Abstract
Aims
Incorporating patient-reported outcomes (PROs) into routine care of atrial fibrillation (AF) enables direct integration of symptoms, function, and health-related quality of life (HRQoL) into practice. We report our initial experience with a system-wide PRO initiative among AF patients.
Methods and results
All patients with AF in our practice undergo PRO assessment with the Toronto AF Severity Scale (AFSS), and generic PROs, prior to electrophysiology clinic visits. We describe the implementation, feasibility, and results of clinic-based, electronic AF PRO collection, and compare AF-specific and generic HRQoL assessments. From October 2016 to February 2019, 1586 unique AF patients initiated 2379 PRO assessments, 2145 of which had all PRO measures completed (90%). The median completion time for all PRO measures per visit was 7.3 min (1st, 3rd quartiles: 6, 10). Overall, 38% of patients were female (n = 589), mean age was 68 (SD 12) years, and mean CHA2DS2-VASc score was 3.8 (SD 2.0). The mean AFSS symptom score was 8.6 (SD 6.6, 1st, 3rd quartiles: 3, 13), and the full range of values was observed (0, 35). Generic PROs of physical function, general health, and depression were impacted at the most severe quartiles of AF symptom score (P < 0.0001 for each vs. AFSS quartile).
Conclusion
Routine clinic-based, PRO collection for AF is feasible in clinical practice and patient time investment was acceptable. Disease-specific AF PROs add value to generic HRQoL instruments. Further research into the relationship between PROs, heart rhythm, and AF burden, as well as PRO-guided management, is necessary to optimize PRO utilization.
Keywords: Atrial fibrillation, Patient-reported outcomes, Feasibility, Toronto Atrial Fibrillation Severity Scale, Quality of life
What’s new?
Patient-reported outcomes (PROs) are increasing recognized to be vital to the assessment of patients with atrial fibrillation (AF).
Few systematic, widespread programmes exist to assess PROs, particularly in AF.
We demonstrate the feasibility of systematic PRO collection in a tertiary care electrophysiology clinic, including both generic and AF-specific PROs.
Disease-specific PROs for AF appear to add to generic PRO instruments.
Further research into the relationship between PROs, heart rhythm, and AF burden, as well as PRO-guided AF management, is necessary to optimize PRO implementation in clinical practice.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in adults and has a substantial effect on morbidity and mortality. Patients with AF also experience a significant reduction in health-related quality of life (HRQoL), comparable to a recent myocardial infarction or heart failure.1 Therefore, treatment of AF is often focused on improving symptoms, which can be best measured using validated, disease-specific, patient-reported outcomes (PROs). Multiple national and international bodies have recommended the use of PROs to guide both research and clinical care for patients with AF.2 However, PROs are included in only a limited number of registered clinical trials of AF,3 and it is not clear the extent to which they are collected in routine clinical practice.
In contrast, PROs have demonstrated added value to disease management across a variety of conditions, including oncology and orthopaedics.4,5 Multiple frameworks have been developed, facilitating treatment decisions aimed at optimizing PROs as primary endpoints, particularly in settings where improving survival is less relevant than improving quality of life. Therefore, PROs represent an opportunity to measure and improve outcomes that often matter most to patients, particularly those with AF.
As part of a unique, health system-wide infrastructure aimed at collecting a wide variety of PROs, we deployed PRO collection to assess AF-specific HRQoL. This report describes the feasibility and initial results of systematically collecting PROs in the electrophysiology clinic setting. The objectives of the analyses were: (i) to understand the feasibility of routine AF PRO collection in an outpatient, clinic setting, including the burden on patients; (ii) to describe disease-specific PROs among AF patients in a tertiary, academic electrophysiology clinic; and (iii) to compare disease-specific and generic PROs among these patients.
