Abstract
Background
Obsessive-compulsive disorder (OCD) is a highly prevalent, severe, and chronic disease. There is a need for alternative strategies for treatment-resistant OCD.
Objective
This review aims to assess the effect of brain stimulation techniques in OCD.
Method
We included papers published in peer-reviewed journals dealing with brain stimulation techniques in OCD. We conducted treatment-specific searches for OCD (Technique AND ((randomized OR randomised) AND control* AND trial) AND (magnetic AND stimulation OR (rTMS OR dTMS)) AND (obsess* OR compuls* OR OCD)) on six databases, i.e., PubMed, Cochrane, Scopus, CINAHL, PsycINFO, and Web of Science to identify randomised controlled trials and ClinicalTrials.gov for possible additional results.
Results
Different add-on stimulation techniques could be effective for severely ill OCD patients unresponsive to drugs and/or behavioural therapy. Most evidence regarded deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS), while there is less evidence regarding transcranial direct current stimulation (tDCS), electroconvulsive therapy, and vagus nerve stimulation (for these last two there are no sham-controlled studies). Low-frequency TMS may be more effective over the supplementary motor area or the orbitofrontal cortex. DBS showed best results when targeting the crossroad between the nucleus accumbens and the ventral capsule or the subthalamic nucleus. Cathodal tDCS may be better than anodal in treating OCD. Limitations. We had to include methodologically inconsistent underpowered studies.
Conclusion
Different brain stimulation techniques are promising as an add-on treatment of refractory OCD, although studies frequently reported inconsistent results. TMS, DBS, and tDCS could possibly find some use with adequate testing, but their standard methodology still needs to be established.
Keywords: Obsessive-compulsive disorder, brain stimulation, deep brain stimulation, direct current transcranial stimulation, transcranial magnetic stimulation, electroconvulsive therapy
1. INTRODUCTION
Obsessive-compulsive disorder (OCD) is a severe psychiatric disorder characterised by obsessive thoughts and/or compulsive acts to a variable degree. It may sometimes be very severe and disabling and run a chronic course. Its prevalence in the US is about 1.2% annually and 2.3% lifetime [1]. It may vary across sampling time, world region and culture, with some countries having lower rates than others (e.g., in Taiwan, the annual prevalence was 0.4% in the early nineties, while other countries ranged from 1.1% [South Korea and New Zealand] to 1.8% [Puerto Rico]) [2]. However, a more recent study found a still lower rate for Taiwan, i.e., 0.00065% [3], while in the same continent, Asia, recent studies showed annual prevalence to vary from 1.8% in Iran [4] to 3% in Singapore [5], and a point prevalence of 3.3% in a southern region of India [6]. In Europe, annual rates are lower than those in the US and more similar to Canadian rates; in Germany, a 0.7% figure was found [7] vs. 0.93% in Canada [8], while a British study found a monthly prevalence of 1.1% [9]. It could also be observed that changes in the nosographic systems (for example, the transition from DSM-IV to DSM-5 or from ICD-9 to -10 or -11) affect the diagnosis and thus the detected prevalence rates.
Obsessions are anxiogenic, undesired, and obstinate thoughts, impulses or images that the patient experiences as ego-dystonic, i.e., as intrusive, distressing, and inappropriate, not in tune with one’s own apparent and perceived feelings.
Compulsions are repetitive and time-consuming behaviours or mental acts that the patient is compelled to endorse often in an attempt to neutralise obsession-induced anxiety [10, 11]. OCD is often associated with markedly impaired interpersonal and occupational functioning [12, 13]. OCD-related disability regards social, psychological and medical aspects of the patient’s life, resulting in remarkable increases of indirect/direct economic/societal costs [14]. The precise aetiology and neurobiology of OCD are currently unclear, but increasing evidence points to an association with orbitofrontal-striatal-pallido-thalamic circuitry dysfunction. Such a circuitry includes the dorsolateral prefrontal (DLPFC), orbitofrontal (OFC), medial prefrontal (MPF), and anterior cingulate (ACC) cortices, the supplementary motor area (SMA), and the basal ganglia [15-17]. The most updated treatment guidelines for OCD propose high-dose selective serotonin reuptake inhibitors (SSRIs; i.e., citalopram, paroxetine, fluvoxamine, sertraline, and fluoxetine) or clomipramine (a tricyclic antidepressant with a marked preference for the serotonin transporter) as first-line treatment strategies; treatment should be carried out over long periods. Furthermore, they benefit from combination with cognitive-behavioural therapy [18, 19]. In OCD-resistant patients, drug treatment has been extended to serotonin- noradrenaline reuptake inhibitors (SNRIs), intravenous clomipramine or citalopram, and/or atypical antipsychotic medication [20, 21]. However, despite a multitude of therapeutic options, about two-thirds of OCD patients fail to reach a satisfactory response, either because they are intolerant to medication untoward effects or because they improve only partially and continue to complain of persistent symptoms that impair their global functioning and well-being [22-24]. Hence, alternative strategies are required to treat severe resistant OCD (defined by non-response to at least one adequate SSRI and/or CBT trial) [25, 26].
Different brain stimulation techniques have been tested in the treatment of refractory OCD, with the aim to change the activity of dysfunctional areas, mainly of the cortico-striato-thalamo-cortical circuitry, thus restoring a level of functioning comparable to what is commonly accepted to represent normal functioning [15, 26].
Severe resistant forms of OCD may benefit from non-invasive techniques, including repetitive transcranial magnetic stimulation (rTMS) [27], deep transcranial magnetic stimulation (dTMS), and transcranial direct current stimulation (tDCS) [27], and the invasive deep brain stimulation (DBS) [28], vagal nerve stimulation (VNS), and electroconvulsive therapy (ECT). Other somatic treatments include ablative stereotactic neurosurgery, which will not be treated here as it is not neurostimulatory. Currently, there is some knowledge of the efficacy of some of these techniques in treatment-resistant OCD, but for each of these, there is no consensus as to the site, frequency, and extent of stimulation, treatment duration and the need for maintenance and no general review of add-on brain stimulation methods for treatment-resistant OCD. This review focuses on the assessment of the efficacy of brain stimulation techniques in OCD patients as emerging from double-blind, sham-controlled trials published in the peer-reviewed literature; where these were not available, we also discussed open trials and case reports. Our aim was to assess the value of each add-on brain stimulation intervention in OCD clinical outcomes (i.e., obsessions and compulsions, as assessed by specific scales).
2. GENERAL METHODOLOGICAL APPROACH
We carried-out specific searches in the PubMed-MedLine-Index Medicus, Cochrane, CINAHL, PsycINFO-PsycARTICLES, Scopus, and Web of Science databases with no time, language or any other restriction. Search strategies will be detailed in the appropriate section for each somatic technique. Inclusion criteria comprised treatment of OCD patients in double-blind conditions vs. sham with a sufficiently appropriate design to obtain a possible clinical response (i.e., protocols with sufficiently long and intense treatment schedules and sample sizes sufficient to obtain meaningful results). We gave priority to randomised control trials (RCTs), which are double-blind trials involving an explicit random assignment to groups, where both raters, investigators and patients have no way of knowing the exact nature of the treatment being administered and have a parallel design. Reviews, meta-analyses and guidelines, case reports, letters, editorials, opinion papers, animal studies, and studies not focusing on or not reporting clinical data that are congruent with our aims (i.e., to investigate efficacy/ effectiveness with the use of appropriate criteria and scales), were not included, but searched in their reference lists for providing additional papers with adequate research data. Papers were individually searched for adherence to our inclusion criteria. Included were sham-controlled trials on OCD patients with data on OCD patients provided separately (i.e., not cumulatively with patients with other disorders) and clinical data at baseline and at the endpoint. Exclusion criteria comprised add-on with no prior stabilisation, missing data, open trials, single-blind trials, case reports, opinion papers-letters-editorials, non-clinical outcomes, unfocused (speaking about other issues, no OCD, including Tourette’s), no TMS (or tDCS, VNS, DBS, ECT, according to the search focusing a given technique), reviews and meta-analyses (the latter were used to identify possible other includible records), and congress/conference abstracts eluding peer-reviewing. Of crossover studies, we included only the proportion of patients who were treated with TMS (tDCS, VNS, DBS, or ECT) or sham (or on-off in the case of DBS) in the first place and counted as the end of the study the time prior to the crossover. Final inclusion criteria for data analysis comprised: single or double-blind design, clearly stated assessment of response (responder rate or percent response on rating scales), randomisation, sufficient time of treatment administration for the expected response to be observed (peculiar to the type of brain stimulation), low risk of bias (as assessed with the Cochrane Risk of Bias Tool [29]), absence or adequate addressing of confounders that could render response not attributable to specific treatments. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [30], applying it to each different database and then making a summary of included papers.
For the specific strategies used for each of the techniques, these will be detailed for each technique in the results section (i.e., strategy, database, and the corresponding output). This is also detailed in Fig. (1)-(5), at the top of each figure for the corresponding technique. The general strategy for all searches was: “((randomized OR randomised) AND control* AND trial) AND (Technique in the forms it can be expressed and truncated as appropriately with an asterisk) AND (obsess* OR compuls* OR OCD)”. We specified each search strategy in Table 1.
Fig. (1).
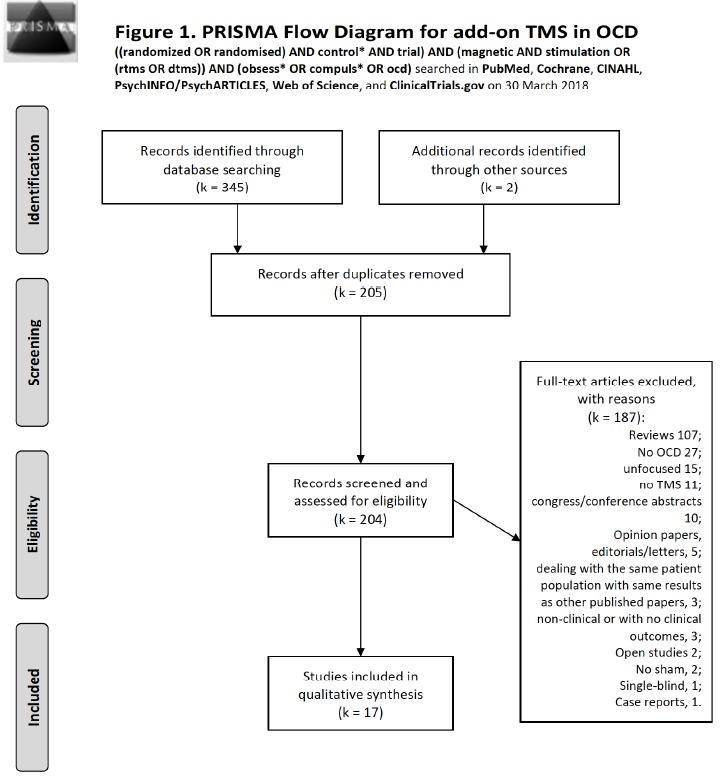
Flow diagram of studies selected for TMS according to the PRISMA statement [30].
Fig. (5).
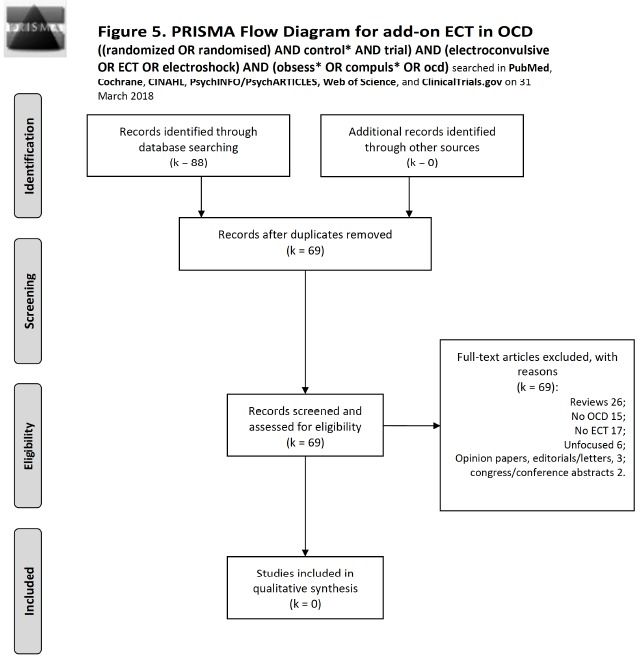
Flow diagram of studies selected for ECT according to the PRISMA statement [30].
Table 1. Search strategies used in identifying studies to include according to the individual brain stimulation technique per database used.
| Technique | Search Strategy | Date of Last Search |
|---|---|---|
| PubMed, Cochrane Library, Scopus, CINAHL, PsycINFO/PsychARTICLES, Web of Science | ||
| rTMS/dTMS | ((randomized OR randomised) AND control* AND trial) AND (magnetic AND stimulation OR (rTMS OR dTMS)) AND (obsess* OR compuls* OR OCD) on all investigated databases. | 30 March 2018 |
| tDCS | ((randomized OR randomised) AND control* AND trial) AND ((transcranial AND direct AND current AND stimulation) OR tDCS) AND (obsess* OR compuls* OR ocd) on all investigated databases. | 30 March 2018 |
| DBS | ((randomized OR randomised) AND control* AND trial) AND (DBS OR “deep brain stimulation” OR Luys[tiab] OR subthalam*) AND (obsess* OR compuls* OR ocd) on all investigated databases. | 30 March 2018 |
| VNS | (randomized OR randomised) AND (“vagus nerve stimulation” OR “vagal nerve stimulation” OR VNS) AND (obsess* OR compuls* OR ocd) on all investigated databases that produced 2 records, both unfocused (1 review and 1 no VNS). | 31 March 2018 |
| ECT | ((randomized OR randomised) AND control* AND trial) AND (electroconvulsive OR ECT OR electroshock) AND (obsess* OR compuls* OR ocd) on all investigated databases. | 31 March 2018 |
| ClinicalTrials.gov | ||
| All | Condition or disease: Obsessive-compulsive disorder AND Other terms: Name of the technique | 31 March 2018 |
All searches were carried out between the 30th and the 31st of March 2018. Search strategies were established prior to undertaking the review. We included randomised controlled trials due to their reliability as possible evidence. When such trials were unavailable, we used data from open trials and case reports or series to discuss the individual techniques, without performing new searches, as many open trials and case reports resulted from our searches.
