Abstract
Repetitive blast traumatic brain injury (TBI) affects numerous soldiers on the battlefield. Mild TBI has been shown to have long-lasting effects with repeated injury. We have investigated effects on neuronal excitability after repetitive, mild TBI in a mouse model of blast-induced brain injury. We exposed mice to mild blast trauma of an average peak overpressure of 14.6 psi, repeated across three consecutive days. While a single exposure did not reveal trauma as indicated by the glial fibrillary acidic protein indicator, three repetitive blasts did show significant increases. As well, mice had an increased indicator of inflammation (Iba-1) and increased tau, tau phosphorylation, and altered cytokine levels in the spleen. Video-electroencephalographic monitoring 48 h after the final blast exposure demonstrated seizures in 50% (12/24) of the mice, most of which were non-convulsive seizures. Long-term monitoring revealed that spontaneous seizures developed in at least 46% (6/13) of the mice. Patch clamp recording of dentate gyrus hippocampus neurons 48 h post-blast TBI demonstrated a shortened latency to the first spike and hyperpolarization of action potential threshold. We also found that evoked excitatory postsynaptic current amplitudes were significantly increased. These findings indicate that mild, repetitive blast exposures cause increases in neuronal excitability and seizures and eventual epilepsy development in some animals. The non-convulsive nature of the seizures suggests that subclinical seizures may occur in individuals experiencing even mild blast events, if repeated.
Keywords: blast; epilepsy; mild traumatic brain injury; repetitive mTBI, seizure; traumatic brain injury
Introduction
Mild traumatic brain injuries (mTBIs) are non-penetrative injuries in which the individual has high responsiveness to challenges necessitating motor, verbal, and eye movement according to the Glasgow Coma Scale.1,2 The mTBI may also be characterized by symptoms such as headaches, slowness in thinking, and dizziness that may not be associated with urgent care.3–6 Consequently, mTBI can go undiagnosed, and for combat soldiers or athletes, may result in little recovery before possible exposure to a repeated TBI. A key concern is that mTBI is exacerbated dramatically by repetitive insults. Repetitive mTBIs are implicated in long-term neurodegenerative changes resulting in post-traumatic stress disorder, cognitive dysfunction, depression, and suicide.7,8
One of the most common types of repetitive mTBIs, besides sports-related head injuries, is the blast-induced TBI. During military operations in Iraq and Afghanistan, mild blast TBI was very common, with 17% of soldiers reporting such incidents, of which 59% were repeated mTBI.9 Blast TBI is caused generally by improvised explosive devices in combat areas.10 Blast waves give rise to substantial oscillating acceleration-deceleration cycles on the head, sometimes referred to as the “bobblehead effect.”11 Soldiers can experience such mTBIs and return to the battlefield without a sufficient recovery period to become exposed to additional TBI. Despite current Department of Defense policy,12 most service officers do not remove themselves from combat operations because of a lack of obvious symptoms.
Blast TBIs have both common and unique features from concussive-type TBI injuries. Like concussive TBIs, activation of astrogliosis, a likely maladaptive inflammatory response and blood–brain barrier breakdown have been reported.9,13 Prominent in blast TBIs are diffuse axonal injury and microvascular damage seen throughout large regions of the brain, likely because of shock-wave shearing forces.14
Because individuals subjected to such mTBIs usually go untreated, there is a growing concern that long-term effects may lead to an increased risk of neurological disorders, including so-called post-traumatic epilepsy (PTE).15,16 Paradoxically, however, PTE has not been reported in shock-wave animal models, and statistics of seizures and epilepsy for members of the armed forces subjected to blast TBIs have not been investigated.
Blast-induced PTE is a relatively new area of neuropathological research, with little exploration of causative factors and possible therapeutic interventions. While animal models of blast TBI have been developed, there has been as yet no investigation of blast-TBI effects on neuronal excitability in animal models. We investigate repetitive blast-type TBI as a mouse model of high relevance to humans. Our primary focus was to document changes in neuronal excitability after blast TBI and to test the hypothesis that such repetitive mTBIs cause changes in the discharge properties of neurons.
Methods
The experimental model
The experimental model was to expose mice to three consecutive days of mild blast TBI (one blast injury per day) or sham treatment. On the fourth day (one day after the last TBI), subset A mice were sacrificed and analyzed for patch clamp recording. Subset B mice were implanted with electroencephalography (EEG) electrodes on the fourth day (one day after the last TBI) and were video-EEG monitored for seizures for 72 h (day 5–7 post-TBI). Some of these mice were also monitored at monthly intervals for spontaneous seizures. Subset C mice were sacrificed seven days post-TBI for Western blot analysis and immunostaining, as described below.
Animals
Male adult (10 weeks old) C57BL/6J mice were group-housed in a 12-h light-dark cycle with food and water ad libitum. For experiments, animals were divided between treatments so that each cage would include animals from all cohorts. During the collection of data, the experimenter was blinded to the animal's treatment. All experiments were undertaken in accordance with Institutional Animal Care and Use Committee at the University of Texas Health San Antonio (UT Health SA) and were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Blast injury model
A blast tube apparatus was used that has been described previously.17–19 The air-driven shock tube (Applied Research Associates, Littleton, CO) was a 17-inch inner diameter tube with a 24-inch driver, an 8-foot long driven section, and a 4-foot expansion cone with a 7.5-degree angle of expansion. The diameter at the opening is ∼36 inches. The shock tube was pressurized using a single 0.016-inch thick aluminum membrane of alloy AL-2200 scored once with a 5-lb weight. Pencil probe gauges (PCB Piezotronics, Depew, NY) were used as a reference sensor to monitor the peak incident overpressure of the blast wave during each blast exposure to mice.
The shock tube produces pressure waves having the characteristic “Friedlander” waveform, with peaking pressures and total energy that are characteristic of a free-field blast wave.20 The average maximum peak incident overpressure was 14.6 psi ± 0.5 (standard deviation [SD]) with a positive phase of approximately 2.82 ± 0.46 milliseconds.
The mice were 10 week old C57BL/6J (Jackson Labs) mice. Mice were anesthetized using ketamine (25–75 mg/kg) and dexmedetomidine (0.25 mg/kg). For the majority of the study, experimental mice underwent three blast exposures over three days, directed head-on in an apparatus that protected the mice from exposure to their torso to prevent confounding blast lung injuries. Control (sham) mice were anesthetized and placed in the shock tube without firing. Mice were monitored to ensure sufficient time for anesthesia to take full effect before the blast wave. During this time and only on the first day of the blast exposure, mice were given a subcutaneous injection of buprenorphine SR, 1.2 mg/kg, in the intrascapular region.
Mice were transported to the blast section and placed in a custom built nine compartment blast holder. Once the animals were safely secured in each compartment, the holder was inserted into the expansion cone of the blast tube with the animals facing directly into the shockwave. Sham groups were placed in the same holder but received no blast exposure. The sham groups remained in the holder for the same amount of time as the blast-treated groups.
Afterward, the mice were given a single subcutaneous injection of atipamezole, 1 mg/kg, for anesthesia reversal. Mice were placed into their recovery and warming chamber until they gained full alertness and mobility. Mice were monitored for pain and distress twice daily. Mice were transported back to UT Health SA after the final blast. In this and other studies using blast trauma, we found three deaths out of 235 mice (1.2%). The day after the last blast trauma, mice were implanted with EEG electrodes as discussed below. During surgery, it was apparent that there was no obvious bleeding or trauma to the skull.
EEG
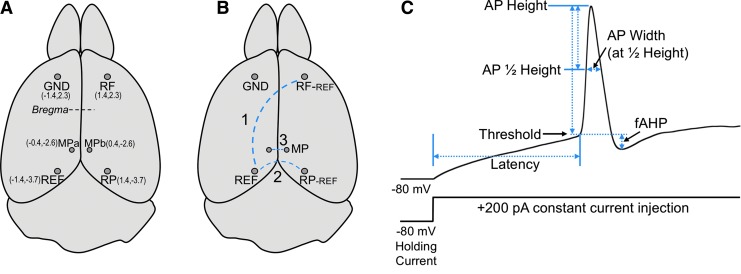
To quantify the occurrence of seizures and study cortical activity, mice were implanted with EEG) electrodes 24 h after the last blast TBI. A tethered EEG system was used. It had a pre-fabricated head mount containing screw electrodes (Pinnacle Technologies) including a ground screw (left frontal), and a reference screw electrode (left parietal) for right frontal (RF, channel 1) and the right parietal (RP, channel 2) electrodes. In addition, a bipolar subcutaneous electrode in the medial parietal region (MP, channel 3) was recorded on a third channel. The position of each electrode (Fig. 1A), and their connections (Fig. 1B) are shown in Figure 1. The head mount and electrodes were further secured to the skull with dental cement (Lang Dental).
FIG. 1.
(A) Dorsal view of mouse brain showing positions of electrode placement. Parentheses indicate absolute position in millimeters relative to medial and bregma (X,Y). The ground electrode (GND) was placed in the left frontal lobe. A reference electrode (REF, in left parietal region) served to record from right frontal (RF) and right parietal (RP) regions. Two electrodes in the medial parietal (MPa and MPb) region served as a bipolar electrode. (B) Recording electrode pairs are indicated by a dashed blue line. (C) Diagram of action potential parameters measured from a 200 pA constant current injection added to a −80 mV holding current. A description of these parameters are detailed in Methods.
During video-EEG recordings, the head mount was connected to a pre-amplifier (Gain 25X; Pinnacle Technologies), which in turn was connected via a commutator to extracellular amplifiers of the Stellate Harmonie acquisition hardware. The system collected coincident video images of the animals with EEG activity. The EEG records were scored using 0.1 to 45 Hz bandpass filter, and also included a 60 Hz notch noise filter. Seizures21–23 were identified visually by the investigator who was blinded to treatments and also by the computer-assisted EEG Stellate Harmonie review software. Post-trauma video/EEG monitoring (n = 24 blast, n = 7 sham) was of 72 h (beginning at day 2 after the last injury). Motor seizures were scored using coincident video recordings. A cohort of mice receiving 3X blast TBI (n = 13) and sham controls (n = 7) was monitored continuously for seizures at monthly intervals for 48–72 h periods.
Whole-cell current-clamp recordings
Mice were anesthetized deeply with inhaled isoflurane (Baxter Healthcare Corporation) before decapitation and whole brain dissection. The brain was placed quickly into ice-cold cutting solution containing (in mM) 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 1 CaCl2, 1 MgCl2, 26 NaHCO3, 206 sucrose, 10 dextrose, and 0.4 vitamin C. The brain was mounted on a cutting platform where coronal brain sections were cut in a Leica vibratome at a thickness of 300 μm.
