Abstract
Because sunlight is essential for human survival, we have developed complex mechanisms for detecting and responding to light stimuli. The eyes and skin are major organs for sensing light and express several light-sensitive opsin receptors. These opsins mediate cellular responses to spectrally-distinct wavelengths of visible and ultraviolet light. How the eyes mediate visual phototransduction is well studied, but less is known about how the skin detects light. Both human and murine skin express a wide array of opsins, with one of the most highly expressed being the functionally elusive opsin 3 (OPN3). In this review we explore light reception, opsin expression and signaling in skin cells; and compile data elucidating potential functions for human OPN3 in skin, with emphasis on recent studies investigating OPN3 regulation of melanin within epidermal melanocytes.
1. INTRODUCTION
Life on earth depends on sunlight; it is a sine qua non condition for human survival. Because sunlight can, arguably, only penetrate skin-deep, its complex effects must be mediated by those organs that light can reach under physiological conditions—our eyes and skin. How light evokes responses in retinal cells to mediate vision has been investigated for many decades (Hisatomi and Tokunaga, 2002; Shichida and Imai, 1998). In addition to rods and cones that mediate vision, the retina also contains cells responsible for the light-dependent entrainment of circadian rhythm (Berson, 2002; Provencio et al., 2000) and other visual and non-visual functions (Berson, 2007; Panda et al., 2002; Sikka et al., 2014). These light-induced responses in the retina are mediated by opsins, a family of light-sensitive G-protein coupled receptors (GPCRs) that covalently bind a retinal chromophore.
Skin, the largest organ of our body, is also constantly exposed to sunlight. While specific skin responses to visible light remain a matter of debate, exposure to solar ultraviolet (UV) radiation has a plethora of short- and long-term effects on skin: DNA damage, oxidative stress, increased pigmentation and photoaging (Cui et al., 2007; Holick, 2008; Honigsmann, 2002; Kiyonaka et al., 2013). Solar UV radiation is comprised of ~95% long wavelength UVA and ~5% short wavelength UVB, each activating distinct signaling pathways in the skin. UVB induces DNA damage, triggering the increase in pigmentation within many hours to days (Cui et al., 2007), while physiological doses of UVA trigger a retinal-dependent G-protein coupled signaling pathway causing immediate pigment darkening via activation of an unknown receptor (Bellono et al., 2013; 2014; Wicks et al., 2011). The retinal-dependence and involvement of G-proteins in the UVA pathway makes it tempting to speculate that the skin, similar to the eye, uses retinal-bound opsin receptors to respond to light. Here we review recent findings related to the expression and function of opsins in skin cells, particularly focusing on elucidating the elusive roles of opsin 3 (OPN3).
2. VISUAL PHOTOTRANSDUCTION
Retinal cells respond to different wavelengths of light via activation of opsins with distinct spectral sensitivities [reviewed in (Terakita, 2005)]. An opsin’s light sensing ability is due to its interaction with a light-sensitive chromophore (Pitt et al., 1955). In an inactive form, the opsin apoprotein forms a covalent bond with an endogenously-produced, vitamin A-derived chromophore, most often 11-cis retinal in mammals. Absorption of photons by the bound retinal induces its isomerization from 11-cis to all-trans conformation, subsequently causing a conformational change in the opsin moiety that renders the opsin active, able to bind a G-protein and potentiate a signaling cascade (T. Hara and R. Hara, 1973; Pitt et al., 1955; Terakita, 2005). Differences in the opsins’ amino acid sequences in the chromophore binding pocket and bond dynamics with the chromophore give rise to an array of different spectral sensitivities across the visible and ultraviolet ranges of light (Menon et al., 2001; Sekharan and Morokuma, 2011; Zhukovsky and Oprian, 1989).
In this review, we focus on five primary opsins found in mammals, opsins 1–5 (OPN1–5). The quintessential opsins that mediate visual phototransduction are blue, green and red cone opsins (OPN1s) and rhodopsin (OPN2). The three OPN1s—blue short wavelength (OPN1-SW1, absorbance maximum λmax ~355–445 nm and OPN1-SW2, λmax ~400–470 nm), green medium wavelength (OPN1-MW, λmax ~527 nm), red long wavelength (OPN1-LW, λmax ~557 nm)—and OPN2 (λmax ~ 500 nm) mediate photonic and scotopic visual responses, respectively. In response to light, OPN1s and OPN2 couple to transducin, a G-protein alpha subunit in the Gαi family (Fung et al., 1981; Manning and Gilman, 1983). Also found in the retina, although not for visual purposes, is melanopsin (OPN4, λmax ~490 nm) which signals via Gαq and mediates pupil size and entrainment of the circadian clock, among other functions (Berson, 2002; Newman et al., 2003; Panda et al., 2002; Provencio et al., 2000; Sikka et al., 2014). Moving beyond the retina, neuropsin (OPN5, λmax ~380 nm), as its name suggests, is present in several tissues including the brain, where it couples to Gαi and may influence circadian rhythms in mice (Buhr et al., 2015; Tarttelin et al., 2003; Yamashita et al., 2010). The remaining opsin, OPN3, was initially identified in the brain where it was aptly named encephalopsin (Blackshaw and Snyder, 1999; Halford et al., 2001), then was later discovered in several other tissues and renamed panopsin (Blackshaw and Snyder, 1999; Halford et al., 2001). Unlike for other members of the opsin family, the G-protein coupling, light sensitivity and function of mammalian OPN3 remain poorly characterized.
3. SKIN PHOTOTRANSDUCTION
Human skin is structured in layers: the uppermost layer, the epidermis, is comprised of keratinocytes surrounding a single basal layer of melanin-producing melanocytes; the dermis comprises a middle fibrous layer; and the subcutis is a cushioning layer upon which the epidermis and dermis rest (Simpson et al., 2011). How deep solar UV radiation penetrates the skin layers depends on its energy: high energy UVA penetrates through both the epidermis and dermis, whereas UVB is primarily confined to the epidermis (Costin and Hearing, 2007; Kanavy and Gerstenblith, 2011; Narayanan et al., 2010) (Fig. 1).
Figure 1: UVB- and UVA-induced melanogenic signaling in epidermal melanocytes.
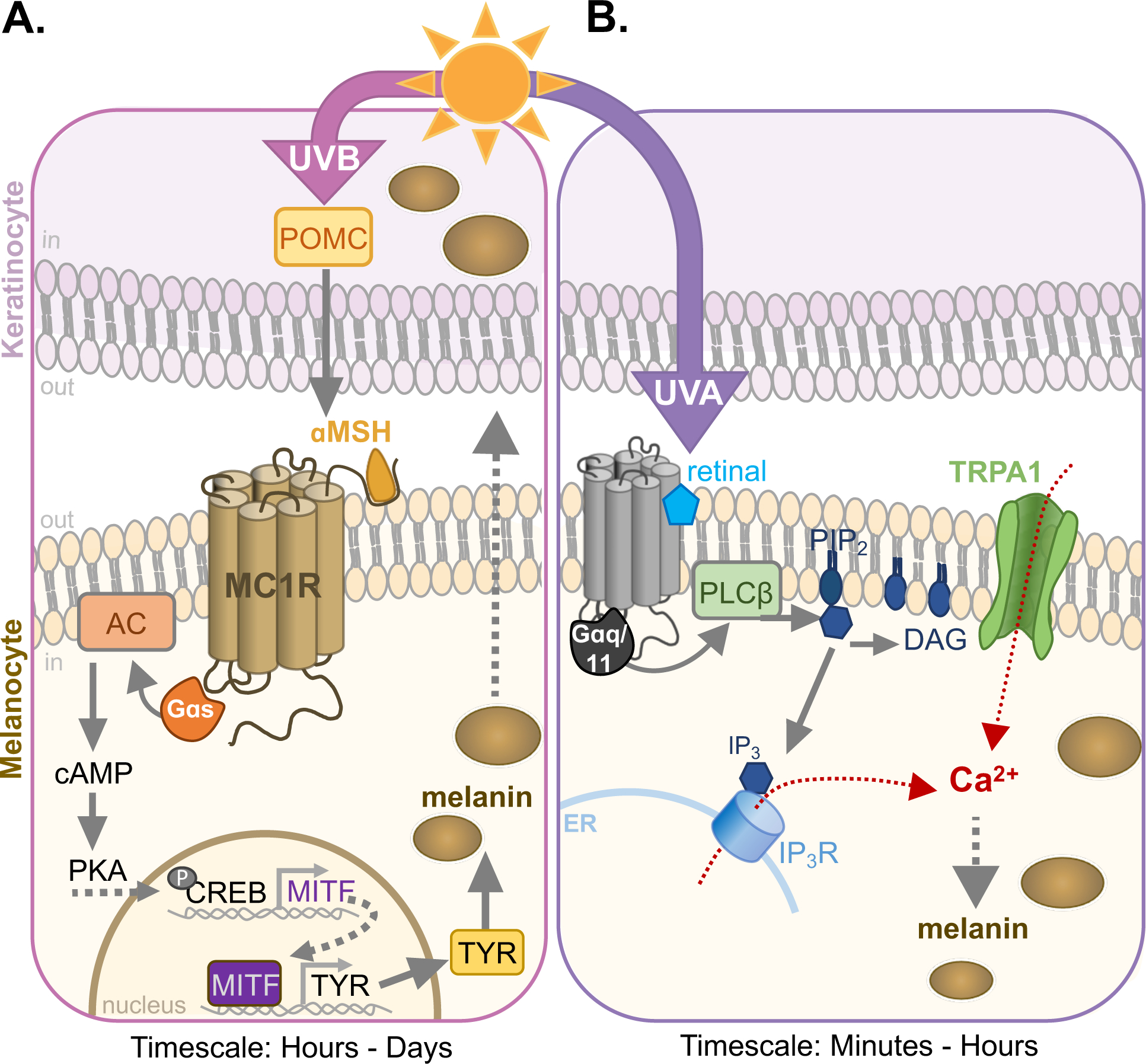
A. UVB induces DNA damage, causing the release of pro-opiomelanocortin (POMC) by keratinocytes and melanocytes. POMC is cleaved to αMSH, the endogenous agonist for the melanocortin-1 receptor (MC1R) in melanocytes. Activation of MC1R signals via Gαs to increase intracellular cAMP levels through activation of adenylyl cyclase (AC). cAMP renders protein kinase A (PKA) catalytically active, phosphorylating cAMP response element binding protein (CREB) which upregulates microphthalmia-associated transcription factor (MITF). The subsequent MITF-driven increase in expression of melanogenic enzymes like tyrosinase (TYR) leads to an increase in melanin within melanosomes (brown circles) and melanin transfer to keratinocytes, which occurs in 12–24 h after UVB exposure.
