Abstract
Driver somatic mutations for aldosterone excess have been found in approximately 90% of aldosterone-producing adenomas (APAs) using an aldosterone synthase (CYP11B2)-guided sequencing approach. In the present study, we identified a novel somatic CACNA1H mutation (c.T4289C, p.I1430T) in an APA without any currently known aldosterone-driver mutations using CYP11B2 immunohistochemistry-guided whole exome sequencing. The CACNA1H gene encodes a voltage dependent T-type calcium channel alpha-1H subunit. Germline variants in this gene are known as a cause of familial hyperaldosteronism IV. Targeted next-generation sequencing detected identical CACNA1H variants in two additional APAs in a cohort of the University of Michigan, resulting in a prevalence of 4% (3/75) in APAs. We tested the functional effect of the variant on adrenal cell aldosterone production and CYP11B2 mRNA expression using the human adrenocortical HAC15 cell line with a doxycycline-inducible CACNA1HI1430T mutation. Doxycycline treatment increased CYP11B2 mRNA levels as well as aldosterone production, supporting a pathologic role of the CACNA1H p.I1430T mutation on the development of primary aldosteronism. In conclusion, somatic CACNA1H mutation is a genetic cause of APAs. Although the prevalence of this mutation is low, this study will provide better understanding of molecular mechanism of inappropriate aldosterone production in APAs.
Keywords: Aldosterone, Primary aldosteronism, Aldosterone-producing adenoma, CYP11B2, Mutation, Calcium channel
INTRODUCTION
Somatic mutations that cause excess aldosterone production have been identified in aldosterone-producing adenomas (APAs). The affected genes include KCNJ51, CACNA1D2, 3, ATP1A14, and ATP2B34. Somatic mutations in these genes alter intracellular ion homeostasis, resulting in increased aldosterone synthase (CYP11B2) expression and enhanced aldosterone production. CYP11B2 is a steroidogenic enzyme that is required for the final steps of aldosterone biosynthesis. Because of the critical role of CYP11B2 in aldosterone production, immunohistochemistry (IHC) for this enzyme allows identification of cells involved in physiologic or pathologic production of aldosterone. To improve the accuracy for detection and subsequent analysis of APA, we recently developed a CYP11B2 IHC-guided targeted next-generation sequencing (NGS) method using formalin-fixed, paraffin-embedded (FFPE) material. Using this approach, somatic mutations in aforementioned aldosterone-driver genes were identified in nearly 90% of APAs5, 6. However, there are still some mutation negative populations of tumors that are confirmed to be APA by CYP11B2 IHC.
In the present study, we performed whole exome sequencing (WES) on a mutation-negative APA by CYP11B2 IHC-guided targeted NGS. WES identified a somatic mutation in CACNA1H, encoding voltage-dependent T-type calcium channel subunit alpha-1H, in which germline variants have been identified in early-onset patients with primary aldosteronism (PA)7. We further investigated the pathophysiologic role of the CACNA1H variant using human adrenocortical cell line HAC15 cells.
MATERIALS AND METHODS
The authors declare that all supporting data are available within the article and its online-only Data Supplement.
Patients
PA patients who underwent unilateral adrenalectomy at the University of Michigan were studied. The diagnosis of PA was made according to the institutional consensus available at the time or the Endocrine Society Clinical Practice guideline8. Patients for mutational analysis were selected based on the availability of archival formalin-fixed paraffin-embedded (FFPE) blocks of resected adrenals. This study was approved by Institutional Review Boards at the University of Michigan. A waiver of informed consent was granted for the use of specimens for the experiments in this study (HUM00083056).