The brain slices were then allowed to recover in extracellular solution at 30°C for 1 h, and then maintained at room temperature until recording. The extracellular solution consisted of (in mM) 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 26 NaHCO3, 10 dextrose, and 0.4 vitamin C. All solutions were bubbled continuously with a mixture of 95%/5% oxygen/carbon dioxide. Intracellular solutions consisted of (in mM) 120 K-gluconate, 10 HEPES, 0.1 EGTA, 20 KCl, 2 MgCl2, 2 Na2ATP, 0.25 Na2GTP, pH adjusted to 7.4 with potassium hydroxide.
Dentate gyrus granule neurons from the central region of the granule cell layer were targeted for patch clamp electrophysiology. Cells were held with a holding current to maintain a resting voltage of −80 mV. Hyperpolarizing input resistance and cell capacitance were measured using a −20 pA current injection step below the holding current. Input resistance (R) was measured from the steady-state voltage change (ΔV) because of the −20 pA current injection (R = ΔV/20 pA). Capacitance (C) was determined from an exponential fit of the hyperpolarizing relaxation time (τ) divided by the input resistance (C = τ/R). Voltage traces were collected using a HEKA EPC10 amplifier and Patchmaster software (HEKA Instruments). During whole-cell current clamp, cells receive both the −80 mV holding current and additional stepwise increases of 20 pA steps (lasting 500 msec) with 10 sec pauses between steps (Fig. 1C).
The individual properties of action potential (AP) waveforms (illustrated in Fig. 1C) were analyzed using AxoGraph software (Kagi, Berkeley, CA). The APs were detected with a derivative threshold of 20 mV/msec. Latency was measured as the time from initiation of current injection to the AP threshold. The AP height was measured from threshold to peak voltage. The AP width was measured at 50% or 10% of the AP peak. Fast-afterhyperpolarization amplitude (fAHP) was measured as the maximum negative peak after an AP (always occurring less than 2 msec after the spike) minus the threshold of AP initiation.
Parameters of AP properties (latency, threshold, height, width, and fAHP) are measured from the first AP during a 200 pA (above holding potential) current injection, and the number of APs evoked was obtained during a 200 pA, 500 msec current injection (AP number). Post-synaptic currents were filtered at 2 kHz frequency and measured at a holding potential of −80 mV.
Immunohistochemistry
Immunohistochemistry was performed to detect glial fibrillary acidic protein (GFAP) and ionized Ca2+ binding adaptor molecule 1 (Iba1), markers of astrocyte and microglia activation, respectively. Seven days after the last blast, mice were euthanized via transcardial perfusion with phosphate buffered saline (PBS) only. Brains were removed, one-half hemisphere of each sample was fixed overnight in 4% paraformaldehyde (Electron Microscopy Sciences), transferred into 30% (w/v) sucrose, and kept at 4°C for three days. Brains were then embedded into histology molds (TedPella) filled with Tissue-Plus O.C.T. compound (Fisher Scientific), rapidly frozen with 2-methylbutane immersed in liquid N2, and stored at −80°C until cryosectioning.
Then 30-micron thick sagittal sections were prepared using an HM 505E Cryostat (MICROM International), mounted on previously gelatin (Sigma) coated Superfrost Plus Microscope Slides (Fisher Scientific), and dried overnight. Sagittal brain sections from the anatomical region that is approximately 2 mm lateral to midline from each mouse brain were selected. Briefly, sections were rehydrated with PBS and their immunoreactivity was enhanced via heat induced antigen retrieval using DIVA decloaker (Biocare Medical). Slides were then washed with PBS twice for 5 min and dipped into 0.25% (v/v) Triton X-100 (Sigma-Aldrich) for another 10 min.
After two more PBS washes, sections were blocked with 5% (v/v) Bovine Serum Albumin (Sigma) for 1 h at room temperature (RT), excess blocking solution removed, and slices incubated with primary antibodies (1:1000 GFAP, Abcam ab53554; 1:500 Iba1, Wako Cat# 019-19741) at 4°C for 20 h. The following day, sections were washed with PBS 5X for 15 min/each and incubated in secondary antibodies (1:200 Thermo Fisher Lot# 1640316 Donkey anti-Goat Alexa Fluor 568; 1:200 Thermo Fisher Lot# 1796684 Chicken anti-Rabbit Alexa Fluor 488) for 1 h at RT. Sections were then washed 3X for 5 min/each, and further incubated in DAPI (Sigma) for 10 min. After a brief wash in distilled H2O, brain sections were mounted with Vectashield antifade mounting medium (Vector Laboratories).
All slides were imaged 24 h after the staining. Images were acquired at 40X magnification (NA 1.3) using a Zeiss LSM710 confocal microscope in the Core Optical Imaging Facility at UT Health SA. Fluorescence settings and parameters were held constant for all sections. The Z stacks at 1 μm intervals were processed using ImageJ (Wayne Rasband, National Institutes of Health). The mean fluorescence intensity analysis was performed using custom macros in ImageJ. In brief, the Z stacked images were combined using maximum intensity projection. The background fluorescence was removed by first masking the cells using the ImageJ plug-in, stack-based spots from ICBM (Indiana Center for Biological Microscopy). The mean gray values were calculated and used for the analysis.
Immunoblotting
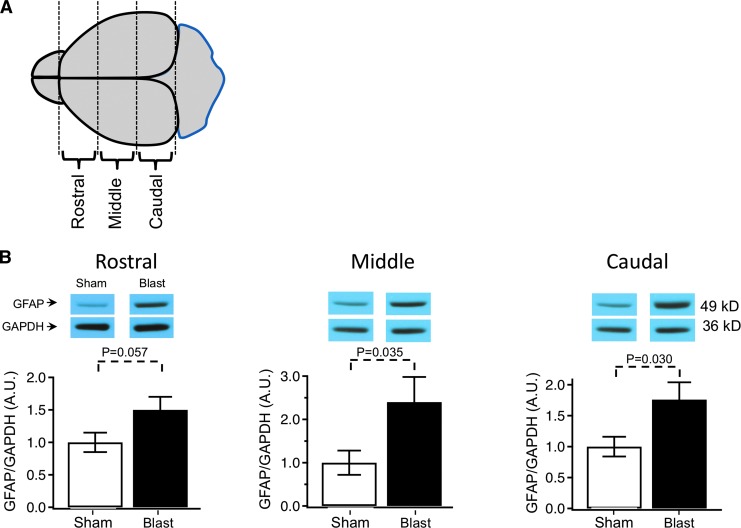
For immunoblotting, brain samples were collected seven days after the last blast and were snap frozen in dry ice. To collect segregated rostral, middle, and caudal tissue samples, olfactory bulbs and cerebellum were removed, and the brain was placed in a brain matrix (Kent Scientific). A razor blade was used to cut the first 3 mm section (rostral), the second 3 mm section (middle), and the last section that was generally 2.6–3 mm (caudal) (Fig. 2A). Samples were homogenized on ice in a solution of radioimmunoprecipitation assay buffer (Thermo Fisher Scientific – 89901), protease inhibitor tablet (Roche – 05892988001), phosphatase inhibitor cocktail 2 (Sigma – P5726), and phosphatase inhibitor cocktail 3 (Sigma – P0044), using an Ultra EZgrind tissue homogenizer (Denville Scientific Inc.) at the lowest speed.
FIG. 2.
(A) Diagram indicating regions of the brain analyzed for Western blot detection. The brain sections included 3 mm rostral, middle, and caudal portions. (B) Glial fibrillary acidic protein (GFAP) expression after repetitive (3X) blast injury measured in the rostral, middle, and caudal regions of the brain. Expression is normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression, and to sham control: rostral: 1 ± 0.15 sham, 1.50 ± 0.20 traumatic brain injury (TBI); middle: 1 ± 0.28 sham, 2.4 ± 0.58 TBI; caudal: 1 ± 0.16 sham, 1.76 ± 0.28 TBI, n = 12 animals for sham and 12 blast TBI. The p values are indicated in bar graphs.
The protein concentration of each sample was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific – 23225). After reduction and denaturation of proteins, samples (25 μg of total protein) were run in 4–20% precast polyacrylamide gel (Bio-rad – 4561096) at 130 V for ∼1 h. Proteins were then transferred to a nitrocellulose membrane (Bio-rad – 10484060) at 100V for 1 h. Membranes were stained with specific antibodies for semi-quantification of GFAP (1:500, Abcam), tau (1:1500, Millipore), p-Tau (S202) (1:100, CP13 provided by Dr. Peter Davis from Albert Einstein Institute), and p-Tau (S396/S404) (1:200, PHF-1 provided by Dr. Peter Davis from Albert Einstein Institute).
Immunoblots bands were revealed via chemiluminescent reaction between horseradish peroxidase-conjugated secondary antibodies and either ECL Western blotting detection reagents (GE healthcare) or Western Lightning Plus-ECL reagents (PerkinElmer). Quantification of bands was performed after exposure in the linear range using ImageJ software (National Institutes of Health). For normalization, the results of every protein of interest were divided by the results of the housekeeping protein β-actin (1:20,000, Sigma) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:25,000, Abcam). The results are presented as group averages and standard error of the mean (SEM) and are standardized to sham averages.
Spleen to body weight ratio and cytokine measurements
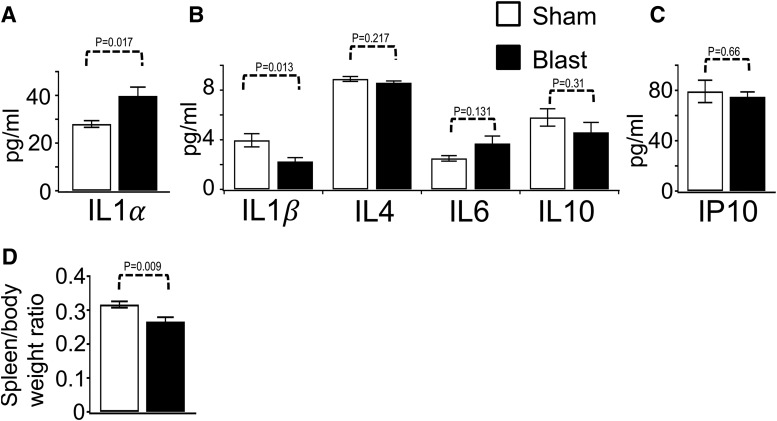
Spleens were dissected immediately after transcardial perfusion with PBS and weighed. Spleen to body weight ratio (relative weight) was calculated based on wet spleen weight and animal's average body weight. For cytokine assessment, a separate cohort of mice was used. Spleens were frozen in dry ice and stored at −80°C until use. The day of the experiment, frozen tissues were thawed briefly, homogenized, centrifuged (14,000 rpm, 4°C, 5 min), and 5 μg of total protein (25 μL per each well) was loaded into a 96 well PCR plate for analysis. Cytokines (IL1α, IL1β, IL4, IL6, IL10, IP10) were determined using the Luminex bead-based Multiplex immunoassay (EMD Millipore) following the manufacturer's directions (Mouse Cytokine/Chemokine Magnetic Bead Panel 96-Well Plate Assay, Cat. # MCYTOMAG-70K).
