Abstract
Background
Food allergy (FA) is a serious, costly and growing health problem worldwide. FA occurs in both children and adults; however, there is a paucity of information on FA prevalence and its clinical features in the adult population, especially in Asia. We sought to assess the prevalence of FAs in Vietnamese adults and the distribution of offending food items among different regions throughout Vietnam.
Methods
A nationwide, cross-sectional, population-based survey was conducted among University students aged 16–50 years. We used a structured, anonymous questionnaire, which was modified from recently published FA epidemiologic studies and based on European Academy of Allergy and Clinical Immunology (EAACI) guidelines, to collect data on FA prevalence, clinical presentations, and implicated food groups. Statistical analysis was performed to generate the prevalence of self-reported and doctor-diagnosed FA and to examine the association of key environmental factors and FA incidence in this population.
Results
Of the 14,500 surveys distributed, a total of 9,039 responses were returned, resulting in a response rate of 62.4%. Among participants who reported food-induced adverse reactions, 48.0% have repeated reactions. 18.0% of the participants perceived FA symptoms, but less than half of them sought medical services for confirmation (37.9%). Stratifying for true FA symptoms, the prevalence of self-reported FA was 11.8% and of doctor-diagnosed FA, 4.6%. The most common doctor-diagnosed FA was to crustacean (3.0%; 95% CI, 2.6–3.3), followed by fish (1.6%; 95% CI, 1.3–1.8), mollusk (1.3%; 95% CI, 1.0–1.5) and beef (1.0%; 95% CI, 0.8–1.2). The prevalence of doctor-diagnosed FA differed among participants living in urban (6.5%) and rural regions (4.9%) (P < 0.001). Atopic family history was the strongest predictor for FA (odds ratio 8.0; 95% CI, 6.2–10.4).
Conclusions
Seafood allergy among adults is predominant in Vietnam, followed by beef, milk, and egg, while peanut, soy, and tree nut allergy are much less common. Populations in rural regions have considerably less FA; however, the protective environmental factors have yet to be identified.
Keywords: Adults, Food allergy, Prevalence, Seafood allergy, Vietnam
Abbreviations: FA, Food allergy; HREC, Human Research Ethics Committee; EAACI, European Academy of Allergy and Clinical Immunology; WAO, World Allergy Organization; IQR, Interquartile range; OR, Odds ratio
Introduction
Food allergy (FA) is defined as abnormal reactions of the human's immune system triggered by food components during food ingestion and/or food exposure process. FA presents with a wide range of clinical manifestations, from a mild skin problem to acute and severe systemic reactions. FA occurs in both children and adults, and is among the most common causes of food-induced anaphylaxis.1 FA lessens the quality of life and imposes a substantial financial burden to its sufferers;2,3 thus, it has been considered a major public health problem in many westernized countries.
Approximately 10% of children and 5% of adults in developed countries experience FA, and this incidence is reported to rise.4 However, very little is known about this epidemic in other parts of the world, especially in developing economies. In Asia, most FA studies focused on children, reporting prevalence rates of 1.11%–7.65%. Epidemiological studies on FA among adults were only conducted in a few Asian countries, and they revealed a prevalence of 18% in China,5 6.4% in Taiwan,6 1.2% in India,7 and 0.21% for wheat allergy in Japan.8 Furthermore, major food triggers even varied from country to country in the region and demonstrated different patterns of food allergens as compared to countries in the West.9
Adulthood FA might be initiated and transitioned from childhood incidence such as in the case of peanut allergy or seafood allergy;10 however, new sensitizations to food allergens in adults were also reported.11 It is well evidenced that long-term exposure to broadly presented allergens from the environment could trigger the development of later FA.12,13 As a result, adults might have different patterns of food allergens and clinical manifestations as compared to the phenotype of FA in children. The study of FA in adults is of importance to provide valuable insight into the nature and development of FA over the course of life.
Our first population-based survey on FA was conducted among Vietnamese adults, comparing two different survey modes: traditional paper-based survey and online survey.14 This study validated the data from two survey modes and proposed the application of the online survey as an economic and validated model for future epidemiological studies. Extended from previous work, in this study a detailed analysis of the paper-based FA survey data was conducted to identify the pattern of FA and food allergens among Vietnamese adults and detect FA risk factors in this population.
Methods
Survey design
A cross-sectional, randomized paper-based survey was conducted from March to December 2016 among university students across 4 different regions of Vietnam. Questionnaires were distributed to the target populations, and most of the answer sheets were collected on the same day. By accepting to answer the questionnaire, a participant consented to the study. The response rate was calculated by dividing the number of returned questionnaires by the total distributed questionnaires. This study was approved by the Human Research Ethics Committee (HREC) at James Cook University (ID: H6437).
Participant recruitment
The minimum sample size of 1,963 participants was required to obtain a precision level of 20%, with p and a confident level of 95%. The study population was recruited using the cluster sampling method. Participants were selected randomly from over 50,000 students at 3 participating universities across Vietnam (Supplemental Figure 1). The participating universities included Nong Lam University, Nha Trang University, and the University of Food Industry. These are multi-disciplinary universities with a wide diversity of student age ranges and backgrounds. The survey at Nong Lam University was conducted at its 3 different campuses in Kon Tum province, Ninh Thuan province, and Ho Chi Minh City.
