Abstract
Aims
Pulsed field ablation (PFA) is a novel, non-thermal modality that selectively ablates myocardium with ultra-short electrical impulses while sparing collateral tissues. In a proof-of-concept study, the safety and feasibility of ventricular PFA were assessed using a prototype steerable, endocardial catheter.
Methods and results
Under general anaesthesia, the left and right ventricles of four healthy swine were ablated using the 12-Fr deflectable PFA catheter and a deflectable sheath guided by electroanatomic mapping. Using the study catheter, electrograms were recorded for each site and pre-ablation and post-ablation pacing thresholds (at 2.0 ms pulse width) were recorded in two of four animals. After euthanasia at 35.5 days, the hearts were submitted for histology. The PFA applications (n = 39) resulted in significant electrogram reduction without ventricular arrhythmias. In ablation sites where it was measured, the pacing thresholds increased by >16.8 mA in the right ventricle (3 sites) and >16.1 mA in the left ventricle (7 sites), with non-capture at maximum amplitude (20 mA) observable in 8 of 10 sites. Gross measurements, available for 28 of 30 ablation sites, revealed average lesion dimensions to be 6.5 ± 1.7 mm deep by 22.6 ± 4.1 mm wide, with a maximum depth and width of 9.4 mm and 28.6 mm, respectively. In the PFA lesions, fibrous tissue homogeneously replaced myocytes with a narrow zone of surrounding myocytolysis and no overlying thrombus. When present, nerve fascicles and vasculature were preserved within surrounding fibrosis.
Conclusion
We demonstrate that endocardial PFA can be focally delivered using this prototype catheter to create homogeneous, myocardium-specific lesions.
Keywords: Ventricular tachycardia, Catheter ablation, Pulsed field ablation, Electroporation, Ventricle
Graphical Abstract
Graphical Abstract.

What’s new?
A proof-of-concept transfemoral focal catheter was used to successfully and safely deliver endocardial pulsed field ablation (PFA) lesions in swine ventricles.
Chronic lesion dimensions were noted to be 6.5 ± 1.7 mm deep and 22.6 ± 4.1 mm wide.
Histology revealed homogeneous fibrosis and evidence of spared nerves and vessels.
Focal endocardial delivery of PFA is feasible and safe and can be considered for ventricular substrate ablation.
Introduction
Pulsed field ablation (PFA) uses sub-second electrical impulses to create lesions by irreversibly electroporating cell membranes. Myocytes have a lower characteristic threshold for irreversible electroporation than surrounding cell types. Thus, in contrast to traditional thermal ablation, the underlying mechanism of PFA offers a degree of tissue-specific safety, sparing supra-threshold tissues within an ablative zone.1–7 Recent preclinical and clinical studies examining its effects on atrial myocardium have demonstrated that large lesions can be created quickly and safely.8–12 We have recently demonstrated that a custom PFA generator with optimized pulse characteristics is able to create durable atrial lesions safely and quickly in humans using an over-the-wire catheter.11,12
On the other hand, there is less data on the use of PFA for ventricular ablation.13–17 These preclinical studies examining ventricular PFA have been largely performed using systems limited by design to a pericardial approach. However, current clinical practice demands endocardial PFA delivery with a deflectable catheter delivered using a transfemoral approach. Accordingly, a prototype, steerable catheter has been designed for performing endocardial ventricular PFA. In this report, we aim to characterize endocardial PFA with this catheter at an exploratory dose in porcine ventricles.
Methods
All preclinical experiments were approved by the Institutional Animal Care and Use Committee at PreClinical Med Device, Covance Laboratories San Carlos, CA, USA. Four female Yorkshire porcine (55–70 kg) were studied.
