Abstract
We have synthesized a series of S-allyl cysteine ester-caffeic acid amide hybrids and evaluated them in order to determine their possible anticancer activity and selectivity in colorectal cancer, which is still one of the leading causes of morbidity and mortality worldwide. All compounds were tested against SW480 human colon adenocarcinoma cells and the non-malignant CHO-K1 cell line. Among the tested compounds, hybrids 6e, 9a, 9b, 9c, and 9e exhibited the highest effect on viability (IC50 SW480-48h= 0.18, 0.12, 0.12, 0.11, and 0.12 mM, respectively) and selectivity (SI = 10.3, 1.5, >83.33, >90.91 and >83.33, respectively) in a time- and concentration-dependent manner. Besides, our results were even better as regards lead compounds (S-allyl cysteine and caffeic acid) and the standard drug (5-FU). Additionally, these five compounds induced mitochondrial depolarization that could be related with an apoptotic process. Moreover, hybrids 6e, 9a, and 9e induced cell cycle arrest in G2/M phase, and compound 9c in S- phase, which suggests that these hybrid compounds could have also a cytostatic effect in SW480 cell line. The SAR analysis showed that hydroxyl groups increased the activity. Besides, there was not a clear relationship between the antitumor properties and the length of the alkyl chain. Since hybrid compounds were much more selective than the conventional drug (5-FU), this makes them promising candidates for further studies against colorectal cancer.
Key Words: S-allyl cysteine, Caffeic acid, Hybrid, Cell death, Colorectal cancer
Introduction
Although colorectal cancer (CRC) can be prevented by healthy lifestyle habits this disease is still one of the leading causes of morbidity and mortality worldwide (1). In the most recent report of GLOBOCAN, the estimated burden of CRC until 2018 has exhibited an important increase through the years, being now the second most lethal cancer, preceded only by lung cancer. In addition, CRC is still the third most commonly diagnosed malignancy worldwide (2,3). Current chemotherapy for CRC involves multi-drug treatments such as FOLFIRI (folic acid/5-FU/irinotecan) and FOLFOX (5-FU/leucovorin/oxaliplatin) which are composed of 5-fluorouracil as the backbone of treatment. Although these therapies are effective, there are many undesirable gastrointestinal and neurological side effects associated with these treatments, which many times result in dose limitations or cessation of the anti-cancer therapy (4,5). Given the toxicity of the current chemotherapy, it is necessary to discover new, more potent and selective agents for treating this disease. According to this, the studies dealing with hybrid compounds have been reported as a promising strategy (6), since these molecules may display a dual activity (7,8).
S-allyl cysteine (SAC), a naturally occurring water-soluble constituent of garlic, has exhibited antioxidant properties both in-vitro (9,10) and in-vivo (11-13). Furthermore, SAC displayed antiproliferative effects on neuroblastoma (14) and melanoma (15). Additionally, in prostate carcinoma cells this compound induced cell cycle arrest at the G0/G1 phases and cell apoptosis with decreased in Bcl-2 expression and increased expression of Bax and caspase 8 (16,17). Although this compound did not show growth-inhibitory effects in HCT15 colon cancer cells or in lung and skin carcinoma cell lines (18), its administration prior to 1,2-dimethylhydrazine (DMH) injection significantly inhibited colonic nuclear damage in female in a dose–dependent manner. These data show that the allyl group coupled to a single sulfur atom might play an important structural role in the inhibition of chemical toxicity and carcinogenesis induced with DMH in the colon (19).
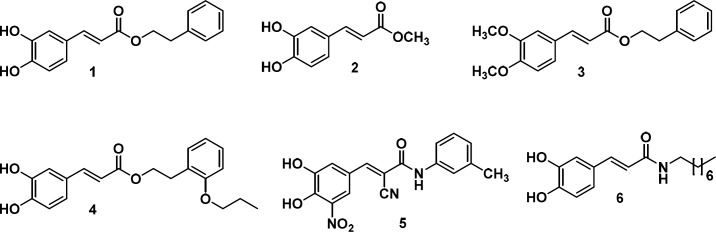
On the other hand, caffeic acid and its derivatives display a broad spectrum of biological properties, among them, antioxidant (20,21), cytotoxic (22), pro-apoptotic, anti-inflammatory, and anti-angiogenic activity (23). Caffeic acid phenylethyl caffeate (CAPE, 1), Methyl caffeate 2 and Phenylethyl dimethyl caffeate 3 (Figure1) significantly inhibited cell growth and syntheses of RNA, DNA, and protein of HT-29 colon adenocarcinoma cells. Moreover, when CAPE was tested in CT26 colon adenocarcinoma cells, it exhibited a decrease in cell viability in a dose–dependent manner, without displaying significant influence on the growth of human umbilical vein epithelial cells (24). Additionally, high anti-proliferative activity was observed when 2´-propoxyl CAPE derivative 4 was tested against Hela and DU-145 human cancer cell lines (25). Furthermore, treatment with CAPE reduced the formation of aberrant crypt foci (ACF) and tumor in the rat colon (26). In addition, caffeic acid was found to be the least effective growth inhibitor of HT-29 cells when compared to its ester analogs, highligting the potency of the derivatives (27). In this sense, cyanoamide derivative 5 showed in-vitro inhibitory activities against human gastric carcinoma cell line BGC-823, human nasopharyngeal carcinoma cell line KB, and human hepatoma cell line BEL-7402 with IC50 values of 5.6 µg/mL, 13.1 µg/mL, and 12.5 µg/mL, respectively (28). Finally, caffeic acid amide 6 and CAPE exhibited proapoptotic activity through activation of caspase-8 (29).
Figure 1.
Caffeic acid derivatives endowed with antitumoral activity
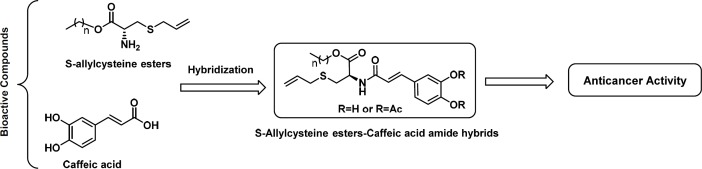
There is an emerging strategy in medicinal chemistry and drug discovery research based on obtaining hybrid molecules that combine two or more structural fragments of the drugs that have a relevant pharmacological action (30,31). These hybrids may display dual activity but do not necessarily act on the same biological target (6,7). We have synthesized several S-allyl cysteine ester–caffeic acid amide hybrids (Figure 2) and their effect on proliferation, mitochondrial membrane permeability, and cell cycle distibution was determined in order to identify posible therapeutic approaches for the treatment of colorectal cancer.
Figure 2.
Design of S-allyl cysteine ester-caffeic acid amide hybrids as anti-cancer agents
Experimental section
Chemical synthesis
General remarks
Microwave reactions were carried out in a CEM Discover microwave reactor in sealed vessels (monowave, maximum power 300 W, temperature control by IR sensor, and fixed temperature). 1H and 13C NMR spectra were recorded on a Varian instrument operating at 300, 600 and 75, 125 MHz, respectively. The signals of the deuterated solvent (CDCl3 or DMSO-D6) were used as reference. Chemical shifts (δ) are expressed in ppm with the solvent peak as reference and TMS as an internal standard; coupling constants (J) are given in Hertz (Hz). Carbon atom types (C, CH, CH2, CH3) were determined by using the DEPT pulse sequence. The signals were assigned using two-dimensional heteronuclear correlations (COSY, HSQC and HMBC). High resolution mass spectra were recorded using electrospray ionization mass spectrometry (ESI-MS). A QTOF Premier instrument with an orthogonal Z-spray-electrospray interface (Waters, Manchester, UK) was used operating in the W-mode. The drying and cone gas was nitrogen set to flow rates of 300 and 30 L/h, respectively. Methanol sample solutions (ca. 1 × 10−5 M) were directly introduced into the ESI spectrometer at a flow rate of 10 µL/min. A capillary voltage of 3.5 kV was used in the positive scan mode, and the cone voltage set to Uc = 10 V. For accurate mass measurements, a 2 mg/L standard solution of leucine enkephalin was introduced via the lock spray needle at a cone voltage set to 85 V and a flow rate of 30 μL/min. IR spectra were recorded on a Spectrum RX I FT-IR system (Perkin-Elmer, Waltham, MA, USA) in KBr disks. Optical rotations were measured (Na-D line) at 25°C using a cell with 1dm path length on a Polartronic (Jasco model p-2000) polarimeter. Silica gel 60 (0.063–0.200 mesh, Merck, Whitehouse Station, NJ, USA) was used for column chromatography, and precoated silica gel plates (Merck 60 F254 0.2 mm) were used for thin layer chromatography (TLC). Monitoring of the reaction progress and product purification was carried out by TLC.
Procedure for the synthesis of S-Allylcysteine ( 2 ): S-Cysteine hydrochloride (1 g, 6.34 mmol) was added to allyl bromide (1.15 g, 823 µL, 9.51 mmol) in 2M NH4OH (20 mL). The resulting mixture was stirred at room temperature for 20h. Then, the reaction mixture was concentrated to precipitate the product as a white solid. The solid was filtered, washed with ethanol (3 × 10 mL) and dried under reduced pressure, affording 818 mg (80%) of compound 2. This compound was used in the following step without further purification.
1H NMR (DMSO-D6, 300 MHz): δ 2.48 (NH2), 2.72 (1H, dd, J = 14.6, 8.0 Hz, S-CH2CHN), 2.87 (1H, dd, J = 14.6, 4.2 Hz, S-CH2CHN), 3.06 (2H, d, J = 7.3 Hz, S-CH2CH=CH2), 3.56 (1H, dd, J = 8.0, 4.2 Hz, -CH-N), 4.29 (-NH2), 4.98-5.13 (2H, m, S-CH2CH=CH2), 5.59-5.75 (2H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 75 MHz): δ 31.33 (S-CH2CHN), 33.93 (S-CH2CH=CH2), 53.45 (CH-N), 118.48 (S-CH2CH=CH2), 133.77 (S-CH2CH=CH2), 171.33 (-C=O)
Procedure for the synthesis of S-Allylcysteine methyl ester ( 3a ):
Thionyl chloride (442.6 mg, 3.72 mmol, 270 µL) was added over 5 min. to dry methanol (15 mL) cooled to -10 °C and the resulting solution was stored for a further 5 min. Then, S-allyl cysteine (500 mg, 3.1 mmol) was added and the mixture was stirred for 10 min. The resulting solution was stored at -10 °C for 2h, kept at room temperature for other 24 h, and then poured into ether (100 mL) and refrigerated for 2 h. The product (488 mg, 90%) separated as colorless needles, was removed by
filtration.
M.p. 114-116 °C; [α]25 + 4.778 (C = 0,013, CHCl3); IR (KBr, cm-1): ν max 3366 (N-H), 1736 (C=O), 1238 (C-O-C); 1H NMR (DMSO-D6, 300 MHz): δ 2.51 (NH2), 2.84 (1H, dd, J = 14.7, 5.0 Hz, S-CH2CHN), 2.93 (1H, dd, J = 14.7, 5.0 Hz, S-CH2CHN), 3.09 (2H, d, J = 7.3 Hz, S-CH2CH=CH2), 3.70 (3H, s, OCH3), 4.14 (1H, dd, J = 7.1, 5.0 Hz, -CH-N), 5.04-5.15 (2H, m, S-CH2CH=CH2), 5.59-5.77 (1H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 75 MHz): δ 30.09 (S-CH2CHN), 34.20 (S-CH2CH=CH2), 52.09 (OCH3), 54.52 (CH-N), 118.86 (S-CH2CH=CH2), 133.37 (S-CH2CH=CH2), 168.96 (-C=O). EIMS: m/z 176,0732 [M + H]+, Calcd for C7H14NO2S: 176.0354.
