Abstract
Natural products isolated from plant sources are well known for their pharmacological potential in diversity of disease treatments such as inflammatory or cancer conditions. Mango (Mangifera indica L.) and Juglans regia are thought to be rich of functional phytochemicals. To clarify the anticancer activity, aqueous extracts of Juglans regia (JR) nut and Mangifera indica L (MI) fruit were exanimated on chronic lymphocytic leukemia (CLL) B lymphocytes and their mitochondria and the results were compared with those of normal B lymphocytes. Cellular parameters such as viability and caspase 3 activity, and mitochondrial parameters such as reactive oxygen species (ROS), mitochondria membrane potential (MMP), mitochondrial swelling, and cytochrome c release were evaluated. Our results demonstrated that the extract of Mangifera indica L increased cytotoxicity and caspase 3 activation through mitochondria pathway only in CLL B lymphocytes and also the extract of Juglans regia did not show cytotoxicity and caspase 3 activation on CLL and healthy B lymphocytes. Our in-vitro findings on isolated mitochondria indicated that mitochondrial ROS formation, MMP collapse, and mitochondrial swelling and cytochrome c release were significantly (p < 0.05) increased after addition of Mangifera indica only in cancerous mitochondria. These results demonstrated that Mangifera indica can act as a promising source for anti-cancer drug candidates by directly and selectively targeting mitochondria and inducing selective mitochondria mediated apoptosis on CLL B lymphocytes.
Key Words: Mangifera indica, Juglans regia, Chronic Lymphocytic Leukemia, Mitochondria, Cytotoxicity, Anticancer activity
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by accumulation of malignant B CD5+ cells, which are resistant to apoptosis (1). Despite standard treatments and more recent targeted therapies,(2) CLL is still incurable. Given that in CLL, multiple pathways are dysregulated (3) and considering that targeting one single pathway is often not sufficient to delete tumor cells, it is therefore necessary to develop novel strategies able to activate multiple apoptotic pathways and, simultaneously inhibit dysregulated survival pathways (4). Studies demonstrated that CLL cells contain significantly more mitochondria than normal lymphocytes. Moreover, the studies demonstrated that mitochondrial content may have therapeutic implications. Also, today mitochondria as main organelles in the cell have good promising targets for treatment of the cancers (5). For many years, medicinal plants have been used to treat different diseases.
The plants and their extracts are therapeutically superior to their single isolated constituents (6). They are generally plentiful, low cost, and relatively nontoxic in clinical practice (6). There are types of herbs, fruits, and their compounds acting as an inhibitor of carcinogen formation, blockers of carcinogen interaction, and suppressor of tumor progression (7).
Mangifera indica (MI) has been an important herb in the medical systems for 4000 years. It is one of the most popular of all tropical fruits (8). Ripe mango fruit is considered to be invigorating and freshening. The juice is restorative tonic and used in heat stroke (8). Mangiferin, being a polyphenolic antioxidant and a glucosyl xanthone, has strong antioxidant, anti-lipid peroxidation, immunomodulation, cardiotonic, hypotensive, wound healing, anti-degenerative, and antidiabetic activities (9). Gallotannins are also phenolics in mango which have shown anti-cancer activity (10). Juglans Regia (J. Regia) belongs to the family Juglandaceae commonly known as walnut tree. J. Regia extract contains ellagitannins containing anti-cancer agent and having anti-inflammatory properties (11). The key chemical composition of walnut is juglone (5 hydroxy-1, 4-naphthoquinone), the toxic compound which is found only in green and fresh walnuts, but such property disappears in dried leaves (12). Other several phenolic compounds with antioxidant properties have been identified in J. Regia leaves (13). Walnut leaves are considered a source of healthcare compounds, and have been extensively used in traditional medicine for treatment of skin inflammations, hyperhidrosis, and ulcers and for its anti-diarrheal, anti-helmintic, antiseptic, and astringent properties (14). Several studies suggest that regular consumption of nuts, mostly walnuts, may have beneficial effects against oxidative stress mediated diseases such as cardiovascular disease and cancer (15).
The objective of the present study was to evaluate the in-vitro cytotoxicity of the aqueous extract of Mangifera indica and Juglans as a routine fruit and nut consumed in many countries against human chronic lymphocytic leukemia cells. We also compared the obtained results of the extracts with those of normal B lymphocytes and mitochondria.
Experimental
Preparation and standardization of extracts
The aqueous extracts Juglans Regia and Mangifera Indica were prepared and standardized according to our published work (16).
Patients
Fifteen CLL patients entered this study. Diagnoses of CLL were performed according to the National Cancer Institute- sponsored Working Group guidelines, and clinical staging was based on the Binet classification. Age-matched controls were obtained from fifteen healthy donors. This study was approved by the Shahid Beheshti University of Medical Sciences research ethics committee and all the patients and healthy donors signed an informed consent form (17).