Questionnaire
Participants were invited to answer a structured, anonymous questionnaire comprised of 2 parts: Part I asked the participant demographic information; Part II consisted of 10 questions on FA (Supplemental Appendix 1). The questionnaire was developed based on the pre-existing, standardized questionnaire of recent epidemiological studies conducted in Asian populations.15,16 The questionnaire was modified taking into consideration the current understanding of general Vietnamese adults about FA definition and its symptoms.
Definitions
Two definitions of FA were used in this study: self-reported FA and doctor-diagnosed FA, based on the most recent European Academy of Allergy and Clinical Immunology (EAACI) guidelines on FA and anaphylaxis.17,18 The suggestive symptoms of FA include: (a) having persistent symptoms towards food ingestion and the co-occurrence of 2 or more different clinical adverse reactions; (b) having typical symptoms for Immunoglobulin E (IgE)-mediated FA, including hives/urticaria or angioedema or gastrointestinal symptoms or anaphylaxis reactions (i.e. a drop in blood pressure, loss of consciousness, chest pain and weak pulse) after food intake.
Self-reported FA was the group of participants who fulfilled the above criteria and reported having FA.
Doctor-diagnosed FA was the group of participants with self-reported FA and self-reported to be clinically confirmed by a medical practitioner.
Food-induced adverse symptoms: any abnormal clinical response that occurs following ingestion of a food or a food component.49
Family history of FA: participant had in their immediate family a member with FA.
Coexisting other allergic diseases: participant had any other allergic diseases including pollen allergy, antibiotic allergy, asthma, eczema.
The lifetime prevalence of self-reported and doctor-diagnosed FA were estimated.
Statistical analysis
For the analysis of generated data, the IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA) was used. A sampling design without replacement was chosen for the estimation of prevalence. The ratio of male to female participants was weighted to fit the natural gender ratio in Vietnam.19 Continuous variables were presented as median and interquartile range (IQR). Categorical data were compared by using Chi-square tests with a 2-tailed P-value. The prevalence was calculated to provide a 95% CI of responses to each criterion. Multiple logistic regression model was used to study the association between multiple risk factors and the incidence of having doctor-diagnosed FA. The significance level was considered at a P-value of <0.05 for all tests.
Results
Demographic features
Table 1 presents the demographic features of the survey. The questionnaire was distributed to 14,500 subjects, with 9,039 subjects responding (response rate 62.4%). The median age and IQR of participants were 20 and 2 years. The survey recruited participants from 5 different regions of Vietnam. There were more participants from South Central Coast (3,753 participants) and South East (4,249 participants) than the remaining areas: North Central Coast (91 participants), the Central Highlands (617 participants), and Mekong Delta (329 participants). Female participation (67.3%) was much higher than male participation in all survey sites.
Table 1.
Demographic features of participants in this survey.
| Variable | n (%) |
|---|---|
| Total questionnaire distributed | 14,500 (100) |
| Number of respondents | 9,039 (62.4) |
| Sex distribution | |
| Male | 2,955 (32.7) |
| Female | 6,084 (67.3) |
| Age median (years) | 20 |
| Interquartile range | 18–20 |
| Age range (years) | |
| 16–20 | 6,802 (75.3) |
| 21–25 | 2,064 (22.8) |
| 26–30 | 88 (1.0) |
| 31–35 | 41 (0.5 |
| Over 35 | 44 (0.5) |
| Number of participants by regions | |
| North Central Coast | 91 (1.0) |
| South Central Coast | 3,753 (41.5) |
| Central Highlands | 617 (6.8) |
| South East | 4,249 (47.0) |
| Mekong Delta | 329 (3.6) |
| Distribution of health service approach in this study by regionsa | |
| North Central Coast | 3 (15.0) |
| South Central Coast | 364 (22.3) |
| Central Highlands | 78 (26.4) |
| South East | 513 (27.5) |
| Mekong Delta | 45 (28.7) |
| Doctor-diagnosed FA | |
| FA to 1 food group | 264 (50.1) |
| FA to 2 different food groups | 117 (22.2) |
| FA to more than 2 different food groups | 146 (27.7) |
Among participants with food-induced adverse symptoms. Percentage was calculated by dividing the number of participant visits to health care services by the total number of participants with food-induced adverse reactions. FA: Food allergy
Reported food-induced adverse reactions and offending food groups
There were 6,563 (72.6%) respondents who experienced adverse clinical symptoms after food intake, with an average of 3.7 symptoms per respondent (Supplemental Table 1). Symptom re-occurrences were reported in 48% of participants (Supplemental Table 2). Gastrointestinal symptoms were the leading complaint with the contribution of diarrhea (16.7%), followed by nausea or vomiting (12.2%) and stomach pain (10.6%) (Supplemental Table 1). Systematic reactions and skin problems were the most common reasons for medical service visit/hospital admission (Supplemental Table 3). The study reported different rates of medical service approach towards health problems across studied regions (Table 1).
The top 3 causative food items belong to the seafood group: crustacean (28%), fish (15.2%), and mollusk (15.1%). Milk (9.5%) and beef (6.8%) were more common offending foods, as compared to peanut (5.0%), wheat (5.0%), tree nut (4.6%), egg (3.8%), and soy (3.3%). Other reactive foods, besides beef, included animal meats (i.e., chicken, duck, dog, and cat), fruits (i.e., mango, papaya, and strawberry), and vegetables (mostly chilli and mushroom), and alcoholic drinks (i.e., beer and wine) accounted for the remaining 10.2% (Supplemental Table 4).
