Abstract
Smooth pursuit eye movements (SPEMs) and saccadic eye movements are both commonly impaired following sport-related concussion (SRC). Typical oculomotor assessments measure individual eye movements in a series of restrictive tests designed to isolate features such as response times. These measures lack ecological validity for athletes because athletes are adept at simple tasks designed for the general population. Yet, because eye movement metrics are sensitive and well-characterized neuroanatomically, it would be valuable to test whether athletes exhibit abnormal eye movements with more challenging tasks.
To address this gap in knowledge, we collected eye-tracking data during a sport-like task to gain insight on gaze behavior during active self-motion. SPEMs and saccadic eye movements were recorded during a sport-like visual task within 24–48 h following SRC. Thirty-six Division I student-athletes were divided into SRC and control (CON) groups. All participants completed two blocks of the Wii Fit© soccer heading game (WF) while wearing a monocular infrared eye tracker. Eye movement classification systems quantified saccadic amplitude (SA), velocity (SV), and count (SC); as well as SPEM velocity (SPV) and amplitude (SPA). Separate Mann-Whitney U tests evaluated SPA and SC and found no significant effects (SPA, p = 0.11; SC, p = 0.10). A multi-variate analysis of variance (MANOVA) for remaining variables revealed SPV was significantly greater in CON (p < 0.05), but the SRC group had greater SA and SV (p < 0.05). These findings suggest that during a sport-like task, to maintain foveation SRC subjects used larger amplitude, faster saccades, but exhibited slower SPEMs. Measuring oculomotor function during ecologically valid, sport-like tasks may serve as a concussion biomarker and provide insights into eye movement control after SRC.
Keywords: concussion, mTBI, oculomotor control, vision
Introduction
Sport-related concussion (SRC) remains a major public health concern.1 The neurological signs and symptoms of concussion vary by the mechanism of the blow (e.g., car accident vs. a head-to-head football tackle), and can be transient and subtle.2,3 As such, it is recommended that clinicians rely on a battery of assessments to define the specific clinical presentation of each SRC. These clinical presentations are commonly based on the most prevalent signs and symptoms that an athlete experiences post-SRC and can range from physical (i.e., headaches, dizziness), cognitive (i.e., attention, memory), affective (i.e., depression, anxiety), and sleep-related abnormalities, which can aid in determining the clinical profile of the injury.2–4 Recently, measurements of the visual system using symptom reporting5,6 or direct measures of oculomotor control7–12 have begun to play a more vital role in the diagnosis of SRC.13,14 Functional magnetic resonance imaging (fMRI) research points to increased activity in networks controlling eye movements, specifically the frontal eye fields and the cerebellar vermis,15 after SRC.16,17 Further, the brainstem, which includes the midbrain, has recently been implicated as a site of potential axonal damage from SRC.18,19 These changes in brain activity or the presence of damage may persist beyond 30 days post-injury but more importantly, eye movement abnormalities such as the eye lagging behind stimuli during pursuit9,20 can persist even after other symptoms resolve. Although these persisting abnormalities, which are readily seen with electroencephalogram (EEG),21 cognition,22 and postural stability,23 are not commonly considered when making clinical decisions, clinicians should be aware of the potential that patients may have lingering issues that could lead to decreased performance in activities of daily living.
Assessments of the visual system range from clinical tests such as the Vestibular Ocular Motor Screening Exam (VOMS)5,6 to the use of eye-tracking technology7–10,24,25 to measure specific eye movement deficits following trauma. Although symptom provocation via the VOMS5,6 remains a cornerstone for immediate diagnosis of SRC, it relies on subjective changes in symptom scores. At times, it is important for clinicians to have access to objective neuromechanical tracking of eye movements to ascertain which are impaired.
Among studies using video oculography to assess smooth pursuit eye movements (SPEMs), the ability to smoothly track an object traveling less than 30 degrees/sec demonstrates that those with SRC lag behind a moving target,9,20 have asymmetry in conjugate eye movements,11,12 have increased initiation latency,11 have few saccadic interruptions during pursuit,20 and show greater variability in tracking accuracy.20 Similarly, in this population, saccadic eye movements, the ballistic eye movements serving to shift foveation, are greater in number,7,8,17,26 are poorly controlled, and/or have variable amplitude and velocity.7–10,26–28 However, the saccadic eye movement deficits are highly dependent upon the task. For example, during antisaccade tasks, where commonly the presentation stimuli occurs on a left or right side off-center and the participants are instructed to move their eyes in the equal and opposite direction of the stimuli,10,11 SRC saccades have greater velocity.10 Yet, during self-paced saccades in which participants complete as many as possible saccades for a set amount of seconds between two stationary targets off-center, SRC have lower velocities compared with controls.10 Thus, the presentation of the stimuli can heavily influence the saccadic outcome. To the authors' best knowledge, no research exists examining SPEMs and saccades during an unrestricted sport-like task. This is partially due to technology deficiencies and the difficulty of correctly classifying the eye movements during these tasks.