CLL and Normal B-lymphocytes isolation
Peripheral blood samples were obtained from CLL patients and healthy donors. Mononuclear cells were isolated from blood and tissue samples by utilizing Ficoll-Paque (GE Healthcare, Waukesha, WI) density gradient centrifugation. After 1 h of incubation at 37 C in 5% CO2, adhesive mononuclear cells were removed. Those non-adherent lymphocytes were thoroughly washed with the Hank′s solution. T lymphocytes were removed using anti-CD3 dynabeads.
The purification of B lymphocytes was assessed by flow cytometry with anti-CD19 antibodies with FACS. This cell preparation contained about 95% CD19 (B lymphocyte antigen) positive cells. It was added stromal cell-derived factor-1 to rescue B-lymphocytes from apoptosis (18).
Cytotoxicity Assay
B-lymphocytes (1×104 cells/well) were incubated in 96-well plates in the presence or absence of extracts for 24 h in a final volume of 50 µL. At the end of the treatment, 20 µL of MTT (5 mg/mL in PBS) was added to each well and incubated for an additional 4 h at 37 ºC. The purple-blue MTT formazan precipitate was dissolved in 100 µL of DMSO and the absorbance was measured at 570 nm on ELISA reader (19).
Caspase 3 Activity
Caspase 3 activity was determined in cell lysate of B-lymphocytes from different treatments using “Sigma’s caspase 3 assay kit (CASP- 3-C)”. In brief, this colorimetric assay is based on the hydrolysis of substrate peptide, Ac-DEVD-pNA, through caspase 3. The released moiety (p-nitroaniline) has a high absorbance at 405 nm. The concentration of the p-nitroaniline (μM) released from the substrate is calculated from the absorbance values at 405 nm or from a calibration curve prepared with defined p-nitroaniline solutions (20).
Succinate Dehydrogenases Activity
The activity of mitochondrial complex II (succinate dehydrogenases or SDH) was assayed by measuring the reduction of3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, 100 µL of mitochondrial suspensions (1mg protein/mL) was incubated with different concentrations of extracts at 37 °C for 1hour; then, 25 µL of 0.4% of MTT was added to the medium and incubated at 37 °C for 30 min. The product of formazan crystals was dissolved in 100 µL DMSO and the absorbance at 570 nm was measured with an ELISA reader (Tecan, Rainbow hermo, Austria) (18, 21).
Mitochondrial Swelling assay
Mitochondria suspensions (at 100 µg protein per well) were incubated in 96-well plates at 25 °C in swelling buffer (140 mmol/L KCl, 10 mmol/L NaCl, 2 mmol/L MgCl2, 0.5 mmol/L KH2PO4, 20 mmol/L HEPES, 0.5 mmol/L EGTA; adjusted to pH 7.2 with KOH) supplemented with 1 mg/mL rotenone and 10 mmol/L succinate. Mitochondrial swelling was measured spectrophotometrically in 1-hour duration. Mitochondrial swelling results in a decrease in absorbance monitored at 540 nm (22).
Mitochondrial ROS formation assay
Briefly, purified mitochondria were isolated and placed in respiration buffer (0.32 mM sucrose, 10 mM Tris, 20 mM Mops, 50 μM EGTA, 0.5 mM MgCl2, 0.1 mM KH2PO4, and 5 mM sodium succinate). Following this step, DCFH-DA was added (final concentration, 10 μM) and then added various concentration of extracts, at 37 °C for an hour. Then, the fluorescence intensity of DCF was measured using Shimadzu RF-5000U fluorescence spectrophotometer at an excitation wavelength of 488 nm and emission wavelength of 527 nm (18).
Mitochondrial MMP Collapse Assay
Briefly the mitochondrial fractions (1000 µg protein /mL) were incubated with 10 µM of rhodamine 123 in MMP assay buffer (220 mM sucrose, 68 mM D-mannitol, 10 mM KCl, 5 mM KH2PO4, 2 mM MgCl2, 50 μM EGTA, 5 mM sodium succinate, 10 mM HEPES, and 2 μM Rotenone) and then various concentrations of the extracts were added at 37 °C for an hour. The fluorescence was monitored using Shimadzu RF-5000U fluorescence spectrophotometer at the excitation and emission wavelength of 490 nm and 535 nm, respectively (23).
Cytochrome c Release Assay
The concentration of cytochrome c was determined through using the Quantikine Human Cytochrome C Immunoassay kit (Minneapolis, Minn). Cytochrome c measurement was performed according to the manufacturer’s instructions (18).
Statistical Analysis
The results were presented as mean ± SD. The assays were performed in triplicate and the mean was used for statistical analysis. Statistical significance was determined using the one-way ANOVA test, followed by the post-hoc Tukey test when appropriate. Statistical significance was set at p < 0.05 and the parameters of mitochondrial dysfunction were analyzed by two-way ANOVA and Bonferonie posttest. All graphs were expressed as mean ± SD and p < 0.05 was considered statistically significant.
