Abstract
Over 40 years ago, abnormal enlargement of the nucleus of tubular epithelial cells was reported in a rare distinct hereditary chronic interstitial nephritis, karyomegalic interstitial nephritis (KIN). Here, we report the second case of systemic karyomegaly with pulmonary manifestations and present a detailed characterization of the karyomegalic cells in lung parenchyma. A 59-year-old woman who was diagnosed with KIN developed renal failure and eventually received a renal transplant later evaluated for chronic and progressive restrictive lung disease. The KIN diagnosis prompted us to carefully examine her lung parenchyma. Karyomegalic cells were identified in the alveolar epithelium, interstitium, as well as, in the vascular wall. Viral serological and biochemical blood analyses were negative. We consider that the pulmonary manifestations of karyomegaly expands the differential diagnosis of interstitial lung disease in patients with KIN.
Keywords: Karyomegaly, Interstitial lung disease, Karyomegalic interstitial nephritis
1. Introduction
Karyomegalic interstitial nephritis (KIN) is a rare cause of hereditary chronic interstitial nephritis, that was first described in 1970s [[1], [2], [3]]. The term KIN was introduced by Michael J. Mihatsch et al. [3]. More than 50 cases of KIN have been reported in the literature [4]. This disease presents as a slowly progressive chronic kidney disease, eventually leading to end-stage renal disease before the age of 50. Karyomegalic cells in renal biopsy specimens are distinguishable from other common causes of chronic tubulointerstitial nephritis. The karyomegalic tubular epithelial cells of the proximal and distal tubules are characterized by markedly enlarged and hyperchromatic nuclei. Autosomal recessive inheritance in families with KIN has been reported by Zhou et al. and mutations in the FAN1 (FANCD2/FANCI-Associated Nuclease 1) gene [5]. FAN1 is involved in the DNA damage response pathway, particularly in the kidney, indicating a potential link between defective DNA repair and chronic kidney disease progression [6].
The only case of systemic karyomegaly with primary pulmonary has been reported in a 33-year-old woman evaluated for chronic and progressive restrictive lung disease and ultimately required single-lung transplantation. A few months after transplantation, graft dysfunction, respiratory decline and renal failure caused her death. At autopsy, karyomegalic cells have been identified in the kidneys and her native lung [7]. Here, we report the second case of systemic karyomegaly with pulmonary manifestations.