B. UVA, detected by an as yet unidentified melanocyte photoreceptor, leads to retinal-dependent activation of Gαq/11, which stimulate phospholipase C-β (PLCβ) to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 binds its cognate receptor IP3R, increasing Ca2+ flux from the endoplasmic reticulum (ER). This pathway also activates transient receptor potential cation channel A1 (TRPA1), leading to Ca2+ influx and a further increase in intracellular Ca2+. Ca2+ from both sources is required for the increase melanin synthesis that can be detected 1 h after exposure (Bellono et al., 2014; 2013; Wicks et al., 2011).
How does skin detect and respond to UV radiation? UVB is directly absorbed by DNA to cross-link pyrimidine bases, causing mutations and subsequent activation of DNA repair pathways (Cadet et al., 2005; Setlow, 2019). The effect of UVB on skin requires genotoxic damage to occur, triggering a signaling cascade on the timescale of hours to days (Cui et al., 2007; Park et al., 1999). The DNA repair mechanism, through a series of molecular steps, leads to production of pro-opiomelanocortin (POMC) in keratinocytes and melanocytes, which is cleaved into a-melanocyte stimulating hormone (αMSH) and other peptide hormones that are secreted (Chakraborty et al., 1996). αMSH is the agonist for the main melanogenic receptor expressed in melanocytes, the Gαs-coupled melanocortin-1 receptor (MC1R). MC1R increases the cytosolic concentration of cyclic adenosine monophosphate (cAMP) through stimulation of adenylyl cyclase (AC). As a second messenger, cAMP activates protein kinase A (PKA), which, in turn, phosphorylates the cAMP responsive element-binding protein (CREB). Phosphorylated CREB stimulates activity of microphthalmia-associated transcription factor (MITF) resulting in an increase in melanogenic enzymes like tyrosinase (TYR), ultimately leading to an increase in melanin (Cronin and Bok, 2016; Cui et al., 2007; Honigsmann, 2002). Melanin is synthesized and stored in specialized organelles called melanosomes and is subsequently transferred to, and dispersed in, keratinocytes, causing the increase in skin pigmentation we know as tanning (Hearing, 2011; Lin and Fisher, 2007; Tran et al., 2008) (Fig. 1A).
How UVA is detected by the skin is less well-understood. Skin exposure to high UVA doses causes immediate pigment darkening on the timescale of minutes to a few hours (Honigsmann, 2002; Pathak et al., 1962), but how physiological doses of UVA are detected and lead to cellular responses in skin is yet to be fully understood. Our group has recently made progress in understanding UVA phototransduction in human epidermal melanocytes via a novel mechanism (Bellono et al., 2014; 2013; Wicks et al., 2011). We found that human epidermal melanocytes exposed to physiological doses of UVA radiation elicit a UVA-specific, retinal-dependent transient increase in cytosolic calcium (Ca2+). Moreover, the Ca2+-mediated response to UVA only occurred in melanocytes, not neighboring keratinocytes (Bellono et al., 2014; 2013; Wicks et al., 2011). We showed that in melanocytes, physiological doses of UVA activate a retinal-dependent, Gαq/11-coupled pathway that stimulates phospholipase C-β (PLCβ) to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) in the endoplasmic reticulum, releasing Ca2+ in the cytosol. This pathway also triggers the opening of transient receptor potential A1 (TRPA1) ion channels at the plasma membrane, further increasing intracellular Ca2+ levels. The combined increase in intracellular Ca2+ from internal and external sources is necessary for the rapid augmentation in melanin levels detected on the order of minutes to hours (Bellono et al., 2014; 2013; Wicks et al., 2011) (Fig. 1B). Although we characterized the molecular steps that mediate the downstream signaling pathway evoked by UVA in melanocytes, the identity of the UVA receptor is yet to be determined. The combined light-sensitivity, G-protein coupling, and retinal-dependence of the UVA-evoked responses led us to hypothesize that UVA could be detected by opsins in melanocytes.
3.1. OPSIN EXPRESSION IN HUMAN SKIN
While dermal photoreception in lower organisms has been extensively studied for more than half a century (Steven, 1963), photoreception in mammalian skin has only recently come into the spotlight. In the early 2000s, Denda and Fuziwara determined that different wavelengths of light have different effects on human epidermal permeability (Denda and Fuziwara, 2008), indicating a potential array of spectrally distinct light receptors within the skin. Shortly thereafter, OPN1s and OPN2 were identified in human epidermal skin (Tsutsumi et al., 2009). OPN2 was found in human epidermal keratinocytes (KERs) (Kim et al., 2013), human basal layer keratinocytes and hair follicle stem cells (Buscone et al., 2017), and, as we found, in primary human epidermal melanocytes (HEMs) (Wicks et al., 2011). To initially test whether opsins were present in HEMs, we used degenerated opsin primers and identified only OPN2 mRNA and subsequently, OPN2 protein (Wicks et al., 2011). To test if OPN2 could sense light in melanocytes, we performed UVA-induced Ca2+ imaging in HEMs with endogenous or reduced levels of OPN2 and saw a decrease in Ca2+ flux when OPN2 was reduced, initially giving hope that OPN2 acts as the UVA-sensitive receptor mediating our signaling cascade (Wicks et al., 2011). However, the differences in spectral sensitivity and G-protein coupling between OPN2 (λmax ~500 nm, canonically coupled to Gαi) and the putative UVA-sensitive GPCR (λ ~320–400 nm, coupled to Gαq/11) suggest that OPN2 does not function by itself as the melanocyte UVA receptor. Rather, OPN2 may work in conjunction with an unidentified UVA-sensitive receptor to mediate UVA phototransduction in HEMs.
Of the five mammalian opsins OPN1–5, OPN4 is the only one known to couple to Gαq, and thus was the most likely candidate for the UVA-sensitive receptor in melanocytes. Using more sensitive techniques, we performed real-time quantitative PCR and RNA-Seq of HEMs and KERs from light- and dark-pigmented donors to determine if, in addition to OPN2, other opsins are present in human epidermal skin. We identified mRNA expression of several opsins in both HEMs and KERs, albeit at different levels, including OPN1-SW, OPN2, OPN3, and OPN5 (Haltaufderhyde et al., 2015; Haltaufderhyde and Oancea, 2014) (Fig. 2). Unfortunately, we did not detect significant expression of OPN4 mRNA, our hypothesized candidate for the UVA receptor. Instead, we found that the most highly expressed opsin was OPN3 in both HEMs and KERs. Because at the time human OPN3 had unknown G-protein coupling and spectral sensitivity, it became the most prospective opsin candidate for the elusive melanocyte UVA sensor. As we now know, OPN3 is not a UVA receptor, but amidst our experiments, we and others have identified novel functions for OPN3 in melanocytes, keratinocytes, dermal fibroblasts and hair follicles within the skin.
Figure 2: Opsin expression in human epidermal melanocytes and keratinocytes.