Results
Viability assay
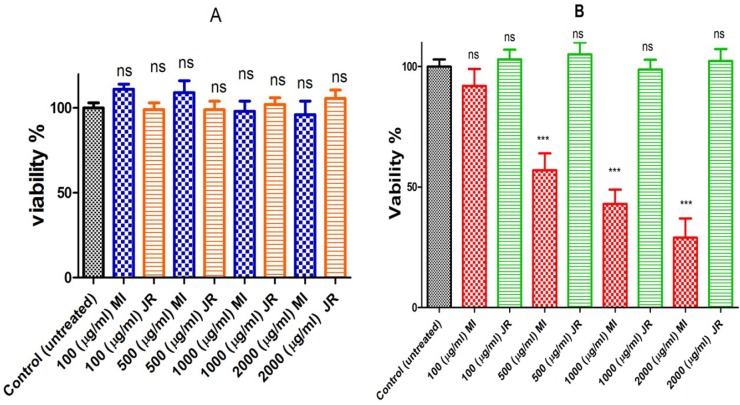
Evaluation of the extracts for potential selective toxicity on B lymphocytes cells obtained from CLL patients was carried out using the MTT assay. A 500 µg/mL concentration of aqueous extract of Mangifera Indica reduced 40% of cancerous B lymphocytes viability following 24-hour exposure, while none of the concentrations of J. Regia did not show any reduction at cancerous B lymphocytes viability at the same time (Figure 1 graph B). Toxicity evaluation in normal lymphocytes obtained from healthy donors revealed no significant decrease in cell viability after exposure to aqueous extracts J. Regia and Mangifera Indica at concentrations of 100-2000 µg/mL (Figure 1 graph A).
Figure 1.
Cell viability. Effect of J. Regia and Mangifera Indica aqueous extracts on cell viability in normal (A) and cancerous B lymphocytes (B). Cells were treated with J. Regia and Mangifera Indica aqueous extracts and cell viability was measured by MTT assay following 24-hours of extracts addition
Values were expressed as mean ± SD of five separate determinations (n = 5).
***: Significant difference in comparison with CLL control (p < 0.001). ns indicates non-significant with control.
Caspase 3 assay
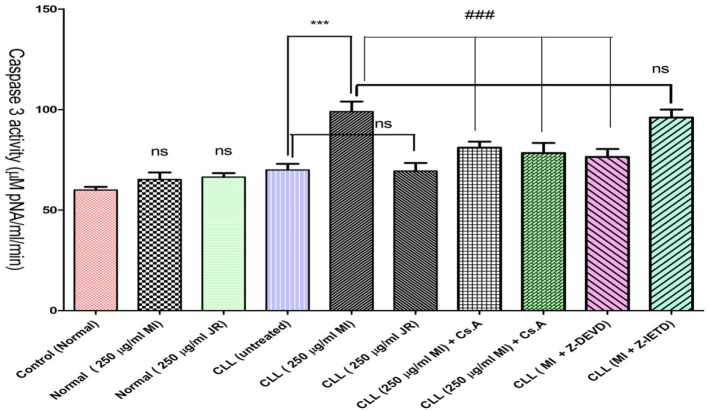
As shown in Figure 2, only Mangifera Indica extract significantly increased the activity of apoptosis final mediator, caspae-3 in CLL. To figure out the upstream mechanism involved in Mangifera Indica extract induced caspase-3 activation we examined the pretreating effect of Z-IETD a caspase 8 inhibitor and cyclosporine A (Cs.A), an MPT pore sealing agent and Butylated hydroxytoluene (BHT), a ROS scavenger on Mangifera Indica extract treated CLL B-lymphocytes. Our results showed that only Cs.A and BHT but not Z-IETD prevented Mangifera Indica extract induced caspase 3 activation (p < 0.001) suggesting that Mangifera Indica extract activates a ROS-mediated mitochondrial intrinsic pathway in cancerous B-lymphocytes which could end in apoptosis.
Figure 2.
Activity of caspae-3 in normal and CLL B-lymphocytes. CLL and healthy B-lymphocytes (106 Cells/mL) were incubated in RPMI 1640 medium in conventional condition (37 °C and 5% CO2-air) following the addition of J. Regia and Mangifera Indica aqueous extracts to both groups. As shown in Figure 2, Mangifera Indica aqueous extract significantly increased the activity of caspae-3 in CLL B-lymphocytes BUT NOT healthy B-lymphocytes. However only Cs.A (5 µM) and BHT (5 µM) but not Z-IETD (10 µM) prevented extract induced caspase 3 activation
Values are expressed as mean ± SD of three separate experiments (n = 5).
