Abstract
Diabetes mellitus has been always one of the most prevalent chronic diseases in the last decades. There exist a wide range of pharmacological agents for controlling this disease. However, these agents fare differently in terms of efficacy and safety. Hence, the aim of this study was to compare dulaglutide and liraglutide, two glucagon-like peptide-1 receptor agonists, in terms of efficacy and safety, drawing on a systematic review and meta-analysis. A systematic review and meta-analysis were carried out in January 2018. The articles were evaluated by two independent investigators and their quality was evaluated using Jadad scale and the Cochrane Collaboration’s tools. Finally, the eligible articles entered the study. HbA1c and FBS were considered as efficacy outcomes. Safety profile was evaluated based on several outcomes such as serious side effects and vital signs. Three articles met the inclusion and exclusion criteria. The results indicated that the mean difference (MD) of HbA1c reduction was -0.10% (95% CI, -0.20% to -0.01%, P=0.03) in the patients who received dulaglutide in comparison with the patients who received liraglutide. In addition, dulaglutide was safer than liraglutide in terms of gastrointestinal problems (RR=0.85, 95% CI, 0.73 to 0.99, P=0.04, I2=55%) and heart rate (RR=-1.14, 95% CI, -1.90 to -0.38, P=0.003, I2=0%). Once-weekly dulaglutide showed a further reduction in HbA1c compared to once-daily liraglutide. However, comparisons between these regimens indicated no significant difference between groups in either FBS reduction or safety profile. Similarly, no statistically significant difference was observed in treatment discontinuation, hypoglycemia events, and vital signs.
Key Words: Diabetes Miletus, Dulaglutide, Liraglutide, Meta-analysis, Systematic review
Introduction
Diabetes mellitus (DM), a chronic metabolic disease, may impair insulin secretion, insulin action, or both. This condition leads to hyperglycemia and micro vascular problems (1). The prevalence of DM has been growing rapidly in the last couple of years. Obesity, ageing population, and lack of physical activities are mainly to blame for this rapid progression (2). DM categorized to type 1 DM and type 2 DM has been branded as ‹Black Death′ in recent years (3). Efficient glycemic control plays essential crucial role in regulating blood glucose balance and preventing micro vascular complications (4, 5). Epidemiological studies reveal that near 90% of DM patients are diagnosed with type 2. Early pharmacological intervention following DM diagnosis has been recommended strongly, and metformin is considered as the drug of choice for the first line treatment. Nevertheless, metformin has failed to change the progressive nature of type 2 DM (6, 7). Several trials have demonstrated that the combination therapy has been more efficacious and is better tolerated than high doses of each anti-diabetic agents (8). In this regard, a recent statement from American Diabetes Association (ADA) and European Association for Study of Diabetes (EASD) recommends combinational therapy for the patients with hemoglobin A1c (HbA1c) > 9%. However, the conventional therapeutic interventions for type 2 DM cannot control hyperglycemia effectively. In addition, several side effects of these agents reduce the patient′s adherence to their medications (9). Hence, it seems necessary to investigate the new pharmacological agents for improving the management of this disease more thoroughly. Recently, new pharmacological agents, i.e. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), have played a considerable role in the treatment of diabetes mellitus (10). GLP-1 RAs are injectable peptides which are structurally and functionally similar to endogenous incretin GLP-1 whose secretion failure has a critical pathophysiologic role in DM (11). However, as they are not neutralized by the dipeptidyl peptidase-4 (DPP-4), their half-life is longer than that of endogenous GLP-1 (12). GLP-1 RAs are categorized as short-acting (exenatide and lixisenatide), and long-acting agents (liraglutide, dulaglutide, albiglutide, and semaglutide) (13). Although all these medications potently decrease HbA1c, some fundamental differences arise among them when such factors as fasting and postprandial hyperglycemia reduction, potency of weight loss, cardiovascular protection efficacy, and adverse events profile are brought to bear (14, 15). In addition, dulaglutide is processing to be entered in Iran drug list and may be in the Iran market in near future. Against this backdrop, the aim of the present study was to compare dulaglutide and liraglutide in terms of efficacy and safety, drawing on a systematic review and meta-analysis.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to carry out the systematic review (16).
To evaluate the efficacy and safety of dulaglutide compared to liraglutide, a systematic review and meta-analysis was carried out.
