Abstract
The recent years saw the advent of promising preclinical strategies that combat the devastating effects of a spinal cord injury (SCI) that are progressing towards clinical trials. However, individually, these treatments produce only modest levels of recovery in animal models of SCI that could hamper their implementation into therapeutic strategies in spinal cord injured humans. Combinational strategies have demonstrated greater beneficial outcomes than their individual components alone by addressing multiple aspects of SCI pathology. Clinical trial designs in the future will eventually also need to align with this notion. The scenario will become increasingly complex as this happens and conversations between basic researchers and clinicians are required to ensure accurate study designs and functional readouts.
Keywords: axon regeneration, clinical trials, combination treatments, reproducibility, spinal cord injury
Subject Categories: Neuroscience, Chemical Biology
This review is comprehensive and timely, and provides to the readers a balanced view of research and clinical findings in spinal cord injury.

Glossary
- Allodynia
A type of neuropathic pain that is the result of increased sensitivity to otherwise innocuous stimuli
- Anterograde tracing
This is a research method which is used to trace axonal projections in the direction of their cell bodies towards their point of termination. The complimentary technique is known as retrograde tracing
- Autologous (transplant)
A transplant is autologous if the recipient also serves as the donor
- Basso, Beattie and Bresnahan (BBB) locomotor rating scale
A 21‐point behavioural analysis scale that is used in spinal cord injury research to assess limb motor skills over a wide range of injury severities
- Chondroitin sulphate proteoglycans (CSPGs)
Components of the extracellular matrix and are composed of a protein core covalently linked to chondroitin sulphate glycosaminoglycan sidechains. CSPGs are known to inhibit axon regeneration
- Chondroitinase ABC (ChABC)
Chondroitinase ABC is a bacterial enzyme that degrades chondroitin sulphate
- Conditioning lesion
These involve lesioning the peripheral branch of adult sensory dorsal root ganglion neurons prior to a central lesion. This increases the regenerative capacity of the central branch of DRG neurons
- Contusion
An injury caused by blunt trauma to the spinal cord
- Corticospinal tract (CST)
One of the most important descending tracts. It controls primary motor activity from the motor cortex
- Dorsal root ganglion (DRG)
A cluster of neurons in dorsal spinal root of a spinal nerve. DRG neurons are pseudo‐unipolar and have a central and peripheral branch
- Excitotoxicity
A pathological process by which neurons are damaged or killed through overstimulation of excitatory neurotransmitter receptors
- Functional electrical stimulation (FES)
FES involves electrophysiological stimulation of spinal cord or peripheral nerves or muscle
- Growth cone
Is the large actin supported extension at the tip of a developing or regenerating neurite/axon
- Induced pluripotent stem cells (iPSCs)
Are pluripotent stem cells that are generated directly from adult cells through the expression of specific transcription factors
- Kinematic analysis
The process of measuring the movement (kinematic) quantities used to describe motion
- Myelin‐associated inhibitors
Components or CNS myelin that are known to be inhibitors of axon growth and regeneration. The three classic myelin‐associated inhibitors are Nogo‐A, myelin‐associated glycoprotein and oligodendrocyte myelin glycoprotein
- Neuroplasticity
Refers to the adaptive changes in neurological function. This can be the result of anatomical or physiological changes
- Neuroprotection
Refers to the relative preservation of neural tissue during ongoing secondary damage
- Neurorehabilitation
Physical therapy that aims to aid recovery of nervous system injury and/or improve compensatory functions
- Neurotrophins
A family of regulatory factors that mediate the survival, differentiation and growth of neurons
- Phase I clinical trial
A clinical trial that includes a small number of human participants to determine the safety of a new drug or invasive medical device; allows the determination of drug dosage or toxicity limits
- Phase II clinical trial
A clinical trial that includes a larger number of human participants than a Phase I trial and is intended to evaluate the efficacy of a treatment; side effects are also monitored
- Propriospinal neuron
These are neurons contained entirely within the spinal cord that interconnect various levels of the cord
- Reticulospinal tract
A descending tract that descends from the reticular formation and is primarily responsible for locomotion and postural control
- Retraction bulb
Dystrophic axonal structures which are a hallmark of a failed growth response
- Transection
A surgical injury created by a fine transverse cut of the spinal cord
Introduction
Through silver staining neurons, Ramon y Cajal discovered that peripheral nervous system (PNS) neurons regenerate after injury, contrasting the minor regenerative response of central spinal cord neurons (Ramon y Cajal, 1928). This then prompted the question: Do central nervous system (CNS) neurons lack the intrinsic capability of regeneration or are there extrinsic factors that influence this dichotomous observation? In actuality, both of these factors come into play when spinal cord neurons are tasked with regenerating after axotomy. Several families of molecules present in the extracellular matrix (ECM) prevent axon growth including chondroitin sulphate proteoglycans (CSPGs), myelin‐associated molecules, ephrins and semaphorins (Miranda et al, 1999; Chen et al, 2000; Willson et al, 2002; Silver & Miller, 2004; Geoffroy & Zheng, 2014; Worzfeld & Offermanns, 2014). Yet even when provided with a growth‐permissive environment, central neurons regenerate feebly compared to their peripheral or immature CNS counterparts, indicating that they also have intrinsic growth limiting factors (Hilton & Bradke, 2017). On the brighter side and nearly 100 years on from Cajal's statement that “in adult centres, the nerve paths are something fixed, ended, immutable; everything may die, nothing may be regenerated” (Ramon‐Cueto et al, 1998), we now know this statement to be not entirely true. Many research groups have reported axon regeneration and functional recovery after experimental spinal cord injury (SCI) following a variety of treatments (Thuret et al, 2006). While this is certainly a feat, many of the experimental approaches are problematic for clinical translation. For those that are, it is also becoming increasingly apparent that addressing singular aspects of the problem won't facilitate successful and functional regeneration after SCI in humans. Conversely, it will likely require the combination of various treatment strategies that address the variety of problems that result after SCI. Many attempts have been made, to varying degrees of success, that combine tissue replacement, removal of inhibitory molecules, supplying neurotrophic factors, manipulation of pro‐regenerative neuronal signalling pathways and neurorehabilitation. This review will provide an update to the many therapeutic interventions after SCI and then will focus on the attempted combinatory approaches, how they could be improved and the road to their clinical translation.
Therapeutic interventions
SCI induces complex processes. SCI first leads to death of cells in the CNS, including neurons, astrocytes, microglia, oligodendrocytes and endothelial cells. In particular, the damage to long axonal projections leads to interruption of descending and ascending pathways that transmit information between the brain and the rest of the body. Secondary damage from vascular changes, acute injury signalling, neuroinflammation, excitotoxicity, demyelination, degeneration, astrogliosis and ECM remodelling exacerbates the initial pathology (Hilton et al, 2017; Bradbury & Burnside, 2019). This unfolds as a temporal cascade of complex biological processes that can last months to years after the injury (Buss et al, 2004; Norenberg et al, 2004; Donnelly & Popovich, 2008). Some degree of spontaneous recovery is observed in experimental animal models and to a lesser extent in humans (Curt et al, 2008; Hilton et al, 2016). However, endogenous repair mechanisms are minor and recovery remains incomplete (Fawcett et al, 2007; Courtine et al, 2008). Based on the pathologies that result from SCI, researchers have identified several targets for the development of potential therapeutic interventions. These can be referred to as the “7 R's” (Fig 1).
Figure 1. The seven targets for therapeutic interventions following spinal cord injury.
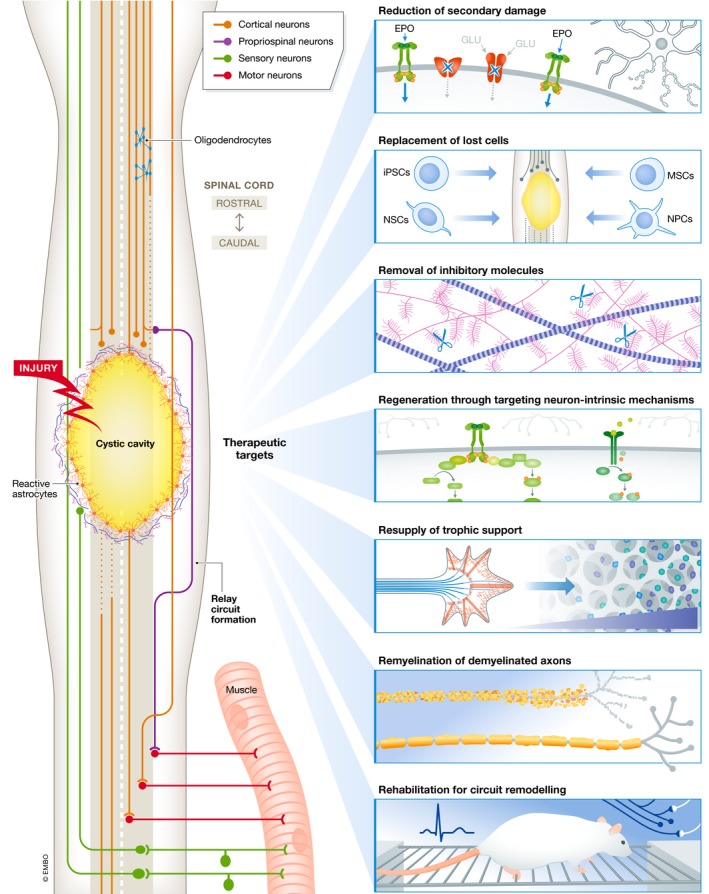
A horizontal plane view through a region of thoracic spinal cord injury depicting some of the features of the pathology. SCI leads to immediate and continued death of neural alongside disruption of descending and ascending fibres. Seven therapeutic targets are present which can improve functional recovery after SCI: neuroprotective strategies to limit ongoing secondary damage resulting in spared tissue; tissue and cellular transplants to replace lost cells and may provide trophic or growth‐permissive environments; removal of inhibitory factors such as CSPGs to allow for enhanced axonal growth; targeting neuron‐intrinsic mechanisms to enhance intrinsic regenerative response which could then be directed through the resupply of trophic support; and remyelination of demyelinated axons may improve axonal conduction. Finally, rehabilitation to function in circuit remodelling and strengthens beneficial connections.
-
1
Reduction of secondary damage (neuroprotection).
-
2
Replacement of cells lost to primary and secondary damage.
-
3
Removal of inhibitory molecules.
-
4
Regeneration to enhance the spontaneous reparative and regenerative responses.
-
5
Resupply of neurotrophic support to improve neuronal survival and direct axonal growth.
-
6
Remyelination of regenerated, replaced or spared (demyelinated) axons.
-
7
Rehabilitation strategies to induce neuroplasticity and/or to shape neuronal connections.
We will discuss these targets and their respective interventions in the following sections.
Reduction: neuroprotection
Substantial efforts have been made to limit secondary damage. Pharmacological agents that suppress the immune system or inhibit key signalling pathways involved in inflammation were the first major strategies applied to patients. These include the non‐steroidal anti‐inflammatory drugs (NSAIDs), minocycline, cyclosporine A and the corticosteroid methylprednisolone (Badhiwala et al, 2018). The use of methylprednisolone remains controversial since clinical trials revealed that it may have no effect or even lead to complications (Bowers et al, 2016). Preclinically, indomethacin (an NSAID) led to tissue sparing and slight functional recovery (Simpson et al, 1991), while minocycline treatment reduced oligodendrocyte as well as neuronal death that improved outcome after cervical spinal cord injury in rats (Stirling et al, 2004). However, preclinical reassessment of indomethacin suggested treatment may be harmful in human SCI, and a phase II clinical trial of minocycline for acute SCI did not report beneficial effects (Guven et al, 1999; Casha et al, 2012). Similar conflicting results have been reported for the immune suppressant cyclosporine A (Chen et al, 2018). Since neuroinflammation has both beneficial and detrimental effects, broad‐spectrum suppression of inflammation may not be efficacious. Neuroprotection can also be achieved through preventing glutamate excitotoxicity by blockade of NMDA receptors by magnesium (Ditor et al, 2007) or gacyclidine (Feldblum et al, 2000), blockade of tetrodotoxin‐sensitive sodium channels using riluzole (Satkunendrarajah et al, 2016), preventing apoptosis using erythropoietin (Baptiste & Fehlings, 2006), inhibition of connexin hemichannels using a mimetic peptide (Mao et al, 2017), mild to moderate hypothermia (Dietrich et al, 2009) and many more strategies (Baptiste & Fehlings, 2006; Thuret et al, 2006). These more discrete manipulations of secondary processes may prove to have greater therapeutic benefit than the broad approaches. Regardless, neuroprotective agents have failed to translate to the clinic. Stroke has a similar neuropathology to SCI, and huge efforts, particularly in the 1990s, were made to develop neuroprotective treatments for stroke. In such attempts, over 1,000 potential therapeutic agents, leading to nearly 200 clinical trials, have resulted in no successful treatments (Minnerup et al, 2012). Worryingly, clinical trials assessing neuroprotective agents for SCI may also reflect this trend. Overall, the preclinical and clinical progression of neuroprotective strategies has often stalled, and the mechanisms by which neuroprotection is conferred is not well understood. Improvements in our understanding as to the mechanisms which underpin the benefits of neuroprotective strategies could better inform their application.
Replacement: cellular transplantation
Cell transplantation for the aim of replacing lost cells has multiple historic origins stretching back to experiments conducted in the laboratory of Ramon y Cajal (Ramon‐Cueto et al, 1998). The seminal works of Anders Björklund and colleagues showed that foetal tissue can promote CNS repair by replacing lost cell types in models of Huntington's and Parkinson's disease (Bjorklund & Lindvall, 2000). This led to the discovery that transplantation of foetal spinal cord tissue into the injured spinal cord results in successful graft survival, differentiation and integration into the host tissue (Bregman et al, 1993). Likewise, experiments by Richard, David and Aguayo revealed that peripheral nerve grafts could provide a permissive conduit for regenerating CNS axons (Richardson et al, 1980, 1984; David & Aguayo, 1981; Benfey & Aguayo, 1982), which has since been thoroughly studied (Cote et al, 2011a). These studies provided critical evidence that CNS axons are capable of regenerating. The grafts support a variety of neuronal types including propriospinal neurons and often neurons from the brain stem (Cote et al, 2011a). It appears that only injured axons regenerate through the grafts and not sprouts from non‐injured axons (Friedman & Aguayo, 1985). Despite these promising findings, there are few studies using PNGs alone which correlate this axonal growth to behavioural or recovery of axonal conduction. The likely reason for this is the remaining challenge that CNS and PNS neurons fail to extend beyond the distal graft–host interface where they encounter the inhibitory environment of the CNS. For example, lack of axon extension and functional improvements were observed in primates after spinal hemisection and PNG transplantation (Levi et al, 2002).
Cell‐based transplantations have largely superseded nerve grafts for several reasons: they can be injected into the spinal cord to fill the lesion site, they are less likely to cause further damage compared to a nerve graft, and cells can be genetically modified ex vivo to secrete specific growth factors (Assinck et al, 2017). The mechanisms behind which cell transplantation confers therapeutic benefit are often multifactorial. This includes direct replacement of damaged neural cells, neuroprotection of the host cells, promoting axon regeneration and synapse formation, and/or promoting myelination of damaged or newly formed axons (Assinck et al, 2017). A variety of cells have been used for SCI transplantation, including mesenchymal stem cells (MSCs), neural progenitor cells (NPCs), Schwann cells, olfactory ensheathing cells (OECs) and induced pluripotent stem cells (iPSCs; Assinck et al, 2017). Each has advantages and disadvantages when compared to one another.
MSCs and NPCs are stem cells, typically harvested from embryonic or foetal tissue, and can differentiate into neurons or glia in vitro (Liu et al, 2000; Billon et al, 2006). Neurons obtained from in vitro differentiation can survive and integrate into the injured rat spinal cord (Deshpande et al, 2006). Whether such differentiation occurs following in vivo transplantation has been less clear in some circumstances considering early investigations reported that majority of transplanted cells remain progenitor‐like or differentiate into glial cells (Vallieres & Sawchenko, 2003; Karimi‐Abdolrezaee et al, 2006). It is now generally accepted that MSCs do not form neurons in vivo (Lu et al, 2004). NPCs, on the other hand, were recently shown to extend “hundreds of thousands of axons into the spinal cord” (Rosenzweig et al, 2018), and injured motor and sensory axons regenerated into appropriate domains of NPC grafts (Dulin et al, 2018). Furthermore, these NPC grafts form host–graft synaptic network formation in patterns paralleling the normal spinal cord (preprint: Ceto et al, 2019). Aside from direct tissue replacement, both MSCs and NPCs confer permissive substrates for growth whereby injured host axons grow into the cellular grafts (Hofstetter et al, 2002; Lu et al, 2003; Ankeny et al, 2004). Injured axons grow into an NPC transplant, mediated by the secretion of various neurotrophic factors from the transplant itself (Lu et al, 2003). Similar to that observed in PNS grafts, regenerating host axons typically terminate their growth at the border of the transplant (Ruff et al, 2012; Assinck et al, 2017). A final consideration is that NPCs may also facilitate growth via paracrine actions. For example, modulation of neuroinflammatory processes may also contribute to the beneficial effects NPCs (Kokaia et al, 2012).
