Abstract
Kidney disease is a major medical problem globally. Chronic kidney disease (CKD) is a progressive loss of kidney function. It causes accumulation of waste and fluid in the body, eventually resulting in kidney failure as well as damaging other organs. Although dialysis and kidney transplantation have been used as primary treatments for renal disease, dialysis does not restore full renal function, and there is a shortage of donor kidneys for transplantation. Recent advances in cell-based therapies have offered a means to augment and restore renal function. Various types of cells have been tested to evaluate their therapeutic effects on injured kidneys. Among various types of cells, amniotic fluid stem cells (AFSCs) share advantages of both embryonic and adult stem cells, such as pluripotent activity, remarkable plasticity, and immunomodulatory effects, which may allow their future therapeutic use as an “off-the-shelf” cell source. AFSC presents advantages of both conventional pluripotent and adult stem cells, such as pluripotent activity, remarkable plasticity, and immunomodulatory effects. This study demonstrates that administration of human-derived AFSC facilitates functional and structural improvement in a rat model of CKD, and suggests that cell therapy with AFSC has potential as a therapeutic strategy to recover renal function in patients with CKD.
Impact Statement
Patients with chronic kidney disease (CKD) have limited treatment options, and renal transplantation is the only definitive treatment method that restores kidney function. However, challenges associated with transplantation, including donor organ shortage, rejection, and life-long immunosuppression, remain a problem. Recently, stem cell-based therapies have been proposed as an alternative approach to augment and restore renal function. In this study, we used human-derived amniotic fluid stem cells (AFSCs) to treat CKD in a rat model and demonstrated that AFSC treatment facilitated positive effects in terms of improvements of renal function.
Keywords: stem cell therapy, amniotic fluid, chronic kidney disease, kidney regeneration
Introduction
Kidney disease is a worldwide public health problem and can manifest in acute and chronic symptoms.1 Acute kidney injury (AKI) often leads to chronic kidney disease (CKD), which can progress to end-stage renal disease (ESRD).2 The current management for renal failure is dialysis or kidney transplantation. Dialysis is limited to removing waste from the body and is the only solution other than kidney transplantation to restore partial kidney function.3,4 Current problems in transplantation include rejection, organ donor shortage, and the need for life-long immunosuppressive therapy.5 Immunosuppression can result in an increased risk of infection and tumor formation. In short, current treatment modalities are inadequate, further increasing the demand for alternative approaches to treat kidney diseases.
One possible approach for treatment of renal injury is to regenerate damaged tissues using therapeutic cells, rather than replacing the damaged organ.6 As such, cell-based therapy is a promising and plausible option for organ repair.7–10 In cell-based therapy, selection of cell sources—whether autologous cells from kidney or nonrenal tissues from patients—is critically important for efficient therapeutic outcomes.
To demonstrate that autologous renal cells are feasible for treatment of renal failure, we previously developed a cell isolation system from human kidneys, established a primary culture expansion platform, and showed that culture-expanded primary human renal cells are able to maintain renal cell phenotypes and function.11 Furthermore, we showed that cell isolation from patients with CKD is possible, and that the expanded renal cells maintained similar renal phenotypes as normal kidneys during extensive cell culture.12 Furthermore, erythropoietin-positive cells purified from cultured renal cells significantly improved renal function compared with unpurified cells in a rat model of CKD.11–14 Although these studies show that autologous cells obtained from CKD patients may be a viable therapeutic approach, the practicality of taking tissue biopsies from these patients is questionable.
Use of alternative cell sources, such as stem cells may be a preferable strategy to treat CKD. We previously demonstrated that amniotic fluid stem cells (AFSCs) share characteristics of both embryonic and adult stem cells. Unlike pluripotent and adult stem cells, AFSCs have low immunogenicity, lack of tumorigenicity, and high proliferative and anti-inflammatory characteristics.15,16 The use of AFSC does not lead to ethical concerns, unlike embryonic stem cells (ESC) or risks of teratoma formation as in the case of ESC and induced pluripotent stem (iPS) cells. Mesenchymal stem cells (MSCs) have been reported to regenerate cells in several renal compartments, including tubular cells, glomerular podocytes, and mesangial and endothelial cells,17,18 through transdifferentiation and secretion of trophic factors. However, they have low cell proliferation capacity.
Therefore, AFSC may be an alternative off-the-shelf cell source for CKD, which eliminates the need for tissue biopsy, cell isolation, and expansion. Several studies have shown the possibility of using AFSC for treating renal failure in AKI models.6 However, it is unknown whether these cells have therapeutic effects in CKD. Thus, this study aimed to examine the potential therapeutic effects of human-derived AFSC for treatment of CKD. We developed a rat model of CKD using a renal ischemia-reperfusion (I/R) method and examined whether AFSC could restore renal function and integrity of renal structures.
Materials and Methods
Human AFSC isolation and culture
We previously have developed techniques for isolation and expansion of AFSC.19,20 In brief, AFSCs were harvested from amniotic fluid procured from the first 2 mL of amniocentesis fluid before retrieving the test specimen. Cells were grown to ∼80% confluence, harvested by trypsinization, and subjected to c-kit selection. AFSCs at passage 13, same donor, were subcultured in a normal growth medium for amniotic cells: α-minimum essential medium supplemented with 15% ESC qualified fetal bovine serum, 18% Chang B (Irvine Scientific, Santa Ana, CA) and 2% Chang C (Irvine Scientific), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine. All cell culture reagents were obtained from Invitrogen (Carlsbad, CA) and used as received unless stated otherwise.
Growth curve and cell population doubling time
To demonstrate the proliferative capacity of AFSCs, P13 cells were plated in 12-well plates with a density of 50,000 cells per well. Cells from three wells were trypsinized daily and counted for 5 days to determine their growth kinetics. The population doubling time of cells was obtained according to the formula DT = T ln2/ln(Xe/Xb),21 where T is the incubation time, Xb is the cell number at the initiation of the culture, and Xe is the cell number at the end of the culture.
