Abstract

The conditions that led to the formation of the first organisms and the ways that life originates from a lifeless chemical soup are poorly understood. The recent hypothesis of “RNA-peptide coevolution” suggests that the current close relationship between amino acids and nucleobases may well have extended to the origin of life. We now show how the interplay between these compound classes can give rise to new self-replicating molecules using a dynamic combinatorial approach. We report two strategies for the fabrication of chimeric amino acid/nucleobase self-replicating macrocycles capable of exponential growth. The first one relies on mixing nucleobase- and peptide-based building blocks, where the ligation of these two gives rise to highly specific chimeric ring structures. The second one starts from peptide nucleic acid (PNA) building blocks in which nucleobases are already linked to amino acids from the start. While previously reported nucleic acid-based self-replicating systems rely on presynthesis of (short) oligonucleotide sequences, self-replication in the present systems start from units containing only a single nucleobase. Self-replication is accompanied by self-assembly, spontaneously giving rise to an ordered one-dimensional arrangement of nucleobase nanostructures.
Introduction
Establishing possible pathways through which life can emerge from inanimate matter is one of the grand challenges in today’s science.1−3 In addressing this challenge the functional and structural characteristics of present-day life provide important guidance. At the same time, the overwhelming complexity of evolved life makes it challenging to extract its essence and identify pathways for its emergence.
Recently, a systems chemistry4,5 view toward the challenges of the origins and synthesis of life is gaining popularity. The facts that many different types of molecules coexisted at the time of life’s origin and that the same applies to present-day life, warrants an exploration of what may emerge upon allowing different compound classes to interact. For example, the notion of peptides and nucleic acids cooperating during the early stages of the emergence of life is becoming increasingly popular6−9 and nucleobase-peptide chimera show unique self-assembly behavior10−12 and can give rise to remarkably complex foldamers.13
Key in the transition of chemistry into biology is the acquisition of function. The core functional characteristics of life are its ability to replicate, to metabolize, and to be spatially segregated from its environment. Where life requires the functional integration of all of these characteristics, most research efforts still focus on one of these aspects in isolation.
Autocatalysis, the ability of systems (molecules, metabolic networks or compartments) to make copies of themselves, is central to all evolutionary scenarios.14−18 Systems where autocatalysis is accompanied by information transfer and heredity are said to be self-replicating. Synthetic systems of self-replicators have been pioneered by von Kiedrowski using short DNA strands.19 Subsequently, self-replicating molecules have been developed that feature most of the other important current biopolymers (i.e., RNA20−24 and peptides25−28) as well as completely synthetic molecules.29−33
A major issue in replicator chemistry is the tendency for self-inhibition through replicator duplex formation. This causes many replicators to exhibit only parabolic growth (i.e., showing a kinetic order in replicator of 1/2) whereas exponential growth (first order in replicator) would be necessary for most scenarios of Darwinian evolution.34−37 Another problem in replicator chemistry is the complexity of the structures associated with most self-replicators, which are unlikely to emerge spontaneously from simple starting materials.
We recently introduced a new approach to self-replication that addresses both of these problems simultaneously.38−40 This approach relies on (i) the creation of a mixture of molecules that continuously interconvert (a dynamic combinatorial library or DCL) and (ii) a self-assembly process that leads to the sequestration of molecules from this mixture, which subsequently get replenished. The combination of these two features is sufficient for the spontaneous and autocatalytic formation of self-replicating molecules. Given that networks of interconverting molecules and self-assembly processes are likely to have been widespread in prebiotic environments, this mechanism provides a likely path for the spontaneous emergence of replicators. Note that the building blocks that give rise to the network of interconverting molecules can be relatively simple,41 while the structure of the emerging replicators can be relatively complex, consisting of many different building blocks connected in a way that is not a priori specified.42 Furthermore, exponential replication is possible upon entering a growth-breakage cycle, in which mechanical energy is utilized to break replicator assemblies exposing more edges from which the assemblies grow.43
A systems approach to the emergence of self-replicating molecules, where different compound classes (i.e., amino acids, peptides, and nucleobases) coexist has thus far received only little attention. Efforts directed at PNA-based replicators, in which an amino acid replaces the phosphate-sugar backbone of DNA/RNA, come closest.44−46 However, PNA remains very similar to DNA/RNA in architecture and behavior.
We now report the spontaneous emergence of new self-replicating molecules from molecular networks in which nucleobases and amino acids are both present. We show that this leads to chimeric replicators, which rely on the assembly of peptides and nucleobases into fibrous aggregates (but do not rely on base-pairing) resulting in the autocatalytic formation of a one-dimensional arrangement of nucleobases. The two different systems constructed herein allow for a direct comparison between replicator mutations. The peptide-nucleobase system shows that mutations are easily accommodated during replication, while in the PNA system, replicator mutation is impeded as it requires a change in ring size. While the building blocks used were not selected for prebiotic relevance, they do illustrate the potential of the assembly driven replication mechanism that might well extend to other types of molecules.
