Abstract
Background
Placebo response appears to be increasing in antidepressant, antipsychotic and various internal medicine trials. A similar trend has been reported for OCD during 1989-1999. Placebo response is generally considered as the extent to which placebo treatment is associated with core symptom improvement. In this analysis, we used Joinpoint regression to assess the time trend of both placebo response and placebo responder rates according to the year of publication with no time restriction in OCD drug trials.
Method
We included drug and/or psychotherapy trials vs. placebo from PubMed, Embase, CINAHL, and PsycINFO retrieved through the search (placebo OR sham) AND (obsessive* OR OCD). We included studies through investigator consensus. We then performed on data of included studies log-linear joinpoint segmented regression models using a p<0.05 cutoff.
Results
We included 113 studies from 112 published papers. Placebo mean annual response rates in OCD studies significantly increased from 1991 to 2017 with an annual percent change (APC) of 0.66%, while placebo mean annual responder rates also significantly increased from 2010 to 2017, with an APC of 5.45%. Drug mean annual response rates in OCD studies significantly increased from 1987 to 2012 with an APC of 0.72%, while the corresponding responder rates did not show statistically significant APC changes between 1984 and 2017.
Conclusion
We observed a tendency for placebo to increase both measures of response in OCD clinical drug trials through the years that tend to approximate the responses shown by drugs. Changes in the type of study (moving from classical head to head comparisons to add-on studies in treatment-resistant populations) and countries involved in experimentation may partially account for some portion of these results. It appears that placebo effects are becoming more elusive and out of control.
Keywords: Obsessive-compulsive disorder, placebo response, placebo effect, publication year
1. INTRODUCTION
A common belief lasting until the early nineties was that obsessive-compulsive disorder (OCD) did not respond to placebo [1]. However, this view rapidly changed as reliable scales developed to measure OCD symptoms.
There are two ways to measure the response of a patient or of a group of patients to a given treatment, drug or placebo, i.e., to consider improvement with respect to a baseline on a predetermined rating scale in terms of points or percentage, hence providing a measure of the percent response of a group, and to define some criteria for responsiveness and provide the percentage of patients who reached or surpassed a given threshold to qualify as treatment responders. A further specification of the latter is the remitter status, based on even stricter criteria. We will call the former “placebo (or drug) effect” to differentiate it from the ambiguous term “placebo (or drug) response”, and term the latter responder rate (to placebo or drug) so to hold the two concepts apart. In OCD, the most commonly used rating scale as a primary outcome measure is the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) [2, 3] and responses to treatments, or treatment effects, as we term them to avoid confusion with number of persons who are considered as treatment responders, are rated as percent variations of scores on the Y-BOCS from baseline at a given time-point. On the other hand, treatment responder rates are considered as the fraction of patients in the sample who are judged on the basis of established criteria, usually an at least 35% (or 25%, according to the study’s author choice) drop of Y-BOCS scores from baseline or 1 or 2 (very much or much improved, respectively) on the Clinical Global Impressions scale, improvement version [4].
There is evidence for the growth of placebo effect (and responder rates) in clinical trials across the years for depression [5] and, less consistently, bipolar disorder [6], although some biases may have influenced the conclusions of this second study [7]. Other psychiatric disorders have not been investigated for this year-of-publication effect specifically, but a multivariate meta-analysis carried-out by Ackerman and Greenland [8] found an increase in the effect of placebo through the years, confirming the impressions of others [9]. This could be due to the host of factors, like trends in the type of patient recruited (regarding illness duration, severity, and comorbidity), type of drug used (the side effect profile of a drug like clomipramine increases the likelihood of drug identification by both clinician and patient and may affect outcome), study site characteristics (that may reflect the characteristics of participating physicians and principal investigators and contribute to large across-sites differences which are usually disregarded in most reports), outcome measures employed, publication bias-file drawer effect, and last, but not least, the fact that the more effective the drug in a study, the more effective the placebo [10]. In fact, a publication year effect has been shown for OCD treatment across the years [11]. However, Ackerman and Greenland [8], who used meta-regression to evaluate placebo-controlled drug trials in OCD, did not include in their remarkable paper, drug trials in paediatric populations did not consider surgical procedures versus sham surgery or psychotherapies versus sham psychological interventions, and failed to consider a considerable number of papers covering a quite long period of time. In fact, they analysed a restricted period of time of placebo-controlled drug trials of three SSRIs and clomipramine in a period spanning from 1989 to 1999 (i.e., after the introduction of the Yale-Brown Obsessive Compulsive Scale, Y-BOCS), with a consequent loss of more than ten years of literature.
Systematic investigations of treatment of OCD started at the dawn of the eighties, with the use of clomipramine [12, 13]; clomipramine [14] and imipramine [15, 16] dominated the scene during the mid-eighties, and it was only during the late eighties that SSRIs, primarily fluvoxamine, were introduced [17, 18]. The first two studies comparing sertraline to placebo appeared in 1990 and yielded contrasting results [19, 20]. The first published trials of fluoxetine versus placebo appeared in 1992 [21], but regarded data that started being gathered in the late eighties [22-24], therefore simultaneous with, if not preceding those of sertraline. It is noteworthy that fluoxetine had received extensive open trials in OCD since 1985 [25], whereas for sertraline, the two aforementioned double-blind studies were the first studies of sertraline in OCD to be published [19, 20]. This publication lag may create a bias in the attempt to clarify whether the effect of a given treatment increased or decreased with time. Unfortunately, most studies do not provide the period during which they were conducted and render it difficult to correct for this bias. Hence, we will consider publication data as a factor despite realising that it does not exactly reflect the period during which the study has been carried out.
Our aim was to extend Ackerman and Greenland’s [8] observations beyond 2002, including also studies that did not use drugs, but other methods as well that could ensure double-blinding. We did not use the same method, but rather a JoinPoint regression.
2. METHODS
We carried-out a general search in the PubMed-MedLine-Index Medicus and Embase-Excerpta Medica and PsycLit-Psychological Abstracts databases using the following strategy: (placebo OR sham) AND (obsessive* OR OCD) with no time, language or any other restriction, but animal studies were subsequently excluded. We did not use the PubMed “Animal studies” function to exclude such studies, because such function often produces unreliable results. Papers were individually searched for adherence to our inclusion criteria. Retrieved relevant papers, comprising reviews and meta-analyses, were searched in their reference lists for providing additional papers with adequate research data. Final inclusion criteria for data analysis comprised: single or double-blind design, clearly stated assessment of response (responder rate or percent response on rating scales), sufficient time of treatment administration for the expected response to be observed, absence or adequate addressing of confounders that could render response not attributable to specific treatments. Specifically, the second part of cross-over studies was discarded if switching from one treatment to another had not a sufficient treatment-free wash-out period to avoid carry-over effects; survival studies were excluded when tapering-off of a combined therapy involved a drug vs. placebo when another drug or treatment was continued; add-ons were given not to patients stabilised on a given drug, but on drug-free or drug naïve populations. In this first report on placebo response in OCD we concentrate on double-blind studies using drugs, hence we excluded studies with psychotherapy or comparing mechanical devices with sham, like deep brain stimulation, electroconvulsive therapy, or deep/repetitive transcranial magnetic stimulation, provided they did not have a placebo and a drug arm. Excluded were also studies focusing on other than clinical outcomes, those carried-out on mixed populations (e.g., OCD and Tourette’s) without providing results specific for each subpopulation, and those with designs such that a placebo effect could not be calculated.
2.1. Statistical Analysis
We analysed temporal trends of placebo and drug response rates/responder proportions through log-linear joinpoint segmented regression models, which identify points corresponding to statistically significant changes over time in the linear slope of the occurring trend [26]. We used annual mean rates of placebo and drug effect (mean placebo and drug-induced improvements and mean placebo/drug responders) as independent variable assuming constant variance (homoscedasticity) without log transformation. We applied a grid search method to fit regression functions with unknown joinpoints assuming a Poisson distribution and uncorrelated errors. We set the minimum/maximum joinpoint number from 0 to 2, and used a permutation test with overall significance level set at p<0.05 and number of randomly permuted datasets of 4,499 to select the best fit. In the final model, each joinpoint indicates a trend change. We reported the estimated annual percent change (APC) for segmented analysis. Joinpoint analyses were performed using the Joinpoint Regression Program, version 3.5, from the US National Cancer Institute (https://surveillance.cancer.gov/joinpoint/).
3. RESULTS
Our PubMed-MedLine-Index Medicus and Embase-Excerpta Medica, CINAHL, and PsycLit-Psychological Abstracts searches yielded 886, 2932, 164, and 994 papers, respectively, as of April 24, 2018. The total output of our research is shown in Fig. (1), which shows also the reasons for exclusion. All studies were searched for possible further includible papers. Included were 113 studies from 112 papers,
Fig. (1).
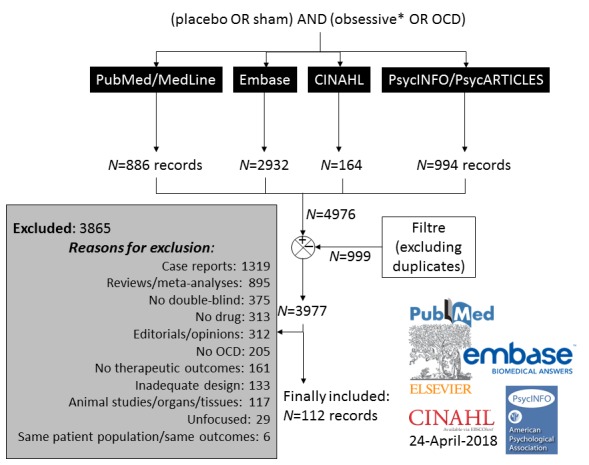
Algorithm of literature search and article selection.
which met criteria for inclusion. The results of the included studies [13-15, 17, 18, 20-24, 28-128] are summarised in Table 1.
Table 1. Placebo and drug responsiveness in OCD studies.
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thorén et al. [13] | 1980 | Karolinska Universitetssjukhuset, Karolinska Institutet, Stockholm, Sweden | 24; 8 placebo vs. 8 clomi vs. 8 nortriptyline | Clomi vs. nortriptyline vs. placebo × 5 wk | ↓ from BL of OCD Scale derived from the CPRS Responders classified according to clinicians’ ratings |
7% OCD Scale | Not given | Clomi 50→150 mg/day Nortriptyline 50→150 mg/day |
42%; 21% OCD Scale |
54.54% including open clomi trial; Nortriptyline: Not given | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Flament et al. [27] | 1985 | Child Psychiatry Branch, National Institute of Mental Health, Bethesda, MD, USA | 19 paediatric patients; 10 received placebo first, 9 clomi | 11-wk Randomised Cross-over (at wk 5) Trial; Clomi vs. placebo | ↓O-C Rating Scale; no response criteria | 10.37% OCR Scale | Chlomi 50→max200 mg/day | 32.6% OCR Scale | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mavissakalian et al. [14] | 1985 | Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA | 12; 5 placebo vs. 7 clomi | RCT clomi vs. placebo ×12 wk | Response: ↓Obsessive-Compulsive Neurotic Scale; Responders: Clinician Rating for OCD (5 points; score 1 on at least 3 points) | 43% O-C Neurotic Scale | 15.53% Clinician Rating for OCD | Clomi 50→max300 mg/day | 35.13% O-C Neurotic Scale | 43% Clinician Rating for OCD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foa et al. [15] | 1987 | Department of Psychiatry, Medical College of Pennsylvania, Philadelphia, PA, USA | 37, 18 (7 scoring high [≥21] on the BDI) placebo vs. 19 (9 scoring high on the BDI) imipramine |
DB RCT imipramine vs. placebo × 6 wk | Effect: ↓MOCI from BL | 3.67% MOCI |
Imipramine 25→max250 mg/day | 7.42% MOCI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perse et al. [17] | 1987 | Anxiety Disorders Center, Department of Psychiatry, University of Wisconsin, Madison, WI, USA | 20 randomised to placebo vs. flexible fluvoxamine doses; 4 drop-outs for various reasons left 16 patients available for the analysis; 8 placebo first, 8 fluvoxamine first | Fluvoxamine vs. placebo DB cross-over trial; placebo run-in × 2 wk → DB fluvoxamine vs. placebo × 8 wk → × 2 wk placebo → × 8 wk DB cross-over | ↓from BL of Maudsley OC Inventory scores for treatment effect; Responder rate: Clinician’s judgement for response | -7% (Maudsley scores ↑) | 19% | Fluvoxamine 50 → max 150 mg/day | 14.54% | 81% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pato et al. [28] | 1988 | Laboratory of Clinical Science, NIMH, Bethesda, MD, USA | 21 responders to clomi ×4 months; 71.42% had significant depression; all received placebo substitution | Clomi substituted by placebo in four days, then placebo ×7 wk; survival study (relapse rates) | Substitution effect: ↑Y-BOCS; ↑CPRS O-C; ↑NIMH-OC; Response: lack of relapse/recurrence; evidence of recurrence: development of “significant symptoms” | 43.7% ↑Y-BOCS; 52.78% ↑CPRS O-C; 43.87% NIMH-OC | 9.52% (OCD symptoms) | Clomi tapering-off (four days, half the dosage first, than all drug substituted with placebo) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodman et al. [18] | 1989 | Department of Psychiatry, Yale University School of Medicine and Connecticut Mental Health Center, Ribicoff Research Facilities, New Haven, CT, USA | 42; 21 placebo vs. 21 fluvoxamine | Multicentre (2 sites), DB RCT fluvoxamine vs. placebo ×6-8 wk | Effect: ↓Y-BOCS score from BL; Response: CGIi 1-2 (used different scale, but rating is similar) | 0% Y-BOCS | 0% | Fluvoxamine started at 50 mg/day → max 300 mg/day | 22.4% Y-BOCS | 42.8% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jenike et al. [29] | 1989 | Harvard Medical School and Massachusetts General Hospital, Boston, MA, USA | 27: 14 placebo vs. 13 clomi | Multicentre (? sites), DB clomi vs. placebo × 10 wk |
↓Y-BOCS scores from BL as treatment effect; no criterion for response, but stratification according to percentages of ↓Y-BOCS | 8.46% ↓Y-BOCS | 20-39% ↓Y-BOCS: 21.42%; ≥40% ↓Y-BOCS: 0% | Clomi 50→200 mg/day →max300 mg/day | 36.93% ↓Y-BOCS | 20-39% ↓Y-BOCS: 76.92%; ≥40% ↓Y-BOCS: 46.15% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chouinard et al. [20] | 1990 | McGill University, Montréal, Québec, Canada | 87, 44 placebo vs. 43 sertraline | Multicentre RCT DB sertraline, flexible doses vs. placebo × 10 wk | Treatment effect: % ↓Y-BOCS score from BL; % ↓NIMH-OC Scale score from BL; Response: CGIi 1-2 | 6.55% Y-BOCS; 6.13% NIMH-OC | 11.364% CGIi | Sertraline 50→200 mg/day | 16.2% Y-BOCS; 15.2% NIMH-OC | 25.581% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Greist et al. [30] | 1990 | Department of Psychiatry, University of Wisconsin, Madison, WI, USA | 31; 16 placebo vs. 15 clomi | Single site part of multicentre (21 sites) study, DB parallel RCT of clomi fixed→ flexible dose vs. placebo ×10 wk | Effect: ↓NIMH-OC and ↓Y-BOCS scores from BL; Response: Patient and Physician Global Evaluation (conceptually similar to CGIi 1-2) | 6.97% ↓Y-BOCS; 8.64% ↓NIMH-OC |
5% “CGIi” | Clomi 25→200 max 300 mg/day | 34.883% ↓Y-BOCS 27.912% ↓NIMH-OC | 45% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jenike et al. [31] | 1990 | Harvard Medical School, Boston, and the OCD Clinic and Research Unit, the Department of Molecular Biology, and the Inpatient Psychiatric Service, Massachusetts General Hospital, Boston, MA, USA | 38; 20 placebo vs. 18 fluvoxamine | DB fluvoxamine vs. placebo × 10 wk | ↓Y-BOCS scores from BL as treatment effect; no criterion for response | 4% ↓Y-BOCS | Fluvoxamine 50→max300 mg/day | 16.81% ↓Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Katz et al. [32] | 1990 | New Drug Development Department-CNS Section, Pharmaceuticals Division, CIBA-GEIGY Corporation, Summit, NJ, USA | 266 with Ham-D <17; 129 placebo vs. 134 clomi | Multicentre (12 sites), DB RCT clomi vs. placebo in pts. with DSM-III OCD and Ham-D <17 or Ham-D 17-21 (subsequently excluded from the analysis due to small sample size) ×10 wk →responders (CGIi ≤3) with treatment confirmed (placebo, N=12, clomi, N=101) ×42 wk | Effect: ↓NIMH-OC scores from BL; response: CGIi 1-2 |
1.96% at week 10; 21.56% at week 52; 24.71% at end-point; NIMH-OC |
9.3% 10 wk; 2.4% wk 52; 16.7% at end-point (wk 70) CGIi | Clomi 20→min 100, target 250, max 300 mg/day | 34.69% at week 10; 55.1% at week 52; 46.94% at end-point, NIMH-OC | 75.37% 10 weeks; 61.2% week 52; 72.2% at end-point (week 70) CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mavissakalian et al. [33] | 1990 | Department of Psychiatry, Ohio State University, Columbus, OH, USA | 25; 12 placebo vs. 13 clomi | DB RCT clomi vs. placebo ×10 wk | Effect: ↓CY-BOCS from BL; response: much improved |