Characterization of AFSC: phenotypic profile and multidifferentiation
To characterize surface antigen profiles of AFSCs, flow cytometric analysis was performed. AFSCs at passage 13 were grown to ∼70% confluence, detached from culture dishes by 0.05% trypsin treatment (Hyclone, South Logan, UT), and washed with phosphate-buffered saline (PBS). For Oct4 immunostaining (R&D BioSciences, Inc., Minneapolis, MN), the cells were fixed with 4% paraformaldehyde in PBS for 10 min and then permeabilized by immersion in 1% Triton X-100 in PBS for 5 min. Assays for surface antigens were carried out with live cells without fixation.
Monoclonal antibodies were obtained from the sources indicated: primary antibodies against CD45, CD73, CD90, and CD105 (BD Biosciences Pharmingen, San Jose, CA) were diluted to the concentration recommended by the supplier (generally 1 μg/mL). FITC-goat antimouse IgG (Beckmann Coulter, Fullerton, CA) or AlexaFluor 488-goat antimouse IgG (Molecular Probes/Invitrogen, Eugene, OR), with a dilution of 1:100, was used as secondary antibody. Immunostained cells were analyzed according to standard protocols using an FACS Calibur analyzer (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Inc., Palo Alto, CA) or an Epics analyzer and CELL Quest software (Beckman Coulter, Brea, CA).
To determine multidifferentiation capability, osteogenic and adipogenic differentiation of AFSC was tested. Cells (0.3 × 106) were plated in a well that had a surface area of 9.5 cm2 and cultured for ∼4–10 days in growth medium until the cultures reached 90% confluency. The expanded cells were then cultured in (1) osteogenic medium (growth medium supplemented with 50 mM ascorbic acid, 0.1 mM dexamethasone, and 10 mM b-glycerolphosphate) and (2) adipogenic medium (growth medium supplemented with 1 mM dexamethasone, 5 mg/mL insulin, 100 mM indomethacin, and 500 mM isobutylmethylxanthine).14–16
All reagents for differentiation were purchased from Sigma-Aldrich (St. Louis, MO). After 2 weeks of culture in the differentiation media, the level of osteogenic and adipogenic differentiation was determined by staining for calcium deposits (Alizarin Red S, alkaline phosphate, or von Kossa) and lipid vacuoles (oil red O), respectively, followed by light microscopic imaging and analysis.22
Creation of renal ischemia model and cell injection
A renal ischemia model was created with minor modifications from our previous method.13 Male nude rats (Hsd:RH-Foxn1rnu) weighing 200–240 g were obtained at 12 weeks of age (Charles River Laboratories, Raleigh, NC). The rats were allowed at least 10 days to acclimate to their new environment before use in any experiment. Procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Wake Forest School of Medicine. Animals were anesthetized by an intraperitoneal injection of sodium pentobarbital (Nembutal; Ovation Pharmaceuticals, Inc., Deerfield, IL) at an initial dose of 50 mg/kg, and anesthesia was maintained using 25 mg/kg/h. The renal pedicle (artery and vein only) was then exposed on each side separately and obstructed for 60 min using a vascular clamp (microserrefine curved 6 mm; Fine Science Tools, Inc., Foster, CA) to induce ischemic injury. Clamps were gently removed, and reperfusion of the kidney was visually confirmed by a return to normal color.
At 1 week after I/R injury, gentamicin was administered for 5 days at 100 mg/kg to establish CKD. Twelve weeks after treatment, AFSCs suspended in PBS were injected (total of 5 × 106 cells per rat) into the upper and lower poles of the renal parenchyma of both kidneys. The cell concentration was chosen based on preliminary studies.23 The vehicle-only control group received bilateral intrarenal injections of PBS. The other group maintained as sham, age-matched control (AMC). Blood was collected for measurements of blood serum creatinine at 1 week postinjection and then every 2 weeks until the end of the study (10 weeks after cell delivery). Serum creatinine was measured using a Beckman Synchron CX5CE chemistry analyzer (Beckman Coulter, Inc.).
Characterization of the harvested kidney tissue: histological, immunohistological, and transmission electron microscopy analyses
At 10 weeks after cell injection (22 weeks after injury), rats were euthanized. Harvested kidney tissues were fixed in 10% paraformaldehyde solution and processed for paraffin embedding. Five micrometer sections were obtained and stained with hematoxylin and eosin and Masson's trichrome. To measure fibrosis, 12 images ( × 200 magnification) were taken from each kidney, and the percentage of blue area (fibrosis) in tissues stained with Masson's trichrome was determined with ImagePro software 6.3 (Media Cybernetics, Bethesda, MD).24
To evaluate the glomerular sclerosis and tubular injury, in a blinded manner, 12 random chosen images per slide were selected. To determine the level of glomerular damage, we measured basement membrane thickness (b), distance between the glomerulus and basement membrane (d), and the internal radius of Bowman's capsules (r) based on periodic acid-Schiff's base-staining ( × 400 magnification images) using ImageJ.
