a low threshold for institution of antiresorptive therapy to supplement other therapeutic approaches, with data general supporting similar efficacy and lessened toxicities with every three monthly, versus monthly, dosing. Still, much work is needed to develop more effective systemic therapeutic approaches to preventing and managing OMs.
Literature acquisition methods
This narrative review was performed based upon literature data obtained from PubMed interrogations using the search terms: “bone metastasis”, “bone metastases”, “thyroid cancer”, “bisphosphonate”, “treatment”, “denosumab”, “cancer” “randomized controlled trials”, “tyrosine kinase inhibitors”.
Funding
This review did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
CRediT authorship contribution statement
Nicole M. Iñiguez-Ariza: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Keith C. Bible: Methodology, Data curation, Formal analysis, Supervision, Writing - original draft, Writing - review & editing. Bart L. Clarke: Conceptualization, Methodology, Data curation, Formal analysis, Supervision.
Declaration of Competing Interest
All authors state that they have no conflicts of interest.
Acknowledgements
The authors are grateful for Ms. Candace Kostelec and to Ms. Bobbi Jebens for exemplary administrative assistance in the preparation and submission of this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2020.100282.
Appendix. Supplementary materials
References
- 1.Durante C., Haddy N., Baudin E., Leboulleux S., Hartl D., Travagli J.P., Caillou B., Ricard M., Lumbroso J.D., De Vathaire F., Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 2.Pittas A.G., Adler M., Fazzari M., Tickoo S., Rosai J., Larson S.M., Robbins R.J. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10:261–268. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 3.Choksi P., Papaleontiou M., Guo C., Worden F., Banerjee M., Haymart M. Skeletal complications and mortality in thyroid cancer: a population-based study. J. Clin. Endocrinol. Metab. 2017;102:1254–1260. doi: 10.1210/jc.2016-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay ID R.M., Sim F.H. In: Sim FH, editor. Raven Press; 1988. pp. 305–317. [Google Scholar]
- 5.Wu D., Gomes Lima C.J., Moreau S.L., Kulkarni K., Zeymo A., Burman K.D., Wartofsky L., Van Nostrand D. Improved survival after multimodal approach with (131)I treatment in patients with bone metastases secondary to differentiated thyroid cancer. Thyroid. 2019;29:971–978. doi: 10.1089/thy.2018.0582. [DOI] [PubMed] [Google Scholar]
- 6.Matta-Coelho C., Simoes-Pereira J., Vilar H., Leite V. Bone metastases from thyroid carcinoma of follicular origin: a single institutional experience. Eur. Thyroid. J. 2019;8:96–101. doi: 10.1159/000494719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernier M.O., Leenhardt L., Hoang C., Aurengo A., Mary J.Y., Menegaux F., Enkaoua E., Turpin G., Chiras J., Saillant G., Hejblum G. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2001;86:1568–1573. doi: 10.1210/jcem.86.4.7390. [DOI] [PubMed] [Google Scholar]
- 8.Fanchiang J.K., Lin J.D., Huang M.J., Shih H.N. Papillary and follicular thyroid carcinomas with bone metastases: a series of 39 cases during a period of 18 years. Changgeng Yi Xue Za Zhi. 1998;21:377–382. [PubMed] [Google Scholar]
- 9.Lin J.D., Huang M.J., Juang J.H., Chao T.C., Huang B.Y., Chen K.W., Chen J.Y., Li K.L., Chen J.F., Ho Y.S. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–1235. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 10.Marcocci C., Pacini F., Elisei R., Schipani E., Ceccarelli C., Miccoli P., Arganini M., Pinchera A. Clinical and biologic behavior of bone metastases from differentiated thyroid carcinoma. Surgery. 1989;106:960–966. [PubMed] [Google Scholar]