General procedure for the synthesis of S-Allyl cysteine esters ( 3b-3e ):
Thionyl chloride (3eq) was added over 5 min. to dry ethyl, propyl, butyl, or pentyl alcohol (15 mL) cooled to -10 °C and the resulting solution was stored for a further 5 min. Then, S-allyl cysteine (500 mg, 3.1 mmol) was added and the resulting mixture was stored at -10 °C for 2 h and the kept at room temperature for a further 24 h. Then the excess of alcohol was removed by distillation. The residue was purified by column chromatography over silica gel eluting with dichloromethane-methanol (95:5 ratio) to obtain S-allyl cysteine ethyl ester, S-allyl cysteine propyl ester, S-allyl cysteine butyl ester and S-allyl cysteine pentyl ester in 60% (352 mg), 71% (447 mg), 621% (418 mg) and 85% (609 mg) yields, respectively. Monitoring the reaction progress and product purification was carried out by TLC.
Ethyl S-prop-2-en-1-ylcysteinate ( 3b ): M.p. 121-123°C; [α]25 + 1.40 (C = 0,62, CHCl3); IR (KBr, cm-1): ν max 3472 (N-H), 1748 (C=O), 1231 (C-O-C); 1H NMR (CDCl3, 600 MHz): δ 0.96 (3H, t, J = 7.2 Hz), 2.63 (NH2), 3.22-3.25 (2H, m, S-CH2CHN), 3.18-3.22 (2H, m, S-CH2CH=CH2), 3.29 (1H, dd, J = 7.5, 5.0 Hz, -CH-N), 4.29 (2H, q, J = 7.0 Hz, OCH2), 5.15 (1H, d, J = 10 Hz, S-CH2CH=CH2), 5.24 (1H, d, J = 18 Hz, S-CH2CH=CH2), 5.74-5.85 (1H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 125 MHz): δ 14.05 (CH3), 30.48 (S-CH2CHN), 35.10 (S-CH2CH=CH2), 52.69 (CH-N), 62.94 (OCH2), 118.51 (S-CH2CH=CH2), 133.37 (S-CH2CH=CH2), 167.95 (-C=O). EIMS: m/z 190,0879 [M + H]+, Calcd for C8H16NO2S: 190.0356.
Propyl S-prop-2-en-1-ylcysteinate ( 3c ): M.p. 115-117 °C; [α]25 + 3.750 (C = 0,015, CHCl3); IR (KBr, cm-1): ν max 3375 (N-H), 1736 (C=O), 1182 (C-O-C); 1H NMR (CDCl3, 300 MHz): δ 0.91 (3H, t, J = 7.5 Hz), 1.53-1.72 (2H, m), 1.98 (NH2), 2.83 (1H, dd, J = 13.5, 5.0 Hz, S-CH2CHN), 2.65 (1H, dd, J = 13.5, 5.0 Hz, S-CH2CHN), 3.11 (2H, d, J = 7.0 Hz, S-CH2CH=CH2), 3.58 (1H, dd, J = 7.4, 5.0 Hz, -CH-N), 4.05 (2H, t, J = 6.7 Hz, OCH2), 5.07-5.16 (2H, m, S-CH2CH=CH2), 5.64-5.82 (1H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 75 MHz): δ 10.41 (CH3), 21.97 (CH2), 35.14 (S-CH2CHN), 35.82 (S-CH2CH=CH2), 54.11 (CH-N), 66.82 (OCH2), 117.61 (S-CH2CH=CH2), 134.01 (S-CH2CH=CH2), 174.15 (-C=O). EIMS: m/z 204,1036 [M + H]+, Calcd for C9H18NO2S: 204.0603.
Butyl S-prop-2-en-1-ylcysteinate ( 3d ): M.p. 101-103°C; [α]25 + 3.40 (C = 0,57, CHCl3); IR (KBr, cm-1): ν max 3396 (N-H), 1750 (C=O), 1233 (C-O-C); 1H NMR (CDCl3, 600 MHz): δ 0.93 (3H, t, J = 7.5 Hz), 1.34-1.43 (2H, m), 1.62-1.70 (2H, m), 2.62 (NH2), 3.19 (2H, d, J = 6.8 Hz, S-CH2CH=CH2), 3.23 (1H, dd, J = 13.9, 7.0 Hz, S-CH2CHN), 3.29 (1H, dd, J = 13.9, 7.0 Hz, S-CH2CHN), 4.17-4.28 (1H, m, -CH-N), 4.38 (2H, t, J = 6.5 Hz, OCH2), 5.14 (1H, d, J = 10 Hz, S-CH2CH=CH2), 5.24 (1H, d, J = 17 Hz, S-CH2CH=CH2), 5.74-5.83 (1H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 125 MHz): δ 13.66 (CH3), 19.02 (2CH2), 30.53 (S-CH2CHN), 35.11 (S-CH2CH=CH2), 52.67 (CH-N), 66.75 (OCH2), 118.49 (S-CH2CH=CH2), 133.38 (S-CH2CH=CH2), 168.08 (-C=O). EIMS: m/z 218,1206 [M + H]+, Calcd for C10H20NO2S: 218.0457.
Pentyl S-prop-2-en-1-ylcysteinate ( 3e ): M.p. 98-100°C; [α]25 + 1.558 (C = 0,018, CHCl3); IR (KBr, cm-1): ν max 3448 (N-H), 1747 (C=O), 1234 (C-O-C); 1H NMR (CDCl3, 600 MHz): δ 0.93 (3H, t, J = 7.0 Hz), 1.31-1.42 (4H, m), 1.60-1.74 (2H, m), 1.82 (NH2), 2.71 (1H, dd, J = 13.5, 5.3 Hz, S-CH2CHN), 2.90 (1H, dd, J = 13.5, 5.3 Hz, S-CH2CHN), 3.18 (2H, d, J = 7.17 Hz, S-CH2CH=CH2), 3.60-3.69 (1H, m, -CH-N), 4.16 (2H, t, J = 6.7 Hz,-OCH2), 5.11-5.20 (2H, m, S-CH2CH=CH2), 5.72-5.89 (1H, m, S-CH2CH=CH2); 13C NMR (CDCl3, 125 MHz): δ 13.70 (CH3), 22.03 (CH2), 27.78 (CH2), 28.01 (CH2), 34.83 (S-CH2CHN), 35.53 (S-CH2CH=CH2), 54.84 (CH-N), 65.04 (OCH2), 117.23 (S-CH2CH=CH2), 134.82 (S-CH2CH=CH2), 173.91 (-C=O); EIMS: m/z 232.1368 [M + H]+, Calcd for C11H22NO2S: 232.1371.
General procedure for condensation using HBTU
A solution of 3,4-diacetoxycaffeic acid (5) or 3,4-disilylated caffeic acid (7) (1 mmol) and triethylamine (4 mmol) in THF (10 mL) was stirred for 15 min. Then, HBTU (1.5 mmol) was added and the resulting mixture was stirred for 10 min. Then, S-allyl cysteine ester (3a-3e) (1.2 mmol) was added and the resulting mixture was allowed to stir for 15 h. The solvent was removed under reduced pressure, and the residue was chromatographed on silica gel. Elution with hexane-ethyl acetate (1:1 ratio) afforded compounds 6a-6e in yields ranging 30-40% [6a, 35% (148 mg); 6b, 39% (170 mg); 6c, 34% (153 mg); 6d, 33% (153 mg) and 6e, 40% (191 mg)] or 8a-8e in yields ranging 50-60% [8a, 50% (283 mg); 8b, 50% (578 mg); 8c, 52% (309 mg); 8d, 50% (304 mg) and 8e, 60% (373 mg)].
Methyl N-{(2E)-3-[3,4-bis(acetyloxy)phenyl]prop-2-enoyl}-S-prop-2-en-1-ylcysteinate ( 6a ): M.p. 107-109°C; [α]25 + 5.292 (C = 0,019, CHCl3); IR (KBr, cm-1): ν max 3394 (N-H), 1770, 1743 and 1662 (C=O), 1259 (C-O-C), 1205 ((C=O)-O); 1H NMR (CDCl3, 300 MHz): δ 2.30 (3H, s, ((CH3-C=O)-O), 2.31 (3H, s, (CH3-C=O)-O), 2.95 (1H, dd, J = 14.0, 5.0 Hz, S-CH2CHN), 3.04 (1H, dd, J = 14.0, 5.0 Hz, S-CH2CHN), 3.13 (2H, d, J = 7.0 Hz, S-CH2CH=CH2), 3.79 (3H, s, OCH3), 4.89-4.98 (1H, m, -CH-N), 5.07-5.17 (2H, m, S-CH2CH=CH2), 5.66-5.82 (1H, m, S-CH2CH=CH2), 6.40 (1H, d, J = 15.6 Hz, –CO–CH=), 6.47 (1H, d, J = 7.5 Hz, -CH-NH-C=O), 7.20 (1H, d, J = 8.3 Hz, Ar-H), 7.39 (1H, d, J = 1.8 Hz, Ar-H), 7.35 (1H, dd, J = 8.3, 1.8 Hz, Ar-H), 7.59 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 75 MHz): δ 20.76 ((CH3-C=O)-O), 20.79 ((CH3-C=O)-O), 32.76 (S-CH2CHN), 35.33 (S-CH2CH=CH2), 52.05 (CH-N), 52.88 (OCH3), 118.19 (C=C-CO-), 121.08 (S-CH2CH=CH2), 122.61 (Ar), 123.99 (Ar), 126.45 (Ar), 129.16 (Ar), 133.64 (Ar), 133.54 (S-CH2CH=CH2), 140.37 (Ar-C=C), 142.50 (Ar-O), 143.29 (Ar-O), 165.09 (-NH-C=O), 168.21 ((CH3-C=O)-O), 168.25 ((CH3-C=O)-O), 171.44 ((NCH-C=O)-O); EIMS: m/z 444.1090 [M + Na]+, Calcd for C20H24NO7S: 444.1093
Ethyl N-{(2E)-3-[3,4-bis(acetyloxy)phenyl]prop-2-enoyl}-S-prop-2-en-1-ylcysteinate ( 6b ): M.p. 96-99°C; [α]25 + 13.60 (C = 0,835, CHCl3); IR (KBr, cm-1): ν max 3317 (N-H), 1774, 1734 and 1655 (C=O), 1265 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 1.31 (3H, t, J = 7.1 Hz), 2.30 (3H, s, ((CH3-C=O)-O), 2.31 (3H, s, (CH3-C=O)-O), 2.96 (1H, dd, J = 14.0, 5.4 Hz, S-CH2CHN), 3.05 (1H, dd, J = 14.0, 5.4 Hz, S-CH2CHN), 3.14 (2H, d, J = 7.02 Hz, S-CH2CH=CH2), 4.26 (2H, q, J = 7.0 Hz, OCH2), 4.90-4.94 (1H, m, -CH-N), 5.10-5.15 (2H, m, S-CH2CH=CH2), 5.71-5.80 (1H, m, S-CH2CH=CH2), 6.41 (1H, d, J = 15.7 Hz, –CO–CH=), 6.51 (1H, d, J = 7.5 Hz, -CH-NH-C=O), 7.21 (1H, d, J = 8.4 Hz, Ar-H), 7.34 (1H, d, J = 2.0 Hz, Ar-H), 7.37 (1H, dd, J = 8.4, 2.0 Hz, Ar-H), 7.58 (1H, d, J = 15.7 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 14.28 (CH3), 20.76 ((CH3-C=O)-O), 20.79 ((CH3-C=O)-O), 32.89 (S-CH2CHN), 35.39 (S-CH2CH=CH2), 52.16 (CH-N), 62.11 (OCH2), 118.03 (S-CH2CH=CH2), 118.13 (C=C-CO-), 121.16 (Ar), 122.61 (Ar), 123.99 (Ar), 126.44 (Ar), 133.73 (S-CH2CH=CH2), 140.30 (Ar-C=C), 142.50 (Ar-O), 143.29 (Ar-O), 165.08 (-NH-C=O), 168.20 ((CH3-C=O)-O), 168.26 ((CH3-C=O)-O), 170.94 ((NCH-C=O)-O); EIMS: m/z 436.1430 [M + H]+, Calcd for C21H26NO7S: 436.1431.