Methods
In 2015, the University of Utah implemented a unique, health system-wide infrastructure to collect structured PROs across medical specialties and outpatient settings. Patients are invited to provide assessments at home prior to their appointment, via e-mail, for those that have a registered address. Alternatively, upon arrival to clinic, administrative staff load the PRO assessment(s) onto a portable tablet computer to be completed by the patient while awaiting their visit. The PRO tools collected during any particular visit are dictated by (i) the provider the patient is registered to see (for disease-specific PROs), and (ii) the interval from last collection of any specific tool (for both disease-specific and generic PROs). Therefore, for any given visit, PRO assessments may include both disease-specific PROs dictated by the provider the patient is seeing, as well as generic PROs based on collection interval for the different assessments. The core assessment of generic PROs is completed by patients at least annually and no more often than weekly; it includes a general health question, a current health visual analogue scale (VAS) assessment, the PROMIS-Bank Physical Function, and the PROMIS-Bank Depression.6 However, the programme includes several hundred disease-specific and generic HRQoL tools; currently, the cardiovascular centre has implemented disease-specific PROs for heart failure (Kansas City Cardiomyopathy Questionnaire) and atrial fibrillation (see below). The data are securely transferred and integrated into the electronic health record within minutes for clinician review during the visit. In addition to responses, the system automatically logs assessment completion rates, with time and date stamps. This system, managed by the University of Utah My Evaluation (mEVAL) Personal Assessment Team, results in a seamless, streamlined, efficient approach yielding near instantaneous PRO assessment for routine, system-wide clinical use. Additional details on this University-wide implementation have been published previously (see Supplementary material online, Appendix Figure A1).7
As part of the 2016 mEVAL deployment in the cardiovascular centre,8 the electrophysiology clinic at the University of Utah began collecting the Toronto AF Severity Scale (AFSS) for all patients scheduled with a clinical cardiac electrophysiology clinician (physician or advanced practice clinician) in the outpatient setting.1 The AFSS is a previously validated and broadly used AF-specific PRO, which includes 20 items across four domains: (i) global well-being; (ii) health care utilization; (iii) AF burden; and (iv) AF symptoms. The last domain, which yields the AFSS symptom score, was used for the current analysis, and includes seven AF-related symptoms on 5-point Likert scales for a possible range of scores from 0 to 35. In order to calculate this domain (and be included in subsequent analyses), patients had to have provided a numerical response to all seven questions in this domain.
Patient cohort
This analysis included patients who (i) had ≥2 prior encounters in our health system (and ≥1 as an outpatient) with an International Classification of Disease (ICD) code for AF [427.31 (9th revision) or I48.0, I48.1, I48.2, I48.91 (10th revision)]; (ii) had an outpatient visit with a University of Utah adult electrophysiology clinician from October 2016 to February 2019; and (iii) initiated at least one AFSS PRO assessment. The analysis excluded patients who, as part of their electrophysiology visit, initiated a PRO core assessment for generic HRQoL, but did not start an AFSS, disease-specific, questionnaire.
The index visit for the cohort was defined as the first electrophysiology visit where an AFSS PRO assessment was initiated. The data sources are derived from the health system’s enterprise data warehouse, and includes all administrative billing encounters with diagnosis codes (inpatient, outpatient, procedural, etc.), as well as medication orders, laboratory results, electrocardiography (ECG) results, and echocardiography results. Clinical comorbidities were calculated using previously validated algorithms for use in administrative data analyses of cardiovascular disease, and include all health system encounters up to and including the baseline visit.9,10 Medication rates were based on orders placed for medications between 90 days before to 30 days after the index visit. Echocardiography and laboratory values were derived from the closest values between 365 days before and 30 days after the index visit. Electrocardiographic findings were derived from the closest ECGs between 30 days before and 1 day after the index visit.
Statistical methods
Categorical variables are summarized as number (percentage), and continuous variables summarized as mean (standard deviation). Univariate comparisons were performed with the χ2 for categorical variables and ANOVA for continuous variables. A two-sided P-value of <0.05 was considered significant. All analyses were performed using R (Version 3.5.2) and RStudio (Version 1.1.463),11 and packages specifically geared to such analyses.12,13 Analysis of the data collected as part of routine clinical care, and subsequent reporting of anonymized, aggregate data, was approved by the University of Utah Institutional Review Board.