1.11% Y-BOCS | 0% Much improved | Clomi 50→2-weeks 200 mg/day →flexible | 54.19% Y-BOCS | 35% Much improved | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Montgomery et al. [34] | 1990 | Imperial College London, St Mary's Hospital Medical School, London UK | 14; 7 placebo first, 7 clomi first | 4-wk DB clomi vs. placebo→cross-over ×4 wk | Criteria for effect or response not specified; assessment with 6-item obsessional scale extracted from CPRS and MADRS | 5.2% (↓from BL of CPRS Obs Scale scores) |
Clomi 75 mg/day | 64.5% (↓from BL of CPRS Obs Scale scores) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| McDougle et al. [35] | 1991 | Connecticut Mental Health Center, New Haven, CT, USA | 30 DR (failure to reach ≥35% ↓ from BL Y-BOCS scores and CGIi>2 and clinician consensus after fluvoxamine × 8 wk) at flexible doses up to 300 mg/day; 9 placebo vs. 11 add-on lithium [study 1]; 5 vs. 5 add-on lithium | DB RCT to add-on lithium vs. placebo ×2 wk to unchanged fluvoxamine (up to 300 mg/day) [study 1, 20 patients]; 4 wk blind placebo followed by 4 wk open lithium [study 2, 10 inpatients] | Y-BOCS (response: ↓≥35% drop from BL and Y-BOCS<16); CGIi 1-2; clinician consensus: all three=marked, 2 of 3=partial, <2 no response) |
Study 1: -5.09% (Y-BOCS scores↑) Study 2: 5.83% (Y-BOCS) |
Studies 1 & 2: 0% marked and partial (Y-BOCS, CGIi, clinician cons.) | Clomi 200-300 mg/day + lithium 900 mg/day →0.5-1.2 mEq in plasma | Study 1: 10.52% (Y-BOCS); Study 2: -10.66% (Y-BOCS scores↑) |
Study 1: 9.091% marked, 9.091% partial (Y-BOCS, CGIi, clinician’s consensus); Study 2: 0% marked and partial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Clomi Collaborative Study Group [36] | 1991 | Clinical Neuropharmacology, Glaxo Inc, Research Triangle Park, NC, USA | 520; 120 placebo vs. 118 clomi (study 1) 129 placebo vs. 134 clomi (study 2) |
Multicentre (9 centres study 1; 12 centres, study 2), clomi vs. placebo × 10 wk; two identical studies | Effect: ↓CY-BOCS from BL; Response ↓≥35% from BL |
3% Y-BOCS study 1 5% Y-BOCS study 2 |
7.5% Y-BOCS study 1 7% Y-BOCS study 2 |
Clomi 25→max300 mg/day | 38% Y-BOCS study 1 44% Y-BOCS study 2 |
51% Y-BOCS study 1 60% Y-BOCS study 2 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DeVeaugh-Geiss et al. [37] | 1992 | Clinical Neuropharmacology, Glaxo Inc, Research Triangle Park, NC, USA | 60 children or adolescents 10-17 years, 29 placebo vs. 31 clomi | Multicentre (5 sites) DB RCT clomi vs. placebo × 8 wk | Effect: ↓Y-BOCS from BL; Response: CGIi 1-2 |
8% Y-BOCS | 17% CGIi | Clomi 25→100, max 200 mg/day | 37% Y-BOCS | 59.8% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mallya et al. [38] | 1992 | McLean Hospital, Harvard Medical School, Belmont, MA, USA | 28 with HAM-D<20; 14 placebo vs. 14 fluvoxamine | RCT fluvoxamine vs. placebo ×10 wk | Effect: ↓Y-BOCS from BL; Response: ≥35%↓Y-BOCS from BL |
5% ↓Y-BOCS |
7% Y-BOCS | Fluvoxamine 50→300 mg/day | 33% ↓Y-BOCS | 43% Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pigott et al. [39] | 1992 | NIH Clinical Center, Rockville Pike, Bethesda, MD, USA | 17 drug-free; 6 placebo vs. 11 trazodone | DB RCT trazodone vs. placebo ×10 wk | Effect: ↓Y-BOCS scores from BL | 10.3% Y-BOCS | Trazodone 50→ 300 mg/day | 12.98%; Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Riddle et al. [21] | 1992 | Yale Child Study Center, Yale University, New Haven, CT, USA | 14 (8.5-16 years); 6 placebo first vs. 7 fluoxetine first | Cross-over randomised study fluoxetine vs. placebo ×20 wk (first 8 wk to one and 12 to the other in random order) | Effect: ↓CY-BOCS scores from BL | 26.72% (week 8) CY-BOCS | Fluoxetine 20 mg/day | 44.03% (week 8) CY-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stein et al. [40] | 1992 | Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York State Psychiatric Institute, New York, NY, USA | 35 with ≥56 on SRON and SROC; comorbidity with depression only if OCD primary and dominating; 21 placebo vs. 14 clomi | Multicentre (2 sites) DB RCT to clomi vs. placebo ×10 wk | Effect: Score ↓OCS, SRON, SROC; Response: CGIi 1-2 | 12% OCS; 33.33% SRON; 31.63% SROC |
19% CGIi | clomi 25→100- 300 mg/day | 29.49% OCS; 40.49% SRON; 29.11% SROC |
50% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grady et al. [41] | 1993 | Department of Psychiatry, Duke, University Medical Center; Durham, NC, USA | 13 DR (fluoxetine) OCD 80 mg/day ×10 wks; order of administration not specified | DB cross-over to add-on buspirone vs. placebo ×8 wk to unchanged fluoxetine (80 mg/day) | Effect: ↓Y-BOCS score from BL; Response: Y-BOCS ↓≥25% from BL and other unusual criteria | -2.9% (↑Y-BOCS scores) | 0% (Y-BOCS) | Buspirone → 60 mg/day added on fluoxetine, 80 mg/die | 3.91% (Y-BOCS) | 7.7% (Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hoehn-Saric et al. [42] | 1993 | Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA | 21 with NIMH-OC≥9, Y-BOCS≥16; Ham-D≤21; 10 placebo vs. 11 clomi | DB CRT clomi vs. placebo ×10 wk | Effect: ↓NIMH-OC and ↓Y-BOCS scores from BL; Response: not investigated | 2.083% NIMH-OC; 5.714% Y-BOCS | Clomi 25→200 mg/day; min 100 max 300 mg/day | 31.521% NIMH-OC; 39.534% Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| McDougle et al. [43] | 1993 | Clinical Neuroscience Research Unit, Yale University School of Medicine, Connecticut Mental Health Center, New Haven, CT, USA | 33 DR (failure to reach ↓≥35% drop from BL Y-BOCS scores after fluvoxamine × 8 wk) at flexible doses up to 300 mg/day; 14 placebo vs. 19 add-on buspirone | RCT to add-on buspirone vs. placebo ×8 wk to unchanged fluvoxamine (up to 300 mg/day) | Y-BOCS (response: ↓≥35% from BL); CGIi 1-2; clinician consensus | 9.09% (Y-BOCS) | 14.29% (Y-BOCS, CGIi, clinician consensus) | Buspirone 15→60 mg/day added on fluvoxamine at the same dose of BL | 4.96% (Y-BOCS) | 10.52% (Y-BOCS, CGIi, clinician’s consensus) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Montgomery et al. [22] | 1993 | Imperial College London, Paterson Wing, St Mary's Hospital Medical School, London UK | 214; 56 placebo vs. 52 fluoxetine, 20 mg/day vs. 52 fluoxetine, 40 mg/day vs. 54 fluoxetine, 60 mg/day | Multicentre (13 sites) 8-wk DB fluoxetine 20, 40 or 60 mg vs. placebo | Treatment effect: ↓Y-BOCS scores Response: ≥↓25% from BL and CGIi 1-2 | 17.5% Y-BOCS | 26% | Fluoxetine 20, 40 and 60 mg/day |
21.6% 20.5% 28.6% Y-BOCS |
36% 48% 47% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| McDougle et al. [44] | 1994 | Department of Psychiatry, Yale University School of Medicine, Yale Child Study Center, New Haven, CT, USA | 34 (failure to reach ↓≥35% drop from BL Y-BOCS scores after fluvoxamine × 7 wk) with or without tics; 17 add-on placebo vs. 17 add-on haloperidol | Double-blind DR to fluvoxamine since 7 wk randomised ×4 wk to add-on haloperidol or placebo | Response: Y-BOCS ↓≥35% from BL and final Y-BOCS≤16; CGIi 1-2; and consensus of clinician. Two criteria met: partial responder; all three met: marked responder | 7.63% | 0% | Fluvoxamine up to 300 mg/day; dose unaltered during trial; add-on haloperidol 2→max10 mg/die (mean, 6.2 mg) | 27.17% | 65% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tollefson et al. [23] | 1994 | Psychopharmacology Division, Eli Lilly & Co., Indianapolis, Ind, USA | 355; 89 placebo vs. 87 fluoxetine 20 mg, vs. 89 fluoxetine 40 mg vs. 90 fluoxetine 60 mg | Multicentre (8 sites), DB fluoxetine at fixed doses vs. placebo × 13 wk | Y-BOCS ↓ from initial score for effect; Y-BOCS ↓≥35% for response |
-1.2% (mean Y-BOCS scores ↑) |
8.5% | Fluoxetine 20 mg/day 40 mg/day 60 mg day |
15.24% 20.26% 25.99% |
32.1% 32.4% 35.1% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tollefson et al.* [24] | 1994 | Psychopharmacology Division, Lilly Research Laboratories, Eli Lilly & Co., Indianapolis, Indiana, USA | 76 responders of the previous study with their treatment confirmed; 6 placebo vs. 23 fluoxetine 20 mg, vs. 21 fluoxetine 40 mg vs. 26 fluoxetine 60 mg | Multicentre (8 sites), DB fluoxetine at fixed doses vs. placebo × 6 months (extension) | Y-BOCS ↓ from initial score for effect; Y-BOCS ↓≥35% for response |
-6.25% (mean Y-BOCS scores ↑), but at BL patients were not OCD |
50% Y-BOCS | Fluoxetine 20 mg/day 40 mg/day 60 mg day |
10.09% 11.32% 23.91% Y-BOCS, but at BL patients were not OCD |
47% Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Greist et al. [45] | 1995 | Dean Foundation for Health, Research, and Education, Madison, Wisconsin, USA | 325; 84 placebo vs. 241 sertraline: 80 sertraline 50 mg/day; 81 sertraline 100 mg/day; 80 sertraline 200 mg/day | Multicentre (11 sites), DB parallel comparison of three dosages of sertraline vs. placebo ×12 wk | Effect: ↓Y-BOCS score from BL; Response: Y-BOCS ↓≥25% from BL CGIi 1-2 |
14.6% ↓Y-BOCS |
30% CGIi |
Sertraline, pooled 50, 100, and 200 mg/day |
23.4% 27.37% 22.83% 33.02% ↓Y-BOCS |
38.9% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fux et al. [46] | 1996 | Ministry of Health Mental Health Center, Faculty of Health Sciences, Ben Gunion University of the Negev, Beersheva, Israel | 13 partial or complete non-responders to clomi/SSRIs or with severe side effects; 6 placebo first, 7 inositol first | Cross-over RCT inositol vs. placebo ×6 wk; cross-over ×6 wk without washout | Response criteria not specifically stated, but data given so that we extrapolated % ↓Y-BOCS scores for response and calculated % of patients who achieved ↓Y-BOCS≥35% (or 25%) from BL for response | 1.55% Y-BOCS; Placebo first at 6 wk: 12.4% | 0% ↓Y-BOCS≥ 35% Placebo first at 6 wk: 0% ↓Y-BOCS≥ 25% |
Oral inositol 18 g/day | 21.104% Y-BOCS; Inositol first at 6 wk: 25.01% | 30.77% ↓Y-BOCS≥ 35%; Inositol first at 6 wk: 28.57% (57.14% ↓Y-BOCS≥ 25%) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodman et al. [47] | 1996 | Department of Psychiatry, University of Florida College of Medicine, Gainsville, FL, USA | 156; 78 placebo vs. 78 fluvoxamine | Multicentre (4 sites), DB RCT fluvoxamine vs. placebo ×10 wk | Effect: ↓Y-BOCS score from BL; ↓NIMH-OC score from BL; Response: CGIi 1-2 |
5.42% Y-BOCS; 3.89% NIMH-OC |
8.6% | Fluvoxamine started at 50 mg/day → max 300 mg/day | 21.14% Y-BOCS; 19.101% NIMH-OC |
43.4% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nakajima et al. [48] | 1996 | Department of Neuropsychiatry, Kyoto Perfectural University of Medicine, Kawaramachi-Hirokoji, Kamigyoku, Kyoto, Japan | 27 fluvoxamine 300 mg/die vs. 34 fluvoxamine 150 mg/die vs. 33 placebo | DSM-III-R OCD patients assigned to one of three groups: high-dose fluvoxamine vs. low-dose fluvoxamine vs. placebo × 8 wk | Y-BOCS scores (%↓Y-BOCS from BL; response ↓≥35% from BL) | 7.25% | 36.36% | Fluvoxamine 150 mg/day 300 mg/day |
28.33%; 29.96% |
79%; 77.8% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zohar et al. [49] | 1996 | Chaim Sheba Medical Centre, Tel-Hashomer, Israel, and Sackler Medical School, Tel Aviv University, Israel | 399; 99 placebo vs. 201 paroxetine vs. 99 clomi | Multicentre-multinational (? sites), DB paroxetine vs. clomi vs. placebo × 12 wk | Y-BOCS ↓≥25% | 18.87% | 35.4% | Paroxetine 20-60 mg/day; Clomi 50-250 mg/day | 30.77%; 32% |
55.1%; 55.3% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jenike et al. [50] | 1997 | Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA | 64; 21 placebo vs. 20 phenelzine vs. 23 fluoxetine | 10-wk fluoxetine vs. phenelzine vs. placebo | ↓Y-BOCS scores from BL as treatment effect | 1% | Phenelzine 60 mg/day, Fluoxetine 80 mg/day |
9.4%; 14.7% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lindsay et al. [51] | 1997 | Clinical Research Unit for Anxiety Disorders, School of Psychiatry, University of New South Wales at St Vincent’s Hospital, Sydney, NSW, Australia | 18 (13 drug-free, 5 unresponsive to clomi or fluoxetine); 9 placebo (anxiety management) vs. 9 CBT | Parallel assignment to CBT (ERP) vs. anxiety management assumed as the placebo (5 1-h sessions per wk × 3 wk); control group also did homework | Treatment effect: ↓Y-BOCS from BL; ↓MOCI from BL; ↓PADUA scores from BL; Response criteria: not provided | -5.93% (↑Y-BOCS); 12.002% MOCI; 15.201% PADUA | CBT in 15 sessions divided in three weeks, about 1 hour per session, for both ERP and anxiety management (hyperventilation and respiration and relaxation control with no cognitive restructuring and exercise at home) | 61.67% Y-BOCS; 43.76% MOCI; 50.99% PADUA |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ushijima et al. [52] | 1997 | Department of Psychiatry, Jikei University School of Medicine, Nishishinbashi, Minato-ku, Tokyo, Japan | 33 150 mg/die fluvoxamine vs. 29 300 mg/die fluvoxamine vs. 42 placebo | Multicentre, DB fluoxetine at fixed doses vs. placebo × 8 wk | Y-BOCS ↓ from initial score for effect; Y-BOCS ↓≥35% for response |
11.66% (mean Y-BOCS ↓) | 38.1% Y-BOCS | Fluvoxamine 150 mg/day 300 mg/day |
23.9% 24.05% (mean Y-BOCS ↓) |
55.17% 51.51% Y-BOCS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fallon et al. [53] | 1998 | Department of Psychiatry, Columbia University, Division of the New York State Psychiatric Institute, New York, NY, USA | 54, 29 DR placebo vs. 25 DR i.v. clomi | DR to oral clomi, randomised to i.v. clomi vs. placebo × 14 days | Effect: %↓Y-BOCS from BL; %↓CGIs from BL; %↓NIMH-OC from BL; response ↓≥25% from BL; CGIi 1-2 |
3.3% Y-BOCS; 0% CGIs; 0% NIMH-OC | 0% CGI; 0% Y-BOCS | Intravenous (i.v.) Clomi 250 mg/day | 11.8% Y-BOCS; 10.1% CGIs; 9.565% NIMH-OC |
20.7% CGIi 21.4% Y-BOCS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Li, J. et al. [54] | 1998 | Mental Health Center of Sichuan province, Mianyang, China | 42; 12 placebo vs. 15 clomi vs. 15 paroxetine | DB CCMD-2 OCD paroxetine 20→80 mg/day vs. 50→300 mg/day vs. placebo ×4 wk | Effect: ↓Y-BOCS from BL; unclear response criteria | 10.06% | 20-80 mg/day paroxetine, 50-300 mg/day clomi |
51.32% 48.71% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| March et al. [55] | 1998 | Departments of Psychiatry and Psychology, Duke University Medical Center, Durham, NC, USA | 189 children and adolescents; 95 placebo vs. 92 sertraline | Multicentre (12 sites), RCT Sertraline flexible doses vs. placebo ×12 wk | Effect: ↓CY-BOCS from BL; Response: ≥25%↓CY-BOCS from BL |
15.31% ↓CY-BOCS | 37% ≥25%↓CY-BOCS | Sertraline 25-50→max200 mg/day | 29.05% ↓CY-BOCS |
53% ≥25%↓CY-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kronig et al. [56] | 1999 | Department of Psychiatry, Hillside Hospital of LIJMC, Glen Oaks, New York, NY, USA | 167; 81 placebo vs. 86 sertraline | Multicentre (10 sites), Double-blind sertraline 50-200 mg/die randomised vs. PLC ×12 wk | CGIi 1-2 (response), ↓Y-BOCS from BL; ↓NIMH from BL (primary outcome) | 17.16% Y-BOCS | 23.45% CGIi | Sertraline 50-200 mg/day | 38.08% | 41.8% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dannon et al. [57] | 2000 | Psychiatry Department, Chaim Sheba Medical Center, Tel Hashomer, Israel | 14 DR to 60 mg/day paroxetine ×≥15 wks (Y-BOCS ↓<25%), 6 placebo vs. 8 pindolol |
DB RCT of DR, ×6 wk to add-on pindolol or placebo | Unresponsiveness: Y-BOCS ↓<25% from BL; Effect of treatment: ↓Y-BOCS scores from BL |
7.69% | Paroxetine 60 mg/day; add-on pindolol 7.5 mg/die | 25.69% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| McDougle et al. [58] | 2000 | Department of Psychiatry, Section of Child and Adolescent Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA | 36 SSRI-refractory; 16 placebo vs. 20 add-on risperidone | Double-blind refractory to clomi, fluvoxamine, sertraline, fluoxetine, and paroxetine (failure to reach ↓≥35% drop from BL Y-BOCS scores), randomised ×6 wk to add-on risperidone or placebo | Response: Y-BOCS ↓≥35% from BL and final Y-BOCS≤16; CGIi 1-2; and consensus of clinician. Two criteria met: partial responder; all three met: marked responder | 9.42% | 0 | Clomi, fluvoxamine, sertraline, fluoxetine, or paroxetine at fixed dose; add-on risperidone 1-6 mg/die (mean, 2.2 mg) | 31.8% | 50% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Geller et al. [59] | 2001 | Pediatric OCD Clinic, McLean Hospital, Belmont, MA, USA |
103 with ≥4 on the CGIs and ≥16 on the CY-BOCS (7-18 years); 32 placebo vs. 71 fluoxetine | Multicentre (21 sites), 13-wk, DB RCT 2:1. Fluoxetine vs. placebo | CY-BOCS scores (response ↓≥40% from BL) | 19.7% | 25% | Fluoxetine, mean 24.6 mg/day | 38.7% | 49% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Montgomery et al. [60] | 2001 | Imperial College of Science, Technology and Medicine, London, UK | 401; 101 placebo vs. 102 20 mg citalopram, vs. 98 40 mg citalopram, vs. 100 60 mg citalopram | Multicentre (53 sites), multinational (12 countries); randomisation to citalopram vs. placebo ×12 wk | Treatment effect: ↓Y-BOCS scores Response: ≥↓25% from BL | 22.05% | 36.6% | Citalopram 20 mg/day 40 mg/day or 60 mg/day |
33.47% 34.23% 40.15% |
57.4%; 52%; 65%; |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Riddle et al. [61] | 2001 | Division of Child and Adolescent Psychiatry, Johns Hopkins Hospital, Baltimore, MD, USA | 120 (8-17 years); 63 placebo vs. 57 fluvoxamine | Multicentre (17 sites) DB RCT fluoxetine vs. placebo × 10 wk | Effect: ↓CY-BOCS scores from BL; Response: CY-BOCS ↓≥25% from BL | 13.63% CY-BOCS | 26% | Fluvoxamine 25→ 50-max 200 mg/day | 24.79% CY-BOCS | 42.1% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Romano et al. [62] | 2001 | Lilly Research Laboratories, Lilly Corporate Center, Indianapolis, IN, USA | 71 responders (out of 130) to 20-wk 20, 40 or 60 mg fluoxetine with ↓≥25% drop from BL; 35 placebo vs. 36 fluoxetine | Multicentre (11 sites), randomisation to fluoxetine as before vs. placebo × 52 wk |