Recent developments in eye-tracking technology such as the EyeLink (SR Research), Pupil Labs, and EyeGuide Focus allow measurement of oculomotor behavior without restricting participants' mobility.29 Whereas traditional methods9,10,25 require being seated and an immobilized head/body, advances now permit active self-motion during eye tracking. This is particularly important in SRC research as it provides researchers the ability to measure eye movements during more challenging ecologically relevant sport-like behaviors. In our previous research in this area,7,8 we demonstrated that during a sport-like task patients with a SRC had greater gaze resultant distance and mean horizontal velocity. This research is promising because of the sensitivity of eye movements and their well-characterized neural mechanisms30,31 but provides little insight into the quality of SPEMs and saccades. The ability to perceive motion is linked to the execution and quality of SPEMs, whereas saccades select regions of interest with the most visual information to center the retina on an object to allow for further pursuit.32 If either of these eye movements is abnormal, it may result in a decline in motion perception.32 Thus, it is now critical to determine more precisely which aspects, specifically SPEMs and saccades, of eye movements are abnormal after SRC during sport-like tasks. This may provide insight regarding where neural disruption is occurring following SRC.
Therefore, the purpose of this article was to investigate smooth pursuit and saccadic eye movements during a sport-like visual task within 24–48 h following SRC. Based on prior data,7–12 we hypothesized that SRC would have slower smooth pursuit amplitude and velocity. Similarly, we hypothesized that saccades would be higher in count along with having greater amplitude and velocity.
Methods
Participants
Thirty-six Division I student-athletes (18 SRC and 18 nearly matched controls) participated (age: 18 ± 1 years; gender: 7 male, 9 female; height: 174 ± 11 cm; weight: 72 ± 20 kg). Participants were divided into two groups: 1) those who were diagnosed with an acute SRC within 24–48 h, and 2) a nearly matched control (CON) group (sport, position, age, sex). The concussion diagnosis was determined by the head team physician within 24 h of the incident using somatic, cognitive, or physical self-reported symptoms following an appropriate mechanism (head or body trauma), as well as the Sport Concussion Assessment Tool-5th edition (SCAT5).3,33
All student-athletes were recruited to participate in this study via a collaboration between the testing facility and the sports medicine staff. These data were collected alongside a larger research study, which the student-athletes participated in on a volunteer basis. Student-athletes were excluded if they self-reported any pre-injury vestibular, visual (excluding corrected myopia or hyperopia via lenses), metabolic, neurological pathology (excluding the existing concussion), attention deficit hyperactivity disorder, learning disabilities, and lower-extremity injury that impaired the ability to maintain upright stance. All student-athletes self-reported being able to clearly see and discern the visual stimuli. All participants signed informed consent documents and all protocols were approved by the University of Nevada, Reno Institutional Review Board.
Procedures
Participants completed two blocks (≈2 min per block) of the Wii Fit© soccer heading game (WF) while wearing a head-mounted monocular eye tracker (H7, 240 Hz, 0.05 degrees precision; Applied Science Laboratory, Medford, MA). The full procedure of this examination has been published in detail in prior articles.7,8 Before the task a 9-point calibration procedure was completed. Stimuli were presented on a 55-in LED TV (48.82 × 28.62 in, 1920 x 1080 pixels, Sharp Aquos HD LED Smart TV; Sharp Electronics Corp., Mahwah, NJ) with the individual 55 in from the screen standing on a Wii Balance Board©. Participants were instructed to interact with the approaching stimulus (soccer ball, 1.25 Hz) while avoiding distractors (panda heads and cleats). Stimuli traveled a total horizontal displacement of 472 pixels (∼21 in). Game score was not incorporated in analyses due to the presence of a lower ceiling effect.
Data analysis
Saccade analysis
Gaze coordinates (eye + head) were exported to MATLAB R2018a (MATLAB 2010; MathWorks, Inc., Natick, MA). The data were transformed into azimuth and elevation. Next, we applied a 5 data-point linear interpolation and a 13 sliding data-point boxcar filter. The filtered data were then differenced across 50–83 msec temporal windows, to identify saccade end-points and determine durations.34 These data were categorized into individual vectors that corresponded to the temporal window (i.e., gaze data at 50-msec window, 51-msec window, 52-msec window …80-msec window). These differenced saccadic data were included if they were within a predefined dispersion threshold (4 degrees ≤ amplitude ≥30 degrees).34,35 From these data, velocity data were calculated as the differenced dispersion datum (at each individual temporal window vector) divided by the temporal window length (i.e., 50–83 msec) of that trial. These data were then filtered via a velocity threshold (75 degrees/sec ≤ velocity ≥500 degrees/sec).34,36 The velocity data from each temporal window were then concatenated into a 34-by-n matrix. An average was calculated across each column to obtain a 1-by-n vector of velocity data.
The same process was conducted for the amplitude data. From this, mean saccadic velocity (SV) was calculated as the average velocity across the vector, whereas mean saccadic amplitude (SA) was calculated as the average amplitude across the vector. Saccadic count (SC) was calculated as the length of each SV array. Typically, SV should be slower and less accurate in disease populations such as Parkinson's37; however, in SRC9–11 this is not always the case and is highly dependent upon the task. It is expected based on our prior research7,8 that the eye typically travels farther and faster during a sport-like task among those with an SRC compared with healthy controls. This may be a result of faster saccadic eye movements. Thus, increased SV is an expected abnormal outcome.
Smooth pursuit analysis
The filtered azimuth and elevation data were parsed to exclude data below the temporal window threshold (<30 msec); this removed microsaccades and fixations.34,38 Because SPEM does not have a set amplitude threshold, 10–30 msec temporal windows also removed amplitudes below 0.66 degrees to filter out fixations and saccades.34,38 A velocity threshold permitted data with velocities between 10 degrees/sec ≤ and ≥30 degrees/sec.34,39,40 We calculated mean SPEM velocity (SPV) and mean SPEM amplitude (SPA) across the data time series. SPV was calculated as the average SPEM velocity across the vector, whereas the SPA was calculated as the average amplitude across the SPEM SPA vector.