Results
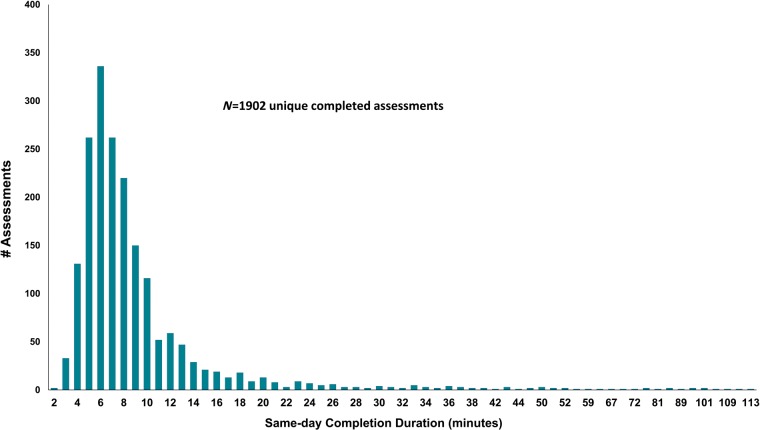
From 4 October 2016 to 4 February 2019, approximately 4568 patients with AF appeared to have had an eligible clinical encounter during which PROs were available (see Supplementary material online, Appendix Table A1). Of these, 1534 AF patients initiated 2379 AFSS PRO assessments (Figure 1, Table 1). This rate is consistent with previously reported completion rates in clinic.8,14 Overall, 2145 encounters (90%) resulted in completion of all planned PRO instruments (AF and generic tools). The median same day completion time was 7.3 min (1st, 3rd quartiles: 6, 10) which included all disease-specific and generic instruments (Figure 2). On average, patients initiated to 1.6 AFSS PRO assessments, and 599 patients started two or more (Supplementary material online, Appendix Figure A2).
Figure 1.
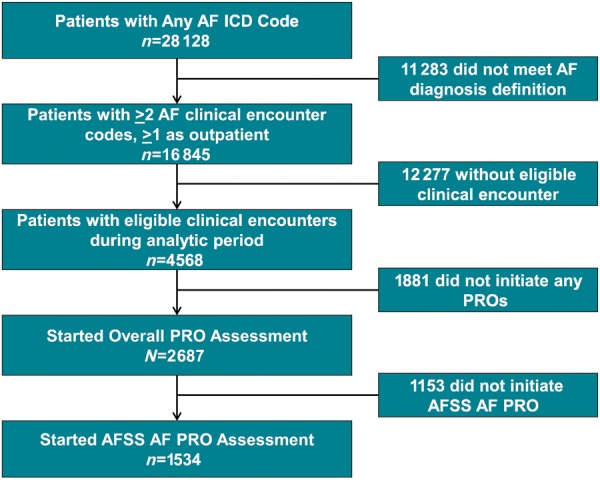
CONSORT diagram of analytic cohort, including primarily all patients who initiated at least one AFSS assessment, but may not necessarily have completed it (n = 1534). The initial cohort was defined by presence of a single diagnostic code for AF and was subsequently limited by a more specific diagnostic definition (see Methods section). AF, atrial fibrillation; AFSS, AF Severity Scale; PRO, patient-reported outcome.
Table 1.
Systematic collection of AF patient-reported outcomes
| Total AFSS assessments initiated | 2379 |
| Total unique AF patients in clinic | 1534 |
| Total AFSS assessments completed | 2145 (90%) |
| Total number PRO questions answereda | 48 902 |
| Same-day completion time (median)a (min) | 7.3 |
AF, atrial fibrillation; AFSS, AF Severity Scale; PRO, patient-reported outcome.
Including all PRO instruments collected (generic measures and disease-specific) during the electrophysiology encounter.
Figure 2.
Distribution of assessment duration among patients who completed all assessments in less than 2 h (1902 of the 1968 same-day assessments, 97%).
