Abstract
Carvacrol (1) and thymol (2) were converted to their alkyl 4-oxobutanoate derivatives (7–20) in three steps, and evaluated for tyrosinase inhibitory activity. The compounds showed structure-dependent activity, with all alkyl 4-oxobutanoates, except 7 and 20, showing better inhibitory activity than the precursor 4-oxobutanoic acids (5 and 6). In general, thymol derivatives exhibited a higher percent inhibitory activity than carvacrol derivatives at 500 μM. Derivatives containing three and four carbon alkyl groups gave the strongest activity (carvacrol derivatives 9–12, IC50 = 128.8–244.1 μM; thymol derivatives 16–19, IC50 = 102.3–191.4 μM).
Keywords: Carvacrol, thymol, alkyl 4-oxobutanoates, tyrosinase inhibition
The major components of oregano and thyme essential oils are the isomeric monoterpene phenols, carvacrol (1) and thymol (2). These phenols exhibit a wide range of biological and pharmacological effects including anticancer, anti-inflammatory, antibacterial, antifungal, anticholinesterase, insecticidal, and antioxidant activities.1–3 Several derivatives of carvacrol and thymol have been synthesized and show similar or enhanced activity relative to the parent phenols. Ester and carbamate derivatives of both compounds showed increased antifungal4 and anticholinesterase5 activities, respectively. Some Schiff base derivatives exhibited similar or better antioxidant activity compared to thymol and ascorbic acid.6 Heterocyclic derivatives of thymol containing pyridazinone,7 pyridone,8 and oxadiazole9 moieties gave moderate to significant antibacterial activity when compared to standard antibiotics. Similarly, oxadiazole and thiadiazole derivatives of carvacrol displayed significant enhancement of insect growth regulation in a moth species,10 and moderate improvement in antioxidant activity11 relative to carvacrol.
Tyrosinase is the rate-limiting enzyme for the biosynthesis of melanin. Although melanin serves to protect the skin from UV damage, overproduction can lead to skin defects such as melasma, freckles and age spots. As a catechol oxidase, tyrosinase is also implicated in the undesirable browning of fruit. Thus, tyrosinase inhibitors are important in the medical, agricultural and cosmeceutical fields, owing to their ability to mitigate fruit browning and skin defects arising from melanin overproduction.12–13 The tyrosinase inhibitory activity of carvacrol and thymol have not been well documented. Satooka indicated that thymol affects the redox processes involved in dopachrome, and subsequently melanin formation, but does not directly affect the tyrosinase enzyme activity.14 However, diesters of carvacrol and thymol incorporating glycolic acid and benzoic or cinnamic acid moieties have shown moderate to potent tyrosinase inhibitory activity when compared with kojic acid,15–16 a commercially used skin whitening agent. In addition, a diester containing a succinoyl moiety coupled with two kojic acid units gave a better than 2-fold increase in inhibitory activity than kojic acid.17 Based on the improvements in tyrosinase inhibitory activities upon structural modification of carvacrol, thymol and kojic acid, it was envisioned that alkyl 4-oxobutanoate derivatives of carvacrol and thymol would give enhancement in activity relative to the parent phenols.
4-Oxobutanoate derivatives of carvacrol and thymol were synthesized in three steps (Figure 1). Carvacrol (1) and thymol (2) were converted to their corresponding ethyl ethers by modification of the procedure reported by Silva et al.18 Treatment of acetonitrile solutions of the phenols with sodium methoxide and bromoethane afforded ethyl carvacrol (3) and ethyl thymol (4) in 60% and 93% yields, respectively. The yields and spectroscopic data for compounds 3 and 4 were in good agreement with literature data.18 With the ethyl ethers in hand, it was anticipated that the 4-oxobutanoic acid moiety could be introduced to the carvacrol/thymol core by Friedel-Crafts acylation with succinic anhydride and aluminum chloride, as reported for butyl thymol.7 However, attempts to synthesize the 4-oxobutanoic acids using succinic anhydride were unsuccessful in our hands. The use of succinoyl chloride, followed by acidic work up gave the 4-oxobutanoic acids 5 and 6 in 68% and 81% yields, correspondingly. While the bis-carvacrol or bis-thymol γ-diketone could have been obtained as a product of the reaction, there was no evidence of its formation. The carvacrol and thymol 4-oxobutanoic acids were each converted to methyl, ethyl, propyl, allyl, butyl, crotyl and benzyl 4-oxobutanoates by O-alkylation, using cesium carbonate and the corresponding alkyl bromides or iodides in acetonitrile.19 The fourteen new alkyl 4-oxobutanoate derivatives of carvacrol and thymol (7–20) were characterized by NMR, IR and HRMS analyses.