To determine the glomerular sclerosis each slide was assed based on the 0–3 scale: (0, normal; 1, mild [small area of glomerular abnormalities]; 2, moderate [<50% necrosis and crest formation]; 3, severe [>50%]).25 Estimation of tubular damage, the score based on the grading of tubular necrosis, loss of brush boarder, cast formation, and tubular dilation on 12 randomly selected samples per animal using the following scoring: 0 (none), 1 (≤10%), 2 (11–25%), 3 (26–45%), 4 (46–75%), and 5 (≥76%).26–28
For immunohistological analysis, paraffin sections were deparaffinized, antigen retrieved, protein blocked, and immunostained with antihuman leukocyte antigen (HLA) class 1 mouse monoclonal antibody (Ab70328) at a 1:500 dilution (Dako North America, Inc., Carpinteria, CA), and kidney injury molecule-1 (KIM-1) (R&D BioSciences, Inc.) at a 1:600 dilution. After incubation with the primary antibodies, tissue sections were incubated with biotin-conjugated secondary antibodies (1:300 dilution) (Vector Laboratories, Burlingame, CA) at room temperature for 30 min, followed by incubation with HRP-conjugated streptavidin (Vector Laboratories) for 30 min at room temperature and further developed with a DAB substrate kit (Vector Laboratories). KIM-1 quantification was performed by blinded observation, at least 10 fields (magnification, × 200) per section for each sample were examined. Images were taken using a light microscope (DM 400B; Leica, Wetzlar, Germany) with digital imaging software (Pro Express 6.3 software).
For transmission electron microscopy (TEM) analysis, kidney tissue was fixed in glutaraldehyde, dehydrated with ethanol, and then fixed in epoxy resin. Sections were stained with uranyl acetate and analyzed using a Philips TEM400 (Philips Eindhoven, Amsterdam, The Netherlands). Podocyte damage quantification was performed by observation, at least four fields (magnification, × 13,000) per section for each sample were examined.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (version 6.01). Results are presented as the mean ± standard deviation. Comparisons between the means in different groups were performed using one-way analysis of variance (ANOVA) followed by post hoc testing using Tukey's multiple comparison test. Differences were considered to be statistically significant when p < 0.05.
Results
Characterization of AFSC
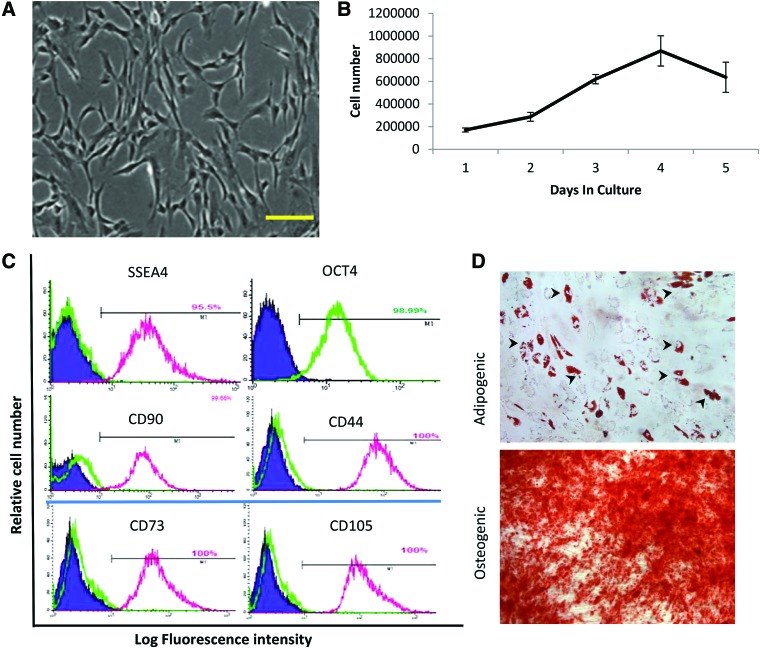
During cell culture, AFSC had a fibroblastic appearance (Fig. 1A). At passage 13, the cells showed high proliferative capability (Fig. 1B). The doubling time for AFSC was 25.83 h. Flow cytometry (Fig. 1C) showed high expression of MSC markers such as CD90, CD44, CD105, and CD73. The ESC markers Oct4 and SSEA4 also were expressed by most cells. In addition, AFSC exhibited the ability for adipogenic and osteogenic differentiation based on oil red O and Alizarin Red S staining, respectively (Fig. 1D).
FIG. 1.
Characterization of human AFSC. (A) Fibroblast-like cell morphology of AFSC at 48 h after seeding. Scale bar: 100 μm. (B) Cell growth curve of AFSC at P13. (C) Phenotypic characterization of AFSC by flow cytometric analysis. Representative histograms showing positive expression of SSEA4, Oct4, CD90, CD44, CD73, CD105 at 13th passage. The respective isotype control is shown as a green line, except for Oct4. (D) Multidifferentiation capability of AFSC showing adipogenic and osteogenic differentiation, revealed by formation of lipid droplets (arrowheads) and Alizarin Red staining, respectively. Scale bar: 100 μm. AFSC, amniotic fluid stem cell. Color images are available online.
The gross appearance and weight changes of the kidney
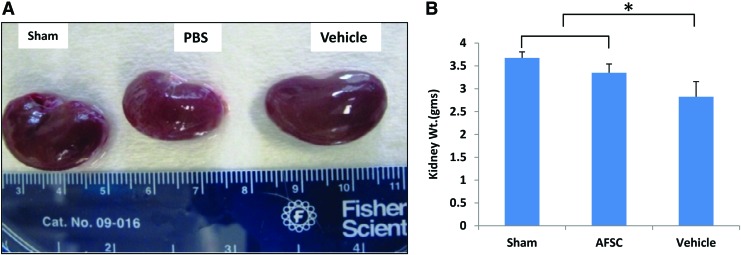
At 22 weeks after injury (10 weeks after treatment), harvested AFSC-treated kidneys showed notable differences in color and surface smoothness more or less similar to the sham kidneys, whereas vehicle kidneys had rough and irregular surfaces (Fig. 2A). The AFSC-treated kidneys (3.35 ± 0.19 g) were significantly higher in mass than vehicle (2.82 ± 0.33 g) with a statistically significant difference (p ≤ 0.01) (Fig. 2B).
FIG. 2.
(A) Harvested kidneys at 22 weeks from rats in one of the three treatment groups. (B) Quantitative comparison of kidney weights in each group. performed by one-way ANOVA followed by Tukey's comparison, p < 0.05, n = 3. Each data point is a mean ± SD of three values. *Statistical significance at p < 0.05 compared with PBS-treated (vehicle) group. ANOVA, analysis of variance; PBS, phosphate-buffered saline; SD, standard deviation. Color images are available online.