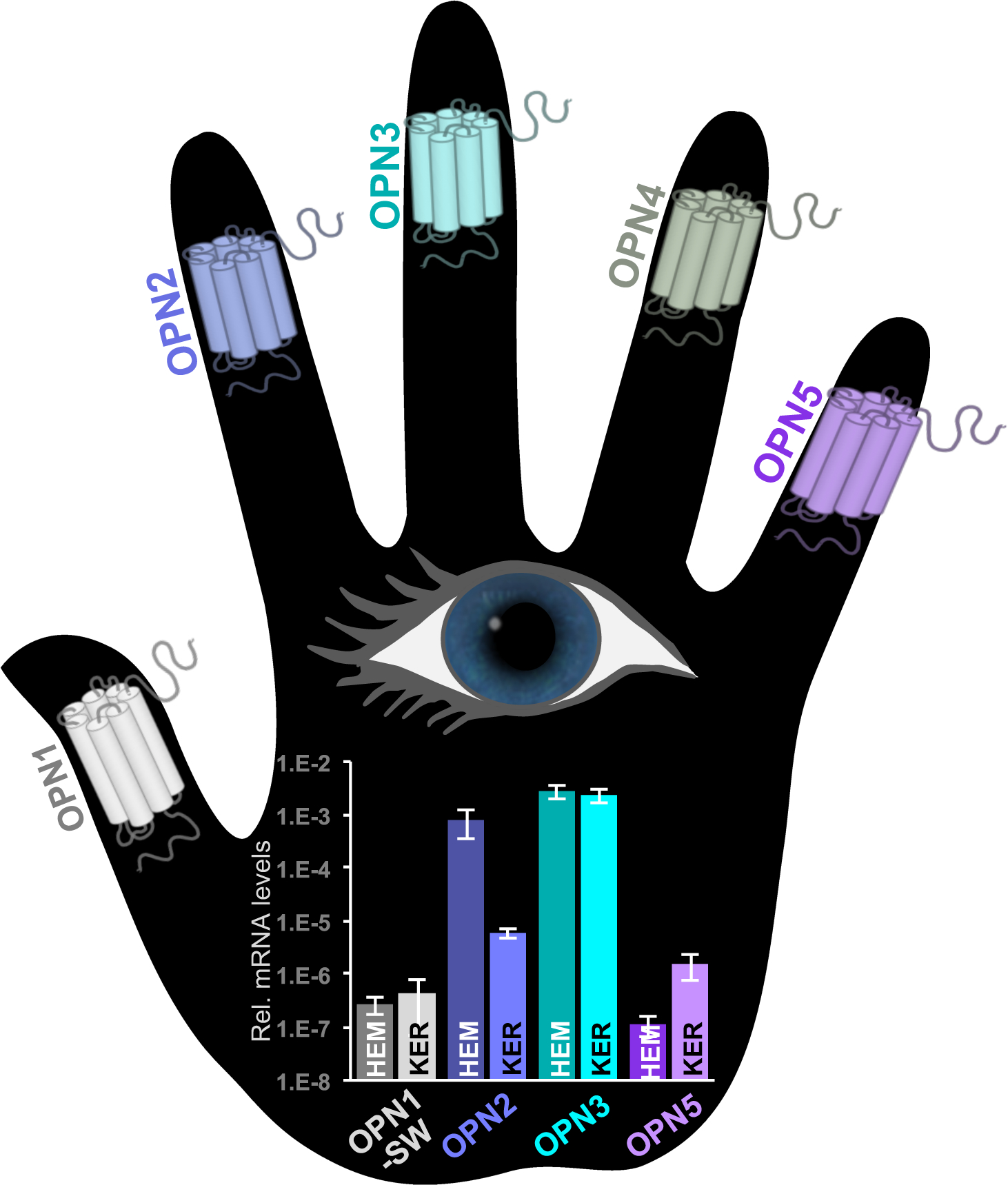
Expression levels in human epidermal melanocytes (HEMs) and human epidermal keratinocytes (KERs) of mRNA encoding OPN1-SW, OPN2, OPN3, OPN5 (OPN4 was not detected) measured by quantitative RT-PCR (Haltaufderhyde et al., 2015). OPN3 is the most highly expressed opsin in both cell types. The known photosensitivity of opsins lends the notion that skin can “see” light. Rel.: relative
3.2. OPSIN EXPRESSION IN MURINE SKIN
Mouse skin shares similarities to human skin: the same layered organization from epidermis, to dermis, to subcutis, but also differences: human skin is thicker (> 100 um) with 5–10 layers of keratinocytes in the epidermis compared to mouse skin (< 25 um), which only has 2–3 layers of keratinocytes; humans have epidermal melanocytes, whereas mice have melanocytes primarily in the hair follicles within the dermis (Khavari, 2006) with some epidermal melanocytes present in the non-hair covered skin of the ears, tail and soles of the feet (Gerson and Szabo, 1968). The first indication that mouse skin may also express opsins came in 2001, when it was demonstrated that mouse skin and cultured murine melanocytes express OPN2 mRNA and protein (Miyashita et al., 2001). Since then, more studies have detected OPN1-SW, OPN4 (de Assis et al., 2016), and OPN5 (Kojima et al., 2011) in normal and malignant mouse melanocytes. Castrucci and her colleagues have led the forefront in studying OPN2 and OPN4 in mouse melanocytes, suggesting that both opsins function as part of local circadian clocks and as potential UVA sensors mediating melanogenesis (de Assis et al., 2017; 2018; Moraes et al., 2017). The differential expression of OPN4 in mouse but not human skin, indicates that some opsins may serve species-specific functions in the skin.
After discovering OPN3 expression in human melanocytes, we wondered if mice also express OPN3 in their skin and, thus, could be used as model organisms to investigate OPN3 function. The lack of reliable antibodies against human and mouse OPN3s represents a significant impediment in evaluating OPN3 expression and function; to overcome this impediment, we have recently generated a novel knock-in mouse model with mCherry inserted in-frame at the C-terminus of OPN3, under the endogenous OPN3 promoter (Olinski et al., (in preparation)). We used this OPN3-mCherry mouse model to perform immunostaining on mouse ears, which contain epidermal melanocytes (Fig. 3). We now show that in mouse skin OPN3 is expressed in epidermal melanocytes, keratinocytes and in a subset of cells in the dermis, likely dermal fibroblasts, as well as in the hair follicle of mouse ears (Fig. 3). The similar OPN3 expression pattern in mouse and human skin lends promise that mouse models can be used to study the function of OPN3 in the skin.
Figure 3: OPN3-mCherry expression in mouse skin.
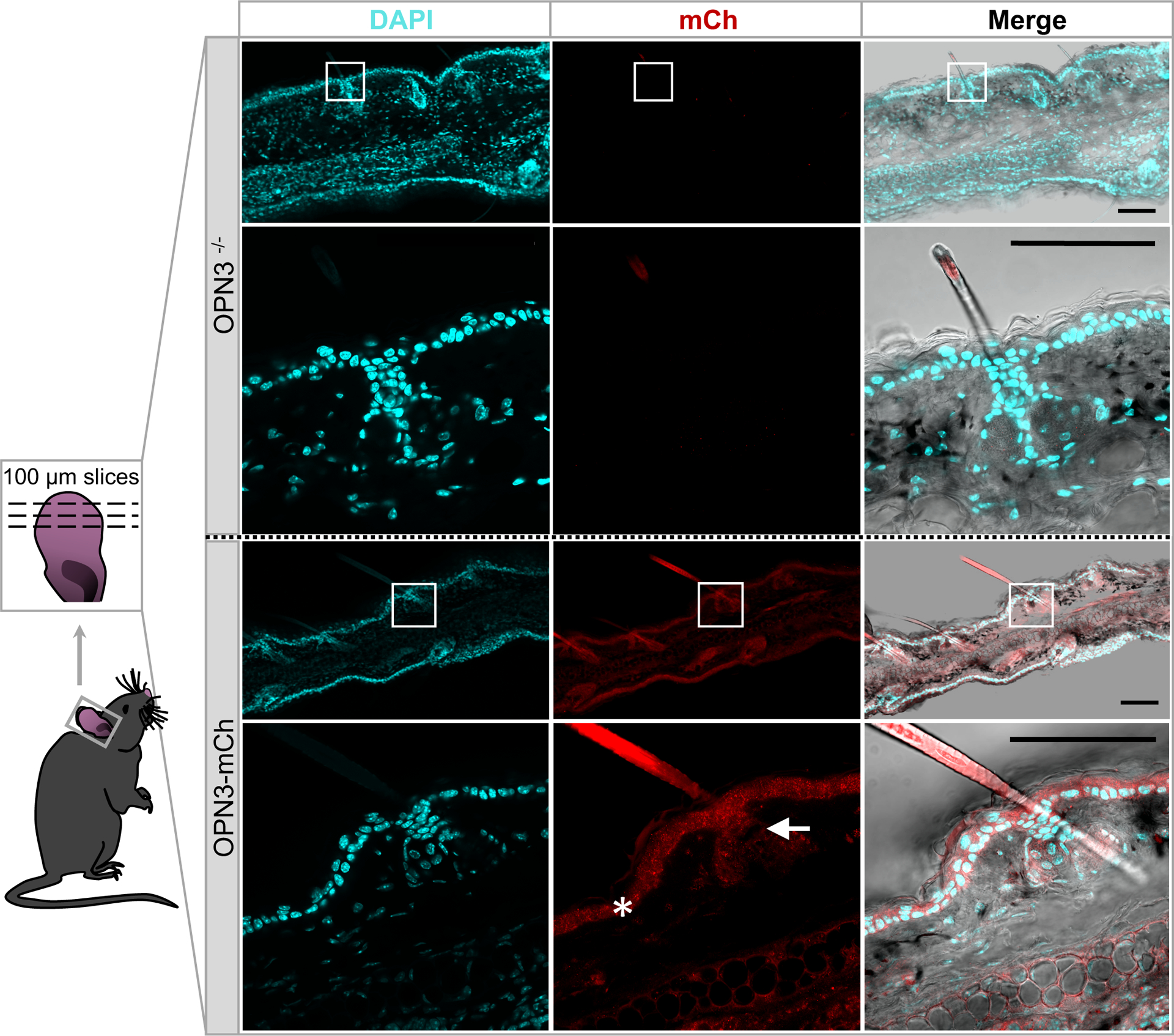
Representative images of horizontal skin sections obtained from ears of OPN3-mCherry (OPN3-mCh) knock-in mice expressing the fusion protein OPN3-mCherry under the endogenous OPN3 promoter (bottom) and OPN3−/− mice, used as a negative control (top). Ear slices obtained from the OPN3-mch knock-in, but not from the OPN3−/−, mouse exhibit mCherry immunostaining in the epidermis (star), hair follicle (arrow), and dermis, indicating OPN3 expression in mouse skin. White boxes indicate the areas shown at higher magnification below. DAPI staining (left column) was used to identify individual cells/nuclei. Scalebars: 100 μm. mCh: mCherry
4. OPN3 PROPERTIES AND FUNCTION IN SKIN
OPN3 was first discovered in 1999 in the mouse brain (Blackshaw and Snyder, 1999). Shortly after, human OPN3 was found to be expressed in a wide array of peripheral tissues including the brain, placenta, retina, liver, heart, lung, skeletal muscle, pancreas (Halford et al., 2001; Haltaufderhyde et al., 2015), and skin (Halford et al., 2001; Haltaufderhyde et al., 2015), affording the colloquial name panopsin. Paradoxically, OPN3 is equipped to be light-sensitive (see below), yet it is expressed in an array of tissues not exposed to light under physiological circumstances. Despite its widespread expression—or possibly because of it—OPN3 has gained limited scientific attention.
4.1. OPN3 TOPOLOGY AND STRUCTURAL FEATURES
To be classified as an opsin, a seven-transmembrane GPCR must possess essential features. All opsins, including OPN3, contain a lysine residue within the seventh transmembrane domain which forms a Schiff base linkage with the retinal chromophore. The retinal is stabilized in a chromophore binding pocket by a negatively charged counterion residue (usually aspartate or glutamate) in the third or fourth transmembrane domain (Nathans, 2002; Sakmar et al., 1989; Zhukovsky and Oprian, 1989) (K299 and D117 for human OPN3; Fig. 4). OPN3 sequence and topology is highly conserved across species, from mosquitoes to pufferfish to mammals.