In this survey, of the 1,629 (18.0%) participants who perceived FA, only 617 subjects (37.9%) sought medical services for their health condition. Of the 617 medical services-seeking participants, 527 (85.4%) were diagnosed to have FA, indicating that 14.6% of the remaining adults might manifest food-induced adverse reactions (e.g., by food toxins) or could not be confirmed due to unavailable diagnostics. Among the doctor-diagnosed FA group, half of the participants reported adverse reactions to only one food item; 22.2% had reactions to 2 different food groups and the remaining 27.7% of FA patients had allergic reactions to more than 2 different food groups (Table 1).
Prevalence of self-reported and doctor-diagnosed FA
The survey data were weighted by gender according to the current distribution of male and female adults aged below 50 years in Vietnam19 to estimate a more accurate prevalence of FA (Supplemental Table 2). As anticipated, the overall prevalence of FA for all survey food groups was more than two-fold in self-reported than in doctor-diagnosed participants (11.8% vs. 4.6%) (Table 2). Crustacean, fish, and mollusk were the top 3 allergy-triggering foods. The pattern of offending foods was the same for both self-reported and doctor-diagnosed group, except for milk. Combining data from crustacean and mollusk allergy indicated a prevalence of 10.0% (95% CI: 9.4–10.6) and 4.2% (95% CI: 3.8–4.6) to shellfish allergy in self-reported and in the doctor-diagnosed group, respectively.
Table 2.
Weighted prevalence of FA in study population.
| Self-reported FA | Doctor-diagnosed FA | |
|---|---|---|
| Any food | 11.80 (11.14–12.47) | 4.55 (4.12–4.98) |
| Crustacean | 6.88 (6.36–7.40) | 2.95 (2.60–3.30) |
| Fish | 3.71 (3.32–4.10) | 1.58 (1.32–1.84) |
| Mollusk | 3.09 (2.73–3.44) | 1.27 (1.04–1.50) |
| Beef | 2.09 (1.80–2.39) | 0.95 (0.75–1.15) |
| Milk | 1.66 (1.40–1.92) | 0.46 (0.32–0.60) |
| Egg | 1.04 (0.83–1.25) | 0.65 (0.49–0.82) |
| Wheat | 1.06 (0.85–1.27) | 0.37 (0.24–0.49) |
| Peanut | 0.89 (0.69–1.08) | 0.32 (0.20–0.44) |
| Soy | 0.81 (0.62–0.99) | 0.31 (0.20–0.42) |
| Tree nut | 0.77 (0.59–0.96) | 0.25 (0.15–0.36) |
| Other foods | 2.05 (1.75–2.34) | 0.66 (0.50–0.83) |
Value reported as % (95% CI). FA: Food allergy. Any food = any food groups other than listed in the questionnaire including "other food". Other foods= other food groups not listed in the questionnaire. Other food commodities reported in the survey are animal meat (i.e. chicken, duck, dog, and cat), fruits (i.e. mango, papaya, and strawberry), vegetables (mostly chili and mushroom), and alcoholic drinks (i.e. beer and wine)
Clinical features of FA
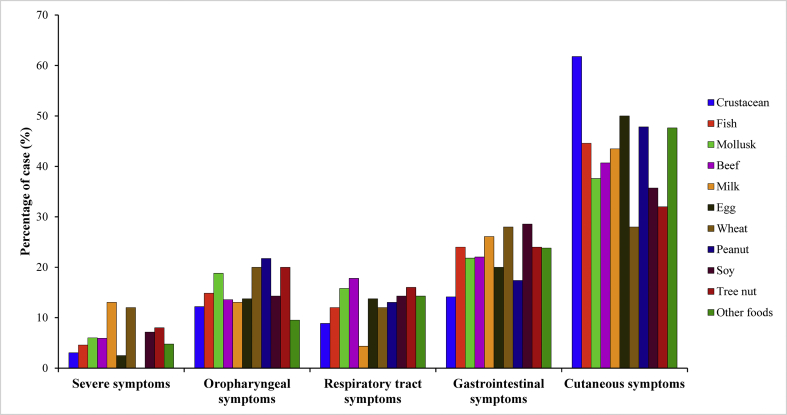
Clinical features of doctor-diagnosed FA participants are presented in Fig. 1. Allergic subjects presented with multiple adverse symptoms involving different organs (an average of 5.5 symptoms per subject). Cutaneous symptoms (hives/urticaria, eczema) were dominant, present in 87.8% of all confirmed FA subjects, followed by gastrointestinal symptoms (diarrhea, nausea/vomiting, and stomach pain). The manifestation of severe reactions (i.e. loss of consciousness, weak pulse, drop in blood pressure, chest pain) was not rare among FA subjects, accounting for up to 38.9% of all affected participants.
Fig. 1.
