Abstract
RNA-binding proteins typically change the fate of RNA, such as stability, translation or processing. Conversely, we recently uncovered that the small non-coding vault RNA 1-1 (vtRNA1-1) directly binds to the autophagic receptor p62/SQSTM1 and changes the protein's function. We refer to this process as ‘riboregulation'. Here, we discuss this newly uncovered vault RNA function against the background of three decades of vault RNA research. We highlight the vtRNA1-1-p62 interaction as an example of riboregulation of a key cellular process.
Keywords: p62, riboregulation, vault RNA 1-1
1. Vault RNAs—small, non-coding and mysterious
Even though vault RNAs have been discovered more than thirty years ago, the molecular function of these abundant, small non-coding RNA polymerase III (Pol III) transcripts has remained unclear [1,2]. Vault RNAs were initially described by Kedersha and Rome as components of 13 MDa ribonucleoprotein assemblies that were identified serendipitously while isolating coated vesicles from rat liver. The ovoid morphology and arch-like structure of these complexes reminiscent of gothic cathedral ceilings prompted their naming as ‘vault particle' or ‘vaults' [1].
Vaults are the largest ribonucleoprotein complexes known to date. They measure 400 × 400 × 700 Å and hence could enclose cellular structures bigger than ribosomes [3,4]. Vaults can reach high copy numbers (10 000–100 000 per cell) in organisms ranging from protists to humans [5–10]. Their structure as well as protein composition are highly conserved, suggesting a fundamental function in eukaryotic cells [11]. The main constituent of the particle is the major vault protein (MVP, 99 kDa), which accounts for more than 70% of the particle mass [12] (reviewed in [13]). Structural studies revealed that the expression of MVP alone suffices for the assembly of vault-like nanoparticles [14,15]. However, full integrity and a morphology indistinguishable from tissue-derived vaults requires co-expression of the two minor vault proteins, vault poly-(adenosine diphosphate-ribose) polymerase (VPARP, 193 kDa) and telomerase-associated protein 1 (TEP1, 290 kDa) [16]. By contrast, the association of vault RNA at the caps of the vault particle does not alter the particle's general morphology [7,12,17,18]. Importantly, most of the vault RNA is not associated with the particle [17], implying that it could well be involved in additional cellular interactions. Like the vault particle, the detailed function and mechanism of action of vault RNAs has remained mysterious over decades.
2. The genomic organization of vault RNA genes
Two vault RNA loci are syntenically conserved across most mammals [11]. In humans, the VTRNA1 locus is situated between the ZMAT2 (zinc finger matrin-type 2) gene and the PCHD (protocadherin) cluster, while the VTRNA2 locus is found in close proximity, between TGFB1 (transforming growth factor beta 1) and SMAD5 (SMAD family member 5) on the same chromosome [11,19–21]. The vault RNA promoters exhibit considerable differences between the two syntenically conserved loci possibly resulting in differential expression patterns of the encoded vault RNAs [11]. So far, no functional relationship between vault RNAs and the syntenically conserved genes has been uncovered. However, with the newly described link between vault RNAs and autophagy (see below), it is noteworthy that members of the protocadherin family have also been described to associate with autophagy-related proteins and to influence lysosome targeting [22]. Additional clues that could explain the syntenic conservation might be uncovered in the future.
The substantial variation in length and sequence of the vault RNA central domain (figure 1) has hampered homology-based searches for vault RNAs in other species [11]. Experimental validation has so far been obtained for a single vault RNA in M. musculus and R. norvegicus [2,24,29], two in R. catesbeiana [2], four paralogues in H. sapiens [7,30] and one each in S. purpuratus [31], T. brucei [32], D. rerio and O. latipes [11]. Interestingly, in S. purpuratus the vault-associated RNA seems to contribute to vault particle integrity [31]. Furthermore, the development of iterative algorithms led to the identification of more than 100 potential vault RNA genes in deuterostome genomes [11]. Strikingly, the vault RNA 5′ and 3′ regions are predicted to form double-stranded structures in all species [2,11]. It has further been speculated that the function of the relatively long rodent vault RNA could encompass the function of several smaller RNA in other species [33].
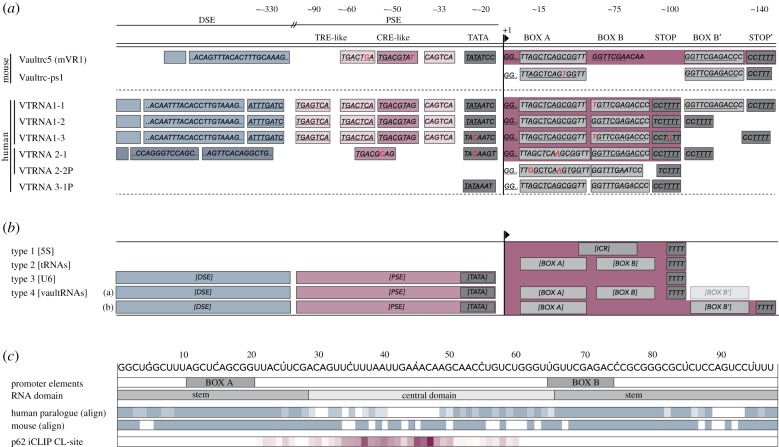
Figure 1.
Vault RNAs are expressed from unusual RNA polymerase III (Pol III) promoters. (a) Overview of vault RNA loci. Transcriptional elements of the human and mouse vault RNA gene family (largely based on [11]). Depicted are key sequence elements of the vault RNA gene promoter and regulatory elements. The transcribed gene body is indicated by dark red background shading. Specific differences between sequence elements of the vault RNAs are highlighted in red. The name and location of sequence elements relative to the transcription start sites are indicated above. Underlined regions indicate canonical transcription factor-binding or termination motifs. (b) Different Pol III promoter types and their features. Polymerase III type 1, 2 and 3 promoters have been previously described [23]. The composite nature of vault Pol III type 4 promoters was initially proposed by [24]. (c) Vault RNA transcript features. Alignment of vault RNA features with its sequence. Top row, numbering from the transcription start site and sequence. Below, location of internal promoter elements within the transcript and structure predictions according to thermodynamic models. Middle, sequence alignment of human vtRNA1-1 to the human vault RNA paralogues or mouse mVR1 according to LocARNA (http://rna.informatik.uni-freiburg.de, v. (4.5.8); [25–27]. Darker shading represents increased conservation. Below, mean cross-link site values in p62 IPs according to individual nucleotide cross-link and immunoprecipitation (iCLIP). Darker shading represents increased cross-linking of p62 to vtRNA1-1 [28]. vtRNA, vault RNA; DSE, distal sequence element; PSE, proximal sequence element; CRE, cAMP responsive element; TRE, tetradecanoylphorbol acetate response element; TATA, TATA box element.
The human genome encodes three vault RNA paralogues at the VTRNA1 locus (VTRNA1-1, VTRNA1-2 and VTRNA1-3 (formerly HVG-1/2/3 [7]) and one at the VTRNA2 locus (VTRNA2-1 (also referred to as pre-miR-886 or CBL3) [19,20]), both located on chromosome 5q31. In addition, two vault RNA pseudogenes––VTRNA2-2P and VTRNA3-1P (formerly HVG-4)––are annotated in the human genome assembly hg38 on chromosomes 2 and X, respectively (figure 1a). The existence of more vault RNA pseudogenes without syntenic conservation has been proposed [11]. For the purpose of simplicity, we refer to the transcripts deriving from the two vault RNA loci as ‘vtRNA1-1’, ‘vtRNA1-2’, ‘vtRNA1-3’ and ‘vtRNA2-1’ in this review.
