Key Points
Question
What is the main reason for cochlear implant revision surgery?
Findings
In this cohort study of 925 patients who underwent cochlear implant surgery from 2001 to 2019, the most common reason for revision was device failure, and no differences in device failure were noted among the different cochlear implant device manufacturers. Flap-associated problems, inner device migration, and postoperative cerebrospinal fluid leakage were other important reasons for cochlear implant revision.
Meaning
By reviewing the reasons for and influencing factors of cochlear implant revision, clinicians can provide realistic counseling to candidates for cochlear implant prior to surgery.
This cohort study uses medical records from October 2001 to March 2019 to analyze the revision surgery rate, reasons for revision surgery, and device failure and survival rates of different device models in recipients of cochlear implant.
Abstract
Importance
Understanding the reasons for cochlear implant (CI) revision surgery and device failure rates is important for clinicians when counseling patients who are considering CI.
Objectives
To analyze the revision surgery rate, reasons for revision surgery, and device failure and survival rates of different device models in recipients of CIs.
Design, Setting, and Participants
In this cohort study, cochlear implants at Samsung Medical Center, a tertiary referral center, were retrospectively reviewed. Patients who underwent CI surgery from October 2001 to March 2019 were included. In the device survival analysis, the first revision surgery was considered the primary event, and the end point of observation was June 1, 2019.
Interventions
Therapeutic and rehabilitative CI surgery.
Main Outcomes and Measures
The revision surgery rate, reasons for revision surgery, and the failure and survival rates of different device models were analyzed. The Kaplan-Meier method and the log-rank test were used to present both the device survival and cumulative survival curves with rates.
Results
In this study, 43 of 925 patients with CIs (4.6%) underwent a revision surgery. Device failure was the most common reason (28 of 43 patients [65%]). Flap-associated problems and migration of the inner device were the next most important reasons (4 of 43 [9.3%] each). Overall, the 10-year cumulative survival rate of CI surgery was 94.4%, and the device survival rate was 96.0%. Thirteen different CI devices from 4 different manufacturers were implanted, and no meaningful differences in device failure were found among CI manufacturers or devices (hazard ratios for cumulative survival: Cochlear, 1.67 [95% CI, 0.72-3.88]; Advanced Bionics, 1.67 [95% CI, 0.61-4.53]; Med-El, reference; hazard ratios for device survival: Cochlear, 1.65 [95% CI, 0.55-4.99]; Advanced Bionics, 1.93 [95% CI, 0.56-6.74]; Med-El, reference). Several recalls were issued by manufacturers during the study period, and after excluding the recalled devices, the device survival rates for 5, 10, and 15 years were 98.2%, 97.7%, and 94.9%, respectively.
Conclusions and Relevance
Generally, implanted devices remain safe and stable for a long time, and no significant differences in survival rates were found between device types or manufacturers. Device failure was the main reason for CI revision, followed by flap-associated problems and migration of the inner device.
Introduction
Cochlear implants (CI), one of the most revolutionary devices available to overcome sensory loss, has successfully helped many patients with hearing loss. Approximately 600 000 people had already received an implant by 2016, and those numbers are increasing rapidly.1 Although CI surgery is relatively safe, with a low incidence of complications,2 both surgical complications and device-associated problems inevitably occur.3 Major surgical complications or device-associated problems necessitate revision surgery or reimplanting.3 According to many institutions’ studies, the revision surgery rate ranges from 5% to 10%,4 and the overall revision rate has declined since 2011.5
Among the various possible reasons for revision surgery, device failure, electrode migration, and flap-associated problems have been the major reasons in previous studies.6 One recent cohort study found that device failure is the most common reason for revision, and flap-associated problems are the most unmanageable reason.6
Prior to CI surgery, delivering accurate information to patients, especially about device survival over time, is important. Because a CI is designed to last a lifetime, the device survival rate is particularly important. Previous studies investigated device failure and survival rates in a variety of ways. The mean time to device failure has been reported to range from 2.1 to 6.2 years,7 and a recent study suggested that the overall device survival rate in 10 years now exceeds 90% because of the rapid evolution of CI devices.6 One study7 also compared different device models’ survival rates, and it found that 5-year device survival ranged from 97.3% to 100%, and 10-year device survival ranged from 88.2% to 100%.