Figure 4: Topology and sequence features of human OPN3.
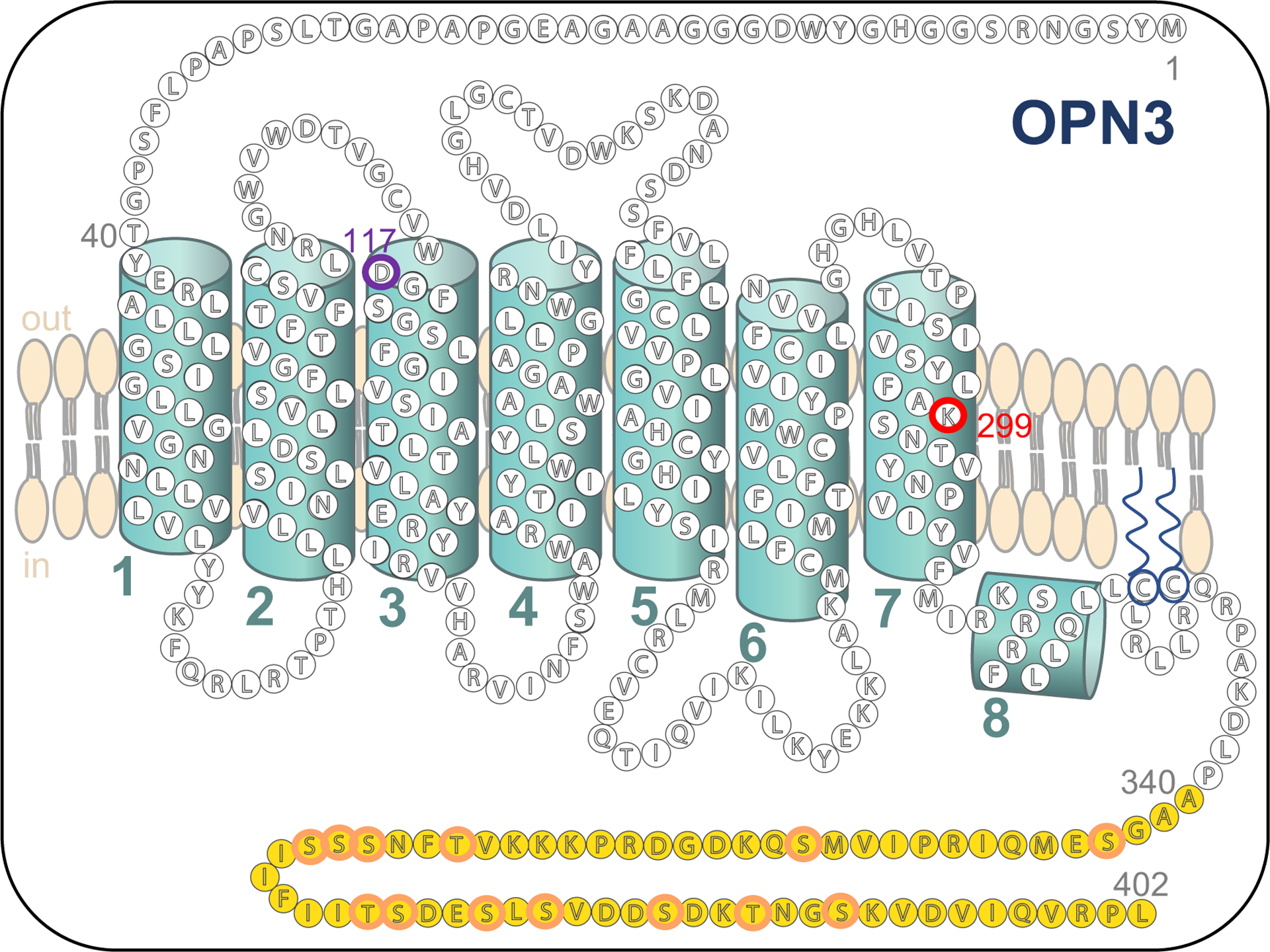
Bead diagram of the secondary structure of human full-length OPN3 based on PredictProtein (Rost et al., 2004). Transmembrane helices are numbered 1–7. The eighth helix is intracellular and is conserved in opsins 1–5. The unique C-terminus is highlighted in yellow; K299 retinal binding residue is outlined in red; D117 negatively charged counterion is outlined in purple; serine and threonine residues within the C-terminus are outlined in orange.
One of the most unique features of OPN3 is its unusually long C-terminus (78 residues; Fig. 4). With the exception of OPN4 with ~100 residues in its C-terminus, the other human opsins have an average C-terminal length of 29 residues. The expansive C-terminus of OPN3 has little homology to that of any GPCR, let alone to any member of the opsin family, yet it remains conserved across species. How the long C-terminus of OPN3 contributes to its function has not been investigated. We examined whether the C-terminus is required for OPN3 trafficking and localization. Full-length OPN3 localizes to both the plasma membrane and to intracellular vesicles (Ozdeslik et al., 2019) and the same localization pattern was observed for C-terminal-truncated OPN3 (Oancea Lab, data not published), suggesting that the unique C-terminus is likely not involved in localization. Based on its residues, the C-terminus may then be involved in OPN3 desensitization, internalization or arrestin binding. The C-terminus contains 13 potential phosphorylation sites (10 serine and 3 threonine residues; Fig. 4) which may play an important role in arrestin-mediated internalization of the receptor (Calebiro et al., 2010). Interestingly, when trying to determine the spectral sensitivity of mosquito and pufferfish OPN3 homologs, Koyanagi et al. found greater protein yields for spectrophotometric analysis when the C-terminus of mosquito OPN3 was removed (Koyanagi et al., 2013). Similarly, when we performed UV-visible spectrometry on human OPN3, our protein yields were far greater when the C-terminus was not present (Ozdeslik et al., 2019). These experimental observations strengthen the idea that the C-terminus of OPN3 across species may have a role in protein lifespan. It is possible that by removing the C-terminus, OPN3 is not internalized as efficiently and high yields of protein can then accumulate over a shorter period of time.
We have found that human melanocytes express OPN3 mRNA encoding the full-length protein and a splice variant missing exon 2 and encoding a truncated peptide comprising only the first three transmembrane domains (Haltaufderhyde et al., 2015). Six other splice variants were found in human placenta, four of which result in truncation after exon 1; one includes two novel exons between exons 1 and 2 encoding a six-exon peptide; and one includes a novel exon replacing exon 2, encoding a protein with identical N- and C-termini as full-length OPN3 but different transmembrane domains and connecting loops (Kasper et al., 2002). It is not clear if splice variants are present in all cell types or if they are tissue specific. The lack of specific anti-OPN3 antibodies makes it difficult to determine whether the peptides encoded by the splice variants are functional proteins or are degraded. The numerous splice variants encoding truncated OPN3 peptides brings into question published results that form conclusions based on OPN3 mRNA expression; if the primers used to detect OPN3 mRNA were based on a small fragment within OPN3, rather than on the full-length coding sequence, the data may be the combined detection of full-length OPN3 and its potentially non-functional splice variants. Until specific OPN3 antibodies become widely available, evidence of the full-length OPN3 coding sequence is a must for inferring the presence of OPN3 protein.
4.2. OPN3 LIGHT ABSORBANCE AND RETINAL BINDING
Based on its GPCR structure and the presence of both a chromophore-binding lysine and negatively charged counterion, OPN3 possesses the necessary features to signal in a light-sensitive and G-protein-dependent manner. However, whether OPN3 homologs across all species absorb light and whether their functions are light-dependent is still a matter of debate.
OPN3 homologs in pufferfish (teleost multiple tissue opsin, pufTMT), mosquito, and zebrafish absorb blue-green light (λmax = 470, 500, and 465 nm, respectively) as measured by UV-visible spectroscopy. These homologs also bind 11-cis retinal which photoisomerizes to all-trans retinal after light irradiation, strongly suggesting that these homologs are bistable (Koyanagi et al., 2013; Sugihara et al., 2016). Koyanagi et al. have also shown that pufTMT and mosquito OPN3 can signal through Gαi/o to decrease cAMP in a light-dependent manner (Koyanagi et al., 2013). Attempts by the same group to determine the spectral sensitivities of anole, chicken, mouse and human OPN3 failed to identify any light absorption, citing low protein yield. Consistently, no light-dependent changes in cAMP were measured for native zebrafish, anole, chicken, mouse or human OPN3s (Koyanagi et al., 2013; Sugihara et al., 2016). These discrepancies across species further fuel the confusion surrounding the ability of mammalian OPN3 to function in a light-dependent manner. Nevertheless, published results seem to agree on the lack of UV-visible absorption and light-induced G-protein coupling for both mouse and human OPN3. Because mammalian OPN3 is phylogenetically distinct, yet closely related to vertebrate visual and non-visual opsins (Terakita, 2005), it possible that it possesses a divergent, non-light sensitive function from other known OPN3 homologs.
Our initial hypothesis that human OPN3 functions as a melanocyte photoreceptor prompted us to measure human OPN3 absorption spectra using UV-visible spectroscopy. To obtain high enough protein yield for accurate spectral measurements, we expressed human OPN3 with a truncated C-terminus, which does not appear to alter spectral characteristics of other OPN3 homologues (Koyanagi et al., 2013; Sugihara et al., 2016) or OPN3 localization. Human OPN3 showed no light absorption in the 300–700 nm range, despite being able to bind retinal (Ozdeslik et al., 2019). One possible explanation is that OPN3 might have undergone selective pressure across species and, at least in humans, may no longer be photosensitive. Such light-independent functions for OPN3 could explain its expression in tissues that are not physiologically exposed to light. Interestingly, despite detecting no light absorption within the visible range, we found that human OPN3 binds 11-cis or all-trans retinal (Ozdeslik et al., 2019), suggesting that the binding of OPN3 to retinal has additional critical roles than just conferring potential light sensitivity. Whether the binding of retinal in human OPN3 has a specific function—such as stabilizing the conformation of the opsin or aiding in any interactions OPN3 may have—is a topic for further investigations.
