Abstract
Background
Respiratory distress syndrome (RDS) is caused by a deficiency or dysfunction of pulmonary surfactant. A variety of surfactant products including protein free synthetic surfactant have been developed and tested in the prevention and treatment of RDS.
Objectives
To assess the effect of prophylactic administration of protein free synthetic surfactant (SS) on mortality, chronic lung disease and other morbidities associated with prematurity in preterm newborns at risk for developing RDS. Subgroup analysis were planned according to the degree of prematurity, surfactant product and dosage schedule.
Search methods
Searches were made of the The Cochrane Library, MEDLINE, OVID, EMBASE, CINAHL from 1966 to 2009. In addition, previous reviews including cross references and abstracts from the Society for Pediatric Research were searched. No language restrictions were applied.
Selection criteria
Randomized and quasi‐randomized controlled trials that compared the effect of protein free SS administered to high risk preterm newborns at or shortly after birth in order to prevent RDS, mortality and complications of prematurity.
Data collection and analysis
Data regarding clinical outcomes was excerpted from the clinical trials by the reviewers. Data were analyzed according to the standards of the Cochrane Neonatal Review Group.
Main results
Studies of prophylactic administration of protein free SS note a variable improvement in the respiratory status and a decrease in respiratory distress syndrome in infants who receive prophylactic protein free SS. The meta‐analysis supports a decrease in the risk of pneumothorax (typical relative risk 0.67, 95% CI 0.50, 0.90), pulmonary interstitial emphysema (typical relative risk 0.68, 95% CI 0.50, 0.93), and neonatal mortality (typical relative risk 0.70, 95% CI 0.58, 0.85). No differences were seen in the risk of intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity and cerebral palsy. The meta‐analysis supports an increase in the risk of patent ductus arteriosus associated with prophylactic SS administration (typical relative risk 1.11, 95% CI 1.00, 1.22), and an increase in the risk of pulmonary hemorrhage (typical relative risk 3.28, 95% CI 1.50, 7.16).
Authors' conclusions
Prophylactic intratracheal administration of protein free synthetic surfactant to infants at risk of developing respiratory distress syndrome has been demonstrated to improve clinical outcome. Infants who receive prophylactic protein free SS have a decreased risk of pneumothorax, a decreased risk of pulmonary interstitial emphysema, and a decreased risk of neonatal mortality. Infants who receive prophylactic protein free SS have an increased risk of developing patent ductus arteriosus and pulmonary hemorrhage.
Plain language summary
Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants
Pulmonary surfactant is a substance that prevents the air sacs of the lungs from collapsing by reducing surface tension. Surfactant is essential to normal lung function in newborn babies. Sometimes it is absent in immature lungs and respiratory distress syndrome (RDS) can develop. Protein free synthetic surfactants have been developed and can be used for babies born prematurely (before 34 weeks) who are at risk of developing RDS. The review of trials found evidence that synthetic surfactant reduced the risk of RDS in babies considered at risk. Babies who receive prophylactic synthetic surfactant have a decreased risk of RDS, pneumothorax (air in the lung cavity) and death. However, babies who receive prophylactic synthetic surfactant have an increased risk of developing lung hemorrhage and patent ductus arteriosus, an open vessel that channels blood from the lungs to the body. Although this can lead to potentially life threatening complications, the overall benefits of surfactant treatment outweigh the risks.
Background
Description of the condition
Respiratory distress syndrome (RDS) is caused by a deficiency or dysfunction of pulmonary surfactant. Surfactant lines the alveolar surface and prevents atelectasis at end expiration. Pulmonary surfactant is predominantly dipalmitoylphosphatidylcholine with lesser amounts of other phospholipids including phosphatidylglycerol (PG), phosphatidylethanolamine, and phosphatidylinositol. Pulmonary surfactant also contains neutral lipids and distinct surfactant proteins. The physiologic function of surfactant includes the ability to lower surface tension, as well as the ability to rapidly adsorb, spread, and reform a monolayer in dynamic conditions associated with the respiratory cycle (Jobe 1993).
Description of the intervention
The first attempts to utilize synthetic surfactants occurred in the 1960s. Investigators attempted to aerosolize dipalmitoylphosphatidylcholine (DPPC) to infants with established respiratory distress syndrome (Robillard 1964; Chu 1967). These investigators could not demonstrate any beneficial effect of surfactant replacement. The poor results were, in part, due to an incomplete understanding of what constitutes pulmonary surfactant. The first successful animal model of surfactant replacement therapy was conducted by Enhorning and coworkers (Enhorning 1972). Enhorning administered a crude natural surfactant extract obtained from lavage of the lungs of mature rabbits directly into the trachea of immature rabbits. Improvement in lung compliance and alveolar expansion was noted. Success in animal models led to widespread clinical trials of surfactant therapy in the newborn.
A wide variety of surfactant products have been formulated and studied in clinical trials. These include synthetic surfactants and animal derived surfactant extracts. Animal derived or "natural" surfactant extracts are derived from animal or human sources. Currently used synthetic surfactants are complex combinations of dipalmitoylphosphatidylcholine and other phospholipids, neutral lipids, lipoprotein, or alcohols. Components of synthetic surfactants are not directly obtained from the extraction of surfactant from animal lung.
The original trials of DPPC alone are not discussed, since neither the surfactant nor the route of administration are considered adequate. Included trials all used complex protein free synthetic surfactants in an attempt to treat infants at high risk of developing respiratory distress syndrome. In these studies, infants were randomized to receive surfactant or control treatment immediately after delivery, prior to the onset of respiratory symptoms. Investigators hoped to decrease the incidence of respiratory distress syndrome and other complications associated with prematurity.
Why it is important to do this review
Multiple systematic reviews have addressed the use of animal derived surfactant preparations or synthetic surfactant preparations in the prevention or treatment of respiratory distress syndrome (Soll 1998a; Soll 1998b; Seger 2009). Systematic reviews have also addressed the benefits of preventive or early treatment (Soll 2001a) and the benefits of different specific surfactant products or treatment strategies (Yost 1999; Soll 2001b; Pfister 2007; Stevens 2007).
The following analysis is an update of the systematic review of randomized controlled trials that compare the prophylactic administration of protein free synthetic surfactant (SS) to control treatment in preterm infants at risk of developing RDS. Results of some of these analyses were previously published in 'Effective Care of the Newborn Infant' (Soll 1992).
Objectives
To assess the effect of prophylactic administration of protein free synthetic surfactant (SS) in preterm infants at risk of developing respiratory distress syndrome (RDS).
Subgroup analyses:
surfactant product : DPPC / HDL, DPPC / PG, DPPC/ hexadecanol/ tyloxapol;
gestational age (infants born at < 28 weeks gestation);
birth weight (<1000g);
single or multiple doses of surfactant.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials comparing prophylactic administration of protein free synthetic surfactant (given prior to the first breath or immediately after delivery room intubation and stabilization) to control treatment (intratracheal administration of normal saline or air placebo).
Types of participants
Premature infants with or without evidence of surfactant deficiency.
Types of interventions
Infants randomized to receive prophylactic administration of protein free synthetic surfactant (pre‐ventilatory or post‐ventilatory) versus control treatment (intratracheal administration of normal saline or air placebo).
Studies which allowed single doses or multiple doses were also included.
Included studies could use any of the following protein free synthetic surfactant preparations: DPPC/high density lipoprotein, powdered DPPC and phosphatidylglycerol, dipalmitoylphosphatidylcholine and phosphatidylglycerol in saline, and Exosurf Neonatal (dipalmitoylphosphatidylcholine, hexadecanol, and tyloxapol).
Types of outcome measures
Primary outcomes
neonatal mortality (mortality < 28 days of age) from any cause;
mortality prior to hospital discharge (from any cause);
bronchopulmonary dysplasia defined as supplemental oxygen at 28‐30 days of age;
chronic lung disease defined as supplemental oxygen at 36 weeks postmenstrual age;
bronchopulmonary dysplasia or death at 28 days of age;
chronic Lung Disease or Death at 36 weeks postmenstrual age.
Secondary outcomes
pneumothorax;
pulmonary interstitial emphysema;
air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum);
pulmonary hemorrhage;
patent ductus arteriosus (PDA) (PDA that has been treated with cyclo‐oxygenase inhibitor or surgery);
culture proven bacterial sepsis;
culture proven fungal sepsis;
necrotizing enterocolitis (NEC) (defined as Bell Stage II or greater);
intraventricular hemorrhage (IVH) [any grade and severe (grade 3 to 4)];
periventricular leukomalacia (PVL);
retinopathy of prematurity (ROP) [all stages and severe (stage 3 or greater)];
rehospitalization;
not assessed at follow‐up;
cerebral palsy (any and moderate/severe cerebral palsy);
neurodevelopmental outcome at approximately two years corrected age (acceptable range 18 months to 28 months) including: cerebral palsy, mental delay (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity), and hearing deficit (aided or < 60 dB on audiometric testing). The composite outcome "neurodevelopmental impairment" was defined as having any one of the aforementioned deficits.
Post hoc analyses were considered for any unexpected adverse effects reported by the studies.
Search methods for identification of studies
The standard search methods of the Cochrane Neonatal Review Group were used.