Schwann cells and OECs are terminally differentiated, myelinating and regeneration promoting cells found in the PNS and the olfactory system, respectively. Like other transplantation studies, both Schwann cells and OECs confer structural and trophic support (Bunge & Pearse, 2003; Barnett & Riddell, 2007). Schwann cells transplanted into the damaged spinal cord of rodents reduce cavitation and promote regeneration of both ascending and descending axons into the graft, and axons became myelinated and are evidenced to be electrophysiologically active (Xu et al, 1995b, 1997; Pinzon et al, 2001; Takami et al, 2002). However, similar to MSC and NPC transplants, in these studies, regenerating axons failed to leave the graft distally to reinnervate the host tissue. Recovery of limb functions was reported by some (Takami et al, 2002) but not all publications (Pearse et al, 2004a). Similar observations have been witnessed for OECs. After injection of OECs into a cervical unilateral lesion site of the corticospinal tract (CST) in rodents, anterograde tracing of lesioned hindlimb CST axons revealed extensive regeneration through the graft and integration with the CST beyond the injury site (Li et al, 1997). Later, it was shown that this intervention could improve respiratory function and climbing ability of the rats (Li et al, 2003). However, it was later challenged whether OECs promote CST regeneration (Lu et al, 2006). Other studies, including veterinary trials, have also shown the ability of OECs to promote robust regeneration of some spinal pathways even beyond the graft to enhance functional recovery (Ramon‐Cueto et al, 1998; Lu et al, 2002; Li et al, 2003; Jeffery et al, 2005; Toft et al, 2007). The benefits of Schwann cells and OECs can be potentiated when delivered together. For example, co‐delivery of the two cell types potentiates long‐distance axonal regeneration through and around guidance channels compared to what is achieved by either the cell type alone (Ramon‐Cueto et al, 1998).
iPSCs may circumvent the ethical issues associated with embryonic or foetal tissue use. NPCs generated from iPSCs have demonstrated beneficial effects after transplantation for animal models of SCI (Nagoshi & Okano, 2018). It remains unclear whether meaningful differentiation into neurons occurs or whether they play a supportive role. As is the case with other pluripotent cells, a critical safety issue for the use of iPSCs is the risk of tumorigenicity. Though promisingly, in marmosets, transplanted iPSC‐NPCs at the subacute phase predominantly differentiated into neurons around the lesion site without tumorigenicity and promoted axonal regrowth and angiogenesis, and preserved myelination area (Kobayashi et al, 2012). Furthermore, one strategy to decrease the risk of tumorigenicity is the addition of gamma‐secretase inhibitors, which removes tumour‐initiating cells and promotes functional recovery in the subacute and chronic phases of SCI in preclinical studies (Okubo et al, 2016, 2018).
Overall, preclinical SCI studies have demonstrated that various cellular transplants are efficacious through many mechanisms. Considering the limitations listed above, there remains much experimentation. The full potential of any cell type for transplantation will likely require the combination with other synergistic therapies.
Removal: targeting chondroitin sulphate proteoglycans
Removal or disruption of CSPGs can be achieved by inhibiting their synthesis, enzymatic degradation, antibody neutralisation or pharmacological targeting of effector molecules (Bradbury & Burnside et al., 2017). For example, xylosyltransferase‐1 (XT‐1) is crucial for the biosynthesis of glycosaminoglycan (GAG) chains of CPSGs. Reduction of XT‐1 expression through using a DNA enzyme for catalytic degradation of XT‐1 mRNA strongly reduced CSPG‐GAGs and allowed for axons from microtransplanted dorsal root ganglions (DRGs) to grow around a dorsal column lesion in rats (Grimpe & Silver, 2004). In a similar study, mRNA‐mediated knockdown of XT‐1 after dorsal column transection in rats significantly reduced proteoglycan expression and increased the length and density of ascending axons through a peripheral nerve graft (Hurtado et al, 2008). This was later replicated in a model of spinal cord contusion where increases in serotonergic innervation caudal to the injury correlated with a reduction in errors during a horizontal ladder test (Oudega et al, 2012).
Enzymatic modification of CSPGs is most commonly achieved with the enzyme chondroitinase ABC (ChABC). Derived from the bacteria Proteus vulgaris, it catalyses the degradation of the glycosidic bonds between CS‐GAGs of CSPGs, liberating them from the CSPG core protein (Prabhakar et al, 2005a, 2005b). Intrathecal infusion of ChABC for 10 days into rats following C4 dorsal column crush lesion degraded CSPGs, increased CST‐axonal regeneration and improved functional scores on several behavioural tests (Bradbury et al, 2002). Using anterograde tracing and immunohistochemical markers for neuroplasticity, ChABC treatment promoted sprouting of intact and injured spinal systems and formed relay networks after SCI (Barritt et al, 2006). The beneficial effects of ChABC delivery have been replicated by many independent laboratories in a variety of injury models (Bradbury & Carter, 2011). The positive results of using ChABC in rodents have been further confirmed in larger animals. In adult cats following thoracic hemisection, for example, ChABC enhanced functional recovery of skilled locomotion and kinematic measurements of hindlimb function (Tester & Howland, 2008). Importantly, in a recent SCI clinical trial in dogs, ChABC resulted in a seemingly modest, yet significantly improved forelimb–hindlimb coordination and early improvements to bladder compliance with no evidence of long‐term adverse effects (Hu et al, 2018). While average group differences are small, 10% of the treated dogs recovered independent ambulation which may represent an estimate of the “true” population effect is in such a large and heterogeneous sample size of 60 dogs (Moon & Bradbury, 2018). Cervical hemisection in rhesus monkeys and intrathecal administration 4 weeks later resulted in increased CST axon growth and formation of synapses from CST axons caudal to the lesion (Rosenzweig et al, 2019). This correlated with improved hand function compared to vehicle‐treated controls. Increasing neuroplasticity may even have long‐lasting effects. A single injection of ChABC into the phrenic motor pool following complete cervical hemidiaphragm paralysis in rats resulted in robust patterned respiratory recovery for more than 1.5 years (Warren et al, 2018). As such, through unmasking of the neuroplasticity that develops after injury, ChABC treatment can ensure rapid and robust functional recovery after a near lifetime of paralysis in rats.
The continuous production of CSPGs after injury may require chronic expression of ChABC. For this reason, a mammalian‐compatible ChABC gene was engineered by modifying N‐glycosylation sites to allow for secretion of ChABC from eukaryotic cells (Muir et al, 2010). This also avoids tissue damage of repeated administration and addresses the possible risks for immune recognition of the bacterial enzyme. Lentiviral vector (LV) gene delivery was used to achieve long‐term expression of this “mammalianised” ChABC (mChABC) gene in the contused rat spinal cord which resulted in large‐scale CSPG degradation and improved behavioural scores following thoracic injury (Bartus et al, 2014). Recently, these findings were replicated in the functional restoration of upper limb function and the strategy further developed to control gene expression (James et al, 2015). An immune‐evasive, doxycycline Tet‐on inducible LV‐mChABC vector enabled temporal control over ChABC expression (Burnside et al, 2018). Such regulatable gene therapy approach could be a viable candidate for clinical translation.
There are endogenous proteins which also may affect matrix composition that could be therapeutically harnessed. These include matrix metalloproteinases (MMPs), a disintegrin and metalloproteases (ADAMs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs; Troeberg & Nagase, 2012). MMPs, ADAMs and ADAMTSs have been implicated to play a beneficial role in CNS injury by degrading CSPGs (Burnside & Bradbury, 2014). For example, knockout of MMP‐9 in mice caused worsened motor deficit after traumatic brain injury (Wang et al, 2000). ADAMs and ADAMTSs are of particular interest as they display CSPG‐specific substrate recognition. ADAMTS4 infusion intrathecally in the contused rat spinal cord resulted in a significant improvement in the Basso, Beattie and Bresnahan (BBB) locomotor rating scale score that was comparable to ChABC infusion (Tauchi et al, 2012). More recently, an astrocyte‐selective AAV‐ADAMTS4 gene therapy resulted in CSPG degradation, decreased lesion size, increased CST axon regeneration, increased caudal serotonergic inputs and significantly improved BBB and error ladder scores following thoracic contusion in rats (Griffin et al, 2020). Given the safety profile of AAV viruses (Hudry & Vandenberghe, 2019), this gene therapy could potentially represent a safer alternative to LV‐ChABC gene therapy. Ultimately, removal of CSPGs has proven to be consistently advantageous for nearly two decades and we anticipate the next steps taken towards clinical translation of CSPG‐targeting therapies.
Removal: targeting myelin‐associated inhibitors
Independent laboratories have reported CNS axon growth and recovery of limb function following the use of anti‐Nogo‐A antibodies (Chen et al, 2000; GrandPre et al, 2000). In non‐human primates, anti‐Nogo‐A antibody therapies promoted the growth of CST tract axons after unilateral dorsal hemisection and regain of fine hand control (Freund et al, 2009). Interestingly, the co‐delivery of anti‐Nogo‐A antibodies and ChABC is more effective than single treatments at enhancing functional recovery after SCI indicating that removal of multiple inhibitory factors is advantageous (Zhao et al, 2013). Other therapeutic targeting of Nogo includes a soluble Nogo‐66 receptor protein (NgR (310) ecto‐FC) that complexes with Nogo‐A. NgR (310) ecto‐FC enhances CST and raphespinal axon growth after dorsal hemisection, and after complete transection in rats significantly enhances the number myelinated fibres in the bridge and increases the area of the bridging tissue between the cord stumps (Li et al, 2004; Guo et al, 2012). The Nogo receptor antagonist NEP1‐40 originally promoted CST and serotonergic fibre growth following thoracic dorsal hemisection in rats with striking results (GrandPre et al, 2002). The promising results obtained from the last three decades of preclinical research investigating Nogo‐A and anti‐Nogo‐A antibodies led to the first‐in‐man intrathecal application of anti‐Nogo‐A antibodies in a phase I human clinical trial of cute spinal cord injury (Kucher et al, 2018), and further trials regarding NOGO intervention are currently ongoing (NCT03935321, NCT03989440).
Regeneration: targeting neuronal intrinsic mechanisms
Intrinsic growth capacity of central neurons declines with age as the neuron differentiates for synaptic functions (Hilton & Bradke, 2017). This is a major reason why they mount a minor regenerative response following an injury. Through studying developing immature neurons and regenerative‐competent peripheral neurons we know that for an axon to regenerate, many diverse and coordinated intracellular mechanisms are required. These include cytoskeleton dynamics, axonal transport and trafficking, signalling and transcription of regenerative programmes, and epigenetic modifications (Curcio & Bradke, 2018; Fawcett & Verhaagen, 2018).
Reactivation of an intrinsic growth programme can be accomplished through conditional lesioning which involves transecting the peripheral branch of adult sensory DRG neurons prior to a central lesion (Richardson & Issa, 1984). Studies correlated cyclic adenosine monophosphate (cAMP) levels in DRG neurons to growth capacity through induction of important pro‐regenerative transcription factors (Qiu et al, 2002). Local administration of cAMP to DRGs can, at least in part, mimic the conditioning lesions (Cai et al, 2001; Neumann et al, 2002; Qiu et al, 2002). Rolipram prevents the hydrolysis of cAMP and sustains elevated levels of cAMP that mimic the effects of a conditioning lesion effect to some extent (Nikulina et al, 2004; Pearse et al, 2004b). cAMP‐mediated gene transcription alone only partially recapitulates the conditioning lesion effect (Blesch et al, 2012), indicating that there are opportunities remaining to discover therapeutic interventions that do so.
Genetic manipulation experiments, including deletion of PTEN or GSK3β, or overexpression of STAT3, KLFs or SOX11, have exemplified that these manipulations to aspects of regenerative programmes can produce a modest regenerative response (Curcio & Bradke, 2018). Converting these interventions into clinically useable alternatives such as pharmacological agents or viral vector gene therapies of these could be a promising avenue (Blackmore et al, 2012; Zukor et al, 2013; Wang et al, 2015). Another strategy is to modulate intracellular signalling through modulation of GTPases. This has been achieved through RhoA‐GTPase inhibitors, C3‐peptides, C3‐ADP‐ribosyltransferase, siRNA and ROCK inhibitors (Wu & Xu, 2016). Blocking of Rho activation using Cethrin (BA‐210; SPRING‐VX‐210) has shown promise in preclinical and multicentre phase I/IIa clinical trials; some patients with one dose reported improvements in an ASIA grade (Fehlings et al, 2011). A further phase 2b/3 study determining efficacy and safety was initiated for patients with acute traumatic cervical SCI (Fehlings et al, 2018), although regrettably it was discontinued shortly after due to lack of efficacy after an interim analysis (Taylor WEBARTICLE, 2018). Similarly, inhibition of the downstream effector Rho kinase (ROCK) by Y27632 and Fasudil in mouse models of SCI resulted in similarly inefficacious effects of RhoA inhibition when delivered acutely (Borisoff et al, 2003; Chan et al, 2005; Watzlawick et al, 2014). While still an intriguing target, one key reason for the unsuccessful trial is that we still understand relatively little about the mode of action of Rho blockade, both for neuronal and non‐neuronal cells. We have only recently gained a deeper understanding of the physiological role of RhoA in axon growth during development and its downstream effectors. RhoA restrains axon initiation through RhoA/myosin‐II‐dependent actin arcs that restrict microtubule advancement in the growth cone (Dupraz et al, 2019). Future research needs to further disentangle the physiological role of RhoA in axon regeneration and how RhoA‐dependent mechanisms in glial cells might affect axon regeneration.
The cytoskeleton plays an important role in axonal growth in development and regeneration. Microtubules underlie the formation of retraction bulbs and are a major contributor to the failure of axonal regeneration; stabilisation of microtubules specifies growth cone formation and protrusion (Erturk et al, 2007; Witte et al, 2008). Stabilisation of microtubules also prevents polarisation and migration of scar‐forming fibroblasts which is associated with decreased deposition on CSPGs (Ruschel et al, 2015). Independent laboratories have shown that systemic administration of low doses of the microtubule‐stabilising drugs epothilone B or D after SCI results in adequate CNS penetration and distribution, reduced deposition of inhibitory proteoglycans, reduced axon die‐back, induced axonal growth and improved motor functions after SCI (Ruschel et al, 2015; Ruschel & Bradke, 2018; Sandner et al, 2018). Considering these drugs are FDA‐approved, easily administered and address both intrinsic and extrinsic determinants of axon regeneration, they represent a promising therapeutic candidate. Further evidence that implicates microtubule dynamics as important regulators of axon development and regeneration comes from studies investigating collapsin response mediator protein 2 (CRMP2). This protein stabilises microtubule polymerisation, while CRMP2 phosphorylation loses its affinity for cytoskeletal proteins, leading to microtubule disorganisation inhibition of axonal growth (Nagai et al, 2016). CRMP2 inactivation can be mediated via a RhoA‐ROCK‐dependent pathway downstream of CSPGs, MAG and Nogo (Rozes Salvador et al, 2016; Curcio & Bradke, 2018). Genetically preventing phosphorylation of CRMP2 promotes axonal regeneration attributed to suppressing microtubule depolymerisation after optic nerve injury (Kondo et al, 2019). Pharmacologically preventing phosphorylation of CRMP2 could be another therapeutic avenue.
Less is known about the role and manipulation of the actin cytoskeleton in axon regeneration. Recently however, the ADF/cofilin family of actin‐regulatory proteins that govern actin retrograde flow and dynamic were shown to promote neurite formation (Tedeschi et al, 2019). Specifically, enhanced actin turnover by ADF/cofilin is critical for axon regeneration in the adult CNS. Thus, actin turnover is a key target for future therapeutic interventions.
We are missing key pieces of the experimental puzzle such as what triggers the switch between growth competent neurons to growth‐restricted mature neurons. Knowing such mechanisms could open a door to new therapeutic interventions. Whole transcriptome sequencing and bioinformatics analysis identified the calcium voltage‐gated channel auxiliary subunit α2δ2 (Cacna2d2) as a one such developmental switch (Tedeschi et al, 2016). Cacna2d2 promotes synapse formation and limits axon growth and regeneration in adult mouse DRG neurons (Tedeschi et al, 2016). Systemic administration of pregabalin to antagonise Cacna2d2 in adult mice after dorsal column lesion resulted in increased axon regeneration by preventing calcium influxes through Cav2 channels (Tedeschi et al, 2016). Further, gabapentinoid administration within a month after human SCI improves motor recovery (Warner et al, 2017), while other anti‐convulsant drugs appear not to show such effects (Warner et al, 2019). Many different interventions are available that influence the intrinsic growth response of axons which seem to represent only a piece of the puzzle. We are yet to discover how to revert neurons to their embryonic growth state.