SPEMs are commonly less accurate (worse or smaller velocity gain),11,20 lag behind the stimuli,9,20 have increased initiation latency,11 and have few saccadic interruptions during pursuit.20 For analysis of these data, because we are unable to calculate gain (stimuli velocity is unknown) we expect in the SRC group slower SPV as the eye may be lagging behind the stimuli.
Statistical analysis
Data were examined to ensure they conformed to normal distributions and were without influential skewness or kurtosis. After review, SPA and SC were considered non-normal for the SRC group. Therefore, two separate Mann-Whitney U tests were run to assess group differences (SRC, CON) for SPA and SC. A single multi-variate analysis of variance model (MANOVA) was used to assess group differences for the remaining measures of interest: SPV, SV, and SA. In the event of a significant interaction, the simple main effects for direction were tested based on independent t tests with appropriate Holm-Bonferroni corrections.41 All trials were averaged to get aggregate values for measures used. Significance was set at alpha = 0.05 for all analyses.
Results
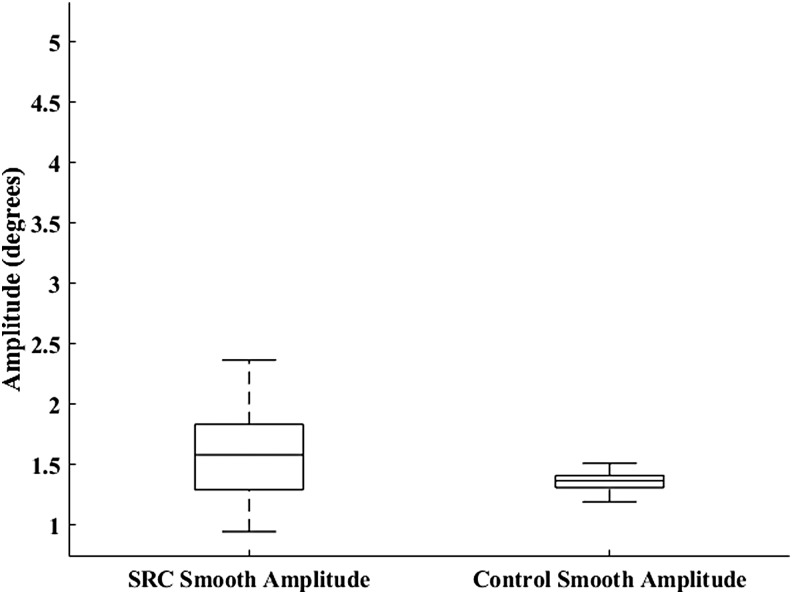
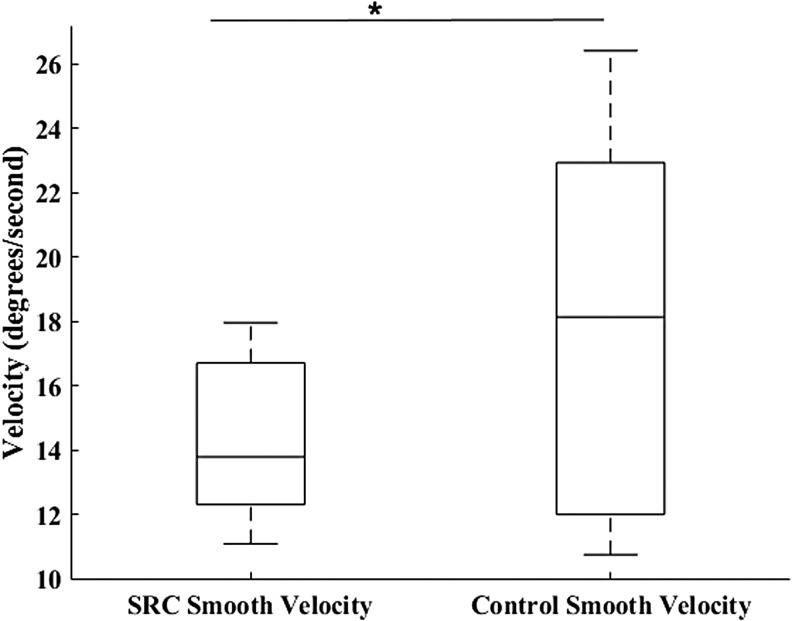
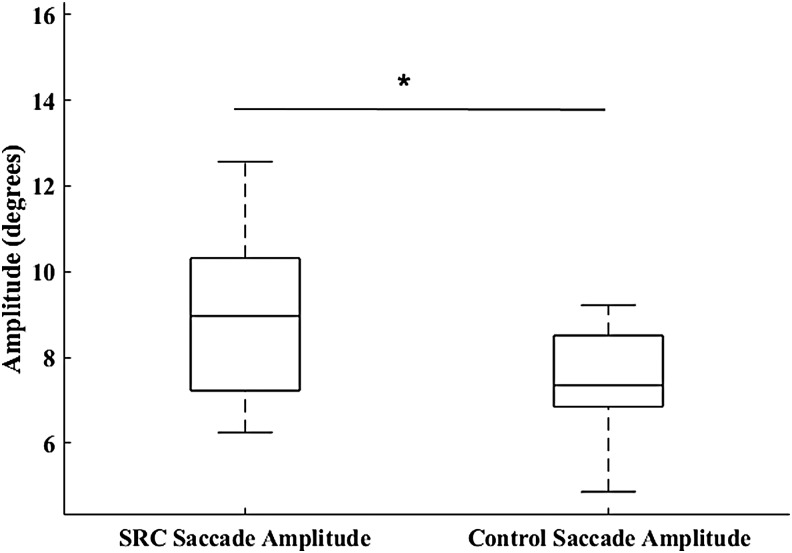
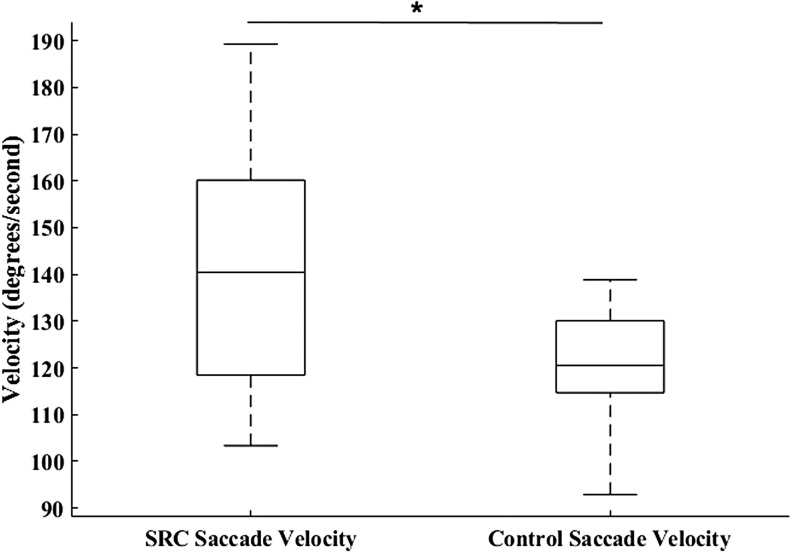
Neither SPA (U = 112, p = 0.114; SRC = 1.84 ± 0.99 degrees, CON = 1.40 ± 0.178 degrees) (Fig. 1) nor SC (U = 110, p = 0.103; SRC = 14.50 ± 7.78, CON = 10.31 ± 6.52) were significantly different between groups. The MANOVA resulted in a significant omnibus effect of group (F3,32 = 4.93, p = 0.006), wherein SPV (p = 0.019; SRC = 14.24 ± 2.31 degrees/sec, CON = 17.86 ± 5.75 degrees/sec, Cohen's d = 0.83) (Fig. 2) was significantly greater in the CON group, and SA (p = 0.008; SRC = 9.19 ± 2.52 degrees, CON = 7.39 ± 1.14 degrees, Cohen's d = 0.92) (Fig. 3) and SV (p = 0.009; SRC = 139.81 ± 25.76 degrees/sec, CON = 120.39 ± 13.36 degrees/sec, Cohen's d = 0.95) (Fig. 4) were significantly greater in the SRC group.
FIG. 1.
Sport-related concussion (SRC) and matched control smooth pursuit eye movement amplitude during the sport-like visual task.
FIG. 2.
Sport-related concussion (SRC) and matched control smooth pursuit eye movement velocity during the sport-like visual task. *p = 0.019.
FIG. 3.
Sport-related concussion (SRC) and matched control saccadic eye movement amplitude during the sport-like visual task. *p < 0.05.
FIG. 4.
Sport-related concussion (SRC) and matched control saccadic eye movement velocity during the sport-like visual task. *p < 0.05.
Discussion