Electronic searches
Published manuscripts: Search included the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2009), MEDLINE (1966 to September 2009), OVID, CINAHL and EMBASE. All languages were included. Search terms: {surfactant OR pulmonary surfactant}, limited to humans and further limited to the age group of newborn infants (infant, newborn) and type of publication (clinical trial). A similar search was performed using the following text words: exosurf, colfoseril, DPPC with similar limits noted above. From the resulting studies randomized or quasi‐randomized controlled studies that fulfill the inclusion criteria were selected.
To identify long‐term neurodevelopmental sequelae, a search using the following keywords was performed: (outcome OR sequelae OR follow‐up OR mental retardation OR cerebral palsy OR hearing OR visual OR motor OR mental OR psychological) AND (surfactant OR pulmonary surfactant) not limited to any age group or language. The bibliography cited in each publication obtained was searched in order to identify additional relevant articles.
Searching other resources
Published abstracts: The abstracts of the Society for Pediatric Research (USA) (published in Pediatric Research) for the years 1985 to 1999 were searched by hand using the following key words: {surfactant OR pulmonary surfactant} AND {respiratory distress syndrome}. Abstracts from 2000 to 2009 were searched electronically through the PAS website (abstractsonline).
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Data collection and analysis
For each included study, information was collected regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was single or multicenter. Information regarding trial participants including birth weight criteria, and other inclusion or exclusion criteria was noted.
Selection of studies
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the previous section were included. Both review authors reviewed the results of the search and separately selected the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
The review authors separately extracted, assessed and coded all data for each study using a form that was designed specifically for this review. Any disagreement was resolved by discussion. For each study, final data was entered into RevMan by one review author (RFS) and then checked by a second review author (EO).
Assessment of risk of bias in included studies
The standard method of the Cochrane Neonatal Review Group were employed. The methodological quality of each trial was reviewed independently by the two review authors (RS and EO). Each identified trial was assessed for methodological quality with respect to a) masking of allocation b) masking of intervention c) completeness of follow‐up d) masking of outcome assessment. This information is included in the 'Characteristics of Included Studies' Table.
For the updated review in 2009, the Risk of Bias table was completed. The two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreement was resolved by discussion.
The Risk of Bias table addressed the following questions:
1. Sequence generation: Was the allocation sequence adequately generated?
For each included study, we described the method used to generate the allocation sequence as: adequate (any truly random process e.g. random number table; computer random number generator); inadequate (any nonrandom process e.g. odd or even date of birth; hospital or clinic record number); or unclear.
2. Allocation concealment: Was allocation adequately concealed?
For each included study, we described the method used to conceal the allocation sequence as: adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes); inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or unclear.
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors and specific outcomes assessed.
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as: adequate (< 20% missing data); inadequate (≥ 20% missing data) or unclear.
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we assessed the possibility of selective outcome reporting bias as: adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported); inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or unclear.
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns regarding other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes; no; or unclear.
Measures of treatment effect
Statistical analyses was performed using Review Manager software. Categorical data was analyzed using relative risk (RR), risk difference (RD) and the number needed to treat (NNT). Continuous data was analyzed using weighted mean difference (WMD). The 95% Confidence interval (CI) was reported on all estimates.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we explored the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc sub group analyses.
Data synthesis
Meta‐analysis was done using Review Manager software (RevMan 5, Cochrane Collaboration). For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
Gestational age (infants born at < 28 weeks gestation)
Birth weight (< 1000 g)
Surfactant product : DPPC / HDL, DPPC / PG, DPPC/ hexadecanol/ tyloxapol
Single or multiple doses of surfactant
Results
Description of studies
Randomized controlled trials which compared the effect of prophylactic protein free synthetic surfactant administration (synthetic surfactant prior to the first breath or immediately after intubation and stabilization in the delivery room) compared to control treatment (sham air treatment or instillation of normal saline) in premature infants thought to be at risk for developing respiratory distress syndrome are included in the analysis.
Results of the search
Studies included in this review: Halliday 1984; Wilkinson 1985; Ten Centre 1987; Bose 1990; Phibbs 1991; Corbet 1991; Stevenson 1992. Details of each study are given in the 'Characteristics of Included Studies' table and references.
Included studies
Halliday and coworkers (Halliday 1984) tested a surfactant composed of a saline suspension of DPPC and high density lipoprotein (HDL). In previous in vitro studies, this surfactant demonstrated the ability to adsorb and spread rapidly. The investigators studied 100 infants between 25 and 33 weeks gestation. Infants were randomized to receive either intratracheal DPPC/HDL or manual ventilation with air. Infants enrolled in this trial were followed for initial complications of prematurity.There were no significant differences in mortality or in the incidence and severity of respiratory distress syndrome between the surfactant treated and control group. However there appeared to be a trend towards improved survival in treated babies of 27 to 29 weeks gestation. The surviving babies were followed up at age two years to evaluate growth and development (Halliday 1986).
A synthetic surfactant composed of DPPC and PG has been tested in the United Kingdom. Morley (Morley 1981) first studied a dry synthetic mixture of DPPC/PG. This study is not included in the review as the subjects were not randomly assigned to the intervention. The infants studied were 34 weeks gestation or less and required resuscitation in the delivery suite. Twenty‐two infants were given DPPC/PG via the endotracheal tube. The comparison group consisted of infants of similar gestational age, resuscitated in the delivery room without the investigators present at the delivery (33 infants). Treated infants required less ventilatory support initially, but no sustained improvement was noted. No deaths were noted in the treatment group, whereas eight control infants died. The results of this clinical experience with dry powdered DPPC/PG have not been corroborated in other studies.
Wilkinson (Wilkinson 1985) conducted a randomized controlled trial with DPPC/PG in both the prevention and treatment of respiratory distress syndrome in infants 31 weeks gestation or less. Infants given prophylactic dry DPPC/PG did not better than untreated controls.
The Ten Centre Collaborative Study (Ten Centre 1987) used a saline suspension of DPPC and PG called artificial lung expanding compound (ALEC). The Ten Centre Study randomized 328 infants between 25 and 29 weeks gestation to either ALEC or 1 ml of saline. This trial was an extension of a trial that originally enrolled infants in Cambridge and Nottingham and was expanded to include infants at eight other centers. Partial results are also reported by Morley (Morley 1988). Treatment was administered in the delivery room by injecting the surfactant suspension into the hypopharynx of the infant. In those infants who failed to breathe spontaneously, intubation and resuscitation were followed by a retreatment via the endotracheal tube. Infants who remained intubated could be given up to three more doses during the first 24 hours of life. No immediate benefit of surfactant treatment was reported. Treated infants demonstrated a significant decrease in the severity of the respiratory distress syndrome, severe intracranial hemorrhage and neonatal mortality. Infants from this trial who were born in Cambridge were followed at nine and 18 months (Morley 1990).
The most widely tested of the complex synthetic surfactant products is Exosurf Neonatal (Burroughs Wellcome). Exosurf Neonatal is composed primarily of DPPC, but also contains hexadecanol (an alcohol) and tyloxapol (a non‐ionic surfactant). Hexadecanol and tyloxapol are not found in naturally occurring surfactant and have been added to aid in the spread and dispersion of the surfactant. Exosurf Neonatal has been successfully tested in animal models (Tooley 1987). The effects of Exosurf Neonatal in animal models is significantly better than saline control, but not equivalent to treatment with a crude natural surfactant extract.
Four randomized controlled trials have evaluated the use of a single intratracheal prophylactic dose of Exosurf Neonatal in infants at risk of developing RDS (Bose 1990; Phibbs 1991; Corbet 1991; Stevenson 1992). The results from these studies are favorable, but not consistent. Phibbs and coworkers (Phibbs 1991) studied Exosurf Neonatal in both the prevention and treatment of respiratory distress syndrome. In the prevention trial, 74 infants of birthweight 700 to 1350 grams were randomized to Exosurf or control treatment. Significant reduction in the requirement for ventilatory support was noted in the 72 hours after treatment. No other substantial clinical benefits were noted. Two large multicenter trials evaluated prophylactic Exosurf Neonatal in a similar population. Bose (Bose 1990) and Corbet (Corbet 1991) studied Exosurf Neonatal in infants with birthweights 700 to1350 grams and 700 to 1100 grams, respectively. Both trials reported early improvement in the need for supplemental oxygen and ventilatory support. In Corbet's study (Corbet 1991), fewer pneumothoraces were noted in treated infants and mortality was significantly decreased. Bose (Bose 1990) did not demonstrate an improvement in overall mortality, but the number of surviving infants without BPD was increased. Stevenson (Stevenson 1992) studied a single dose of prophylactic Exosurf Neonatal in infants with birthweight 500 to 699 grams. Pneumothorax was reduced by slightly more than half. Overall neonatal mortality was not significantly reduced. Other complications of RDS and prematurity were not altered, except that pulmonary hemorrhage occured significantly more frequently in infants receiving synthetic surfactant. Infants enrolled in the Exosurf Neonatal trials were followed at one and two years adjusted age (Corbet 1995, Kraybill 1995, Sell 1995, Walther 1995).
Excluded studies
Six studies identified in the search were considered but excluded (Chu 1967; Milner 1984; Morley 1981; Morley 1988; Morley 1991; Robillard 1964). Details are given in the "Characteristics of Excluded Studies" table.