Procedural details
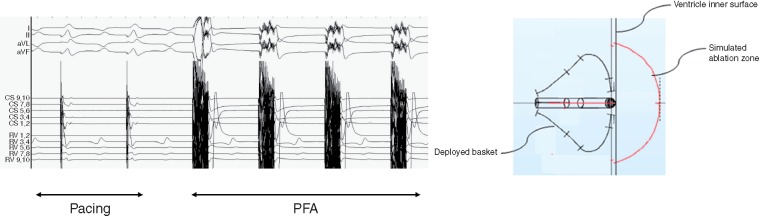
Percutaneous femoral venous access was obtained and a single transseptal puncture was performed after systemic heparin was administered. Additional femoral venous access was obtained to allow for coronary sinus and right ventricular (RV) apical catheter placement. Activated clotting times were maintained over 300 s during the procedure. A 12-Fr deflectable, four-spline multielectrode catheter with a deployable distal end was positioned via a 13-Fr deflectable sheath in both ventricles using intracardiac echocardiography (ICE), fluoroscopy and electroanatomic mapping system guidance (Figure 1). Prior to ablation, the distal end of the catheter was expanded and positioned against tissue to ensure stability. The catheter-tip design was optimized to enhance the electric field distribution for ventricular ablation. Electrogram recording and pacing are possible through the distal electrodes on the catheter. All electrodes including those proximal, are utilized to generate the therapeutic electric field. Ablation was performed using a custom, programmable PFA generator (Farapulse Inc., Menlo Park, CA, USA, described previously).11,12
Figure 1.
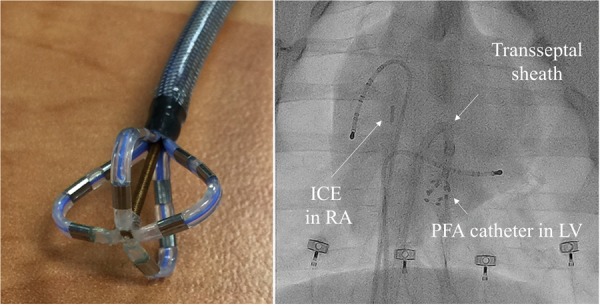
(Left panel) Distal aspect of the ablation catheter in deployed position. (Right panel) Fluoroscopic view demonstrating pacing catheters placed in the right ventricle and coronary sinus along with ICE positioned in the RA. A deflectable transseptal sheath is in position with the ablation catheter positioned in the LV. ICE, intracardiac echocardiography; LV, left ventricle; PFA, pulsed field ablation; RA, right atrium.
The PFA waveform was tuned for the catheter and consisted of a proprietary sequence of biphasic, microsecond-scale pulses delivered in bipolar fashion between catheter electrodes. No surface patch was used. Each delivery spanned six heartbeats with a fixed voltage amplitude of 2200 V. Pulses were synchronized to joint pacing by diagnostic catheters (Biosense Webster) in the coronary sinus and RV apex, using a stimulator (Micropace). Application sites were selected based on the ability to position the catheter in perpendicular or near-perpendicular orientation to tissue (Figure 2 and 3). Wide separation between lesions was sought to facilitate post-sacrifice analysis. Lesions were applied to both the right ventricle (14 sites) and the left ventricle (25 sites). In the right ventricle, five lesions were applied to the RV free wall, three lesions each were applied at the apex and the free wall of the outflow tract, and one lesion each to the following sites: basal septum, septal outflow tract, and the inferior wall. In the left ventricle, nine lesions were applied to the left ventricular free wall, six to the apex, eight to the left ventricular septum, and two to the inferior wall (Figure 2). Pre-ablation and post-ablation pacing thresholds were obtained from the four distal electrodes of the ablation catheter in two of four swine. The animals were recovered and brought back for a repeat procedure at 35.5 days. Electroanatomic mapping of the ventricles was performed using Rhythmia with the Orion multielectrode catheter (Boston Scientific, Cambridge, MA, USA) immediately after ablation and at the repeat procedure to confirm the presence of low-voltage (defined as <0.5 mV) areas in the ventricles. After the repeat procedure, all swine were humanely euthanized.
Figure 2.
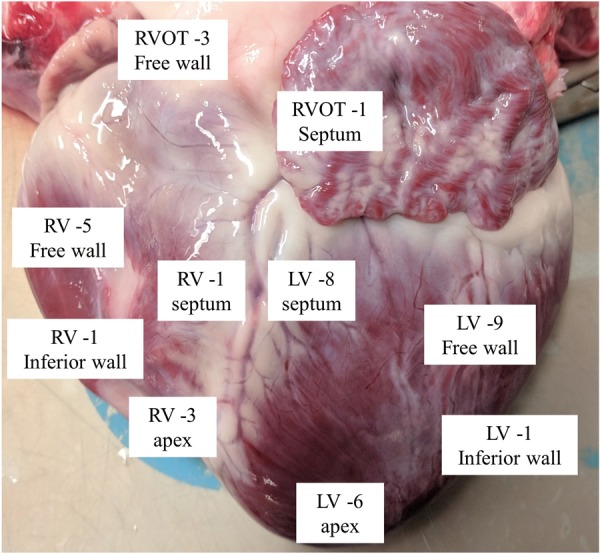
Distribution of applications across both RV and LV. LV, left ventricle; RV, right ventricle; RVOT, right ventricular outflow tract.
