Highlights
-
•
What to look for on MRI of the sacroiliac (SI) joint.
-
•
Incidental findings are common on MRI of the sacroiliac (SI) joint in children.
-
•
There is more to see than sacroiliitis on MRI of the sacroiliac (SI) joint.
-
•
Degeneration, inflammation, tumor and normal variants can be seen on MRI of SI joint.
Abbreviations: AVN, avascular necrosis; BME, bone marrow edema; CRMO, chronic recurrent multifocal osteomyelitis; FOV, field of view; Gd, gadolinium DTPA; HLA-B27, human leukocyte antigen B27; IV, intravenous; JSpA, juvenile spondyloarthritis; MRI, magnetic resonance imaging; TE, echo time; TR, repetition time; TSE, turbo spin echo; SI, sacroiliac; ST, slice thickness; STIR, short tau inversion recovery
Keywords: Magnetic resonance imaging (MRI), Sacroiliac joint, Sacroiliitis, Inflammation, Juvenile spondyloarthritis
Abstract
Purpose
To determine the prevalence of incidental findings on sacroiliac (SI) joint MRI in children clinically suspected of Juvenile Spondyloarthritis (JSpA).
Methods
In this retrospective multi-center study of 540 children clinically suspected of JSpA who underwent MRI of SI joints from February 2012 to May 2018, the prevalence of sacroiliitis and other incidental findings was recorded.
Results
In 106/540 (20 %) children MRI features of sacroiliitis were present. In 228 (42 %) patients MRI showed at least one incidental finding other than sacroiliitis. A total of 271 abnormal findings were reported. The most frequent incidental findings were at lumbosacral spine (158 patients, 29 %) and hip (43 patients, 8 %). The most common incidental finding was axial degenerative changes, seen in 94 patients (17 %). Other less frequent pathologies were: simple (bone) cyst in 15 (2,8 %) patients; enthesitis/tendinitis in 16 (3 %) patients; non-specific focal bone marrow edema (BME) away from SI joints in 10 (1,9 %) patients; ovarian cysts in 7 (1,3 %) patients; BME in the course of chronic recurrent multifocal osteomyelitis (CRMO) in 4 (0,7 %) patients; muscle pathology in 4 (0,7%) patients; benign tumors in 3 (0,6 %) patients; (old) fractures in 3 (0,6 %) patients; bony apophyseal avulsion in 2 (0,4 %) patients and malignant tumors in 2 (0,4 %) patients.
Conclusion
Incidental findings are common on MRI of the SI joints in children clinically suspected of JSpA, particularly at the lumbar spine and hips. They are seen even more frequently than sacroiliitis and can be relevant, as some will have clinical significance or require treatment.
1. Introduction
JSpA represents an important subgroup of chronic arthritis in children [1]. It is defined as a group of seronegative rheumatologic disorders with initial complaints emerging before 16 years of age [[2], [3], [4]]. There is a strong association to human leukocyte antigen (HLA-B27) [5].
New medical treatment options have recently become available to treat inflammation, delay progression of the disease and prevent irreversible damage [[6], [7], [8], [9], [10], [11]]. MRI of the SI joints is increasingly being obtained [11,12], since MRI can depict inflammatory lesions long before radiographic changes become evident [[12], [13], [14], [15]]. MRI of the SI joints may show active as well as structural lesions in sacroiliitis [11].
Most scan protocols of SI joints include part of the lower lumbar spine, hips, pelvis and the muscles and bones of the pelvic girdle. MRI of the SI joints may demonstrate incidental findings in these areas, not associated with JSpA, which might have clinical significance and need to be reported.
The aim of this study was to determine the prevalence of incidental findings demonstrated on MRI of the SI joints in children clinically suspected of JSpA.
2. Materials and methods
This retrospective multicentric study was approved by the institutional ethics committee in all 3 institutions. Informed consent was obtained.
2.1. Study group
All consecutive MRI of the SI joints from February 2012 to May 2018 in children clinically suspected of JSpA.
All MRI scans were collected from three different hospitals (Ghent University Hospital (Belgium); University of Alberta Hospital (Canada); National Institute of Geriatrics (Poland)).
In total 540 pediatric patients were included, 267 (51 %) boys and 264 (49 %) girls with a median age of 14,8 and a mean age of 14,4 (range 0,9–23,1). 180 consecutive patients were included in every single institution. In the Belgian institution (BEL) median age of the patients was 13,5; mean age 13,4; range 4,3–23,1. In the Canadian institution (CAN) median age of the patients was 15,5; mean age 14,8; range 0,9–20,6. In the Polish institution (POL) median age of the patients was 15,3; mean age 14,8; range 4,8–18,4.
2.2. MRI
In Belgium MRI was performed on a 1.5 T MRI unit (Avanto, Siemens Medical, Erlangen, Germany). The SI joints were imaged in a body flexed array coil (Siemens Medical, Erlangen, Germany). Sequence protocol included: semicoronal (along long axis of the sacral bone perpendicular to the S2 vertebral body) T1-weighted turbo spin echo (TSE) (slice thickness (ST): 3 mm; repetition time/echo time (TR/TE): 595/20 ms); semicoronal short tau inversion recovery (STIR) (ST: 3 mm;TR/TE/TI: 5030/67/150 ms); axial STIR related to the pelvis (ST:5 mm; TR/TE/TI: 7540/67/150 ms;). Field of view (FOV) 400 mm × 400 mm from L5 to the lesser trochanter. Contrast-enhanced pulse sequences were also obtained: semicoronal (ST: 3 mm; TR/TE: 558/20 ms) and axial fat saturated T1-weighted TSE (ST: 5 mm; TR/TE: 558/ 9,8 ms) 120 s after intravenous (IV) administration of Gadolinium – DTPA (Gd) contrast (T1/Gd) (Dotarem, 0.1 mmol/kg body weight).