Risk of bias in included studies
Specific methodologic issues regarding the seven studies are discussed below:
Allocation
Randomization: All included studies allocated assigned treatment by randomization. In all seven studies, sealed envelopes with randomly allocated treatment assignments were provided to participating centers.
Blinding
Blinding of treatment: Investigators attempted to blind treatment. Most studies relied on a resuscitation team to administer the randomly allocated treatment. Individuals in this resuscitation team were not responsible for ongoing care of the infant or for study evaluation.
Blinding of outcome assessment: Investigators who are not involved with treatment are assigned to administration of assessment and study outcomes.
Incomplete outcome data
Exclusion after randomization: Minimal exclusions were noted after randomization.
Selective reporting
Not noted in included studies.
Other potential sources of bias
None noted.
Effects of interventions
Prophylactic administration of complex protein free synthetic surfactants in preterm infants at risk for developing RDS has a variable effect on oxygenation and ventilatory requirements. Prophylactic administration of protein free synthetic surfactant extract in preterm infants at risk of developing RDS has the following clinical impact:
PROPHYLACTIC PROTEIN FREE SYNTHETIC SURFACTANT (COMPARISON 1):
PRIMARY OUTCOMES:
NEONATAL MORTALITY (Outcome 1.1): All seven randomized controlled trials reported on the risk of neonatal mortality. The Ten Centre 1987 and Corbet 1991 report a decrease in the risk of neonatal mortality associated with the prophylactic administration of protein free synthetic surfactant. The typical estimate from the meta‐analysis suggests a decrease in the risk of neonatal mortality associated with prophylactic administration of synthetic surfactant (typical relative risk 0.70, 95% CI 0.58 0.85; typical risk difference ‐0.07, 95% CI ‐0.11, ‐0.03).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.1.1 ‐ 1.1.3): Four randomized controlled trials of Exosurf Neonatal reported (Bose 1990; Phibbs 1991; Corbet 1991; Stevenson 1992) on neonatal mortality.The typical estimate from the meta‐analysis suggests a decrease in the risk of neonatal mortality associated with prophylactic administration of Exosurf Neonatal (typical relative risk 0.75, 95% CI 0.60, 0.93; typical risk difference ‐0.06, 95% CI ‐0.11, ‐0.02 ).
Two randomized controlled trials of intratracheal prophylactic dose of DPPC/PG (Wilkinson 1985; Ten Centre 1987) reported on neonatal mortality.The typical estimate from the meta‐analysis suggests a decrease in the risk of neonatal mortality associated with prophylactic administration of DPPC /PG (typical relative risk 0.52, 95% CI 0.33, 0.82); typical risk difference ‐0.12, 95% CI ‐0.21, ‐0.04). 0.52 [0.33, 0.82]
Halliday 1984 conducted a randomized controlled trial with DPPC/HDL in the prevention of respiratory distress syndrome and did not identify any difference in neonatal mortality (typical relative risk 1.04, 95% CI 0.36, 3.01; typical risk difference 0.00, 95% CI ‐0.12, 0.13).
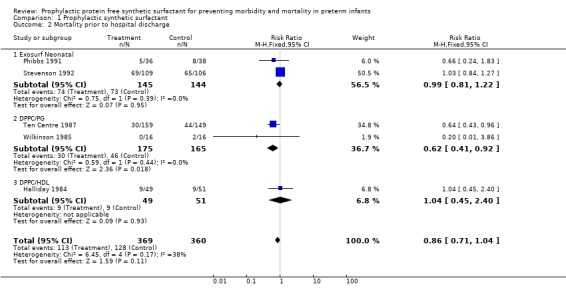
MORTALITY PRIOR TO HOSPITAL DISCHARGE (Outcome 1.2): Five of the trials reported on mortality prior to hospital discharge. The Ten Centre 1987 reported a decrease in the risk of mortality prior to hospital discharge in those infants who received prophylactic synthetic surfactant (typical relative risk 0.64, 95% CI 0.43, 0.96; typical risk difference ‐0.11, 95% CI ‐0.20, ‐0.01). The typical estimate from the meta‐analysis suggests a trend toward decreased risk of mortality prior to hospital discharge in infants who received prophylactic administration of synthetic surfactant (typical relative risk 0.86, 95% CI 0.71, 1.04; typical risk difference ‐0.05, 95% CI ‐0.1, 0.02).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.2.1 ‐ 1.2.3): Two studies of prophylactic Exosurf Neonatal reported on mortality prior to hospital discharge (Phibbs 1991; Stevenson 1992 ). The typical estimate from the meta‐analysis supports no difference in mortality prior to hospital discharge (typical relative risk 0.99, 95% CI 0.81,1.22; typical risk difference ‐0.00 95% CI ‐0.11, 0.10). Two studies of prophylactic DPPC/PG reported on mortality prior to hospital discharge (Wilkinson 1985Ten Centre 1987 ).The typical estimate from the meta‐analysis suggests a decreased risk of mortality prior to hospital discharge (typical relative risk 0.62, 95% CI 0.41, 0.92; typical risk difference ‐0.11, 95% CI ‐0.20, ‐0.02). The study of Halliday and coworkers ( Halliday 1984) with DPPC/HDL demonstrated no difference in mortality prior to discharge (typical relative risk 1.04 95% CI 0.45, 2.40; typical risk difference 0.01, 95% CI ‐0.14, 0.16).
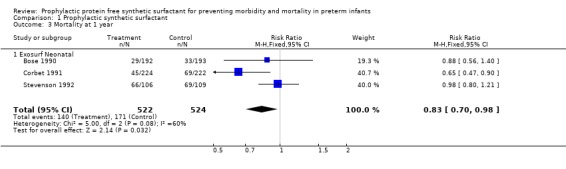
MORTALITY AT ONE YEAR OF AGE (Outcome 1.3): Three of the randomized controlled trials reported on survival at one year of age. Corbet 1991 noted decreased mortality in infants who received Exosurf Neonatal (typical relative risk 0.65, 95% CI 0.47, 0.90; typical risk difference ‐0.11, 95% CI ‐0.19, ‐0.03). The typical estimate from the meta‐analysis suggests a decreased risk of mortality at one year of age in infants receiving prophylactic synthetic surfactant (typical relative risk 0.83, 95% CI 0.70, 0.98; typical risk difference ‐0.06, 95% CI ‐0.11, ‐0.01).
‐ Subgroup analyses: Different surfactant products (Outcome 1.3.1): Only three studies of Exosurf Neonatal reported on mortality at one year of age (Bose 1990; Corbet 1991; Stevenson 1992). The typical estimate from the meta‐analysis suggests a decreased risk of mortality at one year of age in infants receiving prophylactic synthetic surfactant (typical relative risk 0.83, 95% CI 0.70, 0.98; typical risk difference ‐0.06, 95% CI ‐0.11, ‐0.01).
BRONCHOPULMONARY DYSPLASIA (Outcome 1.4): Four randomized trials reported on the risk of bronchopulmonary dysplasia. No individual trial reported a difference in the risk of bronchopulmonary dysplasia. The typical estimate from the meta‐analysis supports no difference in the risk of bronchopulmonary dysplasia (typical relative risk 1.06, 95% CI 0.83 1.36; typical risk difference 0.01, 95% CI ‐0.03, 0.06).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.4.1, 1.4.2): Three randomized controlled trials of Exosurf Neonatal (Bose 1990; Corbet 1991; Stevenson 1992) reported on the risk of bronchopulmpnary dysplasia.The typical estimate from the meta‐analysis supports no difference in the risk of bronchopulmonary dysplasia (typical relative risk 1.05, 95% CI 0.82, 1.36; typical risk difference 0.01, 95% CI ‐0.04, 0.06). Halliday 1984 showed no difference in the risk of bronchopulmonary dysplasia with the prophylactic use of DPPC/HDL (typical risk difference 1.19, 95% CI 0.47, 3.03; typical risk differnce 0.03 95% CI ‐0.11,0.17).
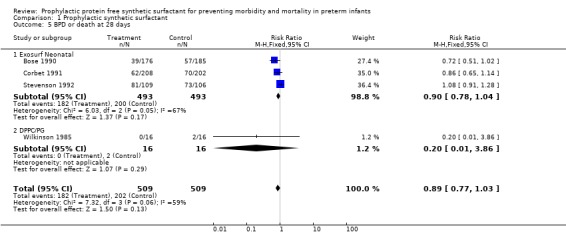
BRONCHOPULMONARY DYSPLASIA OR DEATH (Outcome 1.5): Four of the randomized controlled trials reported the combined outcome of bronchopulmonary dysplasia or death. Bose 1990 reported a trend towards decreased bronchopulmonary dysplasia or death in infants who received prophylactic synthetic surfactant (typical relative risk 0.72, 95% CI 0.51, 1.02; typical risk difference ‐0.09, 95% CI ‐0.18, 0.00). The typical estimate from the meta‐analysis suggests a trend towards decreased risk of bronchopulmonary dysplasia or death at 28 days in infants who received prophylactic synthetic surfactant (typical relative risk 0.89, 95% CI 0.77 1.03; typical risk difference ‐0.04, 95% CI ‐0.10, 0.01).