- 11.Muresan M.M., Olivier P., Leclere J., Sirveaux F., Brunaud L., Klein M., Zarnegar R., Weryha G. Bone metastases from differentiated thyroid carcinoma. Endocr. Relat. Cancer. 2008;15:37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 12.Proye C.A., Dromer D.H., Carnaille B.M., Gontier A.J., Goropoulos A., Carpentier P., Lefebvre J., Decoulx M., Wemeau J.L., Fossati P. Is it still worthwhile to treat bone metastases from differentiated thyroid carcinoma with radioactive iodine? World J. Surg. 1992;16:640–645. doi: 10.1007/BF02067343. discussion 645-646. [DOI] [PubMed] [Google Scholar]
- 13.Schlumberger M., Challeton C., De Vathaire F., Travagli J.P., Gardet P., Lumbroso J.D., Francese C., Fontaine F., Ricard M., Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J. Nucl. Med. 1996;37:598–605. [PubMed] [Google Scholar]
- 14.Xu J.Y., Murphy W.A., Jr., Milton D.R., Jimenez C., Rao S.N., Habra M.A., Waguespack S.G., Dadu R., Gagel R.F., Ying A.K., Cabanillas M.E., Weitzman S.P., Busaidy N.L., Sellin R.V., Grubbs E., Sherman S.I., Hu M.I. Bone metastases and skeletal-related events in medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2016;101:4871–4877. doi: 10.1210/jc.2016-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushchayeva Y.S., Kushchayev S.V., Carroll N.M., Felger E.A., Links T.P., Teytelboym O.M., Bonichon F., Preul M.C., Sonntag V.K., Van Nostrand D., Burman K.D., Boyle L.M. Spinal metastases due to thyroid carcinoma: an analysis of 202 patients. Thyroid. 2014;24:1488–1500. doi: 10.1089/thy.2013.0633. [DOI] [PubMed] [Google Scholar]
- 16.Farooki A., Leung V., Tala H., Tuttle R.M. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2012;97:2433–2439. doi: 10.1210/jc.2012-1169. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y., Ma L. The emerging molecular machinery and therapeutic targets of metastasis. Trends Pharmacol. Sci. 2015;36:349–359. doi: 10.1016/j.tips.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheel C., Onder T., Karnoub A., Weinberg R.A. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 2007;67:11476–11479. doi: 10.1158/0008-5472.CAN-07-1653. discussion 11479-11480. [DOI] [PubMed] [Google Scholar]
- 19.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 20.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Lu X., Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin. Cancer Res. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens L.V., Xu L., Dent G.A., Yang X., Sturge G.C., Craven R.J., Cance W.G. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 1996;3:100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 23.Wexler J.A., Sharretts J. Thyroid and bone. Endocrinol. Metab. Clin. North Am. 2007;36:673–705. doi: 10.1016/j.ecl.2007.04.005. vi. [DOI] [PubMed] [Google Scholar]
- 24.Kessler B.E., Sharma V., Zhou Q., Jing X., Pike L.A., Kerege A.A., Sams S.B., Schweppe R.E. FAK expression, not kinase activity, is a key mediator of thyroid tumorigenesis and pro-tumorigenic processes. Mol. Cancer Res. 2016;14:869–882. doi: 10.1158/1541-7786.MCR-16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K.T., Lin J.D., Chao T.C., Hsueh C., Chang C.A., Weng H.F., Chan E.C. Identifying differentially expressed genes associated with metastasis of follicular thyroid cancer by cDNA expression array. Thyroid. 2001;11:41–46. doi: 10.1089/10507250150500658. [DOI] [PubMed] [Google Scholar]