No relapse | 62% | Fluoxetine 20, 40 or 60 mg/day | 82.5%; only 60 mg/die fluoxetine different from placebo | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atmaca et al. [63] | 2002 | Fırat Üniversitesi Hastanesi, Fırat Tıp Merkezi, Psikiyatri Anabilim Dalı, Elazig, Turkey | 27 DR (one 3-month trial of 25→300 mg/day clomi or 50→300 mg/day fluvoxamine or 20→80 mg/day fluoxetine yielded CGIi ≥3 and Y-BOCS ≥18 and clinician consensus) to SSRIs or clomi ×2 months), 13 placebo vs. 14 DR to quetiapine add-on | Single-blind randomisation of DR patients with stabilised SSRI or clomi to add-on quetiapine or placebo × 8 wk | Treatment effect: ↓Y-BOCS score from BL; ↓CGIs from BL; response: significant improvement, ≥60% ↓Y-BOCS score from BL; partial improvement, ≥30-59% ↓Y-BOCS score from BL plus clinician consensus | 10.08% (↓Y-BOCS); 15.88% (↓CGIs) |
0% | Stable SSRI or clomi; add-on quetiapine flexible, i.e., 50 mg/day → ↑25 mg/day when Y-BOCS did not ↓ by 2 from previous evaluation |
44.4% (↓Y-BOCS); 47.161% (↓CGIs) |
71.4% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Greist et al. [64] | 2002 | Healthcare Technology Systems, University of Wisconsin, Madison, WI, USA | 218 (age 15-80), Y-BOCS≥16, not comorbid with psychoses and Tourette’s; 75 placebo (relaxation) vs.74 computer-guided vs. 69 therapist-guided | Multicentre (8 sites), parallel comparison of three psychotherapies, computer-assisted CBT, therapist-conducted CBT vs. relaxation assumed as the placebo × 10 wk (therapists not raters) | Effect: ↓Self-rated Y-BOCS score from BL; Response: Y-BOCS ↓≥25% from BL CGIi 1-2; PGIi 1-2 | 6.6% ↓Self-rated Y-BOCS Relaxation, 1-h/day ×10 weeks |
15% PGIi; 14% CGIi | Computer-guided CBT, 9 steps: 1-3 education and assessment; 4-9 self ERP ×1 h or more; Clinician-guided CBT, one 1-hour weekly session ×11 weeks ERP + homework |
22.77% 30.16% ↓Self-rated Y-BOCS |
38% PGIi; 38% CGIi 58% PGIi 60% CGIi |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Koran et al. [65] | 2002 | Department of Psychiatry, Stanford, CA, USA | 223 responders to 16 or 52-wk 50-200 mg/day sertraline with Y-BOCS ↓≥25% from BL and CGIi ≤3; 114 placebo vs. 109 sertraline | Multicentre (21 sites), DB randomisation to flexible sertraline or to placebo × 28 wk | No relapse, defined as either ↑Y-BOCS scores ≥5 from randomisation and total score of ≥20 and ↑CGIi score of ≥1; Insufficient clinical response | 76% | Sertraline flexible doses 50-200 mg/day | 91% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liebowitz et al. [66] | 2002 | New York State Psychiatric Institute and Department of Psychiatry at Columbia University, New York, NY, USA |
43 (children 6-18 years); 22 placebo vs. 21 fluoxetine (acute); 7 placebo vs. 11 fluoxetine (extension) | Multicentre (2 sites), RCT fluoxetine vs. placebo ×8 wk (acute phase) → responders extension ×8 wk | CGIi 1 or 2 (response), ↓CY-BOCS from BL (primary outcome) | 22.12% acute; 54.6% extension |
31.81% (CGIi) | Fluoxetine 60-80 mg/die | 34.62% acute; 74.36% extension | 57.14% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Geller et al. [67] | 2003 | Obsessive Compulsive Disorder Program and Pediatric Psychopharmacology Research Program, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA | 8-17 yr-old children who responded to 16-wk 10-60 mg/day paroxetine; 98 placebo vs. 95 paroxetine | Multicentre (? sites); paroxetine-responder children aged 8-17 randomised to paroxetine as before vs. placebo ×16 wk |
CY-BOCS (response ↓≥25% from BL and CGIi 1 or 2); No relapse; relapse defined as ↑CGI by 1 in two follow-up visits or ↑CGI by 2 at any time or CGI≥5 at any time |
56.1% | Paroxetine flexible doses (10-60 mg/die) | 65.3% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hollander et al. [68] | 2003 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 27, 10 placebo vs. 17 clonz | Multicentre (2 sites), DR + drug- naïve patients randomised 2:1 to clonz or placebo × 10 wk |
CGIi 1-2 | 2.6%Y-BOCS | 22% CGIi | Clonz 3-6 mg/day | 7% Y-BOCS | 6.2% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hollander et al. [69] | 2003 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 16 DR (CGIi≥3 after at least two trials of SSRIs, clomi or venlafaxine for 12 wks), 6 placebo vs. 10 add-on risperidone | DB RCT of DR patients to risperidone vs. placebo × 8 wk added on stable previous SSRI, venlafaxine or clomi | Effect: ↓Y-BOCS score from BL Response: Y-BOCS ↓≥25% and CGIs ↓≥2 points from BL |
4.53% Y-BOCS | 0% Y-BOCS and CGIi | Risperidone 0.5→max 3 mg/day add-on to minimum mg/day: 200 clomi, 60 fluoxetine, 150 fluvoxamine, 150 sertraline, 60 citalopram or 325 venlafaxine | 20.89% Y-BOCS | 40% Y-BOCS and CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hollander et al. [70] | 2003 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 89 placebo vs. 88 paroxetine, 20 mg/day, vs. 86 paroxetine, 40 mg/day, vs. 85 paroxetine, 60 mg/day | Multicentre (15 sites), paroxetine, three fixed doses with an about 1:1:1:1 randomisation DB vs. placebo × 12 wk | Effect: ↓Y-BOCS score from BL Response: Y-BOCS ↓≥25% and CGIs ↓≥2 points from BL |
13% Y-BOCS | Not provided | Paroxetine 20, 40, 60 mg/day |
16% 25% 29% Y-BOCS |
Not provided | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hollander et al. [70] | 2003 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 105 responders to paroxetine randomised to placebo (N=52) or to paroxetine (N=53) 20-60 mg/day | Multicentre (15 sites), Paroxetine responders to flexible paroxetine doses (20-60 mg/day) or placebo × 6 months | Non-relapse: relapse defined as return of Y-BOCS to BL values or ≥1↑CGI at any time-point | 41.2% Y-BOCS/ CGIi | Paroxetine 20, 40 or 60 mg/day | 62.3% Y-BOCS/ CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hollander et al. [71] | 2003 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 253; 126 placebo vs. 127 fluvoxamine controlled-release | Multicentre (5? sites) RCT Fluvoxamine controlled-release 100-300. placebo × 12 wk |
Effect: ↓Y-BOCS score from BL Response: CGIi 1 or 2 |
19.01% Y-BOCS | 23% CGIi | Fluvoxamine 100 → 100-300 mg/day | 34.56% Y-BOCS | 44% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bystritsky et al. [72] | 2004 | Department of Psychiatry and Biobehavioral Sciences, Anxiety Disorders Program, University of California at Los Angeles (UCLA) School of Medicine, Los Angeles, CA, USA | 26 DR (unchanged after two adequate antidepressant trials and a course of behavioural psychotherapy), 13 placebo vs. 13 risperidone | DR to clomi or SSRIs with dose unchanged randomised × 6 wk to add-on olanzapine or placebo | Effect: % ↓Y-BOCS; Response: Y-BOCS ↓≥25% from BL | -1.99% (↑Y-BOCS from BL) | 0% (↓≥25% Y-BOCS) | Full-dose clomi, fluoxetine, sertraline, paroxetine; add-on olanzapine 2.5 → 5-20 mg/day |
17.36% (↓Y-BOCS from BL) | 46% (↓≥25% Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Denys et al. [73] | 2004 | Rudolf Magnus Institute of Neuroscience (DD), Department of Psychiatry, University Medical Center Utrecht, Utrecht, The Netherlands | 20 DR to SSRIs to add-on placebo; 20 to add-on quetiapine | DR to SSRIs (failure to achieve ↓≥25% of Y-BOCS scores from BL) randomised to add-on quetiapine vs. placebo × 8 wk | Y-BOCS (response ↓≥35% drop from BL); CGIi 1-2 | 6.8% Y-BOCS; 7,5% CGIi | 10% CGIi | Various SSRIs at various doses plus quietapine (B) 200-300 mg/day | 31.9% Y-BOCS 27,5% CGIi |
40% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fux et al. [74] | 2004 | BeerSheva Mental Health Center, Ben Gurion University of the Negev, Beer-Sheva, Israel | 11 with unsatisfactory response to SSRI during last 2 months: 5 placebo add-on vs. 6 EPA add-on | Cross-over RCT, EPA vs. placebo added-on highest tolerated SSRI dose | Response criteria not specified; assessment performed with Y-BOCS | 32.3% | Various SSRIs at various doses; EPA 2g/day | 28.8% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Geller et al. [75] | 2004 | Pediatric OCD Clinic, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA | 203 (7-17 years); 105 placebo vs. 98 paroxetine | Multicentre (34 sites); paroxetine vs. placebo × 10 wk | Effect: ↓CY-BOCS score from BL; Response: ↓≥25% CY-BOCS from BL |
21.1% | 41.2% | Paroxetine 10-50 mg/die | 36% | 64.9% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kamijima et al. [76] | 2004 | Showa University School of Medicine, Shinagawa-ku, Tokyo, Japan | 188; 94 placebo vs. 94 paroxetine | Multicentre (? sites), DB paroxetine vs. placebo × 12 wk | Effect: ↓Y-BOCS scores from BL; response: ↓≥25% Y-BOCS from BL; CGIi 1-2 | 14.8% Y-BOCS | 23.7% CGIi | Paroxetine 20-50 mg/day | 33.4% Y-BOCS | 50% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shapira et al. [77] | 2004 | Department of Psychiatry, University of Florida, College of Medicine, Gainesville, FL, USA | 44 partial/non responders to fluoxetine, 22 placebo vs. 22 add-on olanzapine | DR (partial/non-responders, i.e., <↓25% Y-BOCS score from BL or <16 total Y-BOCS or symptomatic) to 8-wk double-blind fluoxetine randomised × 6 wk to add-on olanzapine or placebo | Treatment effect: % ↓Y-BOCS scores from BL; Response: Y-BOCS ↓≥25% from BL | 3.8% | 41% ↓≥25% from BL; (18% ↓≥35% Y-BOCS from BL) | Fluoxetine 40 mg/day; Olanzapine 5-10 mg/day | 5.1% | 41% ↓≥25% from BL; (23% ↓≥35% Y-BOCS from BL) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Pediatric OCD Treatment Study (POTS) Team [78] | 2004 | Department of Psychiatry, Duke University Medical College, Durham, NC, USA | 112 children and adolescents (7-17 yrs.) CY-BOCS≥16; 28 placebo vs. 28 sertraline vs. 28 CBT vs. 28 sertraline + CBT placebo | Multicentre (3 sites), balanced, masked RCT (biased by patient preference) of sertraline vs. CBT vs. sertraline + CBT vs. placebo × 12 wk | Effect: ↓CY-BOCS from BL; Response: CY-BOCS≥10 |
14.68% | 3.6% | Sertraline 25→max200 mg/day; 14 CBT sessions with psychoeducation, cognitive training mapping OCD target symptoms and ERP; Sertraline + CBT |
29.79% 46.15% 52.84% |
21.4% 39.3% 53.6% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carey et al. [79] | 2005 | MRC Research Unit on Anxiety Disorders, University of Stellenbosch, Cape Town, South Africa | 42 SSRI-resistant patients defined as not responding to at least two adequate trials (12 wks); 21 SSRI+placebo, 20 SSRI+ quetiapine | Multicentre RCT (5 sites); DR (CGI≥3 or ↓Y-BOCS ≤25% from before treatment) to SSRI randomised to add-on quetiapine vs. placebo × 6 wk | Treatment effect: % ↓Y-BOCS score from BL Response: ↓Y-BOCS ≥25% from BL and CGIi 1-2 |
26% Y-BOCS 22.6% CGIi |
47.6% | Various SSRIs at various doses; Add-on quetiapine, ≈200 mg/day |
26.9% Y-BOCS 21,1% CGIi |
40% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Erzegovesi et al. [80] | 2005 | Department of Neurosciences, San Raffaele Hospital, Vita-Salute San Raffaele University, Milan, Italy | 39 patients stabilised on fluvoxamine 150-300 mg/day; 19 placebo vs. 20 add-on risperidone; 20 DR (10 placebo; 10 risperidone) and 19 responders (9 placebo; 10 risperidone) | DR to fluvoxamine (failure to reach ↓≥35% drop from BL Y-BOCS score) and responders to open fluvoxamine randomised × 6 wk to add-on risperidone or placebo | Y-BOCS scores (response ↓≥35% drop from BL); (Responders and non-responders here refer to open fluvoxamine and not to the add-on) |
13.89%; Responders 27.63%; Non-responders: 6.82% Y-BOCS |
Non-resp 20% Y-BOCS | Fluvoxamine 150-300 mg/die; risperidone 0.5 mg/die | 18.24%; Responders, 3.82%; non-responders, 25.57% |
Non-responders, 50% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fineberg et al. [81] | 2005 | Department of Psychiatry, Queen Elizabeth II Hospital, Hertfordshire, Welwyn Garden City, Department of Psychology, University of Hertfordshire, Hatfield, UK | 21 DR (Y-BOCS ≥18 and ↓≥25% Y-BOCS after ≥12 wks SSRI at max tolerated dose); 10 placebo, 11 quetiapine add-on | Add-on of quetiapine vs. placebo × 16 wk on stable SSRI at max tolerated dose | Effect: %↓Y-BOCS from BL; response ↓≥25% from BL |
6% | 10% | Quetiapine 25→max 400 mg/die + SSRI (citalopram, sertraline, paroxetine) | 14% | 27.27% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foa et al. [82] | 2005 | Center for the Treatment and Study of Anxiety, University of Pennsylvania, Philadelphia, PA, USA | 122, 26 placebo vs. 36 clomi, vs. 29 ERP, vs. 31 clomi + ERP | Multicentre (3 centres), 12-wk RCT; Clomi vs. placebo vs. ERP vs. clomi + ERP | Effect: ↓Y-BOCS from BL Response: CGIi <3 |
11.2% Y-BOCS 6% CGIi |
8% | Clomi 200 mg/day ERP: 2h exposure sessions, 5 times a week + daily exposure and ritual prevention homework max 2h Combined Clomi + ERP |
30.7% Y-BOCS; 19.6% CGIi 55.28% Y-BOCS; 43.75% CGI 58.66% Y-BOCS; 40.82 CGIi |
41.67% 62.07% 67.74% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kobak et al. [83] | 2005 | Dean Foundation For Health, Research and Education, Middleton, WI, USA | 60 with Ham-D <16; 30 placebo vs. 30 St. John’s wort (Hypericum perforatum) | Multicentre (4 sites), DB CRT of flexible Hypericum or placebo × 12 wk | Effect: ↓Y-BOCS scores from BL; response: CGIi 1-2 | 20.665% Y-BOCS | 16.7% CGIi | Hypericum perforatum (Saint John’s wort) flexible doses 600→1800 mg/day | 23.306% Y-BOCS | 17.9% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Koran et al. [84] | 2005 | Department of Psychiatry and Behavioral Sciences, Stanford University Medical Center, Stanford, CA, USA | 23 DR with OCD since at least 3 years, with Y-BOCS ≥20 and poor response to at least two SSRI trials (≥8 wks at full dose); 32 placebo | Add-on to stable antidepressant therapy of morphine, lorazepam or placebo × 7 wk: 2 wk of each in random order; cross-over, but only first part considered (prior to cross-over) | Effect: ↓Y-BOCS score Response: Y-BOCS (response ↓≥25% from BL) |
7% | 0% | Morphine 30 mg/week 1 →15-45 mg/week adjustment Lorazepam 1 mg →0.5-2 mg/week |
27% 6% |
30.43% 17.4% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Li, X. et al. [85] | 2005 | Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, Birmingham, AL, USA | 13 DR to ≥12-wk SSRIs (symptomatic; ≥10 score on Y-BOCS items 1-5; ≥16 total Y-BOCS score); 5 placebo first, 5 haloperidol first, 3 risperidone first | DB cross-over of DR on stable antidepressant, randomised × 9 wk to add-on risperidone, haloperidol or placebo (1 wk placebo→2 wk placebo or haloperidol or risperidone→1 wk placebo→2 wk cross-over→ 1wk placebo→ 2 wk cross-over) | Effect: ↓Y-BOCS from BL; no criteria for response | 23.96% | ≥40 mg fluoxetine, ≥200 mg fluvoxamine, ≥100 mg sertraline; dose unaltered during trial; add-on haloperidol 2 mg/day risperidone 1 mg/day |
48.62% 36.29% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nakatani et al. [86] | 2005 | Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan | 28 DR; 8 placebo + autogenic training vs. 10 behaviour therapy + placebo vs. 10 fluvoxamine + autogenic training | DR patients assigned to one of three groups: behavioural therapy + placebo; fluvoxamine + autogenic training; autogenic training + placebo ×12 wk | Y-BOCS scores (response ↓≥35% from BL), CGIi 1-2; responsiveness to placebo was taken as the response of Y-BOCS scores and the CGIi criterion of the placebo + autogenic training group | 6.88% | 0% | Fluvoxamine 150-200 mg/day | 28.87% | 30% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Buchsbaum et al. [87] | 2006 | Mount Sinai School of Medicine, New York, NY, USA | 16 DR, 6 placebo vs. 10 risperidone | DR to clomi, fluvoxamine, sertraline, fluoxetine, paroxetine, citalopram, and venlafaxine randomised double-blind ×8 wk to add-on risperidone or placebo | Response: Y-BOCS ↓≥25% from BL; CGIi 1-2 | 4.53% | 0% (CGIi) 0% (Y-BOCS) | Actual minimum daily doses: 200 mg clomi, 60 fluoxetine, 150 fluvoxamine, 150 sertraline, 60 citalopram, 325 venlafaxine; add-on risperidone 0.5 → 3 mg/day |
20.89% | 40% (CGIi) 40% (Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| O’Connor et al. [88] | 2006 | Fernand-Seguin Research Centre, Louis-H. Lafontaine Hospital, Montreal, QC, Canada | 21 with Y-BOCS >16; 10 placebo vs. 11 fluvoxamine (first protocol); thereafter, 43 (comprising the first 21) received CBT vs. 10 CBT alone, vs. 12 CBT + stabilised on antidepressant | DB, random allocation to fluvoxamine vs. placebo ×5 months (first protocol); after conclusion or stabilisation (on or off-drug) of another population, all receive CBT; CBT to drug naïve patients, CBT + stabilised on drug, CBT to previous placebo group, CBT to previous fluvoxamine group × 5 months (20 sessions) (second protocol) | Treatment effect: ↓Y-BOCS scores Response: >↓35% Y-BOCS from BL | 6.96% Y-BOCS | 0% | Fluvoxamine 100 → max 300 mg/day; CBT – ERP 1 weekly session for a total of 20 over 5 months; CBT + fluvoxamine |
15.19% 53.33% (not used in analyses) 42.69% Y-BOCS (not used in analyses) |
9% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fineberg et al. [89] | 2007 | Postgraduate Medical School, University of Hertfordshire, Hatfield, UK | 320 responders to 16-wk 10 or 20 mg escitalopram with Y-BOCS ↓≥25% from BL; 157 placebo vs. 163 escitalopram 10 or 20 mg/day | Multicentre (62 sites), multi-national (14 countries), DB RCT escitalopram 10 vs. 20 vs. placebo × 24 wk | No relapse, defined as either an increase in Y-BOCS scores ≥5 from randomisation or as unsatisfactory treatment effect (lack of efficacy) judged by investigator; Responders: Y-BOCS ↓≥25% from BL |
48% no relapse | 72% Y-BOCS | Escitalopram fixed dose 10mg/die or 20mg/die | 77% no relapse | 90% Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stein et al. [90] | 2007 | University of Cape Town, Department of Psychiatry, Groote Schuur Hospital, Cape Town, South Africa | 458; 114 placebo vs. 113 escitalopram 10, 114 escitalopram 20, 117 paroxetine | Double-blind randomized fixed-dose escitalopram × 24 wk to paroxetine or placebo | Primary outcome: ↓Y-BOCS from BL at week 12; secondary: mean Y-BOCS change from BL at week 24; Remission: Y-BOCS ≤10 Response: CGIi 1 or 2; Y-BOCS ↓≥25% from BL |
wk12: 30.54% wk24: 38.51% Y-BOCS | wk12 38.5%; wk24 38% (CGIi); wk12 52%; wk24 50% (Y-BOCS) | Escitalopram 10 mg/die or Escitalopram 20 mg/die or Paroxetine 40 mg/die |
wk12: 42.97% wk24: 51.77% wk12: 45.64 wk24: 51.84% wk12: 42.75 wk24: 54.62% |