Figure 3.
(Left panel) Joint pacing from coronary sinus and right ventricular catheters with synchronized PFA pulses. (Right panel) Figure diagram demonstrating the simulated ablation zone for this specific dose/electrode combination. PFA, pulsed field ablation.
Pathological investigation
Gross examination of all hearts was performed at necropsy and the exterior cardiac surfaces were photographed before the specimens were fixed in formalin. Lesions were then identified, sectioned along their long axes and photographed. All morphometrical measurements were performed with the ImageScope software (Leica Biosystems). The hearts from three swine were submitted for histological examination. One swine receiving two applications per site was excluded from histological analysis due to cost constraints (nine lesions in this swine). Gross measurements were obtained on 28/30 discrete lesions. Two lesions were excluded for technical reasons related to tissue trimming. Histology was obtained on 26 sections and haematoxylin & eosin as well as Masson trichrome staining was performed.
Statistical analyses
Study results are presented using descriptive statistics. For continuous variables, the results include number, mean, standard deviation, and 95% bilateral confidence intervals, where pertinent. Presented data for categorical variables includes the number and percent of subjects in each category. Statistical analyses were performed with SPSS 26.0 software (SPSS Inc., Chicago, IL, USA).
Results
Acute experiments
The mean weight of swine at the time of acute experiments was 56.3 kg. A total of 39 PFA applications were delivered to the ventricles: 14 in the right ventricle and 25 in the left ventricle (Table 1). The first swine received two applications/site and the subsequent three swine received four applications/site. All applications were successfully delivered, and none resulted in waveform discontinuities indicative of arcing. The total elapsed duration of PFA delivery for each animal was <1 min (Figure 3). Importantly, no sustained ventricular arrhythmias occurred in any swine (Table 1).
Table 1.
Summary of ablation protocol and lesion distribution in the right and left ventricle and presence of sustained ventricular arrhythmias
| Animal | Ablation protocol applications/site | Lesion sites (n = 39) |
Sustained VA | |
|---|---|---|---|---|
| RV (n = 14) | LV (n = 25) | |||
| #1 | 2 | 2 | 7 | 0 |
| #2 | 4 | 3 | 5 | 0 |
| #3 | 4 | 4 | 5 | 0 |
| #4 | 4 | 5 | 8 | 0 |
LV, left ventricle; RV, right ventricle; VA, ventricular arrhythmias.
The prototype catheter was connected to allow for pacing and sensing in an arrangement of four bipole pairs correlating with each spline. With perpendicular placement of the catheter tip, the distal electrodes contacted tissue. Electrogram reduction was appreciable following all applications. Post-PFA pacing thresholds were assessed for 10 consecutive sites in two of four swine. Non-capture at maximum output (20 mA @ 2.0 ms) was demonstrated on at least one bipole pair in 8 of 10 sites following PFA (3 of 3 right ventricle, 5 of 7 left ventricle). Thresholds increased by a mean of over 16.8 ± 2.8 mA for the three sites in the right ventricle and over 16.1 ± 2.8 mA for the seven left ventricular sites. As previously described, ICE demonstrated microbubble formation. Mild muscular engagement was present during pulse delivery, although this did not impair catheter-tip stability as seen on ICE and/or fluoroscopy. Following ablation, transient ST-T wave repolarization changes were occasionally seen but were not accompanied by any sustained hypotensive effects.
Remapping study
All four swine completed the follow-up period. Repeat electroanatomic mapping confirmed areas of low voltage (<0.5 mV) corresponding to sites of PFA applications in both ventricles (Supplementary material online, Figure). At necropsy, there was no evidence of pericarditis over any of the lesions and the epicardial surface of the heart and pericardium appeared healthy. Lesions were easy to appreciate over the RV epicardium indicating transmurality in this chamber. The lesions demonstrated a pale appearance with distinct and smooth margins and their endocardial dimensions measured larger than their epicardial dimensions (Figure 4A and B). The endocardial lesions appeared well-healed and were without any evidence of surface disruption or adherent clot. Epicardial lesions were not visible in the left ventricle and lesions in this chamber were visualized only after sectioning (Figure 4C).