- 26.Pecheur I., Peyruchaud O., Serre C.M., Guglielmi J., Voland C., Bourre F., Margue C., Cohen-Solal M., Buffet A., Kieffer N., Clezardin P. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 27.Clement-Demange L., Clezardin P. Emerging therapies in bone metastasis. Curr. Opin. Pharmacol. 2015;22:79–86. doi: 10.1016/j.coph.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer Inst. 2012;104:1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 29.Hofbauer L.C., Rachner T.D., Coleman R.E., Jakob F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014;2:500–512. doi: 10.1016/S2213-8587(13)70203-1. [DOI] [PubMed] [Google Scholar]
- 30.Suva L.J., Washam C., Nicholas R.W., Griffin R.J. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eilon G., Mundy G.R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978;276:726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- 32.Quinn J.M., Athanasou N.A. Tumour infiltrating macrophages are capable of bone resorption. J. Cell. Sci. 1992;101(Pt 3):681–686. doi: 10.1242/jcs.101.3.681. [DOI] [PubMed] [Google Scholar]
- 33.Slook O., Levy S., Slutzky-Shraga I., Tsvetov G., Robenshtok E., Shimon I., Benbassat C., Hirsch D. Long-Term outcomes and prognostic factors in patients with differentiated thyroid carcinoma and bone metastases. Endocr. Pract. 2019;25:427–437. doi: 10.4158/EP-2018-0465. [DOI] [PubMed] [Google Scholar]
- 34.Orr F.W., Lee J., Duivenvoorden W.C., Singh G. Pathophysiologic interactions in skeletal metastasis. Cancer. 2000;88:2912–2918. doi: 10.1002/1097-0142(20000615)88:12+<2912::aid-cncr6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Roodman G.D. Mechanisms of bone metastasis. New Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 36.Subbiah V., Kreitman R.J., Wainberg Z.A., Cho J.Y., Schellens J.H.M., Soria J.C., Wen P.Y., Zielinski C., Cabanillas M.E., Urbanowitz G., Mookerjee B., Wang D., Rangwala F., Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-Mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bible K.C., Ryder M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat. Rev. Clin. Oncol. 2016;13:403–416. doi: 10.1038/nrclinonc.2016.19. [DOI] [PubMed] [Google Scholar]
- 38.Carneiro R.M., Carneiro B.A., Agulnik M., Kopp P.A., Giles F.J. Targeted therapies in advanced differentiated thyroid cancer. Cancer Treat. Rev. 2015;41:690–698. doi: 10.1016/j.ctrv.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Wang E., Karedan T., Perez C.A. New insights in the treatment of radioiodine refractory differentiated thyroid carcinomas: to lenvatinib and beyond. Anticancer Drugs. 2015;26:689–697. doi: 10.1097/CAD.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 40.Klein M., Vignaud J.M., Hennequin V., Toussaint B., Bresler L., Plenat F., Leclere J., Duprez A., Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2001;86:656–658. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 41.Lennard C.M., Patel A., Wilson J., Reinhardt B., Tuman C., Fenton C., Blair E., Francis G.L., Tuttle R.M. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery. 2001;129:552–558. doi: 10.1067/msy.2001.112592. [DOI] [PubMed] [Google Scholar]
- 42.Yu X.M., Lo C.Y., Chan W.F., Lam K.Y., Leung P., Luk J.M. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin. Cancer Res. 2005;11:8063–8069. doi: 10.1158/1078-0432.CCR-05-0646. [DOI] [PubMed] [Google Scholar]
- 43.Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killock D. Genetics: the cancer genome atlas maps papillary thyroid cancer. Nat. Rev. Clin. Oncol. 2014;11:681. doi: 10.1038/nrclinonc.2014.193. [DOI] [PubMed] [Google Scholar]
- 45.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 46.Selvaggi G., Scagliotti G.V. Management of bone metastases in cancer: a review. Crit. Rev. Oncol. Hematol. 2005;56:365–378. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt G.P., Schoenberg S.O., Schmid R., Stahl R., Tiling R., Becker C.R., Reiser M.F., Baur-Melnyk A. Screening for bone metastases: whole-body MRI using a 32-channel system versus dual-modality PET-CT. Eur. Radiol. 2007;17:939–949. doi: 10.1007/s00330-006-0361-8. [DOI] [PubMed] [Google Scholar]