Resupply: trophic support
Neurotrophins facilitate neuron survival, development and function. They may be administered to the damaged spinal cord by direct infusion, biomaterials and ex vivo gene therapy (cell transplantation) or by viral vector expression. Brain‐derived growth factor (BDNF), neurotrophin‐3, (NT‐3), nerve growth factor (NGF), fibroblast growth factor (FGF) and glial cell‐derived growth factor (GDNF), for example, are some of the trophic factors that have been investigated. Different neurotrophic factors have differing effects. For example, NT‐3 elicits growth of CST axons, and NT‐3 and NGF both promote extensive DRG‐origin sensory or axon from the reticular formation and red nucleus (Houweling et al, 1998; Jakeman et al, 1998; McTigue et al, 1998; Oudega & Hagg, 1999; Namiki et al, 2000; Tuszynski et al, 2002; Blesch & Tuszynski, 2003; Shumsky et al, 2003; Zhou & Shine, 2003; Oudega et al, 2019). BDNF, by contrast, has no such effect but rather stimulates sprouting and growth of rubrospinal, reticulospinal, vestibulospinal, raphespinal and motor axons (Nakahara et al, 1996; Tuszynski et al, 1996; Grill et al, 1997; Bradbury et al, 1998; Bregman et al, 2002). There are, however, several issues pertaining to continuous infusions of trophic factors, including damage to the tissue at the infusion site, low stability, limited diffusion and inability to cross the blood–spinal cord barrier. Furthermore, the complexity of the spinal networks conveys another limitation of the use of neurotrophic factors. For example, overexpression of NGF in the dorsal horn resulted in overshooting of targets and formed inappropriate connections, resulting in severe hyperalgesia in rats (Tang et al, 2004). Similar side effects or lack of efficacy have been observed after trophic delivery in human clinical trials of diabetic neuropathy (Apfel, 2002).
Future preclinical investigations should include non‐human primates that include multiple motor and sensory parameters to show whether neurotrophins safe and effective for SCI treatment. It would be invaluable to discover the required combination and temporal administration of growth factors to elicit regrowth of axons through lesions without side effects. Recently, the essential factors required to propel propriospinal axon regeneration across a complete SCI in the adult mouse were discovered (Anderson et al, 2018). The growth capacity of mature descending propriospinal neurons was activated with viral vector‐mediated expression of osteopontin, insulin‐like growth factor 1 (IGF1) and ciliary‐derived neurotrophic factor (CDNF) before a SCI. A growth‐permissive substratum was induced with fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF), and propriospinal axons were chemoattracted with glial‐derived neurotrophic factor (GDNF) delivery via spatially and temporally controlled release from biomaterial depots placed sequentially after SCI (Anderson et al, 2018). These three mechanisms in combination stimulated propriospinal axon regrowth through astrocyte scar borders and across lesion cores of non‐neural tissue that was associated with increased electrophysiological recordings in tissue below the lesion but did not result in functional improvements. It may be possible that functional recovery in these animals may only be evident if combined with rehabilitation to elicit use‐dependent plasticity.
Remyelination
The validity of targeting remyelination after SCI is contentious. The myelin sheaths of myelinated axons increase the speed of transmission of action potentials. The importance of this property is overtly evident in multiple sclerosis; however, researchers have recently questioned whether the contribution of oligodendrocyte support and remyelination in pathophysiology is relevant to functional recovery after SCI (Duncan et al, 2019). After SCI, there is widespread acute oligodendrocyte death and demyelination of axons and it is predicted that remyelination could be important for protecting axons from further degeneration and enhancing conduction (Norenberg et al, 2004; Nave, 2010a, 2010b; Almad et al, 2011). Two approaches are available: transplantation of cells that may directly differentiate into oligodendrocytes and to promote the recruitment and differentiation of endogenous OPCs. Several studies from various laboratories involving transplantation of Schwann cells, OECs and NPCs have demonstrated functional recovery correlates to their ability to promote remyelination (Tetzlaff et al, 2011; Karimi‐Abdolrezaee & Eftekharpour, 2012). Within these experiments, the contribution of remyelination towards functional recovery is unclear as transplanted cells have many potential mechanisms of efficacy (see 3.2. Replacement: Cellular transplantation). Failure of recruitment of OPCs to the SCI lesion site is associated with the inability of axon remyelination and sensitivity to degeneration (Irvine & Blakemore, 2008). Promoting the recruitment and differentiation of OPCs to the lesion site can is a lesser‐explored avenue (Duncan et al, 2019). Various growth factors and hormones can stimulate the differentiation of OPCs in vitro but their in vivo efficacy is unclear (Plemel et al, 2014). More recently however, spontaneous locomotor recovery of stepping following contusive SCI was shown to not require oligodendrocyte remyelination despite the high level of endogenous remyelination that occurs after SCI (Duncan et al, 2018). This finding raises doubts whether therapeutic targeting of oligodendrocyte remyelination is a worthwhile target for clinical translation, a debate we cannot yet conclude at this time. The contribution of Schwann cell myelination may possibly be a more meaningful target. Neuregulin‐1 was found to drive trans‐differentiated of OPCs into Schwann cells, and these cells were essential for Schwann cell‐mediated remyelination following SCI (Bartus et al, 2016). Furthermore, preventing neuregulin‐1/ErbB signalling‐controlled transformation of CNS progenitors into myelinating Schwann cells resulted in negative consequences for functional recovery following spinal cord contusion in mice (Bartus et al, 2019). Another consideration is interspecies differences. The majority of intact axons in chronically injured rats exhibit remyelination, albeit with some structural abnormalities (Powers et al, 2012). Neither was chronic demyelination observed in spared axons after SCI in mice (Lasiene et al, 2008). It is unclear whether this is also the case in humans and primates or whether remyelination contributes to the same functions as it does in rodents, owing to the need for more investigations on this topic.
Rehabilitation: neurorehabilitation, neuronal activity and electrical stimulation
Recent evidence has documented that, in the right context, rehabilitation can play a critical role in regeneration and plasticity (Loy & Bareyre, 2019). Various forms of rehabilitation can enhance the recovery of motor functions after SCI in mice, rats and cats. Rehabilitation is important to prune and refine circuits that are relevant to the motor task (Ichiyama et al, 2008). Moreover, rehabilitation is also reported to stimulate the local production of neurotrophins (Ying et al, 2005; Cote et al, 2011b), modulate multiple neurotransmitter systems (Edgerton et al, 2004) and enhance sprouting of compensatory relay networks (Courtine et al, 2009; van den Brand et al, 2012). Animal models of rehabilitation can come in multiple forms of forced or voluntary physical exercise and environmental enrichment as well as on a spectrum of general to more specific approaches. Increasing general activity of rodents through environmental and social enrichment such as wheel running, multiple housing and the addition of climbing spaces, improved bedding and crawl toys all significantly increase BBB locomotor scores (Berrocal et al, 2007; Loy et al, 2018). Surprisingly, environmental enrichment prior to a SCI in mice and rats mimicked the degree of proprioceptive afferent neurons regeneration and functional recovery to a conditioning lesion (Hutson et al, 2019). The regenerative response correlated with Creb‐binding protein (Cbp)‐mediated histone acetylation and epigenetic changes inducing signalling pathways involved in neuronal activity, calcium modulation and the regenerative programme in proprioceptive neurons (Hutson et al, 2019). A comparative analysis of rats with incomplete SCI subjected to three forced‐exercise rehabilitation paradigms (quadrupedal treadmill training, swim training and stand training) differentially improved recovery after spinal cord injury (Hutchinson et al, 2004). Quadrupedal treadmill‐trained rats showed reduced allodynia and restored sensation; swim training reduced allodynia and stand training had no benefit. This indicates that stand training may not be intensive enough above spontaneous movement. Similarly, quadrupedal treadmill training was superior to hindlimb step training in terms of electrophysiological and kinematic limb analysis and the number of propriospinal labelled neurons above and below the lesion (Shah et al, 2013). Utilising task‐specific rehabilitative strategies such as skilled paw retrieval, horizontal ladder training or hindlimb‐specific training makes intuitive sense in that the best way to relearn a given task is to specifically train for that task. For example, hindlimb‐specific treadmill training can be used after thoracic contusion to significantly improve that lost function although this negatively influenced forelimb–hindlimb inter‐coordination (Griffin et al, 2020). It is apparent that emphasising a particular behaviour can negatively affect the performance of another, which suggests that reassignment of CNS resources is limited (Fawcett & Curt, 2009). For example, rats trained at skilled paw reaching improved in that task but this worsened their performance at walking on ladders, and general environmental enrichment rehabilitation can improve locomotor function, but extinguish skilled tasks (Girgis et al, 2007; Garcia‐Alias et al, 2009). Likewise, spinally transected cats could be trained in weight support or step; however, training one behaviour would extinguish the other (De Leon et al, 1998a, 1998b). We are still rudimental in our understanding of how rehabilitation influences regeneration, neuroplasticity and motor recovery following SCI. Several studies reported exercise‐induced plasticity of various spinal tracts, particularly the serotonergic and reticulospinal tracts (Engesser‐Cesar et al, 2007; Asboth et al, 2018; Loy et al, 2018). Conversely, an equalling number reported no such ability and proposes the role of rehabilitation in shaping newly formed connections by spontaneous mechanisms or plasticity‐promoting treatments (Garcia‐Alias et al, 2009; Maier et al, 2009; Wang et al, 2011a; Alluin et al, 2014). Regardless of these contrasting reports, it is clear that in general, a high‐intensity programme and is key to providing robust motor function improvements in rats (Loy & Bareyre, 2019).
It should be briefly mentioned that very limited recovery after anatomically complete injuries with rehabilitation is observed (Ilha et al, 2011). However, since the spinal circuitry below the lesion remains active, it can be activated through pharmacological and electrical modulation of the central pattern generator and lead to weight‐bearing stepping in rats with completely transected spinal cords (Courtine et al, 2009).
We are also rudimental in our understanding of how electrical stimulation and neuronal activity affect regenerative processes and investigations are ongoing. Brief electrical stimulation accelerates axon outgrowth in models of peripheral nerve injury (Gordon, 2016), and spinal cord stimulation in human SCI patients has shown efficacy in randomised clinical trials over several decades (Sdrulla et al, 2018). Exposure to an electrical field in vitro increased neurite outgrowth of embryonic and adult neurons, and electrical stimulation of the sciatic nerve in vivo increased central DRG axon regeneration comparable to a conditioning lesion (Wood & Willits, 2006; Udina et al, 2008). Conversely, electrical stimulation has also been associated with axon growth inhibition. Electrical activity strongly inhibited axon outgrowth in cultured adult DRG neurons, an effect depended on the L‐type voltage‐gated Ca2+ channel current and involved transcriptional changes (Enes et al, 2010). Knockout of the L‐type voltage‐gated Ca2+ channel current in adult mice was sufficient to boost the growth ability of DRG neurons in vivo after central lesioning (Enes et al, 2010). Likewise, suppressing synaptic function through gabapentinoid‐mediated blockade of the voltage‐gated channel auxiliary subunit α2δ2 also induces axon regeneration the adult CNS (Tedeschi et al, 2016). This counter‐position hypothesises that electrical stimulation suppresses growth by triggering an increase in intracellular calcium, and conversely, a lack of electrical activity within axotomised neurons may recapitulate development and promote axon regeneration (Hilton & Bradke, 2017). It is possible that temporal regulation and the effects of conditioning neurons may account for the contrary evidences and is therefore an exciting prospect for further investigations.
Combinatory approaches
While individual therapies have shown promise in preclinical models, recovery remains incomplete and it is becoming apparent that focusing a singular barrier to repair is not going to facilitate successful and functional recovery of SCI in humans. Conversely, it will require the combination of various treatment strategies that address diverse aspects of SCI pathology. Removal of growth inhibitory molecules as well as boosting the intrinsic growth response will be required. Tissue or cell transplants may be required to replace tissue, act as a permissive bridge and/or facilitate repair. Supplying trophic support will improve survival of cells and direct axonal growth. Lastly, neurorehabilitation will function to augment the functional recovery of remodelling circuits (Tables 1, 2, 3, 4, 5). This section will summarise currently published studies that have combined therapies in SCI models (Fig 2).
Table 1.
Combinations of cell transplants with neurotrophins in preclinical experiments
| Transplant type | Growth factor | Injury model | Outcome | Reference |
|---|---|---|---|---|
| Schwann cells in semipermeable guidance channels | BDNF and NT‐3 delivery via minipump |
Female Fischer rats 160‐190 g Mid‐thoracic transection Acute treatment |
BDNF and NT‐3 infusion enhanced propriospinal axonal regeneration and, more significantly, promoted axonal regeneration of specific distant populations of brain stem neurons into grafts at the mid‐thoracic level in adult rat spinal cord | Xu et al (1995a) |
| Rat intercostal nerve | aFGF in fibrin glue |
Adult Sprague Dawley rats T8 transection Acute treatment |
Hindlimb function improved progressively during the first 6 months, as assessed by two scoring systems. The corticospinal tract regenerated through the grafted area to the lumbar enlargement, as did several bulbospinal pathways | Cheng et al (1996) |
| Rat fibroblasts | Fibroblasts genetically modified to secrete NGF, BDNF, NT‐3 or bFGF |
Adult rats Acute SCI |
Sensory neurites of dorsal root origin extensively penetrated NGF‐, NT‐3‐ and bFGF‐producing grafts, whereas BDNF‐secreting grafts elicited no growth responses. Putative noradrenergic neurites also penetrated NGF‐secreting cell grafts. Local motor and corticospinal motor axons did not penetrate any of the neurotrophic factor‐secreting grafts | Nakahara et al (1996) |
| Rat PNG | IGF, bFGF or TGFβ in gelfoam |
Adult rats, C3 hemisection Chronic treatment (1 month) |
Greatest increase of axonal regeneration by TGFβ | Houle et al (1996) |
| Rat PNG | NT‐3, BDNF or CNTF in gelfoam |
Adult female Sprague Dawley rats (200‐225 g) C2/3 dorsal hemisection Treatment 4 weeks after injury |
Growth factors were required to promote axonal growth into the PN graft. CNTF most effective. Functional testing not done | Ye and Houle (1997) |
| Foetal spinal cord tissue | Gel foam soaked with NT‐3 and BDNF in gelfoam |
Adult male and female Sprague Dawley rats (200–250 g) T6 hemisection Acute treatment |
Application of either transplants or neurotrophic factors partially reverses the axotomy‐induced atrophy in rubrospinal neurons, but that both interventions together reverse the atrophy completely. | Bregman et al (1998) |
| Minced rat PNG | BDNF, NT‐3 and GDNF collagen matrix in cavity |
Adult female rats 150–200 g T10 dorsal hemisection Chronic treatment |
Combination therapy led to sustained regeneration of the CST | Ferguson et al (2001) |
| Autologous PNG | Gelfoam soaked with GDNF |
Adult female Sprague Dawley rats 225–250 g C3 hemisection Acute treatment |
Sevenfold increase in the number of regenerating neurons after GDNF‐treatment. | Dolbeare and Houle (2003) |
| Fibroblasts | Fibroblasts genetically modified to secrete NT‐4/5 |
Adult Fisher 344 rats Dorsal hemisection or complete transections at the mid‐thoracic level |
Motor axons, coerulospinal, reticulospinal and propriospinal axons responded to NT‐4/5 delivery after thoracic spinal cord injury with significantly increased axonal penetration into NT‐4/5 secreting grafts compared to control grafts. Axonal growth beyond NT‐4/5‐producing grafts and functional recovery were not observed | Blesch et al (2004) |
| Fibroblasts | Fibroblasts genetically modified to secrete BDNF and NT‐3 |
Adult female Sprague Dawley rats 225–250 g T8/9 moderate contusion Acute treatment |
BDNF/NT‐3 rats recovered from areflexic bladder earlier showed decreased micturition pressure and fewer episodes of detrusor hyperreflexia, as well as improvements to hindlimb function compared to untreated | Mitsui et al (2005) |
| Human umbilical cord blood cells | BDNF |
Adult male Sprague Dawley Rats 300‐350 g T9 contusion NYU weight‐drop device 10 g weight |
8 weeks after transplantation, the HUCBs with BDNF transplanted group had improved BBB scores, than the other groups | Kuh et al (2005) |
| Bone marrow stromal cells | Lentiviral NT3 expression caudal |
Adult Female Fischer 344 rats Dorsal column lesion C2/C3 Acute treatment |
LV‐NT‐3 allowed regenerating axons to grow beyond a PNG | Taylor et al (2006) |
| Mouse embryonic stem cell‐derived NPCs | NT‐3 + PDGF in fibrin/heparin scaffolds |
Adult female Long–Evans rats 250–275 g T9 dorsal hemisection Subacute treatment (2 weeks) |
Combination enhanced transplanted cell survival and increased the number of NPC‐derived NeuN‐positive neurons 8 weeks after transplantation. All experimental groups treated with NPCs exhibited an increase in behavioural function 4 weeks after transplantation. In a subset of animals, the cells formed tumours | Johnson et al (2010a) |
| Mouse embryonic stem cell‐derived NPCs | NT‐3 + PDGF in fibrin/heparin scaffolds |
Adult Long–Evans female rats 250–275 g Dorsal hemisection T9 Subacute model (2 weeks) |
The combination enhanced the total number of ESNPCs present in the spinal cord lesion 2 weeks after injury. No functional scoring reported. | Johnson et al (2010b) |
| Schwann cells and NSCs | NSCs genetically enhanced expression of NT‐3 in gelfoam |
Adult female Sprague Dawley rats Complete T10 transection Acute treatment |
Significantly improved relay of the cortical motor evoked potential and cortical somatosensory evoked potential as well as ameliorated hindlimb deficits. Neuroprotection and outgrowth of serotonergic firers | Wang et al (2011b) |
| E14 rat NSC human ESC‐derived NSC cell lines 566RSC, HNES7 in fibrin matrix | BDNF, NT‐3, PDGF‐AA, IGF‐1, EGF, bFGF, aFGF, GDNF, GDNF, HGF, calpain inhibitor MDL28170 |
Adult female Fischer 344 rats T3 complete transection 2 mm or C5 hemisection Treatment 2 weeks after injury |
Extensive axonal outgrowth rostral and caudal from the transplant. Human and rat cells performed similarly. Combination greatly improved functional recovery | Lu et al (2012) |
| Adult rat brain NPCs supplemented with EGF, FGF and heparin | PDGF in hyaluronan‐based hydrogel |
Adult female Wistar rats 250–300 g T2 26 g clip compression Acute treatment |
The combination of PDGF with cells protected oligodendrocytes around the lesion. The combination reduces number of errors on ladder task | Mothe et al (2013) |
| Human IPSCs in fibrin matrix | BDNF, NT‐3, PDGF‐AA, IGF‐1, EGF, bFGF, aFGF, GDNF, GDNF, HGF, calpain inhibitor MDL28170 |
Adult female athymic nude rats and adult SCID mice C5 lateral hemisection Treatment 2 weeks after injury |
Extensive outgrowth from grafted cells, but not functional recovery. Few cells were positive for mature markers | Lu et al (2014) |
| Human iPSC‐derived OPCs | PDGF in hyaluronan/methylcellulose hydrogel |
Adult female Sprague Dawley rats 300 g T2 26 g clip compression Acute treatment |
The combination promoted cell survival and differentiation of the cells. No functional recovery observed | Fuhrmann et al (2016) |
Table 2.