Propyl N-{(2E)-3-[3,4-bis(acetyloxy)phenyl]prop-2-enoyl}-S-prop-2-en-1-ylcysteinate ( 6c ): M.p. 80-82°C; [α]25 + 1.442 (C = 0,011, CHCl3); IR (KBr, cm-1): ν max 3315 (N-H), 1766, 1735 and 1658 (C=O), 1209 (C-O-C), 1184 ((C=O)-O); 1H NMR (CDCl3, 300 MHz): δ 0.96 (3H, t, J = 7.5 Hz), 1.62-1.77 (2H, m), 2.29 (3H, s, ((CH3-C=O)-O), 2.30 (3H, s, (CH3-C=O)-O), 2.94 (1H, dd, J = 13.9, 5.3 Hz, S-CH2CHN), 3.04 (1H, dd, J = 13.9, 4.9 Hz, S-CH2CHN), 3.13 (2H, d, J = 7.20 Hz, S-CH2CH=CH2), 4.14 (2H, t, J = 6.7 Hz, OCH2), 4.87-4.97 (1H, m, -CH-N), 5.05-5.18 (2H, m, S-CH2CH=CH2), 5.64-5.82 (1H, m, S-CH2CH=CH2), 6.41 (1H, d, J = 15.6 Hz, –CO–CH=), 6.52 (1H, d, J = 7.5 Hz, -CH-NH-C=O), 7.20 (1H, d, J = 8.3 Hz, Ar-H), 7.24 (1H, d, J = 1.6 Hz, Ar-H), 7.34 (1H, dd, J = 8.3, 1.6 Hz, Ar-H), 7.58 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 75 MHz): δ 10.46 (CH3), 20.77 ((CH3-C=O)-O), 22.00 ((CH3-C=O)-O), 32.86 (S-CH2CHN), 35.37 (S-CH2CH=CH2), 52.16 (CH-N), 67.61 (OCH2), 118.12 (S-CH2CH=CH2), 121.16 (=C-CO-), 122.58 (Ar), 123.97 (Ar), 126.44 (Ar), 129.14 (Ar), 133.66 (S-CH2CH=CH2), 140.25 (Ar-C=), 142.48 (Ar-O), 143.25 (Ar-O), 165.09 (N-C=O), 168.19 ((CH3-C=O)-O), 168.24 ((CH3-C=O)-O), 171.62 ((NCH-C=O)-O); EIMS: m/z 472.1401 [M + Na]+, Calcd for C22H27NO7S-Na: 472.1406.
Butyl N-{(2E)-3-[3,4-bis(acetyloxy)phenyl]prop-2-enoyl}-S-prop-2-en-1-ylcysteinate ( 6d ): M.p. 94-97°C; [α]25 + 8.30 (C = 0,65, CHCl3); IR (KBr, cm-1): ν max 3309 (N-H), 1774, 1742 and 1662 (C=O), 1265 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 0.95 (3H, t, J = 7.2 Hz), 1.36-1.44 (2H, m), 1.61-1.72 (2H, m), 2.30 (3H, s, ((CH3-C=O)-O), 2.31 (3H, s, ((CH3-C=O)-O), 2.95 (1H, dd, J = 13.8, 5.4 Hz, S-CH2CHN), 3.05 (1H, dd, J = 13.9, 5.4 Hz, S-CH2CHN), 3.10-3.16 (2H, m, S-CH2CH=CH2), 4.19 (2H, q, J = 6.9 Hz, OCH2), 4.90-4.96 (1H, m, -CH-N), 5.08-5.15 (2H, m, S-CH2CH=CH2), 5.70-5.79 (1H, m, S-CH2CH=CH2), 6.41 (1H, d, J = 15.6 Hz, –CO–CH=), 6.50 (1H, d, J = 7.2 Hz, -CH-NH-C=O), 7.21 (1H, d, J = 8.3 Hz, Ar-H), 7.35 (1H, s, Ar-H), 7.38 (1H, d, J = 8.3, Ar-H), 7.59 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 13.77 (CH3), 19.19 (CH2), 20.70 ((CH3-C=O)-O), 20.78 ((CH3-C=O)-O), 23.18 (CH2), 32.97 (S-CH2CHN), 35.41 (S-CH2CH=CH2), 52.20 (CH-N), 65.94 (OCH2), 118.01 (S-CH2CH=CH2), 118.11 (=C-CO-), 121.17 (Ar), 122.60 (Ar), 123.98 (Ar), 126.43 (Ar), 133.73 (S-CH2CH=CH2), 140.29 (Ar-C=), 142.51 (Ar-O), 143.29 (Ar-O), 165.09 (N-C=O), 168.18 ((CH3-C=O)-O), 168.23 ((CH3-C=O)-O), 171.02 ((NCH-C=O)-O); EIMS: m/z 464.1743 [M + H]+, Calcd for C23H29NO7S: 464.1745.
Pentyl N-{(2E)-3-[3,4-bis(acetyloxy)phenyl]prop-2-enoyl}-S-prop-2-en-1-ylcysteinate ( 6e ): M.p. 101-103°C; [α]25 + 3.087 (C = 0,0119, CHCl3); IR (KBr, cm-1): ν max 3317 (N-H), 1768, 1743 and 1656 (C=O), 1219 (C-O-C), 1184 ((C=O)-O); 1H NMR (CDCl3, 300 MHz): δ 0.90 (3H, t, J = 7.1 Hz), 1.28-1.39 (4H, m), 1.60-1.74 (2H, m), 2.29 (3H, s, ((CH3-C=O)-O), 2.30 (3H, s, ((CH3-C=O)-O), 2.94 (1H, dd, J = 13.9, 5.3 Hz, S-CH2CHN), 3.05 (1H, dd, J = 13.9, 4.8 Hz, S-CH2CHN), 3.13 (2H, d, J = 7.3 Hz, S-CH2CH=CH2), 4.18 (2H, t, J = 6.8 Hz, OCH2), 4.87-4.96 (1H, m, -CH-N), 5.06-5.10 (2H, m, S-CH2CH=CH2), 5.66-5.82 (1H, m, S-CH2CH=CH2), 6.40 (1H, d, J = 15.6 Hz, –CO–CH=), 6.48 (1H, d, J = 7.4 Hz, -CH-NH-C=O), 7.20 (1H, d, J = 8.4 Hz, Ar-H), 7.34 (1H, d, J = 1.8 Hz, Ar-H), 7.38 (1H, dd, J = 8.4, 1.8 Hz, Ar-H), 7.58 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 75 MHz): δ 14.07 (CH3), 20.77 ((CH3-C=O)-O), 20.80 ((CH3-C=O)-O), 22.37 (CH2), 28.07 (CH2), 28.29 (CH2), 32.89 (S-CH2CHN), 35.41 (S-CH2CH=CH2), 52.18 (CH-N), 66.23 (OCH2), 118.13 (S-CH2CH=CH2), 119.0 (=C-CO-), 121.15 (Ar), 122.60 (Ar), 123.99 (Ar), 126.44 (Ar), 133.67 (S-CH2CH=CH2), 140.29 (Ar-C=C), 142.50 (Ar-O), 143.28 (Ar-O), 165.07 (N-C=O), 168.20 ((CH3-C=O)-O), 168.25 ((CH3-C=O)-O), 171.02 ((NCH-C=O)-O); EIMS: m/z 500.1719 [M + Na]+, Calcd for C24H31NO7S-Na: 500.1719.
Methyl N-[(2E)-3-(3,4-bis{[tert-butyl(dimethyl)silyl]oxy}phenyl)prop-2-enoyl]-S-prop-2-en-1-yl-L-cysteinate (8a): colorless oil; [α]25 + 2.50 (C = 0,50, CHCl3); IR (KBr, cm-1): ν max 3309 (N-H), 1742 and 1666 (C=O), 1424 (Si-C), 1249 (C-O-C), 1202 ((C=O)-O), 1106 (Si-O); 1H NMR (CDCl3, 600 MHz): δ 0.21 (12H, s, -Si-CH3), 0.98 (9H, s, -C-(CH3)3), 1.0 (9H, s, -C-(CH3)3), 2.96 (1H, dd, J = 14.0, 5.0 Hz, S-CH2CHN), 3.04 (1H, dd, J = 14.0, 5.0 Hz, S-CH2CHN), 3.14 (2H, tapp, J = 7.5 Hz, S-CH2CH=CH2), 3.80 (3H, s, OCH3), 4.93-4.98 (1H, m, -CH-N), 5.08-5.16 (2H, m, S-CH2CH=CH2), 5.70-5.80 (1H, m, S-CH2CH=CH2), 6.25 (1H, d, J = 15.5 Hz, –CO–CH=C), 6.35 (1H, d, J = 7.2 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.98-7.02 (2H, m, Ar-H), 7.52 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ -3.92 (-Si-CH3), -3.44 (-Si-CH3), 18.60 (-Si-C-(CH3)3), 18.64 (-Si-C-(CH3)3), 26.03 (-C-(CH3)3), 26.07 (-C-(CH3)3), 32.96 (S-CH2CHN), 35.40 (S-CH2CH=CH2), 52.04 (CH-N), 52.85 (OCH2), 117.58 (Ar), 118.07 (S-CH2CH=CH2), 118.16 (Ar), 120.88 (C=C-CO-), 121.29 (Ar), 122.01 (Ar), 133.73 (S-CH2CH=CH2), 142.07 (Ar-C=C), 147.27 (Ar-O), 149.23 (Ar-O), 165.90 (-NH-C=O), 171.67 (CH-C=O)-O).
EIMS: m/z 566, 2751 [M + H]+, Calcd for C28H48NO5SSi2: 566.1994.
Ethyl N-[(2E)-3-(3,4-bis{[tert-butyl(dimethyl)silyl]oxy}phenyl)prop-2-enoyl]-S-prop-2-en-1-yl-L-cysteinate (8b): colorless oil; [α]25 + 2.30 (C = 0,585, CHCl3); IR (KBr, cm-1): ν max 3293 (N-H), 1750 and 1662 (C=O), Si-C (1416), 1257 (C-O-C), 1209 ((C=O)-O), 1209 (Si-O); 1H NMR (CDCl3, 600 MHz): δ 0.21 (12H, s, -Si-CH3), 0.98 (9H, s, -C-(CH3)3), 1.0 (9H, s, -C-(CH3)3), 1.31 (3H, t, J = 7.2 Hz), 2.96 (1H, dd, J = 13.94, 5.2 Hz, S-CH2CHN), 3.05 (1H, dd, J = 13.94, 5.2 Hz, S-CH2CHN), 3.14 (2H, tapp, J = 6.8 Hz, S-CH2CH=CH2), 4.26 (2H, q, J = 7.1 Hz, OCH2), 4.91-4.96 (1H, m, -CH-N), 5.09-5.16 (2H, m, S-CH2CH=CH2), 5.70-5.79 (1H, m, S-CH2CH=CH2), 6.26 (1H, d, J = 15.6 Hz, –CO–CH=C), 6.36 (1H, d, J = 7.5 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.98-7.02 (2H, m, Ar-H), 7.52 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ -3.92 (-Si-CH3), -3.43 (-Si-CH3), 14.31 (-Si-C-(CH3)3), 18.60 (-Si-C-(CH3)3), 18.64 (-Si-C-(CH3)3), 26.03 (-C-(CH3)3), 26.07 (-C-(CH3)3), 33.03 (S-CH2CHN), 35.45 (S-CH2CH=CH2), 52.15 (CH-N), 62.06 (OCH2), 117.54 (Ar), 118.09 (S-CH2CH=CH2), 120.62 (Ar), 121.29 ((C=C-CO-), 121.99 (Ar), 128.32 (Ar), 133.77 (S-CH2CH=CH2), 138.59 (Ar-C=C), 147.27 (Ar-O), 149.60 (Ar-O), 165.88 (-NH-C=O), 171.17 (CH-C=O)-O). EIMS: m/z 580,2944 [M + H]+, Calcd for C29H50NO5SSi2: 580.2170.