***: Significant difference in comparison with CLL control (p < 0.001).
###: Significant difference in comparison with Mangifera Indica group (p < 0.001). ns indicates non-significant with control.
Mitochondrial Assessment
SDH Activity
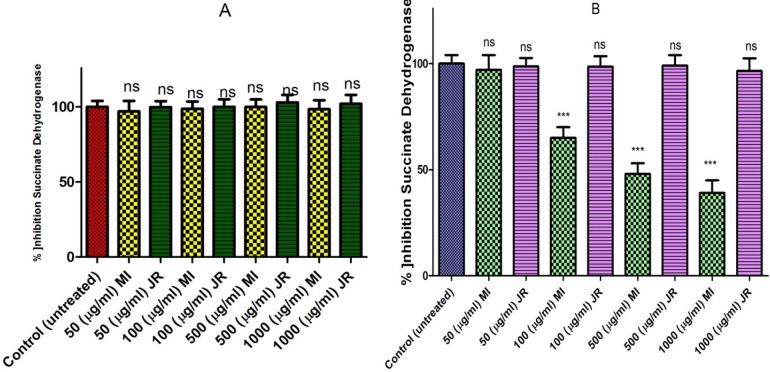
Evaluations of Mangifera Indica and J. Regia extracts for potential activity on mitochondria obtained from B-lymphocytes were carried out by studying the inhibitory effects of these extracts on SDH activity using the MTT assay. Mangifera Indica extract only inhibited succinate dehydrogenase activity in a dose-dependent manner in cancerous BUT NOT healthy mitochondria (Figure 3 graph A and B).
Figure 3.
Effect of J. Regia and Mangifera Indica aqueous extracts on succinate dehydrogenase activity in both normal (A) and cancerous B lymphocytes mitochondria (B). Mitochondria were treated with J. Regia and Mangifera Indica aqueous extracts and succinate dehydrogenase activity was measured by MTT assay following 1-hour of the extract exposure. Values are mean ± SD of three separate experiments
Values presented as mean ± SD of five separate experiments (n = 5).
***: Significant difference in comparison with CLL untreated control (p < 0.001). ns indicates non-significant with control.
ROS formation
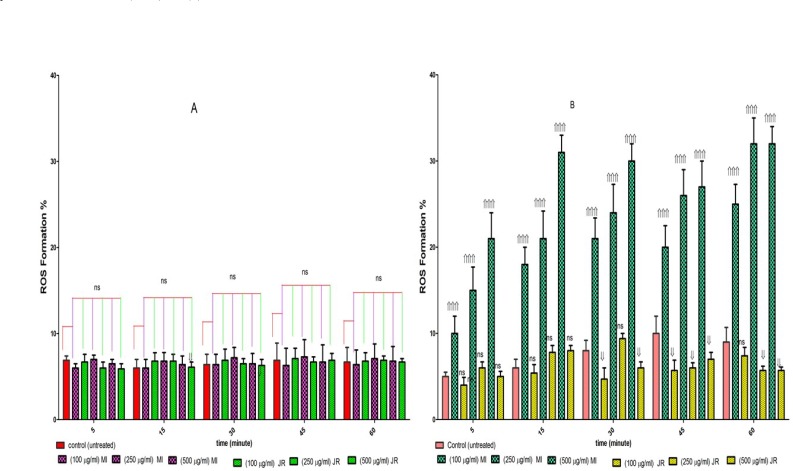
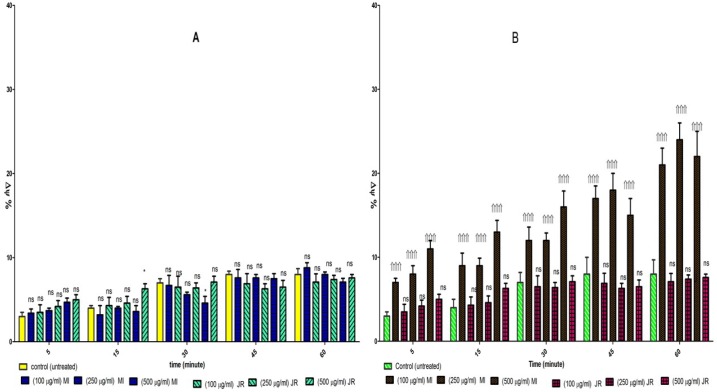
We examined whether the level of ROS in cancerous and normal mitochondria are affected by aqueous extracts J. Regia and Mangifera Indica L. As shown in Figure 4, graph B, only Mangifera Indica treatment at 100, 250, and 500 µg/mL concentrations for 1 h, significantly induced ROS generation (p < 0.05) in cancerous mitochondria. These results suggested that Mangifera Indica induced ROS generation might underlie its effect on promoting CLL cell apoptosis. While these results showed that J. Regia decreased ROS generation cancerous mitochondria. However, as shown in Figure 4 graph A, treatment with both extract at 100, 250, and 500 µg/mL concentrations for 1 hour did not induce ROS generation in normal mitochondria.