Restoration of renal function by AFSC transplantation
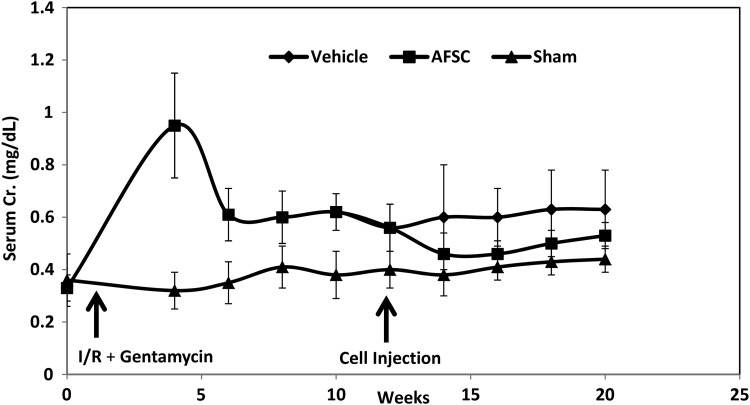
The CKD model was confirmed by an increase in serum creatinine levels for 12 weeks postinjury induction, whereas AMCs maintained low creatinine levels (Fig. 3). Infusion of AFSC facilitated decreased serum creatinine levels at 2 weeks after the cell injection (vehicle: 0.6 ± 0.1 mg/dL, AFSC: 0.46 ± 0.08 mg/dL, p ≤ 0.14, n = 4) with a trend toward improved renal function. The reduction in serum creatinine was maintained up to 10 weeks postcell injection (vehicle: 0.66 ± 0.2 mg/dL, AFSC: 0.53 ± 0.2 mg/dL, p ≤ 0.34, n = 4).
FIG. 3.
Effects of AFSC treatment on CKD. At 12 weeks after I/R and gentamicin treatment, AFSCs were intrarenally injected. At 2 weeks after cell injection, serum creatinine (Cr.) level decreased and was maintained up to 10 weeks compared with vehicle-only group. Age-matched control (sham) rats did not have surgery. n = 4 animals per group. CKD, chronic kidney disease; I/R, ischemia/reperfusion.
Structural recovery by AFSC infusion
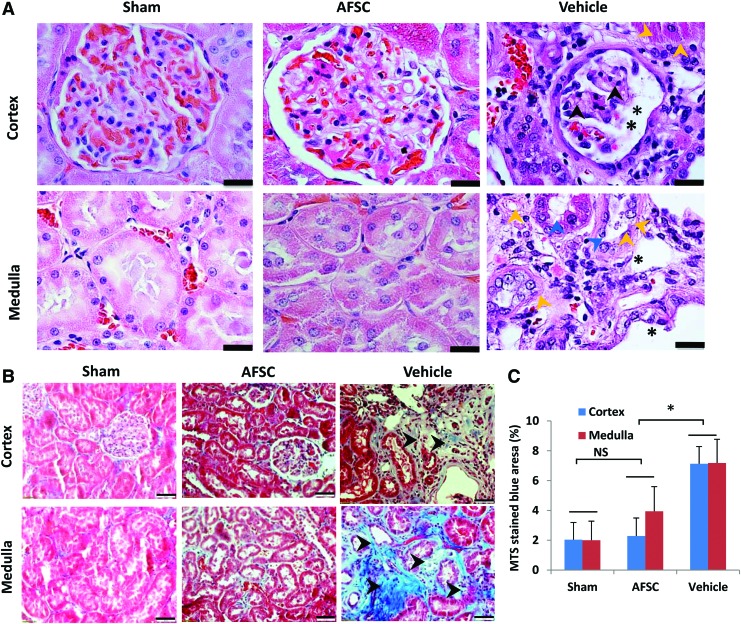
Histological analyses revealed kidney injury, including tubular damage, in control rats (Fig. 4A), whereas AFSC-injected kidneys showed normal tubular vascular structure similar to sham groups. Notably, control tissues showed a fibrotic cortex in medullae, with sclerotic and shrunken glomeruli with widening of Bowman's space in the cortex. In addition, thickened arteries, and dilated tubules were observed on the medulla. However, pathological complications were not found in AFSC-treated kidneys, which were similar to those in the normal kidney (sham).
FIG. 4.
Structural recovery of CKD by AFSC treatment. (A) Glomerular and tubular structures were restored in the AFSC group in the cortex and medulla areas, whereas vehicle tissues show fibrotic cortex (yellow arrowheads). In vehicle group, medullae show loss of brush border in tubules (blue arrowheads) and tubular dilation (black asterisk). Hematoxylin and eosin staining. Scale bar: 20 μm. (B) Masson's trichrome staining: Fibrotic areas (blue color) in the tubulointerstitial and perivascular areas of the renal cortex and medulla appear more prominent (black arrowhead), in vehicle-only group compared with AFSC and sham groups. Scale bar: 50 μm. (C) Quantitative analyses of fibrosis in the cortex and medullary regions of kidney. Collagen deposition (black arrowheads) on vehicle cortex and medulla of kidney. Each data point is a mean ± SD of 12 values.*Statistical significance (p < 0.01) compared with PBS-treated animals. Color images are available online.
In the vehicle group, interstitial fibrosis as indicated by collagen staining (blue) appears to be significantly different from the AFSC-treated groups in both the cortex and medulla (Fig. 4B). Quantitatively, the collagen deposition in cortex and medulla in the AFSC group was significantly lower than in the vehicle group (7.13% ± 1.5% and 2.29% ± 1.2% in cortex region, p < 0.01 and the medulla 7.18% ± 1.5% and 3.94% ± 1.64%, p < 0.01), and no significant difference was observed in AFSCs versus sham for both cortex and medulla (Fig. 4C).