Baseline characteristics of the analysis cohort are shown in Table 2. Overall, 38% of the cohort was female (n = 589) with a mean age of 68 years (SD 12), and a mean CHA2DS2-VASc score of 3.8 (SD 2.0). Prior heart failure was present in 41% (n = 625), and the mean left ventricular ejection fraction among patients with an echocardiogram within the prior year was 57% (SD 12). Overall, 23% (n = 354) were taking an antiarrhythmic medication, and 47% (n = 721) were treated with an oral anticoagulant.
Table 2.
Baseline characteristics of AF patients completing PROs
| Overall (n = 1534) | |
|---|---|
| Age | 68 (12) |
| Female sex | 589 (38.4) |
| Race (%) | |
| American Indian and Alaska Native | 7 (0.5) |
| Asian | 22 (1.4) |
| Black or African American | 7 (0.5) |
| Native Hawaiian/other Pacific Islander | 9 (0.6) |
| White or Caucasian | 1422 (92.7) |
| Medical history (%) | |
| Hypertension | 1165 (75.9) |
| Diabetes mellitus | 443 (28.9) |
| Myocardial infarction | 419 (27.3) |
| Heart failure | 625 (40.7) |
| Peripheral vascular disease | 643 (41.9) |
| History of stroke | 343 (22.4) |
| Dementia | 39 (2.5) |
| Pulmonary disease | 533 (34.7) |
| Cancer | 260 (16.9) |
| Severe liver disease | 28 (1.8) |
| Alcohol | 186 (12.1) |
| Depression | 445 (29.0) |
| Medical therapy (%) | |
| Beta-blocker | 604 (39.4) |
| Non-dihydropyridine calcium-channel blocker | 182 (11.9) |
| Oral anticoagulation | 721 (47.0) |
| Any antiarrhythmic | 354 (23.1) |
| CHA2DS2-VASc score | 3.76 (2.03) |
| Prior cardioversion | 346 (22.6) |
| Prior ablation | 400 (26.1) |
| LVEF | 57.02 (12.06) |
| Baseline ECG in atrial arrhythmia | 220 (27.5) |
| Creatinine | 1.12 (0.67) |
| Haemoglobin | 14.03 (1.98) |
Baseline characteristics, comorbidities, admission data, and laboratory studies.
Values are presented as n (%) or mean (standard deviation), unless otherwise noted.
AF, atrial fibrillation; AFSS, atrial fibrillation severity score; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; PRO, patient-reported outcome.
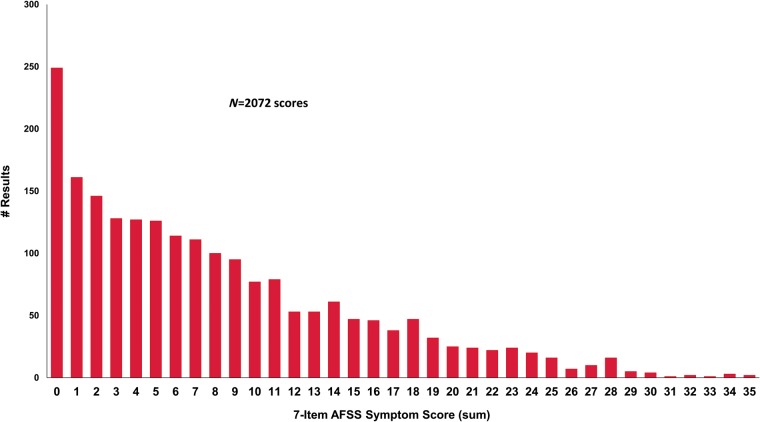
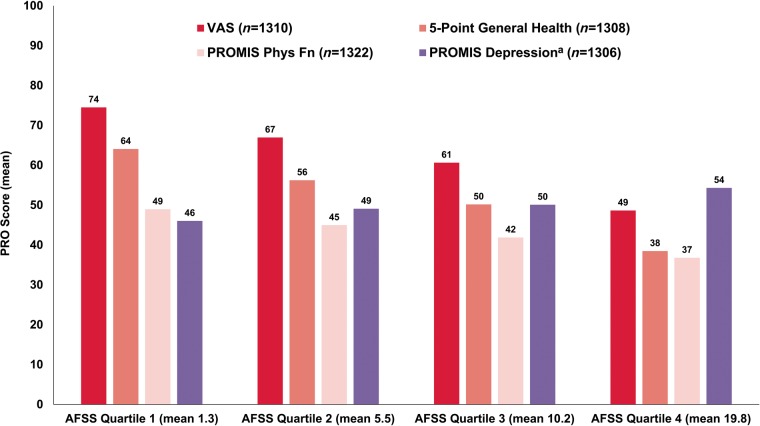
Among the 1534 unique patients who initiated 2379 AFSS assessments, 2072 yielded responses that allowed for calculation of the AFSS symptom score (i.e. all seven symptom questions were answered). The distribution of all AFSS symptom scores is shown in Figure 3. Comparisons between AFSS symptom scores and other generic HRQoL scores are shown in Table 3. The AFSS symptom score was then broken down by quartiles (Figure 4), and compared to generic HRQoL. Overall, general HRQoL measures appeared to degenerate most with highest (most severe) quartile of AFSS symptom score burden (P < 0.0001 for each), with lower magnitude changes in generic HRQoL measures at lower AFSS symptom score quartiles.