Distribution of clinical manifestations among doctor-diagnosed FA participants (n = 506) by food allergens. Clinical symptoms are divided into 5 categories: severe symptoms (loss of consciousness, weak pulse, drop in blood pressure, chest pain); oropharyngeal symptoms (trouble swallowing, itchy mouth or ear canal, odd taste in mouth, swelling of the lips, tongue and/or throat, redness of the skin or around eyes); respiratory tract symptoms (sneezing, nasal congestion or a runny nose, coughing); gastrointestinal symptoms (nausea or vomiting, diarrhoea, stomach pain), and cutaneous symptoms (hives, eczema). Other foods: other food groups that were not listed in the questionnaire. Other food commodities reported in the survey are animal meat (i.e. chicken, duck, dog, and cat), fruits (i.e. mango, papaya, and strawberry), vegetables (mostly chili and mushroom), and alcoholic drinks (i.e. beer and wine)
Influence of demographic factors on the risk of having FA
The influence of demographic factors on FA was analyzed by multivariable logistic regression (Table 3). Predictor variables were gender, family history of FA, and co-existence of other allergic diseases, while the outcome variable was doctor-diagnosed FA. Family history of FA was shown to be the strongest predictor of doctor-diagnosed FA (odds ratio (OR), 8.0, P < 0.001), while co-existing other allergic diseases (P = 0.734) and gender (P = 0.082) did not show any significant associations with doctor-diagnosed FA rate.
Table 3.
Multivariable logistic regression analysis of demographic factors to FA.
| Risk factor, OR (95%CI) | P - value | |
|---|---|---|
| Sex (Female/Male) | 1.2 (1.0–1.5) | 0.082 |
| Family history of FA (Yes/No) | 8.0 (6.2–10.4) | <0.001 |
| Co-existing other allergic diseases (Yes/No) | 1.0 (0.8–1.3) | 0.734 |
Binary logistic regression was performed in SPSS Statistics for Windows to generate ORs. A P-value of <0.05 was considered as statistically significant, and highlighted in bold. FA: Food allergy
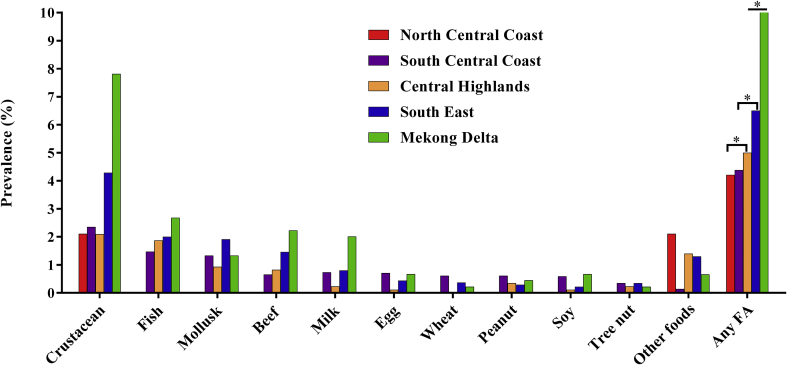
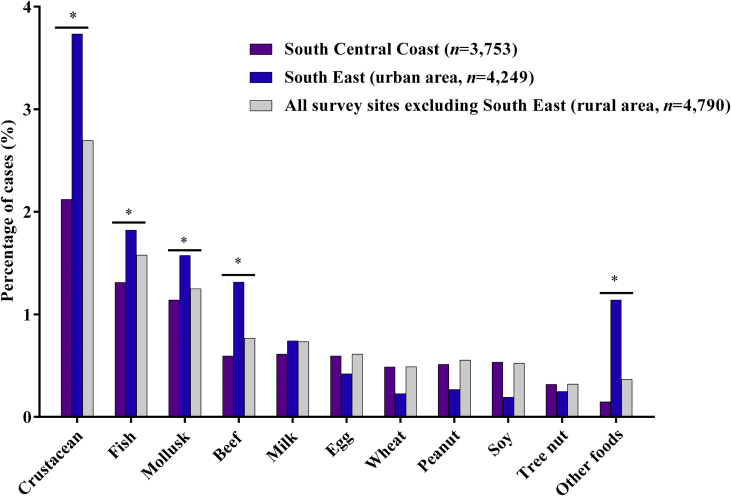
The relationship between living location and the incidence of having a doctor-diagnosed FA was analyzed using Chi-square tests. The difference in overall FA incidence was recorded between the South Central Coast and the South East (P < 0.001), between the Central Highlands and the Mekong Delta (P < 0.05) and between South East and Mekong Delta (P < 0.001) (Fig. 2). Specifically, the prevalence of FA in the Mekong Delta (9.7%) was much higher than in the other study sites: South East region (7.1%), South Central Coast (4.3%), North Central Coast (3.3%) and Central Highlands (4.7%). Taking into consideration the impacts of population density, lifestyle and living environment, participants from the South East — mostly residing in Ho Chi Minh City, the biggest city in Vietnam19— were defined as people living in urban area and participants from other survey sites were considered as living in the rural areas. We observed higher prevalence of crustacean (3.73% vs. 2.69%), fish (1.82% vs. 1.57%), mollusk (1.58% vs. 1.25%), beef (1.32% vs. 0.77%), and other foods allergy (1.14% vs. 0.37%) (P < 0.001) in South East as compared to the other study sites (Fig. 3).
Fig. 2.
Distribution of the prevalence of doctor-diagnosed food allergies across different regions in Vietnam. Statistical significance was recorded between North Central Coast (n = 91) and Central Highlands (n = 617) (P < 0.001), between South Central Coast (n = 3,753) and South East (n = 4,249) (P < 0.001); between South East (n = 4,249) and Mekong Delta (n = 329) (P < 0.001), and between Central Highlands (n = 617) and Mekong Delta (n = 329) (P < 0.05). Other food: other food groups that were not listed in the questionnaire. Other food commodities reported in the survey are animal meat (i.e. chicken, duck, dog, and cat), fruits (i.e. mango, papaya, and strawberry), vegetables (mostly chili and mushroom), and alcoholic drinks (i.e. beer and wine). Any FA: any food groups other than listed in the questionnaire including other foods. ∗ denotes statistical significance (P < 0.001)
Fig. 3.