‐ Subgroup analyses: Different Surfactant Products (Outcomes 1.5.1, 1.5.2): Three studies of Exosurf Neonatal ( Bose 1990; Corbet 1991; Stevenson 1992) reported the combined outcome of bronchopulmonary dysplasia or death. The typical estimate from the meta‐analysis suggests a trend towards decreased risk of bronchopulmonary dysplasia or death at 28 days in infants who received prophylactic Exosurf Neonatal (typical relative risk 0.90, 95% CI 0.78,1.04; typical risk difference ‐0.04, 95% CI ‐0.10, 0.02).
One study of DPPC/PG (Wilkinson 1985) reported the combined outcome of bronchopulmonary dysplasia or death. In this study no significant difference in the risk of bronchopulmonary dysplasia or death at 28 days was noted ( typical relative risk 0.20, 95% CI 0.01, 3.86; typical risk difference ‐0.13 95% CI ‐0.31, 0.06).
SECONDARY OUTCOMES:
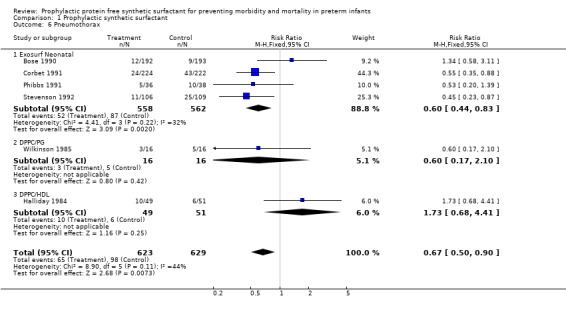
PNEUMOTHORAX (Outcome 1.6): Six of the randomized controlled trials reported on the incidence of pneumothorax associated with prophylactic synthetic surfactant administration. Corbet 1991 and Stevenson 1992 both reported a decrease in the risk of pneumothorax associated with the administration of synthetic surfactant. The typical estimate from the meta‐analysis suggests that prophylactic administration of synthetic surfactant will lead to a significant reduction in the risk of pneumothorax (typical relative risk 0.67, 95% CI 0.50, 0.90; typical risk difference ‐0.05, 95% CI ‐0.09, ‐0.02).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.6.1 ‐ 1.6.3):
Four studies (Bose 1990; Corbet 1991; Phibbs 1991; Stevenson 1992) reported on the effect of Exosurf Neonatal on the risk of pneumothorax.The typical estimate from the meta‐analysis suggests that prophylactic administration of Exosurf Neonatal leads to a significant reduction in the risk of pneumothorax (typical relative risk 0.60, 95% CI 0.44, 0.83; typical risk difference ‐0.06 95% CI ‐0.10, ‐0.02).
One study of DPPC/PG (Wilkinson 1985) reported on the risk of pneumothrorax and showed no significant difference in the risk of pneumothorax (typical relative risk 0.60,95% CI 0.17, 2.10; typical risk difference ‐0.13, 95% CI ‐0.42, 0.17). Only the study using DPPC/HDL (Halliday 1984) showed no statistically significant impact of synthetic surfactant on the risk of pneumothorax (typical relative risk 1.73, 95% CI 0.68, 4.41; typical risk difference 0.09, 95% CI ‐0.06, 0.23).
PULMONARY INTERSTITIAL EMPHYSEMA (Outcome 1.7): Only two of the randomized controlled trials reported on the incidence of pulmonary interstitial emphysema. In the trial of Bose 1990 a significant reduction was noted in the risk of pulmonary interstitial emphysema (typical relative risk 0.50, 95% CI 0.27, 0.95; typical risk difference ‐0.07, 95% CI ‐0.13, ‐0.01). The typical estimate from the meta‐analysis suggests that prophylactic administration of synthetic surfactant will lead to a significant reduction in the risk of pulmonary interstitial emphysema (typical relative risk 0.68, 95% CI 0.50, 0.93; typical risk difference ‐0.06, 95% CI ‐0.11, ‐0.01).
‐ Subgroup analyses: Different surfactant products (Outcome 1.7.1): Two studies of Exosurf Neonatal reported on the risk of pulmonary interstitial emphysema (Bose 1990; Corbet 1991).The typical estimate from the meta‐analysis suggests that prophylactic administration of synthetic surfactant will lead to a significant reduction in the risk of pulmonary interstitial emphysema (typical relative risk 0.68, 95% CI 0.50, 0.93; typical risk difference ‐0.06, 95% CI ‐0.11, ‐0.01).
PATENT DUCTUS ARTERIOSUS (Outcome 1.8): None of the individual trials reported a difference in the risk of patent ductus arteriosus. Typical estimate of the meta‐analysis supports a slight increase in the risk of patent ductus arteriosus associated with prophylactic synthetic surfactant administration (typical relative risk 1.11, 95% CI 1.00, 1.22; typical risk difference 0.05, 95% CI 0.00, 0.10).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.8.1 ‐ 1.8.3): Four studies of prophylactic Exosurf Neonatal reported on the risk of patent ductus arteriosus (Bose 1990; Corbet 1991; Phibbs 1991; Stevenson 1992). Typical estimate of the meta‐analysis supports a slight increase in the risk of patent ductus arteriosus associated with prophylactic use of Exosurf Neonatal (typical relative risk 1.10, 95% CI 0.99, 1.22; typical risk difference 0.05,95% CI ‐0.00, 0.11).Two studies of prophylactic use of DPPC/PG reported on the risk of patent ductus arteriosus (Wilkinson 1985; Ten Centre 1987 ) Overall no effect on the risk of patent ductus arteriosus was demostrated (typical relative risk 1.06, 95% CI 0.74, 1.51; typical risk difference 0.01,95% CI ‐0.08, 0.11).The only study of prophylactic DPPC/HDL (Halliday 1984) showed no difference in the risk of patent ductus arteriosus (typical relative risk 1.78, 95% CI 0.77, 4.16; typical risk difference 0.11, 95% CI ‐0.05, 0.26).
PULMONARY HEMORRHAGE (Outcome 1.9): Four of the seven studies reported on pulmonary hemorrhage. Stevenson 1992 reported an increase in the risk of pulmonary hemorrhage associated with prophylactic synthetic surfactant administration (typical relative risk 6.17, 95% CI 1.41, 26.91; typical risk difference 0.10, 95% CI 0.03, 0.16). The typical estimate from the meta‐analysis suggests that prophylactic administration of synthetic surfactant increases the risk of pulmonary hemorrhage (typical relative risk 3.28, 95% CI 1.50, 7.16; typical risk difference 0.03, 95% CI 0.01, 0.05).
‐ Subgroup analyses: Different surfactant products (Outcome 1.9.1): Only studies of Exosurf Neonatal reported on the risk of pulmonary hemorrhage (Bose 1990; Corbet 1991; Phibbs 1991; Stevenson 1992). The typical estimate from the meta‐analysis suggests that prophylactic administration of Exosurf Neonatal increases the risk of pulmonary hemorrhage (typical relative risk 3.28, 95% CI 1.50, 7.16; typical risk difference 0.03, 95% CI 0.01, 0.05).
NECROTIZING ENTEROCOLITIS (Outcome 1.10): All seven randomized controlled trials reported on the incidence of necrotizing enterocolitis. None of the individual trials reported a difference in the risk of necrotizing enterocolitis and the typical estimate from the meta‐analysis supports no difference in the risk of necrotizing enterocolitis (typical relative risk 1.11, 95% CI 0.78, 1.59; typical risk difference 0.01, 95% CI ‐0.02, 0.03).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.10.1‐ 1.10.3): Four randomized controlled trials of Exosurf Neonatal reported (Bose 1990; Phibbs 1991; Corbet 1991; Stevenson 1992)on the risk of necrotizing enterocolitis.The typical estimate from the meta‐analysis suggests no difference in the risk of necrotizing enterocolitis (typical relative risk 1.35, 95% CI 0.88, 2.09; typical risk difference 0.02, 95% CI ‐0.01, 0.05).
Two studies of prophylactic use of DPPC/PG reported on the risk of necrotizing enterocolitis (Wilkinson 1985; Ten Centre 1987 ) The typical estimate from the meta‐analysis suggests that prophylactic administration of DPPC/PG has no effect on the risk of necrotizing enterocolitis (typical relative risk 0.75, 95% CI 0.39, 1.47; typical risk difference ‐0.03, 95% CI ‐0.09, 0.04). The only study of prophylactic DPPC/HDL (Halliday 1984) showed no difference in the risk of necrotizing enterocolitis (typical relative risk 0.69, 95% CI 0.12, 3.98; typical risk difference ‐0.02 , 95% CI ‐0.10, 0.07).
INTRAVENTRICULAR HEMORRHAGE (Outcome 1.11): Four studies report on the incidence of intraventricular hemorrhage in infants who received prophylactic synthetic surfactant. None of these trials report a difference in the risk of intraventricular hemorrhage and the typical estimate from the meta‐analysis supports no difference in the risk of intraventricular hemorrhage (typical relative risk 0.96, 95% CI 0.81, 1.14; typical risk difference ‐0.01, 95% CI ‐0.07, 0.04).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.11.1, 1.11.2): Three studies of Exosurf Neonatal reported on the incidence of intraventricular hemorrhage (Bose 1990; Corbet 1991; Stevenson 1992). Overall, no effect on the risk of intraventricular hemorrhage was demonstrated (typical relative risk 0.99, 95% CI 0.83, 1.17; typical risk difference ‐0.00, 95% CI ‐0.06, 0.05). The study of Halliday and coworkers (Halliday 1984) with DPPC/HDL demonstrated no significant difference in the risk of intraventricular hemorrhage (typical relative risk 0.30, 95% CI 0.06, 1.36; typical risk difference ‐0.10, 95% CI ‐0.21, 0.01).