In Canada MRI was performed on one of several Siemens 1.5 T MRI units with a body array coil. Sequences included semicoronal T1-weighted TSE (ST 4 mm, typical TR/TE 476/13 ms) and STIR (ST 4 mm, typical TR/TE/TI 4170/50/150 ms), with FOV typically 250 × 250 mm). No post-gadolinium imaging.
In Poland MRI was performed on a 1.5 T MRI unit (Avanto, Siemens Medical, Erlangen, Germany). The SI joints were imaged in a body flexed array coil (Siemens Medical, Erlangen, Germany). Sequence protocol included: Sagittal T2 TSE localizer (TR/TE: 4960/77; FOV 300 layers 26); semicoronal T1 TSE (TR/TE: 644/10; FOV 260 layers 25); semicoronal T1 TSE FS (TR/TE: 600/10; FOV 260 layers 25); semicoronal T2 TSE (TR/TE: 4960/90; FOV 260 layers 25); semicoronal T2 TSE TIRM (TR/TE: 4600/40; FOV 260 layers 25); Semicoronal PD TSE (TR/TE 3630/34; FOV 270 Layers up to 33). No post-gadolinium imaging.
2.3. Image review
The images were collected from three different hospitals (BEL) (CAN) (POL).
The MRI images were reviewed in consensus for the presence of sacroiliitis or other incidental findings in the three different institutions ((ES) (NH) (LJ) in the Belgian institution; (RM) (JJ) in the Canadian institution; (IS) (MZ) in the Polish institution).
A template was provided for the three institutions and contained: date of examination, date of birth, gender, presence of sacroiliitis, presence of JSpA and a list of incidental findings. Other (rare) incidental findings could be manually added to the list.
The presence of active lesions of sacroiliitis was recorded and included capsulitis, joint space enhancement, inflammation at the site of erosion, enthesitis and joint space fluid [16]. The presence of structural lesions of sacroiliitis was also recorded and included sclerosis, fat lesion, erosion, ankylosis and non-bridging bone bud [16]. A global diagnostic impression of sacroiliitis (sacroiliitis yes/no) was recorded. We also looked for incidental findings apart from the SI joint itself (Table 1).
Table 1.
List of the incidental findings demonstrated on MRI of the SI joints.
| Disease | |
|---|---|
| Lumbosacral spine | Degenerative disc of the lower lumbar - lumbosacral spine |
| Facet joint arthrosis/arthritis | |
| Edema pedicle/ spondylolyse with BME | |
| Lumbosacral transitional variant, with or without BME | |
| Spina bifida occulta | |
| Schmorl nodules | |
| Hip joint | Hip fluid (no evidence of synovial proliferation) |
| Hip arthritis (evidence of synovial proliferation) | |
| Hip AVN | |
| Degenerative hip | |
| Simple cyst | Tarlov cyst |
| Ganglion cyst Subchondral cyst |
|
| Bone cyst | |
| BME | Focal bone marrow edema (CRMO/sacroiliitis excluded): aspecific, Posttraumatic or mechanical |
| CRMO | |
| Tumor | Benign tumor |
| Malignant tumor | |
| Enthesitis/tendinitis | Enthesitis/tendinitis gluteus muscle |
| Enthesitis other (not SIJ/not gluteus muscle) | |
| Muscle pathology | Muscle tear |
| Myositis | |
| Muscle strain | |
| Fracture | Old or new |
| Bony apophyseal avulsion | |
| Ovarian cyst | |
| Other |
2.4. Statistical analysis
Statistical analysis was performed using software package SPSS 20.0 for Windows (SPSS, Chicago, IL, USA). Basic descriptive statistics were performed where appropriate.
3. Results
In 106 (20 %) of 540 patients MRI features of sacroiliitis were present (Fig. 1, Fig. 2) (Table 2).