4.3. OPN3 FUNCTION IN HUMAN SKIN
4.3.1. OPN3 in melanocytes
Our initial studies of OPN3 function in melanocytes were designed to test whether OPN3 was the UVA receptor upstream of the Ca2+-melanin cascade in HEMs, but the UVA-induced Ca2+ measurements quickly dismissed this hypothesis. However, HEMs with reduced OPN3 levels had visibly higher melanin levels, surprisingly in the absence of any light stimulation. Our subsequent studies revealed that OPN3 is a negative regulator of melanogenesis, independent of UVA, UVB, blue or green light. We found that OPN3 specifically regulates the MC1R-dependent cAMP melanogenic pathway (Ozdeslik et al., 2019). We showed that human OPN3 couples to Gαi, similar to the Gαi/o coupling of mosquito OPN3 and pufTMT (Koyanagi et al., 2013), and that human OPN3 negatively regulates the MC1R- mediated and Gαs-coupled melanogenic pathway in HEMs. Furthermore, downstream of cAMP, OPN3 negatively regulates MITF and TYR protein levels leading to a decrease in melanin levels, all independent of light stimulation (Fig. 6).
Figure 6: OPN3 negatively regulates MC1R-mediated melanogenesis in human melanocytes.
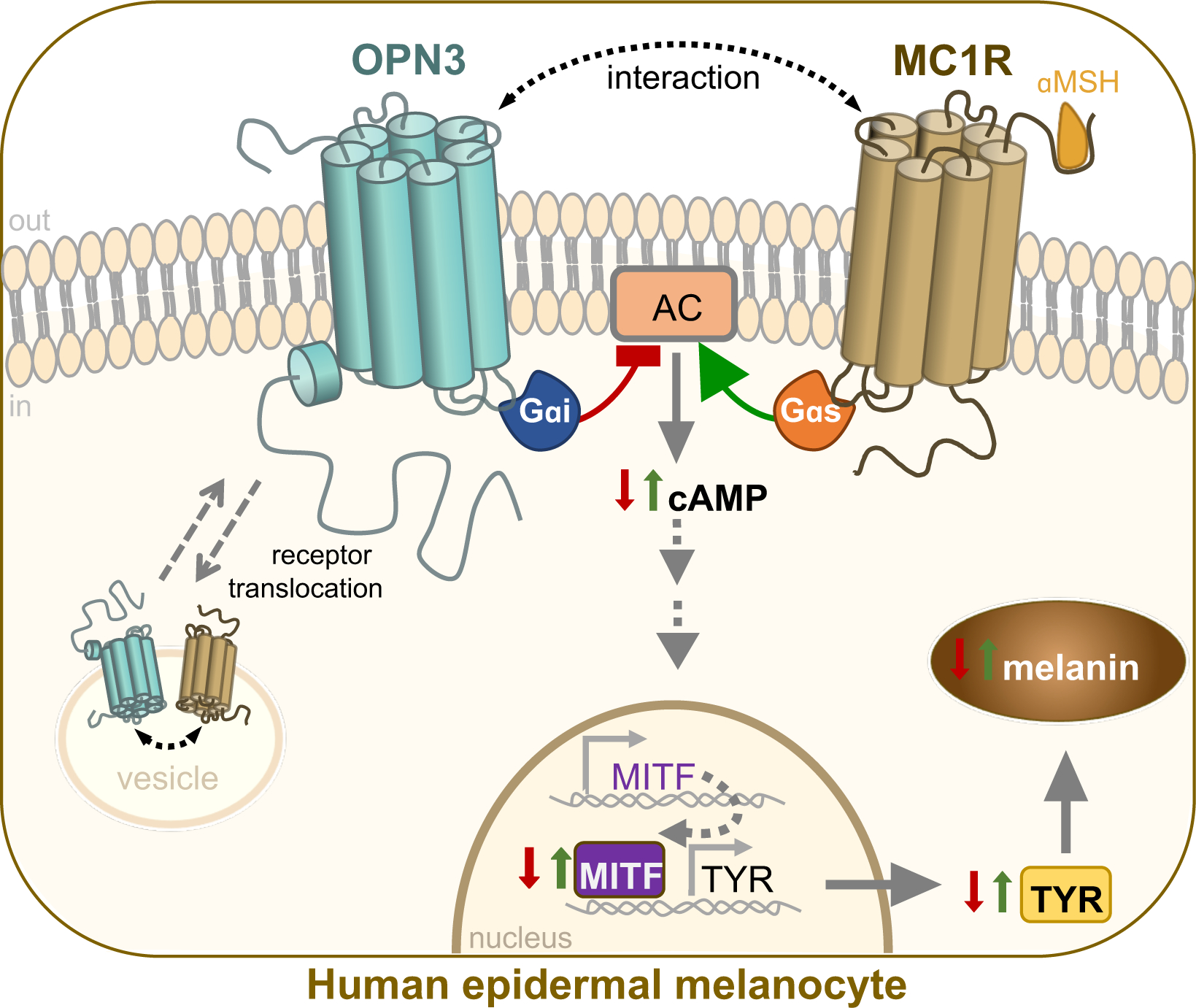
OPN3 couples to Gαi to negatively regulate MC1R-mediated cAMP signaling leading to a decrease in melanin levels in human epidermal melanocytes. Upon αMSH binding and activation, MC1R couples to Gαs, which stimulates adenylyl cyclase (AC) and the production of cAMP. OPN3 acts as a brake on the amount of cAMP produced, therefore regulating downstream levels of microphthalmia-associated transcription factor (MITF) and melanogenic enzyme tyrosinase (TYR). OPN3 can also form a molecular complex with MC1R; both receptors colocalize at the membrane and in intracellular vesicles, suggesting that OPN3 and MC1R translocate as a complex between the cellular compartments.
In addition to the functional relationship between OPN3 and MC1R, we also determined through coimmunoprecipitation that OPN3 and MC1R form a molecular complex. OPN3 and MC1R colocalize on the plasma membrane and in intracellular vesicles and may translocate as part of this complex. We hypothesize that OPN3 interacts with MC1R, either directly or indirectly, to regulate the amount of MC1R available to signal at the plasma membrane. Like other GPCRs with long C-termini, the C-terminus of OPN3 could also act as a binding site for accessory proteins that mediate complex formation with MC1R (Zezula and Freissmuth, 2009). OPN3 may act in a two-pronged manner: (1) OPN3 at the plasma membrane negatively regulates MC1R signaling by coupling to Gαi and (2) OPN3 interacts with MC1R to facilitate translocation of the complex, limiting MC1R signaling by reducing MC1R availability at the plasma membrane (Ozdeslik et al., 2019).
In our experiments, OPN3 did not respond (via cAMP or Ca2+) to external light sources, yet clearly had an effect on melanin levels. These light-independent results are in line with our UV-visible data (Ozdeslik et al., 2019) and the UV-visible experiments from Sugihara et al. indicating no light absorption of human OPN3 (Koyanagi et al., 2013; Sugihara et al., 2016). Although human OPN3 does not appear to be photosensitive, the caveats of our experimental methods must also be considered. OPN3, unlike other known mammalian opsins, might respond to wavelengths—such as infrared— outside of the 300–700 nm range tested in the UV-visible spectroscopic experiments. Regardless of the caveats, our functional studies showed that human OPN3 does not depend on light for its MC1R- mediated function. A light-independent function for OPN3, however, has recently been opposed by a study implicating OPN3 in the positive regulation of a blue-light-dependent pathway in melanocytes (Regazzetti et al., 2018).
Regazzetti et al. reports that blue light (415 nm) increased pigmentation in normal human melanocytes (NHMs) via OPN3 photoactivation (Regazzetti et al., 2018). The study showed that irradiating NHMs, but not keratinocytes, was necessary for an increase in pigmentation. Through quantitative PCR, the study showed that OPN3 mRNA is expressed in NHMs, although its expression did not change with blue light irradiation. Blue light-dependent increases in Ca2+ were diminished when OPN3 was downregulated, which led the investigators to conclude that OPN3 acts as a blue light receptor mediating a Ca2+-dependent melanogenic pathway involving calmodulin-dependent protein kinase II, phosphorylation of CREB, extracellular signal-regulated kinase, p38, upregulation of MITF, and increased expression of the melanogenic enzymes TYR and dopachrome tautomerase in NHMs. However, many of the conclusions are based on Ca2+ measurements that cannot be fully assessed by the reader, as no Ca2+ imaging traces are shown. The study is also missing a direct link between OPN3 and melanin levels, suggesting that further investigations are needed to understand whether human OPN3 may function as a blue-light receptor for this melanogenic pathway.
The only two studies to investigate OPN3 function in human melanocytes have contradictory conclusions (Ozdeslik et al., 2019; Regazzetti et al., 2018): Regazzetti et al. observed OPN3-dependent changes in Ca2+ with and without blue light in NHMs, but Ozdeslik et al. did not detect any OPN3-dependent differences in Ca2+ in HEMs irradiated with blue light, despite both studies using similar LED lamps. In addition, Regazzetti et al. showed decreases in MITF and TYR when OPN3 levels were reduced in NHMs, in direct contradiction with our finding that these levels, along with melanin levels, are increased in HEMs with reduced levels of OPN3. These contradicting results in Ca2+, MITF, TYR, and melanin data could be due to differences in light doses or in the culture methods for the melanocytes used. Regazzetti et al. used a total of 50 J/cm2 of 415 nm light over 33 minutes, whereas we used a total of 200 mJ/cm2 over 10 s. Even efficient light sources release ~75% of their light energy as heat (Ahn et al., 2014), so the total amount and time of light irradiation may be a factor explaining the difference in results, perhaps by temperature-dependent changes in OPN3 or other cellular functions. Both studies used primary human epidermal melanocytes, but Regazzetti et al. used media supplemented with tetradecanoyl phorbol acetate (TPA) and forskolin (FSK), whereas Ozdeslik et al. used media without these additives. Because FSK is known to increase melanogenesis (D’Orazio et al., 2006), and TPA is a non-physiological mitogen that could increase transcription of MITF, TYR and dopachrome tautomerase (Donatien et al., 2004; Prince et al., 2003), their presence in the culture media could affect melanocyte differentiation, melanin levels, and opsin expression. Indeed, we have found expression of OPN1-SW, OPN2, OPN3, and OPN5 in our HEMs (Haltaufderhyde et al., 2015) whereas Regazzetti et al. only detected OPN3 expression in NHMs cultured with TPA and FSK.