wk12 50% wk24 58% (CGIi) wk12 66% wk24 63% (Y-BOCS) wk12 56% wk24 58.5% (CGIi) wk12 70.5% wk24 70.5% (Y-BOCS) wk12 54% wk24 58% (CGIi) wk12 65% wk24 67% (CGIi) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amiaz et al. [91] | 2008 | Division of Psychiatry, Chaim Sheba Medical Center, Tel-Hashomer, Israel | 10 DR to SSRIs or clomi ×2 months; 5 placebo first, 5 naltrexone first | DB cross-over of DR patients to ≥2-month clomi or 2 SSRIs × 5 wk to add-on naltrexone or placebo, × 1 wk to add-on placebo only × 5 wk to cross-over add-on naltrexone or placebo | Treatment effect: ↓Y-BOCS score from BL; ↓CGIs from BL; no response criteria provided | 5.46% (↓Y-BOCS); 18.42% (↓CGIs) |
Stable SSRI or clomi; add-on naltrexone 50 → 100 mg/die |
-16.07% (↑Y-BOCS scores); -10.7% (↑CGIs scores) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kordon et al. [92] | 2008 | Department of Psychiatry and Psychotherapy, University of Luebeck, Luebeck, Germany | 40 DR; 20 placebo vs.20 add-on quetiapine | Multicentre (2 sites), DR to SSRI or clomi × 12 wk (failure to reach ↓≥25% drop from BL Y-BOCS scores) randomised to add-on quetiapine or placebo | Y-BOCS (response ↓≥35% drop from BL); CGIi 1-2 |
15.1% Y-BOCS | 30% CGIi | SSRI or clomi at fixed dose; Quietiapine 400-600 mg/day | 21.6% Y-BOCS | 22% CGIi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Greenberg et al. [93] | 2009 | New York University School of Medicine, Department of Psychiatry, New York, NY, USA | 13 DR (drugs or psychotherapy), with treatment stabilised ×≥12wks, 9 placebo vs. 5 glycine add-on | DB RCT 1:1 of DR to add-on glycine vs. placebo × 12 wk to unchanged regimen (drugs and/or psychotherapy) | Effect: ↓Y-BOCS score from BL; ↓NIMH-OC score from BL;↓CGIs score from BL; Response: Y-BOCS ↓≥35% from BL and CGIi 1-2 |
4.04% (Y-BOCS); 2.44% NIMH-OC; 2.36% (CGIs) |
0% (Y-BOCS plus CGIi) | Glycine powder dissolved in water plus flavour enhancer → 60 g/day added on stabilised treatment (drug and/or psychotherapy) | 24.59% (Y-BOCS); 22.22% NIMH-OC; 13.04% (CGIs) |
40% (Y-BOCS plus CGI) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sayyah et al. [94] | 2009 | Department of Psychiatry, Joondi Shapoor University of Medical Sciences, Ahwaz, Iran | 44 with Y-BOCS≥21; 20 placebo vs. 24 aqueous extract of Echium amoenum | DB, RCT of 500 mg aqueous extract of Echium amœnum × 6 wk | Effect: ↓Y-BOCS scores from BL Response: not provided |
11.31% Y-BOCS | 125 mg aqueous extract of Echium amoenum capsules: 1 morning; 1 afternoon, 2 night | 25.55% Y-BOCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mowla et al. [95] | 2010 | Department of Psychiatry, Bushehr University of Medical Sciences, Bushehr, Iran | 41 Y-BOCS≥18 to: -N=20 Topiramate; or -N=21 placebo; × 12 wks |
12-wk, double-blind, placebo-controlled, randomized trial of 200 mg/day topiramate vs. placebo | Treatment effects: ↓Y-BOCS from BL; response: Y-BOCS ↓≥25% from BL (after 12 weeks) | 2.4% (↓Y-BOCS) | 0% (Y-BOCS ↓≥25% from BL) | Topiramate (initially 25 mg/day, increased in 25-mg increments weekly to a target dose of 200 mg/day | 32% (↓Y-BOCS); | 60% (Y-BOCS ↓≥25% from BL) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storch et al. [96] | 2010 | Department of Pediatrics, Rothman Center for Neuropsychiatry, University of South Florida, St. Petersburg, FL, USA | 30 children and adolescents with OCD (Range 8-17 years); 15 placebo, 15 D-cycloSer | DB RCT of CBT + D-cycloSer vs. CBT + Placebo × 8 wk (10 sessions). 1:1 randomisation | Effect: % ↓ CGI-S, CYBOCS, and ADIS-CSR from BL. no criterion for response |
41% (↓CGI-S), 58% (↓ CY-BOCS), 53% (↓ADIS-CSR) | 25 or 50 mg of D-cycloSer (depending on patient weight) 1 h before psychotherapy, sessions 4-10 | 57%(↓CGI-S) 72% (↓ CYBOCS) 71% (↓ADIS-CSR) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sayyah et al. [97] | 2011 | Education Development Center (EDC), Jundishapur University of Medical Sciences, Ahwaz, Iran | 52 drug naïve OCD patients randomised to celecoxib (N=27) or placebo (N=25) | DB RCT of fluoxetine 20 mg/day + celecoxib 400 mg/day vs. fluoxetine 20 mg/day + placebo × 8 wk | Effect: % ↓Y-BOCS score from BL; no criterion for response | 46.7% (↓Y-BOCS) | Fluoxetine 20 mg/day + Celecoxib 400 mg/day | 66.2% (↓Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Muscatello et al. [98] | 2011 | Section of Psychiatry, Department of Neurosciences, Psychiatric and Anaesthesiological Sciences, Section of Pharmacology, Department of Clinical and Experimental Medicine and Pharmacology, IRCCS Centro Neurolesi “Bonino-Pulejo” University of Messina, Messina, Italy | 30 Y-BOCS≥18 to N=16 aripripazole (15 mg/day) N=14 Placebo |
16-wk, open-label. flexible-dose (up to 30 mg/day), pilot trial | Treatment effects: ↓Y-BOCS score from BL; partial response (pr): Y-BOCS ↓≥25% from BL; complete response (cr): Y-BOCS ↓≥35% from BL; remission (r) (Y-BOCS ≤16 after 16 weeks) | -2.54% (↓Y-BOCS) | 0% (pr); 0% (cr) |
Aripiprazole 15 mg/day added to SSRI | 28.5% (↓Y-BOCS); | 43.7% (pr); 25% (cr) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Berlin et al. [99] | 2011 | Department of Psychiatry, The Mount Sinai School of Medicine, New York, NY, USA | 36 OCD patients with Y-BOCS ≥ 18; 18 randomised to placebo and 18 to topiramate | DB RCT of add-on topiramate (up to 400 mg/d) over continuing SSRI vs. placebo plus SSRIs × 12 wk | Effect: % ↓Y-BOCS score from BL after 12 weeks. No response criteria | 16% (↓Y-BOCS from BL to 12 weeks) | Add-on topiramate titrated over 8 weeks up to 400 mg/day or maximum tolerated dose | 38% (↓Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pakseresht et al. [100] | 2011 | Jundishapur University of Medical Sciences, Ahwaz, Iran | 31 (18-60 y) to -N=15 extract of Valeriana Officinalis L. (765 mg/day) or -N=16 placebo (30 mg/day) × 8 wks (Y-BOCS≥21) |
8-wk double-blind, parallel-group, randomised trial | Treatment effect: ↓Y-BOCS from BL after 8 weeks; no response criteria provided | 23.3% (↓Y-BOCS); | Valeriana Root (Valeriana Officinalis L.) 750 mg/day in three divided doses | 43.3% (↓Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sayyah et al. [101] | 2012 | Jundishapur University of Medical Sciences, Ahwaz, Iran | 23 drug naïve OCD patients; add-on to fluoxetine, 12 ZnSO4, 11 placebo | DB RCT of 20 mg/day fluoxetine + 440 mg/day ZnSO4 vs. 20 mg/day fluoxetine + placebo × 8 wk | Effect: % ↓Y-BOCS score from BL. no criterion for response |
46.84% (↓Y-BOCS) | 20 mg/day Fluoxetine + 440 ZnSO4 mg/day | 54.54% (↓Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sayyah et al. [102] | 2012 | Imam General Hospital, Jundishapur University of Medical Sciences, Ahwaz, Iran | 32 adult outpatients: N=15 10 mg/day aripiprazole N=17 placebo |
12-wk, double-blind RCT | Treatment effects: ↓Y-BOCS score from BL; response: Y-BOCS ↓≥25% after 12 weeks | 17.6% (↓Y-BOCS); | 8.3% (Y-BOCS ↓≥25% after 12 weeks) | Aripiprazole 10 mg/day | 29.5% (↓Y-BOCS); | 53% (Y-BOCS ↓≥25% after 12 weeks) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Afshar et al. [103] | 2012 | Nour Hoospital, Psychosomatic Research Center, Department of Psychiatry, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran | 48 DR OCD patients (SSRI/clomi non-responders); 19 NAC, 20 placebo | DB RCT of add-on →2400 mg/day NAC vs. placebo ×12 wk | Effect: % ↓Y-BOCS score from BL. % ↓ CGI-S from BL Partial response: Y-BOCS ↓≥25% from BL; Response: Y-BOCS ↓≥35% from BL |
20.7% (↓Y-BOCS) 10.4% (↓CGIs) |
Response: 15%; No data on partial response | Initial dosage of 600 mg/d of NAC, which doubled weekly to a maximum dose of 2400 mg/d | 39.2% (↓Y-BOCS); 24.9% (↓CGIs) | Response: 52.6% No data on partial response given |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bruno et al. [104] | 2012 | Section of Psychiatry, Department of Neurosciences, Psychiatric and Anaesthesiological Sciences, University of Messina, Messina, Italy | 33 DR OCD patients (persistent obsessive-compulsive symptoms: despite adequate SSRI trial(s) → Y-BOCS ≥16); 17 lamotrigine, 16 placebo | DB RCT of add-on lamotrigine 100 mg/day vs placebo for 16 wk | Effect: % ↓Y-BOCS score from BL; Partial response: Y-BOCS ↓≥25% from BL; Response: Y-BOCS ↓≥35% from BL |
-1.2% (↓Y-BOCS) | Response: 0% | Add-on lamotrigine increased from 25 mg/day to 100 mg/day at week 4, in increments of 25 mg/week Maximum dose of 100 mg maintained until the end of the trial |
33.8% (↓Y-BOCS) | Partial response: 50% Complete response: 35% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Askari et al. [105] | 2012 | Psychiatric Research Center, Roozbeh Hospital, Tehran University of Medical Sciences, Tehran, Iran | 39 drug naïve OCD patients (Y-BOCS ≥21) randomised to placebo (N=20) or granisetron (N=19) | Multicentre DB RCT of fluvoxamine 100-200 mg/day + granisetron 2 mg/day vs. fluvoxamine 100 - 200 mg/day + placebo × 8 wk | Effect: % ↓Y-BOCS score from BL Partial response: Y-BOCS ↓≥25% from BL Response: Y-BOCS ↓≥35% from BL Remission: Y-BOCS ≤ 16 |
34.7% (↓Y-BOCS) | Partial response: 35%; Complete response: 35%; Remission: 35% | Fluvoxamine 100 mg/day × first 4 weeks, 200 mg/day × next 4 weeks + granisetron 2 mg/day | 59.1% (↓Y-BOCS) | Partial response: 100% Complete response: 100% Remission: 90% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ghaleiha et al. [106] | 2013 | Research Center for Behavioral Disorders and Substance Abuse, Hamadan University of Medical Sciences, Hamadan, Iran | 38 patients with diagnosis of OCD and Y-BOCS score ≥21 randomised to add-on placebo (N=19) or memantine (N=19) | DB RCT of fluvoxamine + memantine vs. fluvoxamine + placebo × 8 wk | Effect: % ↓Y-BOCS score from BL Partial response: Y-BOCS ↓≥25% from BL Response: Y-BOCS ↓≥35% from BL Remission: Y-BOCS ≤16 |
36.9% (↓Y-BOCS) | Partial or complete response: 32% Remission: 32% |
Memantine 10 mg/day for the first week of the trial, then 20 mg/day |
57,9% (↓Y-BOCS) | Partial or complete response: 100% Remission: 89% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storch et al. [107] | 2013 | Department of Pediatrics, University of South Florida, St. Petersburg, FL, USA |
47 children and adolescents with OCD (Range 7-17 years) randomised to RegSert (N=14), SloSert (N=17) or placebo (N=16) + CBT for all | DB RCT of sertraline at standard dosing + CBT or sertraline titrated slowly + CBT or placebo + CBT × 18 wk. Patients randomized in a 1:1:1 fashion. | Effect: % ↓CY-BOCS score from BL. Response: CY-BOCS ↓≥30% from BL Remission: CY-BOCS score <10 |
37.9% (↓Y-BOCS) | Response: 62.5% (CY-BOCS ↓≥30% from BL) Remission: 18.8% (CY-BOCS score below 10) |
RegSert: upward titration from 25 mg/day to 200 mg/day in 5 wk. SloSert: upward titration from 25 mg/day to 200 mg/day in 9 wk |
RegSert: 34.7% (↓Y-BOCS) SloSert: 35.5% (↓Y-BOCS) |
Response: 57.1% for RegSert + CBT; 64.7% for SloSert + CBT; Remission: 42.9% for RegSert + CBT; 23.5% for SloSert + CBT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Haghighi et al. [108] | 2013 | Research Center for Behavioral Disorders and Substances Abuse, Hamadan University of Medical Sciences, Hamadan, Iran | 29 inpatients with diagnosis of OCD and Y-BOCS score ≥21 despite treatment with SSRI or clomi to: memantine (N=14) or placebo (N=15) | DB RCT of add-on memantine 5–10 mg/day vs. placebo × 12 wk | Effect: % ↓Y-BOCS score from BL. % ↓ CGI-S from 4th week; Partial response: Y-BOCS ↓≥25% from BL; Response: Y-BOCS ↓≥35% from BL | 15.8% (↓Y-BOCS) 13.4% (↓CGIs) |
Partial or complete response: 26.6% | Add-on memantine 5–10 mg/day | 32.2% (↓Y-BOCS) 30.2% (↓CGIs) | Partial or complete response: 92.8% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storch et al. [109] | 2013 | Department of Pediatrics, Rothman Center for Neuropsychiatry, University of South Florida, St. Petersburg, FL, USA |
34 DR OCD patients (Y-BOCS≥ 19 despite at least 2 adequate SSRI trials) randomised to placebo (N=17) or paliperidone (N=17) | DB RCT of add-on Paliperidone (up to 9 mg/d) vs. placebo × 8 wk | Effect: % ↓Y-BOCS score from BL. %↓CGI-S from BL Response: Y-BOCS ↓≥35% from BL CGI-i = 1 - 2 |
15.7% (↓Y-BOCS) 18.7% (↓CGI-S) |
Response: 29% (Y-BOCS ↓≥35%) 18% (CGIi =1/2) |
Add-on Paliperidone starting from 3 mg/day and titrated up to 9 mg/day by week 6 unless not tolerated | 29.4% (↓Y-BOCS) 20.1% (↓CGI-S) | Response: 35% (Y-BOCS ↓≥35%) 35% (CGIi=1/2) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rodriguez et al. [110] | 2013 | New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University, College of Physicians and Surgeons, New York, NY, USA | 15 drug free OCD patients with Y-BOCS ≥ 16 who had failed at least one prior SSRI trial and/or CBT: 8 to ketamine and 7 to placebo | DB Crossover RCT of iv ketamine (0.5 mg/kg) vs. iv saline spaced at least 1-wk apart | Effect: ↓OCD-VAS Response: Y-BOCS ↓≥35% from BL | 7.2% (↓OCD-VAS) | Response: 0% | Intravenous infusion of ketamine (0.5 mg/kg) over 40 min | 45.4% (↓OCD-VAS) | Response: 50% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Simpson et al. [111] | 2013 | Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY, USA | 100 patients on SSRI with still clinically significant OCD (Y-BOCS ≥ 16); 97 patients to N=38 Risperidone (up to 4 mg/d) N=40 EX/RP (17 session, 2 wkly) N=19 placebo |
RCT comparing SSRI augmentation with either EX/RP therapy, risperidone (→ max 4.0 mg/d), or pill placebo × 8 wk in two centres | Effect: % ↓Y-BOCS score from BL Response: Y-BOCS ↓≥25% from BL |
10.81% (↓Y-BOCS) | Responders: 15% (Y-BOCS ↓≥25%) |
Add-on risperidone (up to 4 mg/day) EX/RP (17, 2×week, 90 min-sessions) |
EX/RP: 52.2% (↓Y-BOCS). Risperidone: 13.4% (↓Y-BOCS) | EX/RP response: 80% Risperidone response: 22.5% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Park et al. [112] | 2014 | Department of Psychology, University of South Florida, Tampa, FL, USA |
30 children and adolescents with OCD (CY-BOCS≥16) stable on psychotropic medication × ≥ 12 wks: 15 to D-cycloSer; 15 to placebo | DB RCT of ERP + D-cycloSer (25-50 mg) after last 7 sessions vs. ERP + placebo × 10 wk | Effect: %↑ Homework compliance (rated with a 7-point Likert scale ranging from 0 (“did not complete any assigned homework”) to 6 (“completed all homework and made efforts above and beyond assignments”); no criterion for response | 4.7% (↑ homework compliance) | Exposure and response prevention therapy (ERP) + D-cycloSer (25-50 mg depending on weight) after last 7 session | -6% (↑ homework compliance) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grant et al. [113] | 2014 | Pediatrics and Developmental Neuroscience Branch, NIMH, National Institutes of Health, Bethesda, MD, USA | 60 treatment-resistant children and adolescents (7-17 years, CY-BOCS≥20); 30 to riluzole, 30 to placebo | DB RCT of add-on riluzole (up to 100 mg/day) vs. placebo × 12 wk | Effect: % ↓CY-BOCS score from BL. %↓CGI-S from BL %↑ CGAS from BL Response: CY-BOCS ↓≥30% from BL |
22.9% (↓CY-BOCS) 10.7% (↓CGI-S) 18,8% (↑CGAS) |
Response: 18% | Add-on riluzole starting from 10 mg/day and increased daily up to 100 mg/day | 20.1% (↓CY-BOCS) 9.8% (↓CGI-S) 12.8% (↑ CGAS) |
Response: 16% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mataix-Cols et al. [114] | 2014 | King’s College London, Institute of Psychiatry, London, UK | 27 children and adolescents with OCD (CY-BOCS≥16); 13 to D-cycloSer, 14 to placebo | DB RCT of Exposure and response prevention therapy (ERP) + D-cycloSer 50 mg after each session vs. ERP + placebo × 17 wk | Effect: % ↓CY-BOCS score from BL. Response: CY-BOCS ↓≥35% from BL Remission: CY-BOCS score ≤ 10 |
59.3% (↓CY-BOCS) | Response: 64.2% (CY-BOCS ↓≥35% from BL); Remission: 42.8% (CY-BOCS ≤10) | Exposure and response prevention therapy (ERP) + D-cycloSer 50 mg after each session | 60% (↓CY-BOCS) | Response: 61.5% (CY-BOCS ↓≥35% from BL); Remission: 53.8% (CY-BOCS score ≤10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Afshar et al. [115] | 2014 | Isfahan Psychosomatic Research Center, Isfahan University of Medical Sciences, Isfahan, Iran | 31 Y-BOCS≥16 to -N=16 Topiramate (mean dose: 137.5 mg/day) -N=15 Placebo Add-on on current SSRIs |
12-wk, double-blind, placebo-controlled | Treatment effects: ↓Y-BOCS score from BL; response (r): Y-BOCS ↓≥25% from BL after 12 weeks | 8.33% (↓Y-BOCS) after 12 weeks | 14.28% (Y-BOCS ↓≥25% from BL after 12 weeks) | Topiramate (range 100-200, mean dose: 137.5 mg/day), initial dose of 25 mg/day increased by 25 mg weekly to a maximum 200 mg/day | 19.81% (↓Y-BOCS) after 12 weeks | 53.84% (Y-BOCS ↓≥25% from BL to 12 weeks) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sarris et al. [116] | 2015 | Department of Psychiatry, The Melbourne Clinic, The University of Melbourne, Melbourne, VIC, Australia | 34 to: N=18 3g/day NAC or N=16 Placebo (Y-BOCS≥16) |
16-wk, double-blind, placebo-controlled, randomised trial | Treatment effects: ↓Y-BOCS score from BL; response: Y-BOCS ↓≥35% after 16 weeks | 19.5% (↓Y-BOCS); | 27% (Y-BOCS ↓≥35% from BL) | NAC (1.5 g q 12 h) | 21% (↓Y-BOCS) | 20% (Y-BOCS ↓≥35% from BL) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pittenger et al. [117] | 2015 | Departments of Psychiatry and Psychology, Yale Child Study Center, Interdepartmental Neuroscience Program, Yale University School of Medicine, New Haven, CT, USA | 40 DR OCD patients (SSRI/Clomi non-responders): 20 to riluzole, 18 to placebo | DB RCT of add-on riluzole (50 mg/day) vs. placebo × 12 wk | Effect: % ↓Y-BOCS score from BL; Partial response: Y-BOCS ↓≥25% from BL; Response: Y-BOCS ↓≥35% from BL | 11% (↓Y-BOCS) | Partial or complete response: 11% | Add-on riluzole 50 mg/day after 2-week placebo lead-in phase | 15% (↓Y-BOCS) | Partial or complete response: 26,3% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jahanbakhsh et al. [118] | 2016 | Pharmaceutical Research Center, Department of Clinical Pharmacy, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran | 30 OCD patients currently treated with SSRI; 15 to W. somnifera, 15 to placebo | DB RCT of add-on W. somnifera extract 120 mg/day vs. placebo × 6 wk | Effect: % ↓Y-BOCS score from BL no criterion for response |