Statistical analysis
For statistical analysis, IBM SPSS software (version 23.0) was used. Data were checked for normal distribution by Q-Q plot analysis and Shapiro-Wilk normality test. For most samples, differences between blast versus sham were determined using unpaired, two-tailed, Student t test. One-way analysis of variance was used to compare significant differences in Western blots (Figs. 2 and 3) comparing rostral, medial, and caudal tissues. All plots show means and SEMs.
FIG. 3.
Quantification of cytokine and chemokine levels from spleen extracts. (A) IL1α, 28 ± 1.4 sham, 39.8 ± 3.7 traumatic brain injury (TBI). (B) IL1β, 3.96 ± 0.53 sham, 2.25 ± 0.31 TBI; IL4, 8.9 ± 0.2 sham, 8.6 ± 0.14 TBI; IL6, 2.5 ± 0.22 sham, 3.7 ± 0.6 TBI; IL10, 5.8 ± 0.7 sham, 4.6 ± 0.8 TBI. (C) IP10, 79.2 ± 8.9 sham, 74.9 ± 3.9, were measured using Luminex bead based Multiplex immunoassay (n = 7–9 animals for sham or blast TBI). (D) Spleen/body weight ratios were 0.32 ± 0.009 sham, 0.27 ± 0.012 blast; n = 6 sham and 6 blast. Note: Body weight losses because of experimental paradigm were not significantly different. The % change was 6.8 ± 1.2 sham, 5.9 ± 1.1 blast. p = 0.58, n = 6 sham and 6 blast. The p values are indicated on the bar plots.
Results
Increased neuronal excitability in repetitive, blast TBI
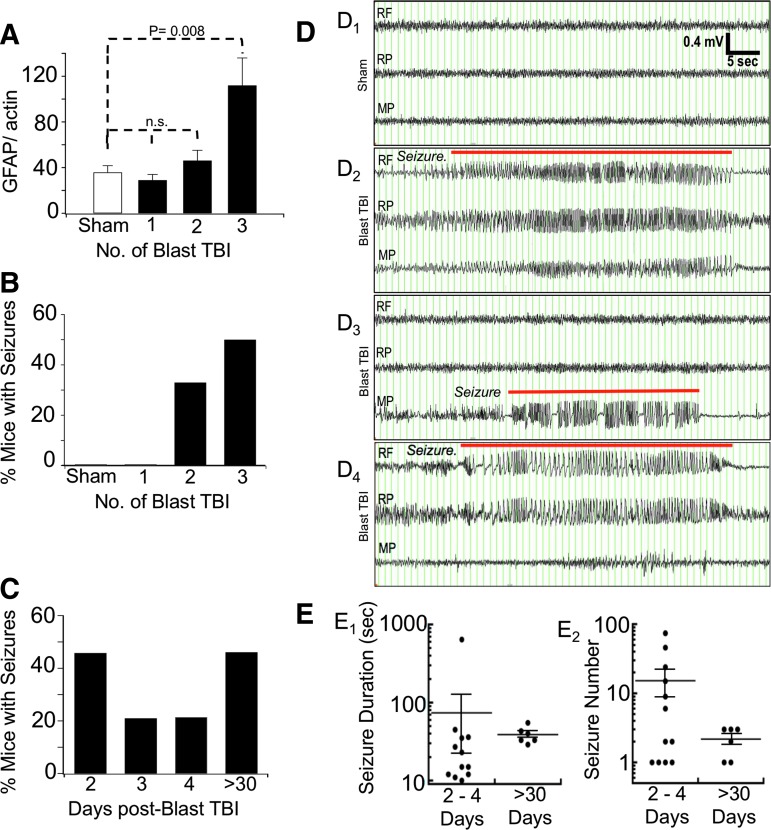
Our goal was to model mild blast TBI injury in mice to observe effects on neuronal excitability. Mice were exposed to an average of 14.6 ± 0.5 psi (100 kPA ± 3.4 kPa) maximum peak overpressure, which has been classified as mild blast TBI (0–145 kPa) in rats and mice using a number of physiological parameters.24,25 We first performed experiments to evaluate the effect of our blast TBI model, with a single exposure, or with repeated second and third exposures using 24-hour intervals between TBI. As an indicator of TBI severity, we assayed GFAP protein levels, which are established to be elevated in numerous TBI models, even in mTBI.26 We found that a single or double blast injury did not result in significant elevations of GFAP (assayed seven days after the last injury) using Western blot detection (Fig. 4A). We found, however, that three repetitive blast waves occurring over three days did show significant elevations of GFAP expression (Fig. 4A).
FIG. 4.
(A) Glial fibrillary acidic protein (GFAP) expression as a function of the number of blast-traumatic brain injury (TBI) injuries. Measurements were made from the rostral region (prefrontal cortex) of the brain. The GFAP/actin expression is shown for: sham n = 5, 35.8 ± 5.9; single TBI (1) n = 5, 29.0 ± 5.0; double TBI (2) n = 5, 46.2 ± 9.0; and triple TBI (3) n = 3, 112.0 ± 24.1, blast TBI. Significance was only observed comparing sham with 3 Blast TBI, p = 0.008. (B) Incidence of seizures in mice after sham (n = 7) was 0%, single TBI (1) was 0%, double TBI (2) was 33% (2/6), and triple TBI (3) was 50% (12/24 seizures). The % of mice with seizures is plotted. Data waere measured from n = 7 sham, 6 single blast, 6 2X blast, and 24 3X blast TBI. (C) The 3X blast TBI mice were measured for incidence of seizures after two (n = 24 mice), three (n = 19 mice), and four days (n = 14 mice) after blast TBI. Spontaneous seizures, >30, were detected after 30 days post-TBI (n = 6/13 mice had spontaneous seizure). (D) Examples of electroencephalography recordings from sham (D1) and blast TBI (D2–D4) treated mice. D2. Example of acute post-TBI multi-lobar seizure with ictal events detected on all electrodes. D3. Example of an acute post-TBI focal seizure. D4. Example of a spontaneous seizure >30 days post-TBI. RF, right frontal; RP, right parietal; MP, medial parietal. Seizure events are overlayed with a red line. A scale bar is located in panel D1. (E) Scatter plot of seizure duration (E1) and seizure number (E2) for mice with post-trauma seizures (2–4 days, n = 12 mice), and mice with post-trauma epilepsy (>30 days, n = 6 mice), for mice exposed to 3X blast trauma. Seizure number is the average number of acute post-trauma seizures per mouse (2–4 days), or the average number of spontaneous seizures per mouse (>30 days, and detected across 10 months). Horizontal line and error bars are mean ± standard error of the means. Average seizure duration was 73.7 ± 51.9 sec (2–4 days), 38.8 ± 3.9 sec (>30 days); average seizure number 15.2 ± 6.6 seizures (2–4 days), 2.2 ± 0.4 seizures (>30 days).
We conducted a similar pilot study to evaluate single and repetitive injury on the generation of seizures. To avoid secondary effects of implanted EEG electrodes during the blast trauma, cortical EEG electrodes were implanted the day following the last blast trauma, and mice were allowed to recover for 24 h. The following day (two days after the last injury) mice were video-EEG monitored for spontaneous seizures for a 72-hour period. The percent of animals that demonstrated seizures are plotted in Fig. 4B. As expected, none of the sham (0/7 mice) displayed seizures. Similar to GFAP measurements, single blast injury mice did not have seizures (0/6 mice). A fraction of mice subjected to two blast TBIs (2/6, Fig. 4B) demonstrated non-convulsive seizures. The two-blast post-TBI seizures were relatively few. One mouse had three seizures averaging 9 ± 0.9 sec, and a second mouse had two seizures averaging 15.5 ± 1.5 sec. The seizures for both mice occurred in a single electrode, indicative of focal seizures.
We analyzed a larger cohort of 24 mice that underwent three blast TBIs (3X blast). We found that 3X blast-TBI caused an increased incidence of seizures (50%, Fig. 4B), with 12 of 24 mice displaying seizures occurring, on average, at a higher frequency (15 ± 6.6 seizures, Fig. 4E2) than 2X blast mice (2.5 ± 0.5). An example of the seizures is shown in Fig. 4D. The seizures generally evolve from low amplitude, low frequency to a higher amplitude and frequency.
Most mice with seizures (9/12) had seizures that are synchronized in all three channels (Fig. 4D2), indicative of seizures occurring across multiple lobes. A subset of mice (3/12 mice with seizures) had seizures that were detected on a single channel (Fig. 4D3). Interestingly, we found only one of 12 mice that displayed convulsions associated with the seizure. The remaining animals, even during obvious electrographic seizures, did not reveal any abnormal movement that was coincident with the seizures. Although some seizures were correlated with a behavioral arrest, many seizures were not associated with any overt altered behavior.
Fig. 4C plots the incidence of post-trauma seizures during the 72 h of EEG monitoring after 3X blast TBI. The incidence of seizures was highest (46%) earliest, at the 24–48 h period post-TBI. For the following two days, seizure incidence declined to 21% of mice. We continued to record EEGs at monthly intervals (48–72 h monitoring per month) for the following 10 months where we observed spontaneous seizures in six of 13 mice, indicating an incidence of post-trauma epilepsy of 46% of mice. Sham control mice (n = 7) did not reveal any seizures during long-term monitoring.
For blast-TBI exposed mice, there was no obvious clustering of a latency period between trauma and spontaneous seizures, because post-blast seizures were detected first between eight weeks to 10 months after TBI. Mice showed only 1–3 spontaneous seizures (Fig. 4E2, all subconvulsive) with an average duration of 29–55 sec (Fig. 4E1) across at least 480 h of video-EEG monitoring (example presented in Fig. 4D4). The low frequency of seizures, within a limited sampling rate of two days/month, leaves the possibility that other animals may have had undetected subconvulsive seizures. In conclusion, we found that repetitive (3X) blast TBI caused non-convulsive seizures. This is the first evidence of post-trauma seizures and post-trauma epilepsy after blast TBI.