Propyl N-[(2E)-3-(3,4-bis{[tert-butyl(dimethyl)silyl]oxy}phenyl)prop-2-enoyl]-S-prop-2-en-1-yl-L-cysteinate (8c): colorless oil; [α]25 + 1.70 (C = 0,665, CHCl3); IR (KBr, cm-1): ν max 3277 (N-H), 1742 and 1662 (C=O), 1416 (Si-C), 1257 (C-O-C), 1209 ((C=O)-O), 1122 (Si-O); 1H NMR (CDCl3, 600 MHz): δ 0.21 (12H, s, -Si-CH3), 0.98 (3H, t, J = 7.0 Hz), 0.99 (9H, s, -C-(CH3)3), 1.0 (9H, s, -C-(CH3)3), 1.67-1.74 (2H, m), 2.95 (1H, dd, J = 14.0, 5.3 Hz, S-CH2CHN), 3.05 (1H, dd, J = 14.0, 5.3 Hz, S-CH2CHN), 3.15 (2H, tapp, J = 6.8 Hz, S-CH2CH=CH2), 4.15 (2H, t, J = 6.6 Hz, OCH2), 4.92-4.97 (1H, m, -CH-N), 5.10-5.15 (2H, m, S-CH2CH=CH2), 5.71-5.79 (1H, m, S-CH2CH=CH2), 6.25 (1H, d, J = 15.5 Hz, –CO–CH=C), 6.37 (1H, d, J = 7.6 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.98-7.02 (2H, m, Ar-H), 7.52 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ -4.06 (-Si-CH3), -3.92 (-Si-CH3), 10.50 (-Si-C-(CH3)3), 18.60 (-Si-C-(CH3)3), 18.64 (-Si-C-(CH3)3), 22.05 (CH2), 26.03 (-C-(CH3)3), 26.06 (-C-(CH3)3), 33.09 (S-CH2CHN), 35.46 (S-CH2CH=CH2), 52.16 (CH-N), 67.60 (OCH2), 117.17 (Ar), 118.10 (S-CH2CH=CH2), 120.62 (Ar), 121.29 ((C=C-CO-), 121.98 (Ar), 128.32 (Ar), 133.76 (S-CH2CH=CH2), 142.05 (Ar-C=C), 147.26 (Ar-O), 149.19 (Ar-O), 165.90 (-NH-C=O), 171.27 (CH-C=O)-O). EIMS: m/z 594, 3136 [M + H]+, Calcd for C30H52NO5SSi2: 594.2331.
Butyl N-[(2E)-3-(3,4-bis{[tert-butyl(dimethyl)silyl]oxy}phenyl)prop-2-enoyl]-S-prop-2-en-1-yl-L-cysteinate (8d): colorless oil; [α]25 + 2.50 (C = 0,64, CHCl3); IR (KBr, cm-1): ν max 3285 (N-H), 1742 and 1655 (C=O), 1416 (Si-C), 1265 (C-O-C), 1209 ((C=O)-O), 1122 (Si-O); 1H NMR (CDCl3, 600 MHz): δ 0.21 (12H, s, -Si-CH3), 0.95 (3H, t, J = 7.1 Hz), 0.98 (9H, s, -C-(CH3)3), 1.0 (9H, s, -C-(CH3)3), 1.34-1.45 (2H, m), 1.61-1.71 (2H, m), 2.95 (1H, dd, J = 13.90, 5.2 Hz, S-CH2CHN), 3.05 (1H, dd, J = 13.90, 5.4 Hz, S-CH2CHN), 3.05 (2H, tapp, J = 6.7 Hz, S-CH2CH=CH2), 4.20 (2H, t, J = 6.7 Hz, OCH2), 4.91-4.96 (1H, m, -CH-N), 5.08-5.016 (2H, m, S-CH2CH=CH2), 5.70-5.79 (1H, m, S-CH2CH=CH2), 6.25 (1H, d, J = 15.5 Hz, –CO–CH=C), 6.36 (1H, d, J = 7.6 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.97-7.02 (2H, m, Ar-H), 7.52 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ -4.02 (-Si-CH3), -3.92 (-Si-CH3), 13.80 (-Si-C-(CH3)3), 18.60 (-Si-C-(CH3)3), 18.64 (-Si-C-(CH3)3), 19.22 (CH2), 26.03 (-C-(CH3)3), 26.07 (-C-(CH3)3), 30.65 (CH2), 33.10 (S-CH2CHN), 35.47 (S-CH2CH=CH2), 52.17 (CH-N), 65.90 (OCH2), 117.68 (Ar), 118.09 (S-CH2CH=CH2), 120.62 (Ar), 121.99 ((C=C-CO-), 123.62 (Ar), 128.32 (Ar), 133.77 (S-CH2CH=CH2), 142.05 (Ar-C=C), 147.27 (Ar-O), 149.20 (Ar-O), 165.90 (-NH-C=O), 171.26 (CH-C=O)-O). EIMS: m/z 609.3315 [M + H]+, Calcd for C31H54NO5SSi2: 609.3293.
Pentyl N-[(2E)-3-(3,4-bis{[tert-butyl(dimethyl)silyl]oxy}phenyl)prop-2-enoyl]-S-prop-2-en-1-yl-L-cysteinate (8f): colorless oil; [α]25 + 2.80 (C = 0,925, CHCl3); IR (KBr, cm-1): ν max 3277 (N-H), 1750 and 1662 (C=O), 1416 (Si-C), 1249 (C-O-C), 1209 ((C=O)-O), 1122 (Si-O); 1H NMR (CDCl3, 600 MHz): δ 0.21 (12H, s, -Si-CH3), 0.91 (3H, t, J = 7.0 Hz), 0.98 (9H, s, -C-(CH3)3), 1.0 (9H, s, -C-(CH3)3), 1.29-1.39 (4H, m), 1.60-1.73 (2H, m), 2.95 (1H, dd, J = 13.90, 5.2 Hz, S-CH2CHN), 3.05 (1H, dd, J = 13.90, 5.4 Hz, S-CH2CHN), 3.15 (2H, tapp, J = 6.9 Hz, S-CH2CH=CH2), 4.18 (2H, t, J = 6.6 Hz, OCH2), 4.91-4.97 (1H, m, -CH-N), 5.10-5.16 (2H, m, S-CH2CH=CH2), 5.70-5.80 (1H, m, S-CH2CH=CH2), 6.24 (1H, d, J = 15.7 Hz, –CO–CH=C), 6.37 (1H, d, J = 7.5 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.98-7.02 (2H, m, Ar-H), 7.52 (1H, d, J = 15.7 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ -4.02 (-Si-CH3), -3.92 (-Si-CH3), 14.09 (-Si-C-(CH3)3), 18.60 (-Si-C-(CH3)3), 18.64 (-Si-C-(CH3)3), 22.40 (CH2), 26.03 (-C-(CH3)3), 26.06 (-C-(CH3)3), 28.12 (CH2), 28.30 (CH2), 33.09 (S-CH2CHN), 35.46 (S-CH2CH=CH2), 52.17 (CH-N), 66.18 (OCH2), 117.68 (Ar), 118.08 (S-CH2CH=CH2), 120.61 (Ar), 121.29 ((C=C-CO-), 121.98 (Ar), 128.32 (Ar), 133.77 (S-CH2CH=CH2), 142.04 (Ar-C=C), 147.26 (Ar-O), 149.19 (Ar-O), 165.88 (-NH-C=O), 171.25 (CH-C=O)-O). EIMS: m/z 623,3478 [M + H]+, Calcd for C32H56NO5SSi2: 623.3431.
Procedure for desprotection of compounds 8a-8e
To a solution of compound 8 (1 mmol) in THF-H2O (1:1) (10 mL) was added KF (4 mmol) and the mixture was stirred for 12 h. Then an aqueous saturated solution of NH4Cl was added and the mixture was extracted with dichloromethane (3 × 10 mL). The combined organic phases were dried over anhydrous MgSO4 and the solvent was evaporated under reduced pressure to afford hybrids 9a-9e in yields ranging 50%-96% [9a, 69% (233 mg); 9b, 50% (176 mg); 9c, 67% (246 mg); 9d, 86% (326 mg) and 9e, 96% (378
mg)].
Methyl N-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-S-2-propen-1-yl-L-cysteinate ( 9a ): M.p. 101-103°C; [α]25 + 10.10 (C = 0,60, CHCl3); IR (KBr, cm-1): ν max 3460 (OH), 3222 (N-H), 1750 and 1665 (C=O), 1265 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 2.93 (1H, dd, J = 14.0, 5.0 Hz, S-CH2CHN), 3.02 (1H, dd, J = 14.10, 5.0 Hz, S-CH2CHN), 3.12 (2H, tapp, J = 7.0 Hz, S-CH2CH=CH2), 3.78 (s, OCH3), 4.86-4.96 (1H, m, -CH-N), 5.04-5.16 (2H, m, S-CH2CH=CH2), 5.67-5.78 (1H, m, S-CH2CH=CH2), 6.26 (1H, d, J = 15.7 Hz, –CO–CH=C), 6.78 (d, J = 7.2 Hz, -CH-NH-C=O), 6.81 (1H, d, J = 8.0 Hz, Ar), 6.86 (1H, dd, J = 8.0, 1.5 Hz, Ar), 7.02 (1H, d, J = 1.5 Hz, Ar), 7.47 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 32.65 (S-CH2CHN), 35.26 (S-CH2CH=CH2), 52.22 (CH-N), 68.13 (OCH3), 114.55 (Ar), 115.54 (Ar), 116.62 ((C=C-CO-), 118.27 (S-CH2CH=CH2), 121.98 (Ar), 127.16 (Ar), 133.59 (S-CH2CH=CH2), 143.13 (Ar-C=C), 144.39 (Ar-O), 146.96 (Ar-O), 167.18 (-NH-C=O), 171.83 (CH-C=O)-O); EIMS: m/z 338.1062 [M + H]+, Calcd for C16H20NO5S: 338.1061.
Ethyl N-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-S-2-propen-1-yl-L-cysteinate ( 9b ): M.p. 110-112°C; [α]25 + 12.70 (C = 0,54, CHCl3); IR (KBr, cm-1): ν max 3467 (OH), 3213 (N-H), 1742 and 1662 (C=O), 1265 (C-O-C), 1202 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 1.29 (3H, t, J = 7.0 Hz), 2.93 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.03 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.13 (2H, tapp, J = 6.2 Hz, S-CH2CH=CH2), 4.24 (2H, t, J = 7.0 Hz, OCH2), 4.86-4.93 (1H, m, -CH-N), 5.07-5.13 (2H, m, S-CH2CH=CH2), 5.67-5.77 (1H, m, S-CH2CH=CH2), 6.26 (1H, d, J = 15.6 Hz, –CO–CH=C), 6.77-6.87 (3H, m, Ar-H, -CH-NH-C=O), 7.01 (1H, s, Ar-H), 7.46 (1H, d, J = 15.6 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 14.24 (CH3), 32.66 (S-CH2CHN), 35.28 (S-CH2CH=CH2), 52.34 (CH-N), 62.41 (OCH2), 114.76 (Ar), 115.57 (Ar), 116.57 ((C=C-CO-), 118.25 (S-CH2CH=CH2), 121.95 (Ar), 127.12 (Ar), 133.59 (S-CH2CH=CH2), 143.18 (Ar-C=C), 144.36 (Ar-O), 147.0 (Ar-O), 167.31 (-NH-C=O), 171.40 (CH-C=O)-O); EIMS: m/z 352.1219 [M + H]+, Calcd for C17H22NO5S: 352.1215.