The purpose of this study was to investigate smooth pursuit and saccadic eye movements during a sport-like visual task within 24–48 h following an SRC. These findings expand on our prior research,7,8 wherein during a sport-like visual task we observed greater overall horizontal and vertical gaze amplitude and velocity. It is important to understand what visual strategies SRC individuals use to perceive visual moving stimuli and what specific eye movement deficits are interrupted following SRC. This may lead to the development of a deeper understanding of how athletes engage in sport-like tasks and what oculomotor control abnormalities are present. These findings may drive future clinical assessments that are not symptom based, such as the VOMS,5 which could lead to targeted rehabilitation of particular impaired eye movements.24,42,43
The key findings of this study are that SRC had greater SA and SV, whereas CON had greater SPV. These findings suggest that during a sport-like task, there were group differences in oculomotor behaviors. This is similar to prior research reporting abnormal oculomotor behavior in SRC groups such as the eye lagging behind a target during pursuit10,20 and increased saccadic velocity.7–10,26,27 However, this is the first study to provide evidence of abnormal oculomotor control following SRC during a sport-like task where SPEMs and saccades are both required to complete the more naturalistic visual task.
Smooth pursuit eye movements
It was hypothesized that the SRC group would have lower SPA and SPV when compared with CON. This hypothesis was partially supported as SPA was not different between groups, although the SRC had significantly lower SPV. Thus, for both groups their eyes moved approximately the same average distance for left and right horizontal directions, but CON movements were faster. This is consistent with prior research9,10 showing that SPEM typically lagged among those with concussions during standard smooth pursuit tasks when compared with healthy controls.
Lag was not directly calculated in the current study as the stimuli velocity was unknown; however, it can be speculated that slower SPEM velocities in the SRC group may indicate that the eye is traveling farther behind the stimuli, whereas the CON subjects were able to consistently maintain foveation on the desired target. Further, the current study's stimuli were more challenging because they were unpredictable, and required target selection and sustained attention.44,45 Decreased attentional resources are caused by concussion10,27,46 and may be associated with inaccurate eye movements28,44; this may aid in explaining the slower SPV in the SRC group. Although attention was not directly measured in the current study, as a common symptom it could have hampered pursuit initiation due to the presence of the conflicting motion stimuli (i.e., other balls, shoes, or pandas in the visual scene).32 This may explain the reduced SPEM velocities and the increased saccadic amplitudes and velocities in the SRC group. This could be a result of poor integration of the temporal data where SRC need additional catch-up saccades to reduce retinal slip during pursuit.