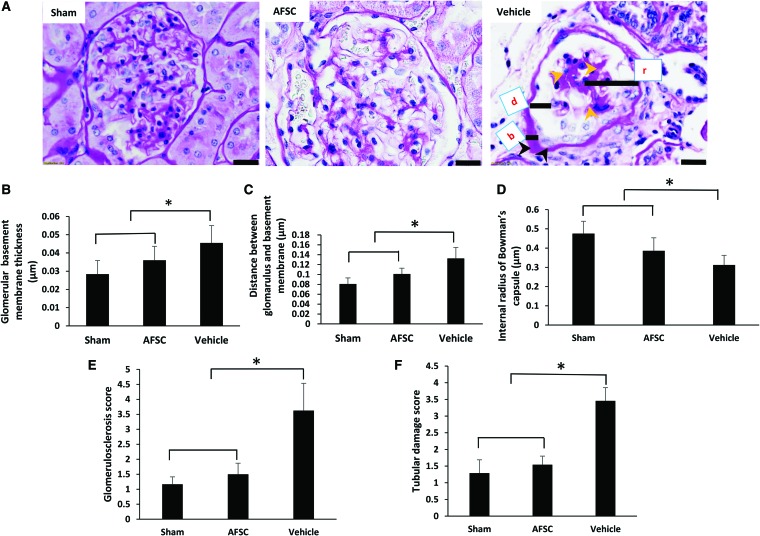
In the vehicle kidneys, severe sclerotic glomeruli filled with a pink cast had the appearance of thyroidization and tubular atrophy compared with AFSC-injected rats (Fig. 5A). Glomerular damage is one of the important pathological phenomena in the diseased kidney, where irregular and thick glomerular basement membrane is primarily observed.30 Our results indicated that the thickness of the basement membrane was comparatively higher in the control group than the AFSC-injected group (p < 0.01) (Fig. 5B). The distance between glomerulus and basement membrane was not significant difference comparatively between vehicle versus AFSC and sham versus AFSC-injected group (Fig. 5C). The internal radius of the Bowman's capsular space also was statistically different between the AFSC- and vehicle kidneys (p < 0.01), similarly sham versus AFSC groups showed significant difference of the Bowman's capsular space (p < 0.01) (Fig. 5D). The quantification of glomerular sclerosis score (Fig. 5E) was significantly higher in vehicle animals than in cells induced animals (3.6 ± 0.90 and 1.5 ± 0.36) (p < 0.01). The tubular damage (Fig. 5F) was significantly higher in PBS-treated animals than in AFSC received animals (3.45 ± 0.39 and 1.54 ± 0.25) (p < 0.01).
FIG. 5.
(A) AFSC-injected kidneys showed fine interstitium in the cortex area and thin basement membrane in the glomerulus, similar to the sham group. However, vehicle group kidneys contained sclerotic glomerulus (yellow arrowhead) and a thick basement membrane (black arrowheads). (B) Glomerular damage was quantified by basement membrane thickness (b). (C) Distance between the glomerulus and the basement membrane (d). (D) Internal radius of Bowman's capsule (r). (E) Quantitative analysis of glomerulosclerosis. (F) Quantitative analysis of tubular damage score. Each data point is a mean ± SD of 12 values. *Statistical significance (p < 0.01) compared with PBS-treated group. Periodic acid-Schiff's staining. Scale bar = 20 μm. Color images are available online.
To examine the levels of tubular damage in the CKD and effects of AFSC injection, expression of KIM-1,31 a kidney injury biomarker, was quantified and compared between the groups. KIM-1 tubular expression was higher in the vehicle kidneys than in the AFSC-injected group (14.0 ± 3.65 and 3.75 ± 1.70) (p < 0.01) and versus AFSC (1.75 ± 0.95 and 3.75 ± 1.7) (Supplementary Fig. S1A, B).
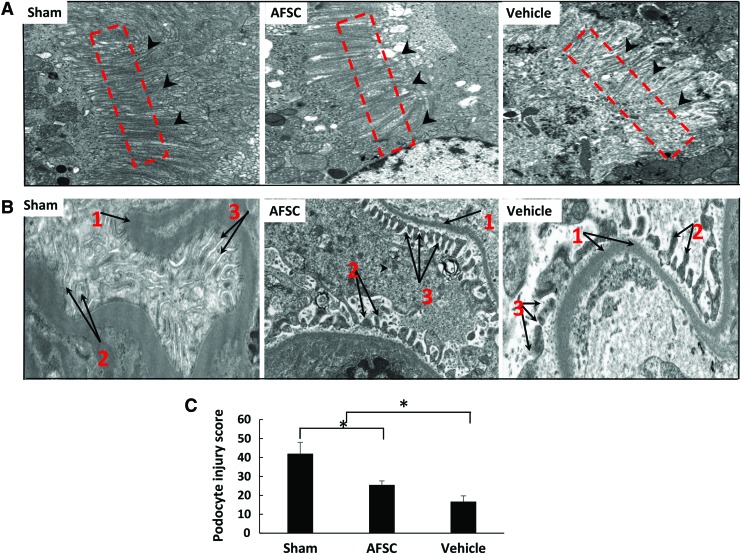
TEM results demonstrate that brush-border microvilli (BBMV) (Fig. 6, red square area) in the AFSC groups appear to be organized (similar to the sham group), whereas control rats showed loss of BBMV (Fig. 6A).32 Severe damage was observed in the glomerular region of vehicle kidneys, loss of pedicles of podocytes (Fig. 6B, 1), and irregularly shaped diaphragms between the pedicles (Fig. 6B, 2). AFSC-treated and sham kidneys showed similar morphologies (Fig. 6A, B). The alteration of podocyte was quantified; vehicle group shows significant damage than treated group kidneys (16.66 ± 3.50 and 25.5 ± 2.12) (p < 0.01) (Fig. 6C).