- 48.Yang H.L., Liu T., Wang X.M., Xu Y., Deng S.M. Diagnosis of bone metastases: a meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur. Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 49.Fayad L.M., Kamel I.R., Kawamoto S., Bluemke D.A., Frassica F.J., Fishman E.K. Distinguishing stress fractures from pathologic fractures: a multimodality approach. Skeletal Radiol. 2005;34:245–259. doi: 10.1007/s00256-004-0872-9. [DOI] [PubMed] [Google Scholar]
- 50.Yuh W.T., Zachar C.K., Barloon T.J., Sato Y., Sickels W.J., Hawes D.R. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology. 1989;172:215–218. doi: 10.1148/radiology.172.1.2740506. [DOI] [PubMed] [Google Scholar]
- 51.Vanel D. MRI of bone metastases: the choice of the sequence. Cancer Imaging. 2004;4:30–35. doi: 10.1102/1470-7330.2003.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito S., Kato K., Ikeda M., Iwano S., Makino N., Tadokoro M., Abe S., Nakano S., Nishino M., Ishigaki T., Naganawa S. Comparison of 18F-FDG PET and bone scintigraphy in detection of bone metastases of thyroid cancer. J. Nucl. Med. 2007;48:889–895. doi: 10.2967/jnumed.106.039479. [DOI] [PubMed] [Google Scholar]
- 53.Savelli G., Maffioli L., Maccauro M., De Deckere E., Bombardieri E. Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q. J. Nucl. Med. 2001;45:27–37. [PubMed] [Google Scholar]
- 54.Sedonja I., Budihna N.V. The benefit of SPECT when added to planar scintigraphy in patients with bone metastases in the spine. Clin. Nucl. Med. 1999;24:407–413. doi: 10.1097/00003072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 55.de Geus-Oei L.F., Oei H.Y., Hennemann G., Krenning E.P. Sensitivity of 123I whole-body scan and thyroglobulin in the detection of metastases or recurrent differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:768–774. doi: 10.1007/s00259-002-0781-x. [DOI] [PubMed] [Google Scholar]
- 56.Schirrmeister H., Buck A., Guhlmann A., Reske S.N. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid. 2001;11:677–683. doi: 10.1089/105072501750362754. [DOI] [PubMed] [Google Scholar]
- 57.Freudenberg L.S., Antoch G., Jentzen W., Pink R., Knust J., Gorges R., Muller S.P., Bockisch A., Debatin J.F., Brandau W. Value of (124)I-PET/CT in staging of patients with differentiated thyroid cancer. Eur. Radiol. 2004;14:2092–2098. doi: 10.1007/s00330-004-2350-0. [DOI] [PubMed] [Google Scholar]
- 58.Chin B.B., Patel P., Cohade C., Ewertz M., Wahl R., Ladenson P. Recombinant human thyrotropin stimulation of fluoro-d-glucose positron emission tomography uptake in well-differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2004;89:91–95. doi: 10.1210/jc.2003-031027. [DOI] [PubMed] [Google Scholar]
- 59.Haentjens P., Casteleyn P.P., Opdecam P. Evaluation of impending fractures and indications for prophylactic fixation of metastases in long bones. Review of the literature. Acta Orthop. Belgica. 1993;59(Suppl 1):6–11. [PubMed] [Google Scholar]
- 60.Satcher R.L., Lin P., Harun N., Feng L., Moon B.S., Lewis V.O. Surgical management of appendicular skeletal metastases in thyroid carcinoma. Int. J. Surg. Oncol. 2012;2012 doi: 10.1155/2012/417086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patchell R.A., Tibbs P.A., Regine W.F., Payne R., Saris S., Kryscio R.J., Mohiuddin M., Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 62.Van Tol K.M., Hew J.M., Jager P.L., Vermey A., Dullaart R.P., Links T.P. Embolization in combination with radioiodine therapy for bone metastases from differentiated thyroid carcinoma. Clin. Endocrinol. 2000;52:653–659. doi: 10.1046/j.1365-2265.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 63.van Tol K.M., Hew J.M., Links T.P. Images in thyroidology. Embolization of a bone metastasis of follicular thyroid carcinoma. Thyroid. 2000;10:621–622. doi: 10.1089/thy.2000.10.621. [DOI] [PubMed] [Google Scholar]