When comparing different species, the vault RNA genes show only limited sequence conservation beyond their internal Pol III type II promoter elements [11]. These comprise the box A and box B motifs, which are typically found in tRNA genes (figure 1) [2,11]. The two internal promoter elements serve as binding sites for the transcription factor TFIIIC, which in turn positions TFIIIB immediately upstream and thereby facilitates Pol III binding to the transcription start site (reviewed in [23]). Generic Pol III type II genes do not include additional upstream promoter sequences. However, mutational analysis of the rat vault RNA gene uncovered additional external promoter elements that contribute to transcription efficiency and regulation [24].
These elements include CRE-like (cAMP response element) and TRE-like (tetradecanoylphorbol acetate response element) motifs as part of a proximal sequence element and further distal sequence elements (figure 1) [2,11]. The proximal CRE-like element is highly conserved between different species [11] and present upstream of all transcribed human paralogues, while it is missing for the vault RNA pseudogenes (figure 1), implying that it could be a determinant of vault RNA expression. CRE- and TRE-like elements are known to bind CREB and AP-1 transcription factor complexes, respectively, which integrate growth factor, nutrient and stress signalling––including bacterial and viral infections––to control key cellular processes such as proliferation, survival and differentiation [34–38]. In fact, the induction of vault RNA transcription upon viral infection [19,20,39,40] and the responsiveness of intracellular vtRNA1-1 levels to starvation [28] indicate that these transcription factor complexes could play a role in regulating vault RNA expression levels. In addition, NF-κB signalling and p65/RELA binding to the distal promoter region of VTRNA1-1 were shown to promote its transcription upon viral infection [39]. Further experiments may determine synergies between, and determinants of, transcription factor binding at the vault RNA loci.
Human VTRNA1-1 as well as other primate vault RNAs harbour a second copy of the box B motif and termination sequence downstream of the transcribed gene body that has been reported to negatively influence transcription depending on the upstream promoter sequences [11,24]. It is a feature that distinguishes the human VTRNA1-1 gene from the other human vault RNA paralogues (figure 1).
Overall, the unique vault RNA Pol III promoter composition including type-2 internal sequences as well as type-3 upstream elements that act synergistically has been suggested to constitute a separate class of Pol III promoters (figure 1b; [24]). Since similar composite promoter arrangements are found in viral RNAs (e.g. EBER in EBV) and the protein MVP is able to self-assemble, the vault complex had been discussed to be an evolutionary relict of an early viral symbiont [20].
3. Expression of vault RNAs
The rat vault RNA shows uneven expression levels in different tissues, with particularly high abundance in the spleen, intestine and heart, and low levels in the brain, liver and kidney [2]. Similarly, the relative expression levels of the vault RNA paralogues vary in different human cell lines. However, vtRNA1-1 represents the predominant vault RNA species deriving from the VTRNA1 locus in most cell lines examined [21]. A higher association of vtRNA1-3 with the vault particle has been reported in multi-drug-resistant cell lines independent of total vtRNA1-1 levels [21], suggesting that vtRNA1-3 is the prime RNA interacting with the vault particle in this context. However, the functional relevance and molecular details of this observation remain to be elucidated.
Besides sequence differences in the promoter region (figure 1) [11,21] and variation in the spacing of internal box A and box B elements [21], epigenetic modifications could contribute to the differential expression of the vault RNA paralogues. Promoter methylation was shown to inversely correlate with the expression levels of vtRNA1-2, vtRNA1-3 and vtRNA2-1 [41,42]. Interestingly, DNA hypermethylation, especially of the VTRNA2-1 gene, further correlated with poor prognosis of some cancers, suggesting a potential role of this non-coding RNA as a tumour suppressor [41–46]. By contrast, the VTRNA1-1 locus does not seem to be subject to methylation in a similar fashion [41]. The proximal promoter regions of all four expressed vault RNA paralogues are nucleosome depleted, facilitating active transcription initiation [47]. Conversely, the distant regulatory elements show differential GpC accessibility––especially for VTRNA1-1––suggesting that epigenetic regulation of these regions could contribute to cell type-specific vault RNA expression [47]. Since vault RNA levels can change profoundly in response to starvation or viral infections, it will be important to decipher the contribution of the various proximal and distal polymerase III promoter elements on the transcriptional regulation of vault RNAs in these contexts.
4. Beyond the primary transcript—from modifications to processing
In addition to transcriptional regulation, post-transcriptional modifications and processing events contribute to the modulation of vault RNA abundance and function. Initial analyses revealed that the rat vault RNA is an uncapped RNA with a 5′ triphosphate (pppG) and an intact 3′ poly-uridine track [2,17]. It has been proposed that DUSP11-mediated dephosphorylation of the 5′ pppG promotes processing and turnover of vault RNA transcripts [48,49]. Accordingly, vault RNA levels are increased in DUSP11 knockout (KO) cells [49]. Interestingly, an infection-dependent reduction in DUSP11 levels results in the accumulation of 5′ pppG vault RNAs that was proposed to trigger an innate immune response via RIG-I like receptors [50]. In addition, uridylation and subsequent binding of the exoribonuclease DIS3L2 to vault RNAs serves as a 3′ directed cytoplasmic quality control and degradation mechanism [51,52].
The most prominently studied modification of vault RNAs is the NSUN2-dependent deposition of 5-methylcytosine (m5C; [53–55]. The m5C modification at C69 of vtRNA1-1 has been demonstrated to regulate vtRNA1-1 processing into smaller regulatory fragments [53,55]. These so-called svRNAs (small-vault RNAs) derive from the primary vtRNA1-1 stem region in a Dicer-dependent way and regulate their target genes (e.g. CYP3A4 and CACNG7/8) in a miRNA-like fashion [53,56] (reviewed in [57]). The abundance of svRNAs 1, 2 and 3 was shown to increase upon NSUN2 depletion and in models of multi-drug resistance, while their relevance under physiological conditions remains unclear [42,56,58]. By contrast, the levels of svRNA4––whose 5′ end starts with the modified C69––decreased upon NSUN2 depletion associated with increased binding of SRSF2 to the primary vtRNA1-1 transcript [55]. The m5C modification and resulting processing into svRNA4 were further proposed to be altered during cell differentiation [55].
Other vault RNA modifications include N6-methyladenosine (m6A [59]) and pseudouridylation (Ψ [60]). It is tempting to speculate that the high similarity between the internal promoter elements of vault and tRNAs genes could result in common RNA processing and modification events. However, a systematic approach to either is pending.
5. Vault RNA structural features
All vault RNAs identified to date are predicted to form distinct stem-loop structures in thermodynamic models [2,11]. RNase H structural probing of cell-derived human vtRNA1-1, vtRNA2-1 or mouse mVR1/VAULTRC5, however, suggests a far more open conformation of the central loop region (figure 1) [20,61]. In line with this, mutational analysis of the mouse vault RNA revealed increased affinity to Tep1 upon destabilization of existing complementarities within the central loop region (G70A, C73U) [61], indicating that a single-stranded nature of the central loop region could favour protein binding. Still, the tertiary structure of vault RNAs remains to be determined.
6. Unleashing non-coding power in autophagy—vault RNA ‘riboregulates' p62
Since its discovery more than thirty years ago, the vault particle has been functionally implicated in drug resistance, apoptosis and nuclear transport (extensively reviewed in [62]). Only few studies focused on vault RNAs as an entity separate from the particle, although the large majority of these transcripts is not associated with it [17]. These studies suggested a role for vault RNAs in viral defence, apoptosis and multi-drug resistance [19,20,39,40,63–65]. Moreover, human vault RNAs can be constituents of microvesicles [66], and a recently identified vault RNA in Trypanosoma brucei has been implicated in mRNA trans-splicing [32]. However, a detailed understanding of their molecular functions and mechanism of action remained to be uncovered.