Propyl N-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-S-2-propen-1-yl-L-cysteinate ( 9c ): M.p. 129-131°C; [α]25 + 12.70 (C = 0,61, CHCl3); IR (KBr, cm-1): ν max 3467 (OH), 3206 (N-H), 1750 and 1662 (C=O), 1257 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 0.95 (3H, t, J = 7.0 Hz), 1.63-1.73 (CH2, m), 2.94 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.03 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.13 (2H, tapp, J = 6.2 Hz, S-CH2CH=CH2), 4.15 (2H, q, J = 7.0 Hz, OCH2), 4.89-4.94 (1H, m, -CH-N), 5.08-5.14 (2H, m, S-CH2CH=CH2), 5.69-5.77 (1H, m, S-CH2CH=CH2), 6.27 (1H, d, J = 15.6 Hz, –CO–CH=C), 6.76 (d, J = 7.2 Hz, -CH-NH-C=O), 6.82 (1H, d, J = 8.0 Hz, Ar), 6.86 (1H, d, J = 8.0 Hz, Ar), 7.02 (1H, s, Ar), 7.48 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 10.48 (CH3), 22.0 (CH2), 32.77 (S-CH2CHN), 35.32 (S-CH2CH=CH2), 52.31 (CH-N), 67.90 (OCH2), 114.74 (Ar), 115.50 (Ar), 116.63 ((C=C-CO-), 118.24 (S-CH2CH=CH2), 121.86 (Ar), 127.15 (Ar), 133.60 (S-CH2CH=CH2), 143.13 (Ar-C=C), 144.33 (Ar-O), 146.97 (Ar-O), 167.15 (-NH-C=O), 171.45 (CH-C=O)-O); EIMS: m/z 366.1375 [M + H]+, Calcd for C18H24NO5S: 366.1377.
Butyl N-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-S-2-propen-1-yl-L-cysteinate ( 9d ): M.p. 119-121°C; [α]25 + 11.0 (C = 0,65, CHCl3); IR (KBr, cm-1): ν max 3467 (OH), 3206 (N-H), 1734 and 1662 (C=O), 1265 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 0.93 (3H, t, J = 7.0 Hz), 1.32-1.46 (CH2, m), 1.57-1.71 (CH2, m), 2.94 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.03 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.13 (2H, tapp, J = 6.2 Hz, S-CH2CH=CH2), 4.19 (2H, q, J = 6.2 Hz, OCH2), 4.88-4.94 (1H, m, -CH-N), 5.07-5.15 (2H, m, S-CH2CH=CH2), 5.66-5.80 (1H, m, S-CH2CH=CH2), 6.27 (1H, d, J = 15.6 Hz, –CO–CH=C), 6.73 (d, J = 7.2 Hz, -CH-NH-C=O), 6.83 (1H, d, J = 8.2 Hz, Ar), 6.88 (1H, d, J = 8.2 Hz, Ar), 7.04 (1H, s, Ar), 7.49 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 13.77 (CH3), 19.19 (CH2), 30.60 (CH2), 32.80 (S-CH2CHN), 35.34 (S-CH2CH=CH2), 52.33 (CH-N), 66.19 (OCH2), 114.77 (Ar), 115.52 (Ar), 116.70 ((C=C-CO-), 118.22 (S-CH2CH=CH2), 121.84 (Ar), 127.19 (Ar), 133.63 (S-CH2CH=CH2), 143.11 (Ar-C=C), 144.38 (Ar-O), 146.96 (Ar-O), 167.10 (-NH-C=O), 171.41 (CH-C=O)-O); EIMS: m/z 380.1532 [M + H]+, Calcd for C19H26NO5S: 380.1536.
Pentyl N-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-S-2-propen-1-yl-L-cysteinate ( 9e ): M.p. 111-113°C; [α]25 + 14.4 (C = 0,80, CHCl3); IR (KBr, cm-1): ν max 3460 (OH), 3206 (N-H), 1734 and 1655 (C=O), 1265 (C-O-C), 1209 ((C=O)-O); 1H NMR (CDCl3, 600 MHz): δ 0.89 (3H, t, J = 6.5 Hz), 1.28-1.37 (CH2, m), 1.61-1.69 (CH2, m), 2.92 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.03 (1H, dd, J = 14.10, 5.2 Hz, S-CH2CHN), 3.13 (2H, tapp, J = 6.2 Hz, S-CH2CH=CH2), 4.17 (2H, q, J = 6.2 Hz, OCH2), 4.87-4.94 (1H, m, -CH-N), 5.07-5.14 (2H, m, S-CH2CH=CH2), 5.68-5.77 (1H, m, S-CH2CH=CH2), 6.25 (1H, d, J = 15.6 Hz, –CO–CH=C), 6.78-6.87 (3H, m), 7.01 (1H, s, Ar), 7.46 (1H, d, J = 15.5 Hz, Ar-CH=C); 13C NMR (CDCl3, 125 MHz): δ 14.06 (CH3), 18.12 (CH2), 21.20 (CH2), 28.06 (CH2), 32.74 (S-CH2CHN), 35.29 (S-CH2CH=CH2), 52.31 (CH-N), 66.47 (OCH2), 114.54 (Ar), 115.47 (Ar), 116.59 ((C=C-CO-), 118.21 (S-CH2CH=CH2), 121.98 (Ar), 127.13 (Ar), 133.60 (S-CH2CH=CH2), 143.11 (Ar-C=C), 144.40 (Ar-O), 146.99 (Ar-O), 167.18 (-NH-C=O), 171.55 (CH-C=O)-O); EIMS: m/z 394.1688 [M + H]+, Calcd for C20H28NO5S: 394.1691.
4.2. Biological activity assays
Cell lines and culture medium
Biological assays were performed using an adenocarcinoma colon cancer cell line (SW480) and non-malignant cells (CHO-K1). These were obtained from the European Collection of Authenticated Cell Cultures (ECACC, England) and maintained in Dulbecco’s Modified Eagle Medium, supplemented with 10% heat-inactivated (56 °C) horse serum, 1% penicillin/streptomycin and 1% non-essential amino acids (Gibco Invitrogen, Carlsbad, USA). For all experiments, horse serum was reduced to 3%, and the medium was supplemented with 5 mg/ml transferrin, 5 ng/mL selenium and 10 mg/ml insulin (ITS-defined medium; Gibco, Invitrogen, Carlsbad, USA) (32).
Cell Viability
The cell viability of the synthesized hybrids, lead, and reference compounds was evaluated through Sulforhodamine B (SRB) assay, a colorimetric test that is based on staining of total cellular protein of adherent cells. The cells were seeded to a final density of 20.000 cells/well in 96-well tissue culture plates and incubated at 37 °C in a humidified atmosphere at 5% CO2. All cultures were allowed to grow for 24 h and afterward they were treated with DMSO (dimethylsulfoxide; vehicle control 1%) or increasing concentrations (0.01–0.1 mM) of the synthesized hybrids, as well as SAC and caffeic acid (Lead compounds) and 5-fluorouracil (5-FU; the standard drug). After treatment, the cells were fixed with trichloroacetic acid (50% v/v) (MERCK) for a period of one h at 4 °C. The cell proteins were determined by staining with 0.4% (w/v) SRB (Sigma-Aldrich, United States), then they were washed with 1% acetic acid for the removal of unbound SRB and left for air-drying. Protein bound SRB was solubilized in 10 mM Tris-base and the absorbance was measured at 492 nm in a microplate reader (Mindray MR-96A) (33). All of the experiments were performed at least in quintuplicate.
Antiproliferative activity
Antiproliferative effect of the most active compounds was also tested through Sulforhodamine B (SRB) assay. Briefly, the cells were seeded to a final density of 2500 cells/well in 96-well tissue culture plates and incubated in the same conditions described for viability. The cultures were allowed to grow for 24 h and then were treated with increasing concentrations of the selected hybrids (0.1 – 0.55 mM, ranges depended on the IC50 -50% inhibitory concentration- values) or DMSO (vehicle control, 1%), for 0, 2, 4, 6, and 8 days. Culture media was replaced every 48 h. After each incubation time, the cells were fixed, stained, and read as previously described for this technique (32).
Measurement of Mitochondrial Membrane Potential (ΔΨm)
Mitochondrial membrane permeability changes were assessed through the fluorescent dye DiOC6 (3,3’-dihexyloxacarbocyanine iodide, Thermo Fisher Scientific, Waltham, MA, USA), and propidium iodide (PI). The cells were seeded to a final density of 2.5 x 105 cells/well in 6-well tissue culture plates and were allowed to grow for 24 h. Then, they were treated with hybrids 6e, 9a, 9b, 9c, and 9e with its respective IC50 (0.18, 0.12, 0.12, 0.11, and 0.12 mM, respectively), being harvested by scrapping at 48 h in the same culture mean, and stained with DiOC6 and PI at room temperature for 30 min in darkness. The cells were collected to analyze 10,000 events by flow cytometry with excitation at 488 nm and detection of the emission with the green (530/15 nm) and the red (610/20 nm) filters. This method allowed us quantifying cells with depolarized mitochondrial membrane (34).
Cell cycle analysis
Cell cycle distribution was analyzed by labelling cells with propidium iodide (PI). Assays were carried out as described by Nicoletti et al. (1991). In brief, cells were seeded in 6-well tissue culture plates at a density of 2.5 x 105 cells/well, incubated at 37 °C in a 5% CO2 atmosphere. The cultures were allowed to grow for 24 h and then were treated for 48 h with 1% DMSO (vehicle control) or hybrids 6e, 9a, 9b, 9c, and 9e with the IC50 for each compound (0.18, 0.12, 0.12, 0.11, and 0.12mM, respectively). After the treatment, the cells were collected by scraping and the centrifuged cell pellet was resuspended with phosphate buffered saline (PBS). The cell suspension was fixed in 1.8 mL 70% ethanol at 4°C overnight, afterward, these were centrifuged, washed twice in PBS and resuspended in 300 µL of PBS containing 0.25 mg/mL RNAse (Type I-A, Sigma-Aldrich, Germany) and 0.1 mg/mL PI. Following the incubation in the dark at room temperature for 30 min, the PI fluorescence of 10,000 cells was analyzed using a FACS Canto II flow cytometer and the software BD FACS Diva 6.1.3. (BD Biosciences, San Jose). PI signal was analyzed with excitation at 488 nm, using a Sapphire laser, and fluorescence was detected at 610nm. The cell clumps were excluded with the PI-Area vs PI-Width signals. The cell cycle model was fixed using the software FlowJo 7.6.2 (Ashland, OR, USA), applying the Dean-Jett-Fox model (34, 35).
Statistical analysis
All experiments were performed at least three times. The data are reported as mean ± SE (standard error). Statistical differences between the control group (non-treated) and treated cells were evaluated by one-way ANOVA followed by the Dunnett′s test. Values with p ≤ 0.05 were considered significant. The data were analyzed with GraphPad Prism version 7.04 for Windows (Graph Pad Software, San Diego, California, USA).