3. RESULTS AND DISCUSSION FOR INDIVIDUAL TECHNIQUES
3.1. TMS: Results
TMS is a non-invasive technique modulating neural excitability through short pulsed electric currents conveyed in a head coil that creates a fast-changing magnetic field, provoking hyperpolarisation or depolarisation of surface cortical neurons [31]. Different data showed increased neural excitability related to high-frequency (5 Hz or more) [32], and reduced excitability to low-frequency stimulation (1 Hz or less) [33, 34]. For transcranial magnetic stimulation (TMS), we used the following search syntax: ((randomized OR randomised) AND control* AND trial) AND (magnetic AND stimulation OR (rTMS OR dTMS)) AND (obsess* OR compuls* OR OCD) on all investigated databases. On PubMed (Medline-Index Medicus), we conducted a search on 30 March 2018; this retrieved 47 records. Of them, no intra- database duplicate was found and 14 were includible. On the same day, the same search for title, abstract and keywords at the Cochrane Database produced 52 records, of which 1 other review, 1 Cochrane review, and 50 trials were observed. Of them, 5 were intra-database duplicates and 29 were new to PubMed, adding 1 to includible. Scopus produced 110 records, 60 were new to previous searches, 1 was intra-database duplicate; it added none to includible. The CINAHL database yielded 8 records, with 1 new to previous searches, no intra-database duplicate and none added to includible. PsycINFO/PsycARTICLES yielded 25 records. There were 0 intra-database duplicates, 6 were new to above searches, and 0 added to includible. The Web of Science database produced 102 records, that had 0 intra-database duplicate, 61 were new to above searches, and added 1 to includible. ClinicalTrials.gov produced 25 records that added none to includible (Search: transcranial magnetic stimulation combined with obsessive-compulsive disorder). Of these, 13 trials were completed, 1 was active and not recruiting, 1 was prematurely terminated due to few subjects recruited, 1 study’s status was unknown, 9 were still recruiting. Of all the studies, 3 had results, one unpublishable, one published [35], and the other was open-label and carried-out on hoarding disorder only. Two records were added that were identified serendipitously and could not be identified through the above searches. One was includible and the other was not. The total number of records was 345. There remained 203 records after excluding duplicates. Reasons for exclusion were: 107 reviews; No OCD 27; unfocused 15; no TMS 11; congress/conference abstracts 10; opinion papers, editorials/letters, 5; dealing with the same patient population with same results as other published papers, 3; 3 were non-clinical or with no clinical outcomes; Open studies 2; no sham, 2; single-blind, 1; case reports, 1. This left 16 records to include. Literature search results according to the PRISMA statement are shown in Fig. (1). Table 2 summarises the included studies and their results. We considered as an adequate design the application of at least one full cycle of rTMS (one month of at least five weekly sessions). We will discuss literature according to the focus area stimulated by the technique.
Table 2. Sham-controlled studies of transcranial magnetic stimulation in OCD patients.
| Study | Active rTMS/dTMS | Sham rTMS/dTMS | rTMS/dTMS Parameters | Psychiatric Comorbidity | Treatment | Results | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age±SD (years) | Female/ male | N | Age±SD (years) | F/M (N) | Brain target | Frequency (Hz)/sessions | % MT | |||||||||||||||||||||||||||||
| rTMS/dTMS studies: High Frequency HF (range 5-20 Hz) | |||||||||||||||||||||||||||||||||||||
| Greenberg et al., 1997 [36] | 12 | 36.9±10.2 | 6/6 | rDLPFC lDLPFC |
20/1 | 80 | 6 current or past major depression | 4 unmedicated 5 fluoxetine, 3 paroxetine |
Mood improvement and reduction of movement urge after right stimulation |
||||||||||||||||||||||||||||
| Sachdev et al., 2007 [39] | 10 | 29.5±9.9 | 3/7 | 8 | 35.8±8.2 | 5/3 | lDLPFC | 10/10 | 110 | None | Augmentation | rTMS over the left DLPFC is ineffective for treatment-resistant OCD | |||||||||||||||||||||||||
| Sarkhel et al., 2010 [32] | 21 | 29.4±6.5 | 11/10 | 21 | 31.9±7.8 | 8/13 | rDLPFC | 10/10 | 110 | Mild depressive symptoms: mean baseline score on the 17-item HAM-D of 12.3±2.4. | Augmentation | High-frequency right prefrontal rTMS does not have any significant effect in OCD | |||||||||||||||||||||||||
| Badawy et al., 2010 [42] | 40 | 26.9±6.7 | 18/22 | 20 | 28.9±5.7 | 13/7 | lDLPFC | 20/15 | None | 20: SSRIs + active rTMS; 20: none + active rTMS; 20 none + sham rTMS | rTMS was not effective as a single treatment but it was effective as add-on treatment | ||||||||||||||||||||||||||
| Mansur et al., 2011 [41] | 13 | 42.1±11.9 | 6/7 | 14 | 39.3±13.9 | 8/6 | rDLPFC | 10/30 | 110 | 23: unipolar depression, 3: bipolar disorder, 7: anxiety disorders, 2: alcohol abuse, 5: motor tics. | Augmentation | Active rTMS over the rDLPFC does not appear to be superior to sham rTMS | |||||||||||||||||||||||||
| Ma et al., 2014 [43] | 25 | 27.12±8.97 | 8/17 | 21 | 29.86±9.42 | 8/13 | Bilateral DLPFC | α-band (8-12 Hz)/10 | 80 | None | Augmentation | αTMS over DLPFC bilaterally could not improve response to drug treatment for OCD patients |
|||||||||||||||||||||||||
| Jahangard et al., 2016 [46] | 5 | 32.40±8.97 | 4/1 | 5 | 33.80 ± 5.81 | 3/2 | Bilateral DLPFC | 20/10 | 100 | None | Augmentation | Y-BOCS scores decreased significantly during the rTMS condition but not during the sham condition |
|||||||||||||||||||||||||
| Study | Active rTMS/dTMS | Sham rTMS/dTMS | rTMS/dTMS Parameters | Psychiatric Comorbidity | Treatment | Results | |||||||||||||||||||||||||||||||
| N | Age±SD (years) | Female/ male | N | Age±SD (years) | F/M (N) | Brain target | Frequency (Hz)/sessions | % MT | |||||||||||||||||||||||||||||
| rTMS/dTMS studies: Low Frequency LF (1 Hz) | |||||||||||||||||||||||||||||||||||||
| Alonso et al., 2001 [37] | 10 | 39.2±13.0 | 8/2 | 8 | 30.3±9.5 | 4/4 | rDLPFC | 1/18 | 110 | None | Partial augmentation, except 2, none+active rTMS; and 1, none +sham rTMS | rTMS failed to produce significant improvement of OCD and was not significantly different from sham | |||||||||||||||||||||||||
| Prasko et al., 2006 [38] | 20 | 28.4±7.4 | 5/15 | 14 | 33.6± 8.4 | 8/6 | lDLPFC | 1/10 | 110 | None | Augmentation | rTMS did not differ from sham rTMS in facilitating the effect of serotonin reuptake inhibitors | |||||||||||||||||||||||||
| Kang et al., 2009 [40] | 10 | 28.6±12.7 | 2/8 | 10 | 26.2±10.5 | 1/9 | rDLPFC, SMA | 1/10 | 110 | 7 patients with MDD | Augmentation | rTMS of rDLPFC and SMA had no therapeutic effect on OCD symptoms | |||||||||||||||||||||||||
| Ruffini et al., 2009 [48] | 16 | 41.5±9.06 | 6/10 | 7 | 39.3±9.55 | 3/4 | lOFC | 1/15 | 80 | None | Augmentation | rTMS produced significant but time-limited improvement in OCD patients compared to sham treatment | |||||||||||||||||||||||||
| Mantovani et al., 2010 [35] | 9 | 39.7±8.6 | 4/5 | 9 | 39.4±10.2 | 3/6 | Pre-SMA | 1/20 | 100 | 10 with moderate non-psychotic MDD | 13: SSRI; 5: support psychotherapy during trial | Low-frequency active rTMS delivered to SMA resulted in more clinical responders compared sham. Differences in response rates were not statistically significant | |||||||||||||||||||||||||
| Gomes et al., 2012 [51] | 12 | 35.5±7.5 | 8/4 | 10 | 37.5±6 | 5/5 | Pre-SMA | 1/10 | 100 | 17 with MDD | Augmentation | Significant difference between active and sham stimulation | |||||||||||||||||||||||||
| Mantovani et al., 2013 [52] | 9 | 39.7±8.6 | 4/5 | 9 | 39.4±10.2 | 3/6 | Pre-SMA | 1/20 | 100 | Mild depressive symptoms | Augmentation | Clinical response rate in 18 patients was 67% (6 out of 9)with active and 22%(2 out of 9) with sham rTMS |
|||||||||||||||||||||||||
| Nauczyciel et al., 2014 [49] | 8 | 40 | 6/2 | 7 | 39 | 6/1 | rOFC | 1/10 | 120 | 6 major depressive disorder | Augmentation | Significant decrease from baseline in the Y-BOCS scores after both active and sham rTMS | |||||||||||||||||||||||||
| Study | Active rTMS/dTMS | Sham rTMS/dTMS | rTMS/dTMS Parameters | Psychiatric Comorbidity | Treatment | Results | |||||||||||||||||||||||||||||||
| N | Age±SD (years) | Female/ male | N | Age±SD (years) | F/M (N) | Brain target | Frequency (Hz)/sessions | % MT | |||||||||||||||||||||||||||||
| rTMS/dTMS studies: Low Frequency LF (1 Hz) | |||||||||||||||||||||||||||||||||||||
| Hawken et al., 2016 [54] | 10 | 33±10 | 11/11 non-specified for each group | 12 | 34±14 | 11/11 non-specified for each group | Bilateral SMA | 1/25 | 110 | Mild depressive symptoms | Augmentation | rTMS over the bilateral SMA improved OCD symptoms; the effect was maintained for at least six weeks after treatment end | |||||||||||||||||||||||||
| Seo et al., 2016 [45] | 14 | 34.6±9.8 | 6/8 | 13 | 36.3±12.5 | 7/6 | rDLPFC | 1/15 | 100 | Active group 12 MDD, sham group 10 MDD | Augmentation | 1 Hz rTMS over the right DLPFC appeared to be superior to sham rTMS for relieving OCD symptoms | |||||||||||||||||||||||||
| Pelissolo et al., 2016 [53] | 20 | 39.1±10.4 | 7/13 | 16 | 42.3±10.6 | 9/7 | Pre-SMA | 1/20 | 100 | Mild depressive symptoms | Augmentation | rTMS applied to the Pre-SMA seems ineffective for the treatment of OCD | |||||||||||||||||||||||||
| rTMS/dTMS studies comparison High vs Low Frequency | |||||||||||||||||||||||||||||||||||||
| Elbeh et al., 2016 [44] | 15 | 26.8±75.2 | 11/4 | 15 | 25.5±4 | 10/5 | rDLPFC | 1/10 | 100 | 29 SSRIs, 12 tricyclics, 4 unmedicated | 1 Hz rTMS has better effect on OCD symptoms than 10 Hz or sham | ||||||||||||||||||||||||||
| 15 | 28.9±73.9 | 9/6 | rDLPFC | 10/10 | 100 | ||||||||||||||||||||||||||||||||
| Carmi et al., 2018 [47] | 7 | 36 ± 2.1 | 7/9 | 8 | 35 ± 3.5 | 7/7 | mPFC, ACC | 20/25 | 100 | None | Augmentation | 20 Hz dTMS over the mPFC-ACC alleviates OCD symptoms, Y-BOCS scores were significantly improved compared to 1 Hz and sham | |||||||||||||||||||||||||
| 8 | 28 ± 3.1 | 4/4 | mPFC, ACC | 1/25 | 110 | ||||||||||||||||||||||||||||||||
Abbreviations: ACC, Anterior Cingulate Cortex; DLPFC, Dorsolateral Prefrontal Cortex; dTMS, Deep Transcranial Magnetic Stimulation; HAM-D, Hamilton Depression Rating Scale; l, left; MDD, Major Depressive Disorder; mPFC, Medial Prefrontal Cortex; MT, Motor Threshold; OCD, Obsessive-Compulsive Disorder; OFC, Orbitofrontal Cortex; r, right; rTMS, Repetitive Transcranial Magnetic Stimulation; SMA, Supplementary Motor Area; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
3.2. TMS: Discussion of Results
3.2.1. DLPFC
Greenberg et al. first investigated during the mid-nineties the effect of the DLPFC rTMS in refractory OCD [36]. They randomised 12 patients to single sessions of active TMS on three separate days; each session consisted of 20 Hz for two seconds applied every minute for 20 minutes at 80% of the motor threshold (MT) conveyed respectively to the right (rDLPFC), left (lDLPFC), and midoccipital (control) sites. RTMS over the rDLPFC significantly improved patient-rated compulsions for 8 hours (but not obsessions), showing a limited
effect on mood enduring 30 min after the end of each session. Nevertheless, subsequent rTMS studies did not confirm these findings. Other two low-frequency (1 Hz) rTMS studies obtained no significant differences between active and sham groups, although showing significant OC symptom reductions in the active group [37, 38]. Other studies investigated the effect of rTMS on prefrontal cortices [29-37]. In a double-blind study, OCD patients were randomised to high-frequency (10 Hz) rTMS or sham rTMS for 10 sessions of daily stimulation over the left DLPFC, no significant effect in OCD symptoms was detected [39]. Another rTMS study over the rDLPFC conducted on 10 treatment-resistant OCD patients did not show significant differences after four weeks of treatment in the active vs. sham group on Y-BOCS scores, although it showed a significant effect of time on symptoms in the active rTMS group [40].