SEVERE INTRAVENTRICULAR HEMORRHAGE (Outcome 1.12): Four trials report on the risk of severe intraventricular hemorrhage (IVH grades III or IV). None of the individual trials support a difference in risk of severe intraventricular hemorrhage and the typical estimate from the meta‐analysis suggests no difference in the risk of severe intraventricular hemorrhage (typical relative risk 1.01, 95% CI 0.75, 1.38; typical risk difference 0.00, 95% CI ‐0.04, 0.04).
‐ Subgroup analyses: Different surfactant products (Outcome 1.121): Four randomized controlled trials of Exosurf Neonatal (Bose 1990; Phibbs 1991; Corbet 1991; Stevenson 1992) reported on the risk of severe intraventricular hemorrhage. The typical estimate from the meta‐analysis suggests no difference in the risk of severe intraventricular hemorrhage (typical relative risk 1.01, 95% CI 0.75, 1.38; typical risk difference 0.00, 95% CI ‐0.04, 0.04).
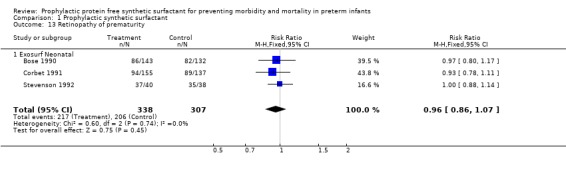
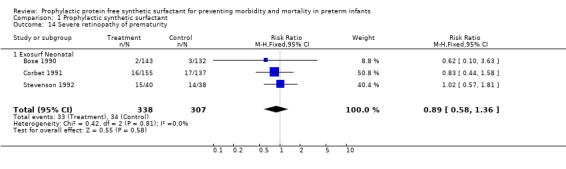
RETINOPATHY OF PREMATURITY(Outcome 1.13 and 1.14): Three trials reported on retinopathy of prematurity in follow‐up evaluation of infants (Bose 1990; Corbet 1991; Stevenson 1992). None of the studies reported a difference in retinopathy of prematurity or in severe retinopathy of prematurity (retinopathy of prematurity, Stage 3 ‐ 4). The meta‐analysis evaluates the risk of retinopathy in surviving infants who were examined. The typical estimate from the meta‐analysis suggests no difference in the risks of any retinopathy (typical relative risk 0.96, 95% CI 0.86, 1.07; typical risk difference ‐0.03, 95% CI ‐0.10, 0.04) or in the risk of severe retinopathy of prematurity (typical relative risk 0.89, 95% CI 0.58, 1.36; typical risk difference ‐0.01, 95% CI ‐0.06, 0.03).
‐ Subgroup analyses: Different surfactant products (Outcomes 1.13.1 and 1.14.1): Three trials of prophylactic Exosurf Neonatal reported on retinopathy of prematurity in follow‐up evaluation of infants (Bose 1990, Corbet 1991, Stevenson 1992).The typical estimate from the meta‐analysis suggests no difference in the risks of any retinopathy (typical relative risk 0.96, 95% CI 0.86, 1.07; typical risk difference ‐0.03, 95% CI ‐0.10, 0.04) or in the risk of severe retinopathy of prematurity (typical relative risk 0.89, 95% CI 0.58, 1.36; typical risk difference ‐0.01, 95% CI ‐0.06, 0.03).
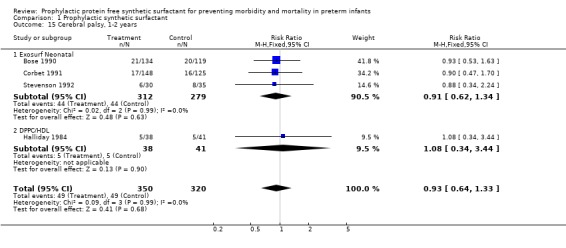
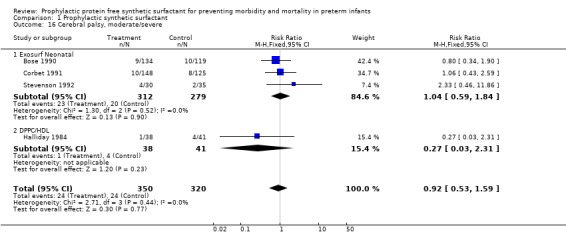
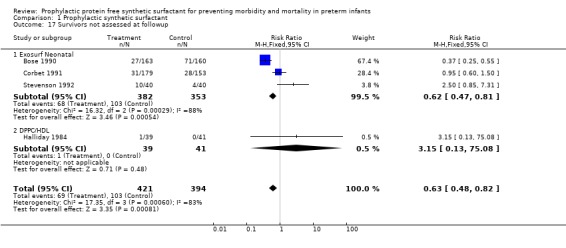
FOLLOW‐UP EVALUATION (Outcomes 1.15 ‐ 1.17): Five of the randomized controlled trials reported on follow‐up of infants enrolled in the trials. Follow‐up from the Ten Centre 1987 Study Group is partially reported by Morley and coworkers (1990). The trials of Bose 1990, Corbet 1991, Stevenson 1992 and Halliday 1984 all reported follow‐up on infants at one to two years of age. Between 80% and 99% of survivors were evaluated in the studies. Somewhat fewer infants who received surfactant failed to return for follow‐up evaluation (typical relative risk 0.63, 95% CI 0.48, 0.82; typical risk difference ‐0.10, 95% CI ‐0.15, ‐0.04). The meta‐analysis suggests no difference in the rates of cerebral palsy at one to two years of age (typical relative risk 0.93, 95% CI 0.64, 1.33; typical risk difference ‐0.01, 95% CI ‐0.07, 0.04). The risk of being severely affected with cerebral palsy is also unchanged by prophylactic synthetic surfactant administration (relative risk 0.92, 95% CI 0.53, 1.59; typical risk difference ‐0.01, 95% CI ‐0.05, 0.03 )
CEREBRAL PALSY 1 ‐ 2 years (Outcomes 1.15.1,1.15.2): Three studies of prophylactic Exosurf Neonatal (Bose 1990; Corbet 1991; Stevenson 1992) reported on the risk of cerebral palsy at one to two years.The meta‐analysis suggests no difference in the rates of cerebral palsy at one to two years of age (typical risk ratio 0.91, 95% CI 0.62, 1.34; typical risk difference ‐0.01 95% CI ‐0.07, 0.04).The study of Halliday and coworkers ( Halliday 1984) with DPPC/HDL reported follow up on infants at 1‐2 years of age. No significant difference was noted in the rates of cerebral palsy at one to two years (typical risk ratio 1.08, 95% CI 0.34, 3.44; typical risk difference 0.01, 95% CI ‐0.14, 0.16 ).
CEREBRAL PALSY 1 ‐ 2 years Moderate/ severe (Outcomes 1.16.1, 1.16.2): Three studies of prophylactic Exosurf Neonatal (Bose 1990; Corbet 1991; Stevenson 1992) reported on the risk of severe cerebral palsy at one to two years.The meta‐analysis suggests no difference in the rates of severe cerebral palsy at one to two years of age (typical risk ratio 1.04, 95% CI 0.59, 1.84; typical risk difference 0.00, 95% CI ‐0.04, 0.04).The study of prophylactic DPPC/HDL (Halliday 1984) showed no difference in the rates of severe cerebral palsy at one to two years (typical risk ratio 0.27, 95% CI 0.03, 2.31; typical risk difference ‐0.07, 95% CI ‐0.18, 0.03).
Other Subgroup analyses: Infants < 1000 grams
None of the studies reported specifically on extremely low birth weight infants (infants < 1000 grams). Individual studies reported on some subpopulations based on gestational age or weight [Halliday reported the mortality rate for infants 27 to 29 weeks; Ten center trial reported the mortality rate by gestational age (25 to 26 weeks vs. 27 to 29 weeks); Stevenson reported the incidence of death and survival without BPD by weight (500‐599; 600‐699 )] (Halliday 1984; Stevenson 1992;Ten Centre 1987). None of these met our a priori definition for subgroup analysis and none were groupable even in a post hoc analysis.
Discussion
Seven randomized controlled trials were identified which compared prophylactic administration of protein free synthetic surfactant to control treatment. Studies used a variety of protein free synthetic surfactants including artificial lung expanding compound, (dipalmitoylphosphatidylcholine and phosphatidylglycerol), dipalmitoylphosphatidylcholine and high density lipoprotein, and Exosurf Neonatal (dipalmitoylphosphatidylcholine, toloxapol, and hexadecanol). The majority of studies evaluated infants 30 weeks gestation or less; however, Halliday 1984 studied infants as old as 33 weeks gestation and Stevenson 1992 focused on the extremely premature infants (birthweight 500 to 699 grams). All studies excluded infants with known lung maturity, but no study required screening for pulmonary immaturity prior to entry into the study. Infants could be enrolled regardless of exposure to antenatal steroids. Only the Ten Centre 1987 allowed for multiple surfactant treatments.