In this study, we investigated the main reasons for CI revision surgery along with demographic and clinical characteristics of patients who have revision surgery (such as inner ear anomaly and reasons for hearing loss) and surgical findings. The revision surgery rate, reasons for revision surgery, and device failure and survival rates of different device models were analyzed.
Methods
In this study, we retrospectively reviewed 925 cochlear implants performed at 1 institution from October 2011 to March 2019. The research was conducted under common ethical rules and was approved by Samsung Medical Center’s institutional review board. All of the enrolled patients agreed to the use of their medical records.
Thirteen different types of inner devices from 4 implant manufacturers were implanted during the included period: the CI 24R, CI 24RE, CI 422, CI 412, CI 522, and CI 532 from Cochlear Corporation; the SONATAti100, CONCERTO, Synchrony, and PULSAR CI 100 from Med-El Corporation; the Clarion CII and HiRes90K from Advanced Bionics Corporation; and the Neuro Zti from Oticon Corporation.
We reviewed all the clinical records of all recipients of cochlear implants to collect demographic records, the date of the first implant, and the manufacturer and device type. For patients who underwent revision surgeries, we also reviewed the reason for hearing loss, underlying diseases, date and reason for revision surgery, neural response telemetry (NRT) or auditory nerve response telemetry (ART) responses, and surgical findings, such as whether the electrode was completely inserted or cerebrospinal fluid (CSF) leakage occurred (Table 1).
Table 1. Review of Characteristics and Surgical Findings in Revision Casesa.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 43 (100) |
| Causative mechanism | |
| Congenital | 31 (72) |
| Acquired | 12 (28) |
| Idiopathic sudden | 5 (12) |
| Meningitis | 2 (5) |
| Vaccination | 1 (2) |
| Chronic otitis media postoperative | 1 (2) |
| High fever | 2 (5) |
| Chemotherapy | 1 (2) |
| Inner-ear anomaly | |
| Absent | 29 (67) |
| Present | 14 (33) |
| Cerebrospinal fluid leakage | |
| Absent | 38 (88) |
| Present | 5 (12) |
| Electrode insertion | |
| Complete insertion | 40 (93) |
| Incomplete insertion | 3 (7) |
| NRT/ART response | |
| Good response | 36 (84) |
| Partial response | 6 (14) |
| No response | 1 (2) |
Abbreviations: ART, auditory nerve response telemetry; NRT, neural response telemetry.
Causative mechanism of hearing loss, underlying diseases, and surgical findings such as cerebrospinal fluid leakage and NRT/ART responses.
The reasons for revision surgeries were classified into 6 categories: device failure, flap-associated problems, migration, hematoma, CSF leakage, and misinsertion. Device failure included not only spontaneous failure of the inner device but also outer reasons, such as trauma. Flap-associated problems included wound infections and seromas.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc) and Stata/SE version 14 (StataCorp) statistical software. Kaplan-Meier plots and the log-rank test were used to show both device survival and cumulative survival curves with rates. We used a Cox proportional hazards regression model to examine the association between revision and manufacturers and 95% confidence intervals (CIs) to describe the precision of the estimates. To analyze survival curves and mean follow-up duration, the first revision surgery was considered as a primary event, and the end point of observation was June 1, 2019. We considered a 2-sided P less than .05 statistically significant.
Results
Among the 925 individuals with CI, 496 (53.6%) were female and 429 (46.4%) were male. The mean age at implantation was 14.3 (range, 0-90) years. Most cases (723 [78.2%]) involved pediatric patients who received implants when they were younger than 20 years old; the other 202 (21.8%) involved adults. Cochlear implants were done unilaterally in 519 patients and bilaterally in 203 patients. By manufacturer, 506 devices were from Cochlear (54.7%), 270 were from Med-El (29.2%), 146 were from Advanced Bionics (15.8%), and 3 were from Oticon (0.3%) (Table 2).