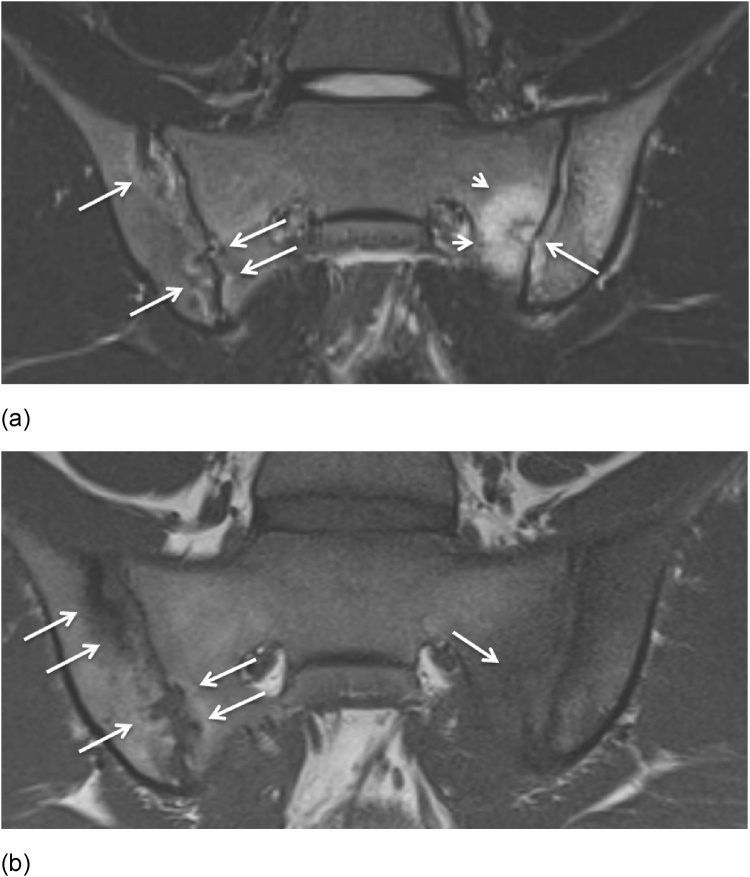
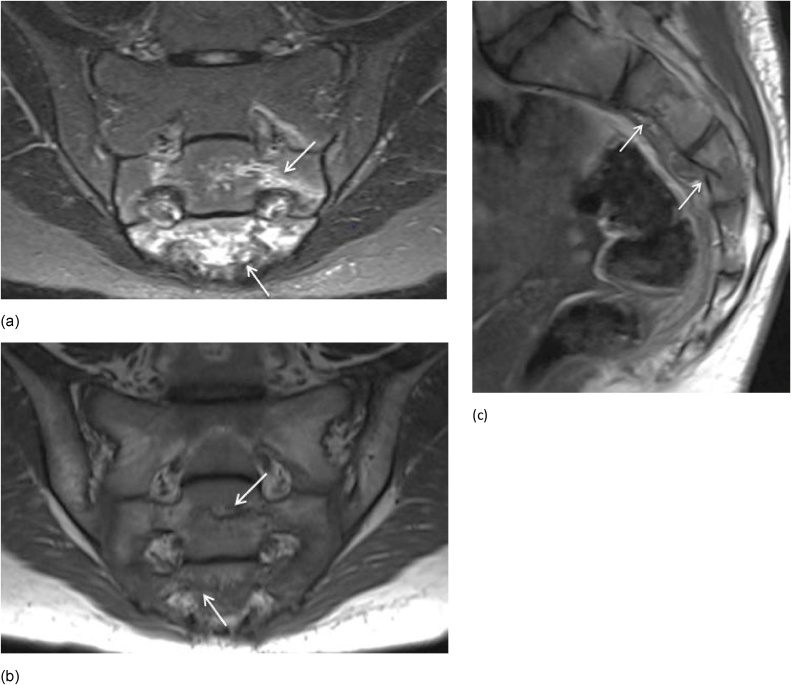
Fig. 1.
Sacroiliitis in a 17-year-old boy. (a) Semicoronal STIR MR image shows erosions on both SI joints (arrows) with extensive surrounding BME at the sacral side of the left SI joint (short arrows). (b) Semicoronal T1-weighted MR image shows erosions on both SI joints and subchondral sclerosis at the iliac side of the right SI joint.
Fig. 2.
Sacroiliitis (active and structural lesions) and CRMO in a 13-year-old boy. (a) Semicoronal STIR MR image shows an active erosion with extensive BME at the iliac side of the right SI joint (short arrow) and moderate BME at the sacral side of the right SI joint (arrow). (b) Axial fat saturated T1-weighted MR image after IV Gd shows synovitis with extensive synovial enhancement (arrows) and capsulitis (short arrow). (c) Axial STIR MR image shows diffuse BME in the proximal femur on both sides (arrows). (d-e) Whole body MRI was performed and confirmed the diagnosis of CRMO with arthritis of multiple joints (short arrow) and diffuse epiphyseal and metaphyseal BME (arrows) on coronal STIR MR images.
Table 2.
The prevalence of sacroiliitis in the three institutions (N = number of patients).
| Institution | Total N | N | % |
|---|---|---|---|
| Belgium | 180 | 40 | 22 |
| Canada | 180 | 29 | 16 |
| Poland | 180 | 37 | 20 |
| Total sacroiliitis | 540 | 106 | 20 |
In 228 (42 %) of all patients MRI showed an incidental finding (one or more, sacroiliitis not included) and a total of 262 abnormal findings were reported. In 312 (58 %) of all patients there were no incidental findings.
The prevalence of the incidental findings (sacroiliitis not included) seen in MRI of the SI joints is presented in Table 3, Table 4, Table 5, Table 6 (Fig. 3, Fig. 4, Fig. 5).
Table 3.
The prevalence of lumbosacral spine disease demonstrated on MRI of the SI joints (N = number of patients).
| Disease | N | % |
|---|---|---|
| Degenerative disc of the lower lumbar - lumbosacral spine | 87 | 161 |
| Lumbosacral transitional variant, no BME | 22 | 4,1 |
| Schmorl nodules | 17 | 3,1 |
| Edema pedicle/ spondylolyse with BME | 12 | 2,2 |
| Facet joint arthrosis/arthritis | 7 | 1,3 |
| Spina bifida occulta | 7 | 1,3 |
| Lumbosacral transitional variant, with BME | 6 | 1,1 |
| Total of lumbosacral spine disease | 158 | 29,2 |
Table 4.
The prevalence of hip disease demonstrated on MRI of the SI joints (N = number of patients).
| Disease | N | % |
|---|---|---|
| Hip fluid (no evidence of synovial proliferation) | 24 | 4,4 |
| Hip arthritis (evidence of synovial proliferation) | 17 | 3,1 |
| Hip avascular necrosis (AVN) | 1 | 0,2 |
| Degenerative hip | 1 | 0,2 |
| Total of hip disease | 43 | 7,9 |
Table 5.