Increased intrinsic excitability and increased post-synaptic currents after blast TBI
Given a relatively high frequency of mice (50%) with acute post-trauma seizures using the 3X blast-TBI model, we analyzed this model for acute post-TBI effects on neuronal excitability using patch clamp electrophysiology from hippocampal brain slices. The day after the last blast injury, we recorded from dentate gyrus granule (DGG) neurons.
These cells were selected for several reasons. First, DGG neurons are normally very quiescent, usually having very negative resting potentials, and so a significant increase in their excitability will disproportionately increase the excitability of the hippocampal formation as a whole; indeed, the dentate gyrus is thought to be the “gatekeeper” against seizures and other forms of temporal-lobe hyperexcitability.27–31
As well, this region of the brain was affected by blast TBI as indicated by Iba1 and GFAP staining (discussed below, Fig. 5). We found that the electrical properties of DG neurons were significantly altered by blast TBIs (Fig. 6A). Whereas we did not detect increased action potential frequency, recordings revealed a significantly reduced latency to the first spike and hyperpolarization of action potential threshold in mice that underwent blast-TBIs. This suggests that in mice that underwent blast TBIs, the DG neurons require a lower excitatory threshold for spiking.
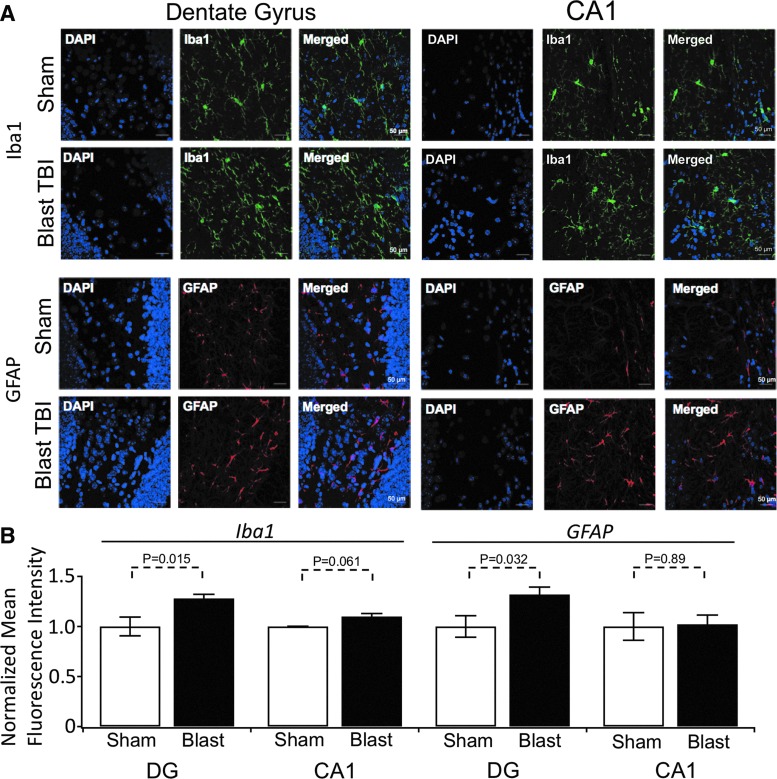
FIG. 5.
Quantification of ionized Ca2+ binding adaptor molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) immunohistochemisty using mean fluorescence intensity analysis. (A) Representative maximum projection images of Iba-1 (top) and GFAP (bottom) labeled optical sections collected from the hippocampal dentate gyrus (left) and CA1 (right) regions of mice receiving sham or blast traumatic brain injury (TBI). Background has been subtracted. Scale bar (50 microns) is shown in the bottom right of each image. (B) Histogram of the average fluorescent intensity values for dentate gyrus (DG) and CA1 regions (fluorescence intensity was integrated for individual experiments). Values were the following for: Iba1 DG staining, sham 1.0 ± 0.09, n = 4, blast 1.28 ± 0.04, n = 6; Iba1 CA1 staining, sham 1.0 ± 0.002, n = 3, blast 1.1 ± 0.03, n = 6. For GFAP DG staining, sham 1.0 ± 0.11, n = 4, blast 1.32 ± 0.07, n = 6; GFAP CA1 staining, sham 1.0 ± 0.14, n = 3, blast 1.0 ± 0.09, n = 6. p values are shown above the plots.
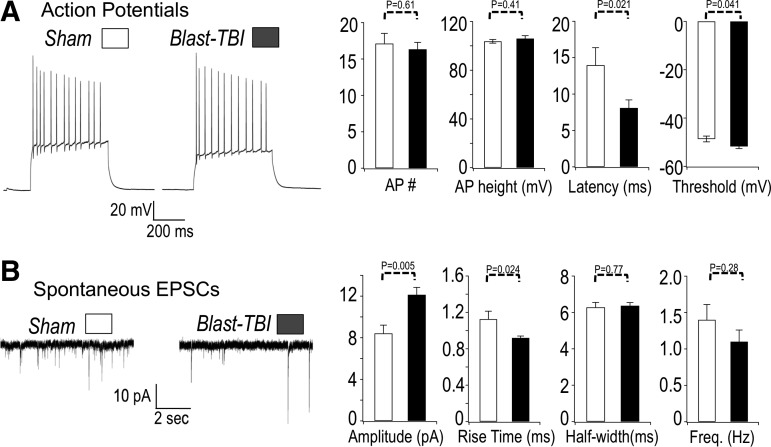
FIG. 6.
(A) Left panel. Sample action potential (AP) recordings from sham-treated (sham) mouse and mice that received three repetitive blast injuries (blast). The APs were measured using whole cell recordings of dentate gyrus granule neurons. The APs were evoked by a 200 pA current injection. Right panel, summary of data. The AP number (per 500 msec current injection): 17.1 ± 1.3 sham, 16.3 ± 0.9 traumatic brain injury (TBI); AP height: 103.7 ± 1.5 mV sham, 106.0 ± 2.4 mV TBI; AP latency (time between current injection and first spike): 14.0 ± 2.6 msec sham, 8.1 ± 1.1 msec TBI; AP threshold: −48.6 ± 1.2 mV sham, −51.7 ± 0.9 mV TBI. n = 25 sham, 32 blast TBI. (B) Blast TBI increases the amplitude and decreases rise time of EPSCs. Left panel. Sample recording of spontaneous excitatory post-synaptic currents in dentate gyrus granule neurons. The excitatory post-synaptic currents (EPSCs) were measured at −80 mV holding potential. Right panel, summary of data. EPSC amplitude: 8.4 ± 0.8 pA sham, 12.1 ± 0.7 pA TBI; EPSC rise time: 1.1 ± 0.08 msec sham, 0.92 ± 0.02 msec TBI; half-width: 6.3 ± 0.3 msec sham, 6.4 ± 0.2 msec TBI. n = 177 events sham, 227 events TBI for above EPSC data. Frequency 1.4 ± 0.2 Hz sham, 1.1 ± 0.1 Hz TBI, n = 10 sham, 15 TBI for frequency data. p values are indicated on the bar plots.
Using a range of current injection protocols, action potential frequency, shape, and other indicators of intrinsic excitability were assayed. Among these, we also detected a sharpening of action potentials in mice that underwent blast TBIs (Table 1). We also recorded post-synaptic currents to determine whether changes in neurotransmission might contribute to alterations in circuit excitability in blast-TBI mice. We indeed detected a significantly increased amplitude of post-synaptic currents and a shorter rise time in blast-TBI mice (Fig. 6B). Other effects on excitatory post-synaptic currents (EPSCs) were not observed, however, including changes on width and frequency.
Table 1.
Action Potential Properties
| Treatment | N | IR (MΩ) | Cap (pF) | Vm (mV) | Threshold (mV) | 50% Width (ms) | 10% width (ms) | Height (mV) | fAHP (mV) |
|---|---|---|---|---|---|---|---|---|---|
| Sham | 25 | 475 ± 25 | 38.5 ± 2 | 88.9 ± 1.1 | −42.5 ± 0.7 | 0.84 ± 0.02 | 1.63 ± 0.03 | 110 ± 1.5 | −16.3 ± 0.6 |
| Blast TBI | 32 | 476 ± 27 | 37.9 ± 2 | 87.7 ± 1.3 | −44.9 ± 0.7 | 0.82 ± 0.02 | 1.57 ± 0.02 | 111 ± 1.7 | −15.5 ± 0.7 |
| p value | 0.97 | 0.84 | 0.53 | 0.02 | 0.29 | 0.05 | 0.75 | 0.38 |
IR, input resistance; Cap, capacitance; Vm, resting membrane potential, fAHP, fast-afterhyperpolarizationl TBI, traumatic brain injury.
In conclusion, the majority of the effects of repetitive blast TBI was an increase in excitability because of reduced time and threshold to action potential spike, and increased EPSC amplitude. The one effect that may be regarded as antiexcitatory was a sharpening of action potentials.
Indicators of inflammation in repetitive, mild blast TBI
Cell damage mediates an immune system response, which itself can result in further neuronal damage, creating a “vicious cycle” of brain damage.32–36 One indicator of such a response is microgliosis.37 To assay for this, we stained brain slices with the microglial marker Iba-1, which has been shown to undergo increased expression after TBI.38 We found repetitive blast TBIs to induce microgliosis, observed as enhanced levels of Iba-1 in the dentate gyrus region of the hippocampus (p = 0.015), and a trend for an increase in CA1 (p = 0.061, Fig. 5). We also investigated astrogliosis using GFAP, and indeed saw that the DG region had a significant increase (p = 0.032, Fig. 5), whereas the CA1 region did not (p = 0.89).
Previous studies have indicated that TBI also provokes changes in peripheral immunity that might affect injury severity.2 We therefore measured splenic weights and observed a significant reduction in the spleen to body weight ratio at day seven post-blast TBI (Fig. 3D). Body weight loss from blast trauma or our experimental paradigm was not significantly different (p = 0.58, Fig. 3 legend). In a separate cohort of animals, we tested a number of cytokines using a bead-based (Luminex) immunoassay using spleen tissue extracts. We detected a significant decrease in IL1β as opposed to a significant increase in IL1α. There was no difference observed in IL4, IL6, IL10, and IP10 levels (Fig. 3A-C).