We recently discovered that vtRNA1-1 binds to and regulates p62-dependent autophagy and aggregate clearance, thereby unravelling a first direct function of this conserved non-coding RNA [28]. Macroautophagy––further referred to as autophagy––is an essential cellular process that entails tethering, degradation and recycling of intracellular cargos including protein aggregates, excess or damaged organelles as well as pathogens. Thereby, autophagy fulfils a key function in cellular homeostasis and provides recycled material as a resource for the anabolic needs of the cell. During the process, cargo is selected and enclosed in double membrane vesicles called autophagosomes which eventually fuse with lysosomes leading to content degradation and release of recycled amino acids, lipids and nucleosides (reviewed in [67–69]).
7. Oligomerization is a key to p62 function in autophagy
The selectivity of autophagic processes is governed by autophagic receptors such as p62. These discriminate between different substrates by the usage of their cargo recognition domains and associate with autophagosomal membranes via a separate domain, thereby targeting intracellular cargo towards growing phagophores (figure 2) [70–74]. p62 plays a pivotal role in the autophagic clearance of intracellular cargos that fail to undergo degradation via the ubiquitin–proteasome system [75–79]. These include aggregation-prone cargos that are difficult to unfold and/or exceed the proteasomal capacity due to their mere size or quantity. As a result, p62 is of paramount importance in situations of acute proteotoxic stress and starvation [80]. Interestingly, p62-dependent assemblies have further been suggested to trigger autophagosome formation, implying a regulatory role in autophagy that was not previously anticipated [75,76,81].
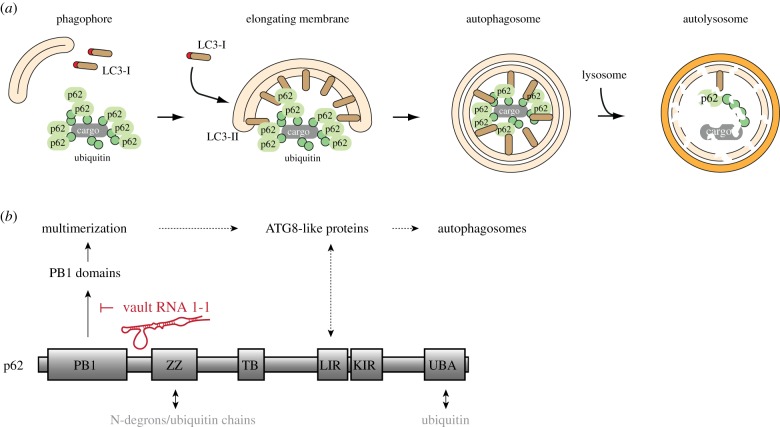
Figure 2.
p62 and its function in selective autophagy and aggregate clearance. (a) Schematic overview of p62-dependent selective autophagy and aggregate clearance. P62 directs intracellular cargo to the phagophore. A double membrane vesicle is formed that fuses with the lysosome to degrade its content. (b) Schematic overview of the p62 domain organization. Cargo-binding sites are indicated below; vtRNA1-1 mediated regulation of p62 effector function is depicted above.
p62 associates with ubiquitin via its C-terminal ubiquitin-associated domain (UBA) and binds to N-arginylated peptides as well as ubiquitin chains via the ZZ-type zinc (ZZ) finger domain (figure 2b) [75,82]. In turn, the LC3-interacting region directly binds to Atg8-like proteins such as LC3 and GABARAP, and thereby facilitates elongation of the autophagic membrane [73,83,84]. Since the intrinsic affinity of p62 for its cargos as well as Atg8-like proteins is rather weak, p62 multimerization is essential to achieving high avidity interactions while maintaining selectivity [84,85]. The interaction of p62 with itself and other autophagic receptors is mediated by the N-terminal Phox and Bem1 (PB1) domain (figure 2b) [71,86]. Strikingly, p62 PB1 domain mutants that are oligomerization-deficient fail to engage in autophagy [87], further emphasizing the importance of oligomerization for the ‘effector' function of p62. The N-terminal linker region between the PB1 and ZZ domains (figure 2) was recently shown to contribute to p62 oligomerization and has been suggested to play an autoregulatory function [71,88]. The ZZ domain of p62 complexes two zinc atoms involving its conserved 4 Cys and 2 Cys-2 His motifs, and folds into a cross-brace zinc finger as also found for other ZZ-type domains [89]. It accommodates a negatively charged surface patch that serves as a binding site for N-arginylated peptides––so-called N-degrons––and synthetic autophagy inducing ligands (figure 2b) [75,89]. In addition, the ZZ domain has been reported to bind to K48- and K63-linked but not linear ubiquitin chains [82]. This intermolecular cross-linking of p62 via poly-ubiquitinated substrates [82], and conformational changes upon cargo binding to the ZZ domain have been reported to support p62 multimerization [75,76]. Yet, the regulation of p62 oligomerization––a key to its ‘effector' function––is far from being fully understood.
We have recently shown that binding of vtRNA1-1 to p62 inhibits the receptor's oligomerization and engagement in autophagy. Mutagenesis of residues R139 and K141 to alanine in the ZZ domain of p62 reduced the interaction with vault RNA1-1. These residues are part of a positive surface patch in close proximity to the N-degron-binding site. Interestingly, we also observed decreased vtRNA1-1 binding to the p62 oligomerization mutant R21A/D68A/D73A (PB1 m) [28]. These data indicate that the both, the ZZ and the PB1 domain, are involved in mediating p62's RNA-binding activity. The involvement of other p62 domains in RNA binding has, however, not yet been excluded. Structural studies of p62 with and without RNA will help to decipher the complex interplay between the autophagy receptor and its ‘riboregulator'. It further remains to be investigated whether vtRNA1-1 inhibits oligomerization of p62 independent of cargo binding, or whether the RNA prevents cargo association or influences cargo specificity, thereby inhibiting oligomerization.
8. ‘Riboregulation' of autophagy—a response to different cues?
Vault RNA levels decrease during starvation [28] and increase profoundly upon different viral infections [19,20,39,40]. In detail, vtRNA1-1 levels diminish about twofold in HuH-7 cells cultured in a minimal medium lacking amino acids and serum [28], whereas the levels of the other vault RNA paralogues remain more stable. The drop in vtRNA1-1 abundance was unaffected by p62 depletion and by treatment with bafilomycin A1, an inhibitor of the proton pump V-ATPase and hence of autophagosome-to-lysosome fusion. These data indicate that the decrease is not a result of RNA co-degradation with p62 during autophagy. Moreover, the starvation-induced decrease of vtRNA1-1 that was ectopically expressed from a heterologous promoter (H1), implies that the regulation of transcript levels is mediated by features present within the transcribed vtRNA1-1 gene body. Still, the detailed mechanism of this regulation remains to be determined.
The reduction of vtRNA1-1 expression during starvation is associated with a decrease in vtRNA1-1 binding to p62 [28]. This decreased RNA binding in turn promotes p62 oligomerization and autophagy, as discussed above. Treatment with bafilomycin A1 resulted in the accumulation of readily formed autophagosomes inside the cell, including enclosed cargo and autophagic receptors. Interestingly, while p62 accumulated upon bafilomycin A1 treatment, the amount of RNA-bound p62 remained constant. This observation further indicates that p62 which is actively involved in autophagy is free of vault RNA. Overall, the available data converge on a model where vtRNA1-1 levels are controlled by starvation and inhibit p62-dependent autophagy (figure 3).