The prevalence of less frequent incidental findings demonstrated on MRI of the SI joints (N = number of patients).
| Disease | N | % |
|---|---|---|
| Simple (bone) cyst | 15 | 2,8 |
| Focal BME (CRMO/sacroiliitis excluded) | 10 | 1,9 |
| Enthesitis/tendinitis gluteus muscle | 8 | 1,5 |
| Enthesitis other (not SIJ/not gluteus muscle) | 8 | 1,5 |
| Ovarian cyst | 7 | 1,3 |
| CRMO | 4 | 0,7 |
| Muscle pathology | 4 | 0,7 |
| Other | 4 | 0,7 |
| Benign tumor | 3 | 0,6 |
| Fracture | 3 | 0,6 |
| Malignant tumor | 2 | 0,4 |
| Bony apophyseal avulsion | 2 | 0,4 |
| Total | 70 | 13,1 |
Table 6.
The prevalence of the incidental findings demonstrated on MRI of the SI joints in the different institutions (N = number of patients).
| Total N | Total % | BEL N | BEL % | CAN N | CAN % | POL N | POL % | |
|---|---|---|---|---|---|---|---|---|
| Degenerative disc of the lower lumbar spine | 87 | 16,1 | 12 | 6,7 | 40 | 22,2 | 35 | 19,4 |
| Hip fluid (no evidence of synovial proliferation) | 24 | 4,4 | 8 | 4,4 | 16 | 8,9 | 0 | 0 |
| Lumbosacral transitional variant, no BME | 22 | 4,1 | 11 | 6,1 | 8 | 4,4 | 3 | 1,7 |
| Schmorl nodules | 17 | 3,1 | 2 | 1,1 | 1 | 0,6 | 14 | 7,8 |
| Arthritis hip (evidence of synovial proliferation) | 17 | 3,1 | 12 | 6,7 | 4 | 2,2 | 1 | 0,6 |
| Simple (bone) cyst | 15 | 2,8 | 7 | 3,9 | 1 | 0,6 | 7 | 3,9 |
| Edema pedicle/ spondylolyse with BME | 12 | 2,2 | 2 | 1,1 | 10 | 5,6 | 0 | 0 |
| BME, non-specific (CRMO excluded) | 10 | 1,9 | 3 | 1,7 | 4 | 2,2 | 3 | 1,7 |
| Enthesitis/tendinitis gluteus muscle | 8 | 1,5 | 8 | 4,4 | 0 | 0 | 0 | 0 |
| Enthesitis other (not SIJ/not gluteus muscle) | 8 | 1,5 | 6 | 3,3 | 2 | 1,1 | 0 | 0 |
| Facet joint arthrosis/arthritis | 7 | 1,3 | 1 | 0,6 | 5 | 2,8 | 1 | 0,6 |
| Spina bifida occulta | 7 | 1,3 | 2 | 1,1 | 3 | 1,7 | 2 | 1,1 |
| Ovarian cyst | 7 | 1,3 | 0 | 0 | 4 | 2,2 | 3 | 1,7 |
| Lumbosacral transitional variant, with BME | 6 | 1,1 | 2 | 1,1 | 4 | 2,2 | 0 | 0 |
| Muscle pathology/ edema | 4 | 0,7 | 1 | 0,6 | 3 | 1,7 | 0 | 0 |
| CRMO | 4 | 0,7 | 3 | 1,7 | 0 | 0 | 1 | 0,6 |
| Other | 4 | 0,7 | 0 | 0 | 4 | 2,2 | 0 | 0 |
| Benign tumor | 3 | 0,6 | 1 | 0,6 | 2 | 1,1 | 0 | 0 |
| Fracture | 3 | 0,6 | 0 | 0 | 3 | 1,7 | 0 | 0 |
| Bony apophyseal avulsion | 2 | 0,4 | 2 | 1,1 | 0 | 0 | 0 | 0 |
| Malignant tumor | 2 | 0,4 | 1 | 0,6 | 0 | 0 | 1 | 0,6 |
| Degenerative hip | 1 | 0,2 | 0 | 0 | 1 | 0,6 | 0 | 0 |
| AVN hip | 1 | 0,2 | 0 | 0 | 1 | 0,6 | 0 | 0 |
| Total pathology | 271 | 84 | 116 | 71 | ||||
| Total patients with incidental findings | 228 | 42 | 72 | 40 | 96 | 53 | 60 | 33 |
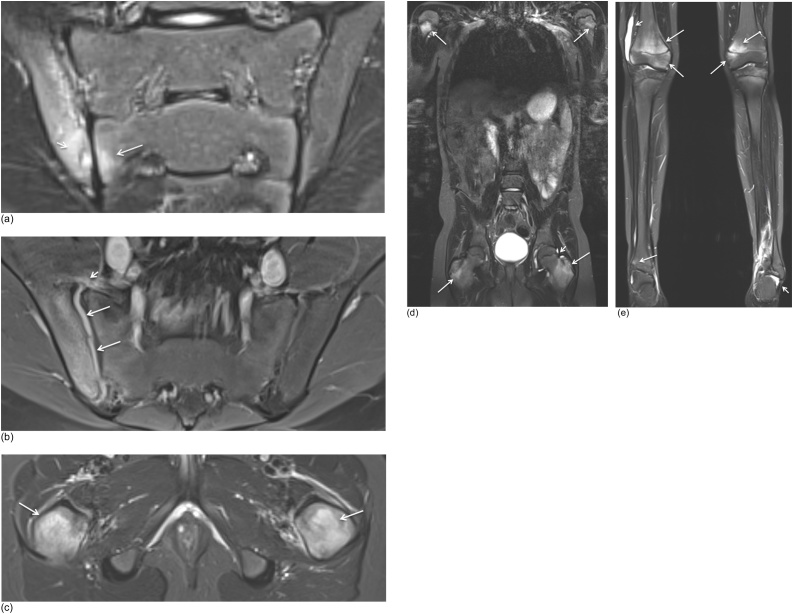
Fig. 3.