The lagging of SPEM behind targets is consistent with prior literature and may account for the finding of decreased SPV in the SRC group. To account for this lagging of SPEM, SRC may require more saccadic eye movements to update the motion of the target on the retina during object tracking rather than using smooth pursuits. During object tracking, a combination of smooth pursuit and saccadic eye movements supports foveating the target.25,47 Success is dependent upon the stimulus velocity and once a lag is detected or an object moves faster than ≈30 degrees/sec, a catch-up saccade is initiated to maintain the target on the fovea.39 Once a saccade is completed, SPEMs continue to track the object until additional saccades are needed to avoid the target leaving the fovea and to minimize retinal slippage.32 Thus, SRC may adopt a visual strategy during object tracking involving larger catch-up saccades to return the image of the stimuli to the fovea to compensate for the reduced SPEM gain. This is speculative, as the specific stimuli position and velocity were not known in the current study and future research is needed that assess this claim.
Saccadic eye movements
It was further hypothesized that the SRC group would have greater SC, SA, and SV when compared with CON. This hypothesis was partially supported as SA and SV in the SRC group was significantly greater than in the CON group, whereas SC was not. These findings indicate that SRC had larger, more rapid saccades but the same number of saccades. This is in partial agreement with prior research showing increased saccadic velocity7–10,26,27 and the number of saccades is typically increased7,8,17,26 following SRC. The lack of significance in our SC data may be due to the SRC eye movements lagging behind the objects due to slower SPV. This would then require larger amplitude saccades to bring the object back into focus on the fovea but may not lead to greater SC.
Overall, by examining the greater SA and SV with the reduction in SPV it can be suggested that the SRC group may have had an inability to properly track objects in a scene and required increased active updates (saccades) to keep an image centered on the fovea. This might indicate that SRC subjects were emphasizing accuracy more than speed to complete the task. The task required participants to move their body to direct the on-screen avatar into the path of the target virtual visual stimulus. In the presence of an impairment such as SRC, a reduced response time and/or motor abnormalities begin to appear during challenging tasks that require a cognitive and motor component.46,48 In fact, an increased working memory load during smooth pursuit tasks makes SPEMs variable and less accurate after concussion.46 This suggests that during more challenging tasks, eye movements become less controlled during attentionally demanding tasks.
This speed-accuracy trade-off is actively controlled by many areas of the brain involved in eye movements such as the frontal eye field,49–51 relayed through the basal ganglia52 with cerebellar inputs for timing.53–55 Further, saccadic eye movements can be modulated by task conditions that can either increase or decrease the saccadic accuracy.56–58 Thus the slower SPV followed by larger and faster saccade in the current study could be a reflection of a speed-accuracy trade-off. This may be caused by the extra concentration of glutamate present in the neurons within the superior colliculus following concussion.59 This area is important for the initiation of saccades and excess of glutamate may elicit greater saccade amplitude and velocity.60 Alternatively, the pars reticula of the substantia nigra maintains constant tonic gamma-aminobutyric acid (GABA)nergic inhibition on the superior colliculus.61 Poor control on the superior colliculus could lead to deleterious eye movements, such as the timely and transient cessation of saccadic initiation.61 These findings are speculative as this study did not directly measure the concentration of glutamate, but they do provide some neurophysiological context behind the abnormal saccadic behavior observed in this study.
Clinically, the results of this study may provide a basis for how medical professionals can approach using sport-like stimuli as an effective way to diagnosis and measure recovery of SRC. Sport-like tasks attempt to replicate on-field situations that are safe and effective to measure. These ecologically valid tasks, such as the one in this article, may provide a more effective way of measuring if an athlete is ready to return to play, unlike assessments that only measure a single domain without regard to holistic systemic function. Unfortunately, the technology required to complete this particular task is expensive; along with the diagnostic validity being unknown, caution must be recommended for immediate implication of this task. Future research should begin to explore diagnostic accuracy along with ways to reduce the cost of such assessments.
This study is not without limitations. First, the visual stimuli used were not controlled by the researchers. Thus, we were unable to calculate measures of saccadic or smooth accuracy (gain). Thus a key measure of interest, accuracy, remains unknown. Future research should study eye movement deficits following SRC during more ecologically valid tasks while having full control over the visual stimuli to assess accuracy of the eye movements. Second, this study used an eye tracker that sampled up to 240 Hz. Saccadic eye movements can extend into the 500 Hz bandwidth,60 thus our data may be subject to false line movements, which were mitigated as much as possible with the filtering techniques. Unfortunately, no known head-mounted eye tracker exists that can sample above 500 Hz, yet we attempted to reduce any errors with the lower sampling frequency by using linear interpolation of the eye data. Future research should incorporate higher-sampling head-mounted eye-tracking technology.
Third, our participant sample included individuals who are exposed to repetitive head impacts (i.e., American rules football players). Some recent evidence suggests that visual accommodation62,63 may decline in players after a single athletic season of repetitive head impacts. These repetitive head impacts were not quantified or controlled for in the current study. Future research should examine if those who incur repetitive head impacts and suffer a concussion have greater visual system deficits following acute SRC. Fourth, no objective visual disturbances or vestibular impairment data were available at the time of testing. Future research should include these data to determine if these oculomotor impairments are subject to existing symptom-based issues. Fifth, no behavioral data were collected from this sport-like task due the presence of a lower-ceiling effect and an inability to control for those who were simply poor at the task but had sufficient oculomotor data. Future research should incorporate behavioral data alongside these important oculomotor findings. Lastly, the total amount of prior concussions was not controlled for in the current study. Future research should evaluate how multiple concussions may negatively impact oculomotor control.