Table 2. Statistical Analyses of Revision Reasons and Device Survival Rates by Devicea.
| Device Model | Mean (SD) Follow-up Duration, y | No. | No. of Revisions | No. of CIs | Rate, No./Total No. (%) | Survival, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Device Failure | Flap-Associated Problem | Migration | Hematoma | CSF Leakage | Misinsertion | Revision | Device Failure | Cumulative | Device | ||||||
| 5-y | 10-y | 5-y | 10-y | ||||||||||||
| Cochlear | |||||||||||||||
| CI 24R | 15.1 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 100 | 100 | 100 | 100 |
| CI 24RE | 10.2 (1.7) | 4 | 1 | 2 | 1 | 0 | 1 | 9 | 159 | 9/159 (5.7) | 4/159 (2.5) | 94.34 | 94.34 | 97.42 | 97.42 |
| CI 422 | 4.6 (1.9) | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 185 | 4/185 (2.2) | 4/185 (2.2) | NA | NA | NA | NA |
| CI 512 | 7.9 (1.4) | 8 | 0 | 2 | 2 | 0 | 1 | 13 | 66 | 13/66 (19.7) | 8/66 (12.1) | 83.33 | NA | 90.24 | NA |
| CI 522 | 1 (0.4) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 30 | 1/30 (3.3) | 1/30 (3.3) | NA | NA | NA | NA |
| CI 532 | 0.5 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | NA | NA | NA | NA |
| Total, No. (%) | 7.5 (4.2) | 17 (63.0) | 1 (3.7) | 4 (14.8) | 3 (11.1) | 0 (0.0) | 2 (7.4) | 27 | 506 | 27/506 (5.3) | 17/506 (3.4) | 94.95 | NA | 96.97 | NA |
| Advanced Bionics | |||||||||||||||
| Clarion CII | 15.5 (0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 100 | 100 | 100 | 100 |
| HiRes 90K | 11.4 (2.2) | 7 | 2 | 0 | 0 | 0 | 0 | 9 | 142 | 9/143 (6.3) | 7/143 (4.9) | 94.32 | 93.60 | 95.67 | 94.94 |
| Total, No. (%) | 11.5 (2.2) | 7 (77.8) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 | 146 | 9/146 (6.2) | 7/146 (4.8) | 94.48 | NA | 95.79 | NA |
| Med-El | |||||||||||||||
| SONATAti100 | 6.8 (0.7) | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 60 | 2/60 (3.3) | 1/60 (1.7) | 96.67 | NA | 98.31 | NA |
| CONCERTO | 3.8 (1.3) | 2 | 1 | 0 | 0 | 1 | 0 | 4 | 178 | 4/178 (2.2) | 2/178 (1.1) | 97.55 | NA | 98.77 | NA |
| PULSAR CI 100 | 6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 100 | NA | 100 | NA |
| Synchrony | 0.6 (0.2) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 31 | 1/31 (3.2) | 1/31 (3.2) | NA | NA | NA | NA |
| Total, No. (%) | 4.1 (2.1) | 4 (57.1) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 7 | 270 | 7/270 (2.6) | 4/270 (1.5) | 96.93 | NA | 98.15 | NA |
| Oticon | |||||||||||||||
| Neuro Zti/Total | 0.5 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 3 | 0 | 0 | NA | NA | NA | NA |
| Total patients who had CIs, No. (%) | 7.1 (4.2) | 28 (65.1) | 4 (9.3) | 4 (9.3) | 3 (7.0) | 2 (4.7) | 2 (4.7) | 43 | 925 | 43/925 (4.6) | 28/925 (3.0) | NA | NA | NA | NA |
| Reason for revision rates, % | NA | 3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.2 | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: CI, cochlear implant; CSF, cerebrospinal fluid; NA, not applicable.
Device failure was the most common reason for revision surgery. Among devices, the revision rate and device failure rate were highest with the CI 512. Among manufacturers, they were highest with Advanced Bionics devices.
Among the 925 individuals with CIs performed, 43 (4.6%) underwent revision surgery. The mean duration from initial surgery to the first revision surgery was 878.67 days (2.4 years; range, 1-5234 days). In the patient group who had revision surgery, the cause of hearing loss was congenital in 31 patients (72%) and acquired in 12 patients (28%). Idiopathic sudden hearing loss (n = 5) was the major reason for acquired hearing loss, and meningitis (n = 2), vaccination (n = 1), chronic otitis media (n = 1), high fever (n = 2), and chemotherapy (n = 1) were the other reasons. In addition, 14 individuals who had revisions (33%) had an inner ear anomaly. During CI surgery, CSF leakage occurred in 5 individuals who had revisions (12%), and the electrode was incompletely inserted in 3 cases (7%). The NRT/ART responses were detected in the operating room. Among the patients who needed revision surgery, 36 (84%) had good responses, 6 (14%) showed partial responses, and 1 patient (2%) showed no response (Table 1).