Figure 1:
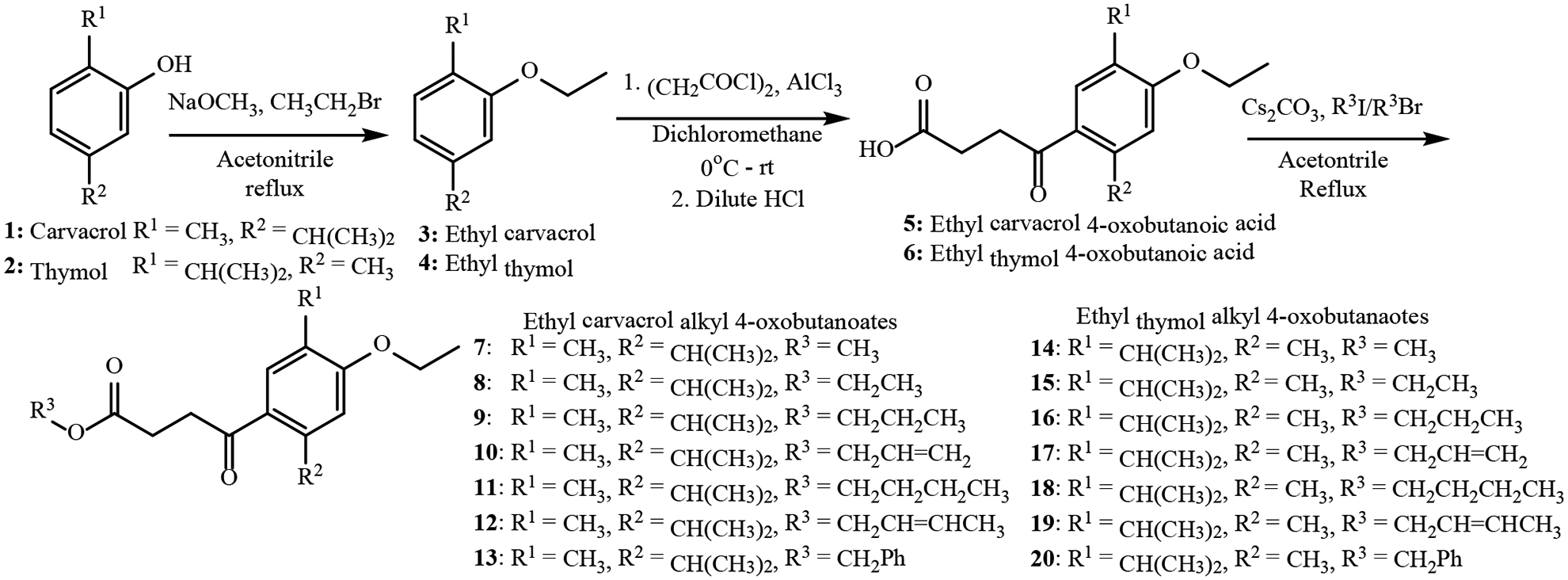
Synthesis of alkyl 4-oxobutanoate derivatives of carvacrol and thymol
Tyrosinase converts L-tyrosine to dopaquinone, which is subsequently converted to dopachrome. The tyrosinase inhibitory assay measures the amount of dopachrome produced in the presence of test compounds.20 In order to determine structure-activity correlations, compounds 1–20 were evaluated at 500 μM for inhibitory activity against mushroom tyrosinase (Table 1). Carvacrol (1) and thymol (2) gave comparable data, with percent inhibition of 35.7 and 29.4%, respectively. For carvacrol there was a 1.6 fold increase in activity for the ethyl ether derivative (3) and a 2.1 fold decrease in activity for the 4-oxobutanoic derivative (5). However, there was no significant difference in activity between thymol and its ethyl ether (4) and 4-oxobutanoic acid (6) derivatives. The similar activity observed for thymol and its ethyl ether derivative is in contrast to a previous study which suggested that the methyl ether derivative of thymol had a lower inhibitory effect than thymol.14 The carvacrol and thymol alkyl 4-oxobutanoates showed better inhibitory activities than the parent 4-oxobutanoic acids, except for the methyl ester derivative of carvacrol, 7, and the benzyl ester derivative of thymol, 20. In general, the activity was enhanced with an increase in the carbon chain length, with esters containing three and four carbon alkyl groups showing the greatest inhibitory activity (%Inhibition = 72.8–100%). The only exception is the carvacrol allyl 4-oxobutanoate (10), which showed significantly lower inhibitory activity (58.2%) than the corresponding propyl 9 (85.6%), butyl 11 (82.3%) and crotyl 12 (72.8%) derivatives. The benzyl ester derivatives showed significantly lower percent inhibition than their three and four carbon counterparts (13 = 47.0%; 20 = 31.3%).