Conclusions
This study is one of the first to provide evidence of abnormal SPEMs and saccades during a sport-like task. The most important findings are that SRC subjects had greater SA and SV, whereas CON subjects had greater smooth pursuit velocity. These findings suggest that during a head-free task, those with SRC may require larger and faster saccadic eye movements to update the retina on the moving object rather than relying primarily on smooth pursuits, but this could be dependent upon the stimuli velocity. It is critical for those involved with the management of SRC to be aware of these potential oculomotor deficits to properly measure and track recovery from SRC.
Funding Information
The authors would like to thank the UNR Clinical and Translational Research - Infrastructure Network (CTR-IN) (P20GM 103440 and U54GM104944) along with the UNR Neuroscience COBRE (P2G0GM103650) for partially supporting and funding this research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. National Center for Injury Prevention and Control. (2003). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- 2. Sussman E.S., Ho A.L., Pendharkar A.V., and Ghajar J. (2016). Clinical evaluation of concussion: the evolving role of oculomotor assessments. Neurosurg. Focus 40, E7. [DOI] [PubMed] [Google Scholar]
- 3. McCrory P., Meeuwisse W., Dvorak J., Aubry M., Bailes J., Broglio S., Cantu R.C., Cassidy D., Echemendia R.J., Castellani R.J., Davis G.A., Ellenbogen R., Emery C., Engebretsen L., Feddermann-Demont N., Giza C.C., Guskiewicz K.M., Herring S., Iverson G.L., Johnston K.M., Kissick J., Kutcher J., Leddy J.J., Maddocks D., Makdissi M., Manley G.T., McCrea M., Meehan W.P., Nagahiro S., Patricios J., Putukian M., Schneider K.J., Sills A., Tator C.H., Turner M., and Vos P.E. (2017). Consensus statement on concussion in sport-the 5(th) International Conference on Concussion in Sport held in Berlin, October 2016. Br. J. Sports Med. 51, 838–847 [DOI] [PubMed] [Google Scholar]
- 4. Broglio S.P., Collins M.W., Williams R.M., Mucha A., and Kontos A.P. (2015). Current and emerging rehabilitation for concussion: a review of the evidence. Clin. Sports Med. 34, 213–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mucha A., Collins M.W., Elbin R.J., Furman J.M., Troutman-Enseki C., DeWolf R.M., Marchetti G., and Kontos A.P. (2014). A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions. Am. J. Sports Med. 42, 2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontos A.P., Deitrick J.M., Collins M.W., and Mucha A. (2017). Review of vestibular and oculomotor screening and concussion rehabilitation. J. Athl. Train. 52, 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray N.G., D'amico N.R., Powell D., and Mormile M.E. (2017). ASB clinical biomechanics award winner 2016: Assessment of gaze stability within 24–48 hours post-concussion. Clinical (PMID ) [DOI] [PubMed] [Google Scholar]
- 8. Murray N.G., Ambati V.N.P., Contreras M.M., Salvatore A.P., and Reed-Jones R.J. (2014). Assessment of oculomotor control and balance post-concussion: a preliminary study for a novel approach to concussion management. Brain Inj. 28, 496–503 [DOI] [PubMed] [Google Scholar]
- 9. Heitger M.H., Anderson T.J., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., and Ardagh M.W. (2004). Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain 127, 575–590 [DOI] [PubMed] [Google Scholar]
- 10. Heitger M.H., Jones R.D., Macleod A.D., Snell D.L., Frampton C.M., and Anderson T.J. (2009). Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132, 2850–2870 [DOI] [PubMed] [Google Scholar]
- 11. Kelly K.M., Kiderman A., Akhavan S., Quigley M.R., Snell E.D., Happ E., Synowiec A.S., Miller E.R., Bauer M.A., Oakes L.P., Eydelman Y., Gallagher C.W., Dinehart T., Schroeder J.H., and Ashmore R.C. (2018). Oculomotor, vestibular, and reaction time effects of sports-related concussion: video-oculography in assessing sports-related concussion. J. Head Trauma Rehabil. 34, 176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samadani U., Ritlop R., Reyes M., Nehrbass E., Li M., Lamm E., Schneider J., Shimunov D., Sava M., Kolecki R., Burris P., Altomare L., Mehmood T., Smith T., Huang J.H., McStay C., Todd S.R., Qian M., Kondziolka D., Wall S., and Huang P. (2015). Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. J. Neurotrauma 32, 548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciuffreda K.J., Ludlam D., and Thiagarajan P. (2011). Oculomotor diagnostic protocol for the mTBI population. Optometry 82, 61–63 [DOI] [PubMed] [Google Scholar]
- 14. Ventura R.E., Balcer L.J., and Galetta S.L. (2014). The neuro-ophthalmology of head trauma. Lancet Neurol. 13, 1006–1016 [DOI] [PubMed] [Google Scholar]
- 15. Kellar D., Newman S., Pestilli F., Cheng H., and Port N.L. (2018). Comparing fMRI activation during smooth pursuit eye movements among contact sport athletes, non-contact sport athletes, and non-athletes. Neuroimage Clin. 18, 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartels A., Logothetis N.K., and Moutoussis K. (2008). fMRI and its interpretations: an illustration on directional selectivity in area V5/MT. Trends Neurosci. 31, 444–453 [DOI] [PubMed] [Google Scholar]