Literature search strategy
To begin with, appropriate keywords were selected based on MeSh terms and a comprehensive review was done in January 2018. The electronic databases including PubMed, Embase, Scopus, Cochrane library, and Web of science as well as non-electronic databases such as library literature were investigated. In addition, Open Gray database, EU CTR database, Google Scholar, and ClinicalTrials.gov database were explored to find unpublished records and conference proceedings. To ensure a more sensitive search, all searches were done based on the two main keywords, i.e. dulaglutide and liraglutide (or their brand names). No limitations such as year of publication or language were set in this conducting search.
Inclusion and Exclusion Criteria
To find eligible articles, the following inclusion and exclusion criteria were applied. The inclusion criteria were (1) study population: patients with type 2 DM; (2) intervention: dulaglutide administration with or without a metformin background therapy; (3) comparison: liraglutide administration with or without a metformin background therapy; (4) efficacy outcome: HbA1C and fasting serum glucose (FSG); (4) safety outcomes: nausea, diarrhea, constipation, gastrointestinal disorders, incidence of serious adverse effects, discontinuation of study, or vital signs; and (5) study design: clinical trials.
The exclusion criteria were: (1) study population: patients with other types of diabetes except type 2 DM; (2) intervention: dulaglutide administration in combination with other antidiabetic agents; (3) comparison: liraglutide administration in combination with other anti-diabetic agents; (4) outcomes: studies assessing irrelevant outcomes; and (5) study design: studies designed inappropriately, studies in which suffering from evident biases and other studies except clinical trial. In addition, studies in outpatient settings were left out of this study.
Study selection and appraisal
Then, the selected articles were transferred to the Mendeley reference manager software and duplicated documents found in different databases were counted out. Then, the full texts of the articles were screened by two reviewers ensure compliance with the inclusion and exclusion criteria and, finally, the data from eligible studies were extracted onto an Excel spreadsheet. In case of disagreement between researchers, a third reviewer was decided on discrepancies of the articles.
The authors independently used the modified Jadad scale to assess the methodological quality of each included study. If two authors had different opinions when assessing and selecting the studies, the consensus was reached by the intervention of a third party. The modified Jadad scale includes 8 items to evaluate as follows: if randomization was done (score range 0–1); if randomization was appropriate (score range −1 to 1); if blinding was done (score range 0–1); if blinding was appropriate (score range −1 to 1); if withdrawals and dropouts were described (score range 0–1); if inclusion and exclusion criteria were described (score range 0–1); if adverse reactions were assessed (score range 0–1); and if the statistical analysis was described (score range 0–1) (17). The score of each study ranges from 0 (the lowest quality) to 8 (the highest quality). Studies were classified as moderate if they had a score of 4 or 5. All included trials were also evaluated using Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials and the Grading of Recommendations Assessment, Development and Evaluation Working Group grading scheme (18, 19).
Data analysis
The data were analyzed using the mean difference (MD) with 95% confidence intervals (95% CIs). RevMan version 5.3.5 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) was used for all data analyses. Meta-analysis was conducted when the trials had an acceptable clinical homogeneity and statistical heterogeneity limit. Heterogeneity was quantified using the Cochran Q test and I2 statistics. A P value < 0.10 for Chi-square testing of the Q statistic or an I2 > 30% was considered as a statistically significant heterogeneity that leads to use random effect and otherwise fixed effect in accordance with the Cochrane methodology (20, 21).