- 64.Eustatia-Rutten C.F., Romijn J.A., Guijt M.J., Vielvoye G.J., van den Berg R., Corssmit E.P., Pereira A.M., Smit J.W. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2003;88:3184–3189. doi: 10.1210/jc.2003-030231. [DOI] [PubMed] [Google Scholar]
- 65.Cazzato R.L., Buy X., Grasso R.F., Luppi G., Faiella E., Quattrocchi C.C., Pantano F., Beomonte Zobel B., Tonini G., Santini D., Palussiere J. Interventional radiologist’s perspective on the management of bone metastatic disease. Eur. J. Surg. Oncol. 2015;41:967–974. doi: 10.1016/j.ejso.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Haugen B.R.M., Alexander E.K., Bible K.C., Doherty G., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G., Sawka A., Schlumberger M., Schuff K.G., Sherman S.I., Sosa J.A., Steward D., Tuttle R.M.M., Wartofsky L. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;2015 doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cazzato R.L., Bonichon F., Buy X., Godbert Y., de Figuereido B.H., Pointillart V., Palussiere J. Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur. J. Surg. Oncol. 2015;41:1247–1255. doi: 10.1016/j.ejso.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Hegg R.M., Kurup A.N., Schmit G.D., Weisbrod A.J., Atwell T.D., Olivier K.R., Moynihan T.J., Callstrom M.R. Cryoablation of sternal metastases for pain palliation and local tumor control. J. Vasc. Intervent. Radiol. 2014;25:1665–1670. doi: 10.1016/j.jvir.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Deschamps F., Farouil G., Ternes N., Gaudin A., Hakime A., Tselikas L., Teriitehau C., Baudin E., Auperin A., de Baere T. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur. Radiol. 2014;24:1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 70.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012;24:112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Simpson W.J. Radioiodine and radiotherapy in the management of thyroid cancers. Otolaryngol. Clin. North Am. 1990;23:509–521. [PubMed] [Google Scholar]
- 72.Pacini F., Cetani F., Miccoli P., Mancusi F., Ceccarelli C., Lippi F., Martino E., Pinchera A. Outcome of 309 patients with metastatic differentiated thyroid carcinoma treated with radioiodine. World J. Surg. 1994;18:600–604. doi: 10.1007/BF00353775. [DOI] [PubMed] [Google Scholar]
- 73.Qiu Z.L., Song H.J., Xu Y.H., Luo Q.Y. Efficacy and survival analysis of 131I therapy for bone metastases from differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011;96:3078–3086. doi: 10.1210/jc.2011-0093. [DOI] [PubMed] [Google Scholar]
- 74.Mazziotti G., Formenti A.M., Panarotto M.B., Arvat E., Chiti A., Cuocolo A., Dottorini M.E., Durante C., Agate L., Filetti S., Felicetti F., Filice A., Pace L., Pellegrino T., Rodari M., Salvatori M., Tranfaglia C., Versari A., Viola D., Frara S., Berruti A., Giustina A., Giubbini R. Real-life management and outcome of thyroid carcinoma-related bone metastases: results from a nationwide multicenter experience. Endocrine. 2018;59:90–101. doi: 10.1007/s12020-017-1455-6. [DOI] [PubMed] [Google Scholar]