Low-frequency (1 Hz) rTMS over the DLPFC appeared to be superior to sham rTMS in relieving OCD symptoms [44, 45]. In two high-frequency DLPFC stimulation studies, active rTMS was not superior to sham [38, 40]. In contrast, other high-frequency studies [42, 46, 47] found significant improvement with active TMS.
Side effects with rTMS included headache, which also occurred in the sham group [47] and was transient [40]; another concern was localised scalp pain [40]. Another side effect was considered to be an improvement in cognition that could have occurred due to the relief of the OC symptom burden [46]. Few studies were laid with attrition bias and just one with reporting bias [42]. Regretfully, this was the study with the larger sample among the considered ones.
3.2.2. SMA
RCT studies showed inconsistent results about the efficacy of rTMS over the DLPFC in refractory OCD, leading to focus on other cortical targets, mainly including the OFC and SMA. Mantovani et al. [35, 50] were the first to conduct studies on SMA stimulation. In a two-week open trial conducted in 10 comorbid OCD-Tourette patients, low-frequency rTMS (1 Hz, 100%MT, for 10 days in two consecutive weeks) correlated with a progressive decrease of Y-BOCS scores [50]. The first randomised sham-controlled study involved four weeks of low-frequency SMA stimulation (1 HZ, 100% MT, 1200 stimuli/day) in refractory OCD patients [35]. This study showed a 25% drop of Y-BOCS scores in the active treatment and a 12% drop in the sham groups, and 67% response rate in active and 22% in sham rTMS [35].
A double-blind sham-controlled study in refractory OCD explored the effects and safety of two-week low-frequency rTMS, first over the rDLPFC (1 Hz, 110% of MT) and then on the SMA (1 Hz, 100% of MT) [30]. It showed significant improvement in OC, anxiety and mood symptoms at treatment end and two weeks later; however, patients in both verum and sham groups responded and there were no significant differences between the two groups [40].
Another study that used low-frequency stimulation was by Gomes et al. [51]. The investigators randomised 22 patients to active (k=12) or sham rTMS (k=10) over the pre-SMA area (bilaterally) for two weeks. Patients were rated before treatment initiation, immediately after treatment cycle completion, and three months thereafter. Three months later, the response rate was 41% with active and 10% with sham treatment, indicating that the verum induced profound, long-term changes in brain activity. Patients receiving active rTMS showed a mean 35% reduction on the Y-BOCS, compared with 6.2% in those receiving the sham. More recently, Mantovani et al. [52] assessed the efficacy of low-frequency rTMS (1 Hz, 100% of MT, 1200 pulses/day for four weeks) stimulation of bilateral SMA in 18 OCD patients. After four weeks of treatment, clinical response rate (defined as Y-BOCS reduction of 25% or more, which is a little less than the 35% drop usually adopted) was 67% in the active and 22% in the sham rTMS group, with a 25% mean reduction on the Y-BOCS in the active group vs. 12% in the sham group. The difference remained significant even after adjusting for baseline depression scores. Clinical improvement in the active rTMS group was correlated with a normalisation of hemispheric asymmetry and with an increase of the right MT. Other recently conducted RCTs in patients with severe and refractory OCD treated with 4-weeks rTMS on the pre-SMA found no significant differences between rTMS and sham stimulation [53]. In a multi-site study [54], rTMS applied bilaterally to the SMA significantly improved clinical OCD symptoms, with a sustained effect for at least six weeks after the end of treatment.
3.2.3. OFC
The other region of interest in the practice of TMS in OCD treatment is the OFC. This region plays a major role in the pathophysiology of OCD, since obsessions and compulsions seem to be mediated by its functional hyperactivity, either bilaterally [55] or restrictedly to its left side [56, 57]. Ruffini et al. [48] conducted the only study of rTMS over the left OFC in drug-resistant OCD patients. In this sham-controlled study, patients were randomly administered real (k=16) or sham (k=7) low-frequency rTMS on weekdays during three consecutive weeks (1 Hz, 80% of the MT). A significant reduction of Y-BOCS scores comparing active versus sham treatment was found at 3 and 10 weeks after the end of the rTMS sessions, but significance was lost after 12 weeks. The intensity of anxiety and depression symptoms was reduced, but no significant differences between the two groups were found. This study suggests that low-frequency rTMS of the OFC may only temporarily improve obsessive-compulsive (OC) symptoms.
A pilot study by Nauczyciel et al. [49] was conducted to assess the efficacy of low-frequency repetitive transcranial magnetic stimulation (rTMS) over the right orbitofrontal cortex (OFC). They found that Y-BOCS scores were significantly reduced from baseline after both active and sham stimulation but observed no difference vs. baseline one month after the end of the second treatment period. These results from RCTs over SMA and OFC are promising, as they obtained statistical significance favouring active over sham low-frequency rTMS.
Overall, rTMS was found to represent a valid alternative for OCD patients who responded poorly to medication, with a quite favourable adverse event profile [58]. Considering globally the results emerging from the studies listed in Table 2, we observe a remarkable convergence with the results of a recent meta-analysis showing rTMS to be efficacious for OCD, underlying the importance of LF-rTMS, OFC, and SMA protocols [58]. We await further developments with the application of deep TMS (dTMS), which could represent a step forward in the direction of non-invasive techniques to supplement current treatment approaches.
3.2.4. The Recent US FDA Approval of dTMS for Treatment-resistant OCD
On August 17, 2018, the US FDA approved dTMS for treatment-resistant OCD. This decision was based on the data of a study showing dTMS to reduce Y-BOCS scores compared to sham stimulation, this was a multicentre trial of 49 patients receiving H7 coil stimulation vs. 51 receiving sham [https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm617244.htm]. In this study [clinicaltrials.gov, study NCT02229903, alias CTP-OCD-01], responders (at least 30% reduction of Y-BOCS scores at endpoint compared to baseline) were 38% in the verum group vs. 11% in the sham. A first study had compared the low (1 Hz) with high (20 Hz) frequency stimulation vs. sham and had found the significant advantage of the high-frequency stimulation over the low and the sham [47]. The results of NCT02229903 have not yet been published.
On the whole, seven TMS studies used low frequencies, eleven high, which were all rTMS, and two (one rTMS and one dTMS) used both with the purpose to compare them. Despite a 20-year history of TMS in OCD, the issue of whether to adopt high or low frequencies and which brain region to target with rTMS is still unresolved.
3.3. Transcranial Direct Current Stimulation (tDCS)
3.3.1. Search Method and Results
For tDCS we used the following search syntax: ((randomized OR randomised) AND control* AND trial) AND ((transcranial AND direct AND current AND stimulation) OR tDCS) AND (obsess* OR compuls* OR ocd) on all investigated databases. On PubMed (MEDLINE), we conducted a search on 30 March 2018; this retrieved 7 records. Of them, there was 0 intra-database duplicate and 1 was includible. Cochrane produced 11 records with 4 intra- database duplicates and 4 new records; none of them but the one already identified by PubMed was includible. Scopus retrieved 17 documents, 10 were new to previous searches, no intra-database duplicate, and none added to includible (it identified the study to include that was already identified by PubMed). CINAHL produced 4 records of which 1 was an intra-database duplicate, adding none to previous searches and to includible. However, it identified the includible article. PsycINFO/PsycARTICLES produced 6 records (0 intra-database duplicates), adding 1 to previous searches and none to includible. The Web of Science produced 20 records (0 intra-database duplicates), adding 12 to previous searches and none to includible; one not includible was identified through other data sources, thus bringing the total number of records to 35 after elimination of inter-database duplicates Fig. (2). Finally, ClinicalTrials.gov searched for obsessive-compulsive disorder (condition or disease) and transcranial direct current stimulation (other term) produced 7 trials, of which 2 were completed, 4 are recruiting, and one enrolling by invitation; none had data or were includible. Of the two completed, one was a comparison of bifocal rTMS vs bifocal tDCS (NCT03284671), still not published, the other was (NCT02329587) double-masked with tDCS, but still not published. The results of our search with reasons of exclusion are shown in Fig. (2). The only study to base on was D’Urso et al. [59] (Table 3). Although this study lacked sham, we included it because it compared anodal with cathodal tDCS polarity over the pre-SMA. A 1-2 weeks of treatment (5-10 sessions) was considered as sufficient to yield meaningful results.
Fig. (2).
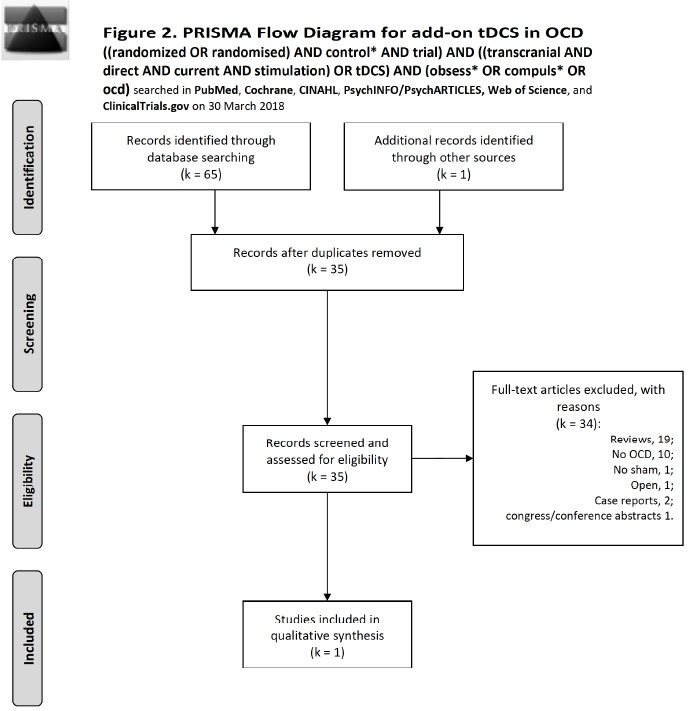
Flow diagram of studies selected for tDCS according to the PRISMA statement [30].
Table 3. Controlled studies of transcranial direct current stimulation in OCD patients.
| Study | N | Age, Years (Range) |
Female/
male (N) |
Design | Observation (Months) | tDCS Target | Results/Conclusions |
|---|---|---|---|---|---|---|---|
| D’Urso et al., 2016 [59] | 12 | 39 (range 20-65) | 7/5 | Patients received initially 10 anodal (n = 6) or cathodal (n = 6) daily for 5 days a week 2 mA/20 min tDCS sessions with the active electrode on the bilateral pre-SMA. If no improvement or no changes: 10 more sessions. In case of symptom worsening after the first 10 sessions: switch to the other polarity for 10 more sessions. |
1 | Pre-SMA, bilaterally | After 10 sessions: 50% of anodal were switched to cathodal; 100% cathodal continued on the same polarity. Cathodal tDCS correlated with significant mean Y-BOCS score decrease. Anodal tDCS showed no pre-post differences. Cathodal, but not anodal pre-SMA tDCS significantly improved OC symptoms. |
tDCS, transcranial Direct Current Stimulation; mA, milliAmpère; OCD, Obsessive-Compulsive Disorder; pre-SMA, pre Supplementary Motor Area; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
3.3.2. dTCS: Discussion of Results
Transcranial direct current stimulation (tDCS) showed promising results in the treatment of different neuropsychiatric disorders [60] and has been proposed for treating refractory OCD [61]. TDCS is considered to represent a non-invasive, low-intensity brain stimulation technique with continuous current over the head applied with scalp electrodes [62], thus influencing brain activity.
This brain stimulation technique is able to modulate neural excitability without triggering action potentials, as the current is always subthreshold, and can facilitate or inhibit neural activities according to electrode polarity [63,64]. dTCS is classified as “anodal” or “cathodal” according to the polarity of the active electrode that is placed over a targeted cortical region to induce local neurophysiological changes, and from there, in connected brain areas [65]. tDCS increases the excitability of the motor cortex and regional cerebral blood flow through anodal stimulation [66] or decreases it through cathodal stimulation [67]. As of now, which areas should be targeted and which parameters should be adopted are matters still to be settled, and neuroimaging may provide a guide [68, 69]. Making treasure of the results of TMS in OCD, it has been hypothesised that applying cathodal tDCS over abnormally hypoactive brain regions (pre-SMA) would decrease OC symptoms by modulating the brain network whose hyperactivity underlies symptoms of OCD. For example, imaging studies revealed an interaction between pre-SMA hypoactivity and deficit in response inhibition, with consequent striatal hyperactivity in patients with OCD [70]. The use of cathodal tDCS over the OFC could be justified by the evidence of OFC hyperactivity in patients with OCD [69]. Targeting the DLPFC is based on studies reporting beneficial clinical effects when stimulating this specific brain region in numerous psychiatric conditions [70]. In one case report, cathodal tDCS proved ineffective when applied to the DLPFC [71], whereas a >40% reduction on the Yale-Brown Obsessive Compulsive Severity Scale was found in two cases of treatment-resistant OCD after anodal tDCS to the pre-SMA [72]. In contrast, D’Urso et al. [59, 73] reported, in a randomised cross-over trial of anodal vs. cathodal tDCS to the pre-SMA, that the cathodal placement was significantly superior to anodal tDCS in reducing OCD symptoms. The results of this study are in the same line with the findings of clinical efficacy of inhibitory rTMS to the pre-SMA [35]. However, it is in contrast with the idea of pre-SMA hypoactivity triggering striatal hyperactivity. tDCS showed efficacy in reducing OCD symptoms, but this effect was reported only in case reports and in non-controlled clinical studies with small sample sizes [74-78]. In a randomised, controlled, partial crossover trial, tDCS over the bilateral pre-SMA significantly reduced OCD symptoms [59]. This effect was polarity-specific since only cathodal and not anodal tDCS showed a positive therapeutic effect. Despite methodological limitations and the heterogeneity of stimulation parameters, tDCS appears to be a promising tool for decreasing OC symptoms as well as comorbid depression and anxiety in patients with treatment-resistant OCD. Few studies investigated the effects of tDCS in OCD, but they showed encouraging results, with some of them reporting a decrease ≥35% in Y-BOCS scores [76]. Sham-controlled studies are needed to confirm these preliminary results.