Table 1:
Tyrosinase inhibitory activities of carvacrol (1)/thymol (2) and their corresponding derivatives 3, 5, 7–13/4, 6, 14–20
| Compound | Carvacrol (1) %Inhibition | Thymol (2) %Inhibition | Carvacrol IC50 (μM) | Thymol IC50 (μM) |
|---|---|---|---|---|
| Parent 1/2 | 35.7±5.4 | 29.4±3.7 | n.d. | n.d. |
| Ethyl ether 3/4 | 57.5±2.5 | 28.8±5.6 | n.d. | n.d. |
| 4-Oxobutanoic acid 5/6 | 16.9±4.0 | 30.7±2.7 | n.d. | n.d. |
| Methyl 4-oxobutanoate 7/14 | 31.7±6.2 | 45.5±2.1 | n.d. | n.d. |
| Ethyl 4-oxobutanoate 8/15 | 50.9±2.1 | 72.0±5.5 | n.d. | 212.3±1.7 |
| Propyl 4-oxobutanoate 9/16 | 85.6±6.6 | 78.8±2.4 | 217.9±4.3 | 154.0±3.7 |
| Allyl 4-oxobutanoate 10/17 | 58.2±2.3 | 100.04±6.7 | 128.8±1.9 | 125.6±3.5 |
| Butyl 4-oxobutanoate 11/18 | 82.3±2.1 | 100±3.9 | 176.5±2.8 | 191.4±2.4 |
| Crotyl 4-oxobutanoate 12/19 | 72.8±3.3 | 99.3±8.0 | 244.1±3.6 | 102.3±.1.8 |
| Benzyl 4-oxobutanoate 13/20 | 47.0±9.0 | 31.3±7.1 | n.d. | n.d. |
Kojic acid (IC50 = 21.8±1.7 μM)
% Inhibition (500 μM); n.d. = not determined (IC50 >500 μM)
IC50 data were obtained for alkyl 4-oxobutanoates (9–12 and 15–19) showing greater than 51% inhibition at 500 μM (Table 1). Kojic acid (IC50 = 21.8 μM), was used as a positive control. The compounds showed moderate inhibitory activity, with IC50 values ranging from 122.8 to 244.1 μM for the carvacrol series (9–12) and 102.3–212.3 μM for the thymol series (15–19). Despite the anomalous percent inhibition of the carvacrol allyl 4-oxobutanoate (10), it showed the highest inhibitory activity among the carvacrol derivatives (IC50 = 128.8 μM). For thymol, the crotyl derivative 19 had the highest inhibitory activity (IC50 = 102.3 μM). While the precise mechanism of action of the 4-oxobutanotes is unknown, the increase in inhibitory activity for the three and four carbon derivatives and decrease for the benzyl group and smaller alkyl groups may indicate interaction with the hydrophobic pocket of the tyrosinase enzyme,12–13 with optimal binding interactions occurring with three and four carbon alkyl groups.
In conclusion, fourteen alkyl 4-oxobutanoate derivatives of carvacrol and thymol were synthesized and evaluated for their tyrosinase inhibitory effects using mushroom tyrosinase. Although all the alkyl 4-oxobutanoate derivatives showed lower inhibitory activity than kojic acid, the correlations between the structure of the alkyl side chain and tyrosinase inhibitory activity are evident. Esters containing three or four carbon atoms in the alkyl chain were the most effective. Further structural modifications of the carvacrol and thymol derivatives could potentially lead to structures with enhanced tyrosinase inhibitory activities.
Supplementary Material
Acknowledgments
We are grateful to Susquehanna University for financial support. The JEOL ECZ 400S NMR spectrometer was acquired by a grant to Susquehanna University from the National Science Foundation (NSF:MRI CHE 1625340). The Waters SYNAPT G2-S QTOFMS system used for HRMS data is located in the RI-INBRE core facility at the University of Rhode Island. The instrument was obtained by a grant (# P20GM103430) from National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Supplementary data
Experimental procedures, tyrosinase inhibitory assay and data (HRMS, IR, 1H and 13C NMR) data for compounds 5–20 are available.