FIG. 6.
Ultrastructural analysis of tubular and podocyte structure by transmission electron microscopy. (A) AFSC-infused proximal tubular BBMV shows regeneration efficiency and less damage compared with vehicle group kidneys. In vehicle group tissues show an irregular and poorly developed BBMV. Magnification: 6800 × . (B) Image of glomerulus in vehicle group kidneys, the pedicle of podocyte (1) irregular and loss most of the areas, the diaphragm between the pedicles of podocytes (2) and more cellular damage when compared with AFSC-infused kidney. (3) Distance between the glomerulus and the basement membrane. AFSC-injected kidney shows less damage on pedicles of podocytes, and diaphragm between the pedicles of podocytes is normal. Magnification: 13,000 × . Quantification of podocyte injury score: Podocytes show significantly higher degree of damage in control (vehicle) group compared with AFSC-induced kidney. (C) Each data point is a mean ± SD of four values. *Statistical significance (p < 0.01) compared with vehicle group. BBMV, brush-border microvilli. Color images are available online.
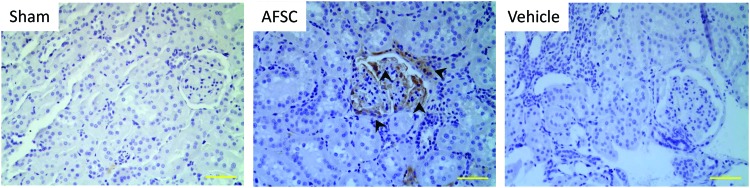
To track infused AFSC within the kidney, tissue sections were immunostained with HLA antibody. AFSCs appeared to engraft into kidneys, and most cells that were positively stained were distributed throughout the glomerular area of the kidney by 10 weeks postinjection (Fig. 7).
FIG. 7.
Tracking of injected AFSC in kidneys. Engrafted AFSC were examined by immunostaining using an antibody against human leukocyte antigen, indicating that the infused cells were recruited into the glomerular areas (arrowheads). In contrast, no positive HLA staining was detected in vehicle group kidneys. Scale bar: 100 μm. Color images are available online.
Discussion
CKD is a major public health problem, which often leads to ESRD.33–35 Although dialysis and kidney transplantation have been used as primary treatments for renal diseases, cell therapy offers a promising alternative treatment strategy.11,13,36,37 As a cell source, AFSC displays important benefits, including pluripotential activity, remarkable plasticity, and immunomodulatory effects.38 This study examined the therapeutic effects of AFSC on the structural and functional improvement of CKD in a rat model. AFSC injection led to a modest improvement of renal function (based on creatinine levels) along with histological findings that AFSC-injected rats showed reduced damage to the glomeruli and improved tubular morphology compared with controls. Our results indicate that AFSC, as an “off-the-shelf” source, may provide an alternative therapeutic strategy to treat patients with CKD.
Various renal failure models have been used to examine therapeutic effects of cell therapy on improving renal function. Previous approaches have demonstrated that AFSC improved renal function in AKI.6,9,19 Huang et al.29 report that treatment with peripheral blood-derived endothelial progenitor cells effectively reduced the progression of CKD, improved angiogenesis, blood flow, and reduced inflammation and fibrosis in a rodent model. Vascular endothelial growth factor (VEGF) AFSCs shows higher protection than amniotic stem cells.39 Our study sought to examine the effect of AFSC in a CKD model since AKI and CKD display distinct renal pathology.40 In an AKI model, tubular damage initially occurs with capillary damage, followed by tubular regeneration and angiogenesis for renal recovery.40 Therefore, most work has examined the effects of administered cells on the amelioration of acute injury. For instance Hauser et al.9 demonstrated that renal function recovered with AFSC administration after glycerol-induced acute injury in mice, and supported functional improvement by preventing apoptosis of tubular cells and inducing proliferation of renal cells. In contrast, CKD shows features such as glomerular and tubular damage, glomerulosclerosis, and fibrosis progression.40
The CKD model our group developed shows pathological changes similar to those in patients with CKD.13 Our results using the CKD model demonstrate that AFSC treatment trended for improved renal function (based on creatinine level) and significant structural recovery. In particular, recovery of glomeruli (Figs. 4A and 5A) and tubular damage (Fig. 4A and Supplementary Fig. S1) and attenuated fibrosis (Fig. 4B, C) were achieved by AFSC administration. These results demonstrate that AFSC treatment has positive effects on functional improvement and structural recovery from CKD, as observed in AKI models in other studies.6,9
Although the mode of action of the AFSC in the injured kidney is still not clear, several mechanisms are possible. First, cells exogenously injected into the kidney may contribute to renal function through a transdifferentiation process, where the engrafted cells differentiate into renal lineages for kidney recovery. Rota et al. reported that infusion of AFSC in mice improved renal function, limited tubular damage, and prolonged survival; injected cells were localized in the tubular area and transdifferentiated into renal cells.38,41 Other studies demonstrated that bone marrow-derived MSCs and AFSC can undergo transdifferentiation into tubular epithelial cells and glomerular-type cells.42,43
Similarly, our histological results also demonstrated that injected AFSCs were recruited into the injury site, particularly the glomerulus region. However, engrafting efficiency appears to be low, and engrafted AFSCs were observed in different sites within the glomeruli. This low efficiency of cell engrafting may correlate with low functional improvement (creatinine level). The human origin of AFSC was confirmed using HLA class I antigen staining and were predominantly observed in the glomerular region.9,33 Stem cell integration into the parenchymal region after the transplantation shows the nephro-protective effect mediated by paracrine mechanism.44,45 It remains to be seen whether injecting more cells or more efficient engraftment of the infused cells enhances improvement of renal function.