We also calculated the revision rates by manufacturer (Table 2). Advanced Bionics had the highest revision rate (9 of 146 patients [6.2%]). The revision rates for Cochlear and Med-El devices were 5.3% (27 of 506 patients) and 2.6% (7 of 270 patients), respectively. Only 3 Oticon devices were implanted, and none of them required revision. In addition, revision rates were analyzed by device type. The revision rate of the CI 512 device was 19.7% (13 of 66 patients), which was the highest rate. The revision rates of the other devices are summarized in Table 2.
The main reasons for revision surgery were analyzed by device (Table 2). Device failure occurred in 28 of 925 patients (3.0%; 28 of 43 revisions [65%]), making it the most common reason for revision CI surgery both overall and for each manufacturer. Flap-associated problems and migration were the second most common reason for revision, occurring in 4 of 925 patients (0.4%; 4 of 43 revisions [9.3%]), followed by hematoma in 3 of 925 patients (0.3%; 3 of 43 revisions [7.0%]). Both CSF leakage and misinsertion occurred in 2 of 925 patients (0.2%; 2 of 43 revisions [4.7%]). Interestingly, flap-associated problems were more commonly observed in patients who received Advanced Bionics devices.
Looking closely at each category, 24 of 28 revisions with device failure were attributable to hard failure and the other 4 to soft failure. Also, 3 of 4 flap-associated problems were attributable to infection and the other to a seroma at the operation site. Analyzing the reasons for reoperation over time, it was found that the device failure had occurred mostly in 2005 and 2011, and the flap-associated problems occurred mainly in this early period (eTable in the Supplement).
We compared the device failure rate by device type and manufacturer (Table 2). Because device failure was the most common reason for revision, the device failure rate and revision rate trended together. Thus, Advanced Bionics had the highest device failure rate (7 of 146 [4.8%]), followed by Cochlear (17 of 506 [3.4%]) and Med-El (4 of 270 [1.5%]). The CI 512 device (8 of 66 [12.1%]) and HiRes90K device (7 of 143 [4.9%]) had higher device failure rates than the other devices.
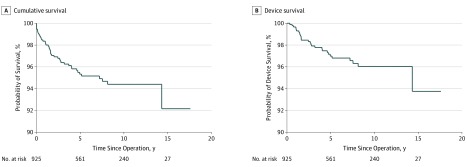
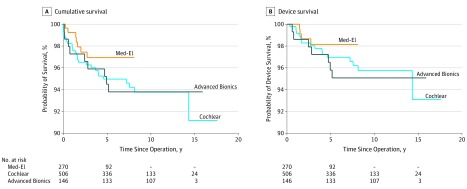
We performed a device survival analysis with the reviewed data. The mean (SD) total follow-up duration was 7.1 (4.2) years, and we also calculated this by manufacturer (Table 2). The overall cumulative survival curve and device survival curve are presented in Figure 1. In addition, the cumulative survival and device survival curves by manufacturer are analyzed in Figure 2. Med-El had the highest 5-year cumulative and device survival. Relative to Med-El, Cochlear (hazard ratio, 1.67 [95% CI, 0.72-3.88]) and Advanced Bionics (hazard ratio, 1.67 [95% CI, 0.61-4.53]) each had a reduction in cumulative survival. Likewise, for device survival, relative to Med-El, Cochlear (hazard ratio, 1.65 [95% CI, 0.55-4.99]) and Advanced Bionics (hazard ratio, 1.93 [95% CI, 0.56-6.74]) each had a reduction in device survival. The wide range of the 95% CIs and the inclusion of the null value indicated that none of the differences in cumulative and device survival are clinically meaningful.
Figure 1. Overall Cumulative and Device Survival of Cochlear Implants.