Results and Discussion
We have constructed molecular networks based on thiol–disulfide chemistry.47 Three families of relatively simple dithiol building blocks were prepared featuring peptides (1; Scheme 1a) or amino-acid nucleobase conjugates (2 and 3). Oxidizing (mixtures of) these dithiols generates DCLs of macrocyclic disulfides with different (compositions and) ring sizes, which interconvert through reaction of the disulfides with residual thiolate anion (Scheme 1b). We previously reported that DCLs made from adenine-containing building block 2a were dominated by a foldamer, consisting of 15 subunits of 2a, accompanied by small amounts of trimers and tetramers (Figure 1a).13 Analysis of DCLs prepared from analogous building blocks 2b, 2c, and 2d, containing thymine, guanine, and cytosine, respectively, led to similar conclusions. Specifically, in the libraries consisting of 2c and 2d, most of the building blocks are converted into the folded 15mer (see the Supporting Information, Figures S34a and S44a).
Scheme 1. (a) Building Block Structures; (b) Oxidation of These Dithiol Building Blocks Gives Rise to Dynamic Combinatorial Libraries of Macrocyclic Disulfides; (c) Assembly of a Specific Ring Size into Stacks Leads to the Autocatalytic Formation of More of These Rings through a Fiber Growth-Breakage Cycle.

Figure 1.
UPLC-MS analysis of DCLs made from different ratios of building blocks 1 and 2a at a total building block concentration of 2.0 mM in 50 mM borate buffer pH 8.2 stirred at 1200 rpm for 25 days. From bottom (a) 100 mol % of 2a to top (k) 100 mol % of 1.
The library made from 2b contains, besides 15mer, a significant amount of trimer and tetramer macrocycles (see the Supporting Information, Figure S21a). In contrast, DCLs made from peptide-functionalized building block 1 readily produces self-replicating hexamers, partially driven by the assembly of the peptide side groups into β-sheets.48 The mechanisms by which 1 replicates is analogous to that shown in Scheme 1c and bears similarities to amyloid formation.43,49
We hypothesized that mixing building blocks 1 and 2 might lead to the formation of mixed macrocycles that retain the ability of the peptides to form β-sheets, while also featuring nucleobases. To test this hypothesis, we dissolved peptide building block 1 and adenine containing building block 2a (1.0 mM each) in 50 mM borate buffer (pH 8.2) in a capped vial and stirred the solution in the presence of air. The DCL reached a stationary distribution after stirring the solution for 3 weeks. Ultra-performance liquid chromatography mass spectrometry (UPLC-MS) analysis revealed that a rather complex and poorly resolved mixture was produced, composed of many mixed macrocycles from trimers up to cyclic 15mers (Figure 1f).
We then prepared similar DCLs at different building block ratios (1:2a = 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, and 10:90; see Figure 1). Interestingly, the DCLs made using ratios of 1:2a of 90:10, 80:20, and 70:30 were dominated by trimer 12(2a)1. The highest yield of this trimer was observed at a ratio of 70:30 (Figure 2a). The almost complete absence of other macrocycles in the latter sample suggests that 12(2a)1 selectively benefits from a special stabilizing effect (vide infra).
Figure 2.
Fraction of trimers (a) 12(2a)1, (b) 12(2b)1, (c) 12(2c)1, and (d) 12(2d)1 obtained in DCLs made from a mixture of building blocks 1 and 2 (2.0 mM total in 50 mM borate buffer at pH 8.2 stirred at 1200 rpm) at different ratios. Change in product distribution with time in DCLs made from (e) 1 (1.4 mM) and 2a (0.6 mM), (f) 1 (1.6 mM) and 2b (0.4 mM), (g) 2c (0.4 mM) and (h) 2d (0.4 mM). Kinetics of formation of mixed trimers (i) 12(2a)1, (j) 12(2b)1, (k) 12(2c)1, and (l) 12(2d)1 in DCLs made from 1 (1.0 mM) and 2 (0.5 mM) in the absence (open symbols) and presence (closed symbols) of 10 or 20 mol % of the corresponding preformed trimer (added at day 3). Seeding experiments were conducted by preparing one mother solution, splitting it in two and adding seed to one of these. Negative staining TEM images of the assemblies formed in DCLs dominated by (m) 12(2a)1, (n) 12(2b)1, (o) 12(2c)1, and (p) 12(2d)1. For additional TEM images, see Figures S146–S149.
To further investigate the nature of the unusual stability of 12(2a)1 we monitored the kinetics of its growth. Upon mixing 1 and 2a a variety of different macrocycles were formed in the first 10 days (Figure 2e). However, after 10 days the rate of growth of 12(2a)1 increased and it became the main product of the library, at the expense of the other macrocycles. The formation of 12(2a)1 was highly depended on mechanical agitation. Nonagitated libraries gave only small amounts of 12(2a)1 even after 20 days (Supporting Information, Figure S1). The sigmoidal growth of 12(2a)1, together with the strong dependence on agitation suggests that this macrocycle is a self-replicator that grows through self-assembly.13 The autocatalytic nature of the formation of 12(2a)1 was confirmed in a seeding experiment. Upon adding 10 mol % of preformed 12(2a)1 to a sample made from 1 and 2a in a 2 to 1 ratio its growth dramatically accelerated (Figure 2i). The rate of emergence of 12(2a)1 is dependent on temperature (see Supporting Information, Figure S159). Analysis by transmission electron microscopy (TEM) of the sample dominated by 12(2a)1 confirmed the presence of fibrous aggregates of lengths ranging from 50 nm to 1 μm, corresponding to 100–2000 nucleobases (Figure 2m). In order to probe whether replication was exponential, rather than parabolic,43 we determined the kinetic order in self-replicator 12(2a)1 by seeding with different amounts of preformed replicator. The initial rate of replication was determined by UPLC analysis (see the Supporting Information, Figure S158 for the kinetic data). The slope of the plot gave a value of the order in replicator 12(2a)1 of 1.24 ± 0.13, consistent with exponential growth (Figure 3). Taken together, these data suggest that 12(2a)1 self-replicates through the fiber growth-breakage mechanisms shown in Scheme 1c, similar to the mechanism we observed previously for replicators formed from only building block 1.
