Abstract
Adipose‐derived mesenchymal stem cell (ADSC)‐based regenerative therapies have shown potential for use in many chronic diseases. Aging diminishes stem cell regenerative potential, yet it is unknown whether stem cells from aged donors cause adverse effects in recipients. ADSCs can be obtained using minimally invasive approaches and possess low immunogenicity. Nevertheless, we found that transplanting ADSCs from old donors, but not those from young donors, induces physical dysfunction in older recipient mice. Using single‐cell transcriptomic analysis, we identified a naturally occurring senescent cell‐like population in ADSCs primarily from old donors that resembles in vitro‐generated senescent cells with regard to a number of key pathways. Our study reveals a previously unrecognized health concern due to ADSCs from old donors and lays the foundation for a new avenue of research to devise interventions to reduce harmful effects of ADSCs from old donors.
Keywords: aging, cellular senescence, frailty, regenerative medicine
Adipose‐derived mesenchymal stem cells (ADSCs) or “preadipocytes” have been increasingly suggested for use in regenerative medicine as a treatment for a wide range of diseases due to their multipotency and accessibility. Older adults represent most likely recipients of ADSC therapies given the high burden of diseases in this population. Since autologous ADSCs are preferred in the clinic, it is essential to understand age‐related changes influencing these cells. Emerging evidence suggests that ADSCs from aged donors have reduced regenerative potential, leading to diminished therapeutic efficacy (Khong et al., 2019; Liu et al., 2017; Ye et al., 2016). However, it is unknown whether transplanting ADSCs from aged donors might cause unexpected or even harmful effects in recipients. This is especially important for older adults, since they tend to be more vulnerable and less resilient to such stresses.
To examine this, we isolated ADSCs from 12 young (6–7 months, referred to as young ADSCs) and 12 old (28–31 months, referred to as old ADSCs) C57BL/6 male mice. We transplanted 1 x 106 ADSCs from young or old donors i.p. into syngeneic 20‐month‐old C57BL/6 male mice (Figure 1a). Four to six weeks after transplantation, we tested maximal walking speed (RotaRod), grip strength, physical endurance (hanging test, treadmill), daily activity, food intake, and body weight change to assess overall physical function in recipients, based on criteria used in clinical practice (Fried et al., 2001). ADSCs from old donors significantly impaired walking speed, grip strength, endurance, and daily activity of older recipient mice after transplantation, compared with mice transplanted with the same number of ADSCs from young donors (Figure 1b‐e). No statistically significant differences were observed in terms of treadmill, body weight change, food intake, or remaining lifespan (Figure S1a‐d), which might be due to an insufficient number of transplanted old ADSCs to cause detectible changes in these tests. Overall, these findings suggest that ADSCs from old donors can induce physical frailty, which is highly associated with morbidity and loss of independence(Ensrud et al., 2009). Thus, regenerative approaches entailing transplantation of ADSCs from aged donors might generate previously unrecognized risks.
Figure 1.