Figure 4.
(A) Epicardial aspect of transmural lesions in the RV, inset-zoomed view of a single lesion. (B) Endocardial aspect of the same lesions in the RV, inset-zoomed view of a single lesion. (C) Section demonstrating non-transmural lesions in the LV. LV, left ventricle; RV, right ventricle.
With this exploratory dose, lesions were noted to be as deep as 9.4 mm with four lesions reaching depths over 8 mm. In general, RV lesions were transmural (10 of 11; 91%) and left ventricular lesions were not (0 of 17; 0%). The average maximum depth for non-transmural lesions was 6.5 ± 1.7 mm. The average transmural lesion penetrated tissue that was 4.8 ± 1.9 mm thick at the time of analysis, with a transmural RV lesion at a maximal depth of 8.5 mm. The average width as measured on the endocardial surface was 22.6 ± 4.1 mm for all lesions with a maximum in one section of 28.6 mm (Table 2).
Table 2.
Distribution of transmural and non-transmural lesions between right and left ventricles and mean lesion dimensions in each group
| Transmural (n = 10) | Non-transmural (n = 18) | P-value | |
|---|---|---|---|
| Chamber | |||
| RV | 10 | 1 | <0.001 |
| LV | 0 | 17 | |
| Lesion depth (mm) | 4.8 + 1.9 | 6.5 + 1.7 | 0.024 |
| Lesion width (mm) | 22.7 + 3.3 | 22.6 + 4.5 | 0.99 |
LV, left ventricle; RV, right ventricle.
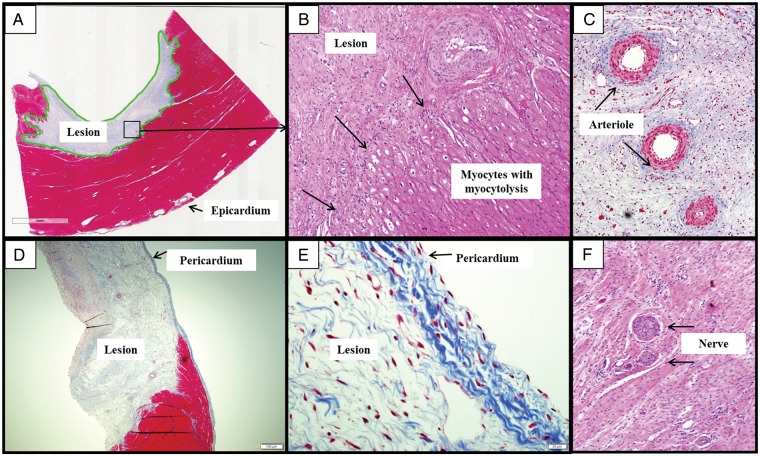
Histology demonstrated complete and homogeneous fibrotic replacement of the myocardium within the lesion consistent with the survival period. No thrombi were seen in any of the sections. Typically, the endothelial cell lining was partially preserved with evidence of disrupted collagen fibres, oedema and some areas of focal thickening. Small areas of haemorrhage, focal areas of lymphocytic infiltration, as well as occasional areas of calcification were seen within the fibrotic area in some sections. Interstitial oedema was also seen in lesions. A narrow zone of myocytolysis marked by vacuolation of cytoplasm, loss of cross-striation, pyknotic nuclei, and intracellular oedema was seen along the border of the lesion and normal myocardium (Figure 5A and B). The adjacent myocardium was noted to be normal and consistent with what was appreciated on gross examination. There was no histological evidence of pericarditis along the epicardial surfaces on any of the lesions (Figure 5D and E). The connective tissue frame of the pericardium was preserved in all sections, including those with lesion transmurality. Finally, in all sections, arterioles within lesions demonstrated preserved endothelial lining. No arteriole demonstrated luminal occlusions or thrombosis. Three sections revealed the presence of viable nerve fascicles fully embedded within the scar (Figure 5C and F). Interestingly, in one section, unablated Purkinje fibres were observed in the border of the PFA lesion.
Figure 5.