Figure 4.
Effect of J. Regia and Mangifera Indica aqueous extracts on ROS formation in both normal (A) and cancerous mitochondria (B). Freshly isolated purified mitochondria were obtained from both healthy donors and CLL patients and then incubated with J. Regia and Mangifera Indica aqueous extracts for 1- hour. ROS was measured spectroflourimetrically by DCF staining. ROS formation % was enhanced by Mangifera Indica extract in comparison to CLL untreated control during 1-hour exposure
Values presented as mean ± SD of five separate experiments (n = 5).
Significant difference in comparison with CLL untreated control (p < 0.001).
Significant difference in comparison with CLL untreated control (p < 0.001). ns indicates non-significant with control.
MMP assay
To search for the mechanisms involved in apoptosis, we examined the effects of J. Regia and Mangifera Indica extracts on mitochondrial membrane potential (∆Ψm) mitochondria isolated from both groups. Addition of the extracts (100, 250 and 500 µg/mL) for 1 h showed decreased ∆Ψm in mitochondria obtained from CLL B lymphocytes only after exposure to Mangifera Indica extracts (Figure. 5, graph B). Addition of the same concentrations of the extracts (100, 250 and 500 µg/mL) on normal mitochondria did not show any significant decrease at ∆Ψm for both extracts (Figure 5, graph A).
Figure 5.
Effect of J. Regia and Mangifera Indica aqueous extracts on ∆Ψm collapse in normal (A) and cancerous mitochondria (B). Freshly isolated purified mitochondria from both healthy and CLL B lymphocytes were treated with different concentrations of extracts (100, 250 and 500 µg/mL) for 1-hour. ∆Ψm was measured by rhodamine 123 staining with spectrofluorescence method. Our data revealed that only Mangifera Indica could induce a significant decrease in ∆Ψm in cancerous mitochondria but not in normal mitochondria
Values presented as mean ± SD of five separate experiments (n = 5).
Significant difference in comparison with CLL untreated control (p < 0.001). ns indicates non-significant with control.
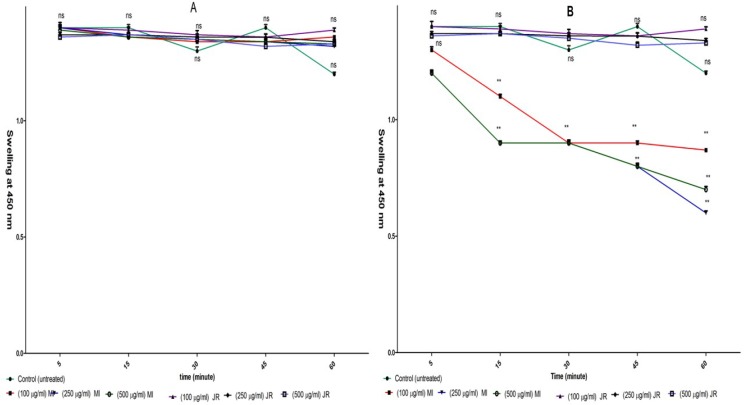
Mitochondrial swelling
Induction of mitochondrial swelling in isolated lymphocyte mitochondria was monitored by following 540 nm absorbance (A540) decrease. Addition Mangifera Indica extract (100, 250 and 500 µg/mL) resulted in an extensive mitochondrial swelling in cancerous mitochondria obtained from B lymphocytes of CLL patients (Figure 6, graph B).
Figure 6.
Effect of J. Regia and Mangifera Indica aqueous extracts on mitochondrial swelling in normal (A) and cancerous mitochondria (B). Only addition of Mangifera Indica aqueous extracts (100, 250 and 500 µg/mL) induced mitochondrial swelling in cancerous BUT NOT normal mitochondria. For swelling evaluation, absorbance of mitochondrial suspensions at 540 nm were monitored every 15 min within 1-hour
Values presented as mean ± SD of five separate experiments (n = 5).
***: Significant difference in comparison with CLL untreated control (p < 0.001). ns indicates non-significant with control.
Addition of the same concentrations (50 and 100 µg/mL) of extract to normal mitochondria did not induce mitochondrial swelling (Figure 5, graph A). Addition J. Regia extract (100, 250 and 500 µg/mL) did not result in mitochondrial swelling in cancerous and normal mitochondria (Figure 6 A and B).