- 75.Ho A.L., Grewal R.K., Leboeuf R., Sherman E.J., Pfister D.G., Deandreis D., Pentlow K.S., Zanzonico P.B., Haque S., Gavane S., Ghossein R.A., Ricarte-Filho J.C., Dominguez J.M., Shen R., Tuttle R.M., Larson S.M., Fagin J.A. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New Engl. J. Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottlieb J.A., Hill C.S., Jr. Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. New Engl. J. Med. 1974;290:193–197. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 77.Shimaoka K., Schoenfeld D.A., DeWys W.D., Creech R.H., DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–2160. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 78.Droz J.P., Schlumberger M., Rougier P., Ghosn M., Gardet P., Parmentier C. Chemotherapy in metastatic nonanaplastic thyroid cancer: experience at the Institut Gustave-Roussy. Tumori. 1990;76:480–483. doi: 10.1177/030089169007600513. [DOI] [PubMed] [Google Scholar]
- 79.Spano J.P., Vano Y., Vignot S., De La Motte Rouge T., Hassani L., Mouawad R., Menegaux F., Khayat D., Leenhardt L. GEMOX regimen in the treatment of metastatic differentiated refractory thyroid carcinoma. Med. Oncol. 2012;29:1421–1428. doi: 10.1007/s12032-011-0070-2. [DOI] [PubMed] [Google Scholar]
- 80.Finlay I.G., Mason M.D., Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005;6:392–400. doi: 10.1016/S1470-2045(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 81.Nilsson S., Larsen R.H., Fossa S.D., Balteskard L., Borch K.W., Westlin J.E., Salberg G., Bruland O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005;11:4451–4459. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 82.Roelofs A.J., Thompson K., Gordon S., Rogers M.J. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res. 2006;12:6222s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 83.Woodward J.K., Coleman R.E., Holen I. Preclinical evidence for the effect of bisphosphonates and cytotoxic drugs on tumor cell invasion. Anticancer Drugs. 2005;16:11–19. doi: 10.1097/00001813-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Yang D.M., Chi C.W., Chang H.M., Wu L.H., Lee T.K., Lin J.D., Chen S.T., Lee C.H. Effects of clodronate on cancer growth and Ca2+ signaling of human thyroid carcinoma cell lines. Anticancer Res. 2004;24:1617–1623. [PubMed] [Google Scholar]
- 85.Valachis A., Polyzos N.P., Coleman R.E., Gnant M., Eidtmann H., Brufsky A.M., Aft R., Tevaarwerk A.J., Swenson K., Lind P., Mauri D. Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. Oncologist. 2013;18:353–361. doi: 10.1634/theoncologist.2012-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Early Breast Cancer Trialists' Collaborative G Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 87.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., Jiang Q., Tadros S., Dansey R., Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henry D., Vadhan-Raj S., Hirsh V., von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G., Smith G., Feng A., Jun S., Dansey R., Yeh H. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support. Care Cancer. 2014;22:679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 89.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q., Jun S., Dansey R., Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 90.Kohno N., Aogi K., Minami H., Nakamura S., Asaga T., Iino Y., Watanabe T., Goessl C., Ohashi Y., Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J. Clin. Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 91.Lipton A., Theriault R.L., Hortobagyi G.N., Simeone J., Knight R.D., Mellars K., Reitsma D.J., Heffernan M., Seaman J.J. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 92.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M.A., Coleman R.E., Reitsma D.J., Chen B.L., Seaman J.J. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 93.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 94.Vitale G., Fonderico F., Martignetti A., Caraglia M., Ciccarelli A., Nuzzo V., Abbruzzese A., Lupoli G. Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br. J. Cancer. 2001;84:1586–1590. doi: 10.1054/bjoc.2001.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orita Y., Sugitani I., Toda K., Manabe J., Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21:31–35. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 96.Orita Y., Sugitani I., Takao S., Toda K., Manabe J., Miyata S. Prospective evaluation of zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Ann. Surg. Oncol. 2015 doi: 10.1245/s10434-015-4497-0. [DOI] [PubMed] [Google Scholar]