Whole exome sequencing on DNA from FFPE
Based on our somatic mutation prevalence study using CYP11B2-IHC guided targeted NGS approach5, 6, whole exome sequencing (WES) was conducted on DNA from a mutation negative APA paired with matched adjacent normal tissue to identify somatic variants. In brief, APA (identified by CYP11B2 IHC9) and adjacent normal adrenal FFPE tissue slides were scraped separately using disposable scalpels. Genomic DNA (gDNA) was isolated using QIAGEN AllPrep DNA/RNA FFPE kit (QIAGEN). WES was performed using standard protocols in our Clinical Laboratory Improvement Amendments (CLIA) compliant sequencing lab10, 11. 500ng of gDNA was sheared using a Covaris S2 to a peak target size of 250 base pairs (bp). Fragmented DNA was concentrated using AMPure beads, followed by end-repair, A-base addition, ligation of the Illumina indexed adapters, and size selection on 3% Nusieve agarose gels (Lonza). Fragments between 300 to 350 bp were recovered, amplified using Illumina index primers, and purified by AMPure beads. One microgram of the library was hybridized to the Agilent SureSelect Human All Exon V4. The targeted exon fragments were captured and enriched following the manufacturer’s protocol (Agilent). Paired-end whole-exome libraries were analysed by an Agilent 2100 Bioanalyzer and DNA 1000 reagents and sequenced using an Illumina HiSeq HiSeq 2500 (Illumina Inc). The primary base call files were converted into FASTQ sequence files using the bcl2fastq converter tool bcl2fastq-1.8.4 in the CASAVA 1.8 pipeline.
Ion torrent targeted NGS
To validate the variants identified by WES, we have developed a new Ampliseq multiplex PCR based panel for targeted NGS using Ion Torrent Sequencing (panel v3). The panel targets the complete coding sequence of the following genes: KCNJ5, CACNA1D, ATP1A1, ATP2B3 CACNA1H and PRKACA. Known oncogenic hotspots in GNAS and CTNNB1 were also targeted. For CACNA1H, the amplicons were redesigned for increased specificity compared to our previous panel, and p.I1430T was better targeted due to the previous version having common SNPs in the binding site. The methods for library preparation and sequencing were performed as described previously9. Somatic variants were identified using our validated pipelines as described previously5, 12. The CACNA1H p.I1430T variant was further confirmed by direct Sanger sequencing as described previously13. The primer sequence is available in online-only Data supplement.
Lentiviral transduction and cell experiments
To assess the effect of newly identified CACNA1H variant p.I1430T, the variant was cloned into the doxycycline-inducible lentiviral vector pCLX-pTF- R1-DEST-R2-EBR6514 via the shuttle vector pENTR1A-GFP-N2 (FR1)15 to generate the lentivector pCLX-pTF-Blast-CACNA1HI1430T. HAC15 cells16 were transduced with lentivirus (HAC15-dox-CACNA1HI1430T) followed by antibiotic selection with blasticidin (1 ¼g/mL). Since the transduction efficiency was relatively low even after antibiotic selection, several clones were isolated using cloning cylinders. A doxycycline-responsive clone (clone 6) was used for further cell experiments. Cells were cultured in DME/F12 medium containing 10% Cosmic Calf Serum, ITS+ Premix, and antibiotics. The detail of cell-based experimental protocols, including quantitative real-rime RT-PCR17–19 and aldosterone quantification by LC-MS/MS (Table S1 and Table S2) are described in online-only Data Supplement.
Statistical analysis
Results of cell-based experiments are given as means ± S.E.M. The data were analyzed with unpaired t test for comparison of samples from two groups. One-way ANOVA method was applied for comparison of samples from three groups. Results were considered significantly different when the P value was less than 0.05. GraphPad Prism ver. 8.0.0 was used for the analyses.
RESULTS
Identification of novel somatic mutations in APA
We performed WES on FFPE DNA from an APA that was found to be negative for somatic mutations previously shown to cause APA using CYP11B2 IHC-guided targeted NGS. This case was reported as Case 5 in our previous publication9. The tumor had distinct heterogeneity in CYP11B2 expression within the tumor. Genomic DNA was selectively isolated from CYP11B2-expressing region and matched adjacent normal adrenal DNA was also used for somatic variant call. For the tumor sample, a total of 103,256,358 reads (85.7% alignment to hg19 reference genome) were obtained for an effective average coverage of 114x per base; for the matched normal sample, a total of 72,114,926 reads (89.6% alignment to hg19 reference genome) provided an effective coverage of 137x per base. No major copy-number variation was observed (Figure S1). One novel somatic mutation in CACNA1H (c.T4289C, p.I1430T, NM_001005407) was identified only in the tumor (Table 1). Although germline CACNA1H variants have been reported as a cause of familial hyperaldosteronism IV (FH-IV)7, 20, somatic mutation in this gene has not been documented in APA.