Summarising, tDCS can be considered as a promising tool for treating refractory OCD [79], but first, we should resolve the anodal vs. cathodal issue and decide which is the best brain area to stimulate. Side effects are few and tolerable, mainly consisting of transient mild headache, itch, tingling and redness at the electrode application site [59]. No risk of bias was encountered for the included study in our analysis [59].
3.4. Deep Brain Stimulation (DBS)
3.4.1. Search Method and Results
For DBS we used the following search syntax: ((randomized OR randomised) AND control* AND trial) AND (DBS OR “deep brain stimulation” OR Luys[tiab] OR subthalam*)
AND (obsess* OR compuls* OR ocd) on all investigated databases, save Cochrane, which does not tolerate [tiab]. On PubMed (MEDLINE), we conducted a search on 30 March 2018; this retrieved 32 records. Of them, 0 were intra-database duplicates and 3 were includible. On the same day, the same search without the [tiab] specification on the Cochrane Database produced 29 records, of which 1 other review, 0 Cochrane reviews, and 28 trials were observed. Of them, 19 were new to the previous search and 2 were intra-database duplicates, adding 0 to includible. Scopus produced with the same strategy 93 records, of which 55 were new to previous searches, 2 were intra-database duplicates and added 1 to includible. The CINAHL database, using the same strategy as PubMed, yielded 8 records, with no intradatabase duplicates, contributing 1 new to previous searches, and contained 0 includible. PsycINFO/PsycARTICLES with the same strategy yielded 20 records, of which 2 were intradatabase duplicates; it contributed 4 new to previous searches and contained none includible. The Web of Science database produced 105 records, had no intra-database duplicates, 74 were new to above searches, and added 0 to includible. The total number of records was 287. There remained 185 records after excluding duplicates. Reasons for exclusion were: 94 reviews; no OCD, 47; opinion papers, editorials/letters, 12; congress/conference abstracts 8; no DBS 5; 2 were non-clinical and 3 with no clinical or with aim- incongruent outcomes; Open studies 2; Unfocused 1; No sham, 1; Surveys, 1; data of OCD patients lumped with those of other patients, 1; case reports, 1; inadequate design such as sample too low to obtain meaningful differences or treatment provided for too short time or at insufficient dosing, 1; technical, 1; animal/cell or tissue studies, 1; dealing with the same patient population with same results as other published papers, 0. This left 4 records to include. The results of our search and the reasons for exclusion are shown in Fig. (3). Table 4 summarises the results and characteristics of included studies. We considered as adequate treatment to obtain valid results a stimulation period of at least two weeks.
Fig. (3).
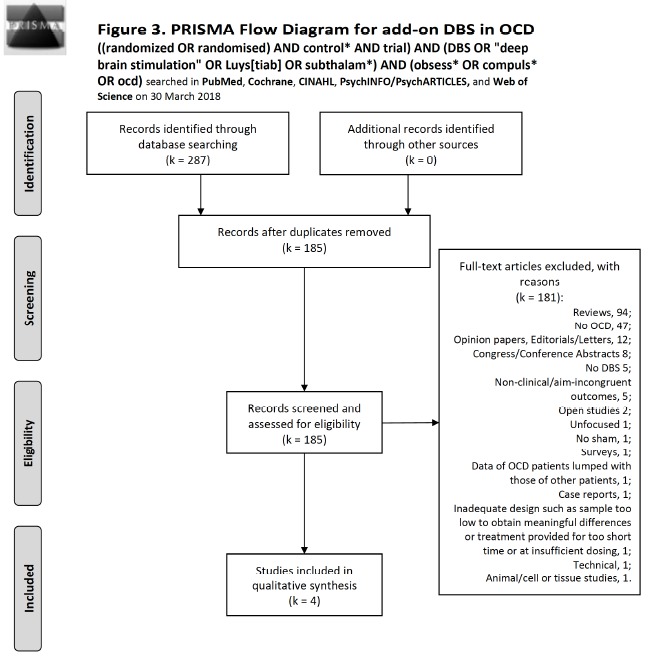
Flow diagram of studies selected for DBS according to the PRISMA statement [30].
Table 4. Sham-controlled studies of DBS in OCD patients.
| Study | N | Age, Years (Range) | Female/ Male (N) | Design | Observation (Months) | DBS Target | Results/Conclusions |
|---|---|---|---|---|---|---|---|
| Mallet et al., 2008 [97] | 17 | 43.05 (29-56) | 7/10 | Randomized, double-blind, crossover study: two 3-month, and a 1-month washout phases | 10 | Subthalamic nucleus | Active (second) vs. sham (first) stimulation of the subthalamic nucleus: Y-BOCS scores was significantly lower. |
| Goodman et al., 2010 [89] | 6 | 36.2 (27-52) | 4/2 | Randomized, staggered-onset study: either 30- or 60-days (blind) stimulation following surgery | 12 | Ventral capsule/ ventral striatum, bilaterally | 12 months of active stimulation: response in 66.7% (≥35% Y-BOCS improvement or Y-BOCS severity ≤16). Sham stimulation: no improvements. |
| Denys et al., 2010 [95] | 16 | 42.56 (21-59) | 7/9 | Three sequential treatment phases: open 8-month treatment phase; double-blind crossover phase with 2-weeks of active or sham stimulation; open 12-month maintenance |
21 | Nucleus accumbens, bilaterally | Bilateral nucleus accumbens DBS may be an effective and safe treatment. Open phase: 46% Y-BOCS decrease; double-blind, sham-controlled phase: active vs. sham treatment showed 25% Y-BOCS score difference |
| Baas et al., 2014 [92] | 8 | 39.2 (27-60)* | 4/4 | Double-blind, crossover study; 2-weeks of active or sham stimulation | 1 | Ventral internal capsule, bilaterally | Decrease in OC, anxiety, and depressive symptoms. |
*Patients were all from Denys et al. [95]. DBS, deep brain stimulation; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
4. DISCUSSION OF DBS RESULTS
Alternatively to ablative neurosurgery, DBS has been proposed as a last-resort option for OCD patients who show resistance to all treatments, with severe symptoms that lead to marked functional impairment [80-82]. DBS involves the implantation of electrodes deep in the brain, the activation of which ensues in electrical stimulation of specific brain sites; this enables focal, adjustable, and reversible neuromodulation. Abnormalities in activity and synaptic connectivity in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and in the striatal circuitry suggested the possible efficacy of DBS in refractory OCD [15, 17, 83]. About 53% of ventral capsule/ventral striatum (VC/VS) or nucleus accumbens (NA) DBS, and 41% subthalamic nucleus (STN) DBS showed OC symptom improvement [84]. The proportion of responders, i.e., patients with a reduction in their symptom severity of at least 35%, is poorly defined, and can vary in the range of 10-61% [85, 86]. Such wide variations may be partly attributed to differences in neuroanatomical targeting, type of electrode, and stimulation protocol.
We here discuss the results of DBS studies in refractory OCD based on specific targeted areas. Considering the invasiveness of the technique, DBS studies have stringent and uniform inclusion and response criteria as compared to TMS studies. This resulted in the inclusion of patients who are only fully refractory to treatment, with at least 5 years of illness, and minimum Y-BOCS score of 25. Treatment response was defined as an improvement of at least 35% of mean Y-BOCS score from baseline.
4.1. Anterior Limb of Internal Capsule (ALIC)
In 1999, the first results were published on bilateral ALIC DBS in 4 patients with OCD [84]. In 3 of 4 patients, beneficial effects were observed but not objectified by Y-BOCS scores. These 4 patients and 2 others were followed up for a period of at least 21 months, at which time, 3 patients were considered as responders, as they showed an at least 35% decrease in symptom severity as evidenced through their scores on the Y-BOCS [85].
Moreover, the real DBS effects (active electrode) seemed to outweigh placebo effects (electrode off), as an average symptom change of 12.5 points (40%) was observed between double-blinded on and off stimulation. In a case of a patient with OCD who was subjected to ALIC DBS, a 79% Y-BOCS reduction at 3-month follow-up and complete remission at 10-month follow-up were reported [86]. However, the initially positive effects of ALIC DBS there obtained, did not receive full replication in a double-blind controlled study [87]. Of the 4 patients, only 1 patient had a decrease of more than 35% in the double-blind phase. Nevertheless, this patient further improved with a 73% Y- BOCS score reduction 8 months later, and another patient improved by 44% after the addition of intensive behavioural therapy.
Decreased OFC activity was observed on positron emission tomographic scans only in these two responders, suggesting that ALIC DBS can improve OCD when it is able to restore the inhibitory function of the ventral cortico-striatal-thalamo-cortical (CSTC) pathway, a part of the salience network.
Based on these first studies in small OCD samples, ALIC DBS seemed to have only modestly positive effects, which warranted exploration of other targets. As high voltages were often needed to achieve positive effects with ALIC DBS, and because the most distal parts of the ALIC electrodes were located in the ventral striatum (VS) and the nucleus accumbens (NAcc), these ventral targets were subsequently explored for treatment of OCD.
4.2. Ventral Capsule/ventral Striatum (VC/VS)
A first open study with DBS (quadripolar) of the bilateral VC and VS was conducted in 10 patients with refractory OCD that started stimulation three weeks later electrode implantation [88]. This study showed that 75% of patients showed clinical response (1/2 showed ≥35% drop of Y-BOCS scores, and 1/4 a drop of 25%) after 36 months.
A randomised double-blind controlled study [89] confirmed these positive results for VC/VS DBS. In this study, six patients with OCD were implanted with bilateral VC/VS electrodes, with three of them receiving the active stimulation, whereas the other three received sham for one month and the verum for another month.
Although Y-BOCS reductions did not significantly differ between sham and verum, clinical improvement was seen in both only when the device was active. At the 1-year follow-up, Y-BOCS dropped by 15.7 points (47%) in the total group, with four of the six patients being classified as DBS- responders. Comorbid depressive symptoms improved with DBS in all 6 patients after one year. The results of two open studies of VC/VS DBS in OCD patients were in the same direction, with one finding an average 22.2 point drop on the Y-BOCS (60%) after two years in four OCD patients [90] and the other a 12.2 point drop (33%) after 15 months in another four patients [91]. Summarising, beneficial effects on OCD symptoms were observed in uncontrolled DBS studies when stimulating the VC/VS. However, patient sample sizes were too small to obtain meaningful results and the only controlled study did not show net benefits of active over sham stimulation at two months post-surgery [89]. A double-blind crossover study, which included half of the sample of another study [95], assigned 8 OCD patients to 2-week periods of bilateral VC/VS active and sham stimulation in random order, found significant decreases in OC symptoms, anxiety, and depression [92]. Still another VC/VS stimulation study was conducted on four Chinese refractory OCD patients [93]. All patients improved in their OC symptoms, depressive symptoms and global functioning, but two developed unpleasant side effects.
Although the efficacy of ALIC and VC/VS DBS appears to be comparable, lower voltages are generally needed to achieve efficacy when stimulating the VC/VS, suggesting that it is the VS that could be central to achieve efficacy for DBS in OCD.
4.3. Nucleus Accumbens (NAcc)
A small sample DBS study on the anterior and VC, and on the NAcc shell, which has been involved in the pathophysiology of OCD [91, 94]. One of four patients showed clinical response with bipolar stimulation of the two-distal electrode leads, and not of internal capsule, which suggested that NAcc stimulation was the effective one. Bilateral vs. right-sided stimulation did not show outcome differences [91]. On this base, Sturm et al. treated with right NAcc DBS three refractory OCD patients that showed symptom improvement based on clinical impression [91]. The same research group failed to replicate this evidence with a double-blind controlled study using unilateral right NAcc DBS [82]. Another double-blind controlled DBS study on the NAcc core showed greater response with active vs. sham stimulation, and more than 50% of subjects showed a mean 72% drop in Y-BOCS scores, and an improvement in anxiety and depressive symptoms [95]. Add-on CBT after a first improvement of 6 points in Y-BOCS could have supported improvement in OC symptoms, including compulsions and avoidant behaviour [95].
DBS of the NAcc core nearly to the internal capsule may be more effective than NAcc shell stimulation [91, 95], possibly based on the neural functional modulation of the same NAcc, and nearby limbic and prefrontal cortices that has been involved in the pathophysiology of OCD. According to this hypothesis, a recent neuroimaging-DBS study on this region demonstrated that clinical improvement correlated with normalisation of the NAcc-prefrontal cortex functional connectivity [96].
4.4. Subthalamic Nucleus (STN)
A double-blind, controlled, multicentre study in refractory OCD patients demonstrated the efficacy of DBS of the STN. OC symptoms significantly improved with active vs. sham STN stimulation (Y-BOCS 32% drop). With three months of open DBS 75% of the sample showed a 25% Y-BOCS drop [97]. These findings were replicated in subsequent case series/reports, although with some inconsistency [93, 96, 98]. OC and affective symptoms may better improve with combined targeting, and double-blinded test of possible combinations suggested that left-lateral stimulations of the NAcc and STN could be more effective than others. However, the laterality of stimulation issue is still unresolved, and it might be that each OCD patient has his/her own requirements [99].
4.5. Inferior Thalamic Peduncle (ITP)
The ITP consists of white matter fibres connecting the thalamus with OFC and may thus be another target for modulation of aberrant activity in the CSTC circuit. One open study investigated ITP DBS in six OCD patients [100]. One year of bilateral bipolar ITP stimulation ensued in a mean 18.3-point Y-BOCS drop (51%).
ITP DBS did not affect comorbid drug use that was present in three of the six patients. Anteromedial globus pallidus internus (GPi) A strong OCD symptom reduction has been reported in four Tourette disorder patients with prominent OCD symptoms after 3-26 months of GPi DBS [101]. Complete OC resolution was obtained in two patients, while the other two showed a >85% reduction in scores on the Obsessive-Compulsive Inventory Scale.
Despite many concerns with the use of DBS, including suicide risk, in the studies conducted on DBS in OCD adverse events were mild and transient and tended to disappear with small setting changes [89]. Risk of bias in studies of DBS in OCD is generally low as regards selection, performance, detection and reporting, but high as regards attrition.