Although some progress has been made, more studies are needed to clarify the function of OPN3 in melanocytes. OPN3 may have several, even opposing, functions because the regulation of melanin is critically important to life. Too much melanin can lead to an increase in oxidative damage as a result of the melanogenic process (Cunha et al., 2012; Jenkins and Grossman, 2013), whereas too little melanin leaves the skin susceptible to radiation-induced DNA damage (D’Orazio et al., 2013; 2006). A complete understanding of the signaling and function of OPN3 in melanocytes and in the skin awaits further study.
4.3.2. OPN3 in keratinocytes, hair follicles, and dermal fibroblasts
In addition to epidermal melanocytes, OPN3 is also expressed in other skin cell types, including epidermal keratinocytes (KERs) (Haltaufderhyde et al., 2015; Regazzetti et al., 2018), hair follicles (Buscone et al., 2017), and dermal fibroblasts (Buscone et al., 2017; Castellano-Pellicena et al., 2018; Lan et al., 2019). Castellano-Pellicena et al. sought to determine OPN3 function in keratinocytes. The study confirmed that OPN3, together with OPN1-SW and OPN5, are expressed in human primary epidermal keratinocytes. Blue light irradiation of keratinocytes with reduced levels of OPN3 resulted in a slight decrease in keratinocyte differentiation, but had no effect on their proliferative capacity, compared to wildtype cells (Castellano-Pellicena et al., 2018). Interestingly, OPN3-dependent keratinocyte differentiation was only detected in response to blue light. Because OPN1-SW is also expressed in keratinocytes and is known to absorb blue light, OPN1-SW may be the actual blue-light sensor that directly or indirectly interacts with OPN3 to regulate downstream effects on keratinocyte differentiation.
Keratinocytes, making up the majority of the human epidermis, accept and distribute melanin transferred from melanocytes (Marks and Seabra, 2001; Raposo and Marks, 2002; X. Wu and Hammer, 2014). Because OPN3 is present in both epidermal melanocytes and keratinocytes (Castellano-Pellicena et al., 2018; Haltaufderhyde et al., 2015), a recent report investigated whether it may play a role in the solar UV-induced transfer of melanosomes from melanocytes to keratinocytes. They detected no change in OPN3 mRNA expression with UV exposure, despite an increase in melanosomal transfer (Hu et al., 2017), a result that is neither causative nor conclusive as to whether OPN3 may have a role in melanin transfer. Whether OPN3 might serve complimentary functions in melanocytes and keratinocytes remain to be investigated.
OPN3 was also shown to be expressed in human anagen hair follicles, particularly in the inner root sheath keratinocytes of terminal and vellus hair follicles by PCR, quantitative PCR and immunofluorescence (Buscone et al., 2017). OPN3 mRNA was also found in follicular stem cells and outer root sheath keratinocytes, although immunostaining of skin samples did not detect OPN3 protein in these locations. Despite this, the study determined the effect of OPN3 downregulation on the transcriptome of outer root sheath keratinocytes and found an inhibitory effect on the metabolic activity of these cells (Buscone et al., 2017). The direct causality, potential mechanism and light-dependence of these effects remains unclear. More research needs to be done to determine the physiological function of OPN3 in the hair follicle; it may be particularly interesting to determine if OPN3 regulates melanogenesis in follicular melanocytes to influence hair color.
A recent report on OPN3 in the skin focused on its potential function in dermal fibroblasts (Lan et al., 2019). Lan et al. confirmed that OPN3 mRNA, as well as OPN1, OPN2, OPN4, and OPN5, are present in dermal fibroblasts (Buscone et al., 2017). Contrary to a study that found no UV-dependent change in OPN3 expression in melanocytes (Hu et al., 2017), Lan et al. observed an increase in OPN3, OPN1 and OPN5 expression in dermal fibroblasts after high UV exposure (10 J/cm2). High UVA doses also increased intracellular Ca2+ through a PLCβ-dependent mechanism, leading to an increase in matrix metalloproteases (MMPs) involved in the photoaging of the skin. Reducing OPN3 levels partially reduced UVA-induced Ca2+ flux and abrogated phosphorylation of CREB and calmodulin-dependent protein kinase II and the upregulation of MMPs. Although the immunostaining for OPN3 looks jarringly cytoplasmic for a GPCR and has disputably high colocalization with every MMP stained for, this study does raise questions as to what role OPN3 may have in dermal fibroblasts.
It is intriguing that Lan et al. suggest that OPN3 acts a UVA receptor in dermal fibroblasts; we have shown that OPN3 does not activate cAMP or Ca2+ cascades in response to UV (or any visible) light in melanocytes. It is possible that OPN3 may act in conjunction with another opsin present in dermal fibroblasts to mediate UVA signaling. The authors do note that UVA exposure does also mediate expression of OPN1 and OPN5, of which OPN5 has been shown to sense UV light (Kojima et al., 2011; Yamashita et al., 2010). Downregulation of OPN3 also reduces the expression of OPN1 and OPN5, suggesting a potential interaction between OPN3, OPN1 and OPN5 which could explain UVA-dependent responses.
There is conflicting data on whether OPN3 signals through Ca2+ (Regazzetti et al., 2018) or cAMP (Ozdeslik et al., 2019) in melanocytes. In dermal fibroblasts, OPN3 may partially regulate a Ca2+ cascade after UVA exposure via PLCβ activation. Because PLCβ can be activated by the βλ-subunits of G proteins upon GPCR activation (Clapham and Neer, 1993; D. Wu et al., 1993), OPN3 may signal both through Gαi-mediated cAMP and Gβλ-mediated Ca2+ in dermal fibroblasts and possibly in other skin cells.
5. CONCLUSIONS AND FUTURE DIRECTIONS
The last decade of research brought skin photodetection and photosensitivity into the spotlight. Although the connection between the ability of our eyes and skin to detect light might seem obvious because light is a physiological stimulus for both, it was the discovery that melanopsin (subsequently named OPN4) is expressed in photosensitive dermal melanophores of Xenopus laevis (Provencio et al., 1998), that brought opsins and dermal photodetection together. The unexpected finding that visual opsins (OPN1s and OPN2) are also expressed in mammalian epidermal melanocytes and keratinocytes, inspired many studies in search of their function in the skin. OPN3 was more recently discovered in mammalian skin, but its high expression in epidermal skin suggests that it might have one or more relevant functions. The OPN3-mediated regulation of melanocyte pigmentation via MC1R regulation reveals an important function for OPN3 and also a new paradigm for how an opsin can signal in a light-independent manner in conjunction with another GPCR. The opsin-mediated effects of blue-light on mammalian skin, suggested by a number of studies, are awaiting further investigations that, perhaps, will uncover novel photodetection and phototransduction mechanisms. In conclusion, despite recent progress, much remains to be understood about how our skin “sees” light.
6. METHODS
Animals and immunohistochemistry.
All animal care and procedures were performed in accordance with the Brown University Institutional Animal Care and Use Committee as in compliance with the National Institutes of Health guide for the care and use of laboratory animals. Animals were housed socially with ad libitum access to food and water in a light- (12 h light/dark cycle) and temperature-controlled (21.5 – 22.5°C) facility. OPN3−/− mice (Buhr et al., 2015) were a generous gift from Dr. K.-W. Yau, Johns Hopkins University. Both male and female mice were used. Ears were removed from the head using sharp scissors after euthanasia. Ears were fixed in 4% paraformaldehyde overnight at 4°C before rinsing in PBS thoroughly and cryopreserving in increasing concentrations of sucrose-PBS solutions (10%, 20%, 30%) over a period of 14 d, or until skin sunk to the bottom of the sucrose solution. Skin was cut into ~4 mm sections and embedded in Tissue-Tek optimal cutting temperature solution (OCT) (Electron Microscopy Sciences), frozen in dry ice, and stored at −80°C until sectioning into 100 μm slices using a cryostat (Leica CM3050S). The skin sections were stained using the CytoVista Tissue and 3D Culture Clearing for 3D Fluorescence Imaging Kit (ThermoFisher Scientific) following manufacturer’s protocols and mounted with VECTASHIELD antifade mounting medium with DAPI (Vector Laboratories). Antibodies used: rabbit anti-mCherry (KeraFAST EMU105, 1:350) and donkey anti-rabbit AF594 (ThermoFisher Scientific, 1:500).
Figure 5: OPN3 characteristics across species.