The overall 5-year cumulative survival rate was 95%, and the 10-year rate was 94%. The overall device 5-year survival rate was 97%, and the 10-year rate was 96%.
Figure 2. Cumulative and Device Survival by Manufacturer.
Both the 5-year cumulative survival and device survival were highest with Med-El devices and lowest with Advanced Bionics devices.
To determine the survival rate more precisely, we calculated overall survival excluding all recalled devices (the CI 512, Clarion II, and HiRes90K). After excluding the recalled device types, the 5-year device survival rate was 98.23%. The 10-year device survival was 97.70%, and 15-year survival was 94.99%, much higher than the rate including all device types. These calculated results more accurately reflect the survival rate for current CI devices than the results that include the recalled devices.
Several patients required multiple revision surgeries, so we also collected the initial reasons for those cases. Nine patients required these revision surgeries, and device failure and flap-associated problems were the most common initial reasons for multiple revision surgeries, followed by CSF leakage, hematoma, and misinsertion. No patient with migration required revision surgery. Three patients underwent 4 revision surgeries, 2 for flap-associated problems and 1 for a CSF leak.
Discussion
In this study, 43 of 925 recipients of CIs (4.6%) required revision surgery. Because it is important to determine which patients are likely to need revision surgery, we have here presented the characteristics and surgical findings of patients who required revision surgery. Among the individuals who had revisions, the most common reason for hearing loss was congenital hearing loss, and the proportion of patients who had revisions who had an inner ear anomaly was relatively high (32.6%). A previous study also found that the success rate for CI among patients with an inner ear malformation was lower than that among patients with normal anatomy.8 The NRT/ART response was good in 83.7% of individuals who had revisions, so most individuals with device failure showed good responses intraoperatively. This result indicates that the NRT/ART response does not correlate directly with device failure.
Five individuals had intraoperative CSF gushers. Postoperative CSF leakage can occur if an intraoperative CSF gusher is inadequately sealed at the electrode entrance site during surgery.9 In this study, postoperative CSF leakage occurred at 2 of the 5 individuals who required more than 1 revision surgery. Therefore, to prevent future CSF leakage and subsequent revision surgery, it is important to manage CSF leaks correctly during surgery, using techniques such as packing muscle or connective tissue around the implant site.
In this study, the total revision rate was 4.6%, and the overall device failure rate was 3.0%. A previous review study calculated 6.0% as the overall revision rate.7 Thus, our institutional revision rate was slightly lower than had been reported previously, and our cumulative survival rate was higher. Device failure has been calculated by many researchers and is consistently the most common reason for revision surgery, which we also found. A recent cohort study also found a device failure rate of 3%, which is consistent with our result.6
In the survival analysis, the overall cumulative survival and device survival rates were more than 95% in 5 years and more than 90% in 15 years. Those rates are higher than those from previous studies and imply that implanted devices remain safe and stable for a long time.6 Moreover, the tendency in all of our survival curves indicates that most revision surgeries are performed near the beginning of the postoperative period. The survival curves dropped right after CI surgery and gradually decreased until they reached a plateau at around 5 years. In this study, the survival rate did not differ significantly among manufacturers, but some differences are still worth discussing. Advanced Bionics had the highest device failure rate and lowest survival rate, primarily because Advanced Bionics voluntarily recalled the Clarion 1.2, Clarion II, and HiRes90K devices in 2004 because of concerns about the possible presence of residual moisture inside or a potential loss of hermeticity.10 Moreover, the Advanced Bionics models used in this study were older and had a longer follow-up duration than the devices from other manufacturers. On the other hand, Med-El had the lowest device failure and revision rates and highest survival rates. Because the first Med-El CI model was approved relatively recently, the models used in this study were newer and had a shorter follow-up duration than the devices from other manufacturers.
Furthermore, Cochlear has also had to recall devices. In 2011, CI 512 was recalled because of a loss of hermeticity in the device components, because water entered through microcracks in the brazing process, leading to the malfunction of the electronic components.7 This supports our finding that CI 512 had the worst device failure, revision, and survival rates among all devices. Moreover, reflecting that recall event, a retrospective review study compared the cumulative failure between the CI 24RE series and the CI 500 series.11 That study confirmed that the cumulative failure percentage of the CI 500 series was almost 10%, whereas that of the CI 24RE series was 0%. After the voluntary recall, the cumulative failure percentage turned out to be 25.0%, which is quite high.