Figure 3.
Determination of the order in replicator 12(2a)1. The initial replication rate is plotted versus the concentration of replicator. The data points correspond to seeding concentrations of 5.0, 7.5, 10, and 12.5% relative to the stock solution (500 μM in building blocks). The error bars denote the standard deviation based on three individual measurements.
Subsequently, analogous mixed DCLs made from peptide 1 and the different nucleobase building blocks 2b – 2d (containing thymine, guanine and cytosine, respectively) were also studied. The results show that in these systems 12(2b)1, 12(2c)1, and 12(2d)1 can also be obtained (Figure 2b–d and f–h, Supporting Information, Figures S21, S34, and S44), albeit with somewhat reduced yields. Seeding experiments demonstrated that all of these mixed trimers are self-replicators (Figure 2g–l). As with 12(2a)1, samples enriched in the mixed trimers all revealed fibrous aggregates when analyzed by TEM (Figure 2n–p). Circular dichroism (CD) spectra on samples dominated by 12(2a)1 showed the bands that are indicative of β-sheets, suggesting that the peptide assembles in this type of secondary structure (see the Supporting Information, Figure S2). A less ordered chiral environment was observed for the other mixed trimer replicators.
The fidelity of replication of these systems is remarkable, particularly for the adenine replicator 12(2a)1, which showed very little tendency to mutate into 1(2a)2 or 13. Thus, mutating a nucleobase unit into a peptide unit or vice versa does not happen frequently. This spurred us to investigate the extent to which the nucleobase could be mutated to another nucleobase. We investigated this question through a series of cross-seeding experiments on libraries made from peptide building block 1 and nucleobase building block 2 mixed in the ratio optimal for producing the corresponding 12(2a)1 replicator. After 3 days of stirring, these samples were seeded with 10 mol % of trimer replicators based on one of the other three nucleobases. As shown in Figure 4, all nucleobase replicators are able to cross-catalyze the formation of any other nucleobase replicator. In most cases the efficiency with which they do so is similar and also similar to the efficiency of autocatalysis upon self-seeding. Only the cytosine replicator 12(2d)1 is less efficient at seeding other replicators and, in turn, also benefits less from being seeded by the other nucleobase replicators. Given the small energy differences involved in base stacking propensity of nucleobases in DNA,50,51 it is hard to fully rationalize the cross-seeding effects.
Figure 4.

Effect of seeding on the growth of 1221 replicators in DCLs prepared from 1 and (a) 2a, (b) 2b, (c) 2c, and (d) 2d in 2 to 1 ratio (1.5 mM total) in the presence of 10 mol % of seed containing 12(2a)1 (red circles), 12(2b)1 (blue triangles), 12(2c)1 (green triangles), or 12(2d)1 (magenta diamonds), which was added at day 3. Seeding experiments were conducted by preparing one mother solution, splitting it in four and adding seed to one of these.
We also performed analogous experiments on DCLs which contained all four nucleobases, which were seeded with one of the four 1221 replicators. These experiments probe the replication fidelity of the individual nucleobase replicators. Unfortunately, we were unable to separate 12(2a)1 and 12(2d)1 in our UPLC analysis. Figure 5 shows that all seeds enhance the formation of the 1221 replicators relative to their spontaneous emergence. However, the extent to which a specific nucleobase replicator is able to selectively enhance the formation of copies of itself seems limited and the incorporation of other nucleobases into trimers occurs readily.
Figure 5.

Effect of seeding on the distribution of 1221 replicators in DCLs prepared from 1 (1.0 mM) and 2a, 2b, 2c, and 2d (0.125 mM each) in the presence of 10 mol % of seed containing 12(2a)1, 12(2b)1, 12(2c)1, or 12(2d)1. Due to co-elution, the amounts of 12(2a)1 and 12(2d)1 could not be separately quantified. The striped areas correspond to the amount of seed.
Taken together, the seeding experiments of Figures 4 and 5 suggest that mutation of the nucleobase residue during replication is facile. Hence, when different nucleobase building blocks are present during replicator growth, the resulting fibers will feature essentially random sequences of nucleobases along the fiber axis.
In order to probe the importance of the nucleobase motif for replication, the nucleobases were replaced by a simple phenyl ring. Thus, building block 2e was synthesized, following a protocol analogous to that of the nucleobase building blocks. The DCLs that were prepared by mixing this building block in different ratios with peptide building block 1 did not provide any indications for the emergence of a self-replicator (see the Supporting Information, Figure S55). Additionally, cross-seeding a DCL prepared from 1 and 2e in a 2:1 ratio with 10 mol % of any of the four nucleobase replicators also failed to promote the growth of 12(2e)1 (see the Supporting Information, Figure S4). These results indicate that, in the context of the present system, the tendency of the canonical nucleobases to form replicators is superior to that of a simple phenyl analog.