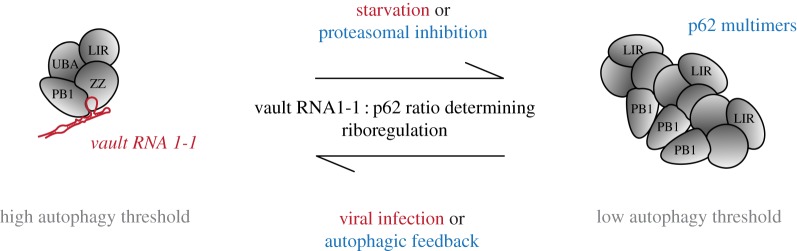
Figure 3.
Riboregulation of p62-dependent autophagy and aggregate clearance by vault RNA 1-1. We propose vtRNA1-1 as negative modulator of autophagy that directly binds to p62 and thereby inhibits p62 oligomerization, a prerequisite for the receptor's involvement in autophagy and aggregate clearance. The vtRNA1-1 to p62 ratio is thereby determining the extend of riboregulation. Conditions that are known to change RNA or protein levels are indicated in red and blue, respectively.
Recently, ZZ domain-specific ligands were shown to potently induce p62-dependent autophagy [75]. VtRNA1-1 KO cells treated with the synthetic p62-ZZ domain ligand XIE62-1004-A activated autophagy significantly stronger than the respective CRISPR control cell lines [28]. This finding further affirmed the role of vtRNA1-1 as a negative ‘riboregulator' of autophagy via p62 and the specificity of this modulation. Since p62-dependent autophagy can be initiated in multiple ways (reviewed in [68]), vtRNA1-1 mediated riboregulation could provide a mechanism to prevent overshooting autophagic activities.
By contrast to starvation, viral infections induce vault RNA expression. This response was observed in human cell culture models that were infected with members of the γ-herpesviridae family, including Epstein–Barr virus (EBV/HHV4) and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8), alpha-herpesvirus (Herpes simplex virus 1; HSV1) or paramyxovirus (Sendai virus (SeV) [19,20,39,40]. A similar increase was seen with human cell lines and mouse lung cells upon influenza A virus exposure [40]. Interestingly, most of these viruses are known to negatively modulate the autophagic flux of their host cells [90].
The transcriptional induction of vault RNAs upon infection has been associated with the expression of latent membrane protein 1 (LMP1) for EBV [39] and non-structural protein NS1 of influenza virus [40], respectively. In both cases high expression levels of vtRNA1-1 fostered an increase in viral load, while prior reduction of cellular vtRNA1-1 levels diminished viral replication in vitro and in vivo. Strikingly, Amort et al. [39] showed that the effect of vtRNA1-1 on virus replication is (i) concentration dependent, (ii) involving the vtRNA1-1 central domain and (iii) independent of MVP. These observations are well in line with the model of p62 riboregulation by vtRNA1-1.
Viruses are known to hijack key regulatory mechanisms of the cell to maximize viral replication while inhibiting cellular defence mechanisms. Upregulation of vtRNA1-1 levels could therefore serve to escape targeted viral degradation via autophagy and subsequent MHC class II antigen presentation [91]. In addition, it might force the cell to enter an anabolic, pro-proliferative state that can be exploited for rapid virus replication and to counteract cellular suicide programmes [92]. Therefore, overexpression of vtRNA1-1 during viral infection and the resulting deregulation of autophagy could serve in multiple ways to turn the host cell into a virus ‘factory' while preventing an immune response (figure 3). Yet, the role of vtRNA1-1 mediated inhibition of autophagy in the context of viral infections has not been explored in much detail.
Furthermore, the molecular pathways that mediate apoptosis resistance in this setting remain to be elucidated [39]. Since p62 is involved in the crosstalk between autophagy and apoptosis (reviewed in [93,94]), increased levels of p62 upon autophagy inhibition via vault RNA 1-1 could contribute to modulate this crosstalk. In addition, the binding of vault RNAs and especially of vault RNA2-1 to protein kinase R (PKR) has been suggested to supress PKR activation upon influenza A infection and induce subsequent antiviral interferon response [40]. It will be interesting to uncover the function of the different vault RNA paralogues and their role in viral infections, as well as other physiological and pathological settings.
p62 levels decrease during autophagy due to phagolysosomal degradation [67] and increase upon proteasome inhibition [95–97]. Such changes in p62 levels could affect its relative ratio to vtRNA1-1 and influence the riboregulation of autophagy (figure 3).
The intracellular levels of p62 can be used as a marker to assess the autophagic state of the cell [67]. As mentioned above, p62 targeted to the autophagosome is ultimately degraded following autophagosome-to-lysosome fusion. In line with this model, we could show that autophagy-engaged p62 is not bound by vtRNA1-1 and consequently does not mediate autophagosomal degradation of the RNA [28]. Nevertheless, the levels of both p62 and vtRNA1-1 decrease upon starvation, suggesting regulatory feedback that will be interesting to unravel.
In contrast with the starvation-induced decrease in p62 levels, proteasome inhibition has been reported to stimulate the Nrf1-dependent transcription of p62 [96]. This stimulation was shown to be essential for cellular survival during proteasome inhibition. Provided the increased levels of p62 shift the vtRNA1-1 to p62 ratio, proteasome inhibition could lead to inefficient vtRNA1-1-mediated riboregulation. This in turn would ensure a rapid sequestration of ubiquitinated proteins into ‘sequestosomes' and allow proteasome-independent aggregate clearance via p62. Indeed, a significantly higher clearance of protein aggregates was observed in vault RNA 1-1 KO cells upon proteasome inhibition compared to the respective control cell lines [28]. This response was p62-dependent, since the expression of the p62 mutant S407A that is unresponsive to ULK1-dependent phosphorylation and the activation of the UBA domain in this context [98], abrogated the effect. Moreover, no difference in vault RNA levels was observed upon proteasome inhibition (R.H. 2017–2020, unpublished observations).
9. The new vaultAge—perspectives
The riboregulation of autophagy by a small non-coding RNA raises several additional perspectives, discussed below.
9.1. Identification of factors that control p62-vtRNA1-1 riboregulation
Identification of p62 and vtRNA1-1 as a riboregulatory effector pair calls for the identification of those cellular and/or viral factors that determine the abundance of both components as well as their interaction. It will be of great interest to assess transcriptional, post-transcriptional and––in the case of p62––translational events that influence intracellular p62 and vault RNA1-1 levels. In addition, post-translational and epi-transcriptomic RNA modifications, respectively, could represent a fast way to control the RNA–protein interaction. While modifications within the binding interface could directly affect ribonucleoprotein complex formation, others that influence conformation or localization could indirectly contribute to the modulation of this interaction.
Vault RNAs modifications have been recently described (see above), and m5C has been functionally implicated in cell differentiation [53,55]. Yet, whether and to what extent these modifications can affect the riboregulation of autophagy remains to be investigated. Conversely, several post-translational modifications are known for p62 [99]. These include the LRRK2-dependent phosphorylation of T138 [100] within the ZZ domain [28]. Interestingly, LRRK2 is involved in the regulation of p62-depenent autophagy, and it has been discussed as a druggable target for Parkinson's disease [101]. The assessment of further modifications that might influence vtRNA binding to p62 will be of great interest, possibly also with regard to designing compounds that exploit the mechanism of ‘riboregulation'.
9.2. Biological scope of p62 riboregulation by vtRNA1-1
It will be interesting to systematically determine the levels of p62 and vault RNA 1-1, and assess their interaction in cellular processes and stress conditions that are highly dependent on autophagy and its clearing function. These include differentiation and development [102], cell-death signalling [103] and cell-cycle regulation [104]. For example, the levels of non-coding RNAs including vault RNAs have been reported to be highly regulated during cellular differentiation [105]. Riboregulation could therefore represent a mechanism to modulate p62 function in this context. Thorough the data-mining of existing multi-omics, datasets might be an effective way for a first assessment of this possibility.