4.6. Vagus Nerve Stimulation (VNS)
4.6.1. Search Method and Results; No Discussion Possible
For VNS, we conducted on 31 March 2018 the search: (randomized OR randomised) AND (“vagus nerve stimulation” OR “vagal nerve stimulation” OR VNS) AND (obsess* OR compuls* OR ocd) that produced 2 records, both unfocused (1 review and 1 no VNS). Cochrane produced no records, Scopus produced 11 records, of which 9 were new to previous searches, CINAHL produced no records, PsycINFO/PsycARTICLES 1 that was the same as the PubMed with no VNS, clinicaltrials.gov yielded 0 records. There were no intra-database duplicates for any of the databases. Database searches for VNS in OCD either yielded no records or they produced reviews dealing with all somatic treatments cumulatively for all psychiatric disorders, but in no review there were mentions about VNS being used for OCD. The total output was 22 records after eliminating duplicates, of which 20 were reviews, 1 was a trial on tDCS, and 1 was a conference abstract (PRISMA statement, Fig. (4).
Fig. (4).
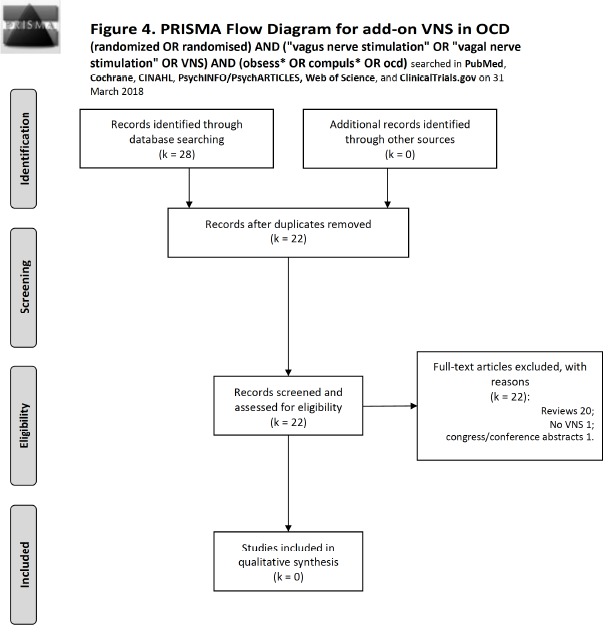
Flow diagram of studies selected for VNS according to the PRISMA statement [30].
4.7. Electroconvulsive Therapy (ECT)
4.7.1. Search Method and Results
For ECT, we conducted on 31 March 2018 the search: ((randomized OR randomised) AND control* AND trial) AND (electroconvulsive OR ECT OR electroshock) AND (obsess* OR compuls* OR ocd). PubMed produced 12 records, none to include. Cochrane produced 5 records, 2 overlapping and 3 new, none to include. Scopus produced 43 records, 35 new to previous searches, none to include. CINAHL produced no records. PsycINFO/PsychARTICLES produced 3 records, 0 new to above searches, and none to include. Web of Science yielded 24 records with 1 intra-database duplicate, 19 new to above searches, adding none to include. Finally, ClinicalTrials.gov produced one record (electroconvulsive therapy combined with obsessive-compulsive disorder) that proved to be unfocused (it was a “Magnetic Seizure Therapy (MST) for Treatment-Resistant Depression, Schizophrenia, and Obsessive Compulsive Disorder” study, still recruiting). Literature search results according to the PRISMA statement are shown in Fig. (5). Since we had reasons to exclude all retrieved records, we chose to discuss the subject by using reviews based on open trials and reported opinions rather than strong evidence-based data. Considered for discussion were ECT trials with at least six sessions (two weeks).
4.8. Discussion of ECT in OCD
Evidence to support the use of ECT in OCD is limited due to small sample sizes and study design issues, like variability in the use of anaesthetics, concomitant pharmacotherapy, and machines. Furthermore, there is a complete lack of controlled trials, and no sham-controlled trials are available for OCD, while there is a bulk of sham-controlled evidence for ECT in depression, for both unilateral and bilateral electrode positioning.
ECT has found no place in treatment guidelines for patients with OCD [102, 103]. Hence, to discuss the subject, we used reviews based on open trials and reported opinions rather than strong evidence-based data. No randomised controlled trial has been conducted to date; however, positive responses to ECT were reported in at least 60% of the cumulative sample [104]. This treatment response rate appears to be rather high and might be mainly contributed by older studies that were conducted prior to the availability of effective drug (clomipramine, SSRIs) and psychological (cognitive-behavioural therapy [CBT]) treatment, i.e., during a period when ECT could have represented first line for any OCD patient. Currently, ECT represents the last resort for all indications, hence the “treatment-resistant” populations that could be legitimately treated with ECT, would lower response rates. In recent years, those OCD patients who receive ECT were previously exposed to inadequate treatment, so treatment-resistance may represent anything between insufficient time of exposure to treatment, wrong drug, or misdiagnosis. An open ECT trial was conducted in nine (four unilateral, five bilateral) treatment-resistant OCD patients [105]. Symptom reduction was 30% on the average and lasted 1-4 months, but by the 6th month, symptoms returned to pre-treatment levels. Unilateral ECT did not differ from bilateral, but with such small numbers no discussion can be made. Authors reported that those patients who displayed obsessive-compulsive personality traits were more resistant to ECT.
Maletzky and colleagues [106] reported long-term efficacy of ECT in 32 patients with “refractory” OCD. Most patients improved considerably for up to one year after the end of the sessions. The improvement of OC symptoms was unrelated to that of depressive symptoms. All patients continued on drug treatment after the end of ECT cycles, while 5 patients obtained maintenance ECT. Favourable effects of ECT on the course of OCD were also reported in case reports, where OCD was usually a co-occurrence with another disorder for which ECT was an indication, i.e., depression, psychosis, catatonia, or resistant, life-threatening anorexia nervosa [63, 107-115]. However, the short duration of the positive effects of ECT cycles prompts us to consider the usefulness of maintenance sessions every 1-8 weeks for 1-3 years. Summarising, ECT is not recommended routinely for OCD, in spite of the results of a recent systematic review that analysed non-randomised and cohort studies, case series, and some single case reports, suggesting beneficial effects of ECT in OCD [104]. Side effects of ECT are well known and mainly involve memory loss, but this has not been reported in the reports we considered here (this does not mean it did not occur). It is not feasible to pool so heterogeneous data to draw firm conclusions; results on ECT in OCD are limited by lack of standardised assessments and lack of consensus of what may be called treatment-resistant OCD.
5. COMBINED, GENERAL DISCUSSION OF SOMATIC (OTHER THAN PHARMACOLOGICAL) TECHNIQUES
Brain stimulation research in OCD suggests that stimulation techniques have some therapeutic potential for severely ill patients who did not achieve satisfactory response to drug treatment or behavioural therapy. In contrast to other strategies, which need some time to show a response, brain stimulation may directly manipulate aberrant brain network circuitry function underlying OCD and may provide for instant adjustment. ECT has no role in the management of OCD, unless comorbid indication are involved. Although non-randomised and cohort studies, case series, and some case report have suggested beneficial effects of ECT in OCD, it is only indicated in patients with OCD comorbid with primary depressive or psychotic disorders.
Few studies investigated the effects of tDCS in OCD, but they showed encouraging results, with some of them reporting symptom improvements, pointing to the need for more investigation. High-frequency rTMS applied over the DLPFC did not appear to be more effective than sham rTMS. On the contrary, low-frequency rTMS applied over the OFC or pre-SMA has proved to be an effective augmentation strategy in OCD. This could be explained by the inhibitory effects of low-frequency rTMS on hyperactive orbitofrontal-striatal circuits that seem to underlie deficient inhibition of irrelevant information and response control in OCD [15].
Although TMS efficacy and tolerability may be similar to those of drug augmentation strategies, TMS applied on a daily basis for a cycle of four weeks has no proven long-term efficacy. Although DBS is invasive, it is a promising device-based intervention for refractory OCD, with long-term responder rates > 60%. Furthermore, as far as now, it has shown good tolerability. Efficacy and tolerability of the various subcortical DBS targets within the corticostriatal network are comparable. Striatal DBS enhances the effects of subsequent behavioural therapy, perhaps by reducing anxiety; it should be investigated whether cortical TMS could do the same by improving the top-down regulation of anxiety. Finally, TMS and DBS trials provide important insights into networks that can be effectively modulated for improving a variety of symptoms. Not only obsessions and compulsions, but also anxiety and depression are reduced with electrical modulation of the orbitofrontal and premotor cortices, of the internal capsule, and the NAcc. Frontostriatal network changes may be critically involved in DBS-induced improvement of OC symptoms, and this knowledge could be used to develop prediction markers, optimise stimulation settings, and design treatment devices that can identify and adjust pathological brain network patterns in OCD.
6. LIMITATIONS
This review is limited by the dearth of studies available for many techniques. For example, there were no studies to include so to discuss adequately ECT and VNS, while for rTMS and DBS included studies were conducted with few patients of each. This might mask any specific positive effects and, considering that the potential for placebo response to machines could be higher than that to pills [116], it could be that a sham procedure could be endowed with a higher placebo response, thus reducing our ability to detect a signal for the verum. A partial response to this doubt could be given by considering the respective effect sizes, and here we presented data based on pre-post statistical significance rather than effect sizes. We also could not carry a meta-analysis due to study heterogeneity, so we conducted a narrative review of each of the physical techniques that were used in the treatment of OCD. Finally, a minority of included studies carried a risk of bias, a fact that lowers the strength of evidence of brain stimulation techniques in OCD.
It should be remarked that studies heretofore available are methodologically diverse, since they include clinically heterogeneous populations with various levels of treatment-resistance. Furthermore, there is still no consensus as to how treatment resistance or refractoriness should be defined and there is no consistency in the scales to adopt for assessing clinical response. In fact, resistance as generally defined, i.e., non-response to at least one adequate SSRI and/or CBT trial [25, 26] appears to be less restrictive than the definitions accepted for depression or schizophrenia, and could represent technique failure rather than true resistance, at least in youths [117]. Moreover, response criteria vary among studies, with some adopting a cut-off of 35% drop of Y-BOCS scores from baseline and other endorsing a 25% cut-off. On top of this, sample sizes in neurostimulation trials are generally small, and this impacts the strength of the conclusions that can be drawn.
GENERAL CONCLUSION AND FUTURE TRENDS
Brain stimulation research in OCD suggests the therapeutic potential for stimulation techniques in the treatment of OCD patients with severe symptoms that impair their quality of life and functioning, who failed to respond adequately to pharmacologic or behavioural therapy, i.e., for those patients who were severe enough and unresponsive to any treatment. Brain stimulation techniques induce reversible modifications in the neural structure and brain functioning of patients and will hopefully target more specifically aberrant brain activity related to OCD. Noninvasive techniques like TMS and tDCS should be first tried before a patient is referred to the more invasive DBS. rTMS has gathered most evidence, but dTMS could replicate positive results and even improve them. TMS may be more effective at low frequencies, around 1 Hz, as it tends to soothe over activation in OCD-relevant neural circuits, in contrast to depression, which responds to stimulation of hypoactive areas in this condition. Cathodal tDCS may be better than anodal in treating OCD. For DBS, multiple targets have been proposed, but the best option appears to be the nucleus accumbens, for which both shell and core have been targeted, especially if combined with stimulation of the ventral capsule. Stimulation of the subthalamic nucleus has also gathered positive results, while for other areas, the evidence is scanty. Summarising, this is an exciting area of development for the treatment of resistant cases of OCD.
To adopt a new treatment for a given condition, we need to test it in a well-defined condition vs. a control treatment, a sham or an established method with fair evidence, with a reliably anticipated expected outcome. If we need to test a neurostimulation technique in treatment-resistant OCD, we first need to define treatment-resistance in OCD patients and refer it to their severity. This could result in some patients with significant comorbidity or with the main diagnosis other than OCD, but with significant OC symptoms, being excluded. This will not allow us to perform real-world studies, hence allowing us to assess efficacy, but not effectiveness. In the future, we need that study designs allow comparability and report data in a meta-analysable way. All this may be reached through consensus among scholars that will define treatment-resistance criteria for OCD patients, appropriateness of study designs and predefined population sizes, so to avoid the study being underpowered, and homogeneity of outcomes. Also, we endorse the adoption of the more restrictive response criterion of an at least 35% drop from baseline in Y-BOCS scores, as 25% criterion may be not strict enough.
ACKNOWLEDGEMENTS
We gratefully acknowledge the contribution of the Librarians of the School of Medicine and Psychology of Sapienza University, Ms. Mimma Ariano, Ms. Felicia Proietti, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo for rendering precious bibliographical material accessible, as well as our Secretary Lucilla Martinelli for her assistance during the writing of this manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work has not been supported by any funding. All authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with the subject matter or materials discussed in the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
STANDARD OF REPORTING
PRISMA guidelines and methodology were followed.