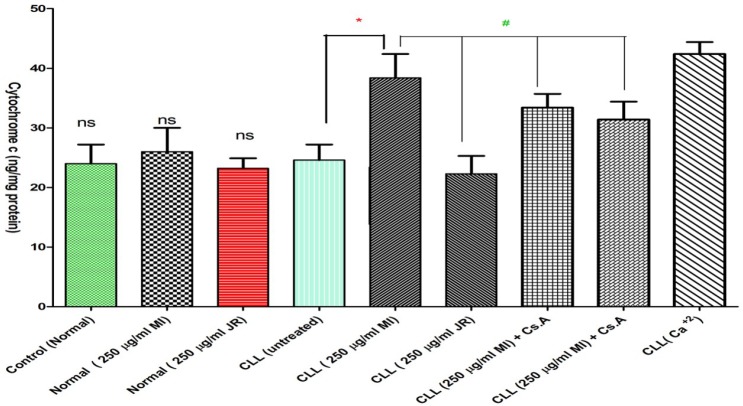
Cytochrome c
Our results demonstrated that Mangifera Indica extract significantly caused mitochondrial swelling and collapse of the mitochondrial membrane potential. These events could result in mitochondrial permeability transition and release of cytochrome c from mitochondria into the incubation buffer. As shown in Figure 7, only Mangifera Indica extract (250 µg/mL) induced significant (p < 0.05) release of cytochrome c on the cancerous mitochondria isolated from CLL patients but not normal healthy donors. Significantly, the pretreatment of extract -treated mitochondria with the MPT inhibitor, cyclosporine A (Cs.A) and ROS scavenger, butylated hydroxyl toluene (BHT) prevented cytochrome c release as compared with sole Mangifera Indica -treated group (250 µg/mL) (p < 0.05), indicating the role of oxidative stress and mitochondrial permeability transition (MPT) pore opening in extract induced cytochrome c release.
Figure 7.
Effect of J. Regia and Mangifera Indica aqueous extracts (250 µg/mL) on the cytochrome c release in the lymphocyte mitochondria isolated from CLL patients’ group. As shown in this figure, only addition of Mangifera Indica aqueous extracts (250 µg/mL) induced cytochrome c release in cancerous BUT NOT normal mitochondria, also pretreatment of BHT or Cs.A significantly prevented cytochrome c release in the cancerous lymphocyte mitochondria. The amount of expelled cytochrome c from mitochondrial fraction into the suspension buffer was determined by man Cytochrome c ELISA kit.
Values presented as mean ± SD of five separate experiments (n = 5).
***: Significant difference in comparison with CLL untreated control (p < 0.001).
###: Significant difference in comparison with CLL Mangifera Indica group (p < 0.001). ns indicates non-significant with control
Discussion
Cancer is the second leading cause of death worldwide. Natural therapies, such as the use of plant-derived products in cancer treatment, may reduce adverse side effects during chemotherapy. Recently, a few plant products are being used to treat cancer. However, a numerous of many plant products exist that have shown very promising anti-cancer properties in-vitro (24). Plants play an important role in cancer prevention, as well as in therapy. Medicinal plants provide new active chemo-preventive molecules (25). The use of therapeutic herbs in developing countries as cures against leukemia is prominent. (7). Our findings showed selective toxicity of Mangifera Indica extract on cancerous B lymphocytes and their mitochondria obtained from CLL patients by MTT assay (Figure 1), while J. Regia extract didn’t show any significant toxicity on CLL lymphocytes and their mitochondria (Figure 1). The results indicate that Mangifera Indica is an excellent source of anti-cancerous agents and may prove fruitful herbal remedy in near future for help to CLL therapy. Also there are several studies on literatures that indicate Mangifera Indica extract and its isolated compounds have anticancer potential in many cancer cell lines (26, 27). Although in our studies we did not observe anticancer activity of J. Regia extract, nevertheless recent studies have shown J. Regia extract has anticancer activity (15, 28, 29). This difference may be due to the kind of species, cancer cell, the content, and interaction of active ingredient in the extract.
The development of therapeutic strategies that target apoptosis in CLL is a very important issue (30). New targets such as mitochondria has facilitated the development of new drugs with a view to improving clinical outcomes for this neoplasm (31). So mitochondria are currently regarded as central organelles in mediating intrinsic death signals and might provide a novel target for new chemotherapeutics (32). Our results showed that Mangifera Indica extract could selectively induce apoptosis in CLL but not healthy normal B-lymphocytes and also showed that this selective apoptosis is initiated from mitochondria (Figure 2). The mitochondrial membrane permeability transition (MPT) is a serious step in induction of apoptosis (33). A mechanism causing the mitochondrial dysfunction is MPT which includes dissipation of the inner membrane potential, osmotic swelling of the matrix, rupture of the outer mitochondrial membrane, release of cytochrome c and other apoptogenic proteins, as well as formation of the caspase-3 activation complex in cytosol (34). In this study, we showed increased ROS formation after exposure to Mangifera Indica extract only in cancerous mitochondria but not normal mitochondria. The mitochondrial respiratory chain is one of the major sources of endogenous ROS (35). Several chemotherapeutic agents have been identified to promote mitochondrial ROS formation (36). ROS are responsible for induction of Ca2 dependent MPT (37, 38). MMP is a universal feature of cell death and is often considered as the “point of no return” in the cascade of the events leading to apoptosis (35, 39). Our results also revealed collapse mitochondria membrane potential (MMP) only on cancerous mitochondria confirming that MMP occurs after Mangifera Indica extract treatment on cancerous (Figure 5). Besides, addition of this extract to cancerous mitochondria also resulted in mitochondrial swelling (Figure 6). Several mechanisms for the release of Cyt c from mitochondria have so far been proposed, including dissociation of this peptide from the inner mitochondrial membrane in response to cardiolipin peroxidation by reactive oxygen species (ROS) (40). Several studies have proposed that the release of cytochrome c occurs due to the rupture of the mitochondrial outer membrane (41). In this study, we showed release of Cyt c only from cancerous but not normal mitochondria after treatment with Mangifera Indica extract. Moreover, Cs A and BHT pretreatment completely blocked the extract-induced release of cytochrome c from the cancerous mitochondria which supports the hypothesis that the apoptosis induction via Mangifera Indica extract is due to an oxidative stress and depends on the opening of the mitochondrial transition pores.