Incidental findings seen on MRI of SI joints in different patients. (a) Semicoronal STIR MR image of a Schmorl nodules (arrow) in a 12-year-old boy. (b) Semicoronal STIR MR image of a lumbosacral transitional variant on the left side without BME (arrow) in a 15-year old girl. (c) Semicoronal STIR MR image of a lumbosacral transitional variant on the right side with discrete BME (arrow) in a 13-year-old. (d) Axial STIR MR image of a muscle tear with hyperintense signal changes of the rectus femoris muscle (arrow) seen on the most inferior image in a 9-year-old boy. (e) Axial STIR MR image shows bone marrow edema of the pedicle on the left side (arrow) suspicious for spondylolysis in a 7-year-old boy. (f) Axial fat saturated T1-weighted MR image after IV Gd of an bony apophyseal avulsion with soft tissue edema and enhacement on the left side (arrow) in a 14-year-old boy. (g) Semicoronal STIR MR image shows an ovarian cyst (arrow) in a 14-year-old girl.
Fig. 4.
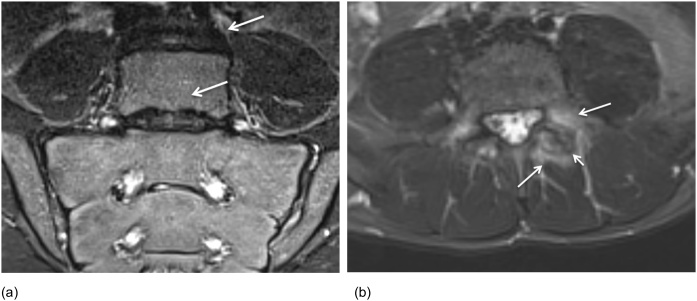
(a-b) Semicoronal STIR and T1 MR image in a 13-year-old girl with sacral fractures shows BME of S2-S3 on STIR (arrows) and transverse fracture lines on T1 (arrows). (c) Additional sagittal T1 was performed and shows disruption of the anterior cortex of S2 and S3 with mild anterior compression on S3 (arrows).
Fig. 5.
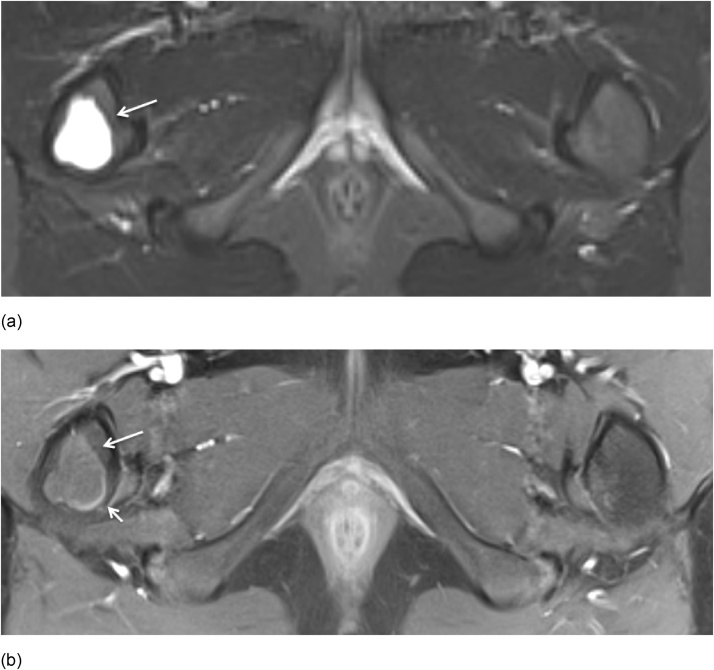
(a-b) Axial STIR and axial fat saturated T1-weighted MR image after IV Gd in a 9-year-old boy. Simple bone cyst was seen as a well-demarcated metaphyeal STIR hyperintense lesion (arrow) and T1 hypo-intense lesion (arrow) with minimal rim enhancement (short arrow) in the right femur.
Of all the incidental findings, axial degenerative changes were the most common. In 87 (16,1 %) patients there was disc degeneration and in 7 (1,3 %) patients there was facet joint degeneration (Fig. 6) (Table 3). Another frequent incidental finding was hip pathology (Fig. 7) with hip joint effusion as the most frequent finding in 24 (4,4 %) patients (Table 4).