REFERENCES
- 1.Ruscio A.M., Stein D.J., Chiu W.T., Kessler R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [http://dx.doi.org/10.1038/mp.2008.94]. [PMID: 18725912]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman M.M., Bland R.C., Canino G.J., Greenwald S., Hwu H.G., Lee C.K., Newman S.C., Oakley-Browne M.A., Rubio-Stipec M., Wickramaratne P.J. The cross national epidemiology of obsessive compulsive disorder. J. Clin. Psychiatry. 1994;55(Suppl.):5–10. [PMID: 8077177]. [PubMed] [Google Scholar]
- 3.Huang L.C., Tsai K.J., Wang H.K., Sung P.S., Wu M.H., Hung K.W., Lin S.H. Prevalence, incidence, and comorbidity of clinically diagnosed obsessive-compulsive disorder in Taiwan: A national population-based study. Psychiatry Res. 2014;220(1-2):335–341. doi: 10.1016/j.psychres.2014.08.011. [http://dx.doi.org/10.1016/j.psychres.2014.08.011]. [PMID: 25169892]. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi M.R., Ghanizadeh A., Rahgozar M., Noorbala A.A., Davidian H., Afzali H.M., Naghavi H.R., Yazdi S.A., Saberi S.M., Mesgarpour B., Akhondzadeh S., Alaghebandrad J., Tehranidoost M. Prevalence of obsessive-compulsive disorder in Iran. BMC Psychiatry. 2004;4:2. doi: 10.1186/1471-244X-4-2. [http://dx.doi.org/10.1186/1471-244X-4-2]. [PMID: 15018627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramaniam M., Abdin E., Vaingankar J.A., Chong S.A. Obsessive--compulsive disorder: prevalence, correlates, help-seeking and quality of life in a multiracial Asian population. Soc. Psychiatry Psychiatr. Epidemiol. 2012;47(12):2035–2043. doi: 10.1007/s00127-012-0507-8. [http://dx.doi.org/10.1007/s00127-012-0507-8]. [PMID: 22526825]. [DOI] [PubMed] [Google Scholar]
- 6.Jaisoorya T.S., Janardhan Reddy Y.C., Nair B.S., Rani A., Menon P.G., Revamma M., Jeevan C.R., Radhakrishnan K.S., Jose V., Thennarasu K. Prevalence and correlates of obsessive-compulsive disorder and subthreshold obsessive-compulsive disorder among college students in Kerala, India. Indian J. Psychiatry. 2017;59(1):56–62. doi: 10.4103/0019-5545.204438. [http://dx.doi.org/10.4103/0019-5545.204438]. [PMID: 28529361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam Y., Meinlschmidt G., Gloster A.T., Lieb R. Obsessive-compulsive disorder in the community: 12-month prevalence, comorbidity and impairment. Soc. Psychiatry Psychiatr. Epidemiol. 2012;47(3):339–349. doi: 10.1007/s00127-010-0337-5. [http://dx.doi.org/10.1007/s00127-010-0337-5]. [PMID: 21287144]. [DOI] [PubMed] [Google Scholar]
- 8.Osland S., Arnold P.D., Pringsheim T. The prevalence of diagnosed obsessive compulsive disorder and associated comorbidities: A population-based Canadian study. Psychiatry Res. 2018;268:137–142. doi: 10.1016/j.psychres.2018.07.018. [http://dx.doi.org/10.1016/j.psychres.2018.07.018]. [PMID: 30025284]. [DOI] [PubMed] [Google Scholar]
- 9.Torres A.R., Prince M.J., Bebbington P.E., Bhugra D., Brugha T.S., Farrell M., Jenkins R., Lewis G., Meltzer H., Singleton N. Obsessive-compulsive disorder: prevalence, comorbidity, impact, and help-seeking in the British National Psychiatric Morbidity Survey of 2000. Am. J. Psychiatry. 2006;163(11):1978–1985. doi: 10.1176/ajp.2006.163.11.1978. [http://dx.doi.org/10.1176/ajp.2006.163.11.1978]. [PMID: 17074950]. [DOI] [PubMed] [Google Scholar]
- 10.Heyman I., Mataix-Cols D., Fineberg N.A. Obsessive-compulsive disorder. BMJ. 2006;333(7565):424–429. doi: 10.1136/bmj.333.7565.424. [http://dx.doi.org/10.1136/bmj.333.7565.424]. [PMID: 16931840]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein D.J. Obsessive-compulsive disorder. Lancet. 2002;360(9330):397–405. doi: 10.1016/S0140-6736(02)09620-4. [http://dx.doi.org/10.1016/S0140-6736(02)09620-4]. [PMID: 12241794]. [DOI] [PubMed] [Google Scholar]
- 12.Markarian Y., Larson M.J., Aldea M.A., Baldwin S.A., Good D., Berkeljon A., Murphy T.K., Storch E.A., McKay D. Multiple pathways to functional impairment in obsessive-compulsive disorder. Clin. Psychol. Rev. 2010;30(1):78–88. doi: 10.1016/j.cpr.2009.09.005. [http://dx.doi.org/10.1016/j.cpr.2009.09.005]. [PMID: 19853982]. [DOI] [PubMed] [Google Scholar]
- 13.Steketee G. Disability and family burden in obsessive-compulsive disorder. Can. J. Psychiatry. 1997;42(9):919–928. doi: 10.1177/070674379704200902. [http://dx.doi.org/10.1177/070674379704200902]. [PMID: 9429061]. [DOI] [PubMed] [Google Scholar]
- 14.DuPont R.L., Rice D.P., Shiraki S., Rowland C.R. Economic costs of obsessive-compulsive disorder. Med. Interface. 1995;8(4):102–109. [PMID: 10141765]. [PubMed] [Google Scholar]
- 15.Del Casale A., Kotzalidis G.D., Rapinesi C., Serata D., Ambrosi E., Simonetti A., Pompili M., Ferracuti S., Tatarelli R., Girardi P. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64(2):61–85. doi: 10.1159/000325223. [http://dx.doi.org/10.1159/000325223]. [PMID: 21701225]. [DOI] [PubMed] [Google Scholar]
- 16.Fineberg N.A., Chamberlain S.R., Hollander E., Boulougouris V., Robbins T.W. Translational approaches to obsessive-compulsive disorder: from animal models to clinical treatment. Br. J. Pharmacol. 2011;164(4):1044–1061. doi: 10.1111/j.1476-5381.2011.01422.x. [http://dx.doi.org/10.1111/j.1476-5381.2011.01422.x]. [PMID: 21486280]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milad M.R., Rauch S.L. 2012. [Google Scholar]
- 18.Koran L.M., Hanna G.L., Hollander E., Nestadt G., Simpson H.B. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am. J. Psychiatry. 2007;164(7) Suppl.:5–53. [PMID: 17849776]. [PubMed] [Google Scholar]
- 19.Stein D.J., Koen N., Fineberg N., Fontenelle L.F., Matsunaga H., Osser D., Simpson H.B.A. 2012 evidence-based algorithm for the pharmacotherapy for obsessive-compulsive disorder. Curr. Psychiatry Rep. 2012;14(3):211–219. doi: 10.1007/s11920-012-0268-9. [http://dx.doi.org/10.1007/s11920-012-0268-9]. [PMID: 22527872]. [DOI] [PubMed] [Google Scholar]
- 20.Bandelow B., Zohar J., Hollander E., Kasper S., Moller H.J. WFSBP task force on treatment guidelines for anxiety, obsessive-compulsive and post-traumatic stress disoders. World federation of societies of biological psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders - first revision. World J. Biol. Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [http://dx.doi.org/10.1080/15622970802465807]. [PMID: 18949648]. [DOI] [PubMed] [Google Scholar]
- 21.Abudy A., Juven-Wetzler A., Zohar J. Pharmacological management of treatment-resistant obsessive-compulsive disorder. CNS Drugs. 2011;25(7):585–596. doi: 10.2165/11587860-000000000-00000. [http://dx.doi.org/10.2165/11587860-000000000-00000]. [PMID: 21699270]. [DOI] [PubMed] [Google Scholar]
- 22.Jenike M.A. Clinical practice. Obsessive-compulsive disorder. N. Engl. J. Med. 2004;350(3):259–265. doi: 10.1056/NEJMcp031002. [http://dx.doi.org/10.1056/NEJMcp031002]. [PMID: 14724305]. [DOI] [PubMed] [Google Scholar]
- 23.Pallanti S., Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(3):400–412. doi: 10.1016/j.pnpbp.2005.11.028. [http://dx.doi.org/10.1016/j.pnpbp.2005.11.028]. [PMID: 16503369]. [DOI] [PubMed] [Google Scholar]
- 24.Simpson H.B., Huppert J.D., Petkova E., Foa E.B., Liebowitz M.R. Response versus remission in obsessive-compulsive disorder. J. Clin. Psychiatry. 2006;67(2):269–276. doi: 10.4088/jcp.v67n0214. [http://dx.doi.org/10.4088/JCP.v67n0214]. [PMID: 16566623]. [DOI] [PubMed] [Google Scholar]
- 25.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [http://dx.doi.org/10.1001/archpsyc.1989.01810110048007]. [PMID: 2684084]. [DOI] [PubMed] [Google Scholar]
- 26.Bais M., Figee M., Denys D. Neuromodulation in obsessive-compulsive disorder. Psychiatr. Clin. North Am. 2014;37(3):393–413. doi: 10.1016/j.psc.2014.06.003. [http://dx.doi.org/10.1016/j.psc.2014.06.003]. [PMID: 25150569]. [DOI] [PubMed] [Google Scholar]
- 27.Saba G., Moukheiber A., Pelissolo A. Transcranial cortical stimulation in the treatment of obsessive-compulsive disorders: efficacy studies. Curr. Psychiatry Rep. 2015;17(5):36. doi: 10.1007/s11920-015-0571-3. [http://dx.doi.org/10.1007/s11920-015-0571-3]. [PMID: 25825002]. [DOI] [PubMed] [Google Scholar]
- 28.Blomstedt P., Sjöberg R.L., Hansson M., Bodlund O., Hariz M.I. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245–e253. doi: 10.1016/j.wneu.2012.10.006. [http://dx.doi.org/10.1016/j.wneu.2012.10.006]. [PMID: 23044000]. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0; The Cochrane Collaboration. 2008 http://handbook-5-1.cochrane.org/https://methods.cochrane.org/bias/assessing-risk-bias-included-studies [http://dx.doi.org/10.1002/9780470712184]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [http://dx.doi.org/10.1371/journal.pmed.1000097]. [PMID: 19621072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker A.T., Jalinous R., Freeston I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [http://dx.doi.org/10.1016/S0140-6736(85)92413-4]. [PMID: 2860322]. [DOI] [PubMed] [Google Scholar]
- 32.Sarkhel S., Sinha V.K., Praharaj S.K. Adjunctive high-frequency right prefrontal repetitive transcranial magnetic stimulation (rTMS) was not effective in obsessive-compulsive disorder but improved secondary depression. J. Anxiety Disord. 2010;24(5):535–539. doi: 10.1016/j.janxdis.2010.03.011. [http://dx.doi.org/10.1016/j.janxdis.2010.03.011]. [PMID: 20392594]. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald P.B., Fountain S., Daskalakis Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [http://dx.doi.org/10.1016/j.clinph.2006.06.712]. [PMID: 16890483]. [DOI] [PubMed] [Google Scholar]
- 34.Speer A.M., Kimbrell T.A., Wassermann E.M.D., Repella D. J.; Willis, M.W.; Herscovitch, P.; Post, R.M. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry. 2000;48(12):1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [http://dx.doi.org/10.1016/S0006-3223(00)01065-9]. [PMID: 11137053]. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A., Simpson H.B., Fallon B.A., Rossi S., Lisanby S.H. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2010;13(2):217–227. doi: 10.1017/S1461145709990435. [http://dx.doi.org/10.1017/S1461145709990435]. [PMID: 19691873]. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg B.D., George M.S., Martin J.D., Benjamin J., Schlaepfer T.E., Altemus M., Wassermann E.M., Post R.M., Murphy D.L. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am. J. Psychiatry. 1997;154(6):867–869. doi: 10.1176/ajp.154.6.867. [http://dx.doi.org/10.1176/ajp.154.6.867]. [PMID: 9167520]. [DOI] [PubMed] [Google Scholar]
- 37.Alonso P., Pujol J., Cardoner N., Benlloch L., Deus J., Menchón J.M., Capdevila A., Vallejo J. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry. 2001;158(7):1143–1145. doi: 10.1176/appi.ajp.158.7.1143. [http://dx.doi.org/10.1176/appi.ajp.158.7.1143]. [PMID: 11431238]. [DOI] [PubMed] [Google Scholar]
- 38.Prasko J., Pasková B., Záleský R., Novák T., Kopecek M., Bares M., Horácek J. The effect of repetitive transcranial magnetic stimulation (rTMS) on symptoms in obsessive compulsive disorder. A randomized, double blind, sham controlled study. Neuroendocrinol. Lett. 2006;27(3):327–332. [PMID: 16816829]. [PubMed] [Google Scholar]
- 39.Sachdev P.S., Loo C.K., Mitchell P.B., McFarquhar T.F., Malhi G.S. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: A double-blind controlled investigation. Psychol. Med. 2007;37(11):1645–1649. doi: 10.1017/S0033291707001092. [http://dx.doi.org/10.1017/S0033291707001092]. [PMID: 17655805]. [DOI] [PubMed] [Google Scholar]
- 40.Kang J.I., Kim C.H., Namkoong K., Lee C.I., Kim S.J. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J. Clin. Psychiatry. 2009;70(12):1645–1651. doi: 10.4088/JCP.08m04500. [http://dx.doi.org/10.4088/JCP.08m04500]. [PMID: 19709504]. [DOI] [PubMed] [Google Scholar]
- 41.Mansur C.G., Myczkowki M.L., de Barros Cabral S. Sartorelli, Mdo.C.; Bellini, B.B.; Dias, A.M.; Bernik, M.A.; Marcolin, M.A. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: A randomized controlled trial. Int. J. Neuropsychopharmacol. 2011;14(10):1389–1397. doi: 10.1017/S1461145711000575. [http://dx.doi.org/10.1017/S1461145711000575]. [PMID: 21557884]. [DOI] [PubMed] [Google Scholar]
- 42.El Badawy A., Sawy H. El, Hay, M.A. Efficacy of repetitive transcranial magnetic stimulation in the management of obsessive compulsive disorder. Egypt. J. Neurol. Psychiat. Neurosurg. 2010;47:1. [Google Scholar]