Results
Study characteristics
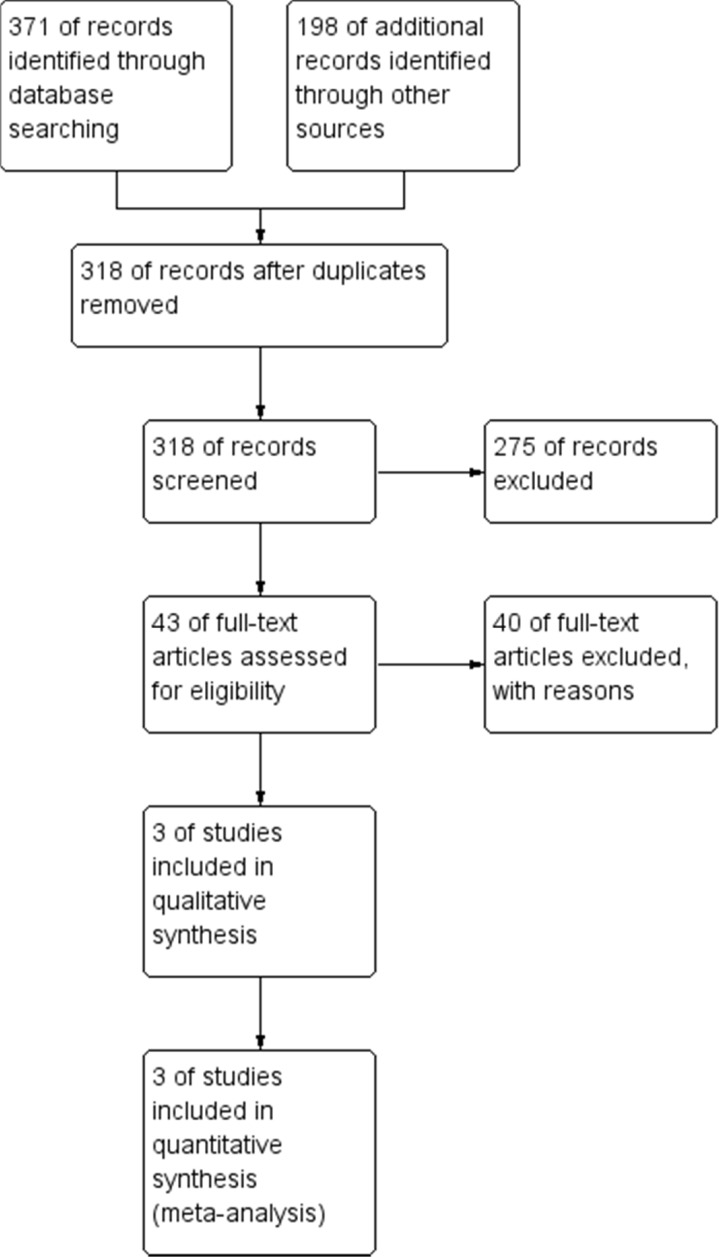
In this comprehensive systematic review and meta-analysis, some databases were searched with appropriate keywords and, overall, 318 articles were included in the study. After primary evaluation by screening the titles and abstracts, according to the inclusion criteria, 43 articles were selected for full-text evaluation. Finally, three articles were selected for final analysis whose characteristics have been presented in Table 1 (22–24). The PRISMA flow chart of this study is given in Figure 1.
Table 1.
Study characteristics
| Authors | Year | Country | Design | Duration (Week) | Sample Size (DLG:LRG) |
Doses (DLG:LRG)
in mg |
Jadad Score |
|---|---|---|---|---|---|---|---|
| Odawara(23) | 2016 | Japan | Parallel | 52 | 280:137 | 0.75:0.9 | 4 |
| Miyagawa(22) | 2015 | Japan | Parallel | 26 | 280:137 | 0.75:0.9 | 4 |
| Dungan(24) | 2014 | International | Parallel | 26 | 299:300 | 1.5:(0.6-1.8) | 3 |
Figure 1.
The PRISMA flow diagram of the study
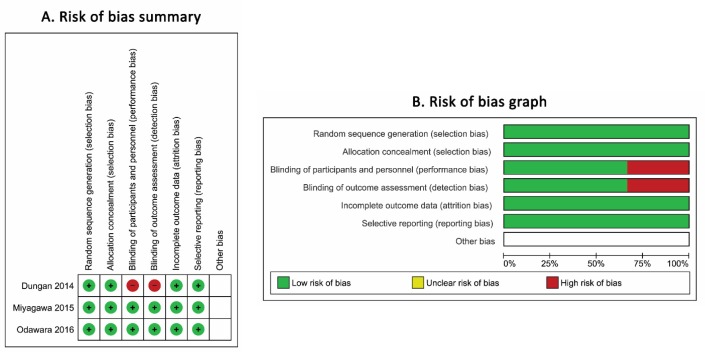
The Jadad score of the selected articles showed that 2 of the included studies have a moderate quality. However, the result of the Cochrane Collaboration’s tool for assessing risk of bias in included randomized trials shows a low bias as illustrated in Figure 2.
Figure 2.