Tau and tau hyperphosphorylation is increased after blast TBI
Given that the blast wave was directed head-on to the mice, we wished to determine whether blast-TBI trauma was localized to specific regions of the brain. To accomplish this, we dissected the brain into 3 mm sections of the rostral, medial, and caudal portions (Fig. 2A), and conducted Western blot detections of GFAP as a marker of trauma. We found significant increases in GFAP expression in medial and caudal portions (Fig. 2B). In the rostral region, the GFAP increases were slightly below significance (p = 0.057, Fig. 2B). These results are consistent with blast injury causing astroglial activation in broad regions of the brain.
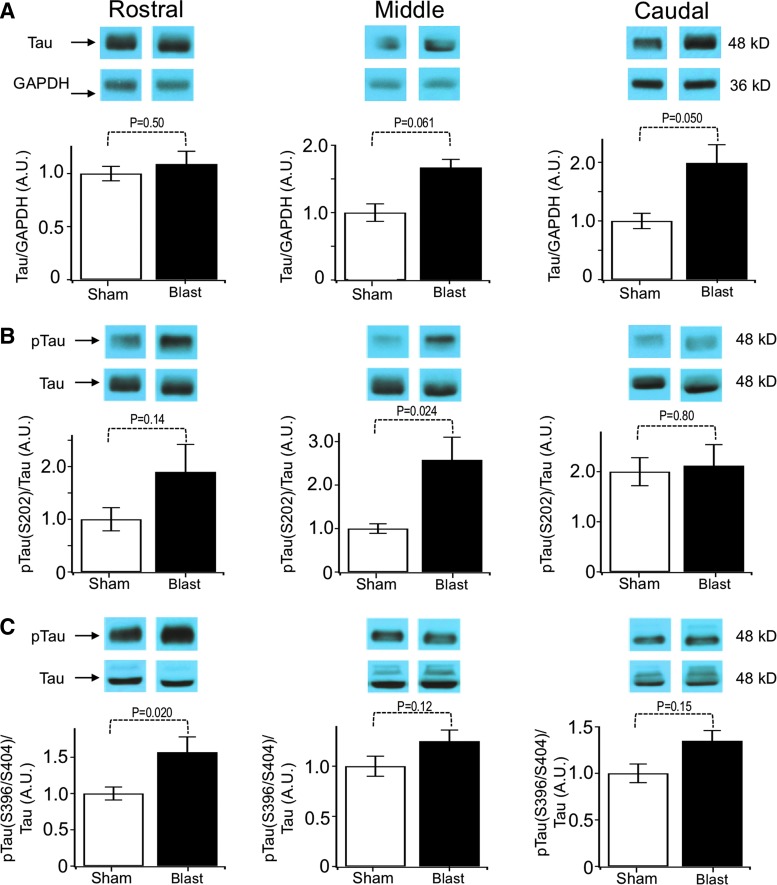
It has become evident recently that increased tau expression commonly accompanies both TBI25,39–41 and epilepsy,42,43 and tau protein might contribute to seizures.44,45 We therefore investigated changes in tau protein and tau hyperphosphorylation in mice that underwent blast TBI. We found that there was a significant increase in total tau expression preferentially localized to caudal regions of the brain (Fig. 7A). Hyperphosphorylation of tau was assayed by normalizing phospho-tau proteins to total tau protein (Figs. 7B, 7C). We found that blast trauma increased phospho-tau in the middle (Ser202, Fig. 7B) and rostral regions (Ser396/Ser404, Fig. 7C).
FIG. 7.
Blast-induced tau hyperphosphorylation. Blots of brain homogenates probed for phosphorylated and total tau in rostral, middle, and caudal portions of brains. Blast animals presented a significant increase in pTau within the rostral third of the brain. (A) Tau expression normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH): rostral: 1 ± 0.07 sham, 1.09 ± 0.11 traumatic brain injury (TBI); middle: 1 ± 0.13 sham, 1.67 ± 0.32 TBI; caudal: 1 ± 0.13 sham, 1.99 ± 0.46 TBI. (B) Quantification of phospho-tau at serine 202 normalized to total tau: rostral: 1 ± 0.22 sham, 1.90 ± 0.52 TBI; middle: 1 ± 0.11 sham, 2.58 ± 0.75 TBI; caudal: 1 ± 0.14 sham, 1.07 ± 0.2 TBI. (C) Quantification of phospho-tau at serine 396 and 404 normalized to total tau: rostral: 1 ± 0.09 sham, 1.57 ± 0.21 TBI; middle: 1 ± 0.11 sham, 1.25 ± 0.12 TBI; caudal: 1 ± 0.10 sham, 1.35 ± 0.21 TBI. All samples are n = 11–12 sham and 11–12 TBI. The p values are indicated in bar graphs.
Given that total tau protein was elevated in the caudal regions of the brain, we also investigated whether phospho-tau could be elevated because of increased levels of tau protein in this region. We therefore normalized phospho-tau to GAPDH, rather than total tau. We indeed found that Ser202 was elevated significantly in the caudal region when normalized to GAPDH (sham 1 ± 0.14, blast 2.2 ± 0.47, p = 0.02, n = 12 sham, 12 blast). Ser396/Ser404 in the caudal region was, however, not elevated (sham 1 ± 0.09, blast 1.3 ± 0.19, p = 0.13, n = 11 sham, 12 blast).
Discussion
Mild repetitive blast injury and post-trauma increases of neuronal excitability
A major finding from these studies is that “mild” blast exposures, when repeated, cause seizures in mice. Such post-traumatic seizures (PTSs) have not been documented before as a symptom of blast-induced TBI. The severity of the seizures was correlated with blast frequency. Thus, a single blast TBI resulted in no seizures, whereas a second blast TBI resulted in few seizures in a third of the mice, and three blast TBIs induced seizures in about half the mice, with the majority of those having high seizure frequency. To date, this is also the first in vivo study of blast TBI effects on neuronal excitability, although it had been known that the risk of epilepsy from non-penetrating or penetrating trauma is correlated with TBI severity.46 This study is consistent with severity being increased with the frequency and number of repetitive blasts.47,48
The time interval between blast events may therefore be an important variable affecting neuronal excitability and epilepsy development that requires further study. This is particularly relevant for sports-related TBI and blast-induced TBI, where TBI severity is difficult to gauge, and therefore individuals may be exposed to multiple TBI-causing events.8,49,50 Indeed, “mTBI” may sometimes be a misnomer, because serious injury may occur without overt symptoms in individuals with multiple mTBIs.7,14,51,52 A recent study showed that servicemen positioned within 10 feet behind fellow combatants firing shoulder-launched weapons suffer repeated “bobble-head” trauma, with each discharge of the weapon inducing another.53
An important issue with experimental comparisons of single to multiple TBIs is that the experimental designs can make interpretation complex.54 In our pilot study, we chose to overlap the last TBI blast of the 1X TBI, 2X TBI models, and 3X TBI models. Thus, the timing between the last TBI and assays for trauma were matched, but the first TBI in the 2X and 3X models occur two and three days, respectively, earlier than the 1X model. The results thus reflect not only the number of trauma events, but also a difference in the timing between the first blast injuries and the assays.
The TBI and resulting sequelae observed are strongly a function of time post-injury, particularly with regard to inflammation, microglia, and astrocyte activation,2,55 which is a caveat in direct comparison of single and multiple TBIs. Indeed, differences between single and repetitive mTBI models might not only reflect the consequence of cumulative injury,7 but also pre-conditioning effects of the first injury.56
The seizures we observed with repetitive mild blast exposures were almost exclusively non-motor seizures. Only one of 12 mice with post-trauma seizures displayed clonus. The post-blast electrographic seizures often coincided with normal movements and lacked obvious evidence of impaired consciousness or behavioral arrest. One might therefore classify these seizures as aware, non-motor seizures, perhaps with a focal onset. Because both cortical hemispheres were involved simultaneously in most animals, it is likely that there was a transient alteration of awareness during the seizure, which we were not able to assess behaviorally with memory tasks as we can easily do in humans.
The observation that non-convulsive post-trauma seizures develop in this animal model raises the concern that combatants exposed to blast TBI may be experiencing repeated, subclinical, undiagnosed seizures, with each additional TBI provoking additional brain damage. Interestingly, a similar concern of subclinical seizures has been raised in Alzheimer disease (AD), because patients with AD and AD mouse models often show non-motor seizures late in the AD condition.57–59
In a study of individuals injured by explosive munitions during the war in the former Yugoslavia, 36% (241/665) of individuals were reported to have abnormal EEG within three days of injury.60 The EEG alterations were reported to be of hypersynchronous, discontinuous, or irregular brain activity with increased theta activity, but it is not clear from this report whether these included seizures.60
Interestingly, a majority of those individuals from the blast injury group demonstrated continued EEG alterations after long-term monitoring for up to one year,60 suggesting potential long term consequences for cortical hyperexcitability and epilepsy development. Because seizures beget further seizures, ultimately leading to epilepsy development,61,62 early management of mild post-TBI trauma and perhaps anti-epileptogenic medications, when available, should be considered as a prophylaxis to prevent blast TBI-induced epilepsy development.
To date, this is the first study to conduct patch clamp recording from brain slices in an in vivo blast-TBI model. Our investigation focused on DG neurons given that we saw significant evidence of brain trauma markers Iba-1 and GFAP in this brain region. We observed a pronounced increase in excitability because of a negative voltage-shift of action potential threshold and an increase in EPSC amplitudes (3). Nevertheless, suprathreshold current injections did not reveal a significant increase in spike frequency.
While a number of studies have investigated concussive injury on DG excitability,63–66 to date, the only other study of blast TBI on neuronal excitability used an in vitro blast exposure model in which pressure waves were impacted onto cultured brain slices, and neuronal activity assayed with microelectrode arrays. Employing what was regarded as a mTBI impact, those studies instead indicated a reduced spike frequency.67 Using a similar approach, other studies identified decreased GluR1 receptor expression and phosphorylation that may underlie reduced long-term potentiation.68,69 These results, however, are not consistent with our in vivo studies showing increased EPSC amplitudes and increased neuronal excitability, consistent with seizure development.
An important caveat for these findings is that the differences in DG neuronal excitability observed between TBI and sham-treated mice may be affected by the additional tissue trauma that is inherent of ex vivo brain tissue preparations. Such potential confounds are difficult to avoid in the absence of in vivo patch clamp techniques.