Distribution of the prevalence of doctor-diagnosed food allergies among 2 major survey sites: South Central Coast (n = 3,753) and South East (n = 4,249). Survey data were combined to generate the prevalence of food allergies for the population living in the rural areas of Vietnam (n = 4,790). Taking into consideration of population density and lifestyles, participations from South East (mostly reside in Ho Chi Minh City, the biggest city in Vietnam) were considered as population living in the urban areas. The comparison of FA prevalence among population living in rural and urban was conducted. Urban population demonstrated a higher risk of being sensitized to seafood, beef and some other foods (P < 0.001). Other foods: other food groups that were not listed in the questionnaire. Other food commodities reported in the survey are animal meat (i.e. chicken, duck, dog, and cat), fruits (i.e. mango, papaya, and strawberry), vegetables (mostly chili and mushroom), and alcoholic drinks (i.e. beer and wine). ∗ denotes statistical significance (P < 0.001)
Discussion
This study determined the lifetime prevalence of doctor-diagnosed FA among Vietnamese adults at 4.6% (95% CI, 4.1–5.0), which is lower than the 6.4% rate previously reported in Taiwan adults.6 The pattern of FA demonstrated seafood as the most common food culprit, consistent with findings in Korean adults20 and the current trend among adults in the United States.21 Our study demonstrated the disparity of FAs across geographic locations (P < 0.001), implying the possible influence of environmental exposures and dietary habits to allergy risk. Additionally, family history of FA was strongly associated with FA (OR, 8.0; 95% CI, 6.2–10.4) but not for other allergic comorbidities or gender factor. These findings would be of great interest to local clinicians, researchers, and policy makers and beneficial for a better management of FA in Vietnam.
We noted a discrepancy between people with suggestive FA symptoms and those who approached medical advice for FA diagnosis. Specifically, less than half of the self-reported FA subjects in this survey ever visited doctors for their medical condition. While most people who visit a medical practitioner are confirmed to have FA, there is a high proportion of people with FA who do not seek advice. These people remain undiagnosed and untreated, leaving them at risk of unexpected FA reactions, which could be fatal. In the current context of Vietnam, the low rates of presentation for suspected allergy symptoms might be explained by insufficient awareness of the general public about FA and/or the possible shortage of medical services providing allergy testing.
Manifestations of FA in adults in the study varied according to the causative allergen (Fig. 1). Among FA events, the major manifestation of FA in Vietnamese adults involved cutaneous symptoms (42.7%). Hives was the major indicator of allergic condition for all food allergens in the study, and this is consistent with previous studies20 and EAACI guidelines.17 The second most frequent FA manifestation was gastrointestinal symptoms, induced more by foods from plant origin than animal origin in this study. We also noticed that plant-origin foods were the major cause of oral allergy syndrome in the doctor-diagnosed FA group. However, milk and wheat were the leading causative food items that evoked severe FA events/anaphylaxis; milk and wheat allergy were reported by 0.46% and 0.37%, respectively. Previous studies showed that most food-induced anaphylaxis in adults were caused by plant foods such as wheat, peanut, and tree nut.22,23 Thus, presenting severe milk-inducing food allergic reactions is rather unusual in adults. Lactose intolerance is predominant in the Asian population,24 and it is undoubtedly presumed as the major suspected reason for any adverse symptoms evoking by milk consumption. However, in a recent investigation of FA in Israeli patients, milk-induced anaphylaxis was reported and confirmed in adults who reported to previously tolerate that food.11 This finding is of importance for clinicians and FA specialists as well as adult patients with milk allergy in addressing the significant risk of anaphylactic and possibly fatal reactions.