2. Case presentation
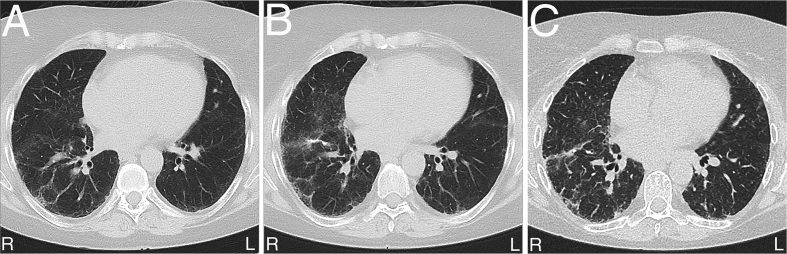
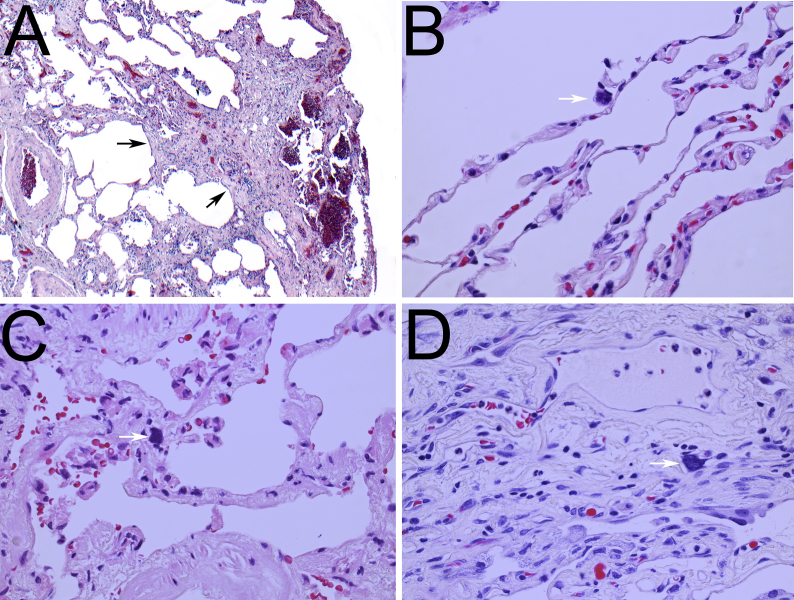
During a trip abroad, a 50-year old woman was evaluated in February 2010 for acute gastroenteritis and elevated levels of creatinine were accidently detected. Serological levels of ANA, ANCA, anti-dsDNA, ENA as well as viral hepatitis were within normal ranges. Urea level was in the range of 16–25 mmol/L. A renal biopsy was later taken due to the elevation in creatinine levels of unknown reason. In March 2010, histomorphological analyses revealed chronic tubulointerstitial nephritis with obvious nuclear changes in the tubular epithelium. These changes were in agreement with KIN (Fig. 1A). Due to development of renal failure, she was started on dialysis in February 2012 and eventually received a renal transplant in July 2015. Increased level of transaminases was detected during her pre-transplantation screening and a liver biopsy was then taken in November 2012, but no pathological change was noted. She later developed respiratory symptoms that were clinically suspected to be due to interstitial pulmonary disease. Non-specific radiological signs included subtle reticular abnormalities, thickened interlobular septa, basal traction bronchiectasis, microcystic subpleural changes, mild ground-glass opacification, and honeycombing (Fig. 2). These radiological signs seen in March 2018 (Fig. 2A) were progressed as detected by subsequent CT-thorax analysis in September 2018 (Fig. 2B) and March 2019 (Fig. 2C), particularly at the right lung lobes. No sign of confluence of inflammation, atelectasis or enlarged mediastinal lymph nodes was observed. Pleural and pericardial spaces were radiologically ordinary. Surgical lung wedge biopsies from the upper and lower lobes of the right lung were undertaken. These showed predominantly patchy established interstitial fibrosis with areas of honeycomb change. Occasional areas of fibroblastic proliferation were present and there was a mild non-specific chronic inflammatory infiltrate, focally with a few eosinophils. Fibrotic changes were more marked in the lower lobe. In terms of a histologic pattern, the features were closest to those of usual interstitial pneumonia (UIP) (Fig. 3A). However, there were also scattered karyomegalic cells, either in the alveolar lining (Fig. 3B), alveolar interstitium (Fig. 3C), or within the perivascular interstitium (Fig. 3D), and the concluding diagnosis was pulmonary involvement by KIN with a UIP pattern of fibrosis.
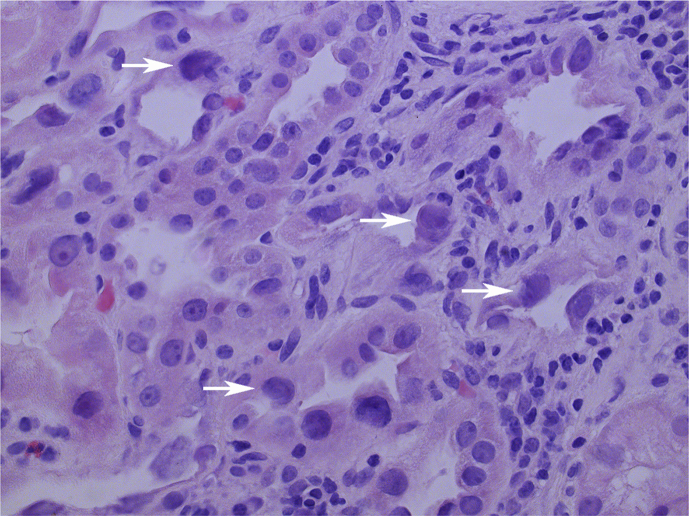
Fig. 1.