Prophylactic use of protein free synthetic surfactants had an inconsistent effect on the immediate respiratory course. Only in the studies of Exosurf Neonatal are there reports of improvement in oxygenation and ventilatory requirements in the 48 to 72 hours after treatment.
The meta‐analysis suggests that prophylactic administration of protein free synthetic surfactant leads to a significant decrease in the risk of pneumothorax, pulmonary interstitial emphysema, and neonatal mortality. The meta‐analysis suggests that for every 100 infants treated prophylactically, there will be five fewer pneumothoraces, six fewer cases of pulmonary interstitial emphysema, and seven fewer neonatal deaths. No impact is noted on the incidence of intraventricular hemorrhage or severe intraventricular hemorrhage.
Although no individual study reported an increase in the incidence of hemodynamically significant patent ductus arteriosus, the meta‐analysis suggests that there is an increase in the risk of patent ductus arteriosus associated with prophylactic protein free synthetic surfactant administration. This risk is of marginal statistical significance. In animals treated with surfactant products, earlier and more severe shunting through the patent ductus arteriosus has been noted. Of interest, the investigators in the trials using Exosurf Neonatal reported an increased incidence in pulmonary hemorrhage. This increased risk of pulmonary hemorrhage is supported by the meta‐analysis. Pulmonary hemorrhage is thought to be hemorrhagic pulmonary edema secondary to massive ductal shunting. Although not reported in the randomized controlled trials of other surfactant products, this outcome was addressed retrospectively in analyses by Raju and coworkers (Raju 1993). The risk of pulmonary hemorrhage appears to occur with both synthetic surfactant products and natural surfactant extracts. In clinical practice, pulmonary hemorrhage may be preventable by aggressive treatment of the ductus arteriosus and appropriate ventilatory management. No other side effects of synthetic surfactant treatment were reported.
The trials included in this review compared prophylatic synthetic surfactant with no surfactant treatment. After the demonstration of the efficacy of surfactant in preventing and treating respiratory distress syndrome, trials were conducted which compared the policies of prophylactic surfactant administration in babies at risk of RDS with selective treatment of babies who develop RDS. These trials were conducted using animal derived surfactant preparations. In these studies, prophylactic animal derived surfactant was noted to be superior to late selective treatment of babies with established RDS (Soll 2001a).
Studies have also evaluated the differences between synthetic surfactant and animal derived surfactant extract. These trials were only done in the context of treating established respiratory distress syndrome. In these studies, the use of animal derived surfactant extract appears superior in decreasing the risk of pneumothorax and increasing survival.
Authors' conclusions
Implications for practice.
Prophylactic administration of protein free synthetic surfactant extract to infants judged to be at risk for developing respiratory distress syndrome has been demonstrated to improve clinical outcome. Infants who receive prophylactic synthetic surfactant have a decreased risk of pneumothorax, a decreased risk of pulmonary interstitial emphysema and a decreased risk of neonatal mortality. No impact is noted on intraventricular hemorrhage (any hemorrhage or severe intraventricular hemorrhage), necrotizing enterocolitis, bronchopulmonary dysplasia, or retinopathy of prematurity. Prophylactic synthetic surfactant may lead to an increase in the incidence of patent ductus arteriosus and pulmonary hemorrhage. However, these complications did not overshadow the impact on overall outcome (neonatal mortality or late mortality).
Implications for research.
Prophylactic protein free synthetic surfactant has been proven to improve clinical outcome. Further placebo controlled trials of prophylactic protein free synthetic surfactant are no longer warranted. The impact of animal derived surfactant extracts has been reviewed elsewhere (Soll 1998a; Soll 1998b; Seger 2009). Trials which compared the prophylactic treatment strategy to treatment of established disease have been conducted using animal derived surfactant extract (Soll 2001a). Preliminary trials of protein containing synthetic surfactant have been conducted (Pfister 2007).
What's new
| Date | Event | Description |
|---|---|---|
| 8 January 2010 | Amended | Minor edits to reference format. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 29 October 2009 | New citation required but conclusions have not changed | New authorship: Roger Soll, Eren Özek |
| 29 October 2009 | New search has been performed | This updates the review "Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants" published in the Cochrane Database of Systematic Reviews, Issue 2, 1998 (Soll 1998a). No new trials identified in updated search March 2009. No change in conclusions. |
| 27 February 2008 | Amended | Converted to new review format. |
| 26 January 1998 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Prophylactic synthetic surfactant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 7 | 1500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.58, 0.85] |
| 1.1 Exosurf Neonatal | 4 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.93] |
| 1.2 DPPC/PG | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.82] |
| 1.3 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.36, 3.01] |
| 2 Mortality prior to hospital discharge | 5 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.04] |
| 2.1 Exosurf Neonatal | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.22] |
| 2.2 DPPC/PG | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.92] |
| 2.3 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.45, 2.40] |
| 3 Mortality at 1 year | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 3.1 Exosurf Neonatal | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 4 Bronchopulmonary dysplasia | 4 | 1086 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.36] |
| 4.1 Exosurf Neonatal | 3 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.36] |
| 4.2 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.47, 3.03] |
| 5 BPD or death at 28 days | 4 | 1018 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 5.1 Exosurf Neonatal | 3 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 5.2 DPPC/PG | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.86] |
| 6 Pneumothorax | 6 | 1252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 6.1 Exosurf Neonatal | 4 | 1120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.83] |
| 6.2 DPPC/PG | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.10] |
| 6.3 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.68, 4.41] |
| 7 Pulmonary interstitial emphysema | 2 | 831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.93] |
| 7.1 Exosurf Neonatal | 2 | 831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.93] |
| 8 Patent ductus arteriosus | 7 | 1560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.22] |
| 8.1 Exosurf Neonatal | 4 | 1120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 8.2 DPPC/PG | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.51] |
| 8.3 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.77, 4.16] |
| 9 Pulmonary hemorrhage | 4 | 1120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [1.50, 7.16] |
| 9.1 Exosurf Neonatal | 4 | 1120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [1.50, 7.16] |
| 10 Necrotizing enterocolitis | 7 | 1543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.59] |
| 10.1 Exosurf Neonatal | 4 | 1120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.88, 2.09] |
| 10.2 DPPC/PG | 2 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.47] |
| 10.3 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 11 Intraventricular hemorrhage | 4 | 1146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 11.1 Exosurf Neonatal | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.17] |
| 11.2 DPPC/HDL | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.36] |
| 12 Severe intraventricular hemorrhage | 4 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.38] |
| 12.1 Exosurf Neonatal | 4 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.38] |
| 13 Retinopathy of prematurity | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 13.1 Exosurf Neonatal | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 14 Severe retinopathy of prematurity | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.36] |
| 14.1 Exosurf Neonatal | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.36] |
| 15 Cerebral palsy, 1‐2 years | 4 | 670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.33] |
| 15.1 Exosurf Neonatal | 3 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 15.2 DPPC/HDL | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.34, 3.44] |
| 16 Cerebral palsy, moderate/severe | 4 | 670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.59] |
| 16.1 Exosurf Neonatal | 3 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.84] |
| 16.2 DPPC/HDL | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.31] |
| 17 Survivors not assessed at followup | 4 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.48, 0.82] |
| 17.1 Exosurf Neonatal | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.81] |
| 17.2 DPPC/HDL | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 75.08] |
1.1. Analysis.

Comparison 1 Prophylactic synthetic surfactant, Outcome 1 Neonatal mortality.
1.2. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 2 Mortality prior to hospital discharge.
1.3. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 3 Mortality at 1 year.
1.4. Analysis.

Comparison 1 Prophylactic synthetic surfactant, Outcome 4 Bronchopulmonary dysplasia.
1.5. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 5 BPD or death at 28 days.
1.6. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 6 Pneumothorax.
1.7. Analysis.
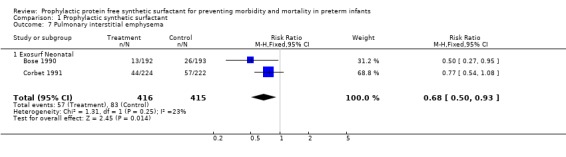
Comparison 1 Prophylactic synthetic surfactant, Outcome 7 Pulmonary interstitial emphysema.
1.8. Analysis.

Comparison 1 Prophylactic synthetic surfactant, Outcome 8 Patent ductus arteriosus.
1.9. Analysis.
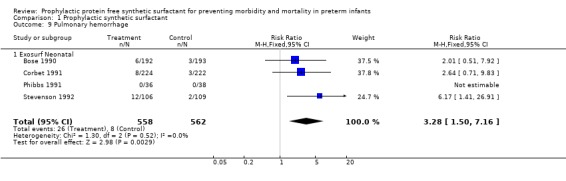
Comparison 1 Prophylactic synthetic surfactant, Outcome 9 Pulmonary hemorrhage.
1.10. Analysis.
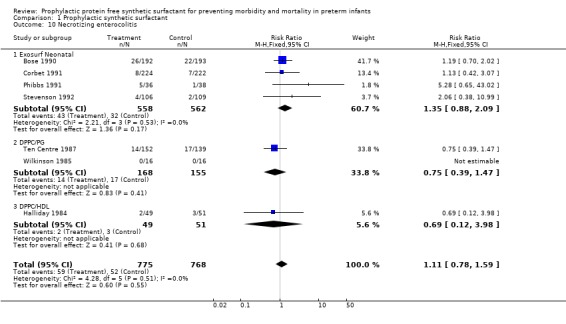
Comparison 1 Prophylactic synthetic surfactant, Outcome 10 Necrotizing enterocolitis.