Paracrine actions on the regenerative capability of growth factors and cytokines (secreted from injected cells) are another possible explanation for our results. Lin et al.46 reported that host renal cells, not injected stem cells, contributed to regeneration of renal structure after renal I/R injury. This finding suggests that restoration of kidney function benefits more from endogenous renal regeneration, possibly through beneficial effects of trophic factors from injected cells, than from transdifferentiation of engrafted cells.
Recent studies have focused on the roles of cell-derived trophic factors in kidney regeneration. Conditioned medium derived from cells, including MSCs and iPS,47 reportedly improved renal function. Beneficial effects of soluble factors on kidney repair were attributed to protection of renal tubules and blood vessels, which facilitated tubulogenesis and angiogenesis.
Among the secreted factors, VEGF and hepatocyte growth factor (HGF) secretion from AFSCs improve renal function through vascularization30,48 and tubular protection from necrosis,49 respectively. The protective effects of MSC transplantation on renal endothelium on ischemia-injured porcine model show the proangiogenic pathways involvement in tissue regeneration.50 Our previous study51 reported that conditioned medium from AFSC contained VEGF and HGF. Attenuation of fibrosis is also an important parameter to evaluate restoration of kidney function in CKD. Another study showed that leukocyte inhibitory factors (LIF) played a crucial role in reducing fibrosis in the AKI model.52 Since Hauser et al.9 demonstrated that AFSC-conditioned medium contained LIF, which49 may explain the antifibrosis outcomes. Thus, the beneficial effects of infused AFSCs on kidney function may be due to trophic factors, including VEGF, HGF, and possibly LIF from the infused cells.
Although a few studies reported that treatment with trophic factors secreted from stem cells is a promising therapy for kidney disease,53 its effectiveness still remains controversial. Some studies have stated that the conditioned medium cannot improve renal function.54 This discrepancy may be due to use of different renal injury models, delivery routes, and doses of the conditioned medium.54 Human amniotic stem cells can be used as an off-the-shelf universal cell source as they have differentiation potential toward different cell types and immunomodulatory properties that make them a potential source for regeneration. However, stem cell delivery needs to be optimized to efficiently use such therapy for clinical purposes. Also, the therapeutic dose of the factors is yet to be determined.
Conclusion
Intrarenal delivery of AFSC promoted recovery from CKD in a rat model, based on assessments of functional and structural aspects. The ability of AFSC to attenuate CKD is possibly due to their migration to sites of injury and differentiation into adult renal cells, or to paracrine effects by trophic factors released from the injected cells. Further studies are required for the optimization of cell number, multiple injection, and molecular mechanism of renal protection. These results suggest that AFSCs should be further explored as a potential clinical treatment option for patients with CKD.
Supplementary Material
Acknowledgments
This study was supported, in part, by the State of North Carolina and the Wake Forest Institute for Regenerative Medicine. We thank Jennifer Huling, Cathy Mathis, Adam Wilson, Tiffaney Bledsoe, Cara Clouse, Cynthia V. Horne and Tsghe Abraha for technical assistance. We also thank Karen Klein, MA, in the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: McClain) for editorial assistance.
Authors' Contributions
Conceptualization and methodology was by S.K.G. and J.J.Y. Animal surgery was performed by S.K.G., M.A., T.H.K., and C.Z. Validation was done by S.J.L., J.D.J., J.A., I.K.K., A.A., and J.J.Y. Formal analysis was performed by S.K.G., I.K.K., and J.D.J. Investigation was by S.K.G., A.A., and J.J.Y. Data curation and writing (original draft preparation) was by S.K.G. Writing (review and editing) was by S.K.G., I.K.K., J.D.J., J.A., A.A., and J.J.Y. A.A. and J.J.Y. were in charge of supervision and project management.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Thomas R., Kanso A., and Sedor J.R. Chronic kidney disease and its complications. Prim Care 35, 329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2014 National Chronic Kidney Disease Fact Sheet. National Center for Chronic Disease Prevention and Health Promotion, 2014. https://www.chronicdisease.org/page/ChronicKidneyDisease
- 3. Collins A.J., Foley R.N., Chavers B., et al. US renal data system 2013 annual data report. Am J Kidney Dis 63, A7, 2014 [DOI] [PubMed] [Google Scholar]
- 4. Coresh J., Selvin E., Stevens L.A., et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038, 2007 [DOI] [PubMed] [Google Scholar]
- 5. van Koppen A., Joles J.A., van Balkom B.W., et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One 7, e38746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rota C., Imberti B., Pozzobon M., et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev 21, 1911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eirin A., and Lerman L.O. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther 5, 83, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung H.C., Ko I.K., Atala A., and Yoo J.J. Cell-based therapy for kidney disease. Korean J Urol 56, 412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hauser P.V., De Fazio R., Bruno S., et al. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 177, 2011, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg L.J., Teng Y.D., and Wrathall J.R. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci 19, 6122, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guimaraes-Souza N.K., Yamaleyeva L.M., AbouShwareb T., Atala A., and Yoo J.J. In vitro reconstitution of human kidney structures for renal cell therapy. Nephrol Dial Transplant 27, 3082, 2012 [DOI] [PubMed] [Google Scholar]
- 12. George S.K., Abolbashari M., Jackson J.D., Aboushwareb T., Atala A., and Yoo J.J. Potential use of autologous renal cells from diseased kidneys for the treatment of renal failure. PLoS One 11, e0164997, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamaleyeva L.M., Guimaraes-Souza N.K., Krane L.S., et al. Cell therapy with human renal cell cultures containing erythropoietin-positive cells improves chronic kidney injury. Stem Cells Transl Med 1, 373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gyabaah K., Aboushwareb T., Guimaraes Souza N., et al. Controlled regulation of erythropoietin by primary cultured renal cells for renal failure induced anemia. J Urol 188, 2000, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Yu S.J., Soncini M., Kaneko Y., Hess D.C., Parolini O., and Borlongan C.V. Amnion: a potent graft source for cell therapy in stroke. Cell Transplant 18, 111, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kaneko Y., Hayashi T., Yu S., et al. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: a new perspective to neuroprotection. J Pineal Res 50, 272, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Poulsom R., Forbes S.J., Hodivala-Dilke K., et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195, 229, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Li B., Morioka T., Uchiyama M., and Oite T. Bone marrow cell infusion ameliorates progressive glomerulosclerosis in an experimental rat model. Kidney Int 69, 323, 2006 [DOI] [PubMed] [Google Scholar]