Finally, our results suggest that Mangifera Indica can act promising source for anti-cancer drug candidate by directly and selectively targeting mitochondria in cancerous cells and thus could induce cell death through ROS mediated mitochondrial pathway which finally ends in cytochrome c release, caspase 3 activation, and apoptosis in cancerous B-lymphocytes isolated from CLL patients. Hence, this extract may be a promising source in the future for the anti-cancer drug development in treatment of CLL.
Acknowledgment
The data provided in this article was extracted from the Pharm D. thesis of Dr. Jalil Rahmati. The thesis was conducted under supervision of Prof. Jalal Pourahmad at Department of Toxicology and Pharmacology, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N. Engl. J. Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Lin TS. New agents in chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 2010;5:29–34. doi: 10.1007/s11899-009-0039-9. [DOI] [PubMed] [Google Scholar]
- 3.Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat. Rev. Cancer . 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 4.Giordano S, Petrelli A. From single-to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr. Med. Chem. 2008;15:422–32. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 5.Carew JS, Huang P. Mitochondrial defects in cancer. Mol. Cancer . 2002;1:1. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantari H, Jalali M, Jalali A, Salimi A, Alhalvachi F, Varga B, Juhasz B, Jakab A, Kemeny-Beke A, Gesztelyi R. Protective effect of Cassia fistula fruit extract on bromobenzene-induced nephrotoxicity in mice. Hum. Exp. Toxicol. . 2011;30:1710–5. doi: 10.1177/0960327110396532. [DOI] [PubMed] [Google Scholar]
- 7.Saedi TA, Md Noor S, Ismail P, Othman F. The effects of herbs and fruits on leukaemia. Evid. Based Complement Alternat. Med. . 2014:2014. doi: 10.1155/2014/494136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah K, Patel M, Patel R, Parmar P. Mangifera indica (mango) Pharmacogn. Rev. 2010;4:42. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khurana RK, Kaur R, Lohan S, Singh KK, Singh B. Mangiferin: a promising anticancer bioactive. Pharm. Pat. Anal. 2016;5:169–81. doi: 10.4155/ppa-2016-0003. [DOI] [PubMed] [Google Scholar]
- 10.Al-Halabi R, Bou Chedid M, Abou Merhi R, El-Hajj H, Zahr H, Schneider-Stock R, Bazarbachi A, Gali-Muhtasib H. Gallotannin inhibits NFĸB signaling and growth of human colon cancer xenografts. Cancer Biol. Ther. 2011;12:59–68. doi: 10.4161/cbt.12.1.15715. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Li D-Y, Wang H-D, Zheng Z-J, Hu J, Li Z-Z. Juglans regia hexane extract exerts antitumor effect, apoptosis induction and cell circle arrest in prostate cancer cells in-vitro. Trop J. Pharm. Res. 2015;14:399–405. [Google Scholar]
- 12.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 13.Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L. Walnut (Juglans regia L) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007;45:2287–95. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Thakur A. Juglone: A therapeutic phytochemical from Juglans regia L. J. Med. Plant. Res. 2011;5:5324–30. [Google Scholar]
- 15.Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010;48:441–7. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 16.Salimi A, Ayatollahi A, Seydi E, Khomeisi N, Pourahmad J. Direct toxicity of amyloid beta peptide on rat brain mitochondria: preventive role of Mangifera indica and Juglans regia. Toxicol. Environ Chem. 2015;97:1057–70. [Google Scholar]
- 17.Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, Pourahmad J. Selective Anticancer Activity of Acacetin Against Chronic Lymphocytic Leukemia Using Both In-vivo and In-Vitro Methods: Key Role of Oxidative Stress and Cancerous Mitochondria. Nutr. Cancer . 2016;68:1404–16. doi: 10.1080/01635581.2016.1235717. [DOI] [PubMed] [Google Scholar]
- 18.Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox. Biol. . 2015;6:461–71. doi: 10.1016/j.redox.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salimi A, Motallebi A, Ayatollahi M, Seydi E, Mohseni AR, Nazemi M, Pourahmad J. Selective toxicity of persian gulf sea cucumber holothuria parva on human chronic lymphocytic leukemia b lymphocytes by direct mitochondrial targeting. Environ. Toxicol. 2016;32:1158. doi: 10.1002/tox.22312. [DOI] [PubMed] [Google Scholar]