- 17. Johnson B., Hallett M., and Slobounov S. (2015). Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology 85, 1163–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirad A.A., Bazarian J.J., Merchant-Borna K., Garcea F.E., Heilbronner S., Paul D., Hintz E.B., van Wijngaarden E., Schifitto G., Wright D.W., Espinoza T.R., and Mahon B.Z. (2019). A common neural signature of brain injury in concussion and subconcussion. Sci. Advances 5, eaau3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruta J., Suh M., Niogi S.N., Mukherjee P., and Ghajar J. (2010). Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 25, 293–305 [DOI] [PubMed] [Google Scholar]
- 21. Munia T.T.K., Gendreau J.L., Verma A.K., Johnson B.D., Romanick M., Tavakolian K., and Fazel-Rezai R. (2016). Preliminary results of residual deficits observed in athletes with concussion history: combined EEG and cognitive study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 41–44 [DOI] [PubMed] [Google Scholar]
- 22. Arciniega H., Kilgore-Gomez A., Harris A., Peterson D.J., McBride J., Fox E., and Berryhill M.E. (2019). Visual working memory deficits in undergraduates with a history of mild traumatic brain injury. Atten. Percept. Psychophys. doi: 10.3758/s13414-019-01774-9 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray N.G., Szekely B., Moran R., Ryan G., Powell D., Munkasy B.A., Buckley T.A., and Guskiewicz K. (2019). Concussion history associated with increased postural control deficits after subsequent injury. Physiol. Meas. 40, 024001. [DOI] [PubMed] [Google Scholar]
- 24. Snegireva N., Derman W., Patricios J., and Welman K.E. (2018). Eye tracking technology in sports-related concussion: a systematic review and meta-analysis. Physiol. Meas. 39, 12TR01. [DOI] [PubMed] [Google Scholar]
- 25. Maruta J., and Ghajar J. (2014). Detecting eye movement abnormalities from concussion, in: Concussion. Niranjan A., and Lunsford L.D. (eds). S. Karger AG: Basel, pps. 226–233 [DOI] [PubMed] [Google Scholar]
- 26. Danna-Dos-Santos A., Mohapatra S., Santos M., and Degani A.M. (2018). Long-term effects of mild traumatic brain injuries to oculomotor tracking performances and reaction times to simple environmental stimuli. Sci. Rep. 8, 4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiCesare C.A., Kiefer A.W., Nalepka P., and Myer G.D. (2017). Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav. Res. Methods 49, 258–266 [DOI] [PubMed] [Google Scholar]
- 28. Cifu D.X., Wares J.R., Hoke K.W., Wetzel P.A., Gitchel G., and Carne W. (2015). Differential eye movements in mild traumatic brain injury versus normal controls. J. Head Trauma Rehabil. 30, 21–28 [DOI] [PubMed] [Google Scholar]
- 29. MacInnes J.J., Iqbal S., Pearson J., and Johnson E.N. (2018). Wearable eye-tracking for research: automated dynamic gaze mapping and accuracy/precision comparisons across devices. bioRxiv, 299925 [Google Scholar]
- 30. Sharpe J.A. (2008). Neurophysiology and neuroanatomy of smooth pursuit: lesion studies. Brain Cogn. 68, 241–254 [DOI] [PubMed] [Google Scholar]
- 31. Thier P., and Ilg U.J. (2005). The neural basis of smooth-pursuit eye movements. Curr. Opin. Neurobiol. 15, 645–652 [DOI] [PubMed] [Google Scholar]
- 32. Schütz A.C., Braun D.I., and Gegenfurtner K.R. (2011). Eye movements and perception: a selective review. J. Vis. 11, pii: . doi: 10.1167/11.5.9 [DOI] [PubMed] [Google Scholar]
- 33. Echemendia R.J., Meeuwisse W., McCrory P., Davis G.A., Putukian M., Leddy J., Makdissi M., John Sullivan S., Broglio S.P., Raftery M., Schneider K., Kissick J., McCrea M., Dvorak J., Sills A.K., Aubry M., Engebretsen L., Loosemore M., Fuller G., Kutcher J., Ellenbogen R., Guskiewicz K., Patricios J., and Herring S. (2017). The Sport Concussion Assessment Tool 5th Edition (SCAT5): background and rationale. Br. J. Sports Med. 51, 848–850 [DOI] [PubMed] [Google Scholar]
- 34. Holmqvist K., Nyström M., Andersson R., Dewhurst R., Jarodzka H., and van de Weijer J. (2011). Eye Tracking: A Comprehensive Guide to Methods and Measures. Oxford University Press [Google Scholar]
- 35. Bahill A.T., Adler D., and Stark L. (1975). Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest. Ophthalmol. 14, 468–469 [PubMed] [Google Scholar]
- 36. Komogortsev O.V., and Karpov A. (2013). Automated classification and scoring of smooth pursuit eye movements in the presence of fixations and saccades. Behav. Res. Methods 45, 203–215 [DOI] [PubMed] [Google Scholar]
- 37. Chan F., Armstrong I.T., Pari G., Riopelle R.J., and Munoz D.P. (2005). Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia 43, 784–796 [DOI] [PubMed] [Google Scholar]
- 38. Otero-Millan J., Troncoso X.G., Macknik S.L., Serrano-Pedraza I., and Martinez-Conde S. (2008). Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J. Vis. 8, 21.1–18 [DOI] [PubMed] [Google Scholar]