Past studies of TBI-induced changes in neuronal electrophysiology have focused mainly on concussion models of various types, including the fluid percussion injury (FPI), controlled cortical impact (CCI), or weight drop models (reviewed70). Generally, a craniotomy precedes the injury for the TBI to be sufficiently severe for seizures or epilepsy to develop. Closed skull techniques such as the weight drop models are less severe, because spontaneous seizures have not yet been reported with this technique.71
Here, the blast injury model we used was a closed skull technique and also unique in that the injury was diffuse, rather than focused on one region of the brain (as in FPI and CCI). Some indicators, such as GFAP, indicate a generalized injury (Fig. 2), whereas tau and phospho-tau effects appeared to be localized to different regions of the brain (Fig. 7). Like other closed skull, mTBI models, we also did not detect seizures with a single injury,71 but did see robust post-trauma seizures with repetitive injuries. Repetitive mTBI is indeed relevant, because those on the battlefield and also in sports who received a diagnosis of mTBI may experience more than one TBI event.8,49,72
Immunologic response to blast TBI
Spleen size reduction, followed by the release of inflammatory cells and subsequent secondary brain injury, has been widely reported in animal stroke models.73 Splenectomy studies support the spleen's contribution to worsening outcome of stroke and brain injuries.74 Our data further point to the importance of this organ in exacerbating the inflammatory response in blast trauma. Specifically, we observed a significant reduction in spleen size (Fig. 3D), an increase in IL1α, and a reduced IL1β as a result of blast TBI (Fig. 3A, 5B). The link between brain injury and the release of inflammatory cytokines by spleen macrophages has been termed as “brain-spleen inflammatory coupling.”2,75,76
Although central and peripheral IL1 have been reported as important contributors to brain injury,77,78 most studies have been focused on the peripheral release of IL1β after injury as the primary mediator of the secondary injury response. Interestingly, our data indicate IL1β was reduced after blast trauma, whereas IL1α was increased. Other splenic cytokines including IL4, IL6, IL10, and IP10 were not changed under our conditions, suggesting IL1α and IL1β may be the more important peripheral targets in blast TBI. Recent reports also indicated IL1α plays a crucial role in post-injury pathogenesis,79 consistent with our observations. Distinct and opposite changes in the levels of IL1α and IL1β might be specific to injuries induced by blast waves.
We note that the underlying mechanisms of IL1α and IL1β secretion involve different pathways, even though their production pathways share some similarities.80 Interestingly, diminished IL1β signaling has been observed after parasympathetic stimulation and linked to the vagus nerve's immunomodulatory role.81–84 Moreover, a vagal response clearly has been observed in blast trauma.60,85–87
Because this study positioned mice to face the blast directly, while shielding the main body, our major hypothesis for spleen/body weight ratio reduction is that it is secondary to brain trauma, rather than direct trauma to viscera. Spleen/body weight ratios were not measured previously in blast trauma, but CCI88 and ischemic brain injury89 also show a reduction of spleen size. This may occur as a consequence of mobilization of macrophage and monocytes out of the spleen.89 Blast injury also causes activation of the autonomic nervous system resulting in enhanced catecholamines,90 which have been shown to decrease spleen size.91
We cannot exclude, however, some contribution of vascular injury as a consequence of sheer stress or central venous pressure changes that were directly the result of blast waves.86 In addition, hemorrhagic infarcts previously have been reported in parenchymatous organs, including the spleen, when the mouse torso is unshielded from blast exposure.92
Mild repetitive blast injury and tau hyperphosphorylation
In contrast to previous studies,93 our results are consistent with Arun and associates94 who reported an increase in total tau after repetitive blast-induced TBI. This is consistent with findings demonstrating increased exosomal and plasma levels of total tau in combat-related repetitive chronic mTBI.95 Most relevant to this study, increased tau expression may contribute to seizures, because reducing tau can eliminate seizures in several epilepsy mouse models.44,45,50 Thus, enhanced expression of tau protein after TBI may be a key contributor to the development of post-traumatic seizures and epilepsies.
Our findings of increased phospho-tau corroborate other studies of increased pathogenic tau forms in brains of rodents that underwent blast-induced TBI.25,93,96 The blast TBI tau hyperphosphorylation was associated with rostral and middle regions of the brain (Fig. 7), which contrasts with enhanced GFAP expression throughout the brain (Fig. 2). Tau pathology is also observed in epilepsy42,43 and been shown to contribute to seizures in epilepsy mouse models.44,45
Like tau pathology, inflammation is not only elevated after seizures, but also contributes to increased excitability and epilepsy.97,98 Thus, it is possible that post-trauma seizures are not only a consequence of, but also contribute to, the maladaptive inflammatory response and tauopathy. Interestingly, Bhaskar and colleagues99 reported LPS-induced inflammation to result in hyperphosphorylation of tau S202 (CP13) and tau S396/S404 (PHF-1). In addition, the LPS-induced hyperphosphorylation of tau was increased in mouse microglial-specific fractalkine receptor (CX3CR1) knockouts, and such increases were dependent on IL1 receptor activation.99 Hence, inflammation and tau phosphorylation are likely to be linked in our experiments as well.
The sites of tau phosphorylation studied here have all been observed to be phosphorylated in physiological situations and to reach abnormally increased levels of phosphorylation in tauopathies linked to AD.100–102 Increased levels of tau S396/S404 phosphorylation result in impairment of the ability of tau to promote microtubule assembly.103 Moreover, the hyperphosphorylation of tau S202 is sufficient to induce tau aggregation.104 Thus, this event may serve as a “seed” to spread tau misfolding and aggregation after TBI, especially after repetitive mTBI.105
Whereas mouse models of repetitive mTBI have been shown to increase levels of tau S202/T205 (AT8) phosphorylation up to six months after injury, the same was not observed with a single trauma.106 Using a blast TBI mice model similar to ours, Huber and coworkers93 observed a significant increase in cortical and hippocampal levels of tau S202 phosphorylation, but not S396/S404. On the other hand, we here report an increase in tau S396/S404 phosphorylation in our mouse blast TBI model.
This apparent contradiction is likely to be related to the difference in the number of injuries, because Huber and colleagues93 only subjected the mice to a single mTBI as opposed to our repetitive mTBI model, and we found obvious brain dysfunction, such as seizures, to only commence on a second or third TBI. There is also a time point difference between studies, because Huber and associates93 collected samples 24 h after injury, whereas our samples were obtained seven days after the last impact.
Conclusion
The conclusion from these studies is that mild blast-induced brain injury, when repeated, results in neuronal hyperexcitability including electrographic seizures and changes in action potential properties and neurotransmission consistent with abnormally elevated neuronal activity. Like other mTBI models, biochemical indicators suggest that mild blast-induced TBI becomes more severe when repeated. Despite the robust electrophysiological consequences of repetitive mTBI, the lack of motor abnormalities associated with the seizures creates a concern that individuals with blast-induced TBI may experience frequent post-trauma seizures that are undetected and untreated and, moreover, subject themselves to further repeated trauma that is likely to be irreversible.
Acknowledgments
We would like to thank the following: Nameer B. Kirma and Nicholas D. Lucio from the Bioanalytics and Single-Cell Facility at UT Health San Antonio for the multiplex immunoassays. We also thank Brian J. Lund at the San Antonio Military Medical Center for advice on use of the blast facility, and Amy E. Pletz for her expert technical assistance. This study was supported by DoD CDMRP grants W81XWH-15-1-0284 (M.S.S. and R.B.) and W81XWH-13-1-0284 (J.D.L.), NSF grant 1456862 (R.B.) and the Morrison Trust Foundation grants (R.B. and M.S.S.)