In addition, p62 plays a pivotal role as a downstream effector for various other cellular pathways (reviewed in [93,94]). While p62 has been described to influence mTORC1 activation in response to amino acids [106,107], we did not observe changes in the phosphorylation of the canonical mTORC1 targets ULK1 and 4E-BP1 upon vault RNA 1-1 depletion [28].
9.3. The role of p62 riboregulation in human disease
p62-dependent autophagy and aggregate clearance has been associated with neurodegenerative diseases, bone disorders and cancer [68,99,108]. Consequently, it will be of interest to explore whether vault RNA1-1-mediated riboregulation is altered under these pathological conditions and whether it could serve as a target for drug development.
Quality control and immunomodulatory functions of autophagy are considered to be tumour suppressive at early stages, while promotion of cellular growth and resistance to stress and drugs are believed to be tumour-promoting facets of autophagy in advanced tumours [93,95,108–110]. While the vault particle and vault RNAs have been previously associated with multi-drug resistance [7,56,63–65,111,112], the functional and molecular links between autophagy and multi-drug resistance are highly influenced by their biological contexts. Therefore, it remains an open question to what extent the riboregulation of p62 oligomerization by vtRNA1-1 and the subsequent modulation of p62-dependent autophagy and aggregate clearance are relevant in these pathological settings.
9.4. The role of the vault particle and MVP in p62-dependent aggregate clearance
Previous work gave no positive indication of the involvement of MVP in the riboregulation of autophagy [28] or in apoptosis resistance upon EBV infection [39], both of which are mediated by vault RNA. With the newly identified role of vtRNA1-1 in autophagy, it will be interesting to see whether the vault particle or MVP are directly or indirectly connected with this cellular process.
9.5. How widespread is riboregulation?
Work on the vtRNA1-1-p62 interaction uncovered an example of riboregulation, a process in which a protein's function is controlled post-translationally by the direct binding of a regulatory RNA [28]. Earlier examples of this emerging biological principle include 6S RNA regulation of RNA polymerase activity in bacteria [113], and the activation of immune receptors or the eIF2α kinase PKR by viral RNAs in mammalian cells [114,115] (reviewed in [116]). With hundreds of recently discovered RNA-binding proteins (RBPs) [117] involved in key cellular functions, we predict the widespread occurrence of riboregulation in the control of cellular processes. Characteristic features of riboregulation include (i) the direct interaction between the regulatory RNA and its target protein, (ii) the regulation of the levels and/or activity of one or both binding components by a biological cue and (iii) a change of the protein's function (in the widest sense) caused by RNA binding. Riboregulation of an RBP by an RNA is distinct from previously described examples of ‘moonlighting', where several metabolic enzymes have been found to moonlight as RBPs and regulate the translation, stability or other aspects of the fate of RNAs [117,118].
To systematically survey for the biological scope of riboregulation, it will be informative to determine the RNA-binding proteomes under various physiological and pathological conditions [117,119–122]. Proteins displaying differential RNA binding can then be studied for their respective RNA-binding partners to uncover functional interactions [123,124]. Thus, the co-evolution of RNAs (including but not limited to non-coding RNAs) with proteins may have constituted a biological regulatory layer that was not previously anticipated.
Supplementary Material
Acknowledgements
We would like to recommend the websites devoted to the vault particle and its components that are maintained by the Rome Lab (https://vaults.arc.ucla.edu/pages/scientists) as well as the McManus Lab (https://mcmanuslab.ucsf.edu/node/256) and thank both labs for their maintenance. We acknowledge Ina Huppertz and Dmytro Dziuba for their helpful input and fruitful discussions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Kedersha NL, Rome LH. 1986. Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. J. Cell Biol. 103, 699–709. ( 10.1083/jcb.103.3.699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kickhoefer VA, Searles RP, Kedersha NL, Garber ME, Johnson DL, Rome LH. 1993. Vault ribonucleoprotein particles from rat and bullfrog contain a related small RNA that is transcribed by RNA polymerase III. J. Biol. Chem. 268, 7868–7873. [PubMed] [Google Scholar]
- 3.Kong LB, Siva AC, Rome LH, Stewart PL. 1999. Structure of the vault, a ubiquitous celular component. Structure 7, 371–379. ( 10.1016/S0969-2126(99)80050-1) [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Kato K, Yamashita E, Sumizawa T, Zhou Y, Yao M, Iwasaki K, Yoshimura M, Tsukihara T. 2009. The structure of rat liver vault at 3.5 Angstrom resolution. Science 323, 384–388. ( 10.1126/science.1164975) [DOI] [PubMed] [Google Scholar]
- 5.Herrmann C, Zimmermann H, Volknandt W. 1997. Analysis of a cDNA encoding the major vault protein from the electric ray Discopyge ommata. Gene 188, 85–90. ( 10.1016/S0378-1119(96)00781-0) [DOI] [PubMed] [Google Scholar]
- 6.Kickhoefer VA, Rome LH. 1994. The sequence of a cDNA encoding the major vault protein from Rattus norvegicus. Gene 151, 257–260. ( 10.1016/0378-1119(94)90667-X) [DOI] [PubMed] [Google Scholar]
- 7.Kickhoefer VA, Rajavel KS, Scheffer GL, Dalton WS, Scheper RJ, Rome LH. 1998. Vaults are up-regulated in multidrug-resistant cancer cell lines. J. Biol. Chem. 273, 8971–8974. ( 10.1074/jbc.273.15.8971) [DOI] [PubMed] [Google Scholar]
- 8.Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC, Scheper RJ. 1995. The drug resistance-related protein LRP is the human major vault protein. Nat. Med. 1, 578–582. ( 10.1038/nm0695-578) [DOI] [PubMed] [Google Scholar]
- 9.Vasu SK, Rome LH. 1995. Dictyostelium vaults: disruption of the major proteins reveals growth and morphological defects and uncovers a new associated protein. J. Biol. Chem. 270, 16 588–16 594. ( 10.1074/jbc.270.28.16588) [DOI] [PubMed] [Google Scholar]
- 10.Vasu SK, Kedersha NL, Rome LH. 1993. cDNA cloning and disruption of the major vault protein alpha gene (mvpA) in Dictyostelium discoideum. J. Biol. Chem. 268, 15 356–15 360. [PubMed] [Google Scholar]
- 11.Stadler PF, et al. 2009. Evolution of vault RNAs. Mol. Biol. Evol. 26, 1975–1991. ( 10.1093/molbev/msp112) [DOI] [PubMed] [Google Scholar]
- 12.Kedersha NL, Heuser JE, Chugani DC, Rome LH. 1991. Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J. Cell Biol. 112, 225–235. ( 10.1083/jcb.112.2.225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rome L, Kedersha N, Chugani D. 1991. Unlocking vaults: organelles in search of a function. Trends Cell Biol. 1, 47–50. ( 10.1016/0962-8924(91)90088-Q) [DOI] [PubMed] [Google Scholar]
- 14.Mrazek J, Toso D, Ryazantsev S, Zhang X, Zhou ZH, Fernandez BC, Kickhoefer VA, Rome LH. 2014. Polyribosomes are molecular 3D nanoprinters that orchestrate the assembly of vault particles. ACS Nano 8, 11 552–11 559. ( 10.1021/nn504778h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephen AG, Raval-Fernandes S, Huynh T, Torres M, Kickhoefer VA, Rome LH. 2001. Assembly of vault-like particles in insect cells expressing only the major vault protein. J. Biol. Chem. 276, 23 217–23 220. ( 10.1074/jbc.C100226200) [DOI] [PubMed] [Google Scholar]