- 39. Fukushima K., Fukushima J., Warabi T., and Barnes G.R. (2013). Cognitive processes involved in smooth pursuit eye movements: behavioral evidence, neural substrate and clinical correlation. Front. Syst. Neurosci. 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spering M., and Gegenfurtner K.R. (2008). Contextual effects on motion perception and smooth pursuit eye movements. Brain Res. 1225, 76–85 [DOI] [PubMed] [Google Scholar]
- 41. Holm S. (1979). A simple sequentially rejective multiple test procedure. Scand. Stat. Theory Appl. 6, 65–70 [Google Scholar]
- 42. Mani R., Asper L., and Khuu S.K. (2018). Deficits in saccades and smooth-pursuit eye movements in adults with traumatic brain injury: a systematic review and meta-analysis. Brain Inj. 32, 1315–1336 [DOI] [PubMed] [Google Scholar]
- 43. Akhand O., Balcer L.J., and Galetta S.L. (2019). Assessment of vision in concussion. Curr. Opin. Neurol. 32, 68–74 [DOI] [PubMed] [Google Scholar]
- 44. Suh M., Kolster R., Sarkar R., McCandliss B., Ghajar J., and Cognitive and Neurobiological Research Consortium. (2006). Deficits in predictive smooth pursuit after mild traumatic brain injury. Neurosci. Lett. 401, 108–113 [DOI] [PubMed] [Google Scholar]
- 45. Hutton S.B., and Tegally D. (2005). The effects of dividing attention on smooth pursuit eye tracking. Exp. Brain Res. 163, 306–313 [DOI] [PubMed] [Google Scholar]
- 46. Stubbs J.L., Corrow S.L., Kiang B.R., Corrow J.C., Pearce H.L., Cheng A.Y., Barton J.J.S., and Panenka W.J. (2019). Working memory load improves diagnostic performance of smooth pursuit eye movement in mild traumatic brain injury patients with protracted recovery. Sci. Rep. 9, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lisberger S.G., Morris E.J., and Tychsen L. (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu. Rev. Neurosci. 10, 97–129 [DOI] [PubMed] [Google Scholar]
- 48. Howell D.R., Oldham J.R., Meehan W.P. 3rd, DiFabio M.S., and Buckley T.A. (2019). Dual-task tandem gait and average walking speed in healthy collegiate athletes. Clin. J. Sport Med. 29, 238–244 [DOI] [PubMed] [Google Scholar]
- 49. Ding L., and Gold J.I. (2012). Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb. Cortex 22, 1052–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forstmann B.U., Anwander A., Schäfer A., Neumann J., Brown S., Wagenmakers E.-J., Bogacz R., and Turner R. (2010). Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc. Natl. Acad. Sci. USA 107, 15916–15920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heitz R.P., and Schall J.D. (2012). Neural mechanisms of speed-accuracy tradeoff. Neuron 76, 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ding L., and Gold J.I. (2010). Caudate encodes multiple computations for perceptual decisions. J. Neurosci. 30, 15747–15759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ratcliff R., Cherian A., and Segraves M. (2003). A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J. Neurophysiol. 90, 1392–1407 [DOI] [PubMed] [Google Scholar]
- 54. Ratcliff R., Hasegawa Y.T., Hasegawa R.P., Smith P.L., and Segraves M.A. (2007). Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J. Neurophysiol. 97, 1756–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Horwitz G.D., and Newsome W.T. (2001). Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J. Neurophysiol. 86, 2543–2558 [DOI] [PubMed] [Google Scholar]
- 56. Gopal A., Jana S., and Murthy A. (2017). Contrasting speed-accuracy tradeoffs for eye and hand movements reveal the optimal nature of saccade kinematics. J. Neurophysiol. 118, 1664–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bronstein A.M., and Kennard C. (1987). Predictive eye saccades are different from visually triggered saccades. Vision Res. 27, 517–520 [DOI] [PubMed] [Google Scholar]
- 58. van Donkelaar P., Siu K.-C., and Walterschied J. (2004). Saccadic output is influenced by limb kinetics during eye—hand coordination. J. Mot. Behav. 36, 245–252 [DOI] [PubMed] [Google Scholar]
- 59. Giza C.C., and Hovda D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, Suppl. 4, S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramat S., Leigh R.J., Zee D.S., and Optican L.M. (2007). What clinical disorders tell us about the neural control of saccadic eye movements. Brain 130, 10–35 [DOI] [PubMed] [Google Scholar]
- 61. Shaikh A.G., Xu-Wilson M., Grill S., and Zee D.S. (2011). “Staircase” square-wave jerks in early Parkinson's disease. Br. J. Ophthalmol. 95, 705–709 [DOI] [PubMed] [Google Scholar]
- 62. Kawata K., Rubin L.H., Lee J.H., Sim T., Takahagi M., Szwanki V., Bellamy A., Darvish K., Assari S., Henderer J.D., Tierney R., and Langford D. (2016). Association of football subconcussive head impacts with ocular near point of convergence. JAMA Ophthalmol. 134, 763–769 [DOI] [PubMed] [Google Scholar]
- 63. Zonner S.W., Ejima K., Fulgar C.C., Charleston C.N., Huibregtse M.E., Bevilacqua Z.W., and Kawata K. (2018). Oculomotor response to cumulative subconcussive head impacts in U.S. high school football players: a pilot longitudinal study. JAMA Ophthalmol. 137, 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]