Figure 3.
Distribution of AFSS symptom scores among all responses, across all patients (repeat measures by same patient included separately). AFSS, AF Severity Scale.
Table 3.
Baseline AF-specific and generic PRO scores at baseline
| Score range | Sample size | Median score | 25th percentile | 75th percentile | |
|---|---|---|---|---|---|
| AFSS symptom scorea | 0–35 | 1308 | 7 | 3 | 13 |
| VAS general health | 0–100 | 1310 | 68 | 49 | 80 |
| General health (5-point) | 10, 30, 50, 70, 90 | 1308 | 50 | 50 | 70 |
| PROMIS—depressiona | 0–100 | 1306 | 50 | 45 | 55 |
| PROMIS—physical function | 0–100 | 1322 | 44 | 37 | 50 |
Baseline scores across different PROs, for unique patients.
AF, atrial fibrillation; AFSS, atrial fibrillation severity score (symptom domain); HRQoL, health-related quality of life; PROMIS, patient-reported outcomes measurement information system [scale ranges 0–100 and normalized to a population mean of 50 and a standard deviation of 10, with higher scores reflecting higher degree of the health status measure examined by the individual domain (e.g. higher score in the fatigue domain means more fatigue)].
Higher scores indicate worse HRQoL (i.e. more AF symptoms and depression); for other tools, higher scores indicate better HRQoL (i.e. more physical function, higher health).
Figure 4.
Simultaneous, generic PROs, stratified by quartile of AFSS symptom score quartile.a See Table 3 for score ranges for each tool. P < 0.001 for comparison of each generic PRO across AFSS quartile. aHigher scores indicate worse HRQoL (i.e. more AF symptoms and depression); for other tools, higher scores indicate better HRQoL (i.e. more physical function, higher health). AF, atrial fibrillation; AFSS, AF Severity Scale; HRQoL, health-related quality of life; PRO, patient-reported outcome.
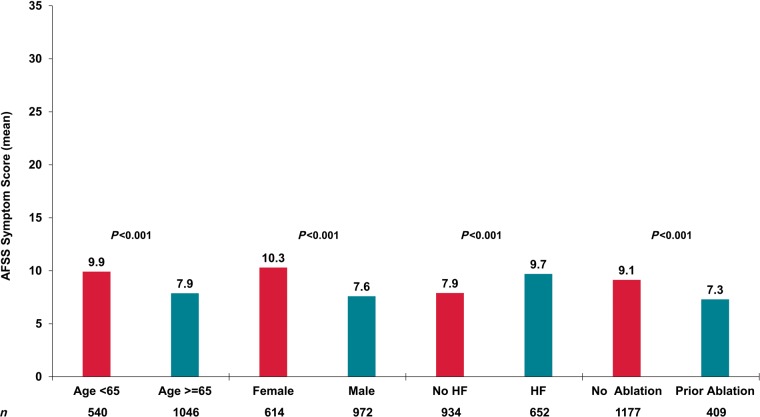
Mean AFSS symptom scores, by subgroups of interest, are shown in Figure 5. Younger patients, those who were female, with a history of heart failure, and no prior ablation, all had worse symptoms compared with their respective referent groups.