Table 1.
Results of whole exome sequencing
| NGS ID | Gene | Type | Base changes | Amino Acid changes | Exon | Ref seq | VF (Tumor) | VF (Normal) |
|---|---|---|---|---|---|---|---|---|
| APA3 | CACNA1H | Missense | c.T4289C | p.I1430T | 22 | NM_001005407 | 54/150(36%) | 0/123(0%) |
VF, variant allele frequency
Prevalence of somatic CACNA1H mutations in APA
To validate the significance of somatic mutations in CACNA1H, targeted NGS using our newly developed panel was performed on eight mutation negative APA samples from eight white patients5. Of them, two had the identical somatic CACNA1H p.1430T mutation (Table 2), resulting in an overall prevalence of 4% (3/75) in APA from PA patients (Table S3). Baseline clinical characteristics of patients with CACNA1H-mutated APA are summarized in Table S4. The histologic characteristics of CACNA1H-mutated APA are shown in Figure 1A–1D. Interestingly, two of them showed distinct heterogeneity of tumor CYP11B2 expression. One is shown in Figure 1 and the other was reported in our previous study as Case 59. The CYP11B2 expressing cells were mainly composed of lipid poor compact cells and mostly negative for CYP17A1, which is required for cortisol synthesis in normal physiology. The CACNA1H variant was identified only in the CYP11B2-positive region of the tumor (Figure 1E and 1F).
Table 2.
Targeted ion torrent NGS results
| NGS ID | Gene | Reference allele | Variant allele | Amino Acid change | FDP | VF (%) |
|---|---|---|---|---|---|---|
| APA3 | CACNA1H | T | C | p.I1430T | 1999 | 34 |
| APA_UM60 | CACNA1H | T | C | p.I1430T | 1994 | 36 |
| APA_UM90 | CACNA1H | T | C | p.I1430T | 1999 | 24 |
There was no evidence of the variants in adjacent normal adrenal tissue or CYP11B2-negative tumor regions by Sanger sequencing or targeted NGS. FDP, flow-corrected read depth; VF, variant allele frequency.
Figure 1. Histologic and genetic heterogeneity of an aldosterone-producing adenoma with somatic CACNA1H mutation.
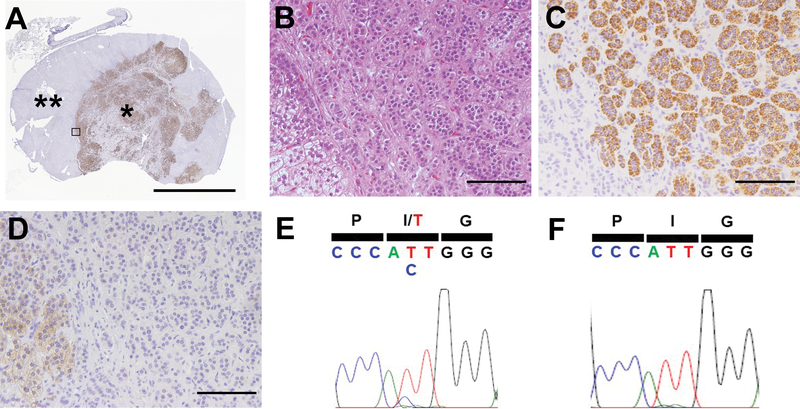
A. Low magnification image showing CYP11B2 IHC for the resected adrenal tumor from a patient with primary aldosteronism (brown, CYP11B2). Boxed area indicates the region where high magnification pictures were captured. Scale bar, 5 mm. B-D. High magnification micrographs of the tumor. Scale bars, 100 ¼m. B. Hematoxylin and eosin staining. C. CYP11B2 IHC. D. CYP17A1 IHC. E and F. Results of Sanger sequencing of CYP11B2-expressing tumor region (*, indicated in A) and CYP11B2-negative tumor region (**, indicated in A), respectively. IHC, immunohistochemistry.