In order to further investigate the importance of the amphiphilicty in the structures of the building blocks, nucleobases were replaced by a simple imidazole moiety to obtain building block 2f. A series of DCLs were set up by mixing building blocks 2f and 1 in different ratios. DCLs made using ratios of 1:2f of 80:20, 70:30, 60:40 were dominated by replicator 12(2f)1 (Figure S139), similar to what was previously observed for the mixture containing 1 and 2a. Moreover, cross-seeding experiments demonstrated that the addition of replicator 12(2a)1 can accelerate the growth of replicator 12(2f)1 (Figure S145). These results suggest that the emergence of the mixed trimer replicator from the binary systems benefits from the presence of a hydrophilic aromatic ring in the monomeric units.
Encouraged by the results described above, we investigated whether we can also obtain self-replicating molecules that feature amino acid and nucleobase subunits without the need for peptide building block 1. We changed our building block design and utilized the PNA motif, linked to an amino acid, while maintaining the aromatic dithiol unit, resulting in building blocks 3a–h. The PNA unit carries one of the four canonical nucleobases, while the amino acid residue is l-lysine or l-histidine. These amino acids were chosen to have different charges: where the amine side chain of lysine is fully protonated at neutral pH, the imidazole ring of histidine is only partially protonated. These building blocks were synthesized using conventional Fmoc/tBu solid-phase peptide synthesis.
We prepared a set of DCLs by dissolving building blocks 3a–h (3.8 mM) separately in 50 mM sodium borate buffer at pH 8.2 under continuous stirring (1200 rpm). Rapid partial oxidation (80% conversion of thiols to disulfides) of the resulting solutions was performed by adding sodium perborate solution (80 mM), followed by slower further oxidation mediated by oxygen present in the air.
We first explored the behavior of DCLs made from the lysine-containing building blocks 3a–d. The corresponding DCL made from adenine building block 3a rapidly became dominated by the cyclic tetramer, which constituted 85% of the library material after 30 min. Over time, the tetramer formed in quantitative yield (see Figure 6a), and the composition remained unchanged for the duration the sample was monitored (6 days). The DCL made from thymine building block 3b behaved somewhat differently. Initially a mixture of cyclic trimer, tetramer, hexamer, and other oligomers was formed, which, after 2 days, gave way to the pentamer macrocycle (Figure 6b). Analogous experiments using cytosine building block 3d gave rise to cyclic trimer (Figure 6c). Rather different behavior was observed for DCLs made from guanine building block 3c, which contained a broad range of macrocycles up to 14mers (Figure S77). The composition remained unchanged for up to 30 days.
Figure 6.

Change in product distribution with time in stirred (1200 rpm) DCLs made from (a) 3a, (b) 3b, and (c) 3d (3.8 mM) in 50 mM borate buffer pH 8.2. Kinetics of formation of: (d) (3a)4, (e) (3b)5, and (f) (3d)3 in DCLs made from the corresponding building block (3.8 mM) in the absence (open symbols) and presence (closed symbols) of 10 mol % of the corresponding preformed seed (added at day 0). All of the DCLs were oxidized to 80% using sodium perborate, with the exception for the seeding experiment of building block 3a (graph 6b), which was performed starting from a 100% reduced library. Negative staining TEM images of the assemblies formed in DCLs: dominated by (g) (3a)4, (h) (3b)5, and (i) (3d)3. Additional TEM images are shown in Figures S150, S151, and S153.
In order to investigate whether macrocycles (3a)4, (3b)5, and (3d)3 are self-replicators, seeding experiments were conducted. UPLC analysis revealed that, upon addition of 10 mol % seed, the formation of these macrocycles is significantly faster compared to the nonseeded control samples (Figure 6d–f). TEM analysis of the samples dominated by these macrocycles revealed the presence of fibrillar assemblies (Figure 6g–i). CD spectra of the samples showed strong induced CD signals for the aromatic dithiol core (peaks observed at 250 and 275 nm) as well as for the nucleobases (300 to 350 nm), suggesting that these experience a chiral microenvironment in the assemblies (see the Supporting Information, Figure S5). Taken together, these data suggest that (3a)4, (3b)5, and (3d)3 are self-replicators. Interestingly, the different nucleobases impose different ring sizes on the replicators. We previously noted that, for peptide-based replicators, the size of the ring is inversely correlated with the strength of the (hydrophobic) interactions between the building blocks in the assemblies.39 Surprisingly, in the present nucleobase system the trend in size of the selected macrocycle (thymine > adenine > cytosine) is exactly the opposite. Judging from water/chloroform partitioning coefficient, the hydrophilicity of the nucleobases follows the trend thymine (0.45) < adenine (0.78) < cytosine (3.00), where the number in parentheses corresponds to log(Cwater/CCHCl3).52 Thus, the mode of assembly of the rings into the stacks appears to involve more than just hydrophobic binding. What these additional interaction patterns are remains obscure as attempts at elucidating the assembly structure have so far been unsuccessful.
Similar to 12(2a)1, the kinetic order in self-replicator (3b)5 (1.21 ± 0.14; Figure 7 and S102) indicates that also (3b)5 is capable of exponential growth.