Histopathological image of renal cortex demonstrates enlarged and hyperchromatic karyomegalic tubular epithelial cells (white arrows). Original magnification x200. Hematoxylin & Eosin stain.
Fig. 2.
Pulmonary radiological changes as detected by CT-thorax analysis (A). Progression of these changes 6 months (B) and 18 months later (C).
Fig. 3.
Images of the lung that show a histological pattern of usual interstitial pneumonia, with subpleural fibrosis and scattered fibroblastic foci (black arrows) (A). Pulmonary parenchyma demonstrates karyomegalic cells (white arrows) within the alveolar epithelium (B), alveolar (C) and perivascular (D) interstitium. (A) Original magnification x40, (B–D) x200. Hematoxylin & Eosin stain.
3. Discussion
This is the second report describing manifestations of karyomegaly in the lung. In addition to karyomegalic cells present within the alveolar epithelium [7], we report the presence of karyomegalic cells even in the alveolar and perivascular interstitium. Notably, pulmonary manifestations of karyomegaly produced significant progressive decline in pulmonary function.
The pathogenesis of systemic karyomegaly is not completely understood. Toxic substances such as ifosfamide [8], trichloroethylene [9] and ochratoxin A [10] have been reported to induce KIN at variably levels in different experimental models. Renal tubule karyomegaly occurs in mice in response to chemical insult, but much less commonly than in the rat [11]. Renal biopsies following the administration of these drugs reveal chronic tubulointerstitial nephritis with atypical tubular epithelial cells showing nuclear enlargement and hyperchromasia, consistent with a diagnosis of KIN. However, human reports of karyomegaly in the kidney associated with chemical exposure are rare, and linked mainly to chemotherapeutic or antiviral therapies [11]. In addition to toxic substances, penicillium nephrotoxins also cause persistent karyomegaly in different animals [12,13].
By exome sequencing, mutations in FAN1 have been identified as a probable cause of KIN [5]. The FAN1 protein has nuclease activity and acts in DNA interstrand cross-link (ICL) repair within the Fanconi anemia partly mediated by DNA damage response pathway. Fan1-deficient mice develop KIN [14,15]. In addition to the kidneys, karyomegaly becomes prominent even in the liver of Fan1-deficient mice developing liver dysfunction with age [16]. In our case, there were no karyomegalic cells in liver biopsies. The ubiquitin-binding zinc finger domain of FAN1 is needed for interaction with FANCD2, which is not required for the initial rapid recruitment of FAN1 to ICLs or for its role in DNA ICL resistance. Epistasis analyses reveal that FAN1 has ICL activities that are independent of the Fanconi anemia proteins and that this activity is redundant with the 5′-3′ exonuclease SNM1A [16]. These experimental results provide insight into the mechanism of FAN1 in ICL repair and demonstrate that the Fan1 mouse model effectively recapitulates the pathological features of human FAN1 deficiency. However, manifestations of karyomegaly in lungs have not been reported in these mouse models of Fan1-deficiency.
Another concern is whether karyomegaly leads to carcinogenic development. Nephrotoxic substances including ochratoxin A induce carcinogenicity in the kidneys [17], in which karyomegaly is prominent in tubular epithelium in experimental models. Nevertheless, renal carcinogenicity following karyomegaly is not consistently associated with renal tubule tumor development in experimental animals. Spontaneous tumor development has not been reported in the Fan1-deficient mouse model either, but this needs to be determined, particularly in aged mice. Thus, renal tubule karyomegaly currently remains an inaccurate predictor of renal tubule neoplasia, and there is no evidence that karyomegalic cells are involved in tumor development as a form of preneoplasia [11].
Our case report indicates that systemic karyomegaly can also manifest in the lung, with a histologic pattern closest to UIP. It is important to realize that the karyomegalic cells in the lung are very few and can be easily overlooked if the pathologist is not aware of the connection with KIN and the presentation of the disease in the lung. Inadequate DNA repair as a result of FAN1 mutations can make the lungs more sensitive to infection and injury. Therefore, patients with KIN diagnoses should be closely monitored as manifestations of at least the lungs and liver that have been reported to exhibit karyomegalic cells in experimental and clinical settings. The characterization of systemic karyomegaly with symptomatic lung involvement expands the differential diagnosis for patients presenting with interstitial lung disease, particularly when non-specific histopathological findings are present.