Effect of CACNA1H mutation on adrenal cell aldosterone production and CYP11B2 mRNA expression
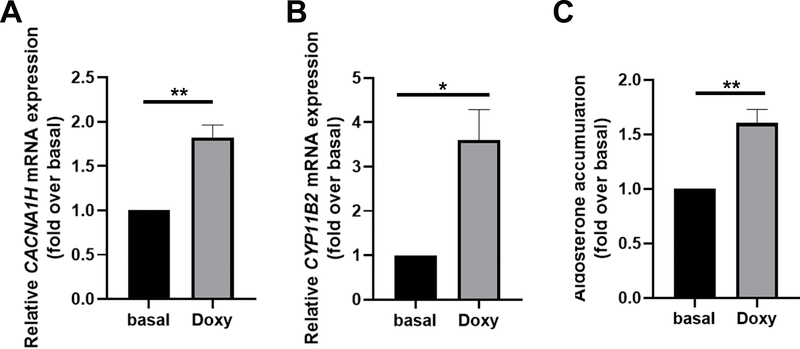
To assess the effect of the CACAN1H p.I1430T mutation on aldosterone production, we developed a human adrenocortical cell line using HAC15 cells with doxycycline-mediated conditional expression of CACNA1HI1430T. Doxycycline treatment successfully induced expression of the CACNA1H transcript carrying the mutation (Figure 2A). Induction of CACNA1HI1430T increased CYP11B2 mRNA expression by 3.6-fold vs basal (no doxycycline treatment) (P<0.05 vs. basal, Figure 2B). LC-MS/MS analysis revealed that the induction of CACNA1HI1430T gene led to increased aldosterone accumulation (1.6-fold vs basal, P<0.01, Figure 2C). CACNA1HI1430T enhanced angiotensin II stimulation of HAC15 cell CYP11B2 mRNA expression (Figure S2A). However, the trend for CACNA1HI1430T enhancement of angiotensin II stimulated aldosterone production did not reach statistical significance due to a large experimental variation in the aldosterone responses between independent experiments (Figure S2B). The results support the concept that the CACNA1H variant is a cause of excess aldosterone production.
Figure 2. Effect of CACNA1HI1430T on aldosterone production and CYP11B2 expression in HAC15 cells.
A. Induction of CACNA1HI1430T in HAC15 cells after treatment of 1 ¼g/mL doxycycline. B. Effect of the induction of CACNA1HI1430T on CYP11B2 mRNA expression. C. Effect of the induction of CACNA1HI1430T on HAC15 cell aldosterone production. Results represent means ± SEM. Experimental data represent a minimum of three independent experiments. Statistical analyses were performed using unpaired t test. *P <0.05 vs basal, ** P <0.01 vs basal.
DISCUSSION
The role of calcium signaling on the regulation of adrenal aldosterone production has been well studied 21. Most of PA-related somatic and germline mutations affect intracellular calcium homeostasis, supporting the importance of calcium signaling pathways in pathologic settings as well as adrenal physiology. Somatic mutations in APA have been found in approximately half of APA using conventional hot-spot Sanger sequencing of DNA isolated from pathology-provided tumor tissue without consideration of its CYP11B2 expression 22. We have recently developed a more targeted method of capturing CYP11B2 expressing tumor cells followed by deep sequencing. This approach led to a nearly 90% detection rate of aldosterone-driver mutations in APAs5, 6. A CYP11B2-guided approach is very important in the analysis of APAs because a discordance between imaging and immunohistochemical findings has been observed in a subset of PA cases 23. In this study, we were able to perform CYP11B2 IHC-guided WES, which identified a novel somatic CACNA1H mutation (p.I1430T). The identical mutation was found in two additional APAs.