- 20.Seydi E, Rajabi M, Salimi A, Pourahmad J. Involvement of mitochondrial-mediated caspase-3 activation and lysosomal labilization in acrylamide-induced liver toxicity. Toxicol. Environ. Chem. 2015;97:563–75. [Google Scholar]
- 21.Talari M, Seydi E, Salimi A, Mohsenifar Z, Kamalinejad M, Pourahmad J. Dracocephalum: novel anticancer plant acting on liver cancer cell mitochondria. Biomed. Res. Int. 2014;2014:892170. doi: 10.1155/2014/892170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salimi A, Saharkhiz MP, Motallebi A, Seydi E, Mohseni AR, Nazemi M, Pourahmad J. Standardized Extract of the Persian Gulf Sponge, Axinella Sinoxea Selectively Induces Apoptosis through Mitochondria in Human Chronic Lymphocytic Leukemia Cells. J. Anal. Oncol. 2015;4:132–40. [Google Scholar]
- 23.Aghvami M, Eshghi P, Zarei MH, Arefi H, Sattari F, Zarghi A, Pourahmad J. Novel Colchicine Analogues Target Mitochondrial PT Pores Using Free Tubulins and Induce ROS-Mediated Apoptosis in Cancerous Lymphocytes. Iran. J. Pharm. Res. 2018;17:1476–87. [PMC free article] [PubMed] [Google Scholar]
- 24.Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008;9:581–91. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachrach ZY. Contribution of selected medicinal plants for cancer prevention and therapy. Acta Fac med. Naiss. 2012;29:117–23. [Google Scholar]
- 26.Noratto GD, Bertoldi MC, Krenek K, Talcott ST, Stringheta PC, Mertens-Talcott SU. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010;58:4104–12. doi: 10.1021/jf903161g. [DOI] [PubMed] [Google Scholar]
- 27.Abdullah A-SH, Mohammed AS, Abdullah R, Mirghani MES, Al-Qubaisi M. Cytotoxic effects of Mangifera indica L kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement Altern. Med. 2014;14:199. doi: 10.1186/1472-6882-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah TI, Sharma E, Shah GA. Anti-proliferative, Cytotoxicity and Anti-oxidant Activity of Juglans regia Extract. Am. J. Cancer Prev. 2015;3:45–50. [Google Scholar]
- 29.Jahanbani R, Ghaffari SM, Salami M, Vahdati K, Sepehri H, Sarvestani NN, Sheibani N, Moosavi-Movahedi AA. Antioxidant and Anticancer Activities of Walnut (Juglans regia L) Protein Hydrolysates Using Different Proteases. Plant Foods Hum. Nutr. 2016;71:402–9. doi: 10.1007/s11130-016-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besbes S, Mirshahi M, Pocard M, Billard C. Strategies targeting apoptosis proteins to improve therapy of chronic lymphocytic leukemia. Blood Rev. 2015;29:345–50. doi: 10.1016/j.blre.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Lu K, Wang X. Therapeutic advancement of chronic lymphocytic leukemia. J. Hematol. Oncol. 2012;5:55. doi: 10.1186/1756-8722-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A Urra F, Cordova-Delgado M, Pessoa-Mahana H, Ramirez-Rodriguez O, Weiss-Lopez B, Ferreira J, Araya-Maturana R. Mitochondria: a promising target for anticancer alkaloids. Curr. Top. Med. Chem. 2013;13:2171–83. doi: 10.2174/15680266113139990150. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J, Zhang S, Wu J, Yu K, Han Y. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One . 2012;7:e46853. doi: 10.1371/journal.pone.0046853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–64. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 35.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science . 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 36.Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P. Inhibition of mitochondrial respiration a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003;278:37832–9. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 37.Byrne AM, Lemasters JJ, Nieminen AL. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert‐butylhydroperoxide in rat hepatocytes. Hepatology . 1999;29:1523–31. doi: 10.1002/hep.510290521. [DOI] [PubMed] [Google Scholar]
- 38.Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca2+‐induced membrane permeability transition in brain mitochondria. J. Neurochem. . 2001;79:1237–45. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- 39.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science . 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 40.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta Bioenerg. 2006;1757:639–47. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl Acad Sci. India Sect B Biol. Sci. 2002;99:1259–63. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]