Declaration of competing interest
None declared.
Acknowledgements
We thank The Swedish Cancer Research, Västra Götalandregionen and The Swedish Society of Pathology for grants (to L.M.A.).
References
- 1.Burry A.F. Extreme dysplasia in renal epithelium of a young woman dying from hepatocarcinoma. J. Pathol. 1974;113(3):147–150. doi: 10.1002/path.1711130303. [DOI] [PubMed] [Google Scholar]
- 2.Sclare G. A case of unexplained karyomegaly. Beitr. Pathol. 1976;157(3):301–306. doi: 10.1016/s0005-8165(76)80089-3. [DOI] [PubMed] [Google Scholar]
- 3.Mihatsch M.J. Systemic karyomegaly associated with chronic interstitial nephritis. A new disease entity? Clin. Nephrol. 1979;12(2):54–62. [PubMed] [Google Scholar]
- 4.Isnard P. Karyomegalic interstitial nephritis: a case report and review of the literature. Medicine (Baltim.) 2016;95(20) doi: 10.1097/MD.0000000000003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012;44(8):910–915. doi: 10.1038/ng.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachaud C. Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability. Science. 2016;351(6275):846–849. doi: 10.1126/science.aad5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagliente D.J. Systemic karyomegaly with primary pulmonary presentation. Hum. Pathol. 2014;45(1):180–184. doi: 10.1016/j.humpath.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Jayasurya R. Karyomegalic interstitial nephropathy following ifosfamide therapy. Indian J. Nephrol. 2016;26(4):294–297. doi: 10.4103/0971-4065.171233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lock E.A., Reed C.J. Trichloroethylene: mechanisms of renal toxicity and renal cancer and relevance to risk assessment. Toxicol. Sci. 2006;91(2):313–331. doi: 10.1093/toxsci/kfj107. [DOI] [PubMed] [Google Scholar]
- 10.Taniai E. Ochratoxin A induces karyomegaly and cell cycle aberrations in renal tubular cells without relation to induction of oxidative stress responses in rats. Toxicol. Lett. 2014;224(1):64–72. doi: 10.1016/j.toxlet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Hard G.C. Critical review of renal tubule karyomegaly in non-clinical safety evaluation studies and its significance for human risk assessment. Crit. Rev. Toxicol. 2018;48(7):575–595. doi: 10.1080/10408444.2018.1503641. [DOI] [PubMed] [Google Scholar]
- 12.Mantle P.G. Persistent karyomegaly caused by Penicillium nephrotoxins in the rat. Proc. Biol. Sci. 1991;246(1317):251–259. doi: 10.1098/rspb.1991.0152. [DOI] [PubMed] [Google Scholar]
- 13.Mantle P.G., McHugh K.M., Fincham J.E. Contrasting nephropathic responses to oral administration of extract of cultured Penicillium polonicum in rat and primate. Toxins. 2010;2(8):2083–2097. doi: 10.3390/toxins2082083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Airik R. A FANCD2/FANCI-associated nuclease 1-knockout model develops karyomegalic interstitial nephritis. J. Am. Soc. Nephrol. 2016;27(12):3552–3559. doi: 10.1681/ASN.2015101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachaud C. Karyomegalic interstitial nephritis and DNA damage-induced polyploidy in Fan1 nuclease-defective knock-in mice. Genes Dev. 2016;30(6):639–644. doi: 10.1101/gad.276287.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thongthip S. Fan1 deficiency results in DNA interstrand cross-link repair defects, enhanced tissue karyomegaly, and organ dysfunction. Genes Dev. 2016;30(6):645–659. doi: 10.1101/gad.276261.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X. Ochratoxin A induces rat renal carcinogenicity with limited induction of oxidative stress responses. Toxicol. Appl. Pharmacol. 2014;280(3):543–549. doi: 10.1016/j.taap.2014.08.030. [DOI] [PubMed] [Google Scholar]