11.1% (↓Y-BOCS) | W. somnifera extract 120 mg/day | 46.2% (↓Y-BOCS) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Paydary et al. [119] | 2016 | Psychiatric Research Center, Roozbeh Psychiatric Hospital, Tehran University of Medical Sciences, Tehran, Iran | 44 drug naïve OCD patients (Y-BOCS ≥21); 22 to NAC add-on, 22 to placebo add-on | Multicentre DB RCT of fluvoxamine 200 mg/day + NAC 2000 mg/day vs. fluvoxamine 200 mg/day + placebo × 10 wk | Effect: % ↓Y-BOCS score from BL Response: Y-BOCS ↓≥35% from BL Remission: Y-BOCS ≤ 16 |
30.3 (↓Y-BOCS) | Response: 22.7% | Fluvoxamine 100 mg/day for × first 4 weeks → 200 mg/day; + NAC 1000 mg/day × 1st week → 2000 mg/day | 39% (↓Y-BOCS) | Response: 54.5% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Khalkhali et al. [120] | 2016 | Department of Psychiatry, Guilan University of Medical Sciences, Rasht, Iran | 53 DR to: -N=26 Lamotrigine -N=27 placebo Y-BOCS≥21 with stable SRIs dosages × at least 3 months before the study |
12-wk, double blind, placebo-controlled RCT of 100 mg/day add-on lamotrigine to SSRIs in DR OCD-patients | Treatment effects: ↓Y-BOCS score from BL; response: Y-BOCS ↓>25% from BL (at week 12) |
17.7% (↓Y-BOCS); | Adjunctive lamotrigine -doses (fixed 100 mg/day) (25→100 mg/day during the first 4 weeks, increments of 25 mg/week) | 32.5% (↓Y-BOCS); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rutrick et al. [121] | 2016 | Adams Clinical Trials, Watertown, MA, USA | 50 DR to N=24 placebo or 7N=26 Mavoglurant (augmentation to SSRIs) (Y-BOCS≥16) |
Multicentre, randomised, DB, add-on phase 2 study × 16 wk | Treatment effects: ↓Y-BOCS from BL; Response (Y-BOCS ↓≥25% from BL) at week 17 | 32.06% (↓Y-BOCS) | 50% (Y-BOCS ↓≥25% from BL) | (4 week up-titration period → 12-weeks fixed-dose 200 mg Mavoglurant q 12 h → 3-weeks tapering-off |
26.59% (↓Y-BOCS) | 34.5% (Y-BOCS ↓≥25% from BL) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Feng et al. [122] | 2016 | Department of Psychiatry, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, China | 360 OCD patients with Y-BOCS ≥ 16 randomised to GROUP A (N=120) GROUP B (N=120) or GROUP C (N=120) | SB RCT of TEAS with CBT + clomi (GROUP A) vs. TEAS with CBT + placebo (GROUP B) vs. sham TEAS with CBT + clomi (GROUP C) × 12 wk | Effect: % ↓Y-BOCS score from BL, Response: Y-BOCS ↓≥35% from BL, Remission: CY-BOCS score ≤ 12 | GROUP B: 45% (↓Y-BOCS) GROUP C: 38.2% (↓Y-BOCS) |
GROUP B: Response: 82.5% Remission: 22.5% GROUP C: Response: 67.5% Remission: 9.2% | GROUP A: Transcutaneous electrical acupoint stimulation combined with CBT + clomi | GROUP A: 59.3% (↓Y-BOCS) | GROUP A: Response: 89.2% Remission: 29.2% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| de Leeuw et al. [123] | 2017 | Altrecht Academic Anxiety Center, Utrecht, The Netherlands | 39 patients with OCD randomised to D-cycloSer (N=19) or placebo (N=20) in add-on | DB RCT of Exposure and response prevention therapy (ERP) + D-cycloSer 125 mg/day vs. ERP + placebo × 8 wk | Effect: % ↓Y-BOCS score from BL. Partial response: Y-BOCS ↓≥25% from BL Response: Y-BOCS ↓≥30% from BL |
17% (↓Y-BOCS) | Partial or complete response: 35% | Exposure and response prevention therapy (ERP) + D-cycloSer 125 mg/day | 25.2% (↓Y-BOCS) | Partial or complete response: 90% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asnaani et al. [124] | 2017 | Department of Psychiatry, Center for the Treatment and Study of Anxiety, University of Pennsylvania, Philadelphia, PA, USA | 100 patients on SRI with Y-BOCS ≥ 16 randomised to risperidone (N=40), EX/PR (N=40) or placebo (N=20) | RCT comparing EX/RP therapy + SSRI, risperidone (0.25 mg/day × 3 days, 0.5 mg/day × 4 days, → ↑0.5 mg/wk to max4.0 mg/day +SSRI vs. pill placebo + SSRI × 8 wk | Effect: ↑ QLESQ-SF score from BL; ↓SAS-SR and ↓SDS score from BL. No response criteria | 11.6% (↑ QLESQ-SF); 5.5% (↓ SAS-SR); 19% (↓ SDS) | SSRI augmentation with either EX/RP therapy (17 twice-weekly, 90-min sessions) or risperidone (0.25 mg/d × 3 days, 0.5 mg/d × 4 days, → ↑0.5 mg/week to max 4.0 mg/day) | EX/RP therapy: 20.6% (↑ QLESQ-SF); 14.8% (↓ SAS-SR); 51.5% (↓ SDS) Risperidone: 5.7% (↑ QLESQ-SF) 4.5% (↓ SAS-SR) 20.6% (↓ SDS) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author(s) | Year |
Affiliation/
Country |
Population (N) | Design | Outcome Measures (Response Criteria) | Placebo Effect (%) | Placebo Responders (%) | Drug(s) or other Treatment than Placebo | Drug Effect (%) | Drug Responders (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ahmadpanah et al.[125] | 2017 | Hamadan University of Medical Sciences (HUMS), Behavioral Disorders and Substance Abuse Research Center, Hamadan, Iran | 43 DR OCD patients (not responding to SSRI or Clomi) to buprenorphine (N=23) or placebo (N=20) | DB RCT of DR patients with stabilised SSRI or clomi to add-on buprenorphine or placebo × 12 wk | Effect: % ↓Y-BOCS score from BL. Partial response: Y-BOCS ↓≥25% from BL Response: Y-BOCS ↓≥35% from BL |
7% (↓Y-BOCS) | Partial or complete response: 25% | Buprenorphine tablets (2-4 g; sublingual) daily | 17,5% (↓Y-BOCS) | Partial or complete response: 39% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modarresi et al. [126] | 2017 | Research Center for Rational Use of Drugs, Tehran University of Medical Sciences, Tehran, Iran | 32 DR OCD patients (failing at least 3 adequate trials of antidepressant, including clomi) and Y-BOCS ≥ 24 randomised to memantine (N=16) or placebo (N=16) | DB RCT of add-on memantine 20 mg/day vs. placebo × 12 wk | Effect: % ↓Y-BOCS score from BL; Response: Y-BOCS ↓≥35% from BL | -0.2% (↓Y-BOCS) | Response: 0% | Add-on memantine 20 mg/day | 31% (↓Y-BOCS) | Response: 73.3% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costa et al. [127] | 2017 | Department & Institute of Psychiatry, University of São Paulo Medical School, São Paulo-SP, Brazil | 40 DR OCD patients (not responding to SSRI or clomi) randomised to NAC (N=18) or placebo (N=22) | DB RCT of add-on NAC (up to 3000 mg/day) vs. placebo × 16 wk | Effect: % ↓Y-BOCS score from BL Response: Y-BOCS ↓≥25% from BL |
12.1% (↓Y-BOCS) | Response: 26.3% | Add-on NAC, 1,200 mg/day for the first week, 2,400 mg in the second week, 3,000 mg/day from the third week on | 16.8% (↓Y-BOCS) | Response: 37.5% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arabzadeh et al. [128] | 2017 | Psychiatric Research Center, Roozbeh Hospital, Tehran University of Medical Sciences, Tehran, Iran | 44 drug naïve OCD patients (Y-BOCS ≥21) randomised to L-carnosine (N=22) or placebo (N=22) | DB RCT of fluvoxamine 100 - 200 mg/day + L‐carnosine 1000 mg/day vs. fluvoxamine 100 - 200 mg/day + placebo × 10 wk | Effect: % ↓Y-BOCS score from BL Partial response: Y-BOCS ↓≥25% from BL; Response: Y-BOCS ↓≥35% from BL Remission: Y-BOCS ≤ 14 |
24.3 (↓Y-BOCS) | Partial: 45.5%; Complete: 9.1%; Remission: 9.1% | Fluvoxamine 100 mg/day × 4 wk→200 mg/day × 6 wk + L‐carnosine 1000 mg/day | 35.3 (↓Y-BOCS) | Partial: 45.5% Complete: 36.4% Remission: 27.3% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abbreviations used: BDI=Beck Depression Inventory; BDZs=benzodiazepines; BL=baseline; BT= Behaviour Therapy; CBT=Cognitive-Behavioural Therapy; CGI=Clinical Global Impressions scale-i=improvement, s=severity; clomi=clomipramine; clonz=clonazepam; CCMD-2=Chinese Criteria of Mental Disorders, 2nd edition; CPRS=Comprehensive Psychopathological Rating Scale, Karolinska institutet; DB=double-blind; DLPFC=dorsolateral prefrontal cortex; DR=drug-resistant/treatment-resistant; D-cycloSer=D-cycloserine; EPA=eicosapentaenoic acid; ERP=exposure and ritual (response) prevention; GAF=Global Assessment of Functioning; GVC=gamma ventral capsulotomy; halo=haloperidol; Ham-D=Hamilton Depression Rating Scale; MOCI=Maudsley Obsessional Compulsive Inventory; MT=motor threshold; NAC=N-Acetylcysteine; NIMH= National Institute of Mental Health Global Obsessive-Compulsive Scale; OCS= Obsessive Compulsive Scale; PGI=Patient’s Global Impressions; RegSert=Standard sertraline dose; SloSert=slowly titrated sertraline; SROC=Self-Rating Obsessive-Compulsive Personality Inventory; SRON=Self-Rating Obsessional Neurotic Scale; wk=weeks; Y-BOCS=Yale-Brown Obsessive-Compulsive Scale; YGTSS, Yale Global Tick Severity Scale; ZnSO4=zinc sulfate; ↑=increase; ↓=reduction; *Patients were not DSM-IV OCD at inclusion, because they had responded to previous trial; hence, actual baseline was factitious and the differences observed were modest for all groups, meaning that treatment continued to work.
Joinpoint regression analyses of the period 1979-2017 showed that placebo mean annual effect rates in OCD studies significantly increased (APC value significantly differing from zero to α = 0.05 level) from 1991 to 2017 with an APC of 0.66% (p=0.04) following a period without statistically significant APC changes Fig. (2). Placebo mean annual responder rates also significantly increased from 2010 to 2017 with an APC of 5.45% (p=0.02) following a period without statistically significant APC changes Fig. (3). Drug mean annual effect rates in OCD studies significantly increased from 1987 to 2012 with an APC of 0.72% (p=0.04) between two periods without statistically significant APC changes Fig. (4). Drug mean annual responder rates did not
Fig. (2).
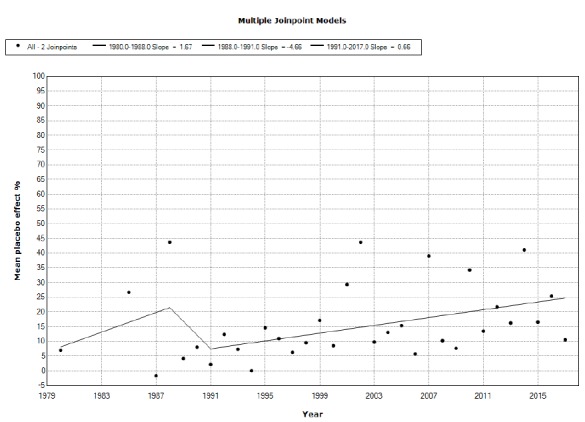
Multiple Join-Point model of the time (year of publication) trend of placebo effect in OCD double-blind trials.
Fig. (3).
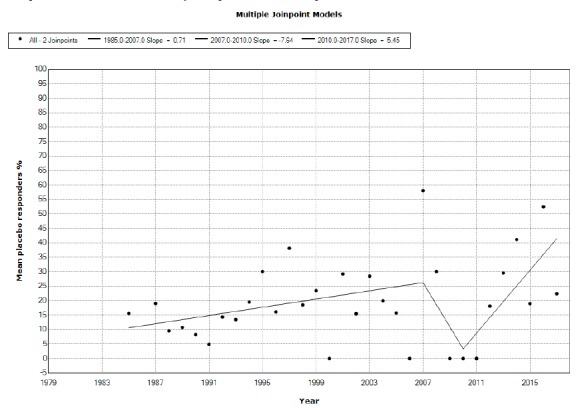
Multiple Join-Point model of the time (year of publication) trend of placebo responder rates in OCD double-blind trials.
Fig. (4).
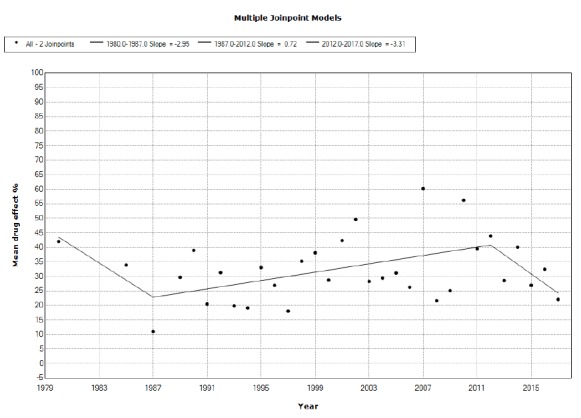
Multiple Join-Point model of the time (year of publication) trend of drug effect in OCD double-blind trials.
show statistically significant APC changes between 1984 and 2017 Fig. (5).
Fig. (5).
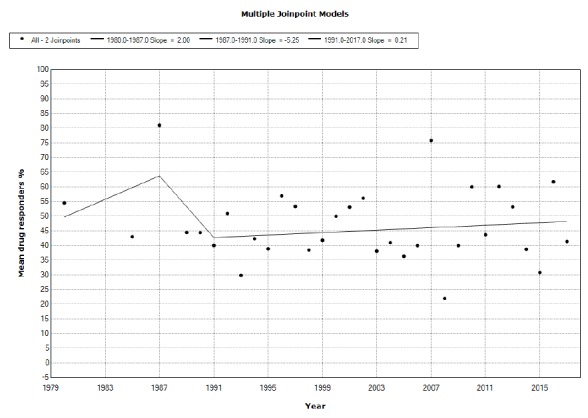
Multiple Join-Point model of the time (year of publication) trend of drug responder rates in OCD double-blind trials.
4. DISCUSSION
In this study, using Joinpoint regression analyses, we found significant increases in the net effect of placebo from 1991 to 2017 and in placebo responder rates from 2010 to 2017. The effects of drug treatment of OCD increased from 1987 to 2012, between two periods of no significant changes (1979-1987 and 2012-2017), while responder rates to drug treatment showed no statistically significant annual changes between 1984 and 2017. We confirmed the finding of a trend towards an increase of placebo effects in OCD drug treatment studies, previously reported by Ackerman and Greenland for the decade 1989 to 1999 in their 2002 meta-regression [8]. Surprisingly, we did not find drugs to increase their effect of responder rates in parallel with placebo. In fact, the increasing placebo effect that was found for other psychiatric (e.g., schizophrenia [129] and depression [130]) and non-psychiatric disorders (e.g., hypertension [131]), and placebo efficacy dragged drug efficacy to higher levels [130]. Here we found a trend towards increased placebo effects and responder rate, with a decrease in the difference between drug and placebo, to the point that the most recent response rates (effects) between placebo and drugs nearly overlap Figs. (2) and 4), while responder rates differ little Figs. (3) and 5); something similar has been described for schizophrenia [132], but not for depression [130]. Furthermore, we also found that effect/responder rates for OCD to both placebo and drugs are low compared to other psychiatric disorders, as recently reported using effect sizes as outcome measures [133]. Why OCD should be stiffer than other psychiatric disorders in responding to treatment may have a response to the pathophysiology of the disorder and to its related personality characteristics. That you can’t teach an old dog new tricks may well apply to this disorder. A therapeutic response may be hampered by too often controlling one’s health state, that prevents a patient from establishing an adequate clinical progress, and people with OCD often display pathological doubt, that prompts them to control for any change all too often and then to doubt for results.
It is not easy to explain the increase with time of placebo effects and responder rates we found here. One explanation that has been offered for similar results in depression is the “baseline inflation” [134], i.e., the tendency to inflate baseline scores of the scale chosen for subject inclusion in a randomised clinical trial (RCT) so to ensure more participants to the sponsor. This, combined with patient expectation and the Hawthorne-like effect of being closely observed, yields better results for drug and placebo alike. However, there has been no increase in baseline Y-BOCS scores of included samples.
Other possible explanations may involve historical factors. In fact, most early studies involved testing the efficacy of clomipramine and SSRIs in patients with OCD, or were survival studies focusing on Kaplan-Meyer curves after switching patients with a benefit on an antidepressant agent to placebo, measuring recurrence/relapse rates. In contrast, later studies increasingly focused on treatment-resistant populations and add-on drugs vs. placebo. It is highly probable that such populations are more resistant to the effects of both drugs and placebo. However, this should have been followed by a decrease in overall responsiveness, which we did not find; on the contrary, both effect and responder rates increased in later years, more so regarding placebo Fig. (2)-5).
Another issue may regard the principal sites involved in the various studies. In antipsychotic drug trials, the increase in placebo response has been observed for North America-based studies, but not for those conducted in the rest of the world or for international studies including US sites [135], and the same phenomenon has been observed for painkiller trials, with sample size and study duration driving the placebo response increase [136]. Sample size in US studies correlated weakly with placebo response in our study (Pearson’s r=0.21). However, treatment duration did correlate strongly (Pearson’s r=0.54). Here we observed a curious phenomenon, i.e., that in studies 1980-2008, US-based studies prevailed over the rest of the world (N=52 vs. N=24), whereas in studies conducted from 2009 on, the rest of the world studies reversed the ratio (N=13, USA vs. N=24, rest of the world). The reversal was driven by Iran (N=17), a country that was not present during the 1980-2009 period. In Iranian studies, the correlation between sample size was much weaker than in US-based studies (r=0.093), perhaps a consequence of the fact that sample sizes in these studies were about 20 each with a much lower standard deviation than in US studies (Table 1). In contrast with US studies, there was a strong negative correlation between treatment duration and response to placebo (r=-0.471). Despite the entity of placebo effect did not differ between US-based and Iran-based studies (Student’s t=1.145; p=0.256, not significant), we feel that the recent upsurge of placebo responder rates and the constant increase of placebo response are linked to the results of Iranian studies. The samples in American studies varied widely, as did the number of sites, while the Iranian studies recruited middle samples and tended to be single-center (Table 1). It has been suggested in antidepressant trials for major depression that two factors that may be linked to reduced ability to detect a signal for an antidepressant are constituted by extremely large and extremely small samples and by multicentricity [137]; a reduced signal is usually linked to increasing placebo response that dampens the drug-placebo difference.
A possibility with longer-term treatment to associate with placebo response could reside in the exacerbating-remitting course that often characterizes OCD [138], especially in paediatric cases [139]. If the interpretation of the placebo effect as a regression to the mean holds true [140], it would ensure that by treating people for more time, there will be an increased probability of spontaneous remission of the disorder, that would be subsequently attributed to placebo. This matches the results of American studies, but is opposite to what Iranian studies tell us.
Still another possibility is a change in the characteristics of included patients. It has been speculated that RCTs tend to include patients repeatedly the same persons who participated in prior antidepressant trials, and this is usually addressed with excluding patients having participated recently in another RCT or having received psychotherapy in recent times. In fact, it was shown that patients with low income may be eager to participate in more than one antidepressant drug trial [141]. These patients would be expected to display a rather uniform behaviour in responding to treatment, thus favouring placebo response and their data tend to be increasingly included in databases, thus affecting results. In OCD, we do not have data at this regard, but the recent change in the classification of OCD spectrum disorders in the DSM-5 [142], which were previously classified amidst the anxiety disorders and included hoarding disorder [143], may have impacted the response of OCD to placebo. However, in this case, we should have expected a join point to occur about 2013, the year of introduction of DSM-5 [142]. In placebo effect, there was no such join point. Placebo effect showed a continuous growth from 1991 onwards, while placebo responder rate had a joinpoint at 2010, when the shift from DSM-IV-TR™ had still to occur. It should be said that DSM-IV-TR™ diagnoses in drug trials continued to be adopted along with DSM-5 diagnoses for some time. At any rate, it appears that in OCD, as in other mental and non-mental disorders, placebo effect and responder rates are puzzlingly increasing and appear to be out of control, and this depends on multiple factors [137, 144], pointing to changing populations included in RCTs (independently from initial severity) and prompting to a revision of the RCT model. Given that this trend is not specifically bound to a single condition, it is possible that it reflects continuing human evolution.