- 43.Ma X., Huang Y., Liao L., Jin Y. A randomized double-blinded sham-controlled trial of α electroencephalogram-guided transcranial magnetic stimulation for obsessive-compulsive disorder. Chin. Med. J. (Engl.) 2014;127(4):601–606. [PMID: 24534207]. [PubMed] [Google Scholar]
- 44.Elbeh K.A.M., Elserogy Y.M.B., Khalifa H.E., Ahmed M.A., Hafez M.H., Khedr E.M. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: Double blind randomized clinical trial. Psychiatry Res. 2016;238:264–269. doi: 10.1016/j.psychres.2016.02.031. [http://dx.doi.org/10.1016/j.psychres.2016.02.031]. [PMID: 27086243]. [DOI] [PubMed] [Google Scholar]
- 45.Seo H.J., Jung Y.E., Lim H.K., Um Y.H., Lee C.U., Chae J.H. Adjunctive low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex in patients with treatment-resistant obsessive-compulsive disorder: A randomized controlled trial. Clin. Psychopharmacol. Neurosci. 2016;14(2):153–160. doi: 10.9758/cpn.2016.14.2.153. [http://dx.doi.org/10.9758/cpn.2016.14.2.153]. [PMID: 27121426]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahangard L., Haghighi M., Shyayganfard M., Ahmadpanah M., Sadeghi Bahmani D., Bajoghli H., Holsboer-Trachsler E., Brand S. Sadeghi, Bahmani, D.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Repetitive transcranial magnetic stimulation improved symptoms of obsessive-compulsive disorder, but also cognitive performance: results from a randomized clinical trial with a cross-over design and sham condition. Neuropsychobiology. 2016;73(4):224–232. doi: 10.1159/000446287. [http://dx.doi.org/10.1159/000446287]. [PMID: 27299900]. [DOI] [PubMed] [Google Scholar]
- 47.Carmi L., Alyagon U., Barnea-Ygael N., Zohar J., Dar R., Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158–165. doi: 10.1016/j.brs.2017.09.004. [http://dx.doi.org/10.1016/j.brs.2017.09.004]. [PMID: 28927961]. [DOI] [PubMed] [Google Scholar]
- 48.Ruffini C., Locatelli M., Lucca A., Benedetti F., Insacco C., Smeraldi E. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: a controlled investigation. Prim. Care Companion J. Clin. Psychiatry. 2009;11(5):226–230. doi: 10.4088/PCC.08m00663. [http://dx.doi.org/10.4088/PCC.08m00663]. [PMID: 19956460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nauczyciel C., Le Jeune F., Naudet F., Douabin S., Esquevin A., Vérin M., Dondaine T., Robert G., Drapier D., Millet B. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl. Psychiatry. 2014;4:e436. doi: 10.1038/tp.2014.62. [http://dx.doi.org/10.1038/tp.2014.62]. [PMID: 25203167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A., Lisanby S.H., Pieraccini F., Ulivelli M., Castrogiovanni P., Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int. J. Neuropsychopharmacol. 2006;9(1):95–100. doi: 10.1017/S1461145705005729. [http://dx.doi.org/10.1017/S1461145705005729]. [PMID: 15982444]. [DOI] [PubMed] [Google Scholar]
- 51.Gomes P.V., Brasil-Neto J.P., Allam N., Rodrigues de Souza E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J. Neuropsychiatry Clin. Neurosci. 2012;24(4):437–443. doi: 10.1176/appi.neuropsych.11100242. [http://dx.doi.org/10.1176/appi.neuropsych.11100242]. [PMID: 23224449]. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani A., Rossi S., Bassi B.D., Simpson H.B., Fallon B.A., Lisanby S.H. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210(3):1026–1032. doi: 10.1016/j.psychres.2013.08.054. [http://dx.doi.org/10.1016/j.psychres.2013.08.054]. [PMID: 24064461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelissolo A., Harika-Germaneau G., Rachid F., Gaudeau-Bosma C., Tanguy M.L., BenAdhira R., Bouaziz N., Popa T., Wassouf I., Saba G., Januel D., Jaafari N. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: a sham-controlled trial. Int. J. Neuropsychopharmacol. 2016;19(8):pyw025. doi: 10.1093/ijnp/pyw025. [http://dx.doi.org/10.1093/ijnp/pyw025]. [PMID: 27207923]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawken E.R., Dilkov D., Kaludiev E., Simek S., Zhang F., Milev R. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: a multi-site study. Int. J. Mol. Sci. 2016;17(3):420. doi: 10.3390/ijms17030420. [http://dx.doi.org/10.3390/ijms17030420]. [PMID: 27011177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alptekin K., Degirmenci B., Kivircik B., Durak H., Yemez B., Derebek E., Tunca Z. Tc-99m HMPAO brain perfusion SPECT in drug-free obsessive-compulsive patients without depression. Psychiatry Res. 2001;107(1):51–56. doi: 10.1016/s0925-4927(01)00086-5. [http://dx.doi.org/10.1016/S0925-4927(01)00086-5]. [PMID: 11472864]. [DOI] [PubMed] [Google Scholar]
- 56.Baxter L.R., Jr, Schwartz J.M., Mazziotta J.C., Phelps M.E., Pahl J.J., Guze B.H., Fairbanks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am. J. Psychiatry. 1988;145(12):1560–1563. doi: 10.1176/ajp.145.12.1560. [http://dx.doi.org/10.1176/ajp.145.12.1560]. [PMID: 3264118]. [DOI] [PubMed] [Google Scholar]
- 57.Swedo S.E., Pietrini P., Leonard H.L., Schapiro M.B., Rettew D.C., Goldberger E.L., Rapoport S.I., Rapoport J.L., Grady C.L. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch. Gen. Psychiatry. 1992;49(9):690–694. doi: 10.1001/archpsyc.1992.01820090018003. [http://dx.doi.org/10.1001/archpsyc.1992.01820090018003]. [PMID: 1514873]. [DOI] [PubMed] [Google Scholar]
- 58.Berlim M.T., Neufeld N.H., Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J. Psychiatr. Res. 2013;47(8):999–1006. doi: 10.1016/j.jpsychires.2013.03.022. [http://dx.doi.org/10.1016/j.jpsychires.2013.03.022]. [PMID: 23615189]. [DOI] [PubMed] [Google Scholar]
- 59.D’Urso G., Brunoni A.R., Mazzaferro M.P., Anastasia A., de Bartolomeis A., Mantovani A. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress. Anxiety. 2016;33(12):1132–1140. doi: 10.1002/da.22578. [http://dx.doi.org/10.1002/da.22578]. [PMID: 27802585]. [DOI] [PubMed] [Google Scholar]
- 60.Tortella G., Casati R., Aparicio L.V., Mantovani A., Senço N., D’Urso G., Brunelin J., Guarienti F., Selingardi P.M., Muszkat D. Junior, Bde.S.; Valiengo, L.; Moffa, A.H.; Simis, M.; Borrione, L.; Brunoni, A.R. Transcranial direct current stimulation in psychiatric disorders. World J. Psychiatry. 2015;5(1):88–102. doi: 10.5498/wjp.v5.i1.88. [http://dx.doi.org/10.5498/wjp.v5.i1.88]. [PMID: 25815258]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senço N.M., Huang Y., D’Urso G., Parra L.C., Bikson M., Mantovani A., Shavitt R.G., Hoexter M.Q., Miguel E.C., Brunoni A.R. Transcranial direct current stimulation in obsessive-compulsive disorder: emerging clinical evidence and considerations for optimal montage of electrodes. Expert Rev. Med. Devices. 2015;12(4):381–391. doi: 10.1586/17434440.2015.1037832. [http://dx.doi.org/10.1586/17434440.2015.1037832]. [PMID: 25982412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner T., Valero-Cabre A., Pascual-Leone A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [http://dx.doi.org/10.1146/annurev.bioeng.9.061206.133100]. [PMID: 17444810]. [DOI] [PubMed] [Google Scholar]
- 63.Bikson M., Inoue M., Akiyama H., Deans J.K., Fox J.E., Miyakawa H., Jefferys J.G. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004;557(Pt 1):175–190. doi: 10.1113/jphysiol.2003.055772. [http://dx.doi.org/10.1113/jphysiol.2003.055772]. [PMID: 14978199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011;21(5):602–617. doi: 10.1080/09602011.2011.557292. [http://dx.doi.org/10.1080/09602011.2011.557292]. [PMID: 21819181]. [DOI] [PubMed] [Google Scholar]
- 65.Shafi M.M., Westover M.B., Fox M.D., Pascual-Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci. 2012;35(6):805–825. doi: 10.1111/j.1460-9568.2012.08035.x. [http://dx.doi.org/10.1111/j.1460-9568.2012.08035.x]. [PMID: 22429242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nitsche M.A., Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [http://dx.doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x]. [PMID: 10990547]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng X., Alsop D.C., Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58(1):26–33. doi: 10.1016/j.neuroimage.2011.06.018. [http://dx.doi.org/10.1016/j.neuroimage.2011.06.018]. [PMID: 21703350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rauch S.L., Jenike M.A., Alpert N.M., Baer L., Breiter H.C., Savage C.R., Fischman A.J. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch. Gen. Psychiatry. 1994;51(1):62–70. doi: 10.1001/archpsyc.1994.03950010062008. [http://dx.doi.org/10.1001/archpsyc.1994.03950010062008]. [PMID: 8279930]. [DOI] [PubMed] [Google Scholar]
- 69.Hou J., Wu W., Lin Y., Wang J., Zhou D., Guo J., Gu S., He M., Ahmed S., Hu J., Qu W., Li H. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI study. J. Affect. Disord. 2012;138(3):313–321. doi: 10.1016/j.jad.2012.01.022. [http://dx.doi.org/10.1016/j.jad.2012.01.022]. [PMID: 22331021]. [DOI] [PubMed] [Google Scholar]
- 70.Lefaucheur J.P., Antal A., Ayache S.S., Benninger D.H., Brunelin J., Cogiamanian F., Cotelli M., De Ridder D., Ferrucci R., Langguth B., Marangolo P., Mylius V., Nitsche M.A., Padberg F., Palm U., Poulet E., Priori A., Rossi S., Schecklmann M., Vanneste S., Ziemann U., Garcia-Larrea L., Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087. [http://dx.doi.org/10.1016/j.clinph.2016.10.087]. [PMID: 27866120]. [DOI] [PubMed] [Google Scholar]
- 71.Volpato C., Piccione F., Cavinato M., Duzzi D., Schiff S., Foscolo L., Venneri A. Modulation of affective symptoms and resting state activity by brain stimulation in a treatment-resistant case of obsessive-compulsive disorder. Neurocase. 2013;19(4):360–370. doi: 10.1080/13554794.2012.667131. [http://dx.doi.org/10.1080/13554794.2012.667131]. [PMID: 22554168]. [DOI] [PubMed] [Google Scholar]
- 72.Narayanaswamy J.C., Jose D., Chhabra H., Agarwal S.M., Shrinivasa B., Hegde A., Bose A., Kalmady S.V., Venkatasubramanian G., Reddy Y.C. Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul. 2015;8(3):655–657. doi: 10.1016/j.brs.2014.12.003. [http://dx.doi.org/10.1016/j.brs.2014.12.003]. [PMID: 25583654]. [DOI] [PubMed] [Google Scholar]
- 73.D’Urso G., Micillo M., Cosentino C., Mantovani A. Differential effects of anodal and cathodal tDCS over the supplementary motor area in OCD patients. Biol. Psychiatry. 2014;75:13S. [Google Scholar]
- 74.Bation R., Poulet E., Haesebaert F., Saoud M., Brunelin J. Transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:153–157. doi: 10.1016/j.pnpbp.2015.10.001. [http://dx.doi.org/10.1016/j.pnpbp.2015.10.001]. [PMID: 26439873]. [DOI] [PubMed] [Google Scholar]
- 75.D’Urso G., Brunoni A.R., Anastasia A., Micillo M., de Bartolomeis A., Mantovani A. Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase. 2015;14:1–5. doi: 10.1080/13554794.2015.1045522. [PMID: 25971992]. [DOI] [PubMed] [Google Scholar]
- 76.Hazari N., Narayanaswamy J.C., Chhabra H., Bose A., Venkatasubramanian G., Reddy Y.C. Response to transcranial direct current stimulation in a case of episodic obsessive compulsive disorder. J. ECT. 2016;32(2):144–146. doi: 10.1097/YCT.0000000000000309. [http://dx.doi.org/10.1097/YCT.0000000000000309]. [PMID: 27008329]. [DOI] [PubMed] [Google Scholar]
- 77.Mondino M., Haesebaert F., Poulet E., Saoud M., Brunelin J. Efficacy of cathodal transcranial direct current stimulation over the left orbitofrontal cortex in a patient with treatment-resistant obsessive-compulsive disorder. J. ECT. 2015;31(4):271–272. doi: 10.1097/YCT.0000000000000218. [http://dx.doi.org/10.1097/YCT.0000000000000218]. [PMID: 25651393]. [DOI] [PubMed] [Google Scholar]
- 78.Palm U., Leitner B., Kirsch B., Behler N., Kumpf U., Wulf L., Padberg F., Hasan A. Prefrontal tDCS and sertraline in obsessive compulsive disorder: a case report and review of the literature. Neurocase. 2017;23(2):173–177. doi: 10.1080/13554794.2017.1319492. [http://dx.doi.org/10.1080/13554794.2017.1319492]. [PMID: 28427306]. [DOI] [PubMed] [Google Scholar]
- 79.Brunelin J., Mondino M., Bation R., Palm U., Saoud M., Poulet E. Transcranial direct current stimulation for obsessive-compulsive disorder: A systematic review. Brain Sci. 2018;8(2):E37. doi: 10.3390/brainsci8020037. [http://dx.doi.org/10.3390/brainsci8020037]. [PMID: 29495298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haynes W.I., Mallet L. High-frequency stimulation of deep brain structures in obsessive-compulsive disorder: the search for a valid circuit. Eur. J. Neurosci. 2010;32(7):1118–1127. doi: 10.1111/j.1460-9568.2010.07418.x. [http://dx.doi.org/10.1111/j.1460-9568.2010.07418.x]. [PMID: 21039951]. [DOI] [PubMed] [Google Scholar]