Risk of bias assessment by the Cochrane Collaboration’s tool
Efficacy
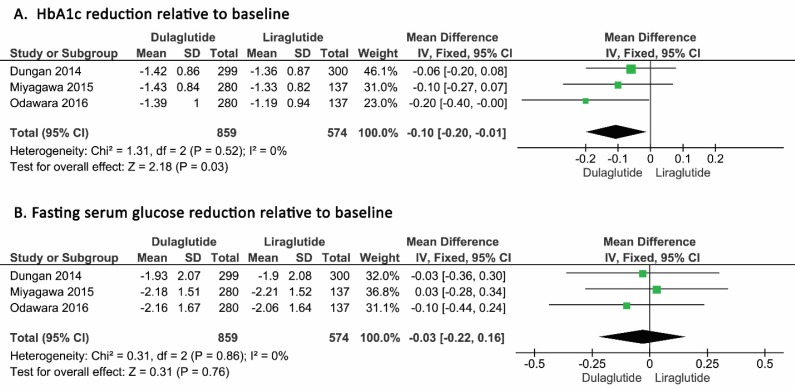
In terms of HbA1C, as heterogeneity among the selected studies based on I2 = 0 was not seen, a fixed model was developed for analysis. After analyzing the 1433 patients, the mean differences of HbA1c reduction was found to be -0.10% (95% CI, -0.20% to -0.01%, P=0.03) in patients who received dulaglutide in comparison with patients who received liraglutide. As shown in the forest plot, this reduction was statistically significant (Figure 3, Panel A).
Figure 3.
Pooled mean difference of HbA1c reduction (A) and FSG reduction (B) in patients who received dulaglutide compared to liraglutide
In terms of FSG, heterogeneity was not seen among the selected studies based on I2 = 0 and a fixed model was developed for analysis.
The mean difference of FSG was 0.03 mmol/L (95% CI, -0.18 mmol/L to -0.24 mmol/L, P=0.76) in the patients who received dulaglutide in comparison with the patients who received liraglutide, and this reduction was not significant as shown in the relevant forest plot (Figure 3, Panel B).
Safety
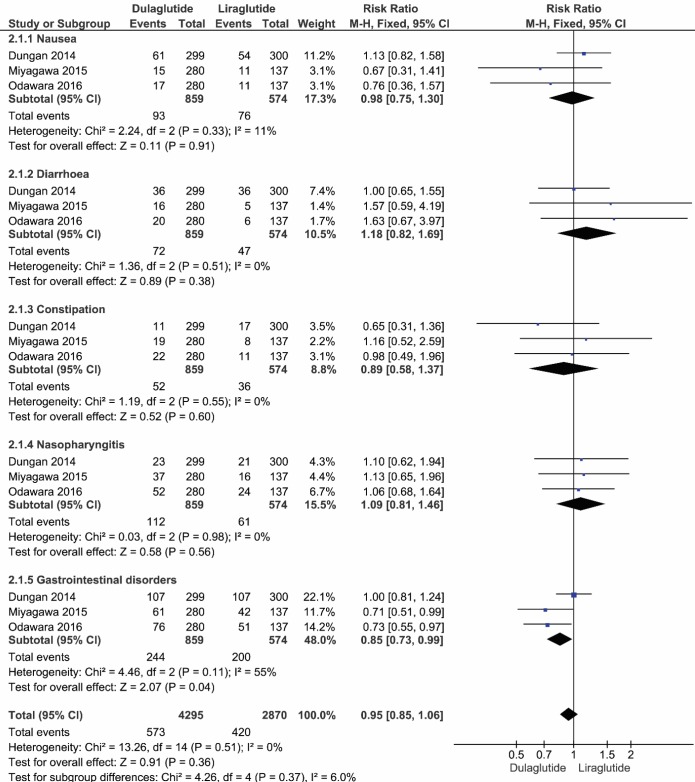
Based on the I2 score, the fixed model was applied to analyze the safety of dulaglutide and liraglutide. The results of 1433 patients showed that the adjusted risk ratio for the incidence of nausea was 0.98 (95% CI, 0.75 to 1.3, P=0.91, I2=11%), which was not statistically significant. Similarly, the adjusted risk ratio for the incidence of diarrhea was not significantly different between these two agents, 1.18 (95% CI, 0.82 to 1.69, P=0.38, I2=0%). Likewise, the adjusted risk ratio of the incidence of constipation, 0.89 (95% CI, 0.58 to 1.37, P = 0.60, I2=0%), and adjusted risk ratio of the incidence of nasopharyngitis, 1.09 (95% CI, 0.81 to 1.46, P=0.56, I2=0%) were not statistically different. However, the adjusted risk ratio of the incidence of gastrointestinal was found to be statistically significant between dulaglutide and liraglutide (RR: 0.85, 95% CI, 0.73 to 0.99, P=0.04, I2=55%). Finally, the pooled data for the incidence of total adverse events showed no significant difference (RR: 0.95, 95% CI, 0.85 to 1.06, P=0.51, I2=0%) (Figure 4).