- 16.Mikyas Y, Makabi M, Raval-Fernandes S, Harrington L, Kickhoefer VA, Rome LH, Stewart PL. 2004. Cryoelectron microscopy imaging of recombinant and tissue derived vaults: localization of the MVP N termini and VPARP. J. Mol. Biol. 344, 91–105. ( 10.1016/j.jmb.2004.09.021) [DOI] [PubMed] [Google Scholar]
- 17.Kickhoefer VA, Poderycki MJ, Chan EKL, Rome LH. 2002. The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J. Biol. Chem. 277, 41 282–41 286. ( 10.1074/jbc.M206980200) [DOI] [PubMed] [Google Scholar]
- 18.Kong LB, Siva AC, Kickhoefer VA, Rome LH, Stewart PL. 2000. RNA location and modeling of a WD40 repeat domain within the vault. RNA N. Y. N 6, 890–900. ( 10.1017/S1355838200000157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mrázek J, Kreutmayer SB, Grässer FA, Polacek N, Hüttenhofer A. 2007. Subtractive hybridization identifies novel differentially expressed ncRNA species in EBV-infected human B cells. Nucleic Acids Res. 35, e73 ( 10.1093/nar/gkm244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandy C, Mrázek J, Stoiber H, Grässer FA, Hüttenhofer A, Polacek N. 2009. Epstein–Barr virus-induced expression of a novel human vault RNA. J. Mol. Biol. 388, 776–784. ( 10.1016/j.jmb.2009.03.031) [DOI] [PubMed] [Google Scholar]
- 21.van Zon A, Mossink MH, Schoester M, Scheffer GL, Scheper RJ, Sonneveld P, Wiemer EA. 2001. Multiple human vault RNAs: expression and association with the vault complex. J. Biol. Chem. 276, 37 715–37 721. ( 10.1074/jbc.M106055200) [DOI] [PubMed] [Google Scholar]
- 22.Hanson HH, Kang S, Fernández-Monreal M, Oung T, Yildirim M, Lee R, Suyama K, Hazan RB, Phillips GR. 2010. LC3-dependent intracellular membrane tubules induced by γ-protocadherins A3 and B2. J. Biol. Chem. 285, 20 982–20 992. ( 10.1074/jbc.M109.092031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schramm L, Hernandez N. 2002. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 16, 2593–2620. ( 10.1101/gad.1018902) [DOI] [PubMed] [Google Scholar]
- 24.Vilalta A, Kickhoefer VA, Rome LH, Johnson DL. 1994. The rat vault RNA gene contains a unique RNA polymerase III promoter composed of both external and internal elements that function synergistically. J. Biol. Chem. 269, 29 752–29 759. [PubMed] [Google Scholar]
- 25.Will S, Reiche K, Hofacker IL, Stadler PF, Backofen R. 2007. Inferring noncoding RNA families and classes by means of genome-scale structure-based clustering. PLoS Comput. Biol. 3, e65 ( 10.1371/journal.pcbi.0030065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Will S, Joshi T, Hofacker IL, Stadler PF, Backofen R. 2012. LocARNA-P: accurate boundary prediction and improved detection of structural RNAs. RNA N. Y. N 18, 900–914. ( 10.1261/rna.029041.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raden M, et al. 2018. Freiburg RNA tools: a central online resource for RNA-focused research and teaching. Nucleic Acids Res. 46, W25–W29. ( 10.1093/nar/gky329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horos R, et al. 2019. The small non-coding vault RNA1-1 acts as a riboregulator of autophagy. Cell 176, 1054–1067.e12. ( 10.1016/j.cell.2019.01.030) [DOI] [PubMed] [Google Scholar]
- 29.Kickhoefer VA, Emre N, Stephen AG, Poderycki MJ, Rome LH. 2003. Identification of conserved vault RNA expression elements and a non-expressed mouse vault RNA gene. Gene 309, 65–70. ( 10.1016/S0378-1119(03)00507-9) [DOI] [PubMed] [Google Scholar]
- 30.Kickhoefer VA, Stephen AG, Harrington L, Robinson MO, Rome LH. 1999. Vaults and telomerase share a common subunit, TEP1. J. Biol. Chem. 274, 32 712–32 717. ( 10.1074/jbc.274.46.32712) [DOI] [PubMed] [Google Scholar]
- 31.Stewart PL, Makabi M, Lang J, Dickey-Sims C, Robertson AJ, Coffman JA, Suprenant KA. 2005. Sea urchin vault structure, composition, and differential localization during development. BMC Dev. Biol. 5, 3 ( 10.1186/1471-213X-5-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolev NG, Rajan KS, Tycowski KT, Toh JY, Shi H, Lei Y, Michaeli S, Tschudi C. 2019. The vault RNA of Trypanosoma brucei plays a role in the production of trans-spliced mRNA. J. Biol. Chem. 294, 15 559–15 574. ( 10.1074/jbc.RA119.008580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Zon A, Mossink MH, Scheper RJ, Sonneveld P, Wiemer EAC. 2003. The vault complex. Cell. Mol. Life Sci. CMLS 60, 1828–1837. ( 10.1007/s00018-003-3030-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. 1987. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49, 729–739. ( 10.1016/0092-8674(87)90611-8) [DOI] [PubMed] [Google Scholar]
- 35.Mayr B, Montminy M. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2, 599 ( 10.1038/35085068) [DOI] [PubMed] [Google Scholar]
- 36.Shaywitz AJ, Greenberg ME. 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821–861. ( 10.1146/annurev.biochem.68.1.821) [DOI] [PubMed] [Google Scholar]
- 37.Sheng M, Greenberg ME. 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485. ( 10.1016/0896-6273(90)90106-P) [DOI] [PubMed] [Google Scholar]
- 38.Wen AY, Sakamoto KM, Miller LS. 2010. The role of the transcription factor CREB in immune function. J. Immunol. Baltim. Md 1950, 6413–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amort M, Nachbauer B, Tuzlak S, Kieser A, Schepers A, Villunger A, Polacek N. 2015. Expression of the vault RNA protects cells from undergoing apoptosis. Nat. Commun. 6, 7030 ( 10.1038/ncomms8030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, et al. 2015. Robust expression of vault RNAs induced by influenza A virus plays a critical role in suppression of PKR-mediated innate immunity. Nucleic Acids Res. 43, 10 321–10 337. ( 10.1093/nar/gkv1078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Søgaard Helbo A, et al. 2015. Hypermethylation of the VTRNA1-3 promoter is associated with poor outcome in lower risk myelodysplastic syndrome patients. Genes 6, 977–990. ( 10.3390/genes6040977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treppendahl MB, et al. 2012. Allelic methylation levels of the noncoding VTRNA2-1 located on chromosome 5q31.1 predict outcome in AML. Blood 119, 206–216. ( 10.1182/blood-2011-06-362541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao J, et al. 2013. DNA methylation-mediated repression of miR-886-3p predicts poor outcome of human small cell lung cancer. Cancer Res. 73, 3326–3335. ( 10.1158/0008-5472) [DOI] [PubMed] [Google Scholar]
- 44.Lee H-S, et al. 2014. Epigenetic silencing of the non-coding RNA nc886 provokes oncogenes during human esophageal tumorigenesis. Oncotarget 5, 3472–3481. ( 10.18632/oncotarget.1927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K-S, et al. 2014. nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget 5, 3944–3955. ( 10.18632/oncotarget.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YS. 2015. A novel type of non-coding RNA, nc886, implicated in tumor sensing and suppression. Genomics Inf. 13, 26–30. ( 10.5808/GI.2015.13.2.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helbo AS, Lay FD, Jones PA, Liang G, Grønbæk K. 2017. Nucleosome positioning and NDR structure at RNA polymerase III promoters. Sci. Rep. 7, 1 ( 10.1038/srep41947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burke JM, Sullivan CS. 2017. DUSP11—an RNA phosphatase that regulates host and viral non-coding RNAs in mammalian cells. RNA Biol. 14, 1457–1465. ( 10.1080/15476286.2017.1306169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke JM, Kincaid RP, Nottingham RM, Lambowitz AM, Sullivan CS. 2016. DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady-state levels of cellular noncoding RNAs. Genes Dev. 30, 2076–2092. ( 10.1101/gad.282616.