Our data contain not only device failure but also many other reasons for revision surgeries. Flap-associated problems were the second most common reason for revision, along with migration, and accounted for a high proportion of revisions, especially with Advanced Bionics devices (22.2%). According to Table 2, as we have discussed, the mean follow-up duration was longest with Advanced Bionics devices. Therefore, this result is highly associated with changes in the size and shape of incisions used for CI surgery. In the past, surgeons made an S-shaped incision, but over time, incision size has been getting smaller, and C-shaped incisions have become the norm, which has decreased the occurrence of flap-associated problems. In other words, flap-associated problems were more likely to occur in the beginning of CI history, with devices such as the old Advanced Bionics devices in this study. In our data, migration, hematoma, and misinsertion occurred only with Cochlear devices, probably because the number of patients with Cochlear devices was large enough for rare problems to appear.
Cerebrospinal fluid leakage was a reason for revision surgery in both ears of 1 patient with Med-El devices, but those instances were highly associated that patient’s inner ears, which both had an incomplete partition type I deformity. Cochlear implants were placed in both ears, and multiple revision surgeries were required, 1 in the left ear and 4 in the right ear. An incomplete partition type I deformity is also known as a cystic cochleovestibular malformation, which means that the cochlea looks like an empty cyst, missing the modiolus and interscalar septa.12 It occurs alongside a dilated large vestibule, but the vestibular aqueduct is rarely enlarged.13 Those structural changes yield a defect in the internal auditory canal that is associated with CSF leakage in 40% to 50% of surgeries.12 Another study also reported that an incomplete partition type I large bony defect in the lamina cribrosa can be a reason for an increase in intraoperative CSF leakage.14 After several revision surgeries, this patient had to have 1 implant extracted because of unmanageable, repetitive CSF leakage.
Nine ears required more than 1 revision surgery, and the reasons for multiple revision surgeries correlated highly with the reason for the initial revision surgery. Flap-associated problems and device failures were the most common reasons for multiple revision surgeries. Given that rate of revision cases caused by flap-associated problems was only 0.4%, a relatively high proportion of patients with flap-associated problems needed multiple revision surgeries, which supports a previous report that flap-associated problems are particularly challenging to solve.6 Two of the patients each required 4 revision surgeries to treat flap-associated problems. One patient had a wound infection after the first surgery, and wound debridement was required. The rotational flap failed after the debridement, so removal and reimplanting surgeries were required. Similarly, the other patient had a wound infection with swelling, which we classified as a flap-associated problem. The swelling at wound site persisted despite being aspirated 3 times. Methicillin-resistant Staphylococcus aureus, a common reason for persistent infection in patients with CI, was isolated in the wound culture. Because methicillin-resistant S aureus is difficult to control because of its resistance to antibiotics,15 the CI was removed to treat the infection, and then reimplanting was done when the infection was gone.
Limitations
This study has a few limitations, including study-design problems, such as inconsistent patient-observation periods and a diversity of patient characteristics. Furthermore, it was difficult to compare different generations of implant devices and recalled devices. In addition, because only 3 Oticon devices were used, comparing them with devices from other manufacturers was impractical. Moreover, the specific reasons for device failure, such as trauma or hermeticity, could not be confirmed because of a lack of information. Those reasons should be considered in a future prospective study.
Conclusions
This study retrospectively reviewed 925 CIs and revision cases to analyze survival rates and reasons for revision. Our institutional 10-year, cumulative survival was 94%, and the device survival was 96%. After excluding recalled device types, the 5-year device survival rate was 98%. The 10-year device survival was 98%, and the 15-year survival was 95%. The most common reason for revision surgery was device failure, which is in line with results from previous studies. Flap-associated problems were the second most common reason, but they have tended to decrease in number because minimal incisions are now being made. Besides, the NRT/ART response did not directly correlate with device failure. Cerebrospinal fluid leakage was also a considerable reason for revision surgery, indicating a need to prevent CSF leakage during surgery, especially in patients with incomplete partition type 1 defects.
eTable. Cause of revision surgery over time. Device failure showed double peak in 2005 and 2011. Flap related problem had tendency to occur at earlier period.