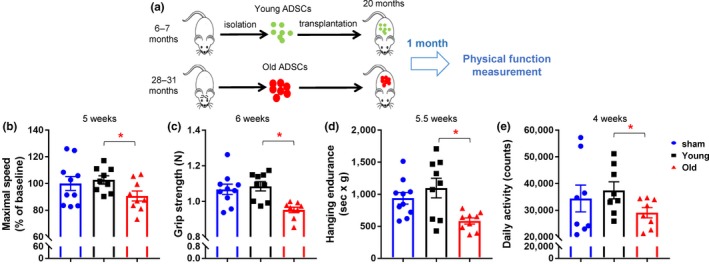
Adipose‐derived mesenchymal stem cell (ADSCs) from old donors impair physical function. (a) Experimental design. (b‐e) Quantification of maximal walking speed (relative to baseline) (b), grip strength (c), hanging endurance (d), daily activity (e) of 21‐month‐old male C57BL/6 mice 4–6 weeks after being injected with 1 × 106 ADSCs from old or young donors or no (sham) ADSCs. For b, c, d, n = 10 for sham, n = 9 for Young, n = 9 for old. For e, n = 8 for all groups. Results are mean ± SEM. *, p < .05; Student's t test
In a previous study, we demonstrated that transplanting a small number of in vitro‐generated senescent ADSCs (stable proliferative arrest caused by various stresses (Campisi & d'Adda di Fagagna, 2007) induces physical dysfunction in mice (Xu et al., 2018). To define a potential naturally occurring senescent cell‐like population, we conducted single‐cell transcriptome (SCT) analysis. We obtained high‐quality single‐cell transcriptomes (5,565 genes and 33,811 polyadenylated RNA transcripts detected per cell on average) from 3,604 and 1,876 ADSCs from young and old donors, respectively. Cdkn1a (p21) and Cdkn2a (p16) are two markers frequently associated with cellular senescence (Campisi & d'Adda di Fagagna, 2007), both of which were highly expressed in cells isolated from the old donors (Figure 2a). We manually set a cutoff of p21 expression level, which is a higher expression level than in 97% of cells from young donors. We considered cells to be p21 high if their p21 levels were higher than this cutoff. We found that 8.9% of cells from old donors were p21 high compared with 3% of cells in young donors (Figure 2a), both of which are similar to the percentages of senescent cells observed in ADSCs isolated from young and old human donors (Xu et al., 2015). We used p21 rather than p16 because the difference of p21 levels between young and old cells was more significant than p16 (Figure 2a,c) and p16 level positively correlates with p21 in p21 high cells (p < .001). In addition, we found that cells from old donors proliferated at a slower rate and contained more SA‐βgal+ and p21 high cells compared with the cells from young donors (Figure S2), which is consistent with an increased abundance of senescent cells among the cells isolated from the old donors. Notably, we documented the transcriptomic signatures of naturally occurring p21 high senescent‐like cells for the first time. By comparing all the distinctly expressed genes within this population using Ingenuity Pathway Analysis (IPA), we observed altered upstream regulators and pathways (Figure 2b) in these naturally occurring p21 high cells that are similar to those of in vitro‐generated “artificial” senescent cells, including Senescent Cell Anti‐Apoptotic Pathways (SCAPs; increased cell survival and decreased apoptosis) (Zhu et al., 2015), NF‐κB (Chien et al., 2011), IL6/JAK (Xu et al., 2015), mTOR (Herranz et al., 2015; Laberge et al., 2015), FOXO (Baar et al., 2017), and HMGB1 (Davalos et al., 2013) pathways. These findings suggest that p21 high cells might contribute to the physical dysfunction and validate the physiological relevance of the in vitro‐generated senescent cells that have been extensively used to study the biology of senescence and for drug screening. To gain more insight into deleterious effects of aging on the ADSCs, we compared transcriptomes of ADSCs isolated from young versus old donors using edgeR (Robinson, McCarthy, & Smyth, 2010). Among 15,565 detected genes, 3,787 were up‐regulated and 948 were down‐regulated (–log10 P value > 4) in the cells from old donors (Figure 2c and Supporting dataset). Several senescence‐associated secretory phenotype (SASP)‐related genes were up‐regulated in cells from old donors (Figure 2c). For validation, we measured secreted protein levels of these genes in conditioned media from young versus old ADSCs by multiplex protein analysis (Xu et al., 2015) . In most cases, the mRNA differences were consistent with differences in secreted proteins (Figure 2d). Using IPA, we detected a number of pathways that were distinctly expressed in ADSCs isolated from young versus old donors (Figure 2e), affording an opportunity to develop interventions that could reverse aged ADSCs into a more “youthful” state, potentially reducing harmful effects and improving regenerative efficacy. One limitation of our study is that we used allogenic transplantation (although the donors and the recipients were syngeneic), which might not fully represent autologous transplantation used in clinic. In future studies, more focused, in‐depth mechanistic insights into how ADSCs from old donors exert their adverse effects need to be obtained. Potential mechanisms include cellular senescence, inflammaging, and resulting sarcopenia. Also, SCT analysis using other senescence‐related markers such as p16, Il6, and Cxcl1 would be helpful for understanding the biology of senescence.
Figure 2.