1.11. Analysis.
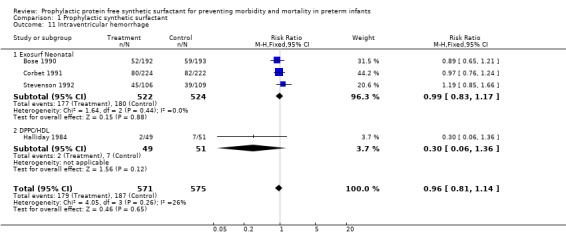
Comparison 1 Prophylactic synthetic surfactant, Outcome 11 Intraventricular hemorrhage.
1.12. Analysis.

Comparison 1 Prophylactic synthetic surfactant, Outcome 12 Severe intraventricular hemorrhage.
1.13. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 13 Retinopathy of prematurity.
1.14. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 14 Severe retinopathy of prematurity.
1.15. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 15 Cerebral palsy, 1‐2 years.
1.16. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 16 Cerebral palsy, moderate/severe.
1.17. Analysis.
Comparison 1 Prophylactic synthetic surfactant, Outcome 17 Survivors not assessed at followup.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bose 1990.
| Methods | Randomized Multicenter Blinding of randomization: yes Blinding of intervention: Attempted (infants treated in screened area by special drug administration team who did not participate in subsequent care) Complete follow‐up: yes Blinding of outcome measurement: yes Stratification by birthweight (700‐1000g, 1001‐1350g) and gender Long‐term follow‐up: Corbet (1995), Kraybill (1995) 80% of survivors evaluated | |
| Participants | Premature infants Inborn Birthweight 700‐1350 grams No proven lung maturity No fetal anomaly or chromosomal abnormality No fetal growth retardation No evidence of hydrops fetalis No proven chorioamnionitis No maternal heroin addiction Infants randomized: Exosurf = 192 Air Placebo = 193 | |
| Interventions | Intubation and intratracheal administration of Exosurf Neonatal (5ml/kg) as soon after birth as possible or intubation and sham air treatment | |
| Outcomes | PRIMARY OUTCOME:
Survival at 28 days of age without bronchopulmonary dysplasia SECONDARY OUTCOMES: Ventilatory requirements Respiratory distress syndrome Complications of prematurity FOLLOW‐UP: Assessed at 1 and 2 years adjusted age |
|
| Notes | Evaluation of BPD, neonatal mortality does not include infants with congenital malformation or congenital pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification by birthweight (700 to 1000 g, 1001 to 1350 g) and gender |
| Allocation concealment? | Low risk | Blinding of randomization: yes |
| Blinding? All outcomes | Low risk | Blinding of intervention: Attempted (infants treated in screened area by special drug administration team who did not participate in subsequent care) Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes Long‐term follow‐up: Corbet (1995), Kraybill (1995) 80% of survivors evaluated |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Corbet 1991.
| Methods | Randomized Multicenter study Blinding of randomization: yes (sealed envelopes) Blinding of intervention: yes (staff not involved in clinical care administered assigned treatment in screened area Complete follow‐up: yes Blinding of outcome measures: yes Stratification based on gestational age and gender Long‐term follow‐up: Corbet (1995), Sell (1995): 82% of survivors evaluated | |
| Participants | Premature infants Birthweight 700 to 1100 grams No proven lung maturity No major congenital malformation No fetal growth retardation No evidence of hydrops fetalis No purulent amnionitis No maternal heroin addition Infants randomized: Exosurf = 224 Air Placebo = 222 | |
| Interventions | Intratracheal Exosurf Neonatal (5ml/kg) or sham treatment (air) in delivery room within 30 minutes after birth | |
| Outcomes | PRIMARY OUTCOME:
Survival at age 28 days without bronchopulmonary dysplasia SECONDARY OUTCOMES: Respiratory distress, mortality, complications of prematurity FOLLOW‐UP: Assessed at 1 year adjusted age |
|
| Notes | Evaluation of BPD, neonatal mortality does not include infants with congenital malformation or congenital pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification based on gestational age and gender |
| Allocation concealment? | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding? All outcomes | Low risk | Blinding of intervention: yes (staff not involved in clinical care administered assigned treatment in screened area Blinding of outcome measures: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes Long‐term follow‐up: Corbet (1995), Sell (1995): 82% of survivors evaluated |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Halliday 1984.
| Methods | Randomized Single Center Blinding of Randomization: yes (sealed envelope) Complete follow‐up: no Blinding of outcome measurement: yes Blinding of Intervention: attempted (separate staff involved in treatment administration) Long‐term follow‐up: Halliday (1986) 99% of survivors evaluated | |
| Participants | Premature infants Gestational age 25 to 33 weeks No evidence of lung maturity No major congenital anomalies Infants randomized: DPPC/HDL = 49 Control = 51 | |
| Interventions | Intratracheal administration of dipalmitdylphosphatidylcholine 30mg/ High‐density lipoprotein 3 mg in 5 ml saline vs. manual ventilation | |
| Outcomes | Respiratory Distress Syndrome
Complications of prematurity FOLLOW‐UP: Assessed at 2 years |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Blinding of Randomization: yes (sealed envelope) |
| Blinding? All outcomes | Low risk | Blinding of Intervention: attempted (separate staff involved in treatment administration) Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Complete follow‐up: no Long‐term follow‐up: Halliday (1986) 99% of survivors evaluated |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Phibbs 1991.
| Methods | Randomized Single center Blinding of randomization: yes (sealed envelopes) Stratification based on gestational age and gender Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measures: no | |
| Participants | Premature infants Gestational age <34 weeks Birthweight 700‐1350 grams No major congenital anomaly Infants randomized: Exosurf = 38 Air Placebo = 39 | |
| Interventions | Intratracheal Exosurf Neonatal (5 ml/kg) or sham treatment (air) in delivery room after intubation and minimal ventilation | |
| Outcomes | PRIMARY OUTCOME:
Respiratory Distress Syndrome
Ventiltory requirements SECONDARY OUTCOMES: Complications of prematurity Neonatal and Respiratory Mortality |
|
| Notes | Infants with congenital malformations excluded from all analyses (2 treatment, 1 control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification based on gestational age and gender |
| Allocation concealment? | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding? All outcomes | High risk | Blinding of intervention: no Blinding of outcome measures: no |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Stevenson 1992.
| Methods | Randomized Multicenter Blinding of randomization: Yes (sealed envelopes) Blinding of intervention: Attempted (treatment administered by a separate dosing team in a screened area) Complete follow‐up: yes Blinding of outcome measurement: yes Stratification by Birthweight: (500 to 599 g, 600 to 699 g) and gender Long‐term follow‐up: Corbet (1995), Walther (1995): 83% of survivors evaluated | |
| Participants | Premature infants Inborn Birthweight 500‐699 grams No proven lung maturity No major congenital malformation No fetal growth retardation No evidence of hydrops fetalis No purulent amnionitis No maternal heroin addiction Infants randomized: Exosurf = 109 Air Placebo = 106 | |
| Interventions | Intratracheal Exosurf Neonatal (5ml/kg) or sham treatment (air) in delivery room shortly after birth | |
| Outcomes | PRIMARY OUTCOME:
Neonatal mortality SECONDARY OUTCOMES: Ventilatory requirements Death due to RDS Bronchopulmonary dysplasia Complications of prematurity FOLLOW‐UP: Assessed at 1 year adjusted age |
|
| Notes | Primary analysis reported as intention to treat (BPD, mortality), other outcomes reported based on treatment administered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification by Birthweight: (500 to 599 g, 600 to 699 g) and gender |
| Allocation concealment? | Low risk | Blinding of randomization: Yes (sealed envelopes) |
| Blinding? All outcomes | Low risk | Blinding of intervention: Attempted (treatment administered by a separate dosing team in a screened area) Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes Long‐term follow‐up: Corbet (1995), Walther (1995): 83% of survivors evaluated |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Ten Centre 1987.
| Methods | Randomized
Multicenter
Blinding of randomization: yes (sealed envelopes)
Blinding of intervention: can't tell
Complete follow‐up: yes
Blinding of outcome measurement: yes
Stratification by gestational age (25 to 26 weeks, 27 to 29 weeks) Long‐term follow‐up: Morley (1990): % survivors followed unclear |
|
| Participants | Premature infants Inborn Gestational age 25 to 29 weeks Infants randomized: ALEC = 164 Saline = 164 | |
| Interventions | Artificial lung expanding compound (dipalmitdylphosphatidylcholine and phosphatidylglycerol in saline) vs. saline administered in the pharynx if intubated, a second dose was given third and fourth doses were given if intubated at 1 and 24 hours of age | |
| Outcomes | PRIMARY OUTCOME:
Mortality SECONDARY OUTCOME: Respiratory Distress Syndrome Complications of prematurity FOLLOW‐UP: Assessed at 9 and 18 months |
|
| Notes | Randomized infants: 328 Ineligible: 20 Total evaluated: ALEC = 159 Saline = 149 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification by gestational age (25 to 26 weeks, 27 to 29 weeks) |
| Allocation concealment? | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding? All outcomes | Unclear risk | Blinding of intervention: can't tell Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Complete follow‐up: yes Long‐term follow‐up: Morley (1990): % survivors followed unclear |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Wilkinson 1985.