Figure 7.
Determination of the order in replicator (3b)5. The initial replication rate is plotted versus the concentration of replicator. The data points correspond to seeding concentrations of 5.0, 7.5, 10, and 12.5% relative to the stock solution (500 μM in building block 3b). The error bars denote the standard deviation based on three individual measurements.
We then extended our exploration to the series of histidine containing building blocks 3e–3h. For adenine building block 3e a tetramer macrocycle was obtained while for thymine and cytosine analogs 3f and 3g, respectively, trimer macrocycles were observed (see the Supporting Information, Figure S101a-c). All of these macrocycles were proven to be self-replicators (Figure S101d–f), adopting ordered supramolecular assemblies (Figures S101g–i and S157), as observed for the lysine containing building blocks.
Of the four nucleobases explored only guanine failed to produce self-replicators. As shown below, DCLs made from building block 3c produced a range of macrocycles, while building block 3g gave rise to tetramer. Guanines are known to assemble into quadruplexes that are stabilized by cations, in particular potassium.53 Indeed, upon addition of 50 mM potassium bromide to the DCL made from 3c a dramatic change in product distribution was observed: the macrocyclic trimer was formed in essentially quantitative yield (Figure 8a). This transformation at the molecular level was accompanied by a change of the macroscopic appearance from a clear solution to a viscous suspension.
Figure 8.
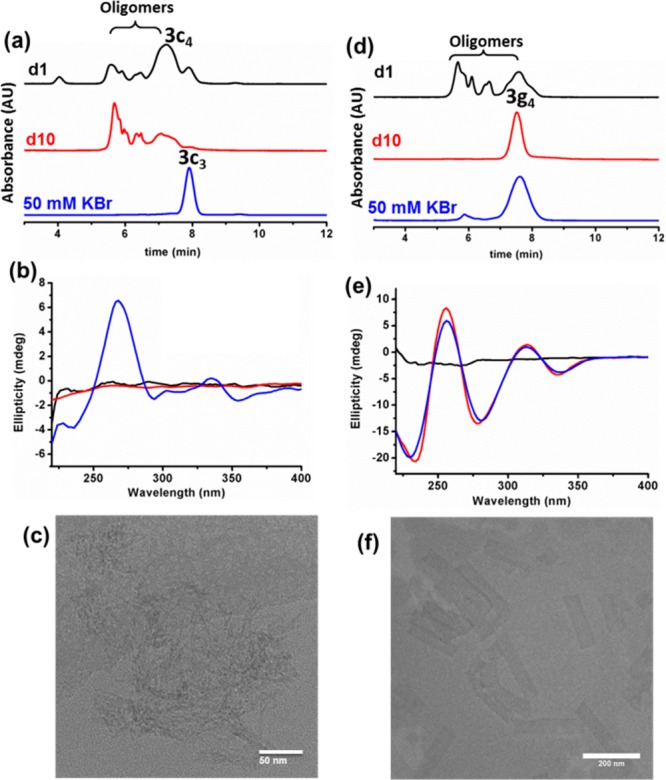
Cation-induced guanine based supramolecular assembly. UPLC analysis of DCLs prepared from (a) 3c and (d) 3g (3.8 mM in 12.5 mM borate buffer pH 8.2) after oxidation (80%) using sodium perborate at day 1 (upper line), day 10 (middle line), and after the addition of 50 mM potassium bromide (KBr). CD spectra of DCLs made from (b) 3c and (e) 3g before (black and red) and after (blue) addition of KBr. (c) Cryo-TEM image of DCL prepared from 3c upon the addition of KBr, corresponding to the trimer and (f) negative stain TEM image of 3g after 10 days of the reaction corresponding to the tetramer. Additional TEM images are shown in Figures S152 and S156.
The CD spectrum changed upon addition of KBr from a low intensity featureless trace to a reveal a strong signal at 268 nm (Figure 8b) typical for CD spectra of G-quadruplexes.54 Bundles of thin fibers were revealed using cryo-TEM (Figure 8c). In the DCL made from 3g, also a family of large oligomers was observed initially. However, over time, these products gave way to the tetramer macrocycle (Figure 8d), which self-assembled into twisted tape-like structures (Figure 8e,f). Addition of KBr did not significantly alter composition of this DCL, nor its nanostructure.
Finally, in order to investigate the possibility to mutate the nucleobases during the replication of the macrocycles based on the PNA building blocks, a series of cross-seeding experiments were performed. Analysis of DCLs made by mixing all four nucleobases revealed broad and overlapping peaks containing mixtures of trimers and tetramers which could not be adequately separated with the available chromatographic techniques (see the Supporting Information, Figure S103). Analysis was drastically improved in the three-component system (A, T, and C). However, seeding experiments failed to induce the amplification of a specific macrocycle (see the Supporting Information, Figure S105). Notably, in the binary system containing A and T, seeding experiments with (3b)5, triggered the autocatalytic formation of a family of mixed pentamers (Figures 9 and S127), whereas a mixture of trimers and tetramers dominated in the system in the absence of seed. Binary mixtures of other nucleobases showed limited response to seeding (see Supporting Information, Figures S116, S120, S123, S127, and S137). Overall, these results suggest that in the PNA system replication with mutation is less efficient compared to the one composed of nucleobase- and peptide-based building blocks, most likely as a consequence of the fact that the individual nucleobases in the PNA system have a strong effect on the nature of the self-replicating macrocycles, each preferring a different ring size.