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Ye X, Dunker W, Song Y, Karijolich J. 2018. RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection. Nat. Commun. 9, 1–14. ( 10.1038/s41467-018-07314-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Łabno A, Warkocki Z, Kuliński T, Krawczyk PS, Bijata K, Tomecki R, Dziembowski A. 2016. Perlman syndrome nuclease DIS3L2 controls cytoplasmic non-coding RNAs and provides surveillance pathway for maturing snRNAs. Nucleic Acids Res. 44, 10 437–10 453. ( 10.1093/nar/gkw649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ustianenko D, Pasulka J, Feketova Z, Bednarik L, Zigackova D, Fortova A, Zavolan M, Vanacova S. 2016. TUT-DIS3L2 is a mammalian surveillance pathway for aberrant structured non-coding RNAs. EMBO J. 35, 2179–2191. ( 10.15252/embj.201694857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussain S, et al. 2013. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 4, 255–261. ( 10.1016/j.celrep.2013.06.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khoddami V, Cairns BR. 2013. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 31, 458–464. ( 10.1038/nbt.2566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sajini AA, et al. 2019. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat. Commun. 10, 1–13. ( 10.1038/s41467-019-10020-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persson H, Kvist A, Vallon-Christersson J, Medstrand P, Borg Å, Rovira C. 2009. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat. Cell Biol. 11, 1268–1271. ( 10.1038/ncb1972) [DOI] [PubMed] [Google Scholar]
- 57.Langenberger D, Çakir MV, Hoffmann S, Stadler PF. 2013. Dicer-processed small RNAs: rules and exceptions. J. Exp. Zoolog. B Mol. Dev. Evol. 320, 35–46. ( 10.1002/jez.b.22481) [DOI] [PubMed] [Google Scholar]
- 58.Lee K, et al. 2011. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 17, 1076–1089. ( 10.1261/rna.2701111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, Sloan KE, Bohnsack MT. 2017. Human METTL16 is a N 6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014. ( 10.15252/embr.201744940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guzzi N, et al. 2018. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell 173, 1204–1216.e26. ( 10.1016/j.cell.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 61.Poderycki MJ, Rome LH, Harrington L, Kickhoefer VA. 2005. The p80 homology region of TEP1 is sufficient for its association with the telomerase and vault RNAs, and the vault particle. Nucleic Acids Res. 33, 893–902. ( 10.1093/nar/gki234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger W, Steiner E, Grusch M, Elbling L, Micksche M. 2009. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cell. Mol. Life Sci. CMLS 66, 43–61. ( 10.1007/s00018-008-8364-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, OuYang H, An X, Liu S. 2018. Vault RNAs partially induces drug resistance of human tumor cells MCF-7 by binding to the RNA/DNA-binding protein PSF and inducing oncogene GAGE6. PLoS ONE 13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Gopinath SCB, Matsugami A, Katahira M, Kumar PKR. 2005. Human vault-associated non-coding RNAs bind to mitoxantrone, a chemotherapeutic compound. Nucleic Acids Res. 33, 4874–4881. ( 10.1093/nar/gki809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gopinath SCB, Wadhwa R, Kumar PKR. 2010. Expression of noncoding vault RNA in human malignant cells and its importance in mitoxantrone resistance. Mol. Cancer Res. 8, 1536–1546. ( 10.1158/1541-7786.MCR-10-0242) [DOI] [PubMed] [Google Scholar]
- 66.Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, Lambowitz AM. 2017. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl. Acad. Sci. USA 114, E8987 ( 10.1073/pnas.1712108114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klionsky DJ, et al. 2016. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222. ( 10.1080/15548627.2015.1100356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahiri V, Hawkins WD, Klionsky DJ. 2019. Watch what you (self-)eat: autophagic mechanisms that modulate metabolism. Cell Metab. 29, 803–826. ( 10.1016/j.cmet.2019.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizushima N. 2018. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 20, 521 ( 10.1038/s41556-018-0092-5) [DOI] [PubMed] [Google Scholar]
- 70.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. 2005. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614. ( 10.1083/jcb.200507002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ciuffa R, Lamark T, Tarafder AK, Guesdon A, Rybina S, Hagen WJH, Johansen T, Sachse C. 2015. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 11, 748–758. ( 10.1016/j.celrep.2015.03.062) [DOI] [PubMed] [Google Scholar]
- 72.Johansen T, Lamark T. 2011. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296. ( 10.4161/auto.7.3.14487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun J-A, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24 131–24 145. ( 10.1074/jbc.M702824200) [DOI] [PubMed] [Google Scholar]
- 74.Zaffagnini G, Martens S. 2016. Mechanisms of selective autophagy. J. Mol. Biol. 428, 1714–1724. ( 10.1016/j.jmb.2016.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cha-Molstad H, et al. 2017. p62/SQSTM1/sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat. Commun. 8, 102 ( 10.1038/s41467-017-00085-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cha-Molstad H, et al. 2018. Regulation of autophagic proteolysis by the N-recognin SQSTM1/p62 of the N-end rule pathway. Autophagy 14, 359–361. ( 10.1080/15548627.2017.1415190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demishtein A, Fraiberg M, Berko D, Tirosh B, Elazar Z, Navon A. 2017. SQSTM1/p62-mediated autophagy compensates for loss of proteasome polyubiquitin recruiting capacity. Autophagy 13, 1697–1708. ( 10.1080/15548627.2017.1356549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dikic I. 2017. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224. ( 10.1146/annurev-biochem-061516-044908) [DOI] [PubMed] [Google Scholar]
- 79.Kageyama S, et al. 2014. Proteasome dysfunction activates autophagy and the Keap1-Nrf2 pathway. J. Biol. Chem. 289, 24 944–24 955. ( 10.1074/jbc.M114.580357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahani MH, Itakura E, Mizushima N. 2014. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10, 431–441. ( 10.4161/auto.27344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turco E, et al. 2019. FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol. Cell 74, 330–346.e11. ( 10.1016/j.molcel.2019.01.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaffagnini G, et al. 2018. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 37 ( 10.15252/embj.201798308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birgisdottir ÅB, Lamark T, Johansen T. 2013. The LIR motif—crucial for selective autophagy. J. Cell Sci. 126, 3237–3247. [DOI] [PubMed] [Google Scholar]
- 84.Danieli A, Martens S. 2018. p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J Cell Sci 131, jcs214304 ( 10.1242/jcs.214304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wurzer B, Zaffagnini G, Fracchiolla D, Turco E, Abert C, Romanov J, Martens S. 2015. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. eLife 4, e08941 ( 10.7554/eLife.08941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamark T, Perander M, Outzen H, Kristiansen K, Øvervatn A, Michaelsen E, Bjørkøy G, Johansen T. 2003. Interaction codes within the family of mammalian Phox and Bem1p domain-containing proteins. J. Biol. Chem. 278, 34 568–34 581. ( 10.1074/jbc.M303221200) [DOI] [PubMed] [Google Scholar]