In our study, seafood allergy clearly accounted for more than half of all FA cases. The perceived shellfish allergy in Vietnamese adults (10.0%) is higher than the rate previously reported in Taiwanese counterparts (7.05%).6 We also demonstrated a doctor-diagnosed shellfish allergy prevalence of 4.2%, which is the highest adult shellfish allergy rate ever reported worldwide.25 Shellfish is a common food source in the Asia Pacific region and has been claimed as the leading allergic food in this region.26 A retrospective survey in Korean patients demonstrated seafood including crustacean, cephalopod, and fish to be the most frequent cause of FA and seafood-induced anaphylaxis in adults (51.1%).20 Similar findings were reported in both Taiwanese children and adults with FA.6 Although there are limited robust studies to investigate the evolution of seafood allergy throughout a life course, we noticed a strikingly high rate of shellfish allergy in both children and adults in Asia.14 In fact, investigations on paediatric shellfish allergy were outnumbered by similar studies in adults. Shellfish allergy was reported in very young children aged 3-months to 6-years in Thailand (0.3%)27 and appeared to surge in other older children. School-age children from Vietnam showed a prevalence of 3.83% to crustacean and 1.03% to mollusk allergy,28 while shellfish allergy rates were 5.12% and 5.23% in Filipino and Singaporean adolescents, respectively.16 There were several hypotheses claimed to elucidate the elevated rate of shellfish allergy in the region including the great abundance and high consumption of this food commodity.9 Furthermore, the tropical climate condition might play a role in favoring the abundance of indoor creatures (e.g., house dust mites and cockroaches)29 that the clinical cross-reactivity of these indoor allergens with allergen molecule in shellfish (e.g. tropomyosin) was widely documented.30,31
Similarly, we found a higher rate of doctor-diagnosed fish allergy (1.58%) in this cohort than previously reported in the United States (0.8%)21 and Canada (0.56%).32 The self-reported fish allergy in Vietnamese adults (3.71) is much higher than in Taiwan (1.17%).6 The identified prevalence of seafood allergy in Vietnamese adults appears to surpass the highest rates established in any published study from North America, Europe, and Asia (i.e. Taiwan).33 One plausible explanation is the availability and abundance of this food commodity in Vietnam as a major source of animal protein.34 Vietnamese consume an average of 33 kg fish per capita per year in comparison to 22 kg in North America and Europe.35 A correlation between seafood consumption rate and seafood allergy prevalence among different survey sites was observed (Fig. 2).36 Another potential cause might be the allergic reaction to Anisakis, a food-borne parasitic nematode frequently contaminating fish.37 Although no specific case of Anisakis infection has been reported in Vietnam, parasite infection via seafood vectors is commonly reported.38,39 The presence of this food-borne allergen seems to be particularly common in raw and undercooked fish, and it was reported to cause infection and allergic reactions in Thailand, Korea, and Japan.39, 40, 41
The current study identified beef as the fourth most common allergy-induced food. A strong correlation of beef allergy with previous tick bites has been previously identified in Australia, Europe, and the United States.42,43 The observed anaphylactic reactions were explained by the production of specific IgE antibodies to galactose-α-1,3-galactose (α-Gal), a carbohydrate present in red meat. While no reports on tick bites in Vietnam have been published, ticks are very common in the region,44 and they could be a new and not yet identified cause of beef allergy in Asia.
FA is thought to be controlled, at least in part, by the interaction between genetic and environmental factors. When family history, atopy, sex, and living location were considered, we observed that family history of FA was the strongest predictor for FA in adults. This finding is in line with previous population-based studies in infants where the investigators revealed that having 2 or more allergic family members increased the risk of having FA in the child (OR, 1.8; 95% CI 1.5–2.3).45 The disparity of male and female participants in the survey as well as the variation in the FA incidence by gender were demonstrated. Female participants seemed to have a higher rate of FA (5.1%) than their male counterparts (3.9%) (Supplemental Table 2). Similar findings were reported in a recent study of North American adults in which female adults were 1.67 times at higher risk of having food allergies than the male participants.46 Furthermore, the geographical location can have a profound impact on allergen exposure, thus increasing the risk of developing atopic conditions.47 In this study, we noted the variation of FA incidence among different geographic regions of Vietnam, with a higher incidence of FA among people living in urban areas compared to rural areas (P < 0.001). This observation supports the hypothesis that there are possible protective influences in the rural environment, and postulated mechanisms include the hygiene hypothesis.48
The major limitation of this study is that the information of doctor-diagnosed FA was self-reported. Furthermore, it is not known if the physicians diagnosing FA in this study group in Vietnam were utilizing the currently available FA diagnostic tests. It would be ideal to confirm the allergic responses in suspected participants combining with other diagnostic methods, including allergen-specific serum IgE quantification and oral food challenge. However, the initial scope of this study was to evaluate the current situation of FA in Vietnam and to approach affected FA patients. The manifestation of true FA among Vietnamese patients is currently under investigation by the authors, using established in vivo and in vitro diagnostics.
This survey gained a slightly lower response rate (62.4%) than previous studies on FA in other Asian countries: 67.9% in Singapore, 81.1% in the Philippines, and 80.2% in Thailand.15 We did not conduct any further investigations on the non-response group. We assume that a paper-based questionnaire survey might not be popular with most Vietnamese, in addition, limited information and/or awareness on FA in the public might influence the response rate. We are also aware that the selection of university students might misrepresent the general Vietnamese adult population. However, a weak correlation of education level to FA incidence was demonstrated in the U.S. adults (OR, 1.06; 95% CI, 1.03–1.09).46 Furthermore, 3 different universities in 5 different geographical regions participated, including over 50,000 students from different age groups and diverse cultural backgrounds. Therefore, the sample selection enabled this study to gain objective and representative data on FA in Vietnam.
Conclusions
This study provides the first population-based data on FA in the adult Vietnamese population. Our findings revealed the dominance of seafood allergy and the commonality of beef allergy as a new allergen source to be reported among adults in the Asian population. This study also suggests that under-diagnosis and under-treatment of FA may occur, owing to low rates of presentation to medical services for FA in Vietnam.
Declarations
Ethics approval
This study was approved by the Human Research Ethics Committee (HREC) at James Cook University (ID: H6437).
Consent for publication
All authors have approved the manuscript for submission.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was funded by grants from the Australian Research Council (ARC), and the National Health and Medical Research Council (ID: 1086656) to AL. TL is the recipient of the Endeavour Postgraduate Scholarship.
Authors’ contributions
TL and AL developed the concept and study design. TT, HH, AV and TL conducted the on-site survey and processed survey data. EM and TL performed the statistical analysis. AL edited the final manuscript. All authors contributed to the development of the manuscript and approved the final version.