References
- 1.Meeran MFN, Javed H, Taee HA, Azimullah S, Ojha SK. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol 2017;8:1–34. DOI: 10.3389/fphar.2017.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghdi Badi H, Addollahi M, Mehrafin A, Ghorbanpour M, Tolyat M, Qaderi A, Ghiaci YM. An overview of two valuable bioactive compounds, thymol and carvacrol, in medicinal plants. J. Med. Plants 2017;16:1–32. [Google Scholar]
- 3.Baser KHC. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des 2008;14:3106–3119. DOI: 10.2174/138161208786404227 [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Jiang S, Yang Y, Fan L, Su F, Ye M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res 2018; DOI: 10.1080/14786419.2018.1480618 [DOI] [PubMed] [Google Scholar]
- 5.Kurt BZ, Gazioglu I, Dag A, Salmas RE, Kayik G, Durdagi S, Sonmez F. Synthesis, anticholinesterase activity and molecular modeling studies of novel carbamate-substituted thymol/carvacrol derivatives. Bioorg. Med. Chem 2017;25:1352–1363. DOI: 10.1016/j.bmc.2016.12.037 [DOI] [PubMed] [Google Scholar]
- 6.Beena DK, Rawat DS. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett 2013;23:641–645. DOI: 10.1016/j.bmcl.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Nagle PS, Pawar YA, Sonawane AE, Bhosale SM, More DH. Docking simulation, synthesis and biological evaluation of novel pyridazinone containing thymol as potential antimicrobial agents. Med. Chem. Res 2014;23:918–926. [Google Scholar]
- 8.Nagle PS, Pawar YA, Sonawane AE, Bhosale SM, More DH. Synthesis and evaluation of antioxidant and antimicrobial properties of thymol containing pyridone moieties. Med. Chem. Res 2012;21:1395–1402. [Google Scholar]
- 9.Vashi BS, Mehta DS, Shah VH. Studies on 1,3,4-oxadiazoles: Preparation and antimicrobial activity of 2-aryl-5-(p-nitroso thymoxy methyl)-1,3,4-oxadiazole. Asian J. Chem 1995;7:847–850. [Google Scholar]
- 10.Bagul SD, Rajput JD, Srivastava C, Bendre RS. Insect growth regulatory activity of carvacrol-based 1,3,4-thiadiazoles and 1,3,4-oxadiazoles. Mol. Divers 2018;22:647–655. DOI: 10.1007/s11030-018-9823-6 [DOI] [PubMed] [Google Scholar]
- 11.Bagul SD, Rajput JD, Karandikar PS, Bendre RS. Synthesis, characterization and antioxidant activity of carvacrol containing novel thiadiazole and oxadiazole moieties. Mod. Chem. Appl 2016;4:1–4. DOI: 10.4172/2329-6798.1000193 [DOI] [Google Scholar]
- 12.Pillaiyar T, Namasivayam V, Manickam M, Jung S. Inhibitors of melanogenesis: an updated review. J. Med. Chem 2018;61:7395–7418. DOI: 10.1021/acs.jmedchem.7b00967 [DOI] [PubMed] [Google Scholar]
- 13.Pillaiyar T, Manickam M, Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem 2017;32:403–425. DOI: 10.1080/14756366.2016.1256882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satooka H, Kubo I. Effects of thymol on mushroom tyrosinase-catalyzed melanin formation. J. Agric. Food Chem 2011;59:8908–8914. DOI: 10.1021/jf2014149 [DOI] [PubMed] [Google Scholar]
- 15.Ashraf Z, Rafiq M, Nadeem H, Hassan M, Afzal S, Waseem M, Afzal K, Latip J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLOS ONE, 2017;12:1–17. DOI: 10.1371/journal.pone.0178069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashraf Z, Rafiq M, Seo S, Kwon KS, Baber MM, Zaidi NS. Kinetic and in silico studies of novel hydroxyl-based thymol analogues as inhibitors of mushroom tyrosinase. Eur. J. Med. Chem 2015;98:203–211. DOI: 10.1016/j.ejmech.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 17.Rho HS, Baek HS, Ahn SM, Kim DH, Chang IS. Synthesis of new anti-melanogenic compounds containing two molecules of kojic acid. Bull. Korean Chem. Soc 2008;29:1569–1571. DOI: DOI: 10.5012/bkcs.2008.29.8.1569 [DOI] [Google Scholar]
- 18.Silva VB, Travassos DL, Nepel A, Barison A, Costa EV, Scotti L, Scotti MT, Mendonca-Junior FJB, La Corte dos Santos R, Cavalcanti, SCH. Synthesis and chemometrics of thymol and carvacrol derivatives as larvicides against Aedes aegypti. J. Arthropod Borne Dis 2017;11:315–330. [PMC free article] [PubMed] [Google Scholar]
- 19.Parrish JP, Dueno SK, Jung KW. Improved Cs2CO3 promoted O-alkylation of acids. Synth. Commun 2000;30:2687–2700. DOI: 10.1080/00397910008086893 [DOI] [Google Scholar]
- 20.Ma H, Xu J, DaSilva NA, Wang L, Wei Z, Guo L, Johnson SL, Lu W, Xu J, Gu Q, Seeram NP. Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma cells. Arch. Dermatol. Res 2017;309:265–274. DOI: 10.1007/s00403-017-1728-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