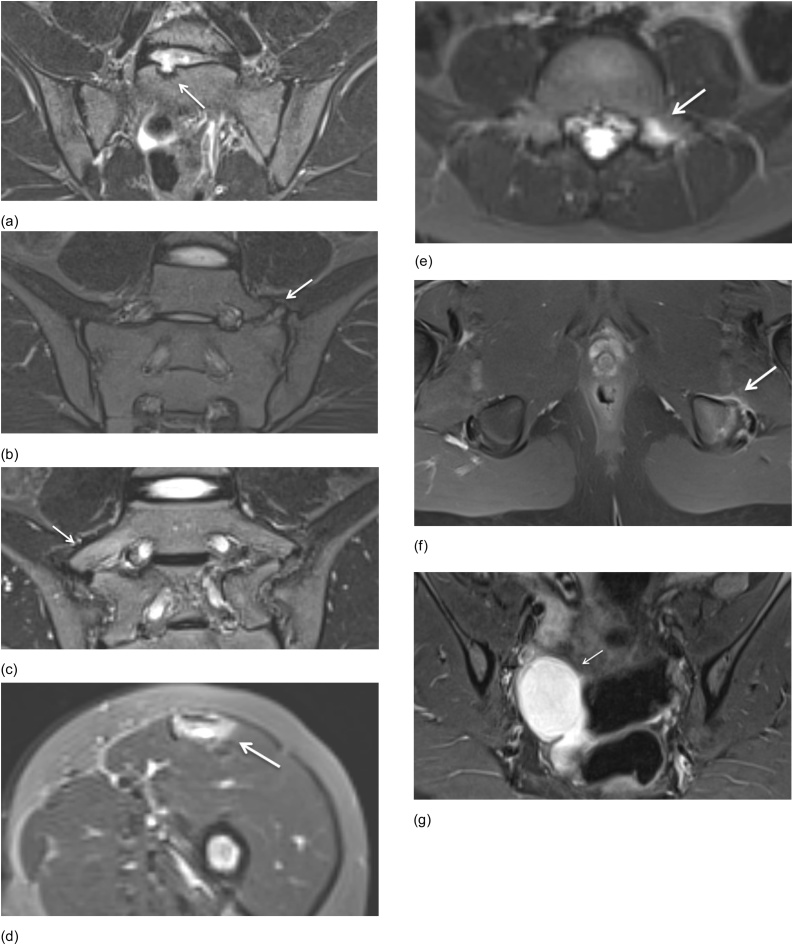
Fig. 6.
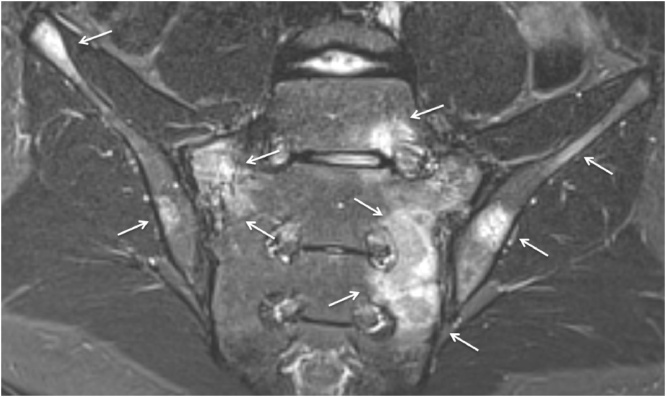
Degenerative changes of the lower lumbar spine. Semicoronal STIR MR images and axial STIR MR image (a) Disc degeneration in a 14-year-old girl shows disc space narrowing, loss of T2 signal within the nucleus pulposus and endplate changes (arrows). (b) Facet arthritis in a 14-year-old girl with surrounding soft tissue inflammation (arrows) with (secondary) degenerative changes with joint space narrowing, hypertrophy of the joint (short arrow) and fluid in the joint (not showed on this image).
Fig. 7.
Hip joint disease. Axial fat saturated T1-weighted MR image after IV Gd and axial STIR MR images. (a) Hip arthritis in a 13-year-old boy shows a joint effusion in the left hip joint with synovial enhancement (arrow) (b) Avascular necrosis (AVN) in a 17-year-old-boy demonstrates discrete T2 hyperintense signal changes in the femoral head on the right side (arrow), AVN was suspected and confirmed. (c) Radiography of the pelvis in the same patient one year later also confirmed the diagnosis of AVN. There is a subchondral fracture, subchondral sclerosis and flattening of the femoral head on the right side (arrow).
There were 3 cases of benign tumor: hemangioma, osteoid osteoma and a non-specific bony lesion of the right iliac wing with benign morphology (no further differentiation possible). There were 2 cases of malignant tumors: Hodgkin lymphoma (Fig. 8) and a large bone tumor of the sacrum (referred to an oncology centre). There were 4 unique cases listed as ‘other’ in Tables 1, 5 and 6 (together 0,7 %): pilonidal cyst (Fig. 9), scoliosis, hypertrophic nerve roots and sequelae of previous infective sacroiliitis.
Fig. 8.
Semicoronal STIR MR images in a 13-year-old boy. Diffuse areas of bone marrow edema (arrows) are present. This patient was ultimately diagnosed with Hodgkin lymphoma.
Fig. 9.
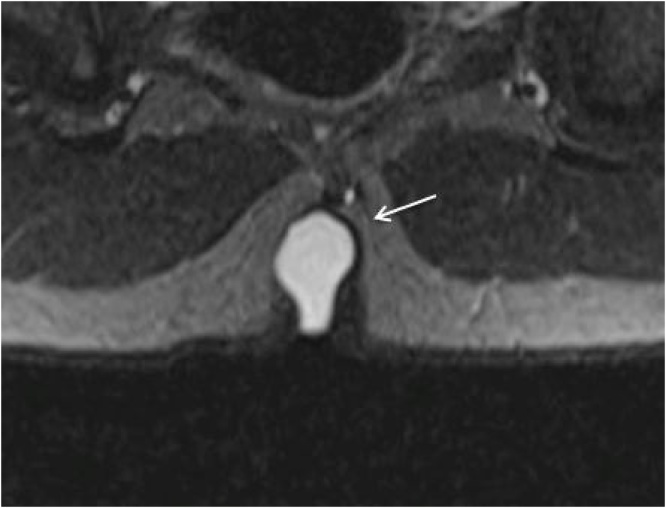
Semicoronal STIR MR image shows a pilonidal cyst (arrow) in a 14-year-old boy in the superior part of the intergluteal cleft.