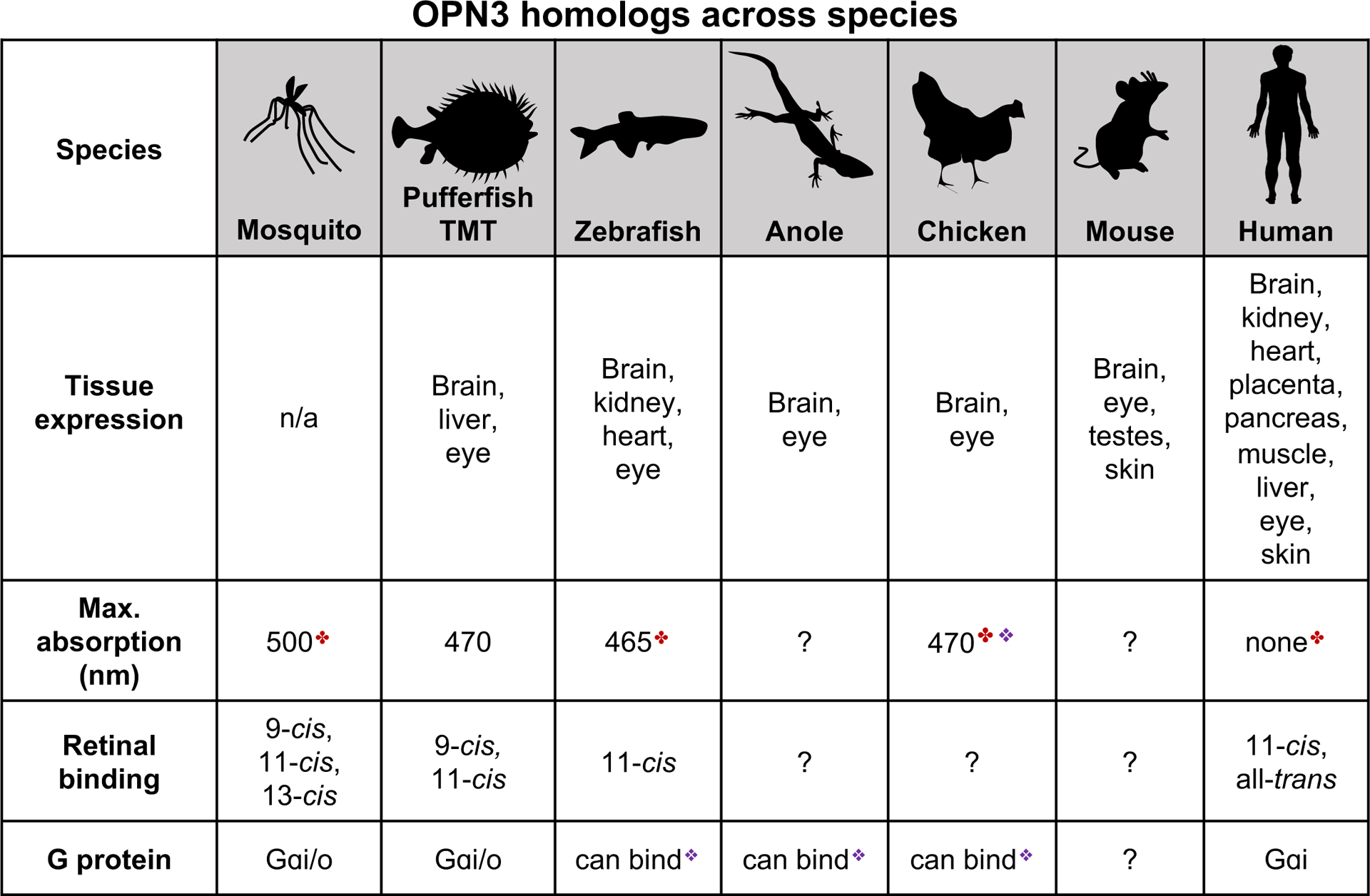
Known characteristics of OPN3 homologs across invertebrate and vertebrate species. ✤spectral sensitivity measured for a C-terminal truncated form of the protein;❖ OPN3 spectral sensitivity or ability to bind G-proteins inferred based on a chimera of OPN3 and the intracellular loop 3 of jellyfish Gαs-coupled opsin. Data summarized from (Blackshaw and Snyder, 1999; Halford et al., 2001; Haltaufderhyde et al., 2015; Koyanagi et al., 2013; Sugihara et al., 2016). TMT: teleost multiple tissue opsin
7. ACKNOWLEDGEMENTS
We thank Dr. K.-W. Yau of Johns Hopkins University for generously providing the OPN3−/− mouse model (Buhr et al., 2015), used as a control for the OPN3-mCherry immunostaining. We also thank present and former members of the Oancea lab, especially Dr. Rana Ozdeslik, for insightful discussions and feedback during the development of the OPN3 project. The research in the laboratory of E.O. as related to this review was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease Grant R01 AR066318 (to E.O.) and the National Institute of General Medical Sciences Training Grant T32 GM077995 (to L.E.O.). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
9. REFERENCES
- Ahn B-L, Jang C-Y, Leigh S-B, Yoo S, Jeong H, 2014. Effect of LED lighting on the cooling and heating loads in office buildings. Applied Energy 113, 1484–1489. [Google Scholar]
- Bellono NW, Kammel LG, Zimmerman AL, Oancea E, 2013. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. U.S.A. 110, 2383–2388. doi: 10.1073/pnas.1215555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Najera JA, Oancea E, 2014. UV light activates a Gαq/11-coupled phototransduction pathway in human melanocytes. J. Gen. Physiol 143, 203–214. doi: 10.1085/jgp.201311094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, 2007. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch - Eur J Physiol 454, 849–855. doi: 10.1007/s00424-007-0242-2 [DOI] [PubMed] [Google Scholar]
- Berson DM, 2002. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 295, 1070–1073. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Snyder SH, 1999. Encephalopsin: A Novel Mammalian Extraretinal Opsin Discretely Localized in the Brain. Journal of Neuroscience 19, 3681–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yue WWS, Ren X, Jiang Z, Liao H-WR, Mei X, Vemaraju S, Nguyen M-T, Reed RR, Lang RA, Yau K-W, Van Gelder RN, 2015. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. U.S.A. 112, 13093–13098. doi: 10.1073/pnas.1516259112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscone S, Mardaryev AN, Raafs B, Bikker JW, Sticht C, Gretz N, Farjo N, Uzunbajakava NE, Botchkareva NV, 2017. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg. Med 45, 487–14. doi: 10.1002/lsm.22673 [DOI] [PubMed] [Google Scholar]
- Cadet J, Sage E, Douki T, 2005. Ultraviolet radiation-mediated damage to cellular DNA. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 571, 3–17. doi: 10.1016/j.mrfmmm.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ, 2010. Signaling by internalized G-protein-coupled receptors. Trends in Pharmacological Sciences 31, 221–228. [DOI] [PubMed] [Google Scholar]
- Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ, 2018. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med 51, 370–382. doi: 10.1002/lsm.23015 [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M, 1996. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochemica et Biophysica Acta 1313, 130–138. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ, 1993. New roles for G-protein By-dimers in transmembrane signalling. Nature 365, 403–406. [DOI] [PubMed] [Google Scholar]
- Costin G-E, Hearing VJ, 2007. Human skin pigmentation: melanocytes modulate skin color in response to stress. The FASEB Journal 21, 976–994. doi: 10.1096/fj.06-6649rev [DOI] [PubMed] [Google Scholar]
- Cronin TW, Bok MJ, 2016. Photoreception and vision in the ultraviolet. Journal of Experimental Biology 219, 2790–2801. doi: 10.1242/jeb.128769 [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE, 2007. Central Role of p53 in the Suntan Response and Pathologic Hyperpigmentation. Cell 128, 853–864. doi: 10.1016/j.cell.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Cunha ES, Kawahara R, Kadowaki MK, Amstalden HG, Noleto GR, Cadena SMSC, Winnischofer SMB, Martinez GR, 2012. Melanogenesis stimulation in B16-F10 melanoma cells induces cell cycle alterations, increased ROS levels and a differential expression of proteins as revealed by proteomic analysis. Experimental Cell Research 318, 1913–1925. doi: 10.1016/j.yexcr.2012.05.019 [DOI] [PubMed] [Google Scholar]
- D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T, 2013. UV radiation and the skin. Int J Mol Sci 14, 12222–12248. doi: 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE, 2006. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 443, 340–344. doi: 10.1038/nature05098 [DOI] [PubMed] [Google Scholar]
- de Assis LVM, Moraes MN, Castrucci A.M. de L., 2017. Heat shock antagonizes UVA-induced responses in murine melanocytes and melanoma cells: an unexpected interaction. Photochem. Photobiol. Sci 16, 633–648. doi: 10.1039/C6PP00330C [DOI] [PubMed] [Google Scholar]
- de Assis LVM, Moraes MN, da Silveira Cruz-Machado S, Castrucci AML, 2016. The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis. BBA - Molecular Cell Research 1863, 1119–1133. doi: 10.1016/j.bbamcr.2016.03.001 [DOI] [PubMed] [Google Scholar]
- de Assis LVM, Moraes MN, Magalhaes-Marques KK, Castrucci A.M. de L., 2018. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. European Journal of Cell Biology 97, 150–162. [DOI] [PubMed] [Google Scholar]
- Denda M, Fuziwara S, 2008. Visible radiation affects epidermal permeability barrier recovery: selective effects of red and blue light. Journal of Investigative Dermatology 128, 1335–1336. doi: 10.1038/sj.jid.5701168 [DOI] [PubMed] [Google Scholar]
- Donatien P, Surleve-Bazelle JE, Thody A, Taieb A, 2004. Growth and differentiation of normal human melanocytes in a TPA-free, cholera toxin-free, low-serum medium and influence of keratinocytes. Arch Dermatol Res 385–392. [DOI] [PubMed] [Google Scholar]
- Fung BK-K, Hurley JB, Stryer L, 1981. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci USA 78, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson DE, Szabo G, 1968. Effect of single gene substitution on the melanocyte system of the C57Bl mouse: Quantitative and qualitative histology. Nature 218, 381–382. [DOI] [PubMed] [Google Scholar]
- Halford S, Freedman MS, Bellingham J, Inglis SL, Poopalasundaram S, Soni BG, Foster RG, Hunt DM, 2001. Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43. Genomics 72, 203–208. doi: 10.1006/geno.2001.6469 [DOI] [PubMed] [Google Scholar]
- Haltaufderhyde K, Özdeşlik RN, Wicks NL, Najera JA, Oancea E, 2015. Opsin expression in human epidermal skin. Photochem. Photobiol 91, 117–123. doi: 10.1111/php.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltaufderhyde KD, Oancea E, 2014. Genome-wide transcriptome analysis of human epidermal melanocytes. Genomics 104, 482–489. doi: 10.1016/j.ygeno.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Hara R, 1973. Isomerization of Retinal catalysed by Retinochrome in the Light. Nature New Biology 242, 39–43. [DOI] [PubMed] [Google Scholar]
- Hearing VJ, 2011. Determination of Melanin Synthetic Pathways 1–4. doi: 10.1038/skinbio.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi O, Tokunaga F, 2002. Molecular evolution of proteins involved in vertebrate phototransduction . Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 133, 509–522. doi: 10.1016/S1096-4959(02)00127-6 [DOI] [PubMed] [Google Scholar]
- Holick MF, 2008. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol. Aspects Med. 29, 361–368. doi: 10.1016/j.mam.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigsmann H, 2002. Erythema and pigmentation. Photodermatology, Photoimmunology and Photomedicine 75–81. [DOI] [PubMed] [Google Scholar]
- Hu Q-M, Yi W-J, Su M-Y, Jiang S, Xu S-Z, Lei TC, 2017. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif 50, e12372–10. doi: 10.1111/cpr.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NC, Grossman D, 2013. Role of melanin in melanocyte dysregulation of reactive oxygen species. BioMed Research International 2013, 908797–3. doi: 10.1155/2013/908797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanavy HE, Gerstenblith MR, 2011. Ultraviolet radiation and melanoma. Semin Cutan Med Surg 30, 222–228. doi: 10.1016/j.sder.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Kasper G, Taudien S, Staub E, Mennerich D, Rieder M, Hinzmann B, Dahl E, Schwidetzky U, Rosenthal A, Rump A, 2002. Different structural organization of the encephalopsin gene in man and mouse. Gene 295, 27–32. [DOI] [PubMed] [Google Scholar]