Results and Discussion
Chemistry
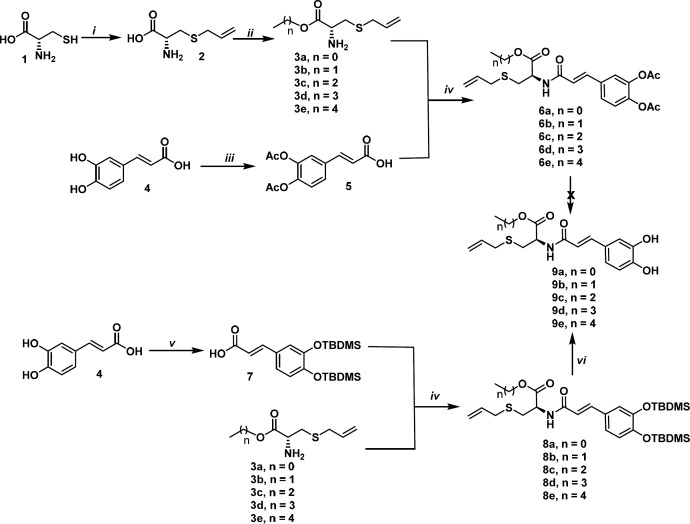
The design of the compounds were based on lipophilic modifications. Allyl cysteine 2 was obtained, in 80% yield, via nucleophilic sustitution between cysteine 1 and allyl bromide (36). Reaction of 1 with the corresponding alcohol in the presence of thionyl chloride (37) afforded, after purification by crystallization or column chromatography, compounds 3a-3e in 60-90% yields. When these compounds were submitted to peptide type-coupling with 3,4-diacetoxycaffeic acid 5 (38) using HBTU as amide bond promoter (39), the compounds 6a-6e were achieved in 30-40% yields.
On the other hand, the phenolic hydroxyl groups of caffeic acid were protected as TBDMS ethers upon the reaction of reacting caffeic with TBDMSCl and imidazole in a solvent-free reaction assisted by microwaves. In this conditions compound 7 was obtained in 50% yield (40). Then, the compound 7 was coupled with allylcysteine esters 3a-3e in the same conditions as above to afford amides 8a-8e in the yields ranging 50-60% (38). Finally, hybrids 9a-9e were obtained by deprotection from compounds 8 (yields 50-96%) (41). Obtention of hybrids 8 by deacetylation of compounds 6 (39, 42) was unsuccessful and a complex mixture was observed in all attempts carried out (Scheme 1).
Scheme 1.
Synthesis of S-allyl cysteine ester-caffeic acid amide hybrids
Reagents and conditions: (i) Allyl bromide, NH4OH, 80% (ii) SOCl2, ROH, -10°C, 60-90%. (iii) Ac2O/NaOH, caffeic acid, 80%. (iv) HBTU, Et3N, THF, 30-40% (6a-6e), 50-60% (8a-8e). (v) TBDMSCl, Imidazole, caffeic acid, MW, 50% (vi) TBAF, Benzoic acid, dioxane, 50-96% (9a-9e).
The structures of the all compounds have been established by a combined study of IR, ESI-MS, 1H-NMR, 13C-NMR, Carbon atom types (C, CH, CH2, CH3), determined by using the DEPT or APT pulse sequence. The signals were assigned using two-dimensional heteronuclear correlations (COSY and HSQC). IR spectra exhibited characteristic absorption peaks corresponding to N-H, C=O, C-O-C and (C=O)-O. ESI-MS spectra showed characteristic [M+H]+ peaks corresponding to their molecular weights. The assignments of all the signals to individual H or C-atoms have been performed on the basis of typical δ-values and J-constants. The 1H-NMR spectra of hybrids 6 and 9 dissolved in CDCl3 showed signals of S-CH2CHN (2.90 and 3.00 ppm), S-CH2CH=CH2 (3.12 ppm), OCH2 or OCH3 (4.2 or 3.8, respectively), -CH-N (4.86-5.03 ppm), S-CH2CH=CH2 (5.10-5.22 ppm), S-CH2CH=CH2 (5.70-5.88), –CO–CH=C (6.26 ppm), -CH-NH-C=O ( 6.78 ppm), Ar-H (6.80-7,62 Ar-H), and Ar-CH=C (7.5 ppm). Additionally, compounds 6 showed signals of acetyl groups ((CH3-C=O)-O (2.30 and 2.31). 13C-NMR spectra of compounds 6 and 8 showed at around 32.60, 35.25, 52.22, 68.13, 116.60, 118.25, 133.60, 143.13, 167.18, 171.80, ppm, corresponding to (S-CH2CHN), (S-CH2CH=CH2), (CH-N), (OCH2 or OCH3), ((C=C-CO-), (S-CH2CH=CH2), (S-CH2CH=CH2), (Ar-C=C), (-NH-C=O), and ((CH-C=O)-O), respectively. Also, compounds 6 exhibited two signals around 20.76 and 20.79 corresponding to acetyl groups ((CH3-C=O)-O).
Biological activity
Effect of S-allyl cysteine ester-caffeic acid amide hybrids on SW480 and CHO-K1 cell viability
In order to assess their effect on the viability, the synthesized S-allyl cysteine ester-caffeic acid amide hybrids were evaluated against SW480 and CHO-K1 cell lines through the sulforhodamine B assay. As shown in Figure 3, the activity was time- and concentration-dependent, with a higher cytotoxic effect on SW480 cells in relation to CHO-K1 cells. Cytotoxicity was reported as 50% inhibitory concentration (IC50 values).
Figures. 3.
Effect of S-allyl cysteine ester - cafeic acid amide hybrids on cell viability of SW480 and CHO-K1 cells, 48 h post-treatment with different concentrations (0.01-0.1mM). Cell viability was calculated using 100% viability of control. Data are presented as the mean ± SE of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
All results regarding to the cytotoxic effect are summarized in Table 1. Among the compounds tested, hybrid 6b was the only one which did not exhibit activity neither at 24 h nor at 48 h after treatment. On the other hand, compounds 6a, 6c, and 6d exhibited good activity against the human colon adenocarcinoma SW480 cells, 24 h after treatment (IC50= 0.67, 1.02, and 2.79 mM, respectively); however, the effect decreased after 48 h as evidenced by the rise in the IC50 (>10 mM). Moreover, compounds 6e and 9a-9e showed the best activity against SW480 cells at 24 h after treatment, with IC50 values ranging from 0.10 to 0.93 mM, besides, the activity was maintained through the time since the IC50 values decreased 48 h after treatment with IC50 values in the range of 0.1-0.18 mM. Additionally, their activity against the normal cell line was significantly lower compared to the reference drug, which gave these compounds great selectivity indexes (6e: 10.3; 9a: 1.5; 9b: >83.33 9c: >90.91 9d: >66.67; 9e: >83.33) after the 48 h treatment. It is important to highlight that compounds 9b-9e were much more selective than the lead compounds and the standard drug (5-FU). Compounds 3a and 3c, which correspond to allyl esters without hybridization, exhibited higher cytotoxicity against CHO-K1 thus displaying low selectivity indexes. The results regarding the activity of our hybrid compounds are in accordance with those reported by Herrera-R et al. (2018), which also found better activity and selectivity indices (SI) with some styrylcoumarin hybrids when tested in SW480 cells (32).
Table 1.
Cytotoxic effect of S-allyl cysteine ester-caffeic acid amide hybrids on SW480 and CHO K1 cell lines
| Compounds |
24 h
|
48 h
|
||||
|---|---|---|---|---|---|---|
|
IC
50
(mM)
CHO-K1 cells |
IC
50
(mM)
SW480 cells |
SI |
IC
50
(mM)
CHO-K1 cells |
IC
50
(mM)
SW480 cells |
SI | |
| 3a | 1.85 | >10 | <0.19 | >10 | >10 | >1.0 |
| 3c | 1.50 | >10 | <0.15 | 1.24 | 1.28 | 0.97 |
| 6a | 2.77 | 0.67 | 4.2 | 7.83 | >10 | <0.78 |
| 6b | 3.84 | >10 | <0.38 | >10 | >10 | >1.0 |
| 6c | 0.27 | 1.02 | 0.26 | 1.25 | >10 | <0.13 |
| 6d | 0.21 | 2.79 | 0.08 | 9.97 | >10 | <1.0 |
| 6e | 0.26 | 0.68 | 0.40 | 1.84**** | 0.18* | 10.3 |
| 9a | 0.47 | 0.24 | 2.0 | 0.18** | 0.12* | 1.5 |
| 9b | >10 | 0.93 | >10.75 | >10**** | 0.12* | >83.33 |
| 9c | 0.12 | 0.10 | 1.2 | >10**** | 0.11* | >90.91 |
| 9d | >10 | 0.11 | >90.91 | >10**** | 0.15* | >66.67 |
| 9e | 0.21 | 0.20 | 1.03 | >10**** | 0.12* | >83.33 |
| S-Allylcysteine | >10 | >10 | >1.0 | >10 | 0.38 | >26.32 |
| Caffeic acid | >10 | 7.06 | >1.42 | >10 | 3.47 | >2.88 |
| 5-Fluorouracil | 0.091 | 0.062 | 1,48 | 0.048 | 0.035 | 1.34 |
The IC50 values were obtained from dose response curves for each compound. Selectivity index was determined as ratio of IC50 against CHO-K1 to that of IC50 against SW480.
In the structure-activity relationship (SAR) study, we noticed a synergistic action of the parent subunits when they are linked to form a single structure in the hybrid, as in the case of the compounds 6e and 9a-9e. Besides, allylcysteine esters such as 3a and 3c show a decreased activity, suggesting that the presence of the acid group is important within the mode of action that could involve the transference of a proton or formation of hydrogen bonds with a receptor (43). In addition, although higher selectivity indexes were achieved when the alkyl chain had two, three, and five carbon atoms (hybrids 9b, 9c and 9e, respectively), there was not a clear relationship between the antitumor activity and the length of the alkyl chain. On the other hand, hybrids with hydroxyl groups (9a-9e) showed better activity than acetylated derivatives (6a-6e), which is in agreement with the reports for several chalcones, coumarins, and caffeic acid esters (44-46), suggesting that this effect could be due to a better molecular recognition ability towards target bioreceptors upon hydrogen bond formation (43), oxidation processes across radical formation, and/or the ability of metal complexation (47).