4. Discussion
There is more to see on MRI of the SI joints in children than the SI joints alone. Our study demonstrated that MRI of the SI joints showed twice as many incidental findings (42 %) than sacroiliitis itself (20 %).
Incidental findings such as these can be important in daily radiology, since their detection may prevent unnecessary or alternative further imaging or require prompt further therapy [11]. In our study lumbosacral spine disease, especially axial degenerative changes, was the most frequent incidental finding. Tumor, infection and fracture were less frequently seen. This may be in part due to the distinct clinical presentation and the low incidence of the latter entities in children. However, given that these non-rheumatological diseases often are unexpected, accurate diagnosis is mandatory for timely and tailored treatment [11].
The incidental findings in our study are also seen in the normal population. Lumbosacral transitional anomaly can be present in 4–30 % of the general population [17] (in our study 5,2 %). By the age of 30 years, 40 % have lumbar intervertebral disc degeneration in general [18], in our study already 14,4 % of pediatric patients show degenerative disc disease.
Spina bifida occulta has an overall prevalence of 12,4 % in the general population [19]. In our study only 1,3% of patients had spina bifida occulta, which may be underestimated due to technical factors, including the lower spatial resolution of MRI than radiographs and the MRI field of view which in many scans only included a limited part of the posterior elements of the lumbosacral spine used in the protocol of SI joints.
The overall prevalence of Schmorl nodules in general population has been reported to be around 3,8 % [20], similar to the 3,1 % rate we found in our study.
Our study shows only limited correspondence between clinical findings and radiological findings. Only 20 % of patients proved to have sacroiliitis on MRI. In some patients, MRI might have been performed to rule out sacroiliitis rather than to confirm it, but this is hazardous since only about half of pediatric patients presenting with inflammatory back pain who ultimately are diagnosed with spondyloarthritis have any MRI abnormalities [12]. A thorough history and physical exam is important and may be helpful for correct diagnosis [11,21,22].
The presence of hip arthritis and enthesitis, characterized as incidental findings here, in patients suspected of JSpA may in fact be closely linked to the primary disease, considering that patients with JSpA more often present with peripheral arthritis and enthesitis, while symptoms involving the spine and SI joints often occur later [12,23].
There were some limitations to our study. First, all patients were imaged due to symptoms, giving us no normal control group. Second, our patient population came from three different hospitals, from different countries, with different MRI protocols and were reviewed by different radiologists. To some extent this is a strength of the study since it demonstrates that incidental findings are consistently seen across a wide range of MRI protocols and observers. However, some differences between the three institutions were substantial (Table 6). There was a notable difference in reporting of degenerative disc disease of the lumbosacral spine, edema pedicle/spondylolyse with BME, hip fluid/arthritis, simple (bone) cyst and enthesitis/tendinitis. Likely the main reason for this is that the Canadian and Polish institutions use a much narrower field of view, in which the hip and groin are mostly not shown, limiting assessment for hip fluid/arthritis and enthesitis/tendinitis. The sagittal localizer used in the Polish institution also facilitates detection of degenerative disc disease at that site. Degenerative disc disease may be less at the Belgian institution since their median age is a bit lower.
Our findings suggest, not surprisingly, that the larger the MRI field of view, the more incidental findings may be seen. Obtaining axial STIR images and a large FOV from L5 to the lesser trochanter when performing MRI of the SI joints can be helpful in a more comprehensive evaluation of inflammatory type back pain. If only semicoronal sequences of the SI joint and narrow field of view axial sequences are obtained, other findings that may be clinically relevant such as hip joint disease and enthesitis may remain undetected [11]. However, a trade-off with wide FOV imaging is decreased resolution at the SI joints, which may limit confidence when the primary question is whether sacroiliitis is present.
Another difference in MRI protocol was use of intravenous contrast. In Belgium Gd was administered to all patients routinely, while in Canada and Poland, no Gd was administered (except for one patient in the Polish institution who had a large sacral bone tumor). This might help account for different prevalence of hip arthritis seen.
5. Conclusion
In conclusion, incidental findings are common on MRI of the SI joints in children clinically suspected of JSpA. They are seen even more frequently than sacroiliitis and can be relevant to symptoms. Reporting of these findings is important, as some will have clinical significance or require treatment. Axial degenerative changes and hip disease were the most common findings. Whether the MRI field of view should be designed to capture these findings is an open question.