Figure 4.
The forest plot showing the incidence of adverse events in patients who received dulaglutide compared to liraglutide
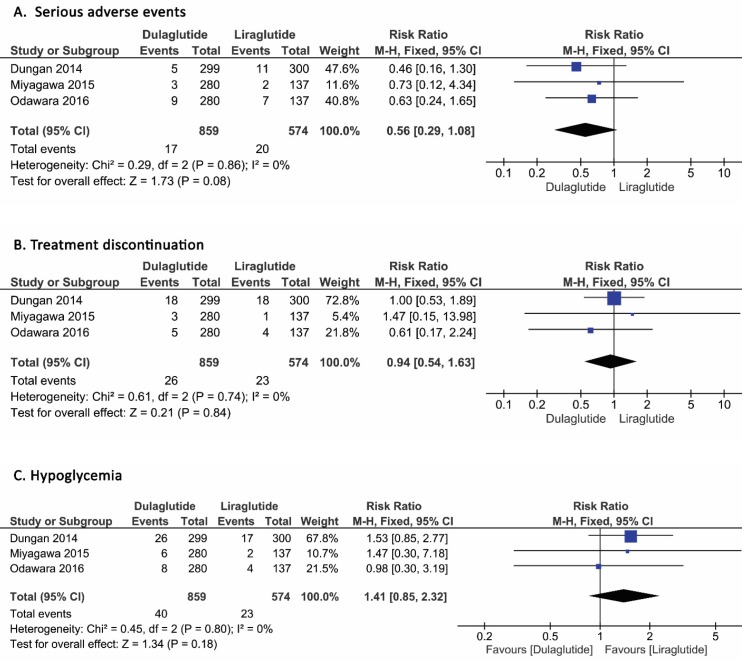
Serious adverse events
The adjusted risk ratio of serious adverse events in 1433 patients on the fixed model was 0.56 (95% CI, 0.29 to 1.08, P=0.08, I2=0%), which, as can be seen in the forest plot, was not different between dulaglutide and liraglutide (Figure 5, Panel A).
Figure 5.
Incidence of serious adverse events (A), Treatment discontinuation (B), and Hypoglycemia (C) in patients who received dulaglutide compared to liraglutide
Discontinuation of Treatment
The discontinuation of treatment was similar between the patients who received dulaglutide and liraglutide.
The adjusted risk ratio of discontinuation of treatment in 1433 patients was 0.94 (95% CI, 0.54 to 1.63, P=0.84, I2=0%) (Figure 5, Panel B).
Hypoglycemia
The adjusted risk ratio of different types of hypoglycemia events in 1433 patients was 1.41 (95% CI, 0.85 to 2.32, P=0.80, I2=0%), which was not different between dulaglutide and liraglutide, based on the fixed model (Figure 5, Panel C).
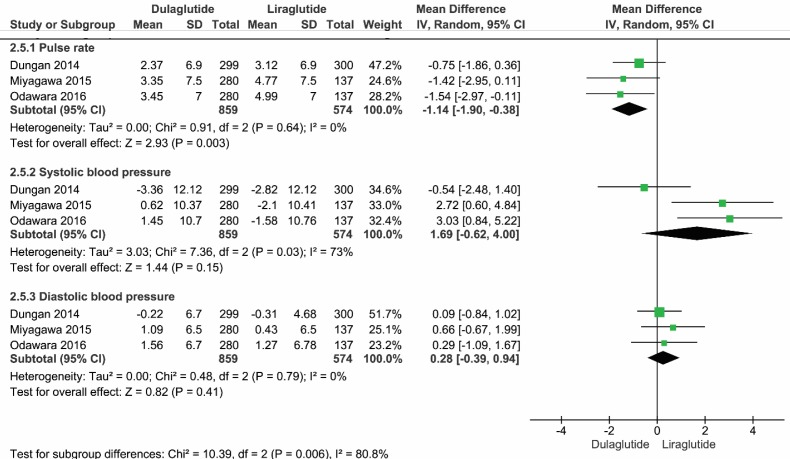
Vital Signs
Due to heterogeneity among the selected studies regarding systolic blood pressure (I2=73%), the random model was utilized to analyze the mean difference of vital signs between dulaglutide and liraglutide. Among vital signs, the mean differences of the heart rate was -1.14 (95% CI, -1.90 to -0.38, P=0.003, I2=0%) in favor of dulaglutide. However, other vital signs, such as systolic blood pressure and diastolic blood pressure, were not significantly different between the two medications
(Figure 6).