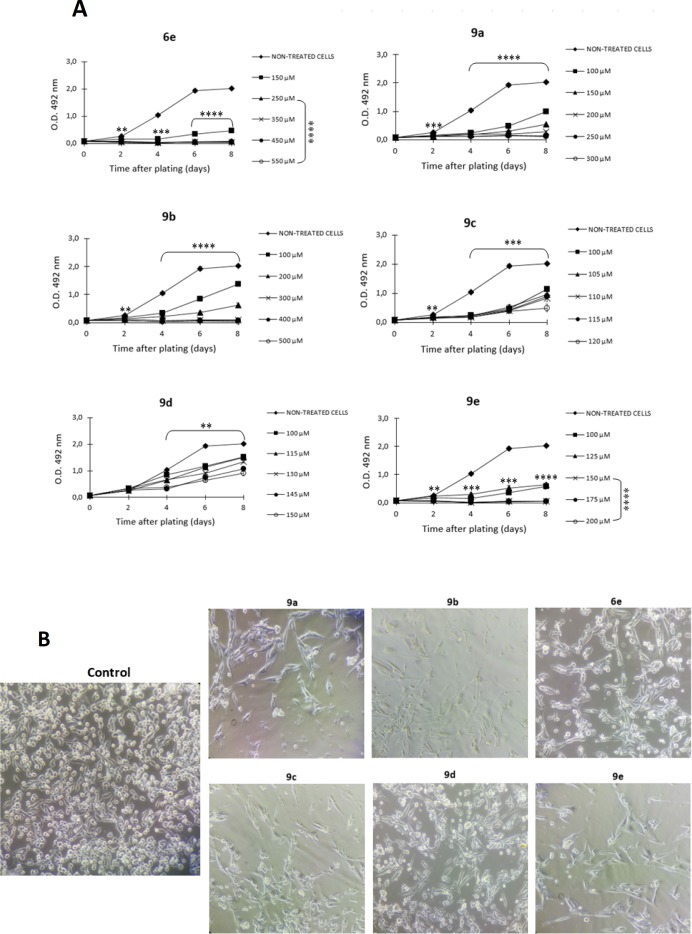
Antiproliferative effect of S-allyl cysteine ester - caffeic acid amide hybrids on SW480 cells
The most active and selective compounds (6e, 9a–9e) were analized through a longer period of time in order to assess if they can display antiproliferative activity. After comparing each treatment with the control, the results indicated that the activity was time- and concentration-dependent. Among the results obtained, hybrids 6e, 9a-9c, and 9e displayed significant antiproliferative activity from day 2 onwards (p ≤ 0.05), even at the lowest concentrations evaluated, while compound 9d required a longer period of time (4 days) to exert activity against SW480 cells (Figure 4A). Additionally, when observed with optical microscope, the cellular morphology of SW480 cells was severely perturbed, exhibiting changes in size and shape after treatment with S-allyl cysteine ester-caffeic acid amide hybrids, while the control displayed normal and healthy shape. Moreover, there was a clear decreased in number of the cells in comparison with the control, indicating either an increasing progression toward cell death or an arrest in cell cycle (Figure 4B).
Figure 4.
Antiproliferative effect of S-allyl cysteine ester - caffeic acid amide hybrids on SW480 colon cancer cell growth. A) Results obtained with sulforhodamine B assay. B) Representative images of SW480 cells 48 h after treatment with the IC50 value (Magnification: 20x). Data are presented as the mean ± SE of at least three independent experiments (**p < 0.01; ***p< 0.001; ****p < 0.0001). Optical Density (O.D.) is directly proportional to cell mass of adherent cells
Changes in mitochondrial membrane potential (ΔΨm) induced by S-allyl cysteine ester - caffeic acid amide hybrids
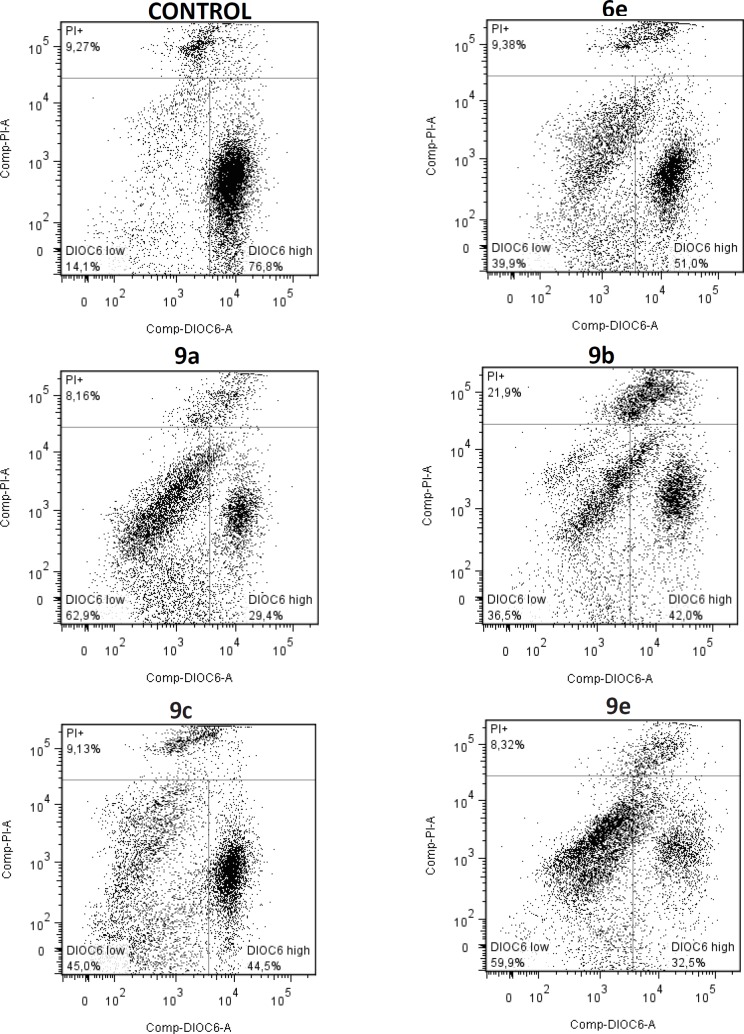
The changes in Mitochondrial Membrane Potential (ΔΨm) could cause mitochondrial dysfunction, a process that is determinant in the execution of the cell death (48). Therefore, to assess the role of mitochondria in SW480 cells treated with our hybrids, the carbocyanine fluorescent dye DiOC6 was used. This dye accumulates in mitochondria due to its large negative membrane potential and it is released to the cytosol after a membrane depolarization (membrane with reduced ΔΨm), staining intracellular membranes (34, 49). According to the results (Figure 5), all hybrids caused a depolarization in the mitochondrial membrane as regards control, as observed in the decrease of the DiOC6 high population. Besides, in all cases, there was an increase in the field of the cells in latency and with loosing membrane polarization (DiOC6 low). Furthermore, there was not a great population with damaged membrane, which can be appreciated in the field of the cells stained with PI. This behavior was similar for all the evaluated compounds; however, hybrids 9a and 9e exhibited the highest activity in this cell line. A possible explanation for this could be related with the ability of hybrids 9a and 9e to induce cell death through a cross-talk between the extrinsic and intrinsic pathways of apoptosis via caspase-8 and Bid activation, which leads to mitochondrial membrane permeabilization (50).
Figure 5.
Mitochondrial membrane potential (ΔΨm) in SW480 cells treated with either the most active hybrids or DMSO (1%) as control. DiOC6 high: live cells with high membrane polarization; DiOC6 low: cells in latency that lose membrane polarization; PI +: cells started to lose membrane polarization and in process of death
S-allyl cysteine ester - caffeic acid amide hybrids induce cell cycle arrest on SW480 cells
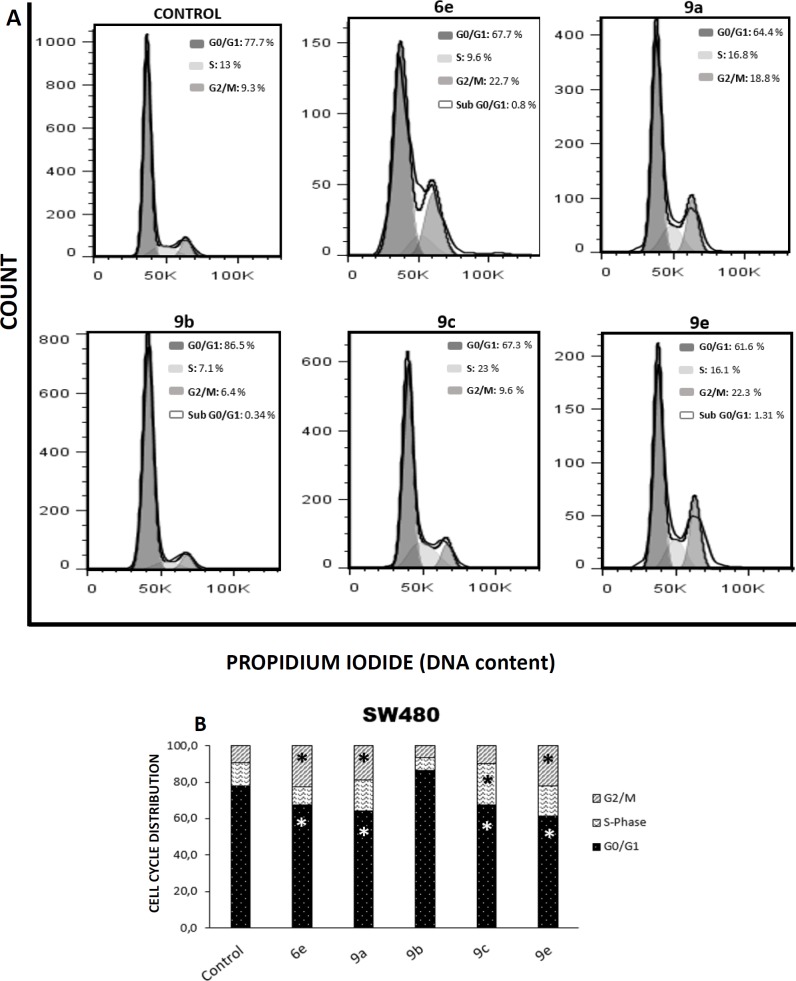
Numerous studies have suggested that cancer progression involves the loss of checkpoint controls that regulate the passage through the cell cycle (51-54), which is critical in cancer pathogenesis and may affect the effectiveness of chemotherapy (17, 55, 56). Thus, we focused on determine whether hybrids 6e, 9a, 9b, 9c, and 9e could have any effect in the regulation of this process. The results obtained show that hybrids 6e, 9a, and 9e caused an arrest in the cell cycle in G2/M phase (22.7, 18.8 and 22.3%, respectively). In contrast, the proportion of the cells in G0/G1 phase significantly decreased with regard to the control (p < 0.05). A similar reduction in G0/G1 phase was observed with compound 9c (67.3%), along with an important increase in the S-phase of the cell cycle (23%). Additionally, compound 9b did not have any impact on the cell cycle. These findings complement our above findings, thus suggesting that our hybrids could have not only a cytotoxic effect but also a cytostatic activity (Figure 6).
Figure 6.
Effect of S-allyl cysteine ester - caffeic acid amide hybrids on cell cycle distribution. (A) Flow cytometry analysis of cell cycle distribution in SW480 cells. (B) The cell cycle distribution in SW480 cells after 48 h of treatment with each hybrid or DMSO 1% (control). p-values lower than 0.05 were considered statistically significant (*p< 0.05)
Conclusion
Our results demonstrate that some S-allyl cysteine ester-caffeic acid amide hybrids may display antiproliferative activity by inducing mitochondrial membrane depolarization and cell cycle arrest in SW480 cells, being even more active than the lead compounds. Besides, according to the results, the evaluated hybrids exhibited more selectivity than 5-FU, the conventional chemotherapeutic drug. The SAR analysis showed that hydroxyl groups increased the activity, besides, there was not a clear relationship between the antitumor properties and the length of the alkyl chain. All these findings make these hybrid compounds promising candidates for further antitumor studies.
Acknowledgment
The authors thank COLCIENCIAS (Grant No. 247-2016, code: 111571249830) and Universidad de Antioquia for financial support.