CRediT authorship contribution statement
E. Schiettecatte: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Visualization, Supervision, Project administration. J.L. Jaremko: Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization, Project administration. I. Sudoł-Szopińska: Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization, Project administration. M. Znajdek: Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization. R. Mandegaran: Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization. V. Swami: Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization. L. Jans: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - review & editing, Visualization, Supervision, Project administration. N. Herregods: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Colbert R.A. Classification of juvenile spondyloarthritis: enthesitis related arthritis and beyond. Nat. Rev. Rheumatol. 2010;6(8):477–485. doi: 10.1038/nrrheum.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin C., MacKenzie J.D., Courtier J.L., Gu J.T., Milojevic D. Magnetic resonance imaging findings in juvenile spondyloarthropathy and effects of treatment observed on subsequent imaging. Pediatr. Rheumatol. 2014;12:25. doi: 10.1186/1546-0096-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scofield R.H., Sestak A.L. Juvenile spondyloarthropathies. Curr. Rheumatol. Rep. 2012;14:395–401. doi: 10.1007/s11926-012-0273-3. [DOI] [PubMed] [Google Scholar]
- 4.Sudoł-Szopińska I., Eshed I., Jans L., Herregods N., Teh J., Vojinovic J. Classification and imaging of juvenile spondyloarthritis. J. Ultrason. 2018;18:224–233. doi: 10.15557/JoU.2018.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herregods N., Jaremko J.L., Baraliakos X., Dehoorne J., Leus A., Verstraete K., Jans L. Limited role of gadolinium to detect active sacroiliitis on MRI in juvenile spondyloarthritis. Skeletal Radiol. 2015;44(11):1637–1646. doi: 10.1007/s00256-015-2211-8. [DOI] [PubMed] [Google Scholar]
- 6.Herregods N., Dehoorne J., Van den Bosch F., Jaremko J.L., Van Vlaenderen J., Joos R., Baraliakos X., Varkas G., Verstraete K., Elewaut D., Jans L. ASAS definition for sacroilitis on MRI in SpA: applicable to children? Pediatr. Rheumatol. Online J. 2017;15:24. doi: 10.1186/s12969-017-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravelli A. Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 8.Haroon N., Inman R.D., Learch T.J., Weisman M.H., Lee M., Rahbar M.H., Ward M.M., Reveille J.D., Gensler L.S. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645–2654. doi: 10.1002/art.38070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse S.M.L., Laxer R.M. New advances in juvenile spondyloarthritis. Nat. Rev. Rheumatol. 2012;8:269–279. doi: 10.1038/nrrheum.2012.37. [DOI] [PubMed] [Google Scholar]
- 10.Baraliakos X., Haibel H., Listing J., Sieper J., Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]
- 11.Jans L., Van Praet L., Elewaut D., Van den Bosch F., Carron P., Jaremko J.L., Behaeghe M., Denis A., Huysse W., Lambrecht V., Verstraete K. MRI of the sacroiliac joints commonly shows non-inflammatory disease in patients clinically suspected of sacroiliitis. Eur. J. Radiol. 2014;83 doi: 10.1016/j.ejrad.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Jaremko J.L., Liu L., Winn N.J., Ellsworth J.E., Lambert R.G. Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J. Rheumatol. 2014;41 doi: 10.3899/jrheum.131064. [DOI] [PubMed] [Google Scholar]
- 13.Eshed I., Bollow M., McGonagle D.G., Tan A.L., Althoff C.E., Asbach P., Hermann K.G. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann. Rheum. Dis. 2007;66:1553–1559. doi: 10.1136/ard.2007.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jans L., Coeman L., Van Praet L., Carron P., Elewaut D., Van den Bosch F. How sensitive and specific are MRI features of sacroiliitis for diagnosis of spondylarthritis in patients with inflammatory back pain? JBR-BTR. 2014;97:202–205. doi: 10.5334/jbr-btr.94. [DOI] [PubMed] [Google Scholar]
- 15.Sieper J., Rudwaleit M., Baraliakos X., Brandt J., Braun J., Burgos-Vargas R., Dougados M., Hermann K.G., Landewé R., Maksymowych W., van der Heijde D. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009;68:ii1–ii44. doi: 10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 16.Maksymowych W.P., Lambert R.G., Østergaard M., Juhl Pedersen S.J. MRI lesions in the sacroiliac joints of patiens with spondyloarthritis: an update of definition and validation by the ASAS MRI wording group. Ann. Rheum. Dis. 2019;0:1–9. doi: 10.1136/annrheumdis-2019-215589. [DOI] [PubMed] [Google Scholar]
- 17.Konin J.P., Walz D.M. Lumbosacral transitional vertebrae: classification, imaging findings, and clinical relevance. Am. J. Neuroradiol. 2010;31:1778–1786. doi: 10.3174/ajnr.A2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung K., Karppinen J., Chan D., Ho D.W., Song Y.Q., Sham P., Cheah K.S., Leong J.C., Luk K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population of one thousand forty-three individuals. Spine. 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 19.Eubanks J.D., Cheruva V.K. Prevalence of sacral Spina Bifida Occulta and its relationship to age, sex, race, and the sacral table angle: an anatomic, Osteologic Study of Three Thousand One Hundred Specimens. Spine. 2009;34(15):1539–1943. doi: 10.1097/BRS.0b013e3181a98560. [DOI] [PubMed] [Google Scholar]
- 20.Sonne-Holm S., Jacobsen S., Rovsing H., Monrad H. The epidemiology of Schmorl’s nodes and their correlation to radiographic degeneration in 4,151 subjects. Eur. Spine J. 2013;22(8):1907–1912. doi: 10.1007/s00586-013-2735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taxter A.J., Chauvin N.A., Weiss P.F. Diagnosis and treatment of low back pain in the pediatric population. Phys. Sportsmed. 2014;42(1):94–104. doi: 10.3810/psm.2014.02.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia N.N., Chow G., Timon S.J., Watts H.G. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J. Pediatr. Orthop. 2008;28(March (2)):230–233. doi: 10.1097/BPO.0b013e3181651bc8. [DOI] [PubMed] [Google Scholar]
- 23.Herregods N., Dehoorne J., Pattyn E., Jaremko J.L., Baraliakos X., Elewaut D., Van Vlaenderen J., Van den Bosch F., Joos R., Verstraete K., Jans L. Diagnositic value of pelvic enthesitis on MRI of the sacroiliac joints in enthesitis related arthritis. Pediatr. Rheumatol. Online J. 2015;13(1):46. doi: 10.1186/s12969-015-0045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]