4.1. Limitations and Strengths
Our study is not a meta-analysis and the overall placebo effect and placebo-responder rates are not weighted for sample size. Furthermore, we did not include studies not using drugs, i.e., somatic treatments vs. sham, and did not distinguish between adult and paediatric studies. Moreover, we did not select our studies based on their quality nor did we address possible sponsor bias. However, our study is the first considering such a wide time period and the first to consider both net placebo effects and responder rates based on clear-cut criteria. Future studies will have to address the above concerns. It has been suggested that mechanical devices [145] and surgery [146] are endowed with a superior placebo effect than drugs, although the evidence is still inconclusive [147], and placebo, despite displaying large effects in depression, was not superior in non-pharmacological than in pharmacological studies in a meta-analysis [148]. Comparison between drug and somatic treatment of OCD will show whether in this disorder there is a strong mechanical device component in placebo response and whether there is an increase of response with the year of publication with these treatments similar to what occurs with drugs.
The fact that the curves of effect and responder rates did not reciprocally correspond for both placebo and drugs may be explained by the fact that some studies did not report one of them (Table 1). Investigators need to report their data more clearly in the future, so to allow other investigators to perform meta-analyses on their data.
The current situation with OCD treatment is that this disorder is either treated with drugs having the ability to block the reuptake of serotonin or with cognitive-behavioral therapy or both, or with attempts to add-on ongoing pharmacotherapy other pharmacological agents having antidopaminergic or antiglutamatergic properties or somatic treatments like deep brain stimulation or transcranial magnetic stimulation. The first studies focused on the efficacy of drugs used classical designs and were carried-out by prestigious institutions. Once the concept that first line treatments were represented by SSRIs/clomipramine, the treatment paradigms shifted towards add-on and somatic treatments, and this may have affected the figures we obtained. The closing gap between placebo and drug treatment must prompt investigators to formulate new hypotheses to test and industries to produce alternatively working drugs.
CONCLUSION
In this Joinpoint regression analysis, we observed an increase of the response to placebo (placebo effect) as well as an increase in responder rates in OCD studies with the year of publication. Changes in study types and sites are apparently related to the results obtained. The gap between response to drug and response to placebo appears to be reducing to an extent that current therapeutic approaches to OCD are becoming questionable and should prompt to seek newer approaches in facing this stubborn disorder.
ACKNOWLEDGEMENTS
We gratefully acknowledge the contribution of the Librarians of the School of Medicine and Psychology of Sapienza University, Ms. Mimma Ariano, Ms. Felicia Proietti, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo for rendering precious bibliographical material accessible, as well as our Secretary Lucilla Martinelli for her assistance during the writing of this manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work has not been supported by any funding. All authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with the subject matter or materials discussed in the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mavissakalian M.R., Jones B., Olson S. Absence of placebo response in obsessive-compulsive disorder. J. Nerv. Ment. Dis. 1990;178(4):268–270. doi: 10.1097/00005053-199004000-00010. [http://dx.doi.org/10.1097/00005053-199004000-00010]. [PMID: 2319236]. [DOI] [PubMed] [Google Scholar]
- 2.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [http://dx.doi.org/10.1001/archpsyc.1989.01810110048007]. [PMID: 2684084]. [DOI] [PubMed] [Google Scholar]
- 3.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Delgado P., Heninger G.R., Charney D.S. The yale-brown obsessive compulsive scale. II. Validity. Arch. Gen. Psychiatry. 1989;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [http://dx.doi.org/10.1001/archpsyc.1989.01810110054008]. [PMID: 2510699]. [DOI] [PubMed] [Google Scholar]
- 4.Guy W. Department of health, education, and welfare, public health service, alcohol, drug abuse, and mental health administration, NIMH psychopharmacology research branch, division of extramural research programs, ECDEU Assessment Manual of Psychopharmacology — Revised (DHEW Publ No ADM 76-338). Rockville, MD, U.S; 1976:218–222. [Google Scholar]
- 5.Walsh B.T., Seidman S.N., Sysko R., Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847. doi: 10.1001/jama.287.14.1840. [http://dx.doi.org/10.1001/jama.287.14.1840]. [PMID: 11939870]. [DOI] [PubMed] [Google Scholar]
- 6.Sysko R., Walsh B.T. A systematic review of placebo response in studies of bipolar mania. J. Clin. Psychiatry. 2007;68(8):1213–1217. doi: 10.4088/jcp.v68n0807. [http://dx.doi.org/10.4088/JCP.v68n0807]. [PMID: 17854245]. [DOI] [PubMed] [Google Scholar]
- 7.Vieta E., Cruz N. Increasing rates of placebo response over time in mania studies. J. Clin. Psychiatry. 2008;69(4):681–682. doi: 10.4088/jcp.v69n0423g. [http://dx.doi.org/10.4088/JCP.v69n0423g]. [PMID: 18507494]. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman D.L., Greenland S. Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J. Clin. Psychopharmacol. 2002;22(3):309–317. doi: 10.1097/00004714-200206000-00012. [http://dx.doi.org/10.1097/00004714-200206000-00012]. [PMID: 12006902]. [DOI] [PubMed] [Google Scholar]
- 9.Fineberg N.A., Hawley C.J., Gale T.M. Are placebo-controlled trials still important for obsessive compulsive disorder? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(3):413–422. doi: 10.1016/j.pnpbp.2005.11.012. [http://dx.doi.org/10.1016/j.pnpbp.2005.11.012]. [PMID: 16413647]. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch I., Sapirstein G. Listening to Prozac but hearing placebo: A meta-analysis of antidepressant medication. In: Kirsch I., editor. How Expectancies Shape Behavior. Washington, DC: American Psychological Association; 1999. pp. 303–320. [http://dx.doi.org/10.1037/10332-012] [Google Scholar]
- 11.Kobak K.A., Greist J.H., Jefferson J.W., Katzelnick D.J., Henk H.J. Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Psychopharmacology (Berl.) 1998;136(3):205–216. doi: 10.1007/s002130050558. [http://dx.doi.org/10.1007/s002130050558]. [PMID: 9566805]. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery S.A. Clomipramine in obsessional neurosis: a placebo controlled trial. Pharmaceut. Med. 1980;1:189–192. [Google Scholar]
- 13.Thorén P., Åsberg M., Cronholm B., Jörnestedt L., Träskman L. Clomipramine treatment of obsessive-compulsive disorder. I. A controlled clinical trial. Arch. Gen. Psychiatry. 1980;37(11):1281–1285. doi: 10.1001/archpsyc.1980.01780240079009. [http://dx.doi.org/10.1001/archpsyc.1980.01780240079009]. [PMID: 7436690]. [DOI] [PubMed] [Google Scholar]
- 14.Mavissakalian M., Turner S.M., Michelson L., Jacob R. Tricyclic antidepressants in obsessive-compulsive disorder: antiobsessional or antidepressant agents? II. Am. J. Psychiatry. 1985;142(5):572–576. doi: 10.1176/ajp.142.5.572. [http://dx.doi.org/10.1176/ajp.142.5.572]. [PMID: 3885761]. [DOI] [PubMed] [Google Scholar]
- 15.Foa E.B., Steketee G., Kozak M.J., Dugger D. Effects of imipramine on depression and obsessive-compulsive symptoms. Psychiatry Res. 1987;21(2):123–136. doi: 10.1016/0165-1781(87)90070-9. [http://dx.doi.org/10.1016/0165-1781(87)90070-9]. [PMID: 3615688]. [DOI] [PubMed] [Google Scholar]
- 16.Foa E.B., Steketee G., Kozak M.J., Dugger D. Imipramine and placebo in the treatment of obsessive-compulsives: their effect on depression and on obsessional symptoms. Psychopharmacol. Bull. 1987;23(1):8–11. [PMID: 3602334]. [PubMed] [Google Scholar]
- 17.Perse T.L., Greist J.H., Jefferson J.W., Rosenfeld R., Dar R. Fluvoxamine treatment of obsessive-compulsive disorder. Am. J. Psychiatry. 1987;144(12):1543–1548. doi: 10.1176/ajp.144.12.1543. [http://dx.doi.org/10.1176/ajp.144.12.1543]. [PMID: 3120604]. [DOI] [PubMed] [Google Scholar]
- 18.Goodman W.K., Price L.H., Rasmussen S.A., Delgado P.L., Heninger G.R., Charney D.S. Efficacy of fluvoxamine in obsessive-compulsive disorder. A double-blind comparison with placebo. Arch. Gen. Psychiatry. 1989;46(1):36–44. doi: 10.1001/archpsyc.1989.01810010038006. [http://dx.doi.org/10.1001/archpsyc.1989.01810010038006]. [PMID: 2491940]. [DOI] [PubMed] [Google Scholar]
- 19.Jenike M.A., Baer L., Summergrad P., Minichiello W.E., Holland A., Seymour R. Vol. 147. erratum, Jenike and Baer; 1990. Sertraline in obsessive-compulsive disorder: a double-blind comparison with placebo. pp. 923–928. 147, 1393. [DOI] [PubMed] [Google Scholar]
- 20.Chouinard G. Sertraline in the treatment of obsessive compulsive disorder: two double-blind, placebo-controlled studies. Int. Clin. Psychopharmacol. 1992;7(Suppl. 2):37–41. doi: 10.1097/00004850-199210002-00007. [http://dx.doi.org/10.1097/00004850-199210002-00007]. [PMID: 1484177]. [DOI] [PubMed] [Google Scholar]
- 21.Riddle M.A., Scahill L., King R.A., Hardin M.T., Anderson G.M., Ort S.I., Smith J.C., Leckman J.F., Cohen D.J. Double-blind, crossover trial of fluoxetine and placebo in children and adolescents with obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(6):1062–1069. doi: 10.1097/00004583-199211000-00011. [http://dx.doi.org/10.1097/00004583-199211000-00011]. [PMID: 1429406]. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery S.A., McIntyre A., Osterheider M., Sarteschi P., Zitterl W., Zohar J., Birkett M., Wood A.J. A double-blind, placebo-controlled study of fluoxetine in patients with DSM-III-R obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 1993;3(2):143–152. doi: 10.1016/0924-977x(93)90266-o. [http://dx.doi.org/10.1016/0924-977X(93)90266-O]. [PMID: 8364350]. [DOI] [PubMed] [Google Scholar]
- 23.Tollefson G.D., Rampey A.H., Jr, Potvin J.H., Jenike M.A., Rush A.J., Dominguez R.A., Koran L.M., Shear M.K., Goodman W., Genduso L.A. A multicenter investigation of fixed-dose fluoxetine in the treatment of obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1994;51:559–567. doi: 10.1001/archpsyc.1994.03950070051010. erratum 51, 864. [DOI] [PubMed] [Google Scholar]
- 24.Tollefson G.D., Birkett M., Koran L., Genduso L. Continuation treatment of OCD: double-blind and open-label experience with fluoxetine. J. Clin. Psychiatry. 1994;55(Suppl.):69–76. [PMID: 7961535]. [PubMed] [Google Scholar]
- 25.Turner S.M., Jacob R.G., Beidel D.C., Himmelhoch J. Fluoxetine treatment of obsessive-compulsive disorder. J. Clin. Psychopharmacol. 1985;5(4):207–212. [http://dx.doi.org/10.1097/00004714-198508000-00003]. [PMID: 3894437]. [PubMed] [Google Scholar]
- 26.Pfeffer C.R., Klerman G.L., Hurt S.W., Lesser M., Peskin J.R., Siefker C.A. Suicidal children grow up: demographic and clinical risk factors for adolescent suicide attempts. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30(4):609–616. doi: 10.1097/00004583-199107000-00013. [http://dx.doi.org/10.1097/00004583-199107000-00013]. [PMID: 1890095]. [DOI] [PubMed] [Google Scholar]
- 27.Flament M.F., Rapoport J.L., Berg C.J., Sceery W., Kilts C., Mellström B., Linnoila M. Clomipramine treatment of childhood obsessive-compulsive disorder. A double-blind controlled study. Arch. Gen. Psychiatry. 1985;42(10):977–983. doi: 10.1001/archpsyc.1985.01790330057007. [http://dx.doi.org/10.1001/archpsyc.1985.01790330057007]. [PMID: 3899048]. [DOI] [PubMed] [Google Scholar]
- 28.Pato M.T., Zohar-Kadouch R., Zohar J., Murphy D.L. Return of symptoms after discontinuation of clomipramine in patients with obsessive-compulsive disorder. Am. J. Psychiatry. 1988;145(12):1521–1525. doi: 10.1176/ajp.145.12.1521. [http://dx.doi.org/10.1176/ajp.145.12.1521]. [PMID: 3057923]. [DOI] [PubMed] [Google Scholar]
- 29.Jenike M.A., Baer L., Summergrad P., Weilburg J.B., Holland A., Seymour R. Obsessive-compulsive disorder: a double-blind, placebo-controlled trial of clomipramine in 27 patients. Am. J. Psychiatry. 1989;146(10):1328–1330. doi: 10.1176/ajp.146.10.1328. [http://dx.doi.org/10.1176/ajp.146.10.1328]. [PMID: 2675643]. [DOI] [PubMed] [Google Scholar]
- 30.Greist J.H., Jefferson J.W., Rosenfeld R., Gutzmann L.D., March J.S., Barklage N.E. Clomipramine and obsessive compulsive disorder: a placebo-controlled double-blind study of 32 patients. J. Clin. Psychiatry. 1990;51(7):292–297. [PMID: 2195006]. [PubMed] [Google Scholar]
- 31.Jenike M.A., Hyman S., Baer L., Holland A., Minichiello W.E., Buttolph L., Summergrad P., Seymour R., Ricciardi J. A controlled trial of fluvoxamine in obsessive-compulsive disorder: implications for a serotonergic theory. Am. J. Psychiatry. 1990;147(9):1209–1215. doi: 10.1176/ajp.147.9.1209. [http://dx.doi.org/10.1176/ajp.147.9.1209]. [PMID: 2143637]. [DOI] [PubMed] [Google Scholar]
- 32.Katz R.J., DeVeaugh-Geiss J., Landau P. Clomipramine in obsessive-compulsive disorder. Biol. Psychiatry. 1990;28(5):401–414. doi: 10.1016/0006-3223(90)90408-t. [http://dx.doi.org/10.1016/0006-3223(90)90408-T]. [PMID: 2207219]. [DOI] [PubMed] [Google Scholar]
- 33.Mavissakalian M.R., Jones B., Olson S., Perel J.M. Clomipramine in obsessive-compulsive disorder: clinical response and plasma levels. J. Clin. Psychopharmacol. 1990;10(4):261–268. [http://dx.doi.org/10.1097/00004714-199008000-00005]. [PMID: 2286699]. [PubMed] [Google Scholar]
- 34.Montgomery S.A., Montgomery D.B., Fineberg N. Early response with clomipramine in obsessive compulsive disorder--a placebo controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14(5):719–727. doi: 10.1016/0278-5846(90)90042-f. [http://dx.doi.org/10.1016/0278-5846(90)90042-F]. [PMID: 2293252]. [DOI] [PubMed] [Google Scholar]
- 35.McDougle C.J., Price L.H., Goodman W.K., Charney D.S., Heninger G.R. A controlled trial of lithium augmentation in fluvoxamine-refractory obsessive-compulsive disorder: lack of efficacy. J. Clin. Psychopharmacol. 1991;11(3):175–184. [http://dx.doi.org/10.1097/00004714-199106000-00005]. [PMID: 1820757]. [PubMed] [Google Scholar]
- 36.Clomipramine in the treatment of patients with obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1991;48(8):730–738. doi: 10.1001/archpsyc.1991.01810320054008. [http://dx.doi.org/10.1001/archpsyc.1991.01810320054008]. [PMID: 1883256]. [DOI] [PubMed] [Google Scholar]
- 37.DeVeaugh-Geiss J., Moroz G., Biederman J., Cantwell D., Fontaine R., Greist J.H., Reichler R., Katz R., Landau P. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(1):45–49. doi: 10.1097/00004583-199201000-00008. [http://dx.doi.org/10.1097/00004583-199201000-00008]. [PMID: 1537780]. [DOI] [PubMed] [Google Scholar]
- 38.Mallya G.K., White K., Waternaux C., Quay S. Short- and long-term treatment with obsessive-compulsive disorder with fluvoxamine. Ann. Clin. Psychiatry. 1992;4:77–80. [http://dx.doi.org/10.3109/10401239209150443]. [Google Scholar]
- 39.Pigott T.A., L’Heureux F., Rubenstein C.S., Bernstein S.E., Hill J.L., Murphy D.L. A double-blind, placebo controlled study of trazodone in patients with obsessive-compulsive disorder. J. Clin. Psychopharmacol. 1992;12(3):156–162. [http://dx.doi.org/10.1097/00004714-199206000-00002]. [PMID: 1629380]. [PubMed] [Google Scholar]
- 40.Stein D.J., Hollander E., Mullen L.S. Comparison of clomipramine, alprazolam and placebo in the treatment of obsessive compulsive disorder. Hum. Psychopharmacol. 1992;7:389–395. [http://dx.doi.org/10.1002/hup.470070604]. [Google Scholar]
- 41.Grady T.A., Pigott T.A., L’Heureux F., Hill J.L., Bernstein S.E., Murphy D.L. Double-blind study of adjuvant buspirone for fluoxetine-treated patients with obsessive-compulsive disorder. Am. J. Psychiatry. 1993;150(5):819–821. doi: 10.1176/ajp.150.5.819. [http://dx.doi.org/10.1176/ajp.150.5.819]. [PMID: 8480832]. [DOI] [PubMed] [Google Scholar]
- 42.Hoehn-Saric R., McLeod D.R., Zimmerli W.D., Hipsley P.A. Symptoms and physiologic manifestations in obsessive compulsive patients before and after treatment with clomipramine. J. Clin. Psychiatry. 1993;54(7):272–276. [PMID: 8335655]. [PubMed] [Google Scholar]
- 43.McDougle C.J., Goodman W.K., Leckman J.F., Holzer J.C., Barr L.C., McCance-Katz E., Heninger G.R., Price L.H. Limited therapeutic effect of addition of buspirone in fluvoxamine-refractory obsessive-compulsive disorder. Am. J. Psychiatry. 1993;150(4):647–649. doi: 10.1176/ajp.150.4.647. [http://dx.doi.org/10.1176/ajp.150.4.647]. [PMID: 8465885]. [DOI] [PubMed] [Google Scholar]
- 44.McDougle C.J., Goodman W.K., Leckman J.F., Lee N.C., Heninger G.R., Price L.H. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Arch. Gen. Psychiatry. 1994;51(4):302–308. doi: 10.1001/archpsyc.1994.03950040046006. [http://dx.doi.org/10.1001/archpsyc.1994.03950040046006]. [PMID: 8161290]. [DOI] [PubMed] [Google Scholar]
- 45.Greist J., Chouinard G., DuBoff E., Halaris A., Kim S.W., Koran L., Liebowitz M., Lydiard R.B., Rasmussen S., White K., Sikes C. Double-blind parallel comparison of three dosages of sertraline and placebo in outpatients with obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1995;52(4):289–295. doi: 10.1001/archpsyc.1995.03950160039008. [http://dx.doi.org/10.1001/archpsyc.1995.03950160039008]. [PMID: 7702445]. [DOI] [PubMed] [Google Scholar]
- 46.Fux M., Levine J., Aviv A., Belmaker R.H. Inositol treatment of obsessive-compulsive disorder. Am. J. Psychiatry. 1996;153(9):1219–1221. doi: 10.1176/ajp.153.9.1219. [http://dx.doi.org/10.1176/ajp.153.9.1219]. [PMID: 8780431]. [DOI] [PubMed] [Google Scholar]