Figure 6.
Forest plot showing the mean difference of vital signs in patients who received dulaglutide compared to liraglutide
Discussion
To the best of the authors′ knowledge, this study is the first systematic review and meta-analysis aiming to compare two long-acting GLP-1 receptor agonists, i.e. dulaglutide and liraglutide, which are prescribed to subjects with type 2 diabetes with or without other hypoglycemic drugs.
The systematic review identified 3 trials reporting results for 26 to 52 weeks (22–24). Based on pooled estimates from 3 included RCTs, once-weekly dulaglutide is associated with more reduction in HbA1c than once-daily liraglutide. This difference between weekly and daily GLP-1 RAs may be attributed to the potential impact of the weekly agents on both FBS and postprandial plasma glucose (PPG), compared to the daily agents that may predominantly regulate PPG (10).
However, there were no statistically significant differences in the FBS reduction outcome. Although FBS is an important indicator of DM management, this indicator reflects the short-term efficacy. Indeed, HbA1c has more reproducibility compared to FBS, reflecting the glucose control condition for the past three months. HbA1c is also identified as a superior predictive factor for diabetic retinopathy and cardiovascular related problems (25, 26). It inevitably follows that dulaglutide has a higher long-term efficacy in glucose management compared to liraglutide.
In terms of safety profile, the results indicated that there were no weight-sparing benefits for either agent, while only dulaglutide was associated with a lower gastrointestinal complications and as well as a reduction in heart rate. Regarding the lower gastrointestinal problems with dulaglutide compared to liraglutide, the most probable mechanism for this phenomenon is the delayed gastric emptying since liraglutide reduces duodenal and small intestine motility (27). The previous randomized trials have revealed that in obese patients with cardiovascular diseases, liraglutide increased the heart rate despite a significant weight reduction and improvement in metabolic profile (28).
The risk of hypoglycemia, which can prove a daunting challenge and obstacle for the treatment of diabetes, was also similar between dulaglutide and liraglutide. However, results should interpret with caution since due to the variability in definitions of hypoglycemia in different studies, a challenge has been identified by the American Diabetes Association (8). No statistically significant difference was observed in treatment of the discontinuation and other vital signs.
Although some systematic reviews, meta-analyses, and network meta-analyses of once-weekly GLP-1 RAs had been previously published (10,29–39), the research question of the current study was more specific than that posed by previous studies. In our analyses, only head-to-head comparisons between dulaglutide and liraglutide have been considered, focusing on the HbA1c reduction and FBS reduction outcomes, which offers more comprehensive evidence for clinicians regarding choice between the two injectable options in the management of type 2 diabetes.
In addition, none of the previously published analyses compared once-weekly dulaglutide with once-daily liraglutide in terms of safety and efficacy except one which has compared dulaglutide and liraglutide indirectly as a part of clinical trial planning (32). Some of the previously published analyses compared GLP-1 RAs with placebo or basal insulin (10, 33). The other available studies assessed the micro vascular effects or cardiovascular events of GLP-1 RAs without attention to HbA1c reduction (36, 37, 39).
Limitation
Limitations regarding the body of evidence and the review process should be taken into account when interpreting our findings. First of all, the number of studies included in this meta-analysis is small; however, we thoroughly searched relevant databases and ensured that no study had been left out.
Second, all included studies received funding from pharmaceutical companies which could skew the results in their favor. Third, as the trials followed the patients up to 52 weeks, the conclusions drawn about the long-term efficacy and safety should not be taken at face value, and the other relevant factors should also be brought to bear on our interpretation of the findings. Fourth, the result of hypoglycemia incidence should be interpreted with caution due to a high heterogeneity in defining hypoglycemic events. Fifth, long-term observational studies are needed to draw safer inferences regarding the safety profile, i.e. rare events, of once-weekly GLP-1 RAs, such as pancreatitis, cardiovascular outcomes, and cancer (40). Sixth, the generalizability of our results is attenuated to conduct sub-group analysis for different populations, such as elderly patients or patients with cardiovascular comorbidities or with a chronic renal disease.