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 2. Nizamutdinov D. and Shapiro L.A. (2017). Overview of traumatic brain injury: an immunological context. Brain Sci. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau K.M., Madden E., Neylan T.C., Seal K.H., and Maguen S. (2016). Assessing for mild TBI among Iraq and Afghanistan veterans: outcomes of injury severity and neurological factors. Brain Inj. 30, 287–294 [DOI] [PubMed] [Google Scholar]
- 4. Carlson K.F., Barnes J.E., Hagel E.M., Taylor B.C., Cifu D.X., and Sayer N.A. (2013). Sensitivity and specificity of traumatic brain injury diagnosis codes in United States Department of Veterans Affairs administrative data. Brain Inj. 27, 640–650 [DOI] [PubMed] [Google Scholar]
- 5. Ganti L., Daneshvar Y., Bodhit A., Ayala S., Patel P.S., Lottenberg L.L., York D., Counsell C., and Peters K.R. (2015). TBI ADAPTER: traumatic brain injury assessment diagnosis advocacy prevention and treatment from the emergency room–a prospective observational study. Mil. Med. 180, 380–386 [DOI] [PubMed] [Google Scholar]
- 6. Verellen R.M. and Cavazos J.E. (2010). Post-traumatic epilepsy: an overview. Therapy 7, 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailes J.E., Dashnaw M.L., Petraglia A.L., and Turner R.C. (2014). Cumulative effects of repetitive mild traumatic brain injury. Prog. Neurol. Surg. 28, 50–62 [DOI] [PubMed] [Google Scholar]
- 8. Bryan C.J. and Clemans T.A. (2013). Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry 70, 686–691 [DOI] [PubMed] [Google Scholar]
- 9. Wilk J.E., Herrell R.K., Wynn G.H., Riviere L.A., and Hoge C.W. (2012). Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom. Med. 74, 249–257 [DOI] [PubMed] [Google Scholar]
- 10. Ling G., Ecklund J.M., and Bandak F.A. (2015). Brain injury from explosive blast: description and clinical management. Handb. Clin. Neurol. 127, 173–180 [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld J.V., McFarlane A.C., Bragge P., Armonda R.A., Grimes J.B., and Ling G.S, 2nd ed. (2013). Blast-related traumatic brain injury. Lancet Neurol. 12, 882–893 [DOI] [PubMed] [Google Scholar]
- 12. Zollman F.S. (2016). Manual of Traumatic Brain Injury : Assessment nd Management. Second edition ed. Demos Medical: New York [Google Scholar]
- 13. Rubovitch V., Ten-Bosch M., Zohar O., Harrison C.R., Tempel-Brami C., Stein E., Hoffer B.J., Balaban C.D., Schreiber S., Chiu W.T., and Pick C.G. (2011). A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 232, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su E. and Bell M. (2016). Diffuse axonal injury, in: Translational Research in Traumatic Brain Injury. Laskowitz D., Grant G. (eds). CRC Press: Boca Raton, FL [Google Scholar]
- 15. Chen J.W., Ruff R.L., Eavey R., and Wasterlain C.G. (2009). Posttraumatic epilepsy and treatment. J. Rehabil. Res. Dev. 46, 685–696 [DOI] [PubMed] [Google Scholar]
- 16. Ling G.S. and Ecklund J.M. (2011). Traumatic brain injury in modern war. Curr. Opin. Anaesthesiol. 24, 124–130 [DOI] [PubMed] [Google Scholar]
- 17. Lund B.J. and Rule G.T. (2014). Pressure wave dosimetry for “Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury”. Invest. Ophthalmol. Vis. Sci. 55, 1348–1349 [DOI] [PubMed] [Google Scholar]
- 18. Walilko T.J., Lowe R.D., Argo T.F., Meegan G.D., Greene N.T., and Tollin D.J. (2016). Experimental Evaluation of blast loadings on the ear and head with and without hearing protection devices, in: Mechanics of Biological Systems and Materials. Korach C.S., Tekalur S.A., and Zavattieri P. (eds). Vol 6 Springer International Publishing: New York [Google Scholar]
- 19. Greene N.T., Alhussaini M.A., Easter J.R., Argo T.F. 4th, Walilko T., and Tollin D.J. (2018). Intracochlear pressure measurements during acoustic shock wave exposure. Hear. Res. 365, 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedlander F.G. (1946). The diffraction of sound pulses; on a paradox in the theory of reflexion. Proc. R. Soc. Lond. A Math. Phys. Sci. 186, 356–367 [DOI] [PubMed] [Google Scholar]
- 21. Lippmann K., Kamintsky L., Kim S.Y., Lublinsky S., Prager O., Nichtweiss J.F., Salar S., Kaufer D., Heinemann U., and Friedman A. (2017). Epileptiform activity and spreading depolarization in the blood-brain barrier-disrupted peri-infarct hippocampus are associated with impaired GABAergic inhibition and synaptic plasticity. J. Cereb. Blood Flow Metab. 37, 1803–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X., Liu J., Liu J., Liu X.L., Jin L.Y., Fan W., Ding J., Peng L.C., Wang Y., and Wang X. (2013). BDNF-TrkB signaling pathway is involved in pentylenetetrazole-evoked progression of epileptiform activity in hippocampal neurons in anesthetized rats. Neurosci. Bull. 29, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keller C.J., Truccolo W., Gale J.T., Eskandar E., Thesen T., Carlson C., Devinsky O., Kuzniecky R., Doyle W.K., Madsen J.R., Schomer D.L., Mehta A.D., Brown E.N., Hochberg L.R., Ulbert I., Halgren E., and Cash S.S. (2010). Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain 133, 1668–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mishra V., Skotak M., Schuetz H., Heller A., Haorah J., and Chandra N. (2016). Primary blast causes mild, moderate, severe and lethal TBI with increasing blast overpressures: experimental rat injury model. Sci. Rep. 6, 26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meabon J.S., Huber B.R., Cross D.J., Richards T.L., Minoshima S., Pagulayan K.F., Li G., Meeker K.D., Kraemer B.C., Petrie E.C., Raskind M.A., Peskind E.R. and Cook D.G. (2016). Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 8, 321ra6. [DOI] [PubMed] [Google Scholar]
- 26. Rhine T., Babcock L., Zhang N., Leach J., and Wade S.L. (2016). Are UCH-L1 and GFAP promising biomarkers for children with mild traumatic brain injury? Brain Inj. 30, 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., and Aldrich R.W. (2005). BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8, 1752–1759 [DOI] [PubMed] [Google Scholar]
- 28. Dudek F.E. and Sutula T.P. (2007). Epileptogenesis in the dentate gyrus: a critical perspective. Prog. Rrain Res. 163, 755–773 [DOI] [PubMed] [Google Scholar]
- 29. Heinemann U., Beck H., Dreier J.P., Ficker E., Stabel J., and Zhang C.L. (1992). The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Supp. 7, 273–280 [PubMed] [Google Scholar]
- 30. Hsu D. (2007). The dentate gyrus as a filter or gate: a look back and a look ahead. Prog. Brain Res. 163, 601–613 [DOI] [PubMed] [Google Scholar]
- 31. Lothman E.W., Stringer J.L., and Bertram E.H. (1992). The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 7, 301–313 [PubMed] [Google Scholar]
- 32. Hammad A., Westacott L., and Zaben M. (2018). The role of the complement system in traumatic brain injury: a review. J. Neuroinflammation 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morganti-Kossmann M.C., Satgunaseelan L., Bye N., and Kossmann T. (2007). Modulation of immune response by head injury. Injury 38, 1392–1400 [DOI] [PubMed] [Google Scholar]
- 34. Shlosberg D., Benifla M., Kaufer D., and Friedman A. (2010). Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 6, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ziebell J.M. and Morganti-Kossmann M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith-Swintosky V.L., Cheo-Isaacs C.T., D'Andrea M.R., Santulli R.J., Darrow A.L., and Andrade-Gordon P. (1997). Protease-activated receptor-2 (PAR-2) is present in the rat hippocampus and is associated with neurodegeneration. J. Neurochem. 69, 1890–1896 [DOI] [PubMed] [Google Scholar]
- 37. Loane D.J. and Byrnes K.R. (2010). Role of microglia in neurotrauma. Neurotherapeutics 7, 366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rowe R.K., Ellis G.I., Harrison J.L., Bachstetter A.D., Corder G.F., Van Eldik L.J., Taylor B.K., Marti F., and Lifshitz J. (2016). Diffuse traumatic brain injury induces prolonged immune dysregulation and potentiates hyperalgesia following a peripheral immune challenge. Mol. Pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C.J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., and McKee A.C. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker K.R. and Tesco G. (2013). Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang W.J., Chen W., Chen L., Guo Y.J., Zeng J.S., Li G.Y., and Tong W.S. (2017). Involvement of tau phosphorylation in traumatic brain injury patients. Acta Neurol. Scand. 135, 622–627 [DOI] [PubMed] [Google Scholar]
- 42. Tai X.Y., Koepp M., Duncan J.S., Fox N., Thompson P., Baxendale S., Liu J.Y., Reeves C., Michalak Z., and Thom M. (2016). Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 139, 2441–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yan X.X., Cai Y., Shelton J., Deng S.H., Luo X.G., Oddo S., Laferla F.M., Cai H., Rose G.M., and Patrylo P.R. (2012). Chronic temporal lobe epilepsy is associated with enhanced Alzheimer-like neuropathology in 3xTg-AD mice. PLoS One 7, e48782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holth J.K., Bomben V.C., Reed J.G., Inoue T., Younkin L., Younkin S.G., Pautler R.G., Botas J., and Noebels J.L. (2013). Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci. 33, 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Z., Hall A.M., Kelinske M., and Roberson E.D. (2014). Seizure resistance without parkinsonism in aged mice after tau reduction. Neurobiol. Aging 35, 2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agrawal A., Timothy J., Pandit L., and Manju M. (2006). Post-traumatic epilepsy: an overview. Clin. Neurol. Neurosurg. 108, 433–439 [DOI] [PubMed] [Google Scholar]
- 47. McCrory P., Davis G., and Makdissi M. (2012). Second impact syndrome or cerebral swelling after sporting head injury. Curr. Sports Med. Rep. 11, 21–23 [DOI] [PubMed] [Google Scholar]
- 48. Wang Y., Wei Y., Oguntayo S., Wilkins W., Arun P., Valiyaveettil M., Song J., Long J.B., and Nambiar M.P. (2011). Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. J. Neurotrauma 28, 2171–2183 [DOI] [PubMed] [Google Scholar]
- 49. Broglio S.P., Eckner J.T., Martini D., Sosnoff J.J., Kutcher J.S., and Randolph C. (2011). Cumulative head impact burden in high school football. J. Neurotrauma 28, 2069–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeVos S.L., Goncharoff D.K., Chen G., Kebodeaux C.S., Yamada K., Stewart F.R., Schuler D.R., Maloney S.E., Wozniak D.F., Rigo F., Bennett C.F., Cirrito J.R., Holtzman D.M., and Miller T.M. (2013). Antisense reduction of tau in adult mice protects against seizures. J. Neurosci 33, 12887–12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buki A., Kovacs N., Czeiter E., Schmid K., Berger R.P., Kobeissy F., Italiano D., Hayes R.L., Tortella F.C., Mezosi E., Schwarcz A., Toth A., Nemes O., and Mondello S. (2015). Minor and repetitive head injury. Adv. Tech. Stand. Neurosurg. 42, 147–192 [DOI] [PubMed] [Google Scholar]
- 52. Ojo J.O., Mouzon B.C., and Crawford F. (2016). Repetitive head trauma, chronic traumatic encephalopathy and tau: challenges in translating from mice to men. Exp. Neurol. 275, 389–404 [DOI] [PubMed] [Google Scholar]
- 53. Sylvia F.R., Drake A.I., and Wester D.C. (2001). Transient vestibular balance dysfunction after primary blast injury. Mil. Med. 166, 918–920 [PubMed] [Google Scholar]
- 54. Bolton-Hall A.N., Hubbard W.B., and Saatman K.E. (2019). Experimental designs for repeated mild traumatic brain injury: challenges and considerations. J. Neurotrauma 36, 1203–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jassam Y.N., Izzy S., Whalen M., McGavern D.B,. and El Khoury J. (2017). Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95, 1246–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokobori S., Mazzeo A.T., Hosein K., Gajavelli S., Dietrich W.D., and Bullock M.R. (2013). Preconditioning for traumatic brain injury. Transl. Stroke Res. 4, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Horvath A., Szucs A., Barcs G., Noebels J.L., and Kamondi A. (2016). Epileptic seizures in Alzheimer disease: a review. Alzheimer Dis. Assoc. Disord. 30, 186–192 [DOI] [PubMed] [Google Scholar]