Figure 9.

Seeding induced the growth of replicators (3a)n(3b)5-n in DCLs prepared from 3a and 3b ([3a] = [3b] = 1.0 mM) in the presence of 10 mol % of seed containing (3b)5 (red circles) or (3a)n(3b)5-n (blue triangles). All of the DCLs were oxidized to 80% using sodium perborate, followed by addition of the seed.
Conclusions
We have shown that exponential replicators featuring nucleobases and amino acids can emerge spontaneously from mixtures of relatively simple building blocks. The autocatalytic supramolecular polymerization of specific nucleobase-containing rings into stacks leads to a linear arrangement of nucleobases. The present system has the advantage over previously reported assemblies of nucleobase analogues55 in that they form autocatalytically. The ease with which these long noncovalent oligomers are produced is in stark contrast to the difficulty in obtaining long oligomers through more conventional nonautocatalytic condensation reactions of (activated) nucleotides. Interactions between nucleobases through hydrogen bonding (i.e., base-pairing) do not appear to play a role in the assembly and replication steps. However, we speculate that the fibrous assemblies might be a stepping stone toward systems in which information transfer occurs through base-pairing interactions. Thus, structures like our replicator fibers might be important for closing the gap between the building blocks of life and the formation of long functional information polymers. Investigations are currently underway aimed at obtaining replicator assemblies featuring nucleobases that are accessible for base-pairing.
Acknowledgments
We are grateful for support from the ERC (AdG 741774), the EU (MCIF 745805 - DSR), NWO (VICI grant), Zernike Dieptestrategie and the Dutch Ministry of Education, Culture and Science (Gravitation program 024.001.035).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b10796.
Synthetic procedures, NMR spectra, UPLC and LC-MS methods, methods of DCL and sample preparation, and mass spectra (PDF)
Author Contributions
† B.L. and C.G.P. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Kauffman S. A. At home in the universe. Math. Soc. Sci. 1997, 1, 94–95. [Google Scholar]
- Schwille P.; Spatz J.; Landfester K.; Bodenschatz E.; Herminghaus S.; Sourjik V.; Erb T. J.; Bastiaens P.; Lipowsky R.; Hyman A.; Dabrock P.; Baret J.-C.; Vidakovic-Koch T.; Bieling P.; Dimova R.; Mutschler H.; Robinson T.; Tang T.-Y. D.; Wegner S.; Sundmacher K. MaxSynBio: avenues towards creating cells from the bottom up. Angew. Chem., Int. Ed. 2018, 57, 13382–13392. 10.1002/anie.201802288. [DOI] [PubMed] [Google Scholar]
- Ruiz-Mirazo K.; Briones C.; de la Escosura A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]
- Ashkenasy G.; Hermans T. M.; Otto S.; Taylor A. F. Systems chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. 10.1039/C7CS00117G. [DOI] [PubMed] [Google Scholar]
- Mattia E.; Otto S. Supramolecular systems chemistry. Nat. Nanotechnol. 2015, 10, 111–119. 10.1038/nnano.2014.337. [DOI] [PubMed] [Google Scholar]
- Williams L. D.Yin and yang: polypeptide and polynucleotide. Origins of Life Community 2012, 2012. [Google Scholar]
- Kunnev D.; Gospodinov A. Possible emergence of sequence specific RNA aminoacylation via peptide intermediary to initiate Darwinian evolution and code through origin of life. Life 2018, 8, 44. 10.3390/life8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. What RNA world? Why a peptide/RNA partnership merits renewed experimental attention. Life 2015, 5, 294–320. 10.3390/life5010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y. S.; Chotera A.; Taran O.; Liang C.; Ashkenasy G.; Lynn D. G. Achieving biopolymer synergy in systems chemistry. Chem. Soc. Rev. 2018, 47, 5530–5530. 10.1039/C8CS90073F. [DOI] [PubMed] [Google Scholar]
- Chotera A.; Sadihov H.; Cohen-Luria R.; Monnard P. A.; Ashkenasy G. Functional assemblies emerging in complex mixtures of peptides and nucleic acid-peptide chimeras. Chem. - Eur. J. 2018, 24, 10128–10135. 10.1002/chem.201800500. [DOI] [PubMed] [Google Scholar]
- Liu P.; Ni R.; Mehta A. K.; Childers W. S.; Lakdawala A.; Pingali S. V.; Thiyagarajan P.; Lynn D. G. Nucleobase-Directed Amyloid Nanotube Assembly. J. Am. Chem. Soc. 2008, 130, 16867–16869. 10.1021/ja807425h. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos N. Peptide-oligonucleotide hybrid molecules for bioactive nanomaterials. Bioconjugate Chem. 2019, 30, 1915–1922. 10.1021/acs.bioconjchem.9b00259. [DOI] [PubMed] [Google Scholar]