Finally, patient-important outcomes, such as quality-adjusted life years, which can be important determinants of health-related quality of life in the patients with type 2 diabetes, were not assessed in our analysis (41).
It seems that the once-weekly dulaglutide should be investigated in terms of cost-effectiveness compared to the once-daily liraglutide to provide stronger evidences for decision-makers to allocate healthcare resources properly.
Conclusion
The current analysis reveals that once-weekly dulaglutide has a greater reduction in HbA1c compared to once-daily liraglutide. However, the comparison between these regimens indicated no significant difference in FBS changes relative to the baseline.
In addition, there were no significant differences in adverse events for either agent, while only dulaglutide was associated with a lower gastrointestinal complication and also a reduction in heart rate. Further studies are needed to make future meta-analysis more robust in terms of the safety and efficacy of these drugs.
References
- 1.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 2.Azadmehr A, Ziaee A, Ghanei L, Fallah Huseini H, Hajiaghaee R, Tavakoli-Far B, Kordafshari G. A Randomized Clinical Trial Study: Anti-Oxidant, Anti-hyperglycemic and Anti-Hyperlipidemic Effects of Olibanum Gum in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2014;13:1003–09. [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson G, Hall GM. Diabetes mellitus: new drugs for a new epidemic. Br. J. Anaesth. 2011;107:65–73. doi: 10.1093/bja/aer120. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature . 2001;414:782–87. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 5.Jung S-H, Chae J-W, Song B-J, Kwona K-I. Bioequivalence Comparison of Two Formulations of Fixed-Dose Combination Glimepiride/Metformin (2/500 mg) Tablets in Healthy Volunteers. Iran. J. Pharm. Res . 2014;13:365–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care . 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farsaei S, Sabzghabaee AM, Zargarzadeh AH, Amini M. Adherence to glyburide and metformin and associated factors in type 2 diabetes in isfahan, iran. Iran. J. Pharm. Res. . 2011;10:933–39. [PMC free article] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters , AL , Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care . 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson S, Douglas I, Stirnadel-Farrant H, Fogarty D, Pokrajac A, Smeeth L, Tomlinson L. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000-2017. BMJ Open. . 2018;8:e022768. doi: 10.1136/bmjopen-2018-022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Wright EE, Kwan AYM, Thompson JC, Syed IA, Korol EE Waser NA, Yu MB, Juneja R. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes. Metab. 2017;19:228–38. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holst JJ, Knop FK, Vilsboll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care . 2011;34:S251–7. doi: 10.2337/dc11-s227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhinehart AS. Adding GLP-1 Receptor Agonist Therapy to Basal Insulin for Postprandial Glucose Control. Clin Diabetes . 2015;33:73–75. doi: 10.2337/diaclin.33.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. . 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormone . 2018;17:333–50. doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- 15.Samson SL, Garber AJ. A Plethora of GLP-1 Agonists: Decisions About What to Use and When. Curr. Diab. Rep. . 2016;16:120. doi: 10.1007/s11892-016-0823-6. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. . 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, Henry J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials . 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.GRADE Working Group. Grading of recommendations assessment, development and evaluation. The GRADE Working Group. [[cited 208 Jun 5]]. Available from: URL: http://www.gradeworkinggroup.org/