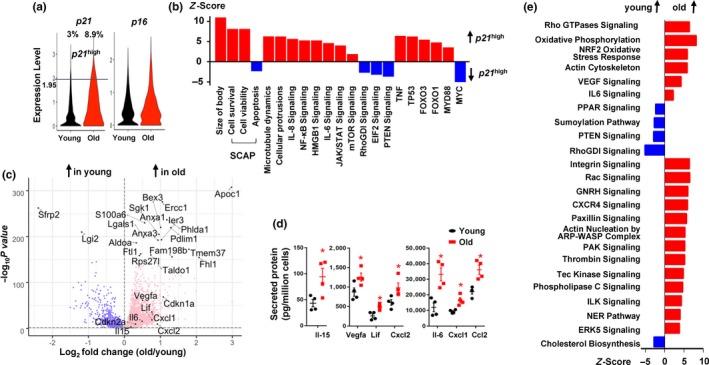
Single‐cell transcriptome analysis. (a) Violin plots for p21 and p16 expression levels in young and old cell populations. (b) IPA analysis of p21 high cells. Positive Z‐score indicates up‐regulation in p21 high cells. (c) Volcano plot of differentially expressed genes between cells from young and old donors. The top 20 most significantly altered genes, and several selected genes are highlighted. –log10 P value and log2(fold change) values are shown. (d) Secreted cytokine levels in conditioned media from ADSCs isolated from young and old donors. n = 4. Results are mean ± SEM. *, p < .05; Student's t test. (e) IPA analysis of canonical pathways enriched in adipose‐derived mesenchymal stem cell (ADSCs) from old versus young donors
In summary, we demonstrate that old ADSCs can impair physical function in older recipients, indicating a potential health concern regarding transplanting aged ADSCs. By SCT, we identified a naturally occurring p21 high senescent‐like cell population. Currently, there is limited knowledge about in vivo senescent cells due to the difficulty in isolating them because of their low numbers and lack of ideal markers. To our knowledge, we obtained the transcriptomic signatures of in vivo p21 high senescent‐like cells for the first time, which could assist in understanding the biology of senescence and drug development. In addition, we uncovered many signaling pathways that vary between ADSCs isolated from young versus old donors. Our study potentially begins new avenues of research to discover whether pharmacological interventions, such as senolytic drugs (Tchkonia & Kirkland, 2018) or anti‐inflammatory drugs, can prevent or reverse dysfunction caused by transplanting ADSCs or even organs from old donors (Lau, Kennedy, Kirkland, & Tullius, 2019) and improve clinical outcomes of transplantation for older patients.
CONFLICT OF INTERESTS
M.X., T.T., and J.L.K, have a financial interest related to this research. Patents on senolytic drugs (including PCT/US2016/041646, filed at the US Patent Office) are held by Mayo Clinic.
AUTHOR CONTRIBUTIONS
M.X. conceived and designed the study. M.X., B.W., V.P.C., C.L.I., C.G., and T.T. performed the mouse studies. B.W., Z.L., M.X., and P.R. contributed to SCT analysis. B.W., L.W., Y.Z., and D.W.R. contributed to the cellular experiments. G.A.K. and J.L.K. contributed to the manuscript preparation. M.X. wrote the manuscript with input from all coauthors. M.X. and J.L.K. oversaw all experimental design, data analysis, and manuscript preparation.
Supporting information
ACKNOWLEDGMENTS
This work was supported by an ISG seed grant from UConn Health (M.X.), the Irene Diamond Fund/AFAR Postdoctoral Transition Award in Aging (M.X.), Glenn Foundation for Medical Research and AFAR Grant for Junior Faculty (M.X.), the Connor Group, Robert J. and Theresa W. Ryan, and NIH grants AG13925, P01AG 62413‐1, and AG49182 (J.L.K).
Wang B, Liu Z, Chen VP, et al. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell. 2020;19:e13106 10.1111/acel.13106
Wang and Liu contributed equally.
Contributor Information
James L. Kirkland, Email: kirkland.james@mayo.edu.
Ming Xu, Email: mixu@uchc.edu.