- 58. Vossel K.A., Beagle A.J., Rabinovici G.D., Shu H., Lee S.E., Naasan G., Hegde M., Cornes S.B., Henry M.L., Nelson A.B., Seeley W.W., Geschwind M.D., Gorno-Tempini M.L., Shih T., Kirsch H.E., Garcia P.A., Miller B.L., and Mucke L. (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 70, 1158–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Noebels J. (2011). A perfect storm: converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia 52, Suppl 1, 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cernak I., Savic J., Ignjatovic D., and Jevtic M. (1999). Blast injury from explosive munitions. J. Trauma 47, 96–103 [DOI] [PubMed] [Google Scholar]
- 61. Blume W.T. (2006). The progression of epilepsy. Epilepsia 47, Suppl 1, 71–78 [DOI] [PubMed] [Google Scholar]
- 62. Hauser W.A. and Lee J.R. (2002). Do seizures beget seizures? Prog. Brain Res. 135, 215–219 [DOI] [PubMed] [Google Scholar]
- 63. Butler C.R., Boychuk J.A., and Smith B.N. (2015). Effects of rapamycin treatment on neurogenesis and synaptic reorganization in the dentate gyrus after controlled cortical impact injury in mice. Front. Syst. Neurosci. 9, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Butler C.R., Boychuk J.A., and Smith B.N. (2016). Differential effects of rapamycin treatment on tonic and phasic GABAergic inhibition in dentate granule cells after focal brain injury in mice. Exp. Neurol. 280, 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Butler C.R., Boychuk J.A., and Smith B.N. (2017). Brain injury-induced synaptic reorganization in hilar inhibitory neurons is differentially suppressed by rapamycin. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mtchedlishvili Z., Lepsveridze E., Xu H., Kharlamov E.A., Lu B., and Kelly K.M. (2010). Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol. Dis. 38, 464–475 [DOI] [PubMed] [Google Scholar]
- 67. Effgen G.B., Vogel E.W. 3rd, Lynch K.A., Lobel A., Hue C.D., Meaney D.F., Bass C.R., and Morrison B. 3rd (2014). Isolated primary blast alters neuronal function with minimal cell death in organotypic hippocampal slice cultures. J. Neurotrauma 31, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 68. Vogel E.W. 3rd, Effgen G.B., Patel T.P., Meaney D.F., Bass C.R., and Morrison B. 3rd (2016). Isolated primary blast inhibits long-term potentiation in organotypic hippocampal slice cultures. J. Neurotrauma 33, 652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vogel E.W. 3rd, Rwema S.H., Meaney D.F., Bass C.R., and Morrison B. 3rd (2017). Primary blast injury depressed hippocampal long-term potentiation through disruption of synaptic proteins. J. Neurotrauma 34, 1063–1073 [DOI] [PubMed] [Google Scholar]
- 70. Briones T.L. (2015). Chapter 3 animal models of traumatic brain injury: is there an optimal model that parallels human brain injury? Annu. Rev. Nurs. Res. 33, 31–73 [DOI] [PubMed] [Google Scholar]
- 71. Golarai G., Greenwood A.C., Feeney D.M., and Connor J.A. (2001). Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 21, 8523–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Miller K.J., Ivins B.J., and Schwab K.A. (2013). Self-reported mild TBI and postconcussive symptoms in a peacetime active duty military population: effect of multiple TBI history versus single mild TBI. J. Head Trauma Rehabil. 28, 31–38 [DOI] [PubMed] [Google Scholar]
- 73. Seifert H.A. and Offner H. (2018). The splenic response to stroke: from rodents to stroke subjects. J. Neuroinflammation 15, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sahota P., Vahidy F., Nguyen C., Bui T.T., Yang B., Parsha K., Garrett J., Bambhroliya A., Barreto A., Grotta J.C., Aronowski J., Rahbar M.H., and Savitz S. (2013). Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int. J. Stroke 8, 60–67 [DOI] [PubMed] [Google Scholar]
- 75. Rasouli J., Lekhraj R., Ozbalik M., Lalezari P., and Casper D. (2011). Brain-spleen inflammatory coupling: a literature review. Einstein J. Biol. Med. 27, 74–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schwulst S.J., Trahanas D.M., Saber R., and Perlman H. (2013). Traumatic brain injury-induced alterations in peripheral immunity. J. Trauma Acute Care Surg. 75, 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Denes A., Wilkinson F., Bigger B., Chu M., Rothwell N.J., and Allan S.M. (2013). Central and haematopoietic interleukin-1 both contribute to ischaemic brain injury in mice. Dis. Model. Mech. 6, 1043–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Murray K.N., Parry-Jones A.R., and Allan S.M. (2015). Interleukin-1 and acute brain injury. Front. Cell. Neurosci. 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luheshi N.M., Kovacs K.J., Lopez-Castejon G., Brough D., and Denes A. (2011). Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J. Neuroinflammation 8, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fettelschoss A., Kistowska M., LeibundGut-Landmann S., Beer H.D., Johansen P., Senti G., Contassot E., Bachmann M.F., French L.E., Oxenius A., and Kundig T.M. (2011). Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proc. Natl. Acad. Sci. U. S. A. 108, 18055–18060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bonaz B., Sinniger V., and Pellissier S. (2017). The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front. Immunol. 8, 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johnston G.R. and Webster N.R. (2009). Cytokines and the immunomodulatory function of the vagus nerve. Br. J. Anaesth. 102, 453–462 [DOI] [PubMed] [Google Scholar]
- 83. Kenney M.J. and Ganta C.K. (2014). Autonomic nervous system and immune system interactions. Compr. Physiol. 4, 1177–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nance D.M. and Sanders V.M. (2007). Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 21, 736–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cernak I., Savic J., Malicevic Z., Zunic G., Radosevic P., Ivanovic I., and Davidovic L. (1996). Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma 40, S100–S104 [DOI] [PubMed] [Google Scholar]
- 86. Suppl, e (U.S.). Committee on Gulf War and Health: Long term effects of blast exposures. (2014). Gulf War and Health: Long-Term Effects of Blast Exposures. National Academies Press: Washington, D.C; [PubMed] [Google Scholar]
- 87. Scott T.E., Kirkman E., Haque M., Gibb I.E., Mahoney P., and Hardman J.G. (2017). Primary blast lung injury - a review. Br. J. Anaesth. 118, 311–316 [DOI] [PubMed] [Google Scholar]
- 88. Walker P.A., Bedi S.S., Shah S.K., Jimenez F., Xue H., Hamilton J.A., Smith P., Thomas C.P., Mays R.W., Pati S., and Cox C.S. Jr. (2012). Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J. Neuroinflammation 9, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kim E., Yang J., Beltran C.D., and Cho S. (2014). Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 34, 1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tumer N., Svetlov S., Whidden M., Kirichenko N., Prima V., Erdos B., Sherman A., Kobeissy F., Yezierski R., Scarpace P.J., Vierck C., and Wang K.K. (2013). Overpressure blast-wave induced brain injury elevates oxidative stress in the hypothalamus and catecholamine biosynthesis in the rat adrenal medulla. Neurosci. Lett. 544, 62–67 [DOI] [PubMed] [Google Scholar]
- 91. Aboud R., Shafii M., and Docherty J.R. (1993). Investigation of the subtypes of alpha 1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br. J. Pharmacol. 109, 80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Koliatsos V.E., Cernak I., Xu L., Song Y., Savonenko A., Crain B.J., Eberhart C.G., Frangakis C.E., Melnikova T., Kim H., and Lee D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–416 [DOI] [PubMed] [Google Scholar]
- 93. Huber B.R., Meabon J.S., Martin T.J., Mourad P.D., Bennett R., Kraemer B.C., Cernak I., Petrie E.C., Emery M.J., Swenson E.R., Mayer C., Mehic E., Peskind E.R., and Cook D.G. (2013). Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 37, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arun P., Abu-Taleb R., Oguntayo S., Tanaka M., Wang Y., Valiyaveettil M., Long J.B., Zhang Y., and Nambiar M.P. (2013). Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: role of compromised cell membrane integrity. Neurosci. Lett. 552, 87–91 [DOI] [PubMed] [Google Scholar]
- 95. Kenney K., Qu B.X., Lai C., Devoto C., Motamedi V., Walker W.C., Levin H.S., Nolen T., Wilde E.A., Diaz-Arrastia R., Gill J., and CENC Multisite Observational Study Investigators. (2018). Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 32, 1276–1284 [DOI] [PubMed] [Google Scholar]
- 96. Du X., West M.B., Cheng W., Ewert D.L., Li W., Saunders D., Towner R.A., Floyd R.A., and Kopke R.D. (2016). Ameliorative effects of antioxidants on the hippocampal accumulation of pathologic tau in a rat model of blast-induced traumatic brain injury. Oxid. Med. Cell. Longev. 2016, 4159357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Janigro D., Iffland P.H., 2nd Marchi, N., and Granata T. (2013). A role for inflammation in status epilepticus is revealed by a review of current therapeutic approaches. Epilepsia 54, Suppl 6, 30–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Terrone G., Salamone A., and Vezzani A. (2017). Inflammation and epilepsy: preclinical findings and potential clinical translation. Curr. Pharm. Des. 23, 5569–5576 [DOI] [PubMed] [Google Scholar]
- 99. Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., and Lamb B.T. (2010). Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mondragon-Rodriguez S., Perry G., Luna-Munoz J., Acevedo-Aquino M.C., and Williams S. (2014). Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer's disease and Down syndrome. Neuropathol. Appl. Neurobiol. 40, 121–135 [DOI] [PubMed] [Google Scholar]
- 101. Regan P., Piers T., Yi J.H., Kim D.H., Huh S., Park S.J., Ryu J.H., Whitcomb D.J., and Cho K. (2015). Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J. Neurosci. 35, 4804–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Simic G., Babic Leko M., Wray S., Harrington C., Delalle I., Jovanov-Milosevic N., Bazadona D., Buee L., de Silva R., Di Giovanni G., Wischik C., and Hof P.R. (2016). Tau protein hyperphosphorylation and aggregation in Alzheimer's disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Evans D.B., Rank K.B., Bhattacharya K., Thomsen D.R., Gurney M.E., and Sharma S.K. (2000). Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau's ability to promote microtubule assembly. J. Biol. Chem. 275, 24977–24983 [DOI] [PubMed] [Google Scholar]
- 104. Despres C., Byrne C., Qi H., Cantrelle F.X., Huvent I., Chambraud B., Baulieu E.E., Jacquot Y., Landrieu I., Lippens G., and Smet-Nocca C. (2017). Identification of the Tau phosphorylation pattern that drives its aggregation. Proc. Natl. Acad. Sci. U. S. A. 114, 9080–9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Edwards G., 3rd Moreno-Gonzalez, I., and Soto C. (2017). Amyloid-beta and tau pathology following repetitive mild traumatic brain injury. Biochem. Biophys. Res. Commun. 483, 1137–1142 [DOI] [PubMed] [Google Scholar]
- 106. Petraglia A.L., Plog B.A., Dayawansa S., Dashnaw M.L., Czerniecka K., Walker C.T., Chen M., Hyrien O., Iliff J.J., Deane R., Huang J.H., and Nedergaard M. (2014). The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg. Neurol. Int. 5, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]