- 19. De Coppi P., Bartsch G., Jr., Siddiqui M.M., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25, 100, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kim J., Jeong S.Y., Ju Y.M., et al. In vitro osteogenic differentiation of human amniotic fluid-derived stem cells on a poly (lactide-co-glycolide)(PLGA)–bladder submucosa matrix (BSM) composite scaffold for bone tissue engineering. Biomed Mater 8, 014107, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Janat-Amsbury M.M., Yockman J.W., Anderson M.L., Kieback D.G., and Kim S.W. Comparison of ID8 MOSE and VEGF-modified ID8 cell lines in an immunocompetent animal model for human ovarian cancer. Anticancer Res 26, 2785, 2006 [PubMed] [Google Scholar]
- 22. Wall M.E., Bernacki S.H., and Loboa E.G. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng 13, 1291, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kelley R., Werdin E.S., Bruce A.T., et al. Tubular cell-enriched subpopulation of primary renal cells improves survival and augments kidney function in rodent model of chronic kidney disease. Am J Physiol Renal Physiol 299, F1026, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Zeisberg E.M., Potenta S.E., Sugimoto H., Zeisberg M., and Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19, 2282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheerin N.S., Abe K., Risley P., and Sacks S.H. Accumulation of immune complexes in glomerular disease is independent of locally synthesized C3. J Am Soc Nephrol 17, 686, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Prophet E.B., and Hardman J.M. Rapid preparation of histologic sections of the whole brain. Tech Bull Regist Med Technol 39, 260, 1969 [PubMed] [Google Scholar]
- 27. Ding J.D., Kelly U., Landowski M., et al. Expression of human complement factor H prevents age-related macular degeneration-like retina damage and kidney abnormalities in aged Cfh knockout mice. Am J Pathol 185, 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pickering M.C., Cook H.T., Warren J., et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31, 424, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Huang T.H., Chen Y.T., Sung P.H., et al. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res 7, 804, 2015 [PMC free article] [PubMed] [Google Scholar]
- 30. Sedrakyan S., Da Sacco S., Milanesi A., et al. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J Am Soc Nephrol 23, 661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ichimura T., Bonventre J.V., Bailly V., et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273, 4135, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Ishida T., Kotani H., Miyao M., et al. Renal impairment with sublethal tubular cell injury in a chronic liver disease mouse model. PLoS One 11, e0146871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun D., Bu L., Liu C., et al. Therapeutic effects of human amniotic fluid-derived stem cells on renal interstitial fibrosis in a murine model of unilateral ureteral obstruction. PLoS One 8, e65042, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiffrin E.L., Lipman M.L., and Mann J.F. Chronic kidney disease: effects on the cardiovascular system. Circulation 116, 85, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Abramson J.L., Jurkovitz C.T., Vaccarino V., Weintraub W.S., and McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int 64, 610, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Atala A. Tissue engineering for the replacement of organ function in the genitourinary system. Am J Transplant 4 Suppl 6, 58, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Bonventre J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14 Suppl 1, S55, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Rota C., Imberti B., Pozzobon M., et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev 21, 1911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mori da Cunha M.G., Zia S., Beckmann D.V., et al. Vascular endothelial growth factor up-regulation in human amniotic fluid stem cell enhances nephroprotection after ischemia-reperfusion injury in the rat. Crit Care Med 45, e86, 2017 [DOI] [PubMed] [Google Scholar]
- 40. Venkatachalam M.A., Weinberg J.M., Kriz W., and Bidani A.K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26, 1765, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morigi M., Introna M., Imberti B., et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem cells 26, 2075, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Da Sacco S., Lemley K.V., Sedrakyan S., et al. A novel source of cultured podocytes. PLoS One 8, e81812, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Togel F., Hu Z., Weiss K., Isaac J., Lange C., and Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289, F31, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Perin L., Giuliani S., Jin D., et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif 40, 936, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leonard E.C., Friedrich J.L., and Basile D.P. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295, F1648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin F., Moran A., and Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115, 1756, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tarng D.C., Tseng W.C., Lee P.Y., Chiou S.H., and Hsieh S.L. Induced pluripotent stem cell-derived conditioned medium attenuates acute kidney injury by downregulating the oxidative stress-related pathway in ischemia-reperfusion rats. Cell Transplant 25, 517, 2016 [DOI] [PubMed] [Google Scholar]
- 48. Togel F., Weiss K., Yang Y., Hu Z., Zhang P., and Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292, F1626, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Chen Y., Qian H., Zhu W., et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev 20, 103, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Baulier E., Favreau F., Le Corf A., et al. Amniotic fluid-derived mesenchymal stem cells prevent fibrosis and preserve renal function in a preclinical porcine model of kidney transplantation. Stem Cells Transl Med 3, 809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skardal A., Mack D., Kapetanovic E., et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1, 792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu Y., Wang Y., Niu Y., et al. Leukemia inhibitory factor attenuates renal fibrosis through Stat3-miR-29c. Am J Physiol Renal Physiol 309, F595, 2015 [DOI] [PubMed] [Google Scholar]
- 53. Bi B., Schmitt R., Israilova M., Nishio H., and Cantley L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18, 2486, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Xing L., Cui R., Peng L., et al. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther 5, 101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.