Acknowledgment
This study received the financial support from the Higher Degree Research Enhancement Scheme (HDRES) of the College of Public Health, Medical and Veterinary Sciences, James Cook University for open access publication.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100102.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pouessel G., Turner P.J., Worm M. Food-induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. 2018;48(12):1584–1593. doi: 10.1111/cea.13287. [DOI] [PubMed] [Google Scholar]
- 2.DunnGalvin A., Koman E., Raver E. An examination of the food allergy quality of life questionnaire performance in a countrywide American sample of children: cross-cultural differences in age and impact in the United States and Europe. J Allergy Clin Immunol Pract. 2017;5(2):363–368 e2. doi: 10.1016/j.jaip.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Ferro M.A., Van Lieshout R.J., Ohayon J., Scott J.G. Emotional and behavioral problems in adolescents and young adults with food allergy. Allergy. 2016;71(4):532–540. doi: 10.1111/all.12829. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.Y., Zhuang Y., Ma T.T., Zhang B., Wang X.Y. Prevalence of self-reported food allergy in six regions of inner Mongolia, northern China: a population-based survey. Med Sci Mon Int Med J Exp Clin Res. 2018;24:1902–1911. doi: 10.12659/MSM.908365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T.C., Tsai T.C., Huang C.F. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42(12):1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 7.Mahesh P.A., Wong G.W., Ogorodova L. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71(7):1010–1019. doi: 10.1111/all.12868. [DOI] [PubMed] [Google Scholar]
- 8.Fujimori A., Yamashita T., Kubota M., Saito H., Takamatsu N., Nambu M. Comparison of the prevalence and characteristics of food hypersensitivity among adolescent and older women. Asia Pac J Clin Nutr. 2016;25(4):858–862. doi: 10.6133/apjcn.092015.39. [DOI] [PubMed] [Google Scholar]
- 9.Lee A.J., Thalayasingam M., Lee B.W. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3(1):3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamdar T.A., Peterson S., Lau C.H., Saltoun C.A., Gupta R.S., Bryce P.J. Prevalence and characteristics of adult-onset food allergy. J Allergy Clin Immunol Pract. 2015;3(1):114–115 e1. doi: 10.1016/j.jaip.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachshon L., Goldberg M.R., Elizur A., Appel M.Y., Levy M.B., Katz Y. Food allergy to previously tolerated foods: course and patient characteristics. Ann Allergy Asthma Immunol. 2018;121(1):77–81. doi: 10.1016/j.anai.2018.04.012. e1. [DOI] [PubMed] [Google Scholar]
- 12.Lopata A.L., Jeebhay M.F. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013;13(3):288–297. doi: 10.1007/s11882-013-0347-y. [DOI] [PubMed] [Google Scholar]
- 13.Werfel T., Asero R., Ballmer-Weber B.K. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70(9):1079–1090. doi: 10.1111/all.12666. [DOI] [PubMed] [Google Scholar]
- 14.Le T.T.K., Tran T.T.B., Ho H.T.M., Vu A.T.L., Lopata A.L. Prevalence of food allergy in Vietnam: comparison of web-based with traditional paper-based survey. World Allergy Organ J. 2018;11(1):16. doi: 10.1186/s40413-018-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connett G.J., Gerez I., Cabrera-Morales E.A. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159(4):384–390. doi: 10.1159/000338940. [DOI] [PubMed] [Google Scholar]
- 16.Shek L.P., Cabrera-Morales E.A., Soh S.E. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126(2):324–331. doi: 10.1016/j.jaci.2010.06.003. 31 e1-7. [DOI] [PubMed] [Google Scholar]
- 17.Muraro A., Werfel T., Hoffmann-Sommergruber K. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69(8):1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 18.Halken S., Muraro A. Global atlas of allergy. In: Akdis A.C., Agache I., editors. Food Allergy. EAACI; Zurich: 2014. pp. 199–201. [Google Scholar]
- 19.Statistical Yearbook of Vietnam 2015 - Population and Employment. Statistical Publishing House; 2016. file:///C:/Users/jc303254/Downloads/02.%20Dan%20so%20-%20Lao%20dong%20(3).pdf [Internet] [cited 20 April 2017]. Available from: [Google Scholar]
- 20.Lee S.-H., Ban G.-Y., Jeong K. A retrospective study of Korean adults with food allergy: differences in phenotypes and causes. Allergy Asthma Immunol Res. 2017;9(6):534–539. doi: 10.4168/aair.2017.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verrill L., Bruns R., Luccioli S. Allergy Asthma Proc; 2015. Prevalence of Self Reported Food Allergy in US Adults: 2001, 2006, and 2010. [DOI] [PubMed] [Google Scholar]
- 22.Asero R., Antonicelli L., Arena A. Causes of food-induced anaphylaxis in Italian adults: a multi-centre study. Int Arch Allergy Immunol. 2009;150(3):271–277. doi: 10.1159/000222679. [DOI] [PubMed] [Google Scholar]
- 23.Parrish C.P., Kim H. Food-induced anaphylaxis: an update. Curr Allergy Asthma Rep. 2018;18(8):41. doi: 10.1007/s11882-018-0795-5. [DOI] [PubMed] [Google Scholar]
- 24.Hegar B., Widodo A. Lactose intolerance in Indonesian children. Asia Pac J Clin Nutr. 2015;24(Suppl 1):S31–S40. doi: 10.6133/apjcn.2015.24.s1.06. [DOI] [PubMed] [Google Scholar]