- 47.Goodman W.K., Kozak M.J., Liebowitz M., White K.L. Treatment of obsessive-compulsive disorder with fluvoxamine: a multicentre, double-blind, placebo-controlled trial. Int. Clin. Psychopharmacol. 1996;11(1):21–29. [http://dx.doi.org/10.1097/00004850-199603000-00003]. [PMID: 8732310]. [PubMed] [Google Scholar]
- 48.Nakajima T., Kudo Y., Yamashita I., Asai M., Kamijima K., Murasaki M., Yamaguchi N., Saito M., Yamawaki S., Nishizono M., Hishikawa Y., Machiyama Y., Yamauchi T., Moriya N., Toru M., Hirose T., Kojima T., Shimizu M., Tamura A., Endo S., Suzuki J., Takemasa K., Uno M., Hasegawa K., Kariya T. Clinical usefulness of Fluvoxamine Maleate (SME3110), a selective serotonin reuptake inhibitor, in the treatment of obsessive compulsive disorder: A double blind, placebo-controlled study investigating the therapeutic dose range and the efficacy of SME3110. J. Clin. Therap. Med. 1996;12(3):409–437. [Rinshou Iyaku]. [Google Scholar]
- 49.Zohar J., Judge R. Paroxetine versus clomipramine in the treatment of obsessive-compulsive disorder. Br. J. Psychiatry. 1996;169(4):468–474. doi: 10.1192/bjp.169.4.468. [http://dx.doi.org/10.1192/bjp.169.4.468]. [PMID: 8894198]. [DOI] [PubMed] [Google Scholar]
- 50.Jenike M.A., Baer L., Minichiello W.E., Rauch S.L., Buttolph M.L. Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am. J. Psychiatry. 1997;154(9):1261–1264. doi: 10.1176/ajp.154.9.1261. [http://dx.doi.org/10.1176/ajp.154.9.1261]. [PMID: 9286186]. [DOI] [PubMed] [Google Scholar]
- 51.Lindsay M., Crino R., Andrews G. Controlled trial of exposure and response prevention in obsessive-compulsive disorder. Br. J. Psychiatry. 1997;171:135–139. doi: 10.1192/bjp.171.2.135. [http://dx.doi.org/10.1192/bjp.171.2.135]. [PMID: 9337948]. [DOI] [PubMed] [Google Scholar]
- 52.Ushijima S., Kamijima K., Asai M., Murasaki M., Nakajima T., Kudo Y., Tashiro N., Kurihara M., Miura S. Clinical evaluation of sertraline hydrochloride, a selective serotonin reuptake inhibitor in the treatment of obsessive- compulsive disorder: A double blind placebo controlled trial. Jpn. J. Neuropsychopharmacol., (Nihon Shinkei Seishin Yakurigaku Zasshi or Nihon Shinkei Seishin Yakuri Gakkai) 1997;19(6):603–623. [Google Scholar]
- 53.Fallon B.A., Liebowitz M.R., Campeas R., Schneier F.R., Marshall R., Davies S., Goetz D., Klein D.F. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch. Gen. Psychiatry. 1998;55(10):918–924. doi: 10.1001/archpsyc.55.10.918. [http://dx.doi.org/10.1001/archpsyc.55.10.918]. [PMID: 9783563]. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Xiang H., Du H. Clinical controlled study of paroxetine and clomipramine in treatment of obsessive- compulsive disorder. Zhonghua jing shen ke za zhi (Chinese Journal of Psychiatry), 1998, 31, 215-217 (李建勋,向虎,杜海英. 帕罗西汀与氯丙咪嗪治疗强迫症的临床对照研究. 中华精神科杂志. 编辑部邮箱 1998年 04期31, 215-217. (1998, (4), 23-25)). (erroneously referred to as: Jianxun L, Hu X, Haiying D. Clinical controlled study of paroxetine and clomipramine in treatment of obsessive- compulsive disorder. Chinese J. Psychiatry. 1998;31:215–217. [Chinese]. [Google Scholar]
- 55.March J.S., Biederman J., Wolkow R., Safferman A., Mardekian J., Cook E.H., Cutler N.R., Dominguez R., Ferguson J., Muller B., Riesenberg R., Rosenthal M., Sallee F.R., Wagner K.D., Steiner H. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. 1998 doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- 56.Kronig M.H., Apter J., Asnis G., Bystritsky A., Curtis G., Ferguson J., Landbloom R., Munjack D., Riesenberg R., Robinson D., Roy-Byrne P., Phillips K., Du Pont I.J. Placebo-controlled, multicenter study of sertraline treatment for obsessive-compulsive disorder. J. Clin. Psychopharmacol. 1999;19(2):172–176. doi: 10.1097/00004714-199904000-00013. [http://dx.doi.org/10.1097/00004714-199904000-00013]. [PMID: 10211919]. [DOI] [PubMed] [Google Scholar]
- 57.Dannon P.N., Sasson Y., Hirschmann S., Iancu I., Grunhaus L.J., Zohar J. Pindolol augmentation in treatment-resistant obsessive compulsive disorder: a double-blind placebo controlled trial. Eur. Neuropsychopharmacol. 2000;10(3):165–169. doi: 10.1016/s0924-977x(00)00065-1. [http://dx.doi.org/10.1016/S0924-977X(00)00065-1]. [PMID: 10793318]. [DOI] [PubMed] [Google Scholar]
- 58.McDougle C.J., Epperson C.N., Pelton G.H., Wasylink S., Price L.H. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2000;57(8):794–801. doi: 10.1001/archpsyc.57.8.794. [http://dx.doi.org/10.1001/archpsyc.57.8.794]. [PMID: 10920469]. [DOI] [PubMed] [Google Scholar]
- 59.Geller D.A., Hoog S.L., Heiligenstein J.H., Ricardi R.K., Tamura R., Kluszynski S., Jacobson J.G. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(7):773–779. doi: 10.1097/00004583-200107000-00011. [http://dx.doi.org/10.1097/00004583-200107000-00011]. [PMID: 11437015]. [DOI] [PubMed] [Google Scholar]
- 60.Montgomery S.A., Kasper S., Stein D.J., Bang H.K., Lemming O.M. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int. Clin. Psychopharmacol. 2001;16(2):75–86. doi: 10.1097/00004850-200103000-00002. [http://dx.doi.org/10.1097/00004850-200103000-00002]. [PMID: 11236072]. [DOI] [PubMed] [Google Scholar]
- 61.Riddle M.A., Reeve E.A., Yaryura-Tobias J.A., Yang H.M., Claghorn J.L., Gaffney G., Greist J.H., Holland D., McConville B.J., Pigott T., Walkup J.T. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(2):222–229. doi: 10.1097/00004583-200102000-00017. [http://dx.doi.org/10.1097/00004583-200102000-00017]. [PMID: 11211371]. [DOI] [PubMed] [Google Scholar]
- 62.Romano S., Goodman W., Tamura R., Gonzales J. Long-term treatment of obsessive-compulsive disorder after an acute response: a comparison of fluoxetine versus placebo. J. Clin. Psychopharmacol. 2001;21(1):46–52. doi: 10.1097/00004714-200102000-00009. [http://dx.doi.org/10.1097/00004714-200102000-00009]. [PMID: 11199947]. [DOI] [PubMed] [Google Scholar]
- 63.Atmaca M., Kuloglu M., Tezcan E., Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive-compulsive disorder: a single-blind, placebo-controlled study. Int. Clin. Psychopharmacol. 2002;17(3):115–119. doi: 10.1097/00004850-200205000-00004. [http://dx.doi.org/10.1097/00004850-200205000-00004]. [PMID: 11981352]. [DOI] [PubMed] [Google Scholar]
- 64.Greist J.H., Marks I.M., Baer L., Kobak K.A., Wenzel K.W., Hirsch M.J., Mantle J.M., Clary C.M. Behavior therapy for obsessive-compulsive disorder guided by a computer or by a clinician compared with relaxation as a control. J. Clin. Psychiatry. 2002;63(2):138–145. doi: 10.4088/jcp.v63n0209. [http://dx.doi.org/10.4088/JCP.v63n0209]. [PMID: 11874215]. [DOI] [PubMed] [Google Scholar]
- 65.Koran L.M., Hackett E., Rubin A., Wolkow R., Robinson D. Efficacy of sertraline in the long-term treatment of obsessive-compulsive disorder. Am. J. Psychiatry. 2002;159(1):88–95. doi: 10.1176/appi.ajp.159.1.88. [http://dx.doi.org/10.1176/appi.ajp.159.1.88]. [PMID: 11772695]. [DOI] [PubMed] [Google Scholar]
- 66.Liebowitz M.R., Turner S.M., Piacentini J., Beidel D.C., Clarvit S.R., Davies S.O., Graae F., Jaffer M., Lin S.H., Sallee F.R., Schmidt A.B., Simpson H.B. Fluoxetine in children and adolescents with OCD: a placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(12):1431–1438. doi: 10.1097/00004583-200212000-00014. [http://dx.doi.org/10.1097/00004583-200212000-00014]. [PMID: 12447029]. [DOI] [PubMed] [Google Scholar]
- 67.Geller D.A., Biederman J., Stewart S.E., Mullin B., Farrell C., Wagner K.D., Emslie G., Carpenter D. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: is the use of exclusion criteria empirically supported in randomized clinical trials? J. Child Adolesc. Psychopharmacol. 2003;13(Suppl. 1):S19–S29. doi: 10.1089/104454603322126313. [http://dx.doi.org/10.1089/104454603322126313]. [PMID: 12880497]. [DOI] [PubMed] [Google Scholar]
- 68.Hollander E., Kaplan A., Stahl S.M. A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J. Biol. Psychiatry. 2003;4(1):30–34. doi: 10.3109/15622970309167908. [http://dx.doi.org/10.3109/15622970309167908]. [PMID: 12582975]. [DOI] [PubMed] [Google Scholar]
- 69.Hollander E., Baldini Rossi N., Sood E., Pallanti S. Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Int. J. Neuropsychopharmacol. 2003;6(4):397–401. doi: 10.1017/S1461145703003730. [http://dx.doi.org/10.1017/S1461145703003730]. [PMID: 14604454]. [DOI] [PubMed] [Google Scholar]
- 70.Hollander E., Allen A., Steiner M., Wheadon D.E., Oakes R., Burnham D.B. Acute and long-term treatment and prevention of relapse of obsessive-compulsive disorder with paroxetine. J. Clin. Psychiatry. 2003;64(9):1113–1121. doi: 10.4088/jcp.v64n0919. [http://dx.doi.org/10.4088/JCP.v64n0919]. [PMID: 14628989]. [DOI] [PubMed] [Google Scholar]
- 71.Hollander E., Koran L.M., Goodman W.K., Greist J.H., Ninan P.T., Yang H., Li D., Barbato L.M. A double-blind, placebo-controlled study of the efficacy and safety of controlled-release fluvoxamine in patients with obsessive-compulsive disorder. J. Clin. Psychiatry. 2003;64(6):640–647. doi: 10.4088/jcp.v64n0604. [http://dx.doi.org/10.4088/JCP.v64n0604]. [PMID: 12823077]. [DOI] [PubMed] [Google Scholar]
- 72.Bystritsky A., Ackerman D.L., Rosen R.M., Vapnik T., Gorbis E., Maidment K.M., Saxena S. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J. Clin. Psychiatry. 2004;65(4):565–568. doi: 10.4088/jcp.v65n0418. [http://dx.doi.org/10.4088/JCP.v65n0418]. [PMID: 15119922]. [DOI] [PubMed] [Google Scholar]
- 73.Denys D., de Geus F., van Megen H.J., Westenberg H.G. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J. Clin. Psychiatry. 2004;65(8):1040–1048. doi: 10.4088/jcp.v65n0803. [http://dx.doi.org/10.4088/JCP.v65n0803]. [PMID: 15323587]. [DOI] [PubMed] [Google Scholar]
- 74.Fux M., Benjamin J., Nemets B. A placebo-controlled cross-over trial of adjunctive EPA in OCD. J. Psychiatr. Res. 2004;38(3):323–325. doi: 10.1016/S0022-3956(03)00077-3. [http://dx.doi.org/10.1016/S0022-3956(03)00077-3]. [PMID: 15003438]. [DOI] [PubMed] [Google Scholar]
- 75.Geller D.A., Wagner K.D., Emslie G., Murphy T., Carpenter D.J., Wetherhold E., Perera P., Machin A., Gardiner C. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(11):1387–1396. doi: 10.1097/01.chi.0000138356.29099.f1. [http://dx.doi.org/10.1097/01.chi.0000138356.29099.f1]. [PMID: 15502598]. [DOI] [PubMed] [Google Scholar]
- 76.Kamijima K., Murasaki M., Asai M., Higuchi T., Nakajima T., Taga C., Matsunaga H. Paroxetine in the treatment of obsessive-compulsive disorder: randomized, double-blind, placebo-controlled study in Japanese patients. Psychiatry Clin. Neurosci. 2004;58(4):427–433. doi: 10.1111/j.1440-1819.2004.01278.x. [http://dx.doi.org/10.1111/j.1440-1819.2004.01278.x]. [PMID: 15298657]. [DOI] [PubMed] [Google Scholar]
- 77.Shapira N.A., Ward H.E., Mandoki M., Murphy T.K., Yang M.C., Blier P., Goodman W.K. A double-blind, placebo-controlled trial of olanzapine addition in fluoxetine-refractory obsessive-compulsive disorder. Biol. Psychiatry. 2004;55(5):553–555. doi: 10.1016/j.biopsych.2003.11.010. [http://dx.doi.org/10.1016/j.biopsych.2003.11.010]. [PMID: 15023585]. [DOI] [PubMed] [Google Scholar]
- 78.Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [http://dx.doi.org/10.1001/jama.292.16.1969]. [PMID: 15507582]. [DOI] [PubMed] [Google Scholar]
- 79.Carey P.D., Vythilingum B., Seedat S., Muller J.E., van Ameringen M., Stein D.J. Quetiapine augmentation of SRIs in treatment refractory obsessive-compulsive disorder: a double-blind, randomised, placebo-controlled study. BMC Psychiatry. 2005;5:5. doi: 10.1186/1471-244X-5-5. [ISRCTN83050762]. [http://dx.doi.org/10.1186/1471-244X-5-5]. [PMID: 15667657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erzegovesi S., Guglielmo E., Siliprandi F., Bellodi L. Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Eur. Neuropsychopharmacol. 2005;15(1):69–74. doi: 10.1016/j.euroneuro.2004.04.004. [http://dx.doi.org/10.1016/j.euroneuro.2004.04.004]. [PMID: 15572275]. [DOI] [PubMed] [Google Scholar]
- 81.Fineberg N.A., Sivakumaran T., Roberts A., Gale T. Adding quetiapine to SRI in treatment-resistant obsessive-compulsive disorder: a randomized controlled treatment study. Int. Clin. Psychopharmacol. 2005;20(4):223–226. doi: 10.1097/00004850-200507000-00005. [http://dx.doi.org/10.1097/00004850-200507000-00005]. [PMID: 15933483]. [DOI] [PubMed] [Google Scholar]
- 82.Foa E.B., Liebowitz M.R., Kozak M.J., Davies S., Campeas R., Franklin M.E., Huppert J.D., Kjernisted K., Rowan V., Schmidt A.B., Simpson H.B., Tu X. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am. J. Psychiatry. 2005;162(1):151–161. doi: 10.1176/appi.ajp.162.1.151. [http://dx.doi.org/10.1176/appi.ajp.162.1.151]. [PMID: 15625214]. [DOI] [PubMed] [Google Scholar]
- 83.Kobak K.A., Taylor L.V., Bystritsky A., Kohlenberg C.J., Greist J.H., Tucker P., Warner G., Futterer R., Vapnik T. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int. Clin. Psychopharmacol. 2005;20(6):299–304. doi: 10.1097/00004850-200511000-00003. [http://dx.doi.org/10.1097/00004850-200511000-00003]. [PMID: 16192837]. [DOI] [PubMed] [Google Scholar]
- 84.Koran L.M., Aboujaoude E., Bullock K.D., Franz B., Gamel N., Elliott M. Double-blind treatment with oral morphine in treatment-resistant obsessive-compulsive disorder. J. Clin. Psychiatry. 2005;66(3):353–359. doi: 10.4088/jcp.v66n0312. [http://dx.doi.org/10.4088/JCP.v66n0312]. [PMID: 15766302]. [DOI] [PubMed] [Google Scholar]
- 85.Li X., May R.S., Tolbert L.C., Jackson W.T., Flournoy J.M., Baxter L.R. Risperidone and haloperidol augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder: a crossover study. J. Clin. Psychiatry. 2005;66(6):736–743. doi: 10.4088/jcp.v66n0610. [http://dx.doi.org/10.4088/JCP.v66n0610]. [PMID: 15960567]. [DOI] [PubMed] [Google Scholar]
- 86.Nakatani E., Nakagawa A., Nakao T., Yoshizato C., Nabeyama M., Kudo A., Isomura K., Kato N., Yoshioka K., Kawamoto M. A randomized controlled trial of Japanese patients with obsessive-compulsive disorder--effectiveness of behavior therapy and fluvoxamine. Psychother. Psychosom. 2005;74(5):269–276. doi: 10.1159/000086317. [http://dx.doi.org/10.1159/000086317]. [PMID: 16088264]. [DOI] [PubMed] [Google Scholar]
- 87.Buchsbaum M.S., Hollander E., Pallanti S., Baldini Rossi N., Platholi J., Newmark R., Bloom R., Sood E. Positron emission tomography imaging of risperidone augmentation in serotonin reuptake inhibitor-refractory patients. Neuropsychobiology. 2006;53(3):157–168. doi: 10.1159/000093342. [http://dx.doi.org/10.1159/000093342]. [PMID: 16707915]. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor K.P., Aardema F., Robillard S., Guay S., Pélissier M.C., Todorov C., Borgeat F., Leblanc V., Grenier S., Doucet P. Cognitive behaviour therapy and medication in the treatment of obsessive-compulsive disorder. Acta Psychiatr. Scand. 2006;113(5):408–419. doi: 10.1111/j.1600-0447.2006.00767.x. [http://dx.doi.org/10.1111/j.1600-0447.2006.00767.x]. [PMID: 16603032]. [DOI] [PubMed] [Google Scholar]
- 89.Fineberg N.A., Tonnoir B., Lemming O., Stein D.J. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2007;17(6-7):430–439. doi: 10.1016/j.euroneuro.2006.11.005. [http://dx.doi.org/10.1016/j.euroneuro.2006.11.005]. [PMID: 17240120]. [DOI] [PubMed] [Google Scholar]
- 90.Stein D.J., Andersen E.W., Tonnoir B., Fineberg N. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr. Med. Res. Opin. 2007;23(4):701–711. doi: 10.1185/030079907x178838. [http://dx.doi.org/10.1185/030079907X178838]. [PMID: 17407626]. [DOI] [PubMed] [Google Scholar]
- 91.Amiaz R., Fostick L., Gershon A., Zohar J. Naltrexone augmentation in OCD: a double-blind placebo-controlled cross-over study. Eur. Neuropsychopharmacol. 2008;18(6):455–461. doi: 10.1016/j.euroneuro.2008.01.006. [http://dx.doi.org/10.1016/j.euroneuro.2008.01.006]. [PMID: 18353618]. [DOI] [PubMed] [Google Scholar]
- 92.Kordon A., Wahl K., Koch N., Zurowski B., Anlauf M., Vielhaber K., Kahl K.G., Broocks A., Voderholzer U., Hohagen F. Quetiapine addition to serotonin reuptake inhibitors in patients with severe obsessive-compulsive disorder: a double-blind, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2008;28(5):550–554. doi: 10.1097/JCP.0b013e318185e735. [http://dx.doi.org/10.1097/JCP.0b013e318185e735]. [PMID: 18794652]. [DOI] [PubMed] [Google Scholar]
- 93.Greenberg W.M., Benedict M.M., Doerfer J., Perrin M., Panek L., Cleveland W.L., Javitt D.C. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J. Psychiatr. Res. 2009;43(6):664–670. doi: 10.1016/j.jpsychires.2008.10.007. [http://dx.doi.org/10.1016/j.jpsychires.2008.10.007]. [PMID: 19046587]. [DOI] [PubMed] [Google Scholar]
- 94.Sayyah M., Boostani H., Pakseresht S., Malaieri A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(8):1513–1516. doi: 10.1016/j.pnpbp.2009.08.021. [http://dx.doi.org/10.1016/j.pnpbp.2009.08.021]. [PMID: 19737592]. [DOI] [PubMed] [Google Scholar]
- 95.Mowla A., Khajeian A.M., Sahraian A., Chohedri A.H., Kashkoli F. Topiramate augmentation in resistant OCD: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15(11):613–617. doi: 10.1017/S1092852912000065. [http://dx.doi.org/10.1017/S1092852912000065]. [PMID: 24726048]. [DOI] [PubMed] [Google Scholar]
- 96.Storch E.A., Murphy T.K., Goodman W.K., Geffken G.R., Lewin A.B., Henin A., Micco J.A., Sprich S., Wilhelm S., Bengtson M., Geller D.A. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol. Psychiatry. 2010;68(11):1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [http://dx.doi.org/10.1016/j.biopsych.2010.07.015]. [PMID: 20817153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sayyah M., Boostani H., Pakseresht S., Malayeri A. A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res. 2011;189(3):403–406. doi: 10.1016/j.psychres.2011.01.019. [http://dx.doi.org/10.1016/j.psychres.2011.01.019]. [PMID: 21329988]. [DOI] [PubMed] [Google Scholar]