DATA AVAILABILITY STATEMENT
Raw data are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.11295950.v1
REFERENCES
- Baar, M. P. , Brandt, R. M. C. , Putavet, D. A. , Klein, J. D. D. , Derks, K. W. J. , & Bourgeois, B. R. M. … de Keizer, P. L. J. (2017). Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell, 169, 132–147, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi, J. , & d'Adda di Fagagna, F. (2007). Cellular senescence: When bad things happen to good cells. Nature Reviews Molecular Cell Biology, 8, 729–740. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Chien, Y. , Scuoppo, C. , Wang, X. , Fang, X. , Balgley, B. , Bolden, J. E. , … Lowe, S. W. (2011). Control of the senescence‐associated secretory phenotype by NF‐kappaB promotes senescence and enhances chemosensitivity. Genes & Development, 25, 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos, A. R. , Kawahara, M. , Malhotra, G. K. , Schaum, N. , Huang, J. , Ved, U. , … Campisi, J. (2013). p53‐dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. Journal of Cell Biology, 201, 613–629. 10.1083/jcb.201206006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud, K. E. , Ewing, S. K. , Cawthon, P. M. , Fink, H. A. , Taylor, B. C. , Cauley, J. A. , … Osteoporotic Fractures in Men Research Group (2009). A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society, 57, 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen, C. M. , Walston, J. , Newman, A. B. , Hirsch, C. , Gottdiener, J. , … McBurnie, M. A. (2001). Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56, M146–156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Herranz, N. , Gallage, S. , Mellone, M. , Wuestefeld, T. , Klotz, S. , Hanley, C. J. , … Gil, J. (2015). mTOR regulates MAPKAPK2 translation to control the senescence‐associated secretory phenotype. Nature Cell Biology, 17, 1205–1217. 10.1038/ncb3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong, S. M. L. , Lee, M. , Kosaric, N. , Khong, D. M. , Dong, Y. , Hopfner, U. , … Gurtner, G. C. (2019). Single‐cell transcriptomics of human mesenchymal stem cells reveal age‐related cellular subpopulation depletion and impaired regenerative function. Stem Cells, 37, 240–246. 10.1002/stem.2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge, R.‐M. , Sun, Y. U. , Orjalo, A. V. , Patil, C. K. , Freund, A. , Zhou, L. , … Campisi, J. (2015). MTOR regulates the pro‐tumorigenic senescence‐associated secretory phenotype by promoting IL1A translation. Nature Cell Biology, 17, 1049–1061. 10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, A. , Kennedy, B. K. , Kirkland, J. L. , & Tullius, S. G. (2019). Mixing old and young: Enhancing rejuvenation and accelerating aging. The Journal of Clinical Investigation, 129, 4–11. 10.1172/JCI123946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Lei, H. , Dong, P. , Fu, X. , Yang, Z. , Yang, Y. , … Xiao, R. (2017). Adipose‐derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplantation, 26, 1505–1519. 10.1177/0963689717721221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M. D. , McCarthy, D. J. , & Smyth, G. K. (2010). edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26, 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia, T. , & Kirkland, J. L. (2018). Aging, cell senescence, and chronic disease: Emerging therapeutic strategies. JAMA, 320, 1319–1320. 10.1001/jama.2018.12440 [DOI] [PubMed] [Google Scholar]
- Xu, M. , Pirtskhalava, T. , Farr, J. N. , Weigand, B. M. , Palmer, A. K. , Weivoda, M. M. , … Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature Medicine, 24, 1246–1256. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Tchkonia, T. , Ding, H. , Ogrodnik, M. , Lubbers, E. R. , Pirtskhalava, T. , … Kirkland, J. L. (2015). JAK inhibition alleviates the cellular senescence‐associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America, 112, E6301–6310. 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, X. , Liao, C. , Liu, G. , Xu, Y. , Tan, J. , & Song, Z. (2016). Age‐related changes in the regenerative potential of adipose‐derived stem cells isolated from the prominent fat pads in human lower eyelids. PLoS One, 11, e0166590 10.1371/journal.pone.0166590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Tchkonia, T. , Pirtskhalava, T. , Gower, A. C. , Ding, H. , Giorgadze, N. , … Kirkland, J. L. (2015). The Achilles' heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell, 14, 644–658. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.11295950.v1