- Khavari PA, 2006. Modelling cancer in human skin tissue. Nat Rev Cancer 6, 270–280. doi: 10.1038/nrc1838 [DOI] [PubMed] [Google Scholar]
- Kim H-J, Son ED, Jung J-Y, Choi H, Lee TR, Shin DW, 2013. Violet light down-regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PlosONE 8, e73678. doi: 10.1371/journal.pone.0073678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaka S, Kajimoto T, Sakaguchi R, Shinmi D, Omatsu-Kanbe M, Matsuura H, Imamura H, Yoshizaki T, Hamachi I, Morii T, Mori Y, 2013. Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells. Nat. Methods 10, 1232–1238. doi: 10.1038/nmeth.2690 [DOI] [PubMed] [Google Scholar]
- Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y, 2011. UV-sensitive photoreceptor protein OPN5 in humans and mice. PlosONE 6, e26388–e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Takada E, Nagata T, Tsukamoto H, Terakita A, 2013. Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc. Natl. Acad. Sci. U.S.A. 110, 4998–5003. doi: 10.1073/pnas.1219416110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Wang Y, Lu H, 2019. OPN3 is a key regulator of UVA-induced photoaging in human dermal fibroblast cells. Br. J. Dermatol. bjd 18410–27. doi: 10.1111/bjd.18410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Fisher DE, 2007. Melanocyte biology and skin pigmentation. Nature 445, 843–850. doi: 10.1038/nature05660 [DOI] [PubMed] [Google Scholar]
- Manning DR, Gilman AG, 1983. The Regulatory Components of Adenylate Cyclase and Transducin. Journal of Biological Chemistry 258, 7059–7063. [PubMed] [Google Scholar]
- Marks MS, Seabra MC, 2001. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2, 738–748. [DOI] [PubMed] [Google Scholar]
- Menon ST, Han M, Sakmar TP, 2001. Rhodopsin: Structural Basis of Molecular Physiology. Physiological Reviews 81, 1659–1681. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Moriya T, Kubota T, Yamada K, Asami K, 2001. Expression of opsin molecule in cultured murine melanocyte. J. Investig. Dermatol. Symp. Proc 6, 54–57. doi: 10.1046/j.0022-202x.2001.00018.x [DOI] [PubMed] [Google Scholar]
- Moraes MN, de Assis LVM, Magalhaes-Marques KK, Poletini MO, de Lima LHRG, Castrucci AM, 2017. Melanopsin, a Canonical Light Receptor, Mediates Thermal Activation of Clock Genes. Sci Rep 1–9. doi: 10.1038/s41598-017-13939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan DL, Saladi RN, Fox JL, 2010. Ultraviolet radiation and skin cancer. Int J Dermatol 49, 978–986. doi: 10.1111/j.1365-4632.2010.04474.x [DOI] [PubMed] [Google Scholar]
- Nathans J, 2002. Determinants of visual pigment absorbance: identification of the retinylidene Schiff’s base counterion in bovine rhodopsin. Biochemistry 29, 9746–9752. doi: 10.1021/bi00493a034 [DOI] [PubMed] [Google Scholar]
- Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR, 2003. Melanopsin forms a functional short-wavelength photopigment. Biochemistry 42, 12734–12738. doi: 10.1021/bi035418z [DOI] [PubMed] [Google Scholar]
- Olinski LE, Tsuda A, Kauer J, Oancea E Endogenous opsin 3 (OPN3) expression in the brain of a novel knock-in mouse model. (in preparation) [DOI] [PMC free article] [PubMed]
- Ozdeslik RN, Olinski L, Trieu ML, Oprian DD, Oancea E, 2019. The human non-visual opsin OPN3 regulates pigmentation of epidermal melanocytes through interaction with MC1R. Proc Natl Acad Sci USA 116, 11508–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA, 2002. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216. doi: 10.1126/science.1076848 [DOI] [PubMed] [Google Scholar]
- Park SB, Suh DH, Youn JI, 1999. A long-term time course of colorimetric evaluation of ultraviolet light-induced skin reactions. Clinical and Experimental Biology 24, 315–320. [DOI] [PubMed] [Google Scholar]
- Pathak MA, Riley FJ, Fitzpatrick TB, Curwen WL, 1962. Melanin formation in human skin induced by long-wave ultra-violet and visible light. Nature 193, 148–150. doi: 10.1038/193148a0 [DOI] [PubMed] [Google Scholar]
- Pitt GAJ, Collins FD, Morton RA, Stok P, 1955. Studies on rhodopsin. 8. Retinylidenemethylamine, an indicator yellow analogue. Biochem. J 59, 122–1315. doi: 10.1042/bj0590122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince S, T, W., PA, H., SH, K., 2003. Stimulation of Melanogenesis by Tetradecanoylphorbol 13- acetate (TPA) in Mouse Melanocytes and Neural Crest Cells. Pigment Cell Research 16, 26–34. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD, 1998. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA 95, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD, 2000. A novel human opsin in the inner retina. J. Neurosci 20, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS, 2002. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic 3, 237–248. [DOI] [PubMed] [Google Scholar]
- Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, De Donatis GM, Chignon-Sicard B, Rocchi S, Passeron T, 2018. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. Journal of Investigative Dermatology 138, 171–178. doi: 10.1016/j.jid.2017.07.833 [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J, 2004. The PredictProtein server. Nucleic Acids Research 32, W321–W326. doi: 10.1093/nar/gkh377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmar TP, RR F, Khorana HG, 1989. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci USA 86, 8309–8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekharan S, Morokuma K, 2011. Why 11- cis-Retinal? Why Not 7- cis-, 9- cis-, or 13- cis-Retinal in the Eye? J. Am. Chem. Soc 133, 19052–19055. doi: 10.1021/ja208789h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, 2019. Cyclobutane-Type Pyrimidine Dimers in Polynucleotides. Science 153, 379–386. [DOI] [PubMed] [Google Scholar]
- Shichida Y, Imai H, 1998. Visual pigment: G-protein-coupled receptor for light signals. Cell. Mol. Life Sci. 54, 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, Steppan J, Kim JH, Barodka V, Myers AC, Santhanam L, Nyhan D, Halushka MK, Koehler RC, Snyder SH, Shimoda LA, Berkowitz DE, 2014. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci USA 111, 17977–17982. doi: 10.1073/pnas.1420258111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ, 2011. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 565–580. doi: 10.1038/nrm3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven DM, 1963. The Dermal Light Sense. Biol. Rev 38, 204–240. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Nagata T, Mason B, Koyanagi M, Terakita A, 2016. Absorption Characteristics of Vertebrate Non-Visual Opsin, Opn3. PlosONE 11, e0161215–15. doi: 10.1371/journal.pone.0161215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ, 2003. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 554, 410–416. [DOI] [PubMed] [Google Scholar]
- Terakita A, 2005. The opsins. Genome Biol. 6, 213–213.9. doi: 10.1186/gb-2005-6-3-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T-NT, Schulman J, Fisher DE, 2008. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell & Melanoma Research 21, 509–516. doi: 10.1111/j.1755-148X.2008.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Ikeyama K, Denda S, Nakanishi J, Fuziwara S, Aoki H, Denda M, 2009. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp. Dermatol 18, 567–570. doi: 10.1111/j.1600-0625.2009.00851.x [DOI] [PubMed] [Google Scholar]
- Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E, 2011. UVA Phototransduction Drives Early Melanin Synthesis in Human Melanocytes. Current Biology 21, 1906–1911. doi: 10.1016/j.cub.2011.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Katz A, Simon MI, 1993. Activation of phospholipase C B2 by the a and By subunits of trimeric GTP-binding protein. Proc Natl Acad Sci USA 90, 5297–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hammer JA, 2014. Melanosome transfer: it is best to give and receive. Curr. Opin. Cell Biol. 29, 1–7. doi: 10.1016/j.ceb.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y, 2010. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. U.S.A. 107, 22084–22089. doi: 10.1073/pnas.1012498107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezula J, Freissmuth M, 2009. The A2A-adenosine receptor: a GPCR with unique features? British Journal of Pharmacology 153, S184–S190. doi: 10.1038/sj.bjp.0707674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukovsky EA, Oprian DD, 1989. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science 246, 928–930. [DOI] [PubMed] [Google Scholar]