The CACNA1H gene encodes voltage-gated T-type calcium channel alpha subunit Cav3.2 which expresses in the adrenal zona glomerulosa7. Several germline CACNA1H variants have been reported in PA patients7, 20. This condition is currently considered as FH-IV (OMIM #617027). Scholl et al.7 initially reported recurrent gain-of-function CACNA1H mutation (p.M1549V) in young-onset PA patients. The CACNA1H p.M1549V variant is located in the S6 segment of repeat III of Cav3.2. Impaired channel inactivation and a shift of activation to less depolarizing potentials were observed in whole-call patch clamp experiments using HEK293T cells transfected with CACNA1HM1549V7. This leads to increased calcium influx in adrenal cells. In vitro studies using human adrenocortical HAC15 cells conditionally expressing CACNA1HM1549V further revealed increased CYP11B2 mRNA levels and enhanced aldosterone production 24.
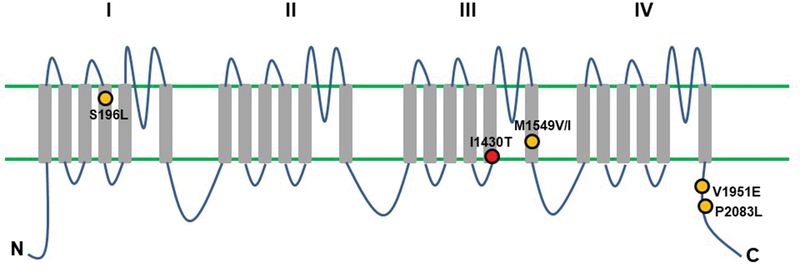
The CACNA1H p.I1430T variant, which was recurrently identified in our cohort, lies in the S5 segment of repeat III (Figure 3). HAC15 cells with induced expression of CACNA1H p.I1430T displayed increased aldosterone production as well as CYP11B2 mRNA expression, supporting a pathologic role of this variant. Functional characterization of any newly identified variant is very important. Of note, many of the somatic mutations thus far identified in APAs have not been functionally validated. Specifically, somatic mutations in CACNA1D are widely spread throughout the channel and their impact on aldosterone production is not clear. For example, a germline CACNA1D p.V401L variant has also been identified in a patient with autism spectrum disorders and epilepsy without any endocrine abnormality25. In addition, extensive electrophysiological analysis of the gating properties of CACNA1D p.M1354I, which was previously identified in an APA2, demonstrated an absence of any gain-of-function effect, suggesting non-pathogenic role of the variant on aldosterone production26.
Figure 3. CACNA1H mutations identified in patients with primary aldosteronism.
Transmembrane structure of Cav3.2 is described. Cav3.2 contains four homologous repeats (I-IV). Each domain contains six transmembrane segments (S1-S6). Orange circles indicate previously reported gain-of-function germline CACNA1H variants7, 20. The red circle indicates the somatic CACNA1H mutation identified in this study. The p.I1430T mutation is located in S5 of repeat III.
The prevalence of somatic CACNA1H mutations in APA appears to be relatively low with similar prevalence to that seen for ATP2B3 mutations. Interestingly, two out of three CACNA1H-mutated APAs showed distinct heterogeneity of CYP11B2 and CYP17A1 expression and the somatic CACNA1H mutation was observed only in CYP11B2-expressing area of the tumor, suggesting a unique developmental mechanism of CACNA1H-mutated APA. The effect of the CACNA1H mutation on cell proliferation or apoptosis is also of great interest and will need to be addressed in future studies
In conclusion, we identified a recurrent somatic CACNA1H mutation in a subset of APA. The CACNA1H p.I1430T mutation was functionally characterized as having the ability to increase CYP11B2 mRNA expression and adrenal cell aldosterone production. The significance of somatic CACNA1H mutation will need to be further validated in other cohorts.
PERSPECTIVES
A recurrent somatic CACNA1H mutation was found in APAs without any other known aldosterone-driver gene mutation. The pathologic role of the variant in enhanced aldosterone production was confirmed by in vitro studies using a mutant gene inducible cell model. Although CACNA1H-mutated APA represents a small subset of APAs, the identification of somatic CACNA1H mutations provides better understanding of molecular pathogenesis of APA.