Combinations of cell transplants with anti‐inhibitory therapies in preclinical experiments
| Transplant type | Anti‐inhibitory therapy | Injury model | Outcome | Reference |
|---|---|---|---|---|
| Human Schwann cells | IN‐1 antibody + aFGF–fibrin glue |
Adult female athymic nude rats 145–165 g T8 transection Acute treatment |
Human SC grafts alone do not support the regeneration of injured CST fibres and do not prevent die‐back. Grafts plus IN‐1 antibody‐containing supernatant support some sprouting but die‐back continues. Grafts plus aFGF–fibrin glue support regeneration of some fibres into the grafts and reduce die‐back | Guest et al (1997) |
| PNG | BDNF or ChABC |
Adult female Sprague Dawley rats 200–250 g T11 hemisection Acute treatment |
BDNF did not improve axonal regeneration; however, ChABC resulted in significant increase in the number of regenerated Clarke's nucleus neurons | Yick et al (2000) |
| Schwann cell‐seeded channels | ChABC intraparenchymal infusion |
Adult female Fischer rats T8 hemisection Acute treatment |
Significant anatomical evidence of regeneration through the graft compared with that seen without ChABC treatment | Chau et al (2004) |
| Schwann cell matrigel guidance channels + olfactory ensheathing glia grafts | ChABC osmotic pump delivery |
Adult female Fischer 344 rats 165–180 g Complete thoracic T8 transection Acute treatment |
Increased 5HT fibres exiting bridge caudally. Functional recovery which was absent without ChABC application | Fouad et al (2005) |
| NSCs | ChABC pretreatment |
Adult female Sprague Dawley rats 230–250 g T10 contusion (10 g weight drop) Acute treatment |
Combined treatment significantly induced the outgrowth of a greater number of growth‐associated protein‐43‐positive fibres at the lesion epicentre, compared with NSPC transplantation alone | Ikegami et al (2005) |
| PNG | ChABC intrathecal infusion at lesion site |
Adult female Sprague Dawley rats 225–250 g C5 dorsal quadrant aspiration Acute treatment |
Combination promotes significant axonal regeneration beyond the distal end of a PN bridge back into the spinal cord and that regenerating axons can mediate the return of useful function of the affected limb | Houle et al (2006) |
| Schwann cell‐filled guidance channels + OEC implant | ChABC intraspinal injections |
Adult female Fischer 344 rats Complete transection Acute treatment |
Regeneration of many fibre tracts and the combination was associated with significantly improved locomotor recovery | Vavrek et al (2007) |
| PNG | Intraspinal ChABC microinjection |
Adult female Sprague Dawley rats 225–250 g C5 dorsal quadrant aspiration Acute treatment |
More regenerating axons to exit a PNG and reenter spinal cord tissue than saline injections | Tom and Houle (2008) |
| PNG | mRNA‐mediated knockdown of XT‐1 | Adult female Sprague Dawley rats (200–225 g). Thoracic dorsal transection | 1.4‐fold reduction in GAG‐side chains of chondroitin sulphate or heparin sulphates‐PGs. Ninefold increase in length and a fourfold increase in density of ascending axons growing through the nerve graft and scar tissue present at the rostral spinal cord | Hurtado et al (2008) |
| NPCs | ChABC + EGF, bFGF and PDGF‐AA |
Adult female Wistar rats 250 g T7 Clip compression Chronic treatment |
Combined strategy promoted the axonal integrity and plasticity of the corticospinal tract and enhanced the plasticity of descending serotonergic pathways and significantly improved neurobehavioural recovery | Karimi‐Abdolrezaee et al (2010) |
| PNG | ChABC injections at day 1 and 1 week later |
Adult female Sprague Dawley rats 240–300 g C2 lateral hemisection Acute treatment |
Combination with a peripheral nerve autograft, ChABC treatment resulted in lengthy regeneration of serotonin‐containing axons and other bulbospinal fibres and remarkable recovery of diaphragmatic function compared to alone | Alilain et al (2011) |
| Adult rat NPCs | Nogo‐66 receptor protein (blocker) + bFGF, EGF, PDGF via osmotic pump |
Adult female Sprague Dawley 200–300 g T8 complete transection Acute treatment |
Transplanted cells survive longer with the growth factors; NgR had no effect on their survival. NgR increases myelination. Combination did not improve sprouting or functional recovery | Guo et al (2012) |
| PNG + fibrin glue + aFGF | ChABC microinjection rostral and caudal |
Adult male Sprague Dawley rats 225–250 g T8 transection Acute treatment |
Remarkably lengthy regeneration of certain subtypes of brainstem and propriospinal axons across the injury site. Restoration of supraspinal bladder control | Lee et al (2013) |
| PNG | ChABC + acidified FGF intraspinal injection mixture |
Adult Sprague Dawley rats 225–250 g Severe T8 contusion 250 KDyne Acute and chronic treatment |
The combination enhanced integration between host astrocytes and graft Schwann cells, allowing for robust growth. Axons did not enter/exit the graft without ChABC and aFGF. Limited compared to the acute scenario. Combination leads to functional improvements | DePaul et al (2017) |
Table 3.
Combinations of anti‐inhibitory therapies with growth factors in preclinical experiments
| Anti‐inhibitory therapy | Growth factor | Injury model | Outcome | Reference |
|---|---|---|---|---|
| Thermostabilised ChABC | NT‐3 lipid microtubes embedded in agarose gel for both molecules |
Adult male Sprague Dawley rats T10 dorsal hemisection Acute treatment |
Animals treated with ChABC in combination with sustained NT‐3 delivery showed significant improvement in locomotor function and enhanced growth of cholera toxin B subunit‐positive sensory axons and sprouting of serotonergic fibres | Lee et al (2010) |
| ChABC intraspinal injections | NT‐3 and NR2D expression |
Adult female Sprague Dawley rats 200 g T8 Lateral Hemisection Acute treatment |
Animals receiving combined therapy displayed the most improved body stability and inter‐limb coordination. Only animals with the full combination treatment recovered consistent multisynaptic responses in these motor neurons indicating formation of a detour pathway around the injury site | Garcia‐Alias et al (2011) |
| Nogo‐66 receptor protein (blocker) |
bFGF, EGF, PDGF via osmotic pump +adult rat NSCPs |
Adult female Sprague Dawley 200–300 g T8 complete transection Acute treatment |
Transplanted cells survive longer with the growth factors; NgR had no effect on their survival. NgR increases myelination. Combination did not improve sprouting or functional recovery | Guo et al (2012) |
| ChABC | NGF in electrospun scaffold |
Adult female Sprague Dawley rats T9/T10 complete transection Acute treatment |
Improved BBB scores compared to implant only | Colello et al (2016) |
| NEP1‐40 (Nogo antagonist), ephrin‐B3 and Sema4D receptor |
Collagen‐binding BDNF and NT‐3 cAMP in functionalised collagen scaffold |
Adult female Sprague Dawley rats 200–230 g T10 complete transection Acute treatment |
Full combinatorial therapy exhibited the greatest advantage in reducing the volume of cavitation, facilitating axonal regeneration and promoting neuronal generation. Neurons generated in the lesion area could form the neuronal relay and enhance the locomotion recovery | Li et al (2016) |
| Anti‐Nogo‐A | NT‐3 delivery by nanoparticles |
Adult female Sprague Dawley rats T1/2 clip compression Acute treatment |
Increased anatomical improvements in both treatments individually. Only functional improvements in the combination group | Elliott Donaghue et al (2016) |
Table 4.
Combination treatments involving targeting the intrinsic growth response in preclinical experiments
| Therapy | Combination | Injury model | Outcome | Reference |
|---|---|---|---|---|
| Rolipram, cAMP | Rat Schwann cell transplantation |
Adult female Fischer 344 rats 160–180 g T8 moderate contusion injury Acute treatment |
The combination of rolipram and cAMP had the greatest effect on cAMP levels, axonal sparing, myelination and locomotor function | Pearse et al (2004b) |
| Rolipram (minipump drug delivery) | Rat Schwann cells expressing D15A (BDNF + NT3) |
Adult female Fischer 344 rats 180–200 g T8 moderate contusion injury MASCIS weight drop Subacute treatment (2 weeks) |
Compared to the single treatments, the combination led to the largest SC grafts, the highest numbers of serotonergic fibres in the grafts, and increased numbers of axons from the reticular formation below the lesion/implant area and provided the greatest improvement in hindlimb function | Flora et al (2013) |
| Scar‐targeted liposomes containing docetaxel | BDNF, aFGF |
Adult female Sprague Dawley 220–230 g T10 contusion 50 mm Height MASCIS impactor Acute treatment |
The combined application of GFs and DTX supported neuroregeneration by improving neuronal survival and plasticity, rendering a more permissive extracellular matrix environment with improved regeneration potential. In addition, our combination therapy promoted axonal regeneration via moderation of microtubule function and mitochondrial transport along the regenerating axon. Significantly improved BBB score | Wang et al (2018) |
| Taxol and cetuximab in collagen scaffold | Cetuximab (EGFR signalling antagonist) |
Adult female Sprague Dawley rats 190–210 g Complete T10 transection Acute treatment |
Combined functional scaffold implantation significantly increased neural regeneration to reconnect the neural network and improved functional recovery | Fan et al (2018) |
Table 5.
Combinations of therapies with rehabilitation in preclinical experiments
| Combination | Form of rehabilitation | Injury model | Outcome | Reference |
|---|---|---|---|---|
| Autologous bone marrow stem cells | Swim training 60 min a day 6 days/week |
Adult male Wistar rats 350 g NYU impactor contusion: 10 g 25 mm height Cell transplant 48 h after injury |
The combination of bone marrow stem cell therapy (CD45 (+)/CD34 (−)) and exercise training resulted in significant functional improvement in acute spinal cord injury | Carvalho et al (2008) |
| ChABC infusion | Forepaw reaching and grasping rehabilitation |
Adult male Lister Hooded rats 250–300 g C4 dorsal funiculus cut Acute ChABC treatment |
Synergistic effect compared to either intervention alone | Garcia‐Alias et al (2009) |
| Anti‐Nogo‐A antibody | Bipedal and quadrupedal treadmill training—1 week after injury |
Adult female Sprague Dawley rats 200–250 g T‐Lesion T8 Acute treatment |
Lack of synergistic effect with the combination | Maier et al (2009) |
| Single lumbar ChABC injection | Voluntary wheel running |
Adult female C57BL/6 mice Moderate contusion Ohio State ESCID impactor ChABC treatment 1 week after injury |
Rehabilitation did not improve functional recovery | Jakeman et al (2011) |
| 5 ChABC intraspinal injections over 10 days beginning 1 month after injury | Task‐specific paw reaching beginning 1 month after injury |
Adult male Lister Hooded rats 150–200 g C4 dorsal hemisection Chronic treatment |
Significant improvement to paw reaching task with combination but only after ChABC infusion | Wang et al (2011a) |
| Anti‐Nogo‐A infusion into intrathecal space for 2 weeks followed by 5 intraspinal ChABC injections over 10 days |
Multitask rehabilitation: Seed‐reaching task and ladder walking beginning 4 weeks after the lesion |
Adult male Lister Hooded rats 150–200 g C4 dorsal hemisection |
Both single treatments produced increases in sprouting and axon regeneration, but the combination treatment produced greater increases | Zhao et al (2013) |
| ChABC, PDGF, bFGF, EGF subarachanoid infusion for 7 days | Daily quadrupedal treadmill training 15 min a day for 3 weeks |
Adult female Wistar rats 250–275 g Rat T7 23.8 g clip compression 1 min. Acute treatment 4 days after injury |
Combined therapy significantly enhanced the neuroanatomical plasticity of major descending spinal tracts such as corticospinal and serotonergic‐spinal pathways. Structural changes did not translate to an additional long‐term improvement of locomotor parameters | Alluin et al (2014) |
| AAV10‐NT‐3 intraspinal injections |
Spinal electromagnetic stimulation every 2 days (2.8 T, 0.2 Hz, 35 min) Swimming and exercise ball training |
Adult female Sprague Dawley rats 210 g Rat T10 contusion 150 KDyne IH impactor Acute and chronic treatments; sequential electrical stimulation |
Acutely, the combination significantly improves electrophysiology recordings, narrow beam task, error ladder task and Catwalk gait parameters Chronic treatment also improved electrophysiology recordings when all treatments are combined. |
Petrosyan et al (2015) |
| ChABC intrathecal infusion 6 weeks after injury for 7 days | Quadrupedal treadmill exercise weeks 6–14; 30 min a day, 5 days a week |
Adult female Sprague Dawley rats 200–220 g Severe 250 KDyne contusion IH impactor Acute treatment |
Increases in spared tissue and neuronal fibre regeneration. No associated improvement to motor functions. | Shinozaki et al (2016) |
| Anti‐Nogo‐A 2‐week continuous infusion | Sequential (3 weeks after injury/1 week after last treatment) hindlimb bipedal treadmill training for 8 weeks, 5 days a week |
Adult female Sprague Dawley rats 200–250 g T‐Shaped lesion Transection of dorsomedial, dorsolateral, ventromedial CST |
Sequential training showed superior recovery of motor function. No improvement when treated in parallel | Chen et al (2017) |
Figure 2. Combinatory therapies for spinal cord injury.
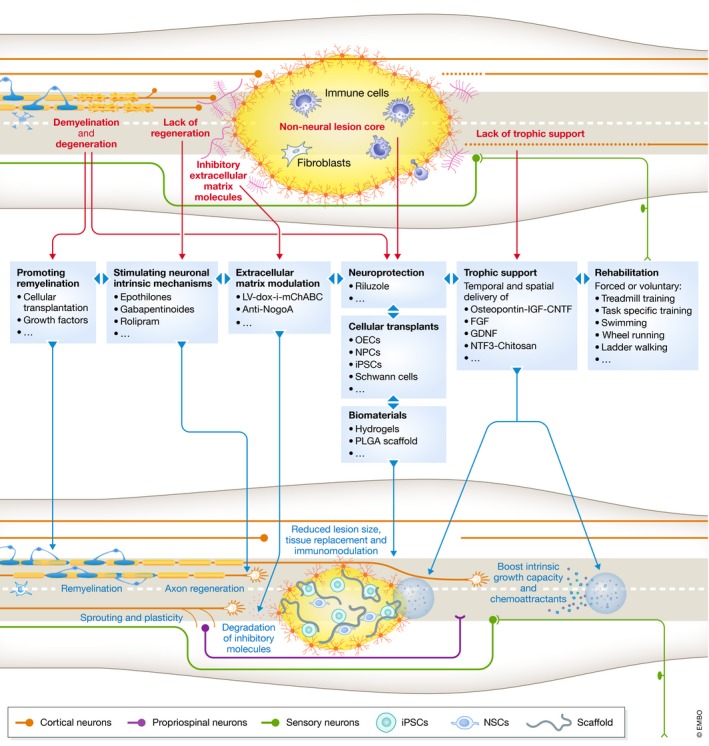
Experimental spinal cord injury research has resulted in a multitude of individual therapeutic possibilities yet recovery after injury remains incomplete. Combinatory approaches to address the seven targets will need to be a focus of translation studies. Tissue and cellular transplants will replace lost cells, among other regenerative functions. Removal of inhibitory factors such as CSPGs allows for enhanced axonal growth. Targeting neuron‐intrinsic mechanisms enhance intrinsic regenerative response which can then be directed through resupply of trophic support. Remyelination of demyelinated axons may improve axonal conduction and survival. Finally, rehabilitation functions in circuit remodelling and strengthens beneficial connections.