- 25.Ruethers T., Taki A.C., Johnston E.B. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Lee A.J., Gerez I., Shek L.P., Lee B.W. Shellfish allergy--an Asia-Pacific perspective. Asian Pac J Allergy Immunol. 2012;30(1):3–10. [PubMed] [Google Scholar]
- 27.Santadusit S., Atthapaisalsarudee S., Vichyanond P. Prevalence of adverse food reactions and food allergy among Thai children. J Med Assoc Thai. 2005;88(Suppl 8):S27–S32. [PubMed] [Google Scholar]
- 28.Le T.T.K., Nguyen D.H., Vu A.T.L., Ruethers T., Taki A.C., Lopata A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr Allergy Immunol. 2019;30:348–355. doi: 10.1111/pai.13022. [DOI] [PubMed] [Google Scholar]
- 29.D'Amato G., Holgate S.T., Pawankar R. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ J. 2015;8(1):25. doi: 10.1186/s40413-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popescu F.D. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;5(2):31–50. doi: 10.5662/wjm.v5.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng G., Luo W., Wu Z. A cross-sectional observational study on allergen-specific IgE positivity in a southeast coastal versus a southwest inland region of China. Sci Rep. 2017;7(1):9593. doi: 10.1038/s41598-017-10109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soller L., Ben-Shoshan M., Harrington D.W. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130(4):986–988. doi: 10.1016/j.jaci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Moonesinghe H., Mackenzie H., Venter C. Prevalence of fish and shellfish allergy: a systematic review. Ann Allergy Asthma Immunol. 2016;117(3):264–272 e4. doi: 10.1016/j.anai.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Hop L.T. Programs to improve production and consumption of animal source foods and malnutrition in Vietnam. J Nutr. 2003;133(11) doi: 10.1093/jn/133.11.4006S. 4006S-9S. [DOI] [PubMed] [Google Scholar]
- 35.Per Capita Consumption of Fish and Shellfish for Human Food, by Region and Country. FAOSTAT; 2013. http://www.fao.org/faostat/en/#data/BL [Internet] [cited 10/04/2017]. Available from: [Google Scholar]
- 36.Lopata A.L., Kleine-Tebbe J., Kamath S.D. Allergens and molecular diagnostics of shellfish allergy: Part 22 of the Series Molecular Allergology. Allergo J Int. 2016;25(7):210–218. doi: 10.1007/s40629-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieuwenhuizen N.E., Lopata A.L. Allergic reactions to Anisakis found in fish. Curr Allergy Asthma Rep. 2014;14(8):455. doi: 10.1007/s11882-014-0455-3. [DOI] [PubMed] [Google Scholar]
- 38.Hung N.M., Dung do T., Lan Anh N.T. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasites Vectors. 2015;8:21. doi: 10.1186/s13071-015-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuchjangreed C., Hamzah Z., Suntornthiticharoen P., Sorosjinda-Nunthawarasilp P. Anisakids in marine fish from the coast of chon buri province, Thailand. Southeast Asian J Trop Med Publ Health. 2006;37(Suppl 3):35–39. [PubMed] [Google Scholar]
- 40.Baird F.J., Gasser R.B., Jabbar A., Lopata A.L. Foodborne anisakiasis and allergy. Mol Cell Probes. 2014;28(4):167–174. doi: 10.1016/j.mcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Choi S.J., Lee J.C., Kim M.J., Hur G.Y., Shin S.Y., Park H.S. The clinical characteristics of Anisakis allergy in Korea. Korean J Intern Med. 2009;24(2):160–163. doi: 10.3904/kjim.2009.24.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripathi A., Commins S.P., Heymann P.W., Platts-Mills T.A. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(3):259–265. doi: 10.1016/j.jaip.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wuerdeman M.F., Harrison J.M. A case of tick-bite-induced red meat allergy. Mil Med. 2014;179(4):e473–e475. doi: 10.7205/MILMED-D-13-00369. [DOI] [PubMed] [Google Scholar]
- 44.Apanaskevich M.A., Apanaskevich D.A. Description of new dermacentor (Acari: ixodidae) species from Malaysia and Vietnam. J Med Entomol. 2015;52(2):156–162. doi: 10.1093/jme/tjv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koplin J.J., Allen K.J., Gurrin L.C. The impact of family history of allergy on risk of food allergy: a population-based study of infants. Int J Environ Res Publ Health. 2013;10(11):5364–5377. doi: 10.3390/ijerph10115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta R.S., Warren C.M., Smith B.M. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1) doi: 10.1001/jamanetworkopen.2018.5630. e185630-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oktaria V., Dharmage S.C., Burgess J.A. Association between latitude and allergic diseases: a longitudinal study from childhood to middle-age. Ann Allergy Asthma Immunol. 2013;110(2):80–85. doi: 10.1016/j.anai.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Gupta R.S., Singh A.M., Walkner M. Hygiene factors associated with childhood food allergy and asthma. Allergy Asthma Proc. 2016;37(6):140–146. doi: 10.2500/aap.2016.37.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson S.G., Hourihane J.O., Bousquet J., Bruijnzeel-Koomen C., Dreborg S., Haahtela T. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56(9):813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.