- Liu B.; Pappas C. G.; Zangrando E.; Demitri N.; Chmielewski P. J.; Otto S. Complex molecules that fold like proteins can emerge spontaneously. J. Am. Chem. Soc. 2019, 141, 1685–1689. 10.1021/jacs.8b11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross A. Causation and the origin of life. Metabolism or replication first?. Origins Life Evol. Biospheres 2004, 34, 307–321. 10.1023/B:ORIG.0000016446.51012.bc. [DOI] [PubMed] [Google Scholar]
- Kosikova T.; Philp D. Exploring the emergence of complexity using synthetic replicators. Chem. Soc. Rev. 2017, 46, 7274–7305. 10.1039/C7CS00123A. [DOI] [PubMed] [Google Scholar]
- Bissette A. J.; Fletcher S. P. Mechanisms of autocatalysis. Angew. Chem., Int. Ed. 2013, 52, 12800–12826. 10.1002/anie.201303822. [DOI] [PubMed] [Google Scholar]
- Patzke V.; von Kiedrowski G. Self-replicating systems. Arkivoc 2007, 293–310. [Google Scholar]
- Duim H.; Otto S. Towards open-ended evolution in self-replicating molecular systems. Beilstein J. Org. Chem. 2017, 13, 1189–1203. 10.3762/bjoc.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonkiedrowski G. A self-replicating hexadeoxynucleotide. Angew. Chem., Int. Ed. Engl. 1986, 25, 932–935. 10.1002/anie.198609322. [DOI] [Google Scholar]
- Paul N.; Joyce G. F. A self-replicating ligase ribozyme. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 12733–12740. 10.1073/pnas.202471099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. A.; Joyce G. F. Self-sustained replication of an RNA enzyme. Science 2009, 323, 1229–1232. 10.1126/science.1167856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden E. J.; von Kiedrowski G.; Lehman N. Systems chemistry on ribozyme self-construction: evidence for anabolic autocatalysis in a recombination network. Angew. Chem., Int. Ed. 2008, 47, 8424–8428. 10.1002/anie.200802177. [DOI] [PubMed] [Google Scholar]
- Vaidya N.; Manapat M. L.; Chen I. A.; Xulvi-Brunet R.; Hayden E. J.; Lehman N. Spontaneous network formation among cooperative RNA replicators. Nature 2012, 491, 72–77. 10.1038/nature11549. [DOI] [PubMed] [Google Scholar]
- Jayathilaka T. S.; Lehman N. Spontaneous covalent self-assembly of the azoarcus ribozyme from five fragments. ChemBioChem 2018, 19, 217–220. 10.1002/cbic.201700591. [DOI] [PubMed] [Google Scholar]
- Lee D. H.; Granja J. R.; Martinez J. A.; Severin K.; Ghadiri M. R. A self-replicating peptide. Nature 1996, 382, 525–528. 10.1038/382525a0. [DOI] [PubMed] [Google Scholar]
- Yao S.; Ghosh I.; Zutshi R.; Chmielewski J. A pH-modulated, self-replicating peptide. J. Am. Chem. Soc. 1997, 119, 10559–10560. 10.1021/ja9710619. [DOI] [Google Scholar]
- Rubinov B.; Wagner N.; Rapaport H.; Ashkenasy G. Self-replicating amphiphilic beta-sheet peptides. Angew. Chem., Int. Ed. 2009, 48, 6683–6686. 10.1002/anie.200902790. [DOI] [PubMed] [Google Scholar]
- Nanda J.; Rubinov B.; Ivnitski D.; Mukherjee R.; Shtelman E.; Motro Y.; Miller Y.; Wagner N.; Cohen-Luria R.; Ashkenasy G. Emergence of native peptide sequences in prebiotic replication networks. Nat. Commun. 2017, 8, 434. 10.1038/s41467-017-00463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjivikua T.; Ballester P.; Rebek J. A self-replicating system. J. Am. Chem. Soc. 1990, 112, 1249–1250. 10.1021/ja00159a057. [DOI] [Google Scholar]
- Dieckmann A.; Beniken S.; Lorenz C. D.; Doltsinis N. L.; von Kiedrowski G. Elucidating the origin of diastereoselectivity in a self-replicating system: selfishness versus altruism. Chem. - Eur. J. 2011, 17, 468–480. 10.1002/chem.201002325. [DOI] [PubMed] [Google Scholar]
- Bottero I.; Huck J.; Kosikova T.; Philp D. A synthetic replicator drives a propagating reaction-diffusion front. J. Am. Chem. Soc. 2016, 138, 6723–6726. 10.1021/jacs.6b03372. [DOI] [PubMed] [Google Scholar]
- Sadownik J. W.; Kosikova T.; Philp D. Generating system-level responses from a network of simple synthetic replicators. J. Am. Chem. Soc. 2017, 139, 17565–17573. 10.1021/jacs.7b09735. [DOI] [PubMed] [Google Scholar]
- Kosikova T.; Philp D. Two synthetic replicators compete to process a dynamic reagent pool. J. Am. Chem. Soc. 2019, 141, 3059–3072. 10.1021/jacs.8b12077. [DOI] [PubMed] [Google Scholar]
- von Kiedrowski G. In Bioorganic chemistry frontiers; Springer: Berlin, 1993; pp 113–146. [Google Scholar]
- Szathmáry E.; Gladkih I. Sub-exponential growth and coexistence of non-enzymatically replicating templates. J. Theor. Biol. 1989, 138, 55–58. 10.1016/S0022-5193(89)80177-8. [DOI] [PubMed] [Google Scholar]