Combining cellular transplants with neurotrophins
Early attempts at transplantation of cells into the damaged spinal cord promoted some degree of axonal regeneration into the graft. However, axons fail to grow beyond the graft, transplants typically lack appropriate orientation, and the hostile adult spinal cord environment limits transplant survival and/or differentiation. The logical addition of neurotrophins was one of the first combinatory strategies to address these problems and many studies have since been conducted over the last three decades (Table 1). The first attempt at this strategy utilised Schwann cells in semipermeable guidance channels in combination with BDNF and NT‐3 delivery via a minipump in a thoracic transection model (Xu et al, 1995a). BDNF and NT‐3 infusion enhanced propriospinal axonal regeneration and, more significantly, promoted axonal regeneration of specific distant populations of brain stem neurons into grafts at the mid‐thoracic level in adult rat spinal cord. In the following years, many studies reported that combining growth factor delivery with a PNG is capable of allowing axons to extend beyond the graft, although this was often not associated with improvement to behavioural measures (Cheng et al, 1996, 2004; Ye & Houle, 1997; Dolbeare & Houle, 2003). This may reflect a lack of beneficial integration of the cells and a limitation of PNGs over other transplant types. Many combinatory strategies for PNGs have utilised FGF (Table 1). It appears that FGF is an important growth factor to include with cellular transplantation. Firstly, FGF tends to stimulate axonal elongation in a rectilinear rather than a branching pattern, which is critical for long‐distance regrowth. Secondly, FGF tends to act on reactive astrocytes to cause them to change their shapes into a more primitive bipolar morphology, which is critical to allow axons to pass the graft–host interface (Zhou et al, 2018).
It is apparent that the transplant and growth factor delivery methods are important considerations and they need to be spatially and temporally organised. Cell and drug delivery by injection or loaded gelfoam are substandard due to washing away of cells and short‐lived release of the factors, respectively. Osmotic pumps ensure long‐lasting local delivery but are invasive and can cause local tissue damage. Ex vivo gene therapy delivery of cells to secrete growth factors is limited for the recurring reason that the high concentration gradient of growth factors at the transplant site does not allow axons to leave the graft (Table 1; Nakahara et al, 1996; Blesch et al, 2004; Mitsui et al, 2005). This may possibly be overcome by further expression of growth factors beyond the graft such as what was observed after lentiviral NT‐3 administration beyond a bone marrow stromal cell transplant (Taylor et al, 2006). Non‐regulated viral vector gene expression of growth factors can allow for spatial expression of specific growth factors but is limited in that continued delivery after resolution is likely to be disadvantageous. The development of various natural and synthetic polymer biomaterials is advancing as a promising way to circumvent the limitations of the other delivery methods although they each also have benefits and limitations (Fuhrmann et al, 2017).
Fibrin matrix‐delivery of two human ESC‐derived NPCs with a cocktail of ten growth factors after severe SCI allowed for extensive axonal outgrowth of the grafted cells rostral and caudal from the transplant and the combination improved functional recovery (Lu et al, 2012). This experiment was later repeated using human IPSCs. A similar level of extensive axonal outgrowth from the grafted cells was present, although in this case there was no improvement to motor functions which may imply an inability of IPSCs to integrate into the hose tissue to form functional connections (Table 1; Lu et al, 2014). Extensive axon growth is not always beneficial as axons may overshoot and oversupply targets, leading to spasticity, neuropathic pain and worsened motor outcome. Another issue of the combination of transplants with growth factors is that growth factors may enhance uncontrolled differentiation of NSCs into astrocytes which may lead to allodynia or pluripotent cells to overproliferate or form tumours (Hofstetter et al, 2005). For example, in a subset of animals, the combination of ESC‐derived NPCs combined with platelet‐derived growth factor (PDGF) and NT‐3 led to tumour formation by 8 weeks after transplantation (Johnson et al, 2010a). Therefore, terminally differentiated cells such as Schwann cells or OECs may be a more suitable option. There is currently no gold standard cell type or growth factors, and combinations of the two for clinical translation and so it may be short‐sighted to progress with clinical trials before this information is attained.
Combination of cellular transplants with anti‐inhibitory therapies
Removing inhibitory elements through various means in conjunction with cellular transplantation has been investigated to enable regeneration in a more permissive environment (Table 2). Through such interventions, regenerating axons are capable of exiting the graft and are likely to form functional synaptic contacts. The first of these studies was in the form of administration of the IN‐1 antibody (anti‐Nogo‐A antibody) with human Schwann cells with aFGF–fibrin glue after acute thoracic transection in rats. Inclusion of the antibody and aFGF were necessary to support sprouting and reduce axon die‐back (Guest et al, 1997). Studies utilising ChABC later became a major focus in subsequent attempts that led to axonal growth beyond the grafts of Schwann cells, PNGs, OECs and NPCs (Chau et al, 2004; Houle et al, 2006; Vavrek et al, 2007; Tom & Houle, 2008; Alilain et al, 2011; Lee et al, 2013; DePaul et al, 2017). Unlike the combination of PNGs with neurotrophins, combinations with PNGs with ChABC appear to have much more pronounced effects on regeneration and functional recovery, indicating that unmasking neuroplasticity is crucial for the formation of functional connections (Yick et al, 2000; Houle et al, 2006; Alilain et al, 2011; Lee et al, 2013; DePaul et al, 2017). Likewise, similar subjective conclusions could be drawn for Schwann cells, OECs or NPCs, although direct comparisons to these relative roles have not been drawn (Table 2; Chau et al, 2004; Fouad et al, 2005; Vavrek et al, 2007; Karimi‐Abdolrezaee et al, 2010). Rather, growth factors do play an important role in the survival of the graft. Co‐treatment of Nogo receptor blocking peptide (NgR) with delivery of bFGF, EGF and PDGF with adult NSCPs could overcome substantial cell loss; the growth factors increased the survival of the transplanted cells while NgR had no effect on their survival, and NgR increased axonal regeneration and myelination (Guo et al, 2012). In another study, only the combination of NPCs with ChABC and the growth factors EGF, bFGF and PDGF‐AA could significantly improve neurobehavioural recovery without enhancing aberrant synaptic connectivity (Table 2; Karimi‐Abdolrezaee et al, 2010). Yet, all of these results were achieved from infusions or microinjections of ChABC enzyme which has suboptimal stability and activity in vivo compared to gene therapy approaches (Lee et al, 2010). The combination of the dox‐inducible LV‐mChABC vector with other therapies is yet to be reported. It would be interesting to combine such tuning of plasticity and delivery of growth factors.
Combining anti‐inhibitory therapies with neurotrophins
Combing neurotrophins with anti‐inhibitory therapies was conceived to promote axonal growth in a newly permissive environment (Table 3). The first attempt used ChABC with NT‐3‐loaded lipid microtubes embedded in agarose gel following T10 dorsal hemisection in rats (Lee et al, 2010). Animals treated with ChABC in combination with sustained NT‐3 delivery showed a significant improvement in locomotor function and enhanced growth of cholera toxin B subunit‐positive sensory axons as well as sprouting of serotonergic fibres. This result was replicated by an independent group in which animals receiving combined therapy displayed the most improved body stability and inter‐limb coordination (Garcia‐Alias et al, 2011). Only animals with the full combination treatment recovered consistent multisynaptic responses in these motor neurons indicating formation of a detour pathway around the injury site.
Similar to ChABC, anti‐Nogo‐A antibody in combination with NT‐3 delivery by nanoparticles in an adult rat clip compression model led to increased anatomical improvements in both treatments individually but only functional improvements in the combination group (Elliott Donaghue et al, 2016). This combinatory approach may be limited in the context of complete transection injuries since increasing neuronal plasticity through anti‐inhibitory therapies largely relies on spared circuitry and the formation of relay networks (Bradbury & McMahon, 2006). For example, in a model of complete transection, co‐treatment of Nogo receptor blocking peptide (NgR) with delivery of bFGF, EGF and PDGF with adult NSCPs did not have an effect on functional recovery or regeneration (Table 3; Guo et al, 2012). The growth factors, not the NgR, improved survival of the transplanted cells, while the NgR enhanced axonal regeneration and myelination. However, this is not always the case. Impressively, the combination of ChABC with neurotrophin delivery has also reported to be therapeutic in complete transection models in which ChABC typically has a very limited therapeutic benefit. ChABC and NGF delivery from an electrospun scaffold following thoracic complete transection in rats displayed a significant improvement in BBB functional recovery and that the electrospun matrix is an important factor in this observation (Colello et al, 2016). Likewise, antagonism of Nogo, EphrinB3 and sema4D receptor combined with the growth factors BDNF, NT‐3 and cAMP administration after complete transection reduced the volume of cavitation, facilitated axonal regeneration and promoted neuronal generation leading to enhanced locomotion recovery (Table 3; Li et al, 2016). How does this occur? In the latter study, it was argued that new‐born neurons generated at the lesion area could form the neuronal relay and enhance the locomotion recovery, but this is yet to be confirmed. There clearly are many variables between these experiments that complicate the interpretations of the outcomes, but further investigations and replications should result in more clarification.
Combination treatments involving targeting the intrinsic growth response
A less explored area of research is enhancing the regenerative programme of injured neurons through targeting intracellular pathways and mechanisms as a combinatory therapy. Excitingly, this could prove to be a promising avenue for recovery after SCI (Table 4). Activation of cAMP signalling by rolipram and further cAMP injections combined with Schwann cells transplantation to the injury site following spinal cord contusion in rats further increased axonal sparing, myelination and locomotor function compared to the individual components (Pearse et al, 2004b). It was apparent that rolipram was responsible for most of the beneficial effects and the transplants alone had a minimal effect (only significant improvements to myelination). This suggested that growth factors might be required to promote axonal growth through the graft. Indeed, rolipram combined with the further addition of modified Schwann cells to secrete a bifunctional neurotrophic molecule (D15A) with both NT‐3 and BDNF activity increased numbers of axons originating from the reticular formation below the lesion/implant area and provided the greatest improvement in hindlimb function (Flora et al, 2013). Similar to the previous study, rolipram was the main contributor to the beneficial effects compared to a minor improvement observed by BDNF + NT‐3. This indicates that activating the intrinsic regenerative programme is an approach that may be superior to cell‐ or growth factor‐based therapies (Table 4).
Microtubule stabilisation is fast becoming an attractive therapeutic option for promoting intrinsic regenerative capacity as well as limiting the deposition of inhibitory molecules in the injured CNS. Recently, enhancing and directing the growth of docetaxel‐stabilised microtubules by the co‐treatment of the growth factors BDNF and aFGF in liposome hydrogels in a rat thoracic contusion injury model was achieved (Wang et al, 2018). This application supported regeneration by improving neuronal survival and plasticity, and rendering a more permissive extracellular matrix environment, leading to significantly improvement of BBB score points compared to untreated controls (Wang et al, 2018). Co‐administration of taxol with the EGFR signalling antagonist cetuximab verified therapeutic effects towards inhibition of scar deposition and promotion of neuronal differentiation, axonal outgrowth and functional recovery in a severe SCI model in rats (Table 4; Fan et al, 2018). The precise role of cetuximab in this scenario is unclear, although it was demonstrated to promote the differentiation of injury‐activated endogenous‐NSC differentiation into neurons. There is clearly need for more investigations to continue in this area of research. The CRMP family of proteins regulate aspects of neurite growth through stabilising microtubules and phosphorylation of CRMP2 by GSK3β reduces CRMP2's binding affinity for tubulin heterodimers leading to microtubule depolymerisation (Fukata et al, 2002). Interestingly, preventing CRMP2 phosphorylation by pharmacological inhibition of GSK3β was found to be a critical mediator of the neurotrophic response in DRG neurite outgrowth (Nagai et al, 2016). This suggests that targeting actin dynamics may also potentiate the efficacy of neurotrophic delivery which could be an attractive strategy.
Combinations of therapies with rehabilitation
Despite the many efforts made by patients, physiotherapists and trial designers, rehabilitation as a treatment for SCI achieves a modest recovery at best. Could combining rehabilitation with therapies that aim to restore intrinsic growth capacity and/or remove inhibitory factors be more effective? Plasticity‐promoting therapies including ChABC can restore neuroplasticity to a level it appears in early childhood and promote compensatory sprouting of spared fibres to form new neural connections. Yet, there is frequently a mismatch between anatomical and functional recovery. For example, treatment of rat cervical spinal cord injuries with ChABC produced a modest recovery in CST function, as measured by skilled paw function despite a large observable increase in axonal regeneration and neuronal plasticity (Garcia‐Alias et al, 2008). Are new neuronal connections ineffective if the host does not know how to use them? Promoting plasticity by itself may not be sufficient to promote functional recovery if it leads to random new connections. The formation of appropriate connections in the spinal cord and brain may need to be driven by appropriate rehabilitation. Indeed, subsequent studies have shown that ChABC treatment combined with rehabilitation leads to enhanced recovery through opening a window during which rehabilitation becomes more effective in the newly plastic environment (Fawcett & Curt, 2009; Garcia‐Alias et al, 2009; Wang et al, 2011a; Shinozaki et al, 2016). Therefore, utilising appropriate rehabilitation regimes is emerging as a promising avenue for shaping the new circuits created by therapies into useful new connections (Table 5). However, the timing of the treatment and rehabilitation appears to be critical. In one experiment involving thoracic spinal cord contusion in rats, the animals developed an abnormal stepping pattern when rehabilitation was included in parallel with the treatment of anti‐Nogo‐A antibody (Maier et al, 2009). Similarly, synchronous treatment of anti‐Nogo‐A antibody with rehabilitation in a model of stroke resulted in “chaotic hyperinnervation” leading the researchers to hypothesise that rehabilitation at an early stage was reinforcing erroneous connections (Wahl et al, 2014). Instead, sequential rehabilitation regimes are astonishingly effective and in some cases can almost completely restore specific motor tasks (Table 5; Garcia‐Alias et al, 2009; Chen et al, 2017). More recently, endogenous CSPG degradation by an astrocyte‐selective AAV‐ADAMTS4 gene therapy combined with hindlimb rehabilitation enhanced functional recovery following rat thoracic SCI compared to either intervention alone (Griffin et al, 2020).
Current reports for the role of rehabilitation with cell transplantation are limited to one preclinical study which enlisted autologous bone marrow stem cells with swim training where a difference to functional parameters between groups was observed (Carvalho et al, 2008). As we discussed above, considering swim training is less effective than high‐intensity quadrupedal training, it would be of interest to test cellular transplantation with other regimes of rehabilitation. Rather, combining rehabilitation with cell transplants in human has progressed immediately to trials (Table 6). These trials have demonstrated that cell transplantation into the injury site seems to be safe and appears to only show beneficial effects for patients when combined with rehabilitation further highlighting the importance of combinatory approaches in the clinical setting (Li et al, 2015; Zhu et al, 2016; Anderson et al, 2017). Similarly, current reports for the role of rehabilitation with neurotrophic factors are limited to two preclinical studies. First, acute AAV‐mediated expression of NT‐3 combined with sequential spinal electromagnetic stimulation rehabilitation, swim training and exercise ball training improved electrophysiology recordings, narrow beam task, error ladder task and several Catwalk gait analysis parameters (Table 5; Petrosyan et al, 2015). This result was only observed with the combination of electrical stimulation plus exercise or electrical stimulation plus exercise plus AAV‐NT3, whereas any singular therapy had no effect. This indicates that electrical activity in the spinal cord may be a requirement for activity‐induced neuroplasticity. Second, intrathecal administration of ChABC, PDGF, bFGF and EGF combined with daily quadrupedal treadmill training leads to enhanced neuroanatomical plasticity of CST and the serotonergic‐spinal pathway, although these changes failed to translate to functional improvements (Alluin et al, 2014). Considering there are many variables between these two studies, at the stage it is difficult to draw larger conclusions.
Table 6.