Figure 5.
Mean unadjusted AFSS symptom scores in important subgroups. AFSS, AF Severity Scale.
Discussion
While many have called for increasing use of PROs in clinical care and for quality assessment,15 there are minimal insights into how to accomplish this or of its clinical value. In this analysis of systematic AF PRO implementation in a tertiary-care electrophysiology clinic, there are several important findings. First, health-system wide collection and integration of PRO data into the electronic medical record is feasible within the structure of a busy, electrophysiology practice. Second, the burden of collection to patients appears acceptable and manageable, as evidenced by (i) ascertainment rates comparable to other, similar endeavours8,14; (ii) high completion rates among initiated assessments; and (iii) short overall completion times. Finally, concurrent collection of generic HRQoL metrics demonstrated internal consistency with AF symptom status, while adding valuable additional, disease-specific HRQoL data.16 Patients with higher AFSS scores appeared to receive more rhythm-control therapies during follow-up.
Patient-reported outcomes have been validated in several cardiovascular diseases, including AF,1 and major clinical trials of AF ablation have demonstrated favourable effects on HRQoL via PROs.17 However, such outcomes are not routinely collected nor used to guide clinical management of AF despite the fact that a major goal in the treatment of AF is improving HRQoL and/or symptoms. Through a clinic-based, streamlined, infrastructure, we have demonstrated feasibility of PRO measurement and integration into standard clinic processes. Our data from concomitant measurement of generic PROs, demonstrates the internal validity of AF HRQoL but also highlights the additive value of disease-specific AF PROs to generic HRQoL measures. Changes in generic HRQoL was most pronounced among patients with the most severe AF symptom score, and may be less sensitive to changes at the lower end of the spectrum.
Our data confirm and extend the experience recently reported by electrophysiologists at the Cleveland Clinic.14 In their published experience, the AFSS was deployed via electronic e-mail invitation to selected patients undergoing catheter ablation for AF. They demonstrated the feasibility of this approach. Notably, the ascertainment and completion rates reported by the Cleveland Clinic were similar to those described in our Utah mEVAL cohort. However, there are also some notable differences between the Cleveland Clinic implementation and Utah’s mEVAL. First, we targeted PRO collection to all patients with an encounter in our clinic, not just those undergoing interventions. The data collected in patients who are not undergoing interventions for AF are extremely valuable when evaluating the impact of interventions on HRQoL. Second, mEVAL also measures generic and disease-specific PROs in other settings, allowing examination of patients with multimorbidity in a single platform through unified underlying PRO collection. Third, the streamlined integration between the mEVAL PRO system and other electronic data repositories, via an enterprise data warehouse, allows for near real-time assessments of a variety of clinical characteristics (see Table 2) that provide context to interpretation of PROs, and also facilitates triggered PRO collection based on procedural (or other) encounter codes.
There remain several steps in order to fully-implement PROs into routine management of AF. While several other AF assessments besides the AFSS have been published, such as the Mayo AF-Specific Symptom Inventory (MAFSI) and Atrial fibrillation Effect on QualiTy-of-life (AFEQT),18,19 none is as well validated as tools for other disease states such as heart failure.20 Determining important metrics and management processes, such as minimal important differences, ‘actionable’ symptom scores, and tailored treatment plans remain under development for AF PROs. Additionally, PROs represent only one aspect of AF care, and other outcome measures (e.g. hospitalization, stroke, heart failure) will need to be integrated into management decisions. Further analyses from our cohort, and others, will focus on appropriate processes and outcomes of PRO-guided AF management. Finally, very little attention has been given to sharing PRO results with patients and understanding the impact on engagement, adherence to recommendations or satisfaction with care. Further studies are needed to better-evaluate these opportunities to improve care.