Supplementary Material
Novelty and Significance
What Is New?
A recurrent somatic mutation in CACNA1H, which encodes a voltage dependent T-type calcium channel alpha-1H subunit, was identified in a subset of aldosterone-producing adenomas.
In vitro studies suggest that the CACNA1H p.I1430T variant has a pathologic potential to increase aldosterone production and CYP11B2 expression.
What Is Relevant?
Genetic alterations in the CACNA1H gene can cause not only familial hyperaldosteronism type IV (germline mutation) but also aldosterone-producing adenoma (somatic mutation).
Summary
Somatic CACNA1H mutation is a genetic cause for a rare subset of aldosterone-producing adenomas. Our study expands our understanding of molecular mechanisms leading to the inappropriate aldosterone production in aldosterone-producing adenomas.
ACKNOWLEDGEMENTS
We would like to thank Michelle Vinco and Farah Keyoumarsi at the University of Michigan for assistance in slide preparation. We also would like to thank Marcin Cieslik, Fengyun Su, Rui Wang, Xuhong Cao, and Arul M. Chinnaiyan at the University of Michigan for whole exome sequencing.
SOURCES OF FUNDING
This work was supported by grants from American Heart Association (17SDG33660447 to K. Nanba, 14SDG17990000 to T. Else and 16POST29900003 to N. G. Hattangady), National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 to W.E. Rainey), and National Heart, Lung, and Blood Institute (HL130106 to T. Else). S.A. Tomlins was supported by the A. Alfred Taubman Medical Research Institute and a National Cancer Institute Grant CA46592 (to the Michigan Cancer Center Core).
Footnotes
DISCLOSURES
S.A. Tomlins is co-founder of, equity holder in, and current employee of Strata Oncology.
References
- 1.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–60. [DOI] [PubMed] [Google Scholar]
- 3.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–4, 444e1–2. [DOI] [PubMed] [Google Scholar]
- 5.Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA and Rainey WE. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab. 2018;103:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension. 2019;73:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M and Young WF Jr, The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–916. [DOI] [PubMed] [Google Scholar]
- 9.Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA and Rainey WE. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2016;101:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell. 2015;162:454. [DOI] [PubMed] [Google Scholar]
- 12.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K and Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112:E4591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanba K, Omata K, Tomlins SA, Giordano TJ, Hammer GD, Rainey WE and Else T. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol. 2016;175:K1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giry-Laterriere M, Cherpin O, Kim YS, Jensen J and Salmon P. Polyswitch lentivectors: “all-in-one” lentiviral vectors for drug-inducible gene expression, live selection, and recombination cloning. Hum Gene Ther. 2011;22:1255–67. [DOI] [PubMed] [Google Scholar]
- 15.Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK and Kaufman PD. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar J, Key RE and Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab. 2008;93:4542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanba K, Chen AX, Turcu AF and Rainey WE. H295R expression of melanocortin 2 receptor accessory protein results in ACTH responsiveness. J Mol Endocrinol. 2016;56:69–76. [DOI] [PubMed] [Google Scholar]
- 18.Pezzi V, Mathis JM, Rainey WE and Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87:181–9. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 20.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, Lory P and Zennaro MC. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine. 2016;13:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattangady NG, Olala LO, Bollag WB and Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–61. [DOI] [PubMed] [Google Scholar]
- 23.Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ and Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reimer EN, Walenda G, Seidel E and Scholl UI. CACNA1H(M1549V) Mutant Calcium Channel Causes Autonomous Aldosterone Production in HAC15 Cells and Is Inhibited by Mibefradil. Endocrinology. 2016;157:3016–22. [DOI] [PubMed] [Google Scholar]
- 25.Pinggera A, Mackenroth L, Rump A, Schallner J, Beleggia F, Wollnik B and Striessnig J. New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum Mol Genet. 2017;26:2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinggera A, Negro G, Tuluc P, Brown MJ, Lieb A and Striessnig J. Gating defects of disease-causing de novo mutations in Cav1.3 Ca(2+) channels. Channels (Austin). 2018;12:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.