(A) A well-demarcated, non-transmural lesion in the left ventricle (MT, 3×). (B) Junction of fibrotic lesions and normal myocardium (H&E, 10×). (C) Thickening of arteriolar walls (black arrows) with well-preserved endothelial lining and spared lumen as seen within the core of an ablation lesion. Note: no thrombi in the vessels (MT, 10×). D) A transmural fibrotic lesion in right ventricle (MT, 2×). (E) Flat mesothelial cells lining of pericardium are seen to be well-preserved (MT, 40×). (F) Two well-preserved and viable nerve fascicles seen within fibrous tissue of an ablation lesion. H&E, haematoxylin and eosin; MT, masons trichrome.
Discussion
In this in vivo study, we demonstrate that ventricular endocardial delivery of focal PFA is both safe and feasible. At this first exploratory dose, the study catheter was able to create lesions of sufficient width and depth, with maximum dimensions of 28.6 mm wide and 9.4 mm deep. In addition, we were also able to demonstrate sparing of arterioles and nerves within the lesion, confirming PFA’s known myocardial specificity.
Our current understanding of ventricular lesions created with this energy source is derived from previous preclinical reports utilizing a monophasic pulse from an external defibrillator.13–17 The authors of these landmark reports applied circular multielectrode or linear suction devices to the epicardial surface of swine ventricles and demonstrated that large PFA lesions can be created safely. These reports have cumulatively established that: (i) deep and wide ventricular lesions can be created, (ii) this can be achieved with sparing of epicardial coronary arteries, and that (iii) there is a dose-dependent effect, i.e. lesion dimensions increase with the amount of energy delivered.
However, it is important to note that the means of energy delivery was via either open surgical access, thoracotomy or subxiphoid pericardial access. Given that transvenous, endocardial procedures are much more common in clinical practice; these epicardial-only delivery strategies have significantly limited clinical viability in the treatment of ventricular arrhythmias. Additionally, the pulses in these reports were delivered using a monophasic and monopolar system. These delivery parameters are limited by the disadvantages of creating significant muscular stimulation that can lead to catheter instability, making it less suitable for endocardial PFA delivery.
In this report, we assessed whether focal PFA-based endocardial ablation can be achieved in the ventricles using a deflectable catheter. This approach is most closely aligned with clinical workflows for ventricular substrate ablation. The availability of a deflectable focal PFA catheter is important, as endocardial targets often require the ablation element to be manoeuvred to specific ventricular locations. In addition, the use of a generator that has been shown to safely deliver biphasic PFA in humans is an added advantage of the system tested in this study. This study was a proof-of-concept and an initial dose exploration, supporting the titration of a dose prescription after the first subject and driven by a need to understand dimensional and pathological characteristics of PFA lesions.
Our ability to create focal lesions in the ventricle up to depths of 9 mm is encouraging and serves as the basis for additional dosing studies. Although this was a small study, the lesion dimensions achieved here are adequate for this approach to be considered for clinical ventricular scar ablation. Homogeneous and large PFA lesions could allow for rapid and confluent ablation of scar without sequestered myocytes or lingering conductive channels. The ability to observe electrogram reduction and demonstrate changes in pacing threshold were helpful at this early stage but these parameters need to be further characterized and correlated to lesion size. The biphasic delivery was also noteworthy for its ability to ablate ventricular substrate without arrhythmogenic effects in the highly sensitive porcine model (indeed, when radiofrequency energy is delivered to porcine ventricular myocardium, complex ventricular ectopy, including ventricular fibrillation, almost invariably occurs). Results from this study should inform improvements to the waveform and further study of dose–response behaviour, including variables such as catheter orientation and repeat applications.
The catheter was localized with a commercial mapping system, which was primarily used to document acute lesion creation and prevent lesion overlap. Acutely, low voltage post-ablation was appreciated and qualitatively appeared to extend beyond the profile of the catheter tip. Similar to our experience with this biphasic system in preclinical atrial studies, albeit with a different catheter, muscular contractions did occur but were greatly reduced when compared to older monophasic waveforms. Minor repolarization changes and microbubble formation reflected our prior observations and have proven to not be clinically relevant.