- 98.Muscatello M.R., Bruno A., Pandolfo G., Micò U., Scimeca G., Romeo V.M., Santoro V., Settineri S., Spina E., Zoccali R.A. Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 2011;31(2):174–179. doi: 10.1097/JCP.0b013e31820e3db6. [http://dx.doi.org/10.1097/JCP.0b013e31820e3db6]. [PMID: 21346614]. [DOI] [PubMed] [Google Scholar]
- 99.Berlin H.A., Koran L.M., Jenike M.A., Shapira N.A., Chaplin W., Pallanti S., Hollander E. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J. Clin. Psychiatry. 2011;72(5):716–721. doi: 10.4088/JCP.09m05266gre. [http://dx.doi.org/10.4088/JCP.09m05266gre]. [PMID: 20816027]. [DOI] [PubMed] [Google Scholar]
- 100.Pakseresht S., Boostani H., Sayyah M. Extract of valerian root (Valeriana officinalis L.) vs. placebo in treatment of obsessive-compulsive disorder: a randomized double-blind study. J. Complement. Integr. Med. 2011;8(1):32. doi: 10.2202/1553-3840.1465. [http://dx.doi.org/10.2202/1553-3840.1465]. [PMID: 22718671]. [DOI] [PubMed] [Google Scholar]
- 101.Sayyah M., Olapour A., Saeedabad Ys., Yazdan P.R., Malayeri A. Evaluation of oral zinc sulfate effect on obsessive-compulsive disorder: a randomized placebo-controlled clinical trial. Nutrition. 2012;28(9):892–895. doi: 10.1016/j.nut.2011.11.027. [http://dx.doi.org/10.1016/j.nut.2011.11.027]. [PMID: 22465904]. [DOI] [PubMed] [Google Scholar]
- 102.Sayyah M., Sayyah M., Boostani H., Ghaffari S.M., Hoseini A. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double blind clinical trial). Depress. Anxiety. 2012;29(10):850–854. doi: 10.1002/da.21996. [http://dx.doi.org/10.1002/da.21996]. [PMID: 22933237]. [DOI] [PubMed] [Google Scholar]
- 103.Afshar H., Roohafza H., Mohammad-Beigi H., Haghighi M., Jahangard L., Shokouh P., Sadeghi M., Hafezian H. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2012;32(6):797–803. doi: 10.1097/JCP.0b013e318272677d. [http://dx.doi.org/10.1097/JCP.0b013e318272677d]. [PMID: 23131885]. [DOI] [PubMed] [Google Scholar]
- 104.Bruno A., Micò U., Pandolfo G., Mallamace D., Abenavoli E., Di Nardo F., D’Arrigo C., Spina E., Zoccali R.A., Muscatello M.R. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J. Psychopharmacol. (Oxford) 2012;26(11):1456–1462. doi: 10.1177/0269881111431751. [http://dx.doi.org/10.1177/0269881111431751]. [PMID: 22351381]. [DOI] [PubMed] [Google Scholar]
- 105.Askari N., Moin M., Sanati M., Tajdini M., Hosseini S.M., Modabbernia A., Najand B., Salimi S., Tabrizi M., Ashrafi M., Hajiaghaee R., Akhondzadeh S. Granisetron adjunct to fluvoxamine for moderate to severe obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2012;26(10):883–892. doi: 10.2165/11635850-000000000-00000. [http://dx.doi.org/10.2165/11635850-000000000-00000]. [PMID: 22873680]. [DOI] [PubMed] [Google Scholar]
- 106.Ghaleiha A., Entezari N., Modabbernia A., Najand B., Askari N., Tabrizi M., Ashrafi M., Hajiaghaee R., Akhondzadeh S. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J. Psychiatr. Res. 2013;47(2):175–180. doi: 10.1016/j.jpsychires.2012.09.015. [http://dx.doi.org/10.1016/j.jpsychires.2012.09.015]. [PMID: 23063327]. [DOI] [PubMed] [Google Scholar]
- 107.Storch E.A., Bussing R., Small B.J., Geffken G.R., McNamara J.P., Rahman O., Lewin A.B., Garvan C.S., Goodman W.K., Murphy T.K. Randomized, placebo-controlled trial of cognitive-behavioral therapy alone or combined with sertraline in the treatment of pediatric obsessive-compulsive disorder. Behav. Res. Ther. 2013;51(12):823–829. doi: 10.1016/j.brat.2013.09.007. [http://dx.doi.org/10.1016/j.brat.2013.09.007]. [PMID: 24184429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haghighi M., Jahangard L., Mohammad-Beigi H., Bajoghli H., Hafezian H., Rahimi A., Afshar H., Holsboer-Trachsler E., Brand S. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl.) 2013;228(4):633–640. doi: 10.1007/s00213-013-3067-z. [http://dx.doi.org/10.1007/s00213-013-3067-z]. [PMID: 23525525]. [DOI] [PubMed] [Google Scholar]
- 109.Storch E.A., Goddard A.W., Grant J.E., De Nadai A.S., Goodman W.K., Mutch P.J., Medlock C., Odlaug B., McDougle C.J., Murphy T.K. Double-blind, placebo-controlled, pilot trial of paliperidone augmentation in serotonin reuptake inhibitor-resistant obsessive-compulsive disorder. J. Clin. Psychiatry. 2013;74(6):e527–e532. doi: 10.4088/JCP.12m08278. [http://dx.doi.org/10.4088/JCP.12m08278]. [PMID: 23842022]. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez C.I., Kegeles L.S., Levinson A., Feng T., Marcus S.M., Vermes D., Flood P., Simpson H.B. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475–2483. doi: 10.1038/npp.2013.150. [http://dx.doi.org/10.1038/npp.2013.150]. [PMID: 23783065]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simpson H.B., Foa E.B., Liebowitz M.R., Huppert J.D., Cahill S., Maher M.J., McLean C.P., Bender J., Jr, Marcus S.M., Williams M.T., Weaver J., Vermes D., Van Meter P.E., Rodriguez C.I., Powers M., Pinto A., Imms P., Hahn C.G., Campeas R. Cognitive-behavioral therapy vs. risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2013;70(11):1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. [http://dx.doi.org/10.1001/jamapsychiatry.2013.1932]. [PMID: 24026523]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park J.M., Small B.J., Geller D.A., Murphy T.K., Lewin A.B., Storch E.A. Does D-cycloserine augmentation of CBT improve therapeutic homework compliance for pediatric obsessive-compulsive disorder? J. Child Fam. Stud. 2014;23(5):863–871. doi: 10.1007/s10826-013-9742-1. [http://dx.doi.org/10.1007/s10826-013-9742-1]. [PMID: 24999301]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grant P.J., Joseph L.A., Farmer C.A., Luckenbaugh D.A., Lougee L.C., Zarate C.A., Jr, Swedo S.E. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39(6):1453–1459. doi: 10.1038/npp.2013.343. [http://dx.doi.org/10.1038/npp.2013.343]. [PMID: 24356715]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mataix-Cols D., Turner C., Monzani B., Isomura K., Murphy C., Krebs G., Heyman I. Cognitive-behavioural therapy with post-session D-cycloserine augmentation for paediatric obsessive-compulsive disorder: pilot randomised controlled trial. Br. J. Psychiatry. 2014;204(1):77–78. doi: 10.1192/bjp.bp.113.126284. [http://dx.doi.org/10.1192/bjp.bp.113.126284]. [PMID: 24262813]. [DOI] [PubMed] [Google Scholar]
- 115.Afshar H., Akuchekian S., Mahaky B., Zarean E. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J. Res. Med. Sci. 2014;19(10):976–981. [PMID: 25538783]. [PMC free article] [PubMed] [Google Scholar]
- 116.Sarris J., Oliver G., Camfield D.A., Dean O.M., Dowling N., Smith D.J., Murphy J., Menon R., Berk M., Blair-West S., Ng C.H. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: a 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29(9):801–809. doi: 10.1007/s40263-015-0272-9. [http://dx.doi.org/10.1007/s40263-015-0272-9]. [PMID: 26374743]. [DOI] [PubMed] [Google Scholar]
- 117.Pittenger C., Bloch M.H., Wasylink S., Billingslea E., Simpson R., Jakubovski E., Kelmendi B., Sanacora G., Coric V. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial. J. Clin. Psychiatry. 2015;76(8):1075–1084. doi: 10.4088/JCP.14m09123. [http://dx.doi.org/10.4088/JCP.14m09123]. [PMID: 26214725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jahanbakhsh S.P., Manteghi A.A., Emami S.A., Mahyari S., Gholampour B., Mohammadpour A.H., Sahebkar A. Evaluation of the efficacy of Withania somnifera (Ashwagandha) root extract in patients with obsessive-compulsive disorder: A randomized double-blind placebo-controlled trial. Complement. Ther. Med. 2016;27:25–29. doi: 10.1016/j.ctim.2016.03.018. [http://dx.doi.org/10.1016/j.ctim.2016.03.018]. [PMID: 27515872]. [DOI] [PubMed] [Google Scholar]
- 119.Paydary K., Akamaloo A., Ahmadipour A., Pishgar F., Emamzadehfard S., Akhondzadeh S. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2016;41(2):214–219. doi: 10.1111/jcpt.12370. [http://dx.doi.org/10.1111/jcpt.12370]. [PMID: 26931055]. [DOI] [PubMed] [Google Scholar]
- 120.Khalkhali M., Aram S., Zarrabi H., Kafie M., Heidarzadeh A. Lamotrigine augmentation versus placebo in serotonin reuptake inhibitors-resistant obsessive-compulsive disorder: a randomized controlled trial. Iran. J. Psychiatry. 2016;11(2):104–114. [PMID: 27437007]. [PMC free article] [PubMed] [Google Scholar]
- 121.Rutrick D., Stein D.J., Subramanian G., Smith B., Fava M., Hasler G., Cha J.H., Gasparini F., Donchev T., Ocwieja M., Johns D., Gomez-Mancilla B. Mavoglurant augmentation in OCD patients resistant to selective serotonin reuptake inhibitors: a proof-of-concept, randomized, placebo-controlled, phase 2 study. Adv. Ther. 2017;34(2):524–541. doi: 10.1007/s12325-016-0468-5. [http://dx.doi.org/10.1007/s12325-016-0468-5]. [PMID: 28044255]. [DOI] [PubMed] [Google Scholar]
- 122.Feng B., Zhang Z.J., Zhu R.M., Yuan G.Z., Luo L.Y., McAlonan G.M., Xu F.Z., Chen J., Liu L.Y., Lv Y.Y., Wong H.K., Zhang Y., Zhu L.X. Transcutaneous electrical acupoint stimulation as an adjunct therapy for obsessive-compulsive disorder: A randomized controlled study. J. Psychiatr. Res. 2016;80:30–37. doi: 10.1016/j.jpsychires.2016.05.015. [http://dx.doi.org/10.1016/j.jpsychires.2016.05.015]. [PMID: 27281260]. [DOI] [PubMed] [Google Scholar]
- 123.de Leeuw A.S., van Megen H.J., Kahn R.S., Westenberg H.G. d-cycloserine addition to exposure sessions in the treatment of patients with obsessive-compulsive disorder. Eur. Psychiatry. 2017;40:38–44. doi: 10.1016/j.eurpsy.2016.06.011. [http://dx.doi.org/10.1016/j.eurpsy.2016.06.011]. [PMID: 27837671]. [DOI] [PubMed] [Google Scholar]
- 124.Asnaani A., Kaczkurkin A.N., Alpert E., McLean C.P., Simpson H.B., Foa E.B. The effect of treatment on quality of life and functioning in OCD. Compr. Psychiatry. 2017;73:7–14. doi: 10.1016/j.comppsych.2016.10.004. [http://dx.doi.org/10.1016/j.comppsych.2016.10.004]. [PMID: 27838572]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ahmadpanah M., Reihani A., Ghaleiha A., Soltanian A., Haghighi M., Jahangard L., Sadeghi Bahmani D., Holsboer-Trachsler E., Brand S. Buprenorphine augmentation improved symptoms of OCD, compared to placebo - Results from a randomized, double-blind and placebo-controlled clinical trial. J. Psychiatr. Res. 2017;94:23–28. doi: 10.1016/j.jpsychires.2017.06.004. [http://dx.doi.org/10.1016/j.jpsychires.2017.06.004]. [PMID: 28647677]. [DOI] [PubMed] [Google Scholar]
- 126.Modarresi A., Sayyah M., Razooghi S., Eslami K., Javadi M., Kouti L. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2017;51(6):263–269. doi: 10.1055/s-0043-120268. [http://dx.doi.org/10.1055/s-0043-120268] [PMID: 29100251] [DOI] [PubMed] [Google Scholar]
- 127.Costa D.L.C., Diniz J.B., Requena G., Joaquim M.A., Pittenger C., Bloch M.H., Miguel E.C., Shavitt R.G. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J. Clin. Psychiatry. 2017;78(7):e766–e773. doi: 10.4088/JCP.16m11101. [http://dx.doi.org/10.4088/JCP.16m11101]. [PMID: 28617566]. [DOI] [PubMed] [Google Scholar]
- 128.Arabzadeh S., Shahhossenie M., Mesgarpour B., Rezaei F., Shalbafan M.R., Ghiasi Z., Akhondzadeh S. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: A randomized double-blind study. Hum. Psychopharmacol. 2017;32(4) doi: 10.1002/hup.2584. [http://dx.doi.org/10.1002/hup.2584]. [PMID: 28485008]. [DOI] [PubMed] [Google Scholar]
- 129.Alphs L., Benedetti F., Fleischhacker W.W., Kane J.M. Placebo-related effects in clinical trials in schizophrenia: what is driving this phenomenon and what can be done to minimize it? Int. J. Neuropsychopharmacol. 2012;15(7):1003–1014. doi: 10.1017/S1461145711001738. [http://dx.doi.org/10.1017/S1461145711001738]. [PMID: 22217384]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khan A., Fahl M.K., Faucett J., Khan S.S., Brown W.A. Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013. World Psychiatry. 2017;16(2):181–192. doi: 10.1002/wps.20421. [http://dx.doi.org/10.1002/wps.20421]. [PMID: 28498591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan A., Fahl M.K., Schilling J., Brown W.A. Does the rising placebo response impact antihypertensive clinical trial outcomes? An analysis of data from the Food and Drug Administration 1990-2016. PLoS One. 2018;13(2):e0193043. doi: 10.1371/journal.pone.0193043. [http://dx.doi.org/10.1371/journal.pone.0193043]. [PMID: 29489874]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rutherford B.R., Pott E., Tandler J.M., Wall M.M., Roose S.P., Lieberman J.A. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71(12):1409–1421. doi: 10.1001/jamapsychiatry.2014.1319. [http://dx.doi.org/10.1001/jamapsychiatry.2014.1319]. [PMID: 25321611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sugarman M.A., Kirsch I., Huppert J.D. Obsessive-compulsive disorder has a reduced placebo (and antidepressant) response compared to other anxiety disorders: A meta-analysis. J. Affect. Disord. 2017;218:217–226. doi: 10.1016/j.jad.2017.04.068. [http://dx.doi.org/10.1016/j.jad.2017.04.068]. [PMID: 28477500]. [DOI] [PubMed] [Google Scholar]
- 134.Kasper S., Dold M. Factors contributing to the increasing placebo response in antidepressant trials. World Psychiatry. 2015;14(3):304–306. doi: 10.1002/wps.20245. [http://dx.doi.org/10.1002/wps.20245]. [PMID: 26407782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khin N.A., Chen Y.F., Yang Y., Yang P., Laughren T.P. Exploratory analyses of efficacy data from schizophrenia trials in support of new drug applications submitted to the US Food and Drug Administration. J. Clin. Psychiatry. 2012;73(6):856–864. doi: 10.4088/JCP.11r07539. [http://dx.doi.org/10.4088/JCP.11r07539]. [PMID: 22687813]. [DOI] [PubMed] [Google Scholar]
- 136.Tuttle A.H., Tohyama S., Ramsay T., Kimmelman J., Schweinhardt P., Bennett G.J., Mogil J.S. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156(12):2616–2626. doi: 10.1097/j.pain.0000000000000333. [http://dx.doi.org/10.1097/j.pain.0000000000000333]. [PMID: 26307858]. [DOI] [PubMed] [Google Scholar]
- 137.Mancini M., Wade A.G., Perugi G., Lenox-Smith A., Schacht A. Impact of patient selection and study characteristics on signal detection in placebo-controlled trials with antidepressants. J. Psychiatr. Res. 2014;51:21–29. doi: 10.1016/j.jpsychires.2014.01.001. [http://dx.doi.org/10.1016/j.jpsychires.2014.01.001]. [PMID: 24462042]. [DOI] [PubMed] [Google Scholar]
- 138.Perugi G., Akiskal H.S., Gemignani A., Pfanner C., Presta S., Milanfranchi A., Lensi P., Ravagli S., Maremmani I., Cassano G.B. Episodic course in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 1998;248(5):240–244. doi: 10.1007/s004060050044. [http://dx.doi.org/10.1007/s004060050044]. [PMID: 9840370]. [DOI] [PubMed] [Google Scholar]
- 139.Lin H., Yeh C.B., Peterson B.S., Scahill L., Grantz H., Findley D.B., Katsovich L., Otka J., Lombroso P.J., King R.A., Leckman J.F. Assessment of symptom exacerbations in a longitudinal study of children with Tourette’s syndrome or obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(9):1070–1077. doi: 10.1097/00004583-200209000-00007. [http://dx.doi.org/10.1097/00004583-200209000-00007]. [PMID: 12218428]. [DOI] [PubMed] [Google Scholar]
- 140.Hróbjartsson A., Gøtzsche P.C. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N. Engl. J. Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. [http://dx.doi.org/10.1056/NEJM200105243442106]. [PMID: 11372012]. [DOI] [PubMed] [Google Scholar]
- 141.Busch A.B., He Y., Zelevinsky K., O’Malley A.J. Predicting Participation in Psychiatric Randomized Controlled Trials: Insights From the STEP-BD. Psychiatr. Serv. 2015;66(8):817–823. doi: 10.1176/appi.ps.201300557. [http://dx.doi.org/10.1176/appi.ps.201300557]. [PMID: 25828873]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 5th; (DSM-5®). Arlington, VA: American Psychiatric Publishing, Inc; 2013. [Google Scholar]
- 143.American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th; –Text Revision, (DSM-IV-TR™). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 144.Fountoulakis K.N., McIntyre R.S., Carvalho A.F. From randomized controlled trials of antidepressant drugs to the meta-analytic synthesis of evidence: methodological aspects lead to discrepant findings. Curr. Neuropharmacol. 2015;13(5):605–615. doi: 10.2174/1570159X13666150630174343. [http://dx.doi.org/10.2174/1570159X13666150630174343]. [PMID: 26467410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaptchuk T.J., Stason W.B., Davis R.B., Legedza A.R., Schnyer R.N., Kerr C.E., Stone D.A., Nam B.H., Kirsch I., Goldman R.H. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ. 2006;332(7538):391–397. doi: 10.1136/bmj.38726.603310.55. [http://dx.doi.org/10.1136/bmj.38726.603310.55]. [PMID: 16452103]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wartolowska K., Judge A., Hopewell S., Collins G.S., Dean B.J., Rombach I., Brindley D., Savulescu J., Beard D.J., Carr A.J. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. doi: 10.1136/bmj.g3253. [http://dx.doi.org/10.1136/bmj.g3253]. [PMID: 24850821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaptchuk T.J., Goldman P., Stone D.A., Stason W.B. Do medical devices have enhanced placebo effects? J. Clin. Epidemiol. 2000;53(8):786–792. doi: 10.1016/s0895-4356(00)00206-7. [http://dx.doi.org/10.1016/S0895-4356(00)00206-7]. [PMID: 10942860]. [DOI] [PubMed] [Google Scholar]
- 148.Brunoni A.R., Lopes M., Kaptchuk T.J., Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One. 2009;4(3):e4824. doi: 10.1371/journal.pone.0004824. [http://dx.doi.org/10.1371/journal.pone.0004824]. [PMID: 19293925]. [DOI] [PMC free article] [PubMed] [Google Scholar]