- Szathmáry E. Simple growth laws and selection consequences. Trends. Ecol. Evol. 1991, 6, 366–370. 10.1016/0169-5347(91)90228-P. [DOI] [PubMed] [Google Scholar]
- Lifson S.; Lifson H. Coexistence and Darwinian selection among replicators: response to the preceding paper by Scheuring and Szathmáry. J. Theor. Biol. 2001, 212, 107–109. 10.1006/jtbi.2001.2361. [DOI] [PubMed] [Google Scholar]
- Carnall J. M. A.; Waudby C. A.; Belenguer A. M.; Stuart M. C. A.; Peyralans J. J. P.; Otto S. Mechanosensitive self-replication driven by self-organization. Science 2010, 327, 1502–1506. 10.1126/science.1182767. [DOI] [PubMed] [Google Scholar]
- Malakoutikhah M.; Peyralans J. J. P.; Colomb-Delsuc M.; Fanlo-Virgos H.; Stuart M. C. A.; Otto S. Uncovering the selection criteria for the emergence of multi-building-block replicators from dynamic combinatorial libraries. J. Am. Chem. Soc. 2013, 135, 18406–18417. 10.1021/ja4067805. [DOI] [PubMed] [Google Scholar]
- Leonetti G.; Otto S. Solvent composition dictates emergence in dynamic molecular networks containing competing replicators. J. Am. Chem. Soc. 2015, 137, 2067–2072. 10.1021/ja512644f. [DOI] [PubMed] [Google Scholar]
- Bartolec B.; Altay M.; Otto S. Template-promoted self-replication in dynamic combinatorial libraries made from a simple building block. Chem. Commun. 2018, 54, 13096–13098. 10.1039/C8CC06253F. [DOI] [PubMed] [Google Scholar]
- Sadownik J. W.; Mattia E.; Nowak P.; Otto S. Diversification of self-replicating molecules. Nat. Chem. 2016, 8, 264–269. 10.1038/nchem.2419. [DOI] [PubMed] [Google Scholar]
- Colomb-Delsuc M.; Mattia E.; Sadownik J. W.; Otto S. Exponential self-replication enabled through a fibre elongation/breakage mechanism. Nat. Commun. 2015, 6, 8427. 10.1038/ncomms8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura Y.; Beierle J. M.; Leman L. J.; Orgel L. E.; Ghadiri M. R. Self-assembling sequence-adaptive peptide nucleic acids. Science 2009, 325, 73–77. 10.1126/science.1174577. [DOI] [PubMed] [Google Scholar]
- Singhal A.; Nielsen P. E. Cross-catalytic peptide nucleic acid (PNA) replication based on templated ligation. Org. Biomol. Chem. 2014, 12, 6901–6907. 10.1039/C4OB01158A. [DOI] [PubMed] [Google Scholar]
- Ploger T. A.; von Kiedrowski G. A self-replicating peptide nucleic acid. Org. Biomol. Chem. 2014, 12, 6908–6914. 10.1039/C4OB01168F. [DOI] [PubMed] [Google Scholar]
- Otto S.; Furlan R. L. E.; Sanders J. K. M. Dynamic combinatorial libraries of macrocyclic disulfides in water. J. Am. Chem. Soc. 2000, 122, 12063–12064. 10.1021/ja005507o. [DOI] [Google Scholar]
- Frederix P.; Ide J.; Altay Y.; Schaeffer G.; Surin M.; Beljonne D.; Bondarenko A. S.; Jansen T. L. C.; Otto S.; Marrink S. J. Structural and spectroscopic properties of assemblies of self-replicating peptide macrocycles. ACS Nano 2017, 11, 7858–7868. 10.1021/acsnano.7b02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L.; Serpell C. J.; Tuite M. F.; Xue W. F. The molecular lifecycle of amyloid - Mechanism of assembly, mesoscopic organisation, polymorphism, suprastructures, and biological consequences. Biochim. Biophys. Acta, Proteins Proteomics 2019, 1867, 140257. 10.1016/j.bbapap.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Jafilan S.; Klein L.; Hyun C.; Florian J. Intramolecular base stacking of dinucleoside monophosphate anions in aqueous solution. J. Phys. Chem. B 2012, 116, 3613–3618. 10.1021/jp209986y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito S.; Peyret N.; SantaLucia J. Thermodynamic parameters for DNA sequences with dangling ends. Nucleic Acids Res. 2000, 28, 1929–1934. 10.1093/nar/28.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. M.; Wolfenden R. Affinities of nucleic-acid bases for solvent water. Biochemistry 1981, 20, 3024–3028. 10.1021/bi00514a006. [DOI] [PubMed] [Google Scholar]
- Peters G. M.; Davis J. T. Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs. Chem. Soc. Rev. 2016, 45, 3188–3206. 10.1039/C6CS00183A. [DOI] [PubMed] [Google Scholar]
- Davis J. T. G-quartets 40 years later: From 5 ′-GMP to molecular biology and supramolecular chemistry. Angew. Chem., Int. Ed. 2004, 43, 668–698. 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- Cafferty B. J.; Gallego I.; Chen M. C.; Farley K. I.; Eritja R.; Hud N. V. Efficient self-assembly in water of long noncovalent polymers by nucleobase analogues. J. Am. Chem. Soc. 2013, 135, 2447–2450. 10.1021/ja312155v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