| Methods | Randomized Single Center Blinding of randomization: yes (sealed envelopes) Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: yes Stratification based on gender | |
| Participants | Premature infants Gestational age < 31 weeks Intubated in delivery room Infants randomized: DPPC/PG = 16 Control = 16 | |
| Interventions | Intratracheal administration of dry powdered dipalmitoylphosphatidylcholine and phosphatidylglycerol vs. routine resuscitation | |
| Outcomes | Requirement for respiratory support
Complications of respiratory distress syndrome Follow‐up : for a minimum 2 years |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratification based on gender |
| Allocation concealment? | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding? All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: yes |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chu 1967 | Utilized ineffective surfactant prepartion (DPPC alone) and ineffective mode of delivery |
| Milner 1984 | Reports respiratory compliance only |
| Morley 1981 | Treatment allocation not random |
| Morley 1988 | Infants less than 30 weeks gestation included in Ten Centre Study |
| Morley 1991 | Reports respiratory compliance only |
| Robillard 1964 | Utilized ineffective surfactant preparation (DPPC alone) and ineffective mode of delivery |
Contributions of authors
R. Soll wrote the original review including performing the search, extrating data, completing the analysis and writing the review.
E. Ozek performed the updated search, checked the data entry and re‐wrote the text to reflect the new structure and the results of the subgroup analyses.
Sources of support
Internal sources
Neonatal Collaborative Review Group, NIH Contract N01‐MD‐6‐3253, USA.
External sources
[Information not provided], Not specified.
Declarations of interest
Dr. R. Soll has acted as a consultant and invited speaker for several of the pharmaceutical companies which manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs‐Welcome).
Edited (no change to conclusions)
References
References to studies included in this review
Bose 1990 {published data only}
- Bose C, Corbet A, Bose G, Garcia‐Prats J, Lombardy L, Wold D, Donlon D, Long W. Improved outcome at 28 days of age for very low birthweight infants treated with a single dose of a synthetic surfactant. Journal of Pediatrics 1990;117:947‐53. [DOI] [PubMed] [Google Scholar]
- Corbet A, Long W, Schumacher R, et al. Double‐blind developmental evaluation at one year corrected age of 597 premature infants with birthweights from 500 to 1350 grams enrolled in three placebo controlled trials of prophylactic synthetic surfactant. Journal of Pediatrics 1995;126:S5‐12. [DOI] [PubMed] [Google Scholar]
- Kraybill EN, Bose C, Corbet A, Garcia‐Prats J, Asbill D, Edwards K, Long W. Double‐blind evaluation of developmental and health status to age 2 years of infants weighing 700 to 1350 grams treated prophylactically at birth with a single dose of synthetic surfactant or air placebo. Journal of Pediatrics 1995;126:S33‐42. [DOI] [PubMed] [Google Scholar]
Corbet 1991 {published data only}
- Corbet A, Bucciarelli R, Goldman S, Mammel M, Wold D, Long W, US Exosurf Pediatric Study Group 1. Decreased mortality rate among small premature infants treated at birth with a single dose of synthetic surfactant: a multicenter controlled trial. Journal of Pediatrics 1991;118:277‐84. [DOI] [PubMed] [Google Scholar]
- Corbet A, Long W, Schumacher R, et al. Double‐blind developmental evaluation at one year corrected age of 597 premature infants with birthweights from 500 to 1350 grams enrolled in three placebo controlled trials of prophylactic synthetic surfactant. Journal of Pediatrics 1995;126:S5‐12. [DOI] [PubMed] [Google Scholar]
- Sell M, Cotton R, Hirata T, Guthrie R, Leblanc M, Mammel M, Long W. One year follow‐up of 273 infants with birthweights of 700 to 1100 grams after prophylactic treatment of respiratory distress syndrome with synthetic surfactant or air placebo. Journal of Pediatrics 1995;126:S20‐25. [DOI] [PubMed] [Google Scholar]
Halliday 1984 {published data only}
- Halliday HL, McClure G, Reid MM. Growth and development two years after artificial surfactant replacement at birth. Early Human Development 1986;13:323‐7. [DOI] [PubMed] [Google Scholar]
- Halliday HL, Reid MM, Meban C, McClure G, Lappin TRJ, Thomas PS. Controlled trial of artificial surfactant to prevent respiratory distress syndrome. Lancet 1984;1:476‐8. [DOI] [PubMed] [Google Scholar]
Phibbs 1991 {published data only}
- Phibbs RH, Ballard RA, Clements JA, Heilbron DC, Phibbs CS, Schlueter MA, Sniderman SH, Tooley WH, Wakeley A. Initial clinical trial of Exosurf, a protein‐free synthetic surfactant, for the prophylaxis and early treatment of hyaline membrane disease. Pediatrics 1991;88:1‐9. [PubMed] [Google Scholar]
Stevenson 1992 {published data only}
- Stevenson D, Walther F, Long W, Sell M, Pauly T, Gong A, Easa D, Pramanik A, LeBlanc M, Anday E, Dhanireddy R, Burchfield D, Corbet A, American Exosurf Neonatal Study Group 1. Controlled trial of a single dose of synthetic surfactant at birth in premature infants weighing 500 to 699 grams. Journal of Pediatrics 1992;120:S3‐S12. [DOI] [PubMed] [Google Scholar]
- Walther FJ, Mullett M, Schumacher R, Sundell H, Easa D, Long W. One year follow‐up of 66 premature infants weighing 500 to 699 grams treated with a single dose of synthetic surfactant or air placebo at birth: results of a double‐blind trial. Journal of Pediatrics 1995;126:S13‐9. [DOI] [PubMed] [Google Scholar]
Ten Centre 1987 {published data only}
- Morley CJ, Morley R. Follow‐up of premature babies treated with artificial surfactant (ALEC). Archives of Disease in Childhood 1990;65:667‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Centre Study Group. Ten centre trial of artificial surfactant (artificial lung expanding compound) in very premature babies. British Medical Journal 1987;294:991‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 1985 {published data only}
- Wilkinson AR, Jenkins PA, Jeffrey JA. Two controlled trials of dry artificial surfactant: early effects and later outcome in babies with surfactant deficiency. Lancet 1985;2:287‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chu 1967 {published data only}
- Chu J, Clements JA, Cotten EK, Klaus MH, Sweet AY, Tooley WH. Neonatal Pulmonary Ischemia. Pediatrics 1967;40 Suppl:709‐82. [PubMed] [Google Scholar]
Milner 1984 {published data only}
- Milner AD, Vyas H, Hopkin IE. Effect of exogenous surfactant on total respiratory system compliance. Archives of Disease in Childhood 1984;59:369‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 1981 {published data only}
- Morley CJ, Bangham AD, Miller N, Davis JA. Dry artificial surfactant and its effect on very premature babies. Lancet 1981;i:64‐8. [DOI] [PubMed] [Google Scholar]
Morley 1988 {published data only}
- Morley CJ, Greenough A, Miller NG, Bangham AD, Pool J, Wood S, South M, Davis JA, Vyas H. Randomized trial of artificial surfactant (ALEC) gien at birth to babies from 23 to 34 weeks gestation. Early Human Development 1988;17:41‐54. [DOI] [PubMed] [Google Scholar]
Morley 1991 {published data only}
- Morley CJ, Greenough A. Respiratory compliance in premature babies treated with artificial surfactant (ALEC). Archives of Disease in Childhood 1991;68:467‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robillard 1964 {published data only}
- Robillard E, Alarie Y, Dagenais‐Perusse P, Baril E, Guilbeault A. Micro‐aerosol administration of synthetic dipalmitoyl lecithin in the respiratory distress syndrome: A preliminary report. Canadian Medical Association Journal 1964;90:55‐7. [PMC free article] [PubMed] [Google Scholar]
Additional references
Enhorning 1972
- Enhorning G, Robertson B. Lung expansion in the premature rabbit fetus after tracheal deposition of surfactant. Pediatrics 1972;50:58‐66. [PubMed] [Google Scholar]
Jobe 1993
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328:861‐8. [DOI] [PubMed] [Google Scholar]
Pfister 2007
- Pfister RH, Soll R, Wiswell TE. Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Raju 1993
- Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous surfactant. Journal of Pediatrics 1993;123:603‐10. [DOI] [PubMed] [Google Scholar]
Seger 2009
- Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 1998a
- Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 1998b
- Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2001a
- Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000510] [DOI] [PubMed] [Google Scholar]
Soll 2001b
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000144] [DOI] [PubMed] [Google Scholar]
Stevens 2007
- Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tooley 1987
- Tooley WH, Clements JA, Maramatsu K, Brown CL, Schlueter MA. Lung function in prematurely delivered rabbits treated with a synthetic surfactant. American Review of Respiratory Disease 1987;136:651‐6. [DOI] [PubMed] [Google Scholar]
Yost 1999
- Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001456] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Soll 1992
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:325‐58. [Google Scholar]
Soll 1998
- Soll RF. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD001079] [DOI] [PubMed] [Google Scholar]