References
- 1.Cullington H, Kitterick P, DeBold L, et al. Personalised long-term follow-up of cochlear implant patients using remote care, compared with those on the standard care pathway: study protocol for a feasibility randomised controlled trial. BMJ Open. 2016;6(5):e011342. doi: 10.1136/bmjopen-2016-011342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(3):177-182. doi: 10.1016/j.anorl.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 3.Olgun Y, Bayrak AF, Catli T, et al. Pediatric cochlear implant revision surgery and reimplantation: an analysis of 957 cases. Int J Pediatr Otorhinolaryngol. 2014;78(10):1642-1647. doi: 10.1016/j.ijporl.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 4.Cullen RD, Fayad JN, Luxford WM, Buchman CA. Revision cochlear implant surgery in children. Otol Neurotol. 2008;29(2):214-220. doi: 10.1097/MAO.0b013e3181635e9a [DOI] [PubMed] [Google Scholar]
- 5.Stevens SM, Dougherty H, Wenstrup L, et al. Is hard failure still a common indication for revision surgery in adult cochlear implant recipients? Otol Neurotol. 2019;40(3):321-327. doi: 10.1097/MAO.0000000000002118 [DOI] [PubMed] [Google Scholar]
- 6.Karamert R, Düzlü M, Tutar H, et al. Assessment of cochlear implant revision surgeries in a cohort of 802 patients. Otol Neurotol. 2019;40(4):464-470. doi: 10.1097/MAO.0000000000002152 [DOI] [PubMed] [Google Scholar]
- 7.Lane C, Zimmerman K, Agrawal S, Parnes L. Cochlear implant failures and reimplantation: a 30-year analysis and literature review. Laryngoscope. 2019. doi: 10.1002/lary.28071 [DOI] [PubMed] [Google Scholar]
- 8.Demir B, Cesur S, Sahin A, Binnetoglu A, Ciprut A, Batman C. Outcomes of cochlear implantation in children with inner ear malformations. Eur Arch Otorhinolaryngol. 2019;276(9):2397-2403. doi: 10.1007/s00405-019-05475-9 [DOI] [PubMed] [Google Scholar]
- 9.Eftekharian A, Amizadeh M. Cerebrospinal fluid gusher in cochlear implantation. Cochlear Implants Int. 2014;15(3):179-184. doi: 10.1179/1754762814Y.0000000069 [DOI] [PubMed] [Google Scholar]
- 10.Health Canada Archived—notification to CLARION 1.2 cochlear implant users—advanced bionics—for the public. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2004/14133a-eng.php. Accessed July 3, 2018.
- 11.Hildrew DM, Molony TB. Nucleus N5 CI500 series implant recall: hard failure rate at a major cochlear implantation center. Laryngoscope. 2013;123(11):2829-2833. doi: 10.1002/lary.24149 [DOI] [PubMed] [Google Scholar]
- 12.Berrettini S, Forli F, De Vito A, Bruschini L, Quaranta N. Cochlear implant in incomplete partition type I. Acta Otorhinolaryngol Ital. 2013;33(1):56-62. [PMC free article] [PubMed] [Google Scholar]
- 13.Sennaroglu L. Cochlear implantation in inner ear malformations—a review article. Cochlear Implants Int. 2010;11(1):4-41. doi: 10.1002/cii.416 [DOI] [PubMed] [Google Scholar]
- 14.Kontorinis G, Goetz F, Giourgas A, Lenarz T, Lanfermann H, Giesemann AM. Radiological diagnosis of incomplete partition type I versus type II: significance for cochlear implantation. Eur Radiol. 2012;22(3):525-532. doi: 10.1007/s00330-011-2301-5 [DOI] [PubMed] [Google Scholar]
- 15.Im GJ, An YS, Choi J, Song JJ, Chae SW, Jung HH. Analysis of bacterial biofilms on a cochlear implant following methicillin-resistant staphylococcus aureus infection. J Audiol Otol. 2015;19(3):172-177. doi: 10.7874/jao.2015.19.3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Cause of revision surgery over time. Device failure showed double peak in 2005 and 2011. Flap related problem had tendency to occur at earlier period.