Limitations
This preclinical evaluation was performed in healthy swine using an initial, exploratory dosing prescription. Extrapolation of findings to human myocardium should not be assumed. Furthermore, PFA of ventricular myocardium with heterogeneous fibrosis/scar may behave completely differently; dedicated studies in ventricular disease models are necessary. Interpretation of findings must also be made while keeping the small sample size in mind. A lack of detailed pathological examination of other organs limits the completeness of the safety assessment. Electroanatomic mapping was performed without force-sensing capabilities while constrained by small porcine anatomy, making interpretation of voltage maps difficult and requiring further study. Lesions were mostly created with a perpendicular catheter–tissue orientation and therefore may not properly reflect PFA lesions with other catheter–tissue orientations. Larger and more detailed studies with dedicated safety assessments over a range of doses are necessary and planned.
Conclusions
In this initial study of an exploratory dose, we demonstrate that endocardial focal PFA can be delivered using a prototype catheter. Myocardium-specific lesions were rapidly and safely created, with dimensions that are relevant for ablation of ventricular tachycardia in the clinical setting. Further studies are needed to understand PFA’s role in the treatment of ventricular arrhythmias as well as other focal targets for ablation.
Conflict of interest: V.Y.R.: Farapulse: stock, consultant; Boston Scientific: consultant. Conflicts with other medical companies not related to this manuscript are listed in the Supplementary material online. J.K.: Farapulse: grant support, consultant; conflicts with other medical companies not related to this manuscript are listed in the Supplementary material online. S.R.D.: Farapulse: stock. R.V., R.B., and E.D.B.: employees of Farapulse. E.D.: consultant.
Supplementary Material
Funding
This study was supported by Farapulse Inc., the manufacturer of the prototype catheter studied in this manuscript.
References
- 1. Davalos RV, Mir IL, Rubinsky B.. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–31. [DOI] [PubMed] [Google Scholar]
- 2. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B.. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 2006;53:1409–15. [DOI] [PubMed] [Google Scholar]
- 3. Rubinsky B, Onik G, Mikus P.. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat 2007;6:37–48. [DOI] [PubMed] [Google Scholar]
- 4. Maor E, Ivorra A, Rubinsky B.. Non thermal irreversible electroporation: novel technology for vascular smooth muscle cells ablation. PLoS One 2009;4:e4757.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li W, Fan Q, Ji Z, Qiu X, Li Z.. The effects of irreversible electroporation on nerves. PLoS One 2011;6:e18331.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaminska I, Kotulska M, Stecka A, Saczko J, Drag-Zalesinska M, Wysocka T. et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen Physiol Biophys 2012;31:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M.. Cell membrane electroporation—Part 1: the phenomenon. IEEE Trans Dielectr Electr Insul 2012;28:14–23. [Google Scholar]
- 8. Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E. et al. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754–64. [DOI] [PubMed] [Google Scholar]
- 9. Witt CM, Sugrue A, Padmanabhan D, Vaidya V, Gruba S, Rohl J. et al. Intrapulmonary vein ablation without stenosis: a novel balloon-based direct current electroporation approach. J Am Heart Assoc 2018;7:pii: e009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH.. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm 2015;12:1838–44. [DOI] [PubMed] [Google Scholar]
- 11. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H. et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I. et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol 2018;4:987–95. [DOI] [PubMed] [Google Scholar]
- 13. Neven K, van Driel V, van Wessel H, van Es R, Du Pré B, Doevendans PA. et al. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ Arrhythm Electrophysiol 2014;7:913–9. [DOI] [PubMed] [Google Scholar]
- 14. Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F.. Myocardial lesion size after epicardial electroporation catheter ablation after subxiphoid puncture. Circ Arrhythm Electrophysiol 2014;7:728–33. [DOI] [PubMed] [Google Scholar]
- 15. Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F.. Epicardial linear electroporation ablation and lesion size. Heart Rhythm 2014;11:1465–70. [DOI] [PubMed] [Google Scholar]
- 16. Wittkampf FHM, van Driel VJ, van Wessel H, Neven KGEJ, Gründeman PF, Vink A. et al. Myocardial lesion depth with circular electroporation ablation. Circ Arrhythm Electrophysiol 2012;5:581–6. [DOI] [PubMed] [Google Scholar]
- 17. Du Pré BC, van Driel VJ, van Wessel H, Loh P, Doevendans PA, Goldschmeding R. et al. Minimal coronary artery damage by myocardial electroporation ablation. Europace 2013;15:144–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.