- 97.Xu JY M.W., Milton D.R. Medullary Thyroid Cancer with Bone Metastases and the Effect of Antiresorptive Agents on Skeletal Related Events: Experience at MD Anderson Cancer Center Endocrine Society's 98th Annual Meeting and Expo; April 3, 2016; Boston; 2016. 2016. [Google Scholar]
- 98.Wells S.A., Jr., Asa S.L., Dralle H., Elisei R., Evans D.B., Gagel R.F., Lee N., Machens A., Moley J.F., Pacini F., Raue F., Frank-Raue K., Robinson B., Rosenthal M.S., Santoro M., Schlumberger M., Shah M., Waguespack S.G., American Thyroid Association Guidelines Task Force on Medullary Thyroid C Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puhaindran M.E., Farooki A., Steensma M.R., Hameed M., Healey J.H., Boland P.J. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J. Bone Joint Surg. Am. 2011;93:1235–1242. doi: 10.2106/JBJS.J.01199. [DOI] [PubMed] [Google Scholar]
- 100.Owosho A.A., Liang S.T.Y., Sax A.Z., Wu K., Yom S.K., Huryn J.M., Estilo C.L. Medication-related osteonecrosis of the jaw: an update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018;125:440–445. doi: 10.1016/j.oooo.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chu T., Teng J., Jiang L., Zhong H., Han B. Lung cancer-derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem. Biophys. Res. Commun. 2014;443:962–968. doi: 10.1016/j.bbrc.2013.12.076. [DOI] [PubMed] [Google Scholar]
- 102.Cabanillas M.E., Hu M.I., Jimenez C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat–and with which drug–those are the questions. J. Clin. Endocrinol. Metab. 2014;99:4390–4396. doi: 10.1210/jc.2014-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elisei R., Schlumberger M.J., Muller S.P., Schoffski P., Brose M.S., Shah M.H., Licitra L., Jarzab B., Medvedev V., Kreissl M.C., Niederle B., Cohen E.E., Wirth L.J., Ali H., Hessel C., Yaron Y., Ball D., Nelkin B., Sherman S.I. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wells S.A., Jr., Robinson B.G., Gagel R.F., Dralle H., Fagin J.A., Santoro M., Baudin E., Elisei R., Jarzab B., Vasselli J.R., Read J., Langmuir P., Ryan A.J., Schlumberger M.J. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brose M.S., Nutting C.M., Jarzab B., Elisei R., Siena S., Bastholt L., Fouchardiere C., Pacini F., Paschke R., Shong Y.K., Sherman S.I., Smit J.W., Chung J., Kappeler C., Pena C., Molnar I., Schlumberger M.J. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., Gianoukakis A.G., Kiyota N., Taylor M.H., Kim S.B., Krzyzanowska M.K., Dutcus C.E., de las Heras B., Zhu J., Sherman S.I. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 107.Shah MH D.S.J., Wirth L., Menefee M.E., Liu S., Geyer S W.J., Villalona M., Cabanillas M. Cabozantinib in patients with radioiodine-refractory differentiated thyroid cancer who progressed on prior VEGFR-targeted therapy: results of NCI- and ITOG-sponsored multicenter phase II clinical trial. 85th Annual Meeting of the American Thyroid Association; Lake Buena Vista, FL; 2015. 2015. [Google Scholar]