Current published clinical trials for spinal cord injury
| Name of therapy | Mechanism | References | Key findings | Current status of trials |
|---|---|---|---|---|
| Methylprednisolone |
Neuroprotection: Anti‐inflammatory corticosteroid |
Bracken et al (1984, 1990, 1997), Matsumoto et al (2001) | Ultimately, no convincing improvement to motor and sensory functions. May lead infection, pulmonary, gastrointestinal complications |
Completed Clinical use in some countries but not all |
| GM‐1 ganglioside (Sygen) | Neuroprotection and regenerative properties | Geisler et al (1991, 2001) | No statistical significance between trial groups achieved |
Completed Rarely used in the clinical setting |
| Thyrotropin‐releasing hormone (TRH) |
Neuroprotection: Prevents apoptosis |
Pitts et al (1995) | No validity for clinical use |
Completed No current clinical use |
| Nimodipine |
Neuroprotection: Calcium‐channel blocker |
Pointillart et al (2000) | No significant difference among all groups in the trial |
Completed No current clinical use |
| GK‐11 (gacyclidine) |
Neuroprotection: NMDA receptor antagonist |
Tadie (2003) | No statistical significance between trial groups achieved |
Phase II completed No current clinical use |
| Transplantation of peripheral nerve graft and aFGF–fibrin glue |
Tissue replacement: Repair/regeneration |
Cheng et al (2004), Wu et al (2008, 2011) |
Treatment is well tolerated and safe Significant improvement in ASIA scores Impetus for a phase III trial |
Three phase I/II trials completed No current clinical use |
| Transplantation of autologous activated macrophages |
Tissue replacement: Repair/regeneration |
Knoller et al (2005) | Appears to be safe. Three patients improved ASIA score |
Phase I completed Phase II suspended for financial reasons |
| Human adipose tissue‐derived mesenchymal stem cells |
Tissue replacement: Repair/regeneration |
Ra et al (2011) |
Safe and tolerable; Impetus for subsequent trials |
Phase I completed Phase II trials underway Further trials underway |
| Cethrin (BA‐210; SPRING‐VX‐210) |
Repair/regeneration: RhoA blocker |
Fehlings et al (2011) |
Safe and tolerable Improved ASIA motor scores in cervical SCI patients Impetus for subsequent trial |
Phase I/IIa completed Phase IIb/III underway No current clinical use |
| Minocycline |
Neuroprotection: Tetracycline antibiotic |
Casha et al (2012) | No statistical significance between trial groups achieved |
Phase II completed Phase III RCT ongoing No current clinical use |
| Granulocyte‐stimulating factor | Neuroprotection | Takahashi et al (2012) |
Safe and tolerable ASIA score increased by one point in 9 of 16 patients |
Phase I/IIa Completed No current clinical use |
| Transplantation of autologous Schwann cells |
Tissue replacement: Repair/regeneration |
Review of 2 trials: Guest et al (2013) Anderson et al (2017) |
Initial trial reports from Iran and China suggest clinical safety Recovery of function motor and sensory function in many patients although more characterisation is needed |
Phase I completed No current clinical use |
| Riluzole |
Neuroprotection: Sodium‐channel blocker |
Grossman et al (2014) |
Safe and tolerable. Improved ASIA motor score Impetus for subsequent trial |
Phase I completed Phase IIB/III trials discontinued No current clinical use |
| Umbilical cord mesenchymal stem cell transplantation |
Tissue replacement: Repair/regeneration |
Cheng et al (2014) Zhu et al (2016) |
Significant improvements in some patients | Phase II completed |
| Olfactory ensheathing cells |
Tissue replacement: Repair/regeneration |
Meta‐analysis of eleven articles: Li et al (2015) |
OEC transplantation appears to be safe Evidence for efficacy is modest Further RCTs required to determine benefit |
Trials completed in Portugal, China and Australia Patients can pay for treatment in Portugal and China |
| Autologous mesenchymal stem cells |
Tissue replacement: Repair/regeneration |
Oh et al (2016) | Safe but has a very weak therapeutic effect | Phase III clinical Trial completed |
| Autologous bone marrow stem cell transplantation |
Tissue replacement: Repair/regeneration |
Satti et al (2016) | Safe and tolerable | Phase I completed |
| HuCNS‐SC (human foetal neural stem cells) |
Tissue replacement: Repair/Regeneration |
Levi et al (2018) | No safety concerns | Phase I/II for thoracic and cervical SCI complete |
| ATI‐355 (anti‐Nogo‐A antibody) |
Repair/regeneration: Blocks inhibitory Nogo |
Kucher et al (2018) |
Safe and tolerable Impetus for subsequent trial |
Phase I complete Phase II underway No current clinical use |
Rehabilitation is limited in complete injuries, yet considering neuronal networks below the lesion site remain active, combining treadmill rehabilitation with neurotransmitter administration and electrical modulations in the lumbar spinal cord can lead to the re‐establishment of walking ability in completely transected rats, a major step towards future therapy for completely paralysed patients (van den Brand et al, 2012). The use of electrical stimulation with rehabilitation in humans has been practised in the clinic for many decades. This can be achieved by functional electrical stimulation (FES; on the skin), epidural electrical stimulation (EES; stimulation of the spinal cord directly). FES has recently made large strides towards helping people with SCI. A major breakthrough showed that EES in combination with intense rehabilitation and neurotransmitter administration could help to restore walking function in three individuals with varying levels of incomplete SCIs (Wagner et al, 2018). This may be the result of the precise timing of the stimulation rather than continuous stimulation which may facilitate the naturally occurring signals. However, this study did not include a group of rehabilitation without stimulation and it is possible that the high‐intensity orthotic‐assisted rehabilitation programme is the main therapeutic component.
Towards clinical translation: current status
Many therapeutic strategies have been discussed in terms of their potential to address the human condition since their conception, yet relatively few SCI clinical trials have been completed (Table 6). For those that have reached clinical trials, none have reached general clinical application, aside from some countries that offer medical tourism for non‐validated therapies. Trials to overcome the devastating effects of SCI can be categorised into neuroprotective and regenerative approaches. Unfortunately, neuroprotection trials for human SCI have largely failed (Kim et al, 2017; Santamaria A.J., 2017). For example, trials from the neuroprotective interventions methylprednisolone, GM‐1 and gacyclidine failed to show sufficient statistical evidence for efficacy to be approved for clinical use (Table 6). Neuroregeneration/repair trials have emerged more recently, yet only a small number of treatments have reached the stage of human testing. These include anti‐Nogo‐A antibody therapy and Cethrin, and various cell‐based therapies, which have each completed phase I/IIa trials with no apparent adverse effects and some indication of functional improvements (Fehlings et al, 2011; Kucher et al, 2018). Trials of cell transplant therapies remain controversial in terms of ethical, safety and efficacy, but there is, at least, evidence that transplants may improve some sensory/motor and bladder functions (Fan et al, 2017). An exciting future prospect is for the human application of ChABC, which is yet to reach clinical trial; however, the evidence from the recent trials of SCI in dogs and rhesus monkeys supports ongoing preclinical efforts (Hu et al, 2018; Rosenzweig et al, 2019). The drive towards translating the promising results of ChABC to being clinically available is currently led by a collective team named the CHASE‐IT (ChABC for Spinal Injury Therapy) consortium. A focus of the consortium is now optimising the LV‐ChABC gene therapy approach to making it more clinically safe by using an AAV vector instead and with gene regulation (International Spinal Research Trust WEBARTICLE, 2019). Such approach would allow for robust, prolonged and controllable expression of mChABC in the human spinal cord which may exceed the efficacy achieved by infusion of the native enzyme. We are on the cusp of observing auspicious therapeutic candidates entering human trials; however, the road towards their clinical translation is arduous, and many factors need to be considered.
Problems for translation
One major problem towards clinical translation is the lack of robustness and reproducibility in preclinical studies (Schaffran & Bradke, 2019). With this notion, the “Facilities of Research Excellence‐Spinal Cord Injury” (FORE‐SCI) programme was developed to replicate key studies in the SCI field (Steward et al, 2012). Out of 12 studies selected to be replicated, it is perhaps worrying that only one study was fully replicated, six were not and the rest had mixed results or were only partially replicated. Therefore, it is imperative that a confirmatory replication of key aspects of the original study by an independent laboratory is conducted before the therapy progresses to the clinical stage. In academic research however, replication studies are rarely performed due to a lack of funding, the absence of recognition for performing such studies or problems with publishing. This is something that needs to change for us as a community to progress. This will further become more complicated when multiple strategies are combined.
Combinatorial approaches are likely to require tailored experimental design and analytical frameworks. The complexity of the combinatory treatments may project deceivingly incremental increases in efficacy. It is difficult to discern the involvement of the individual treatment entity when there are many variables that plague the reproducibility between studies and between laboratories. Facing a slew of possible therapies and combinations of such, it is a good idea to wait until we have more data before trials are initiated. What is the best cell type and biomaterial for tissue replacement? What are the ethical concerns or potential for harm when obtaining these cells? What growth factor or combination of growth factors is the best? What is the best method for removing inhibitory factors? Is the removal of multiple types of inhibitory factors required? Is the experimental model relevant to the human condition that the therapy is intended for? The experimental model should as closely represent the human condition, fine hemisection manipulations, for example, do not. Contusion and compression injuries are certainly more clinically relevant, though this does not make them the best choice for every experiment. Another consideration is that the spinal cord of humans is much thicker, has different anatomy, and drug penetration is very different. It would be preferential for therapies to be tested in multiple models, ideally including a larger animal (primate or porcine), to broaden our understanding of the limitations of the treatment. Another major problem is that outcome measures of motor improvements in humans are relatively insensitive compared to animal behavioural tests. The ASIA impairment scale is outdated, and a 5‐point scale ranging from full movement to no movement is underperforming. However, FORE‐SCI groups are developing additional tests of sensory and motor function that should allow more sensitive testing of outcomes after SCI in clinical trials. Another important consideration is that non‐motor system directly affects quality of life and often out‐ranks in importance to patients (Simpson et al, 2012). Often multiple aspects of health or motor functions are not reported in studies and so we would not see these kinds of side effects that could manifest in humans. Careful analysis of alterations in not only the locomotor system but also sensory, autonomic and possibly cognitive function should also be assessed in relevant animal models prior to entering human clinical trials. The complexity of clinical trial designs featuring combination therapies will also be an important factor to take into consideration during therapeutic translation (Fawcett et al, 2007). Ultimately, preclinical researchers and trial designers need to communicate better and more often. Trials must be large enough to prove efficacy, reflecting the parameters and outcomes of the preclinical studies.
Conclusions
SCI results in many problems that need to be addressed in order to discover a cure for the condition. Many promising individual therapies have been developed and are progressing towards clinical trials. However, many in vivo studies fail to be replicated and often fail to translate clinically which is unacceptable to individuals with SCI anxiously awaiting therapeutic options. The reproducibility of results from promising candidates must be assured before progressing to human clinical trials. Many combinational strategies have demonstrated greater beneficial outcomes than the individual components alone by addressing multiple aspects of the pathology, but these need further characterisation to ensure meaningful efficacy beyond their individual components. Clinical trials in the future will eventually need to focus on combining the best therapies that aim to address as many of the problems as possible. The scenario will become increasingly complex as this happens and conversation between basic researchers and clinicians must happen to ensure accurate study design and functional readouts.
Pending issues.
(i) What is the ideal combination of treatments?
(ii) How to assure the reproducibility of preclinical research?
(iii) What is the efficacy of combinatory treatments in larger animal models?
(iv) Are these feasible, safe and efficient for clinical translation?
(v) How to proceed with clinical trials involving combinatory therapies?
Conflict of interest
H. Witte, A. Ertürk, F. Hellal and F. Bradke filed a patent on the use of microtubule‐stabilising compounds for the treatment of lesion of CNS axons (European Patent no. 1858498; European patent application EP 11 00 9155.0; U.S. patent application 11/908,118). The authors declare no competing financial interests.
For more information
- (i)
- (ii)
- (iii)
- (iv)
- (v)
- (vi)
- (vii)
- (viii)
Acknowledgements
We would like to greatly thank Dr Brett Hilton and Dr Emily Burnside for critically reading and editing the manuscript. This work was supported by IRP, WfL, DFG, ERANET AXON REPAIR and ERANET RATER SCI (F.B.). F.B. is a member of the excellence cluster ImmunoSensation2, the SFBs 1089 and 1158, and is a recipient of the Roger de Spoelberch Prize.
EMBO Mol Med (2020) 12: e11505
See the Glossary for abbreviations used in this article.
Contributor Information
Jarred M Griffin, Email: jarred.griffin@dzne.de.
Frank Bradke, Email: frank.bradke@dzne.de.
References
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J (2011) Functional regeneration of respiratory pathways after spinal cord injury. Nature 475: 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluin O, Delivet‐Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi‐Abdolrezaee S (2014) Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS ONE 9: e111072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, Sahinkaya FR, McTigue DM (2011) Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 8: 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf‐Lavi E et al (2017) Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma 34: 2950–2963 [DOI] [PubMed] [Google Scholar]
- Anderson MA, O'Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B et al (2018) Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561: 396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, McTigue DM, Jakeman LB (2004) Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol 190: 17–31 [DOI] [PubMed] [Google Scholar]
- Apfel SC (2002) Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 50: 393–413 [DOI] [PubMed] [Google Scholar]
- Asboth L, Friedli L, Beauparlant J, Martinez‐Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P et al (2018) Cortico‐reticulo‐spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci 21: 576–588 [DOI] [PubMed] [Google Scholar]
- Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W (2017) Cell transplantation therapy for spinal cord injury. Nat Neurosci 20: 637–647 [DOI] [PubMed] [Google Scholar]
- Badhiwala JH, Wilson JR, Kwon BK, Casha S, Fehlings MG (2018) A review of clinical trials in spinal cord injury including biomarkers. J Neurotrauma 35: 1906–1917 [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG (2006) Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23: 318–334 [DOI] [PubMed] [Google Scholar]
- Barnett SC, Riddell JS (2007) Olfactory ensheathing cell transplantation as a strategy for spinal cord repair–what can it achieve? Nat Clin Pract Neurol 3: 152–161 [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ (2006) Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci 26: 10856–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez‐Munoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ (2014) Large‐scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci 34: 4822–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, Galino J, James ND, Hernandez‐Miranda LR, Dawes JM, Fricker FR, Garratt AN, McMahon SB, Ramer MS, Birchmeier C et al (2016) Neuregulin‐1 controls an endogenous repair mechanism after spinal cord injury. Brain 139: 1394–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, Burnside ER, Galino J, James ND, Bennett DLH, Bradbury EJ (2019) ErbB receptor signaling directly controls oligodendrocyte progenitor cell transformation and spontaneous remyelination after spinal cord injury. Glia 67: 1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey M, Aguayo AJ (1982) Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 296: 150–152 [DOI] [PubMed] [Google Scholar]
- Berrocal Y, Pearse DD, Singh A, Andrade CM, McBroom JS, Puentes R, Eaton MJ (2007) Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma 24: 1761–1772 [DOI] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Raff M (2006) Generation and characterization of oligodendrocytes from lineage‐selectable embryonic stem cells in vitro. Methods Mol Biol 330: 15–32 [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3: 537–544 [DOI] [PubMed] [Google Scholar]
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL (2012) Kruppel‐like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci USA 109: 7517–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH (2003) Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol 467: 403–417 [DOI] [PubMed] [Google Scholar]
- Blesch A, Yang H, Weidner N, Hoang A, Otero D (2004) Axonal responses to cellularly delivered NT‐4/5 after spinal cord injury. Mol Cell Neurosci 27: 190–201 [DOI] [PubMed] [Google Scholar]
- Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH (2012) Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp‐mediated effects. Exp Neurol 235: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W (2003) Suppression of Rho‐kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 22: 405–416 [DOI] [PubMed] [Google Scholar]
- Bowers CA, Kundu B, Hawryluk GW (2016) Methylprednisolone for acute spinal cord injury: an increasingly philosophical debate. Neural Regen Res 11: 882–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL Jr et al (1984) Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251: 45–52 [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo‐Summers L, Maroon J et al (1990) A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal‐cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 322: 1405–1411 [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo‐Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF et al (1997) Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277: 1597–1604 [PubMed] [Google Scholar]
- Bradbury EJ, King VR, Simmons LJ, Priestley JV, McMahon SB (1998) NT‐3, but not BDNF, prevents atrophy and death of axotomized spinal cord projection neurons. Eur J Neurosci 10: 3058–3068 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB (2006) Spinal cord repair strategies: why do they work? Nat Rev Neurosci 7: 644–653 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM (2011) Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84: 306–316 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnside ER (2017) Strategies to overcome the inhibitory environment of the spinal cord In Aospine master series: spinal cord injury and regeneration, Vialle LR. (ed.), pp 1–15. Nature Communications [Google Scholar]
- Bradbury EJ, Burnside ER (2019) Moving beyond the glial scar for spinal cord repair. Nat Commun 10: 3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S et al (2012) Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336: 1182–1185 [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel‐Bagden E, Reier PJ, Dai HN, McAtee M, Gao D (1993) Recovery of function after spinal cord injury: mechanisms underlying transplant‐mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol 123: 3–16 [DOI] [PubMed] [Google Scholar]
- Bregman BS, Broude E, McAtee M, Kelley MS (1998) Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp Neurol 149: 13–27 [DOI] [PubMed] [Google Scholar]
- Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, Sandhu F (2002) Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res 137: 257–273 [DOI] [PubMed] [Google Scholar]
- Bunge MB, Pearse DD (2003) Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev 40: 55–62 [DOI] [PubMed] [Google Scholar]