References
- 1.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018:1–31. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut . 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Pointet AL, Taieb J. Cáncer de colon. EMC - Tratado De Med . 2017;21:1–7. [Google Scholar]
- 5.McQuade RM, Bornstein JC, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and sideeffects. Int. J. Clin. Med. . 2014;5:393–406. [Google Scholar]
- 6.Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. . 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 7.Tsogoeva SB. Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini Rev. Med. Chem. 2010;10:773. doi: 10.2174/138955710791608280. [DOI] [PubMed] [Google Scholar]
- 8.Shaveta , Mishra S, Singh P. Hybrid molecules: the privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016;124:500–36. doi: 10.1016/j.ejmech.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Kim KM, Chun SB, Koo MS, Choi WJ, Kim TW, Kwon YG, Chung HT, Billiar TR, Kim YM. Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic. Biol. Med. 2001;30:747–56. doi: 10.1016/s0891-5849(01)00460-9. [DOI] [PubMed] [Google Scholar]
- 10.Ho SE, Ide N, Lau BH. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine . 2001;8:39–46. doi: 10.1078/0944-7113-00005. [DOI] [PubMed] [Google Scholar]
- 11.Numagami Y, Ohnishi ST. S-allylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. J. Nutr. 2001;131:1100S–5S. doi: 10.1093/jn/131.3.1100S. [DOI] [PubMed] [Google Scholar]
- 12.Numagami Y, Sato S, Ohnishi ST. Attenuation of rat ischemic brain damage by aged garlic extracts: a possible protecting mechanism as antioxidants. Neurochem. Int. 1996;29:135–143. doi: 10.1016/0197-0186(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 13.Mostafa MG, Mima T, Ohnishi ST, Mori K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000;66:148–51. doi: 10.1055/s-2000-11124. [DOI] [PubMed] [Google Scholar]
- 14.Welch C, Wuarin L, Sidell N. Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in-vitro. Cancer Lett. 1992;63 doi: 10.1016/0304-3835(92)90263-u. [DOI] [PubMed] [Google Scholar]
- 15.Takeyama H, Hoon DS, Saxton RE, Morton DL, Irie RF. Growth inhibition and modulation of cell markers of melanoma by S-allyl cysteine. Oncology . 1993;50 doi: 10.1159/000227149. [DOI] [PubMed] [Google Scholar]
- 16.Pinto JT, Qiao C, Xing J, Rivlin RS, Protomastro ML, Weissler ML, Tao Y, Thaler H, Heston WD. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. . 1997;66:398–405. doi: 10.1093/ajcn/66.2.398. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Li M, Chen K, Yang J, Chen R, Wang T, Liu J, Yang W, Ye Z. S-allylcysteine induces cell cycle arrest and apoptosis in androgen-independent human prostate cancer cells. Mol. Med. Rep. 2012;5:439–43. doi: 10.3892/mmr.2011.658. [DOI] [PubMed] [Google Scholar]
- 18.Shirin H, Pinto JT, Kawabata Y, Soh JW, Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR, Weinstein IB. Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res. 2001;61:725–31. [PubMed] [Google Scholar]
- 19.Sumiyoshi H, Wargovich MJ. Chemoprevention of 1,2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds. Cancer Res. . 1990;50:5084–7. [PubMed] [Google Scholar]
- 20.Hung CC, Tsai WJ, Kuo LM, Kuo YH. Evaluation of caffeic acid amide analogues as anti-platelet aggregation and anti-oxidative agents. Bioorg. Med. Chem. . 2005;13:1791–7. doi: 10.1016/j.bmc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 21.Son S, Lewis BA. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J. Agric. Food Chem. . 2002;50:468–472. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 22.Otero E, Robledo S, Díaz S, Carda M, Muñoz D, Paños J, Vélez ID, Cardona W. Synthesis and leishmanicidal activity of cinnamic acid esters: structure–activity relationship. Med. Chem. Res. . 2014;23:1378–86. [Google Scholar]
- 23.De P, Baltas M, Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011;18:1672–1703. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]
- 24.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, Shieh CJ, Shiao MS, Chen YJ. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003;51:7907–7912. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, Yang F, Zhang M, Lam C, Qiao Y, Xiao J, Zhang D, Ge Y, Fu L, Xie D. Antiproliferative activity and SARs of caffeic acid esters with mono-substituted phenylethanols moiety. Bioorg. Med. Chem. Lett. 2017;27:131–4. doi: 10.1016/j.bmcl.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia . 2002;Suppl. 1 73:S38–S43. doi: 10.1016/s0367-326x(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 27.Rao CV, Desai D, Kaul B, Amin S, Reddy BS. Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chem. Biol. Interact. . 1992;84:277–290. doi: 10.1016/0009-2797(92)90129-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Li HB, Xia CN, Zheng XM, Hu WX. The synthesis and biological evaluation of some caffeic acid amide derivatives: E-2-cyano-(3-substituted phenyl)acrylamides. Bioorg. Med. Chem. Lett. 2009;19:1861–1865. doi: 10.1016/j.bmcl.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 29.Hung MW, Shiao MS, Tsai LC, Chang GG, Chang TC. Apoptotic effect of caffeic acid phenethyl ester and its ester and amide analogues in human cervical cancer ME180 cells. Anticancer Res. 2003;23:4773–80. [PubMed] [Google Scholar]
- 30.Gupta A, Saha P, Descôteaux C, Leblanc V, Asselin E, Bérubé G. Design, synthesis and biological evaluation of estradiol-chlorambucil hybrids as anticancer agents. Bioor. Med. Chem. Lett. 2010;20:1614–8. doi: 10.1016/j.bmcl.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 31.Provencher-Mandeville J, Descôteaux C, Mandal SK, Leblanc V, Asselin E, Bérubé G. Synthesis of 17beta-estradiol-platinum(II) hybrid molecules showing cytotoxic activity on breast cancer cell lines. Bioor. Med. Chem. Lett. 2008;18:2282–7. doi: 10.1016/j.bmcl.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Herrera-R A, Castrillón W, Otero E, Ruiz E, Carda M, Agut R, Naranjo T, Moreno G, Maldonado ME, Cardona-G W. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med. Chem. Res. 2018;27:1893–905. [Google Scholar]
- 33.Pérez JM, Maldonado ME, Rojano B, Alzate F, Sáez J, Cardona W. Comparative Antioxidant, Antiproliferative and Apoptotic Effects of Ilex laurina and Ilex paraguariensis on Colon Cancer Cells. Trop. J. Pharm. Res. 2014;13:1279–86. [Google Scholar]
- 34.García-Gutiérrez N, Maldonado-Celis ME, Rojas-López M, Loarca-Piña GF, Campos-Vega R. The fermented non-digestible fraction of spent coffee grounds induces apoptosis in human colon cancer cells (SW480) J. Funct. Foods . 2017;30:237–46. [Google Scholar]
- 35.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods . 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 36.Goudreau N1, Brochu C, Cameron DR, Duceppe JS, Faucher AM, Ferland JM, Grand-Maître C, Poirier M, Simoneau B, Tsantrizos YS. Potent inhibitors of the hepatitis C virus NS3 protease: design and synthesis of macrocyclic substrate-based beta-strand mimics. J. Org. Chem. . 2004;69:6185–201. doi: 10.1021/jo049288r. [DOI] [PubMed] [Google Scholar]
- 37.Grigg R, Armstrong P. X Y ZH systems as potential - dipoles Part 25 Intramolecular cycloadditon reactions of pyridoxal imines of ε-alkenyl α-amino esthers A possible new approach to pyridoxal enzyme inhibition. Tetrahedron . 1989;45:7581–6. [Google Scholar]
- 38.Touaibia M, Guay M. Natural Product Total Synthesis in the Organic Laboratory: Total Synthesis of Caffeic Acid Phenethyl Ester (CAPE), A Potent 5-Lipoxygenase Inhibitor from Honeybee Hives. J. Chem. Edu. . 2011;88:473. [Google Scholar]
- 39.Kwak SY, Lee S, Yang JK, Lee YS. Antioxidative activities of caffeoyl-proline dipeptides. Food Chem. 2012;130:847. [Google Scholar]
- 40.Bastos E, Ciscato L, Baader W. Microwave‐Assisted Protection of Phenols as tert‐Butyldimethylsilyl (TBDMS) Ethers Under Solvent‐Free Conditions. Synth. Commun. 2005;35:1501–9. [Google Scholar]
- 41.Sartori G, Ballini R, Bigi F, Bosica G, Maggi R, Righi P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004;104:199–250. doi: 10.1021/cr0200769. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc LM, Paré AF, Jean-François J, Hébert MJG, Surette ME, Touaibia M. Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analogues. Molecules . 2012;17:14637–50. doi: 10.3390/molecules171214637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick GL. An Introduction to Medicinal Chemistry. fifth ed. Oxford University Press; 2013. pp. 1–14. [Google Scholar]
- 44.Aponte JC, Castillo D, Estevez Y, Gonzalez G, Arevalo J, Hammond GB, Sauvain M. In-vitro and in-vivo anti-Leishmania activity of polysubstituted synthetic chalcones. Bioorg. Med. Chem. Lett. . 2010;20:100–103. doi: 10.1016/j.bmcl.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Brenzan MA, Nakamura CV, Dias Filho BP, Ueda-Nakamura T, Young MC, Côrrea AG, Alvim J Jr, dos Santos AO, Cortez DA. Structure-activity relationship of (-) mammea A/BB derivatives against Leishmania amazonensis. Biomed. Pharmacother. . 2008;62:651–8. doi: 10.1016/j.biopha.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Otero E, García E, Palacios G, Yepes LM, Carda M, Agut R, Vélez ID, Cardona WI, Robledo SM. Triclosan-caffeic acid hybrids: Synthesis, leishmanicidal, trypanocidal and cytotoxic activities. Eur. J. Med. Chem. . 2017;141:73–83. doi: 10.1016/j.ejmech.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 47.Rajan P, Vedernikova I, Cos P, Berghe DV, Augustyns K, Haemers A. Synthesis and evaluation of caffeic acid amides as antioxidants. Bioorg. Med. Chem. Lett. 2001;11:215–57. doi: 10.1016/s0960-894x(00)00630-2. [DOI] [PubMed] [Google Scholar]
- 48.Sithara T, Arun KB, Syama HP, Reshmitha TR, Nisha P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017;8:640. doi: 10.3389/fphar.2017.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maldonado-Celisa ME1, Roussia S, Foltzer-Jourdainne C, Gossé F, Lobstein A, Habold C, Roessner A, Schneider-Stock R, Raul F. Modulation by polyamines of apoptotic pathways triggered by procyanidins in human metastatic SW620 cells. Cell. Mol. Life Sci. 2008;65:1425–34. doi: 10.1007/s00018-008-8023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maldonado-Celis ME, Bousserouel S, Gossé F, Minker C, Lobstein A, Raul F. Differential induction of apoptosis by apple procyanidins in TRAIL-sensitive human colon tumor cells and derived TRAIL-resistant metastatic cells. J. Cancer Mol. 2009;5:21–30. [Google Scholar]
- 51.Caldon CE, Sutherland RL, Musgrove E. Cell cycle proteins in epithelial cell differentiation: implications for breast cancer. Cell Cycle . 2010;9:1918–28. doi: 10.4161/cc.9.10.11474. [DOI] [PubMed] [Google Scholar]
- 52.Zhang K, Wu J, Wu X, Wang X, Wang Y, Zhou N, Kuo ML, Liu X, Zhou B, Chang L, Ann D, Yen Y. p53R2 inhibits the proliferation of human cancer cells in association with cell-cycle arrest. Mol. Cancer Ther. 2011;10:269–78. doi: 10.1158/1535-7163.MCT-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooke GN, Culley RL, Dart DA, Mann DJ, Gaughan L, McCracken SR, Robson CN, Spencer-Dene B, Gamble SC, Powell SM, Wait R, Waxman J, Walker MM, Bevan CL. FUS/TLS is a novel mediator of androgen-dependent cell-cycle progression and prostate cancer growth. Cancer Res. 2011;7:914–24. doi: 10.1158/0008-5472.CAN-10-0874. [DOI] [PubMed] [Google Scholar]
- 54.Li B, Shi XB, Nori D, Chao CK, Chen AM, Valicenti R, White Rde V. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate . 2011;71:567–74. doi: 10.1002/pros.21272. [DOI] [PubMed] [Google Scholar]
- 55.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. . 2009;8:547–66. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 56.Malumbres M1, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer . 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]