Limitations
These data are from real-world clinical practice, and may be susceptible to informative missingness; irregular data collection intervals may also influence ascertainment and completeness. Additionally, this is a single-centre experience within a highly motivated health system; application to other settings is unknown. Even in this setting, ascertainment rates could be improved, and the reasons for lack of an assessment are not immediately clear. Lastly, the impact of this data on current care patterns, and future management, remains the goal of future studies.
Conclusions
Collection of systematic, clinic-based assessments of PROs for AF is feasible in real-world clinical practice. Patient time investment, a critical metric of feasibility, appears reasonable. Generic HRQoL appeared to reflect AFSS symptom score among the most symptomatic, but the disease-specific PRO appeared most sensitive across the spectrum of AF-related symptoms. Further research into the relationship between PROs and AF rhythm, as well as PRO-guided AF management, is necessary to optimize both patient-reported and clinical outcomes.
Conflict of interest: The following relationships exist related to this presentation: B.A.S. reports research support from NIH/NHLBI, AHA/PCORI, Boston Scientific, Janssen, BMS/Pfizer; and consulting to BMS/Pfizer, Biosense-Webster, and Merit Medical. M.G.C.: research funding from Wavelet Health, Biotronik, Medtronic, Boston Scientific. R.U.S. was supported by a Career Development Award from the NHLBI (K08 HL136850). J.P.P. reports funding for clinical research from Abbott Medical, ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, and Verily; and consultant to Allergan, Bayer, Johnson & Johnson, Medtronic, Sanofi, and Phillips. No other authors declared conflict of interest.
Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL143156 (to B.A.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
References
- 1. Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM. et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steinberg BA, Dorian P, Anstrom KJ, Hess R, Mark DB, Noseworthy PA. et al. Patient-reported outcomes in atrial fibrillation research: results of a Clinicaltrials.gov analysis. JACC Clin Electrophysiol 2019;5:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hung M, Baumhauer JF, Licari FW, Bounsanga J, Voss MW, Saltzman CL.. Responsiveness of the PROMIS and FAAM instruments in foot and ankle orthopedic population. Foot Ankle Int 2019;40:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krohe M, Tang DH, Klooster B, Revicki D, Galipeau N, Cella D.. Content validity of the National Comprehensive Cancer Network—Functional Assessment of Cancer Therapy—Breast Cancer Symptom Index (NFBSI-16) and Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function Short Form with advanced breast cancer patients. Health Qual Life Outcomes 2019;17:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S. et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biber J, Ose D, Reese J, Gardiner A, Facelli J, Spuhl J. et al. Patient reported outcomes—experiences with implementation in a University Health Care setting. J Patient Rep Outcomes 2018;2:34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stehlik J, Rodriguez-Correa C, Spertus JA, Biber J, Nativi-Nicolau J, Zickmund S. et al. Implementation of real-time assessment of patient-reported outcomes in a heart failure clinic: a feasibility study. J Card Fail 2017;23:813–6. [DOI] [PubMed] [Google Scholar]
- 9. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF.. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–5. [DOI] [PubMed] [Google Scholar]
- 10. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 11. R: A Language and Environment for Statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 12. Yoshida K, Bohn J.. Tableone: Create ‘Table 1’ to Describe Baseline Characteristics (R Package). 2018. [Google Scholar]
- 13. Wasey JO. icd: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes. R Package Version 3.3. 2018. [Google Scholar]
- 14. Hussein AA, Lindsay B, Madden R, Martin D, Saliba WI, Tarakji KG. et al. New model of automated patient-reported outcomes applied in atrial fibrillation. Circ Arrhythm Electrophysiol 2019;12:e006986. [DOI] [PubMed] [Google Scholar]
- 15. Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT. et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018;319:483–94. [DOI] [PubMed] [Google Scholar]
- 16. Bjorkenheim A, Brandes A, Magnuson A, Chemnitz A, Edvardsson N, Poci D.. Patient-reported outcomes in relation to continuously monitored rhythm before and during 2 years after atrial fibrillation ablation using a disease-specific and a generic instrument. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH. et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM. et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55:2308–16. [DOI] [PubMed] [Google Scholar]
- 19. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR. et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 20. Green CP, Porter CB, Bresnahan DR, Spertus JA.. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.