- Burnside ER, Bradbury EJ (2014) Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol Appl Neurobiol 40: 26–59 [DOI] [PubMed] [Google Scholar]
- Burnside ER, De Winter F, Didangelos A, James ND, Andreica EC, Layard‐Horsfall H, Muir EM, Verhaagen J, Bradbury EJ (2018) Immune‐evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB (2004) Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127: 34–44 [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21: 4731–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KA, Cunha RC, Vialle EN, Osiecki R, Moreira GH, Simeoni RB, Francisco JC, Guarita‐Souza LC, Oliveira L, Zocche L et al (2008) Functional outcome of bone marrow stem cells (CD45(+)/CD34(‐)) after cell therapy in acute spinal cord injury: in exercise training and in sedentary rats. Transplant Proc 40: 847–849 [DOI] [PubMed] [Google Scholar]
- Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ (2012) Results of a phase II placebo‐controlled randomized trial of minocycline in acute spinal cord injury. Brain 135: 1224–1236 [DOI] [PubMed] [Google Scholar]
- Ceto S, Sekiguchi K, Takashima Y, Nimmerjahn A, Tuszynski M (2019) Calcium imaging reveals host‐graft synaptic network formation in spinal cord injury. bioRxiv 10.1101/795583 [PREPRINT] [DOI]
- Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W (2005) Dose‐dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol 196: 352–364 [DOI] [PubMed] [Google Scholar]
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM (2004) Chondroitinase ABC enhances axonal regrowth through Schwann cell‐seeded guidance channels after spinal cord injury. FASEB J 18: 194–196 [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo‐A is a myelin‐associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN‐1. Nature 403: 434–439 [DOI] [PubMed] [Google Scholar]
- Chen K, Marsh BC, Cowan M, Al'Joboori YD, Gigout S, Smith CC, Messenger N, Gamper N, Schwab ME, Ichiyama RM (2017) Sequential therapy of anti‐Nogo‐A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol 292: 135–144 [DOI] [PubMed] [Google Scholar]
- Chen ZR, Ma Y, Guo HH, Lu ZD, Jin QH (2018) Therapeutic efficacy of cyclosporin A against spinal cord injury in rats with hyperglycemia. Mol Med Rep 17: 4369–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273: 510–513 [DOI] [PubMed] [Google Scholar]
- Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH (2004) Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine 1976) 29: E284–E288 [DOI] [PubMed] [Google Scholar]
- Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y (2014) Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med 12: 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello RJ, Chow WN, Bigbee JW, Lin C, Dalton D, Brown D, Jha BS, Mathern BE, Lee KD, Simpson DG (2016) The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote axon regrowth following spinal cord injury. J Tissue Eng Regen Med 10: 656–668 [DOI] [PubMed] [Google Scholar]
- Cote MP, Amin AA, Tom VJ, Houle JD (2011a) Peripheral nerve grafts support regeneration after spinal cord injury. Neurotherapeutics 8: 294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD (2011b) Activity‐dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma 28: 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV (2008) Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I et al (2009) Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M, Bradke F (2018) Axon regeneration in the central nervous system: facing the challenges from the inside. Annu Rev Cell Dev Biol 34: 495–521 [DOI] [PubMed] [Google Scholar]
- Curt A, Van Hedel HJ, Klaus D, Dietz V, Group E‐SS (2008) Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 25: 677–685 [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214: 931–933 [DOI] [PubMed] [Google Scholar]
- DePaul MA, Lin CY, Silver J, Lee YS (2017) Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci Rep 7: 9018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A et al (2006) Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol 60: 32–44 [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM (2009) Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma 26: 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditor DS, John SM, Roy J, Marx JC, Kittmer C, Weaver LC (2007) Effects of polyethylene glycol and magnesium sulfate administration on clinically relevant neurological outcomes after spinal cord injury in the rat. J Neurosci Res 85: 1458–1467 [DOI] [PubMed] [Google Scholar]
- Dolbeare D, Houle JD (2003) Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line‐derived neurotrophic factor (GDNF). J Neurotrauma 20: 1251–1261 [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209: 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin JN, Adler AF, Kumamaru H, Poplawski GHD, Lee‐Kubli C, Strobl H, Gibbs D, Kadoya K, Fawcett JW, Lu P et al (2018) Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun 9: 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Liu J, Moulson A, Plemel JR, Tetzlaff W (2018) Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat Commun 9: 3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Plemel JR, Tetzlaff W (2019) The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia [DOI] [PubMed] [Google Scholar]
- Dupraz S, Hilton BJ, Husch A, Santos TE, Coles CH, Stern S, Brakebusch C, Bradke F (2019) RhoA controls axon extension independent of specification in the developing brain. Curr Biol [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR (2004) Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 27: 145–167 [DOI] [PubMed] [Google Scholar]
- Elliott Donaghue I, Tator CH, Shoichet MS (2016) Local delivery of neurotrophin‐3 and anti‐NogoA promotes repair after spinal cord injury. Tissue Eng Part A 22: 733–741 [DOI] [PubMed] [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa‐Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic S, Hofmann F, Stein V et al (2010) Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol 20: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Engesser‐Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ (2007) Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci 25: 1931–1939 [DOI] [PubMed] [Google Scholar]
- Erturk A, Hellal F, Enes J, Bradke F (2007) Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci 27: 9169–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang JZ, Lin XM, Zhang L (2017) Stem cell transplantation for spinal cord injury: a meta‐analysis of treatment effectiveness and safety. Neural Regen Res 12: 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li X, Zhao Y, Xiao Z, Xue W, Sun J, Li X, Zhuang Y, Chen Y, Dai J (2018) Cetuximab and Taxol co‐modified collagen scaffolds show combination effects for the repair of acute spinal cord injury. Biomater Sci 6: 1723–1734 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J et al (2007) Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45: 190–205 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A (2009) Damage control in the nervous system: rehabilitation in a plastic environment. Nat Med 15: 735–736 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Verhaagen J (2018) Intrinsic determinants of axon regeneration. Dev Neurobiol 78: 890–897 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A et al (2011) A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28: 787–796 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Kim KD, Aarabi B, Rizzo M, Bond LM, McKerracher L, Vaccaro AR, Okonkwo DO (2018) Rho inhibitor VX‐210 in acute traumatic subaxial cervical spinal cord injury: design of the spinal cord injury rho inhibition investigation (SPRING) clinical trial. J Neurotrauma 35: 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Arnaud S, Simon M, Rabin O, D'Arbigny P (2000) Efficacy of a new neuroprotective agent, gacyclidine, in a model of rat spinal cord injury. J Neurotrauma 17: 1079–1093 [DOI] [PubMed] [Google Scholar]
- Ferguson IA, Koide T, Rush RA (2001) Stimulation of corticospinal tract regeneration in the chronically injured spinal cord. Eur J Neurosci 13: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Flora G, Joseph G, Patel S, Singh A, Bleicher D, Barakat DJ, Louro J, Fenton S, Garg M, Bunge MB et al (2013) Combining neurotrophin‐transduced schwann cells and rolipram to promote functional recovery from subacute spinal cord injury. Cell Transplant 22: 2203–2217 [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD (2005) Combining Schwann cell bridges and olfactory‐ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci 25: 1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM (2009) Anti‐Nogo‐A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates–re‐examination and extension of behavioral data. Eur J Neurosci 29: 983–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B, Aguayo AJ (1985) Injured neurons in the olfactory bulb of the adult rat grow axons along grafts of peripheral nerve. J Neurosci 5: 1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, Nagy A, Tator CH, Morshead CM, Shoichet MS (2016) Injectable hydrogel promotes early survival of induced pluripotent stem cell‐derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83: 23–36 [DOI] [PubMed] [Google Scholar]
- Fuhrmann T, Anandakumaran PN, Shoichet MS (2017) combinatorial therapies after spinal cord injury: how can biomaterials help?. Adv Healthc Mater 6 [DOI] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H et al (2002) CRMP‐2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 4: 583–591 [DOI] [PubMed] [Google Scholar]
- Garcia‐Alias G, Lin R, Akrimi SF, Story D, Bradbury EJ, Fawcett JW (2008) Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp Neurol 210: 331–338 [DOI] [PubMed] [Google Scholar]
- Garcia‐Alias G, Barkhuysen S, Buckle M, Fawcett JW (2009) Chondroitinase ABC treatment opens a window of opportunity for task‐specific rehabilitation. Nat Neurosci 12: 1145–1151 [DOI] [PubMed] [Google Scholar]
- Garcia‐Alias G, Petrosyan HA, Schnell L, Horner PJ, Bowers WJ, Mendell LM, Fawcett JW, Arvanian VL (2011) Chondroitinase ABC combined with neurotrophin NT‐3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. J Neurosci 31: 17788–17799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FH, Dorsey FC, Coleman WP (1991) Recovery of motor function after spinal‐cord injury–a randomized, placebo‐controlled trial with GM‐1 ganglioside. N Engl J Med 324: 1829–1838 [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D, Sygen Study G (2001) The Sygen multicenter acute spinal cord injury study. Spine 26: S87–S98 [DOI] [PubMed] [Google Scholar]
- Geoffroy CG, Zheng B (2014) Myelin‐associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol 27: 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K (2007) Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 130: 2993–3003 [DOI] [PubMed] [Google Scholar]
- Gordon T (2016) Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 13: 295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403: 439–444 [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM (2002) Nogo‐66 receptor antagonist peptide promotes axonal regeneration. Nature 417: 547–551 [DOI] [PubMed] [Google Scholar]
- Griffin JM, Fackelmeier B, Clemett CA, Fong DM, Mouravlev A, Young D, O'Carroll SJ (2020) Astrocyte‐selective AAV‐ADAMTS4 gene therapy combined with hindlimb rehabilitation promotes functional recovery after spinal cord injury. Exp Neurol 327: 113232 [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH (1997) Cellular delivery of neurotrophin‐3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci 17: 5560–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimpe B, Silver J (2004) A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci 24: 1393–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B et al (2014) A prospective, multicenter, phase I matched‐comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma 31: 239–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, Hesse D, Schnell L, Schwab ME, Bunge MB, Bunge RP (1997) Influence of IN‐1 antibody and acidic FGF‐fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J Neurosci Res 50: 888–905 [DOI] [PubMed] [Google Scholar]
- Guest J, Santamaria AJ, Benavides FD (2013) Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant 18: 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zahir T, Mothe A, Shoichet MS, Morshead CM, Katayama Y, Tator CH (2012) The effect of growth factors and soluble Nogo‐66 receptor protein on transplanted neural stem/progenitor survival and axonal regeneration after complete transection of rat spinal cord. Cell Transplant 21: 1177–1197 [DOI] [PubMed] [Google Scholar]
- Guven MB, Cirak B, Yuceer N, Ozveren F (1999) Is indomethacin harmful in spinal cord injury treatment? An experimental study. Pediatr Neurosurg 31: 189–193 [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Anenberg E, Harrison TC, Boyd JD, Murphy TH, Tetzlaff W (2016) Re‐establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci 36: 4080–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton BJ, Bradke F (2017) Can injured adult CNS axons regenerate by recapitulating development? Development 144: 3417–3429 [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Moulson AJ, Tetzlaff W (2017) Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett 652: 3–10 [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 99: 2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld‐Hallin Z, Kurpad SN, Frisen J, Olson L (2005) Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci 8: 346–353 [DOI] [PubMed] [Google Scholar]
- Houle JD, Ye JH, Kane CJ (1996) Axonal regeneration by chronically injured supraspinal neurons can be enhanced by exposure to insulin‐like growth factor, basic fibroblast growth factor or transforming growth factor beta. Restor Neurol Neurosci 10: 205–215 [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J (2006) Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci 26: 7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling DA, van Asseldonk JT, Lankhorst AJ, Hamers FP, Martin D, Bar PR, Joosten EA (1998) Local application of collagen containing brain‐derived neurotrophic factor decreases the loss of function after spinal cord injury in the adult rat. Neurosci Lett 251: 193–196 [DOI] [PubMed] [Google Scholar]
- Hu HZ, Granger N, Pai SB, Bellamkonda RV, Jeffery ND (2018) Therapeutic efficacy of microtube‐embedded chondroitinase ABC in a canine clinical model of spinal cord injury. Brain 141: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Hudry E, Vandenberghe LH (2019) Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron 102: 263 [DOI] [PubMed] [Google Scholar]
- Hurtado A, Podinin H, Oudega M, Grimpe B (2008) Deoxyribozyme‐mediated knockdown of xylosyltransferase‐1 mRNA promotes axon growth in the adult rat spinal cord. Brain 131: 2596–2605 [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez‐Pinilla F, Crowe MJ, Ying Z, Basso DM (2004) Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127: 1403–1414 [DOI] [PubMed] [Google Scholar]
- Hutson TH, Kathe C, Palmisano I, Bartholdi K, Hervera A, De Virgiliis F, McLachlan E, Zhou L, Kong G, Barraud Q et al (2019) Cbp‐dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci Transl Med 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR (2008) Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci 28: 7370–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Nakamura M, Yamane J, Katoh H, Okada S, Iwanami A, Watanabe K, Ishii K, Kato F, Fujita H et al (2005) Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth‐associated protein‐43‐positive fibers after rat spinal cord injury. Eur J Neurosci 22: 3036–3046 [DOI] [PubMed] [Google Scholar]
- Ilha J, Centenaro LA, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT et al (2011) The beneficial effects of treadmill step training on activity‐dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res 36: 1046–1055 [DOI] [PubMed] [Google Scholar]
- International Spinal Research Trust WEBARTICLE (2019) CHASE‐IT chondroitinase ABC for spinal injury therapy. https://www.spinal-research.org/chase-it
- Irvine KA, Blakemore WF (2008) Remyelination protects axons from demyelination‐associated axon degeneration. Brain 131: 1464–1477 [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Wei P, Guan Z, Stokes BT (1998) Brain‐derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol 154: 170–184 [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Hoschouer EL, Basso DM (2011) Injured mice at the gym: review, results and considerations for combining chondroitinase and locomotor exercise to enhance recovery after spinal cord injury. Brain Res Bull 84: 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Shea J, Muir EM, Verhaagen J, Schneider BL, Bradbury EJ (2015) Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp Neurol 271: 131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery ND, Lakatos A, Franklin RJ (2005) Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma 22: 1282–1293 [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama‐Elbert SE (2010a) Tissue‐engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter 6: 5127–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, Shiu A, Sakiyama‐Elbert SE (2010b) Controlled release of neurotrophin‐3 and platelet‐derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant 19: 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG (2006) Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26: 3377–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG (2010) Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 30: 1657–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi‐Abdolrezaee S, Eftekharpour E (2012) Stem cells and spinal cord injury repair. Adv Exp Med Biol 760: 53–73 [DOI] [PubMed] [Google Scholar]
- Kim YH, Ha KY, Kim SI (2017) Spinal cord injury and related clinical trials. Clin Orthop Surg 9: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M et al (2005) Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine 3: 173–181 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K et al (2012) Pre‐evaluated safe human iPSC‐derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One 7: e52787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O (2012) Cross‐talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 15: 1078–1087 [DOI] [PubMed] [Google Scholar]
- Kondo S, Takahashi K, Kinoshita Y, Nagai J, Wakatsuki S, Araki T, Goshima Y, Ohshima T (2019) Genetic inhibition of CRMP2 phosphorylation at serine 522 promotes axonal regeneration after optic nerve injury. Sci Rep 9: 7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucher K, Johns D, Maier D, Abel R, Badke A, Baron H, Thietje R, Casha S, Meindl R, Gomez‐Mancilla B et al (2018) First‐in‐man intrathecal application of neurite growth‐promoting anti‐nogo‐a antibodies in acute spinal cord injury. Neurorehabil Neural Repair 32: 578–589 [DOI] [PubMed] [Google Scholar]
- Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y (2005) Functional recovery after human umbilical cord blood cells transplantation with brain‐derived neutrophic factor into the spinal cord injured rat. Acta Neurochir 147: 985–992; discussion 992 [DOI] [PubMed] [Google Scholar]
- Lasiene J, Shupe L, Perlmutter S, Horner P (2008) No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci 28: 3887–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV (2010) Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci USA 107: 3340–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J (2013) Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci 33: 10591–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR (1998a) Full weight‐bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 80: 83–91 [DOI] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR (1998b) Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 79: 1329–1340 [DOI] [PubMed] [Google Scholar]
- Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M (2002) Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg 96: 197–205 [DOI] [PubMed] [Google Scholar]
- Levi AD, Okonkwo DO, Park P, Jenkins AL III, Kurpad SN, Parr AM, Ganju A, Aarabi B, Kim D, Casha S et al (2018) Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery 82: 562–575 [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G (1997) Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277: 2000–2002 [DOI] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G (2003) Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci 23: 727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E et al (2004) Blockade of Nogo‐66, myelin‐associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo‐66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci 24: 10511–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Adnan H, Xu B, Wang J, Wang C, Li F, Tang K (2015) Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: a systematic review and meta‐analysis. Eur Spine J 24: 919–930 [DOI] [PubMed] [Google Scholar]
- Li X, Han J, Zhao Y, Ding W, Wei J, Li J, Han S, Shang X, Wang B, Chen B et al (2016) Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater 30: 233–245 [DOI] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW (2000) Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA 97: 6126–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy K, Schmalz A, Hoche T, Jacobi A, Kreutzfeldt M, Merkler D, Bareyre FM (2018) Enhanced voluntary exercise improves functional recovery following spinal cord injury by impacting the local neuroglial injury response and supporting the rewiring of supraspinal circuits. J Neurotrauma 35: 2904–2915 [DOI] [PubMed] [Google Scholar]