- 81.Karas P.J., Lee S., Jimenez-Shahed J., Goodman W.K., Viswanathan A., Sheth S.A. Deep brain stimulation for obsessive compulsive disorder: evolution of surgical stimulation target parallels changing model of dysfunctional brain circuits. Front. Neurosci. 2019;12:998. doi: 10.3389/fnins.2018.00998. [http://dx.doi.org/10.3389/fnins.2018.00998]. [PMID: 30670945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huff W., Lenartz D., Schormann M., Lee S.H., Kuhn J., Koulousakis A., Mai J., Daumann J., Maarouf M., Klosterkötter J., Sturm V. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin. Neurol. Neurosurg. 2010;112(2):137–143. doi: 10.1016/j.clineuro.2009.11.006. [http://dx.doi.org/10.1016/j.clineuro.2009.11.006]. [PMID: 20006424]. [DOI] [PubMed] [Google Scholar]
- 83.Greenberg B.D., Gabriels L.A., Malone D.A., Jr, Rezai A.R., Friehs G.M., Okun M.S., Shapira N.A., Foote K.D., Cosyns P.R., Kubu C.S., Malloy P.F., Salloway S.P., Giftakis J.E., Rise M.T., Machado A.G., Baker K.B., Stypulkowski P.H., Goodman W.K., Rasmussen S.A., Nuttin B.J. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol. Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [http://dx.doi.org/10.1038/mp.2008.55]. [PMID: 18490925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nuttin B., Cosyns P., Demeulemeester H., Gybels J., Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354(9189):1526. doi: 10.1016/S0140-6736(99)02376-4. [http://dx.doi.org/10.1016/S0140-6736(99)02376-4]. [PMID: 10551504]. [DOI] [PubMed] [Google Scholar]
- 85.Nuttin B.J., Gabriëls L.A., Cosyns P.R., Meyerson B.A., Andréewitch S., Sunaert S.G., Maes A.F., Dupont P.J., Gybels J.M., Gielen F., Demeulemeester H.G. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. [http://dx.doi.org/10.1227/01.NEU.0000064565.49299.9A]. [PMID: 12762871]. [DOI] [PubMed] [Google Scholar]
- 86.Anderson D., Ahmed A. Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. Case report. J. Neurosurg. 2003;98(5):1104–1108. doi: 10.3171/jns.2003.98.5.1104. [http://dx.doi.org/10.3171/jns.2003.98.5.1104]. [PMID: 12744372]. [DOI] [PubMed] [Google Scholar]
- 87.Abelson J.L., Curtis G.C., Sagher O., Albucher R.C., Harrigan M., Taylor S.F., Martis B., Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol. Psychiatry. 2005;57(5):510–516. doi: 10.1016/j.biopsych.2004.11.042. [http://dx.doi.org/10.1016/j.biopsych.2004.11.042]. [PMID: 15737666]. [DOI] [PubMed] [Google Scholar]
- 88.Greenberg B.D., Malone D.A., Friehs G.M., Rezai A.R., Kubu C.S., Malloy P.F., Salloway S.P., Okun M.S., Goodman W.K., Rasmussen S.A. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–2393. doi: 10.1038/sj.npp.1301165. [http://dx.doi.org/10.1038/sj.npp.1301165]. [PMID: 16855529]. [DOI] [PubMed] [Google Scholar]
- 89.Goodman W.K., Foote K.D., Greenberg B.D., Ricciuti N., Bauer R., Ward H., Shapira N.A., Wu S.S., Hill C.L., Rasmussen S.A., Okun M.S. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol. Psychiatry. 2010;67(6):535–542. doi: 10.1016/j.biopsych.2009.11.028. [http://dx.doi.org/10.1016/j.biopsych.2009.11.028]. [PMID: 20116047]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roh D., Chang W.S., Chang J.W., Kim C.H. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res. 2012;200(2-3):1067–1070. doi: 10.1016/j.psychres.2012.06.018. [http://dx.doi.org/10.1016/j.psychres.2012.06.018]. [PMID: 22784468]. [DOI] [PubMed] [Google Scholar]
- 91.Sturm V., Lenartz D., Koulousakis A., Treuer H., Herholz K., Klein J.C., Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J. Chem. Neuroanat. 2003;26(4):293–299. doi: 10.1016/j.jchemneu.2003.09.003. [http://dx.doi.org/10.1016/j.jchemneu.2003.09.003]. [PMID: 14729131]. [DOI] [PubMed] [Google Scholar]
- 92.Baas J.M., Klumpers F., Mantione M.H., Figee M., Vulink N.C., Schuurman P.R., Mazaheri A., Denys D. No impact of deep brain stimulation on fear-potentiated startle in obsessive-compulsive disorder. Front. Behav. Neurosci. 2014;8:305. doi: 10.3389/fnbeh.2014.00305. [http://dx.doi.org/10.3389/fnbeh.2014.00305]. [PMID: 25249953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsai H.C., Chang C.H., Pan J.I., Hsieh H.J., Tsai S.T., Hung H.Y., Chen S.Y. Pilot study of deep brain stimulation in refractory obsessive-compulsive disorder ethnic Chinese patients. Psychiatry Clin. Neurosci. 2012;66(4):303–312. doi: 10.1111/j.1440-1819.2012.02352.x. [http://dx.doi.org/10.1111/j.1440-1819.2012.02352.x]. [PMID: 22624735]. [DOI] [PubMed] [Google Scholar]
- 94.Saxena S., Rauch S.L. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr. Clin. North Am. 2000;23(3):563–586. doi: 10.1016/s0193-953x(05)70181-7. [http://dx.doi.org/10.1016/S0193-953X(05)70181-7]. [PMID: 10986728]. [DOI] [PubMed] [Google Scholar]
- 95.Denys D., Mantione M., Figee M., van den Munckhof P., Koerselman F., Westenberg H., Bosch A., Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2010;67(10):1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [http://dx.doi.org/10.1001/archgenpsychiatry.2010.122]. [PMID: 20921122]. [DOI] [PubMed] [Google Scholar]
- 96.Figee M., Luigjes J., Smolders R., Valencia-Alfonso C.E., van Wingen G., de Kwaasteniet B., Mantione M., Ooms P., de Koning P., Vulink N., Levar N., Droge L., van den Munckhof P., Schuurman P.R., Nederveen A., van den Brink W., Mazaheri A., Vink M., Denys D. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat. Neurosci. 2013;16(4):386–387. doi: 10.1038/nn.3344. [http://dx.doi.org/10.1038/nn.3344]. [PMID: 23434914]. [DOI] [PubMed] [Google Scholar]
- 97.Mallet L., Polosan M., Jaafari N., Baup N., Welter M.L., Fontaine D., du Montcel S.T., Yelnik J., Chéreau I., Arbus C., Raoul S., Aouizerate B., Damier P., Chabardès S., Czernecki V., Ardouin C., Krebs M.O., Bardinet E., Chaynes P., Burbaud P., Cornu P., Derost P., Bougerol T., Bataille B., Mattei V., Dormont D., Devaux B., Vérin M., Houeto J.L., Pollak P., Benabid A.L., Agid Y., Krack P., Millet B., Pelissolo A. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 2008;359(20):2121–2134. doi: 10.1056/NEJMoa0708514. [http://dx.doi.org/10.1056/NEJMoa0708514]. [PMID: 19005196]. [DOI] [PubMed] [Google Scholar]
- 98.Chabardès S., Polosan M., Krack P., Bastin J., Krainik A., David O., Bougerol T., Benabid A.L. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg. 2013;80(3-4):31.e1–31.e8. doi: 10.1016/j.wneu.2012.03.010. [http://dx.doi.org/10.1016/j.wneu.2012.03.010]. [PMID: 22469523]. [DOI] [PubMed] [Google Scholar]
- 99.Barcia J.A., Reyes L., Arza R., Saceda J., Avecillas J., Yáñez R., García-Albea J., Ortiz T., López-Ibor M.I., López-Ibor J.J. Deep brain stimulation for obsessive-compulsive disorder: is the side relevant? Stereotact. Funct. Neurosurg. 2014;92(1):31–36. doi: 10.1159/000353187. [http://dx.doi.org/10.1159/000353187]. [PMID: 24216976]. [DOI] [PubMed] [Google Scholar]
- 100.Jiménez F., Nicolini H., Lozano A.M., Piedimonte F., Salín R., Velasco F. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80(3-4):30.e17–30.e25. doi: 10.1016/j.wneu.2012.07.010. [http://dx.doi.org/10.1016/j.wneu.2012.07.010]. [PMID: 22824558]. [DOI] [PubMed] [Google Scholar]
- 101.Nair G., Evans A., Bear R.E., Velakoulis D., Bittar R.G. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J. Clin. Neurosci. 2014;21(5):815–821. doi: 10.1016/j.jocn.2013.10.003. [http://dx.doi.org/10.1016/j.jocn.2013.10.003]. [PMID: 24524950]. [DOI] [PubMed] [Google Scholar]
- 102.National Institute for Health and Care Excellence (NICE) Obsessive- compulsive disorder: core interventions in the treatment of obsessive- compulsive disorder and body dysmorphic disorder Obsessive- compulsive disorder: core interventions in the treatment of obsessive- compulsive disorder and body dysmorphic disorder. National Institute for Health and Care Excellence. 2005 http://www.nice.org.uk/guidance/CG31 [PubMed]
- 103.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. American Psychiatric Association. 2007 http://psychiatryonline.org/guidelines.aspx [PubMed]
- 104.Fontenelle L.F., Coutinho E.S., Lins-Martins N.M., Fitzgerald P.B., Fujiwara H., Yücel M. Electroconvulsive therapy for obsessive-compulsive disorder: A systematic review. J. Clin. Psychiatry. 2015;76(7):949–957. doi: 10.4088/JCP.14r09129. [http://dx.doi.org/10.4088/JCP.14r09129]. [PMID: 25844888]. [DOI] [PubMed] [Google Scholar]
- 105.Khanna S., Gangadhar B.N., Sinha V., Rajendra P.N., Channabasavanna S.M. Electroconvulsive therapy in obsessive-compulsive disorder. Convuls. Ther. 1988;4(4):314–320. [PMID: 11940981]. [PubMed] [Google Scholar]
- 106.Maletzky B., McFarland B., Burt A. Refractory obsessive compulsive disorder and ECT. Convuls. Ther. 1994;10(1):34–42. [PMID: 8055290]. [PubMed] [Google Scholar]
- 107.Casey D.A., Davis M.H. Obsessive-compulsive disorder responsive to electroconvulsive therapy in an elderly woman. South. Med. J. 1994;87(8):862–864. doi: 10.1097/00007611-199408000-00027. [http://dx.doi.org/10.1097/00007611-199408000-00027]. [PMID: 8052907]. [DOI] [PubMed] [Google Scholar]
- 108.Thomas S.G., Kellner C.H. Remission of major depression and obsessive-compulsive disorder after a single unilateral ECT. J. ECT. 2003;19(1):50–51. doi: 10.1097/00124509-200303000-00011. [http://dx.doi.org/10.1097/00124509-200303000-00011]. [PMID: 12621279]. [DOI] [PubMed] [Google Scholar]
- 109.Strassnig M., Riedel M., Müller N. Electroconvulsive therapy in a patient with Tourette’s syndrome and co-morbid Obsessive Compulsive Disorder. World J. Biol. Psychiatry. 2004;5(3):164–166. doi: 10.1080/15622970410029930. [http://dx.doi.org/10.1080/15622970410029930]. [PMID: 15346542]. [DOI] [PubMed] [Google Scholar]
- 110.Chaves M.P., Crippa J.A., Morais S.L., Zuardi A.W. Electroconvulsive therapy for coexistent schizophrenia and obsessive-compulsive disorder. J. Clin. Psychiatry. 2005;66(4):542–543. doi: 10.4088/jcp.v66n0420c. [http://dx.doi.org/10.4088/JCP.v66n0420c]. [PMID: 15816802]. [DOI] [PubMed] [Google Scholar]
- 111.Hanisch F., Friedemann J., Piro J., Gutmann P. Maintenance electroconvulsive therapy for comorbid pharmacotherapy-refractory obsessive-compulsive and schizoaffective disorder. Eur. J. Med. Res. 2009;14(8):367–368. doi: 10.1186/2047-783X-14-8-367. [http://dx.doi.org/10.1186/2047-783X-14-8-367]. [PMID: 19666398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loi S., Bonwick R. Electroconvulsive therapy for treatment of late-onset obsessive compulsive disorder. Int. Psychogeriatr. 2010;22(5):830–831. doi: 10.1017/S1041610210000451. [http://dx.doi.org/10.1017/S1041610210000451]. [PMID: 20370958]. [DOI] [PubMed] [Google Scholar]
- 113.Makhinson M., Furst B.A., Shuff M.K., Kwon G.E. Successful treatment of co-occurring catatonia and obsessive-compulsive disorder with concurrent electroconvulsive therapy and benzodiazepine administration. J. ECT. 2012;28(3):e35–e36. doi: 10.1097/YCT.0b013e318254c2ea. [http://dx.doi.org/10.1097/YCT.0b013e318254c2ea]. [PMID: 22914637]. [DOI] [PubMed] [Google Scholar]
- 114.Raveendranathan D., Srinivasaraju R., Ratheesh A., Math S.B., Reddy Y.C. Treatment-refractory OCD responding to maintenance electroconvulsive therapy. J. Neuropsychiatry Clin. Neurosci. 2012;24(2):E16–E17. doi: 10.1176/appi.neuropsych.11060124. [http://dx.doi.org/10.1176/appi.neuropsych.11060124]. [PMID: 22772680]. [DOI] [PubMed] [Google Scholar]
- 115.Bülbül F., Copoglu U.S., Alpak G., Unal A., Tastan M.F., Savas H.A. Maintenance therapy with electroconvulsive therapy in a patient with a codiagnosis of bipolar disorder and obsessive-compulsive disorder. J. ECT. 2013;29(2):e21–e22. doi: 10.1097/YCT.0b013e31827b4e76. [http://dx.doi.org/10.1097/YCT.0b013e31827b4e76]. [PMID: 23519221]. [DOI] [PubMed] [Google Scholar]
- 116.Schwitzgebel R.K., Traugott M. Initial note on the placebo effect of machines. Behav. Sci. 1968;13(4):267–273. doi: 10.1002/bs.3830130402. [http://dx.doi.org/10.1002/bs.3830130402]. [PMID: 5663895]. [DOI] [PubMed] [Google Scholar]
- 117.Krebs G., Isomura K., Lang K., Jassi A., Heyman I., Diamond H., Advani J., Turner C. Mataix-Cols, D. How resistant is ‘treatment-resistant’ obsessive-compulsive disorder in youth? Br. J. Clin. Psychol. 2015;54(1):63–75. doi: 10.1111/bjc.12061. [DOI] [PubMed] [Google Scholar]