- 87.Itakura E, Mizushima N. 2011. p62 targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 192, 17–27. ( 10.1083/jcb.201009067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, et al. 2018. ZZ-dependent regulation of p62/SQSTM1 in autophagy. Nat. Commun. 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon DH, Park OH, Kim L, Jung YO, Park Y, Jeong H, Hyun J, Kim YK, Song HK. 2018. Insights into degradation mechanism of N-end rule substrates by p62/SQSTM1 autophagy adapter. Nat. Commun. 9, 3291 ( 10.1038/s41467-018-05825-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson WT. 2015. Viruses and the autophagy pathway. Virology 479–480, 450–456. ( 10.1016/j.virol.2015.03.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh JE, Lee HK. 2013. Autophagy as an innate immune modulator. Immune Netw. 13, 1–9. ( 10.4110/in.2013.13.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eisenreich W, Rudel T, Heesemann J, Goebel W. 2019. How viral and intracellular bacterial pathogens reprogram the metabolism of host cells to allow their intracellular replication. Front. Cell. Infect. Microbiol. 9 ( 10.3389/fcimb.2019.00042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moscat J, Diaz-Meco MT. 2009. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137, 1001–1004. ( 10.1016/j.cell.2009.05.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moscat J, Karin M, Diaz-Meco MT. 2016. p62 in cancer: signaling adaptor beyond autophagy. Cell 167, 606–609. ( 10.1016/j.cell.2016.09.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puissant A, Fenouille N, Auberger P. 2012. When autophagy meets cancer through p62/SQSTM1. Am. J. Cancer Res. 2, 397–413. [PMC free article] [PubMed] [Google Scholar]
- 96.Sha Z, Schnell HM, Ruoff K, Goldberg A. 2018. Rapid induction of p62 and GABARAPL1 upon proteasome inhibition promotes survival before autophagy activation. J Cell Biol 217, 1757–1776. ( 10.1083/jcb.201708168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thompson HGR, Harris JW, Wold BJ, Lin F, Brody JP. 2003. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 22, 2322–2333. ( 10.1038/sj.onc.1206325) [DOI] [PubMed] [Google Scholar]
- 98.Lim J, et al. 2015. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 11, e1004987 ( 10.1371/journal.pgen.1004987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sánchez-Martín P, Komatsu M. 2018. p62/SQSTM1––steering the cell through health and disease. J. Cell Sci. 131, jcs222836 ( 10.1242/jcs.222836) [DOI] [PubMed] [Google Scholar]
- 100.Kalogeropulou AF, Zhao J, Bolliger MF, Memou A, Narasimha S, Molitor TP, Wilson WH, Rideout HJ, Nichols RJ. 2018. P62/SQSTM1 is a novel leucine-rich repeat kinase 2 (LRRK2) substrate that enhances neuronal toxicity. Biochem. J. 475, 1271–1293. ( 10.1042/BCJ20170699) [DOI] [PubMed] [Google Scholar]
- 101.Manzoni C, Lewis PA. 2017. LRRK2 and autophagy. Adv. Neurobiol. 14, 89–105. ( 10.1007/978-3-319-49969-7_5) [DOI] [PubMed] [Google Scholar]
- 102.Mizushima N, Levine B. 2010. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12, 823–830. ( 10.1038/ncb0910-823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. 2014. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94. ( 10.1038/nrm3735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mathiassen SG, De Zio D, Cecconi F. 2017. Autophagy and the cell cycle: a complex landscape. Front. Oncol. 7, 51 ( 10.3389/fonc.2017.00051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skreka K, et al. 2012. Identification of differentially expressed non-coding RNAs in embryonic stem cell neural differentiation. Nucleic Acids Res. 40, 6001–6015. ( 10.1093/nar/gks311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. 2011. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 44, 134–146. ( 10.1016/j.molcel.2011.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. 2013. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell 51, 283–296. ( 10.1016/j.molcel.2013.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denk H, Stumptner C, Abuja PM, Zatloukal K. 2019. Sequestosome 1/p62-related pathways as therapeutic targets in hepatocellular carcinoma. Expert Opin. Ther. Targets 23, 393–406. ( 10.1080/14728222.2019.1601703) [DOI] [PubMed] [Google Scholar]
- 109.Islam MA, Sooro MA, Zhang P. 2018. Autophagic regulation of p62 is critical for cancer therapy. Int. J. Mol. Sci. 19, 1405 ( 10.3390/ijms19051405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rusten TE, Stenmark H. 2010. p62, an autophagy hero or culprit? Nat. Cell Biol. 12, 207–209. ( 10.1038/ncb0310-207) [DOI] [PubMed] [Google Scholar]
- 111.Mossink MH, van Zon A, Fränzel-Luiten E, Schoester M, Kickhoefer VA, Scheffer GL, Scheper RJ, Sonneveld P, Wiemer EAC. 2002. Disruption of the murine major vault protein (MVP/LRP) gene does not induce hypersensitivity to cytostatics. Cancer Res. 62, 7298–7304. ( 10.1016/s0378-1119(02)00789-8) [DOI] [PubMed] [Google Scholar]
- 112.Mossink MH, van Zon A, Scheper RJ, Sonneveld P, Wiemer EA. 2003. Vaults: a ribonucleoprotein particle involved in drug resistance? Oncogene 22, 7458–7467. ( 10.1038/sj.onc.1206947) [DOI] [PubMed] [Google Scholar]
- 113.Wassarman KM, Storz G. 2000. 6S RNA regulates E. coli RNA polymerase activity. Cell 101, 613–623. ( 10.1016/S0092-8674(00)80873-9) [DOI] [PubMed] [Google Scholar]
- 114.Kato H, Takahasi K, Fujita T. 2011. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immunol. Rev. 243, 91–98. ( 10.1111/j.1600-065X.2011.01052.x) [DOI] [PubMed] [Google Scholar]
- 115.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62, 379–390. ( 10.1016/0092-8674(90)90374-N) [DOI] [PubMed] [Google Scholar]
- 116.Beckmann BM, Castello A, Medenbach J. 2016. The expanding universe of ribonucleoproteins: of novel RNA-binding proteins and unconventional interactions. Pflugers Arch. 468, 1029–1040. ( 10.1007/s00424-016-1819-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hentze MW, Castello A, Schwarzl T, Preiss T. 2018. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 19, 327–341. ( 10.1038/nrm.2017.130) [DOI] [PubMed] [Google Scholar]
- 118.Ciesla J. 2006. Metabolic enzymes that bind RNA: yet another level of cellular regulatory network? Acta Biochim. Pol. 53, 11–32. ( 10.18388/abp.2006_3360) [DOI] [PubMed] [Google Scholar]
- 119.Asencio C, Chatterjee A, Hentze MW. 2018. Silica-based solid-phase extraction of cross-linked nucleic acid–bound proteins. Life Sci. Alliance 1, e201800088 ( 10.26508/lsa.201800088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baltz AG, et al. 2012. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 46, 674–690. ( 10.1016/j.molcel.2012.05.021) [DOI] [PubMed] [Google Scholar]
- 121.Castello A, et al. 2012. Insights into RNA Biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406. ( 10.1016/j.cell.2012.04.031) [DOI] [PubMed] [Google Scholar]
- 122.Perez-Perri JI, Rogell B, Schwarzl T, Stein F, Zhou Y, Rettel M, Brosig A, Hentze MW. 2018. Discovery of RNA-binding proteins and characterization of their dynamic responses by enhanced RNA interactome capture. Nat. Commun. 9, 4408 ( 10.1038/s41467-018-06557-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huppertz I, Attig J, D'Ambrogio A, Easton LE, Sibley CR, Sugimoto Y, Tajnik M, König J, Ule J. 2014. iCLIP: protein–RNA interactions at nucleotide resolution. Methods San Diego Calif 65, 274–287. ( 10.1016/j.ymeth.2013.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Nostrand EL, et al. 2016. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat. Methods 13, 508–514. ( 10.1038/nmeth.3810) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.