- 19.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials . 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes. Metab. . 2015;17:974–83. doi: 10.1111/dom.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes. Metab. . 2016;180:249–57. doi: 10.1111/dom.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dungan KM, Povedano ST, Forst T, González JGGG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet . 2014;384:1349–57. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 25.McCance DR, Hanson RL, Charles MA, Jacobsson LT, Pettitt DJ, Bennett PH, Knowler WC. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ . 1994;308:1323–8. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann. Intern. Med. . 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 27.Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y, Yasu T, Harasawa H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. . 2017;43:430–7. doi: 10.1016/j.diabet.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, Nielsen OW, Haugaard SB, Sajadieh A. Effects of Liraglutide on Heart Rate and Heart Rate Variability: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Diabetes Care . 2017;40:117–24. doi: 10.2337/dc16-1580. [DOI] [PubMed] [Google Scholar]
- 29.Karagiannis T, Liakos A, Bekiari E, Athanasiadou E, Paschos P, Vasilakou D, Mainou M, Rika M, Boura P, Matthews DR, Tsapas A. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonists for the management of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. . 2015;17:1065–74. doi: 10.1111/dom.12541. [DOI] [PubMed] [Google Scholar]
- 30.Htike ZZ, Francesco Zaccardi M, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and Safety of Glucagon-like peptide-1 receptor agonists in type 2 diabetes Systematic review and mixed-treatment comparison analysis GLP-1RAs treatments in diabetes. Diabetes Obes. Metab. . 2017;19:524–36. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X-W, Zhang X, Xu B, Kang L. Comparative safety and efficacy of insulin degludec with insulin glargine in type 2 and type 1 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetol. . 2018;55:429–41. doi: 10.1007/s00592-018-1107-1. [DOI] [PubMed] [Google Scholar]
- 32.Fahrbach JL, Fu H, Shurzinske L, Skrivanek Z, Martin S. Network meta-analysis accurately predicted the outcome of a subsequent randomised trial comparing once weekly dulaglutide 15 mg and once daily liraglutide 1-8 mg. Int. J. Clin. Pract. . 2016;70:218–21. doi: 10.1111/ijcp.12775. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Zhang Y, Quan X, Yang Z, Zeng X, Ji L, Sun F, Zhan S. Efficacy and acceptability of glycemic control of glucagon-like peptide-1 receptor agonists among type 2 diabetes: A systematic review and network meta-analysis. PLoS One . 2016;11:1–25. doi: 10.1371/journal.pone.0154206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Zhang M, Zhang Y, Tong N. Efficacy and safety of dulaglutide in patients with type 2 diabetes: A meta-analysis and systematic review. Sci. Rep. . 2016;6:1–11. doi: 10.1038/srep18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai X, Gao X, Yang W, Ji L. Comparison between insulin degludec/liraglutide treatment and insulin glargine/lixisenatide treatment in type 2 diabetes: a systematic review and meta-analysis. Expert. Opin. Pharmacother. . 2017;18:1789–98. doi: 10.1080/14656566.2017.1400011. [DOI] [PubMed] [Google Scholar]
- 36.Dicembrini I, Nreu B, Scatena A, Andreozzi F, Sesti G, Mannucci E, Monami M. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetol. . 2017;54:933–41. doi: 10.1007/s00592-017-1031-9. [DOI] [PubMed] [Google Scholar]
- 37.Monami M, Nreu B, Scatena A, Giannini S, Andreozzi F, Sesti G, Mannucci E. Glucagon-like peptide-1 receptor agonists and atrial fibrillation: a systematic review and meta-analysis of randomised controlled trials. J. Endocrinol. Invest. . 2017;40:1251–8. doi: 10.1007/s40618-017-0698-7. [DOI] [PubMed] [Google Scholar]
- 38.Monami M, Dicembrini I, Nreu B, Andreozzi F, Sesti G, Mannucci E. Predictors of response to glucagon-like peptide-1 receptor agonists: a meta-analysis and systematic review of randomized controlled trials. Acta Diabetol. . 2017;54:1101–14. doi: 10.1007/s00592-017-1054-2. [DOI] [PubMed] [Google Scholar]
- 39.Monami M, Zannoni S, Pala L, Silverii A, Andreozzi F, Sesti G, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on mortality and cardiovascular events: A comprehensive meta-analysis of randomized controlled trials. Int. J. Cardiol. . 2017;240:414–21. doi: 10.1016/j.ijcard.2017.03.163. [DOI] [PubMed] [Google Scholar]
- 40.Giorda CB, Sacerdote C, Nada E, Marafetti L, Baldi I, Gnavi R. Incretin-based therapies and acute pancreatitis risk: a systematic review and meta-analysis of observational studies. Endocrine . 2015;48:461–71. doi: 10.1007/s12020-014-0386-8. [DOI] [PubMed] [Google Scholar]
- 41.Javanbakht M, Abolhasani F, Mashayekhi A, Baradaran HR, Jahangiri noudeh Y. Health related quality of life in patients with type 2 diabetes mellitus in Iran: a national survey. PLoS One . 2012;7:e44526. doi: 10.1371/journal.pone.0044526. [DOI] [PMC free article] [PubMed] [Google Scholar]