- 108.Cabanillas M.E., Waguespack S.G., Bronstein Y., Williams M.D., Feng L., Hernandez M., Lopez A., Sherman S.I., Busaidy N.L. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J. Clin. Endocrinol. Metab. 2010;95:2588–2595. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 109.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: revised Recist guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 110.Hamaoka T., Madewell J.E., Podoloff D.A., Hortobagyi G.N., Ueno N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 111.Hayward J.L., Carbone P.P., Heusen J.C., Kumaoka S., Segaloff A., Rubens R.D. Assessment of response to therapy in advanced breast cancer. Br. J. Cancer. 1977;35:292–298. doi: 10.1038/bjc.1977.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayward J.L., Carbone P.P., Rubens R.D., Heuson J.C., Kumaoka S., Segaloff A. Assessment of response to therapy in advanced breast cancer (an amendment) Br. J. Cancer. 1978;38:201. doi: 10.1038/bjc.1978.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jasim S., Nathan M.A., Bible K.C. “Pseudo-progression” in advanced thyroid cancer in response to kinase inhibitor therapy. Endocrine. 2017;57:187–188. doi: 10.1007/s12020-017-1321-6. [DOI] [PubMed] [Google Scholar]
- 114.Choi Y.M., Kim W.G., Kwon H., Jeon M.J., Lee J.J., Ryu J.S., Hong E.G., Kim T.Y., Shong Y.K., Kim W.B. Early prognostic factors at the time of diagnosis of bone metastasis in patients with bone metastases of differentiated thyroid carcinoma. Eur. J. Endocrinol. 2016 doi: 10.1530/EJE-16-0237. [DOI] [PubMed] [Google Scholar]
- 115.Gayed I., Vu T., Johnson M., Macapinlac H., Podoloff D. Comparison of bone and 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography in the evaluation of bony metastases in lung cancer. Mol. Imaging Biol. 2003;5:26–31. doi: 10.1016/s1536-1632(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 116.Marom E.M., McAdams H.P., Erasmus J.J., Goodman P.C., Culhane D.K., Coleman R.E., Herndon J.E., Patz E.F., Jr. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803–809. doi: 10.1148/radiology.212.3.r99se21803. [DOI] [PubMed] [Google Scholar]
- 117.Yang S.N., Liang J.A., Lin F.J., Kao C.H., Lin C.C., Lee C.C. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99 m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J. Cancer Res. Clin. Oncol. 2002;128:325–328. doi: 10.1007/s00432-002-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falkmer U., Jarhult J., Wersall P., Cavallin-Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol. 2003;42:620–633. doi: 10.1080/02841860310014895. [DOI] [PubMed] [Google Scholar]
- 119.Frassica D.A.General principles of external beam radiation therapy for skeletal metastases. Clin. Orthop. Relat. Res. 2003:S158–164. [DOI] [PubMed]
- 120.Kougioumtzopoulou A., Zygogianni A., Liakouli Z., Kypraiou E., Kouloulias V. The role of radiotherapy in bone metastases: a critical review of current literature. Eur. J. Cancer Care. 2017 doi: 10.1111/ecc.12724. [DOI] [PubMed] [Google Scholar]
- 121.Wowra B., Muacevic A., Zausinger S., Tonn J.C. Radiosurgery for spinal malignant tumors. Dtsch. Arztebl. Int. 2009;106:106–112. doi: 10.3238/arztebl.2009.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kotecha R., Angelov L., Barnett G.H., Reddy C.A., Suh J.H., Murphy E.S., Neyman G., Chao S.T. Calvarial and skull base metastases: expanding the clinical utility of gamma knife surgery. J. Neurosurg. 2014;121(Suppl:91–101) doi: 10.3171/2014.7.GKS141272. [DOI] [PubMed] [Google Scholar]
- 123.Rief H., Chaudhri N., Tonndorf-Martini E., Bruckner T., Rieken S., Bostel T., Forster R., Schlampp I., Debus J., Sterzing F. Intensity-modulated radiotherapy versus proton radiotherapy versus carbon ion radiotherapy for spinal bone metastases: a treatment planning study. J. Appl. Clin. Med. Phys. 2015;16:5618. doi: 10.1120/jacmp.v16i6.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prince E.A., Ahn S.H. Interventional management of vertebral body metastases. Semin. Intervent. Radiol. 2013;30:278–281. doi: 10.1055/s-0033-1353480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.