- Loy K, Bareyre FM (2019) Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise‐induced neuroplasticity. Neural Regen Res 14: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Feron F, Mackay‐Sim A, Waite PM (2002) Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 125: 14–21 [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH (2003) Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 181: 115–129 [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH (2004) Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res 77: 174–191 [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Culbertson M, Graham L, Roskams AJ, Tuszynski MH (2006) Olfactory ensheathing cells do not exhibit unique migratory or axonal growth‐promoting properties after spinal cord injury. J Neurosci 26: 11120–11130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA et al (2012) Long‐distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150: 1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J et al (2014) Long‐distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier IC, Ichiyama RM, Courtine G, Schnell L, Lavrov I, Edgerton VR, Schwab ME (2009) Differential effects of anti‐Nogo‐A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain 132: 1426–1440 [DOI] [PubMed] [Google Scholar]
- Mao Y, Nguyen T, Tonkin RS, Lees JG, Warren C, O'Carroll SJ, Nicholson LFB, Green CR, Moalem‐Taylor G, Gorrie CA (2017) Characterisation of Peptide5 systemic administration for treating traumatic spinal cord injured rats. Exp Brain Res 235: 3033–3048 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H (2001) Early complications of high‐dose methylprednisolone sodium succinate treatment in the follow‐up of acute cervical spinal cord injury. Spine 1976) 26: 426–430 [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH (1998) Neurotrophin‐3 and brain‐derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci 18: 5354–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13: 11753–11772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR (1999) Induction of Eph B3 after spinal cord injury. Exp Neurol 156: 218–222 [DOI] [PubMed] [Google Scholar]
- Mitsui T, Fischer I, Shumsky JS, Murray M (2005) Transplants of fibroblasts expressing BDNF and NT‐3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol 194: 410–431 [DOI] [PubMed] [Google Scholar]
- Moon LDF, Bradbury EJ (2018) “Chase”: in dogged pursuit of a therapy for spinal cord injury. Brain 141: 941–943 [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS (2013) Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan‐based hydrogel. Biomaterials 34: 3775–3783 [DOI] [PubMed] [Google Scholar]
- Muir EM, Fyfe I, Gardiner S, Li L, Warren P, Fawcett JW, Keynes RJ, Rogers JH (2010) Modification of N‐glycosylation sites allows secretion of bacterial chondroitinase ABC from mammalian cells. J Biotechnol 145: 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Owada K, Kitamura Y, Goshima Y, Ohshima T (2016) Inhibition of CRMP2 phosphorylation repairs CNS by regulating neurotrophic and inhibitory responses. Exp Neurol 277: 283–295 [DOI] [PubMed] [Google Scholar]
- Nagoshi N, Okano H (2018) iPSC‐derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci 75: 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara Y, Gage FH, Tuszynski MH (1996) Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT‐3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant 5: 191–204 [DOI] [PubMed] [Google Scholar]
- Namiki J, Kojima A, Tator CH (2000) Effect of brain‐derived neurotrophic factor, nerve growth factor, and neurotrophin‐3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma 17: 1219–1231 [DOI] [PubMed] [Google Scholar]
- Nave KA (2010a) Myelination and support of axonal integrity by glia. Nature 468: 244–252 [DOI] [PubMed] [Google Scholar]
- Nave KA (2010b) Myelination and the trophic support of long axons. Nat Rev Neurosci 11: 275–283 [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier‐Lavigne M, Basbaum AI (2002) Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34: 885–893 [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT (2004) The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA 101: 8786–8790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A (2004) The pathology of human spinal cord injury: defining the problems. J Neurotrauma 21: 429–440 [DOI] [PubMed] [Google Scholar]
- Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR (2016) A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 78: 436–447; discussion 447 [DOI] [PubMed] [Google Scholar]
- Okubo T, Iwanami A, Kohyama J, Itakura G, Kawabata S, Nishiyama Y, Sugai K, Ozaki M, Iida T, Matsubayashi K et al (2016) Pretreatment with a gamma‐secretase inhibitor prevents tumor‐like overgrowth in human iPSC‐derived transplants for spinal cord injury. Stem Cell Rep 7: 649–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Nagoshi N, Kohyama J, Tsuji O, Shinozaki M, Shibata S, Kase Y, Matsumoto M, Nakamura M, Okano H (2018) Treatment with a gamma‐secretase inhibitor promotes functional recovery in human iPSC‐ derived transplants for chronic spinal cord injury. Stem Cell Rep 11: 1416–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Hagg T (1999) Neurotrophins promote regeneration of sensory axons in the adult rat spinal cord. Brain Res 818: 431–438 [DOI] [PubMed] [Google Scholar]
- Oudega M, Chao OY, Avison DL, Bronson RT, Buchser WJ, Hurtado A, Grimpe B (2012) Systemic administration of a deoxyribozyme to xylosyltransferase‐1 mRNA promotes recovery after a spinal cord contusion injury. Exp Neurol 237: 170–179 [DOI] [PubMed] [Google Scholar]
- Oudega M, Hao P, Shang J, Haggerty AE, Wang Z, Sun J, Liebl DJ, Shi Y, Cheng L, Duan H et al (2019) Validation study of neurotrophin‐3‐releasing chitosan facilitation of neural tissue generation in the severely injured adult rat spinal cord. Exp Neurol 312: 51–62 [DOI] [PubMed] [Google Scholar]
- Pearse DD, Marcillo AE, Oudega M, Lynch MP, Wood PM, Bunge MB (2004a) Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin‐10 enhance recovery? J Neurotrauma 21: 1223–1239 [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB (2004b) cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10: 610–616 [DOI] [PubMed] [Google Scholar]
- Petrosyan HA, Alessi V, Hunanyan AS, Sisto SA, Arvanian VL (2015) Spinal electro‐magnetic stimulation combined with transgene delivery of neurotrophin NT‐3 and exercise: novel combination therapy for spinal contusion injury. J Neurophysiol 114: 2923–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon A, Calancie B, Oudega M, Noga BR (2001) Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat thoracic spinal cord. J Neurosci Res 64: 533–541 [DOI] [PubMed] [Google Scholar]
- Pitts LH, Ross A, Chase GA, Faden AI (1995) Treatment with thyrotropin‐releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma 12: 235–243 [DOI] [PubMed] [Google Scholar]
- Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W (2014) Remyelination after spinal cord injury: is it a target for repair? Prog Neurogibol 117: 54–72 [DOI] [PubMed] [Google Scholar]
- Pointillart V, Petitjean ME, Wiart L, Vital JM, Lassie P, Thicoipe M, Dabadie P (2000) Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 38: 71–76 [DOI] [PubMed] [Google Scholar]
- Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, Horner PJ (2012) Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J Neurosci 32: 5120–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar V, Capila I, Bosques CJ, Pojasek K, Sasisekharan R (2005a) Chondroitinase ABC I from Proteus vulgaris: cloning, recombinant expression and active site identification. Biochem J 386: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar V, Raman R, Capila I, Bosques CJ, Pojasek K, Sasisekharan R (2005b) Biochemical characterization of the chondroitinase ABC I active site. Biochem J 390: 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34: 895–903 [DOI] [PubMed] [Google Scholar]
- Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ et al (2011) Safety of intravenous infusion of human adipose tissue‐derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20: 1297–1308 [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S (1928) Degeneration and regeneration of the nervous system (translated by R. M. May). Oxford University Press; [Google Scholar]
- Ramon‐Cueto A, Plant GW, Avila J, Bunge MB (1998) Long‐distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci 18: 3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ (1980) Axons from CNS neurons regenerate into PNS grafts. Nature 284: 264–265 [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM (1984) Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309: 791–793 [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Aguayo AJ (1984) Regeneration of long spinal axons in the rat. J Neurocytol 13: 165–182 [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S et al (2018) Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 24: 484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Salegio EA, Liang JJ, Weber JL, Weinholtz CA, Brock JH, Moseanko R, Hawbecker S, Pender R, Cruzen CL et al (2019) Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci 22: 1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozes Salvador V, Heredia F, Berardo A, Palandri A, Wojnacki J, Vivinetto AL, Sheikh KA, Caceres A, Lopez PH (2016) Anti‐glycan antibodies halt axon regeneration in a model of Guillain Barre Syndrome axonal neuropathy by inducing microtubule disorganization via RhoA‐ROCK‐dependent inactivation of CRMP‐2. Exp Neurol 278: 42–53 [DOI] [PubMed] [Google Scholar]
- Ruff CA, Wilcox JT, Fehlings MG (2012) Cell‐based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol 235: 78–90 [DOI] [PubMed] [Google Scholar]
- Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K et al (2015) Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschel J, Bradke F (2018) Systemic administration of epothilone D improves functional recovery of walking after rat spinal cord contusion injury. Exp Neurol 306: 243–249 [DOI] [PubMed] [Google Scholar]
- Sandner B, Puttagunta R, Motsch M, Bradke F, Ruschel J, Blesch A, Weidner N (2018) Systemic epothilone D improves hindlimb function after spinal cord contusion injury in rats. Exp Neurol 306: 250–259 [DOI] [PubMed] [Google Scholar]
- Santamaria AJ, Guest JD (2017) The current status of neuroprotection for spinal cord injury. Cham: Springer; [Google Scholar]
- Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG (2016) Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol 276: 59–71 [DOI] [PubMed] [Google Scholar]
- Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA (2016) Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 18: 518–522 [DOI] [PubMed] [Google Scholar]
- Schaffran B, Bradke F (2019) Reproducibility ‐ The key towards clinical implementation of spinal cord injury treatments? Exp Neurol 313: 135–136 [DOI] [PubMed] [Google Scholar]
- Sdrulla AD, Guan Y, Raja SN (2018) Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract 18: 1048–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Garcia‐Alias G, Choe J, Gad P, Gerasimenko Y, Tillakaratne N, Zhong H, Roy RR, Edgerton VR (2013) Use of quadrupedal step training to re‐engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 136: 3362–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki M, Iwanami A, Fujiyoshi K, Tashiro S, Kitamura K, Shibata S, Fujita H, Nakamura M, Okano H (2016) Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res 113: 37–47 [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M (2003) Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT‐3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol 184: 114–130 [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5: 146–156 [DOI] [PubMed] [Google Scholar]
- Simpson RK Jr, Baskin DS, Dudley AW, Bogue L, Rothenberg F (1991) The influence of long‐term nifedipine or indomethacin therapy on neurologic recovery from experimental spinal cord injury. J Spinal Disord 4: 420–427 [DOI] [PubMed] [Google Scholar]
- Simpson LA, Eng JJ, Hsieh JT, Wolfe DL, Spinal Cord Injury Rehabilitation Evidence Scire Research T (2012) The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma 29: 1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Popovich PG, Dietrich WD, Kleitman N (2012) Replication and reproducibility in spinal cord injury research. Exp Neurol 233: 597–605 [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W (2004) Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24: 2182–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadie M, Gaviria M, Mathe J‐F, Menthonnex P, Loubert G, Lagarrigue J, Saint‐Marc C, Argenson C, Kempf C, D'Arbigny Pet al., , , (2003) Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis 15: 363–376 [Google Scholar]
- Takahashi H, Yamazaki M, Okawa A, Sakuma T, Kato K, Hashimoto M, Hayashi K, Furuya T, Fujiyoshi T, Kawabe J et al (2012) Neuroprotective therapy using granulocyte colony‐stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur Spine J 21: 2580–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB (2002) Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci 22: 6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM (2004) Semaphorin3A inhibits nerve growth factor‐induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci 24: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K (2012) The endogenous proteoglycan‐degrading enzyme ADAMTS‐4 promotes functional recovery after spinal cord injury. J Neuroinflamm 9: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A (2006) Neurotrophin‐3 gradients established by lentiviral gene delivery promote short‐distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci 26: 9713–9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WEBARTICLE (2018) Vertex halts phase 2b spinal injury trial for futility. https://www.fiercebiotech.com/biotech/vertex-halts-phase-2b-spinal-injury-trial-for-futility
- Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F (2016) The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92: 419–434 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Curcio M, Laskowski CJ, Schaffran B, Flynn KC, Santos TE, Stern S, Hilton BJ, Larson MJE et al (2019) ADF/cofilin‐mediated actin turnover promotes axon regeneration in the adult CNS. Neuron [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Howland DR (2008) Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol 209: 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi‐Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ et al (2011) A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28: 1611–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7: 628–643 [DOI] [PubMed] [Google Scholar]
- Toft A, Scott DT, Barnett SC, Riddell JS (2007) Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain 130: 970–984 [DOI] [PubMed] [Google Scholar]
- Tom VJ, Houle JD (2008) Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol 211: 315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeberg L, Nagase H (2012) Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta 1824: 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A (1996) Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol 137: 157–173 [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, McKay HM, Blesch A (2002) Spontaneous and augmented growth of axons in the primate spinal cord: effects of local injury and nerve growth factor‐secreting cell grafts. J Comp Neurol 449: 88–101 [DOI] [PubMed] [Google Scholar]
- Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K (2008) Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol 210: 238–247 [DOI] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE (2003) Bone marrow‐derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci 23: 5197–5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrek R, Pearse DD, Fouad K (2007) Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma 24: 1667–1673 [DOI] [PubMed] [Google Scholar]
- Wagner FB, Mignardot JB, Le Goff‐Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seanez I, Caban M, Pirondini E et al (2018) Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563: 65–71 [DOI] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F et al (2014) Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 344: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH (2000) Effects of matrix metalloproteinase‐9 gene knock‐out on morphological and motor outcomes after traumatic brain injury. J Neurosci 20: 7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW (2011a) Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31: 9332–9344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Zeng YS, Wu JL, Li Y, Teng YD (2011b) Cograft of neural stem cells and schwann cells overexpressing TrkC and neurotrophin‐3 respectively after rat spinal cord transection. Biomaterials 32: 7454–7468 [DOI] [PubMed] [Google Scholar]
- Wang Z, Reynolds A, Kirry A, Nienhaus C, Blackmore MG (2015) Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J Neurosci 35: 3139–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang H, Xu H, Zhao Y, Li Z, Li J, Wang H, Zhuge D, Guo X, Xu H et al (2018) Novel multi‐drug delivery hydrogel using scar‐homing liposomes improves spinal cord injury repair. Theranostics 8: 4429–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FM, Cragg JJ, Jutzeler CR, Rohrich F, Weidner N, Saur M, Maier DD, Schuld C, Sites E, Curt A et al (2017) Early administration of gabapentinoids improves motor recovery after human spinal cord injury. Cell Rep 18: 1614–1618 [DOI] [PubMed] [Google Scholar]
- Warner FM, Jutzeler CR, Cragg JJ, Tong B, Grassner L, Bradke F, Geisler F, Kramer JK (2019) The effect of non‐gabapentinoid anticonvulsants on sensorimotor recovery after human spinal cord injury. CNS Drugs 33: 503–511 [DOI] [PubMed] [Google Scholar]
- Warren PM, Steiger SC, Dick TE, MacFarlane PM, Alilain WJ, Silver J (2018) Rapid and robust restoration of breathing long after spinal cord injury. Nat Commun 9: 4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzlawick R, Sena ES, Dirnagl U, Brommer B, Kopp MA, Macleod MR, Howells DW, Schwab JM (2014) Effect and reporting bias of RhoA/ROCK‐blockade intervention on locomotor recovery after spinal cord injury: a systematic review and meta‐analysis. JAMA Neurol 71: 91–99 [DOI] [PubMed] [Google Scholar]
- Willson CA, Irizarry‐Ramirez M, Gaskins HE, Cruz‐Orengo L, Figueroa JD, Whittemore SR, Miranda JD (2002) Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant 11: 229–239 [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F (2008) Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180: 619–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M, Willits RK (2006) Short‐duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 27: 328–331 [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Offermanns S (2014) Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discovery 13: 603–621 [DOI] [PubMed] [Google Scholar]
- Wu JC, Huang WC, Tsai YA, Chen YC, Cheng H (2008) Nerve repair using acidic fibroblast growth factor in human cervical spinal cord injury: a preliminary Phase I clinical study. J Neurosurg Spine 8: 208–214 [DOI] [PubMed] [Google Scholar]
- Wu JC, Huang WC, Chen YC, Tu TH, Tsai YA, Huang SF, Huang HC, Cheng H (2011) Acidic fibroblast growth factor for repair of human spinal cord injury: a clinical trial. J Neurosurg Spine 15: 216–227 [DOI] [PubMed] [Google Scholar]
- Wu X, Xu XM (2016) RhoA/Rho kinase in spinal cord injury. Neural Regen Res 11: 23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB (1995a) A combination of BDNF and NT‐3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol 134: 261–272 [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB (1995b) Axonal regeneration into Schwann cell‐seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol 351: 145–160 [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB (1997) Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol 26: 1–16 [DOI] [PubMed] [Google Scholar]
- Ye JH, Houle JD (1997) Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol 143: 70–81 [DOI] [PubMed] [Google Scholar]
- Yick LW, Wu W, So KF, Yip HK, Shum DK (2000) Chondroitinase ABC promotes axonal regeneration of Clarke's neurons after spinal cord injury. Neuroreport 11: 1063–1067 [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez‐Pinilla F (2005) Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol 193: 411–419 [DOI] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW (2013) Combination treatment with anti‐Nogo‐A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci 38: 2946–2961 [DOI] [PubMed] [Google Scholar]
- Zhou L, Shine HD (2003) Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J Neurosci Res 74: 221–226 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Li J, Li X, Xiao J (2018) Fibroblast growth factors in the management of spinal cord injury. J Cell Mol Med 22: 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Poon W, Liu Y, Leung GK, Wong Y, Feng Y, Ng SCP, Tsang KS, Sun DTF, Yeung DK et al (2016) Phase I‐II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant 25: 1925–1943 [DOI] [PubMed] [Google Scholar]
- Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z (2013) Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci 33: 15350–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]


