Abstract
T-lymphocytes have a multifaceted role in ischemic stroke, but the majority of studies have been conducted in young mice, which may limit the translational value of these findings. Previous studies have shown that aging results in T cell dysfunction, leading to enhanced production of pro-inflammatory cytokines and chemokines, including interferon gamma (IFN-γ) and interferon-gamma-inducible protein (IP-10). This study assessed the role of T cells and pro-inflammatory factors on histologic and functional outcomes in an aged mouse model. Levels of IP-10 were measured in the brain and serum of young and aged male mice following middle cerebral artery occlusion (MCAo) or sham surgery. Additionally, IP-10 levels were evaluated in stroke patients. To directly determine the role of brain-infiltrating T cells after stroke, a separate cohort of aged male and female animals received either an anti-CD4 depletion antibody or IgG isotype control at 72 and 96 hours following experimental stroke. Behavioral assessments were performed on day 7 post-MCAo. CD4 T cell depletion resulted in improved behavioral outcomes, despite the lack of differences in infarct size between the isotype control and anti-CD4 antibody treated stroke groups. Circulating IP-10 levels were increased in both humans and mice with age and stroke, and depletion of CD4 T cells led to a reduction in IFN-γ and IP-10 levels in mice. Since anti-CD4 treatment was administered three days after stroke onset, targeting this inflammatory pathway may be beneficial to aged stroke patients who present outside of the current time window for thrombolysis and thrombectomy.
Keywords: Stroke, Inflammation, Age, CXCL10, CD4 T cells
1. Introduction
Stroke is a leading cause of death and disability in the USA. Given that nearly two-thirds of all strokes occur in elderly populations, it is imperative that aged animals be examined in preclinical studies for appropriate translational relevance when assessing potential therapeutic strategies. We previously found that aged mice have significantly slower and less complete recovery after stroke, despite smaller infarct volumes (Manwani et al., 2011). Thus, infarct volume alone cannot explain the poorer recovery seen in aged mice. Importantly, the post-stroke immunological response differs with aging and contributes to functional outcome (Ritzel et al., 2016).
The inflammatory role of T cells following stroke has been well documented. Specifically, CD4 T cells are recruited within 24 hours after ischemic injury, with peak infiltration occurring between days 3 and 4 (Stevens et al., 2002). Following ischemia, CD4 T cells secrete IFN-γ, which stimulates the secretion of IP-10 from a variety of cells (Seifert et al., 2014). IP-10 in turn stimulates CD4 T cells to secrete even more IFN-γ and other pro-inflammatory cytokines. Therefore, CD4 cells play an important role in propagating the pro-inflammatory cycle (“CD4-IFN-γ-CXCL10”) following stroke.
Dampening the CD4+ T cell driven pro-inflammatory response provides therapeutic benefit following stroke in young animals (Seifert et al., 2012). Specifically, genetic deletion of CD4 T cells and neutralization of IFN-γ have been found to reduce infarct volume, suppress IP- 10 expression, and decrease T cell infiltration (Gu et al., 2012; Seifert et al., 2014). Since aging itself has been shown to profoundly alter T cell function (Ritzel et al., 2016), it is critical to assess the effects of CD4 T cells on ischemic stroke pathology in aged animals. This study examined the pro-inflammatory cytokine profile in aged populations following stroke. We directly evaluated the role of CD4 T cells via antibody depletion given at the time of peak parenchymal T cell infiltration.
2. Materials and methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas Health & Science Center and were performed in accordance with National Institutes of Health guidelines. Young C57Bl/6 male mice (8-10 weeks) and aged mice (18-20 months) from Charles River were used for this study. Animals were aged in house to control for dietary factors. Food and water were provided ad libitum.
Animal Cohort
Cohort 1:
To investigate the acute effects of age and stroke on central and peripheral CD4 cytokine/chemokine levels (CXCL10, IFN-y), young (n=14) and aged (n=14) mice underwent ischemia or sham surgery and were euthanized 72 hours following. Brain and plasma IP-10 levels were measured.
Cohort 2:
To investigate the effects of transient CD4 T depletion on post-stroke recovery in aged male and female mice (n=20 male & 11 females) were randomly assigned to receive anti-CD4 antibody or IgG isotype control on days 3 and 4 post-stroke. Mice were euthanized on day 7 following behavioral assessments. Brain and plasma were collected for infarct analysis and cytokine measurement.
Middle cerebral artery occlusion (MCAo)
The MCAo model was used in young and aged mice as described previously (Harris et al., 2016). In brief, transient focal ischemia was induced under isoflurane anesthesia by occlusion of right MCA; the filament was not inserted into the MCA for sham mice. The suture diameter was 0.21mm for young mice and 0.23mm diameter for aged animals with a silicone coated tip at a length 5-6 mm. The MCA was occluded for 60 minutes, followed by reperfusion in both young and aged mice. All studies were performed blinded and animals were randomly assigned to stroke/sham and treatment group by computer generated block randomization.
CD4+ T cell depletion
Mice were injected intraperitoneally (i.p.) with 5mg/kg of an anti-CD4 depletion antibody (clone GK 1.5, eBioscience, San Diego, CA) or IgG isotype control (BD Biosciences PharMingen). The dose was based on prior literature that found that i.p. injection of this antibody (0.2mg/kg/day on 2 consecutive days and 5mg/kg a week apart) leads to depletion of CD4 T lymphocytes in the peripheral blood in mice (Mochimaru et al., 2008). In order to ensure adequate depletion, we administered 5mg/kg on days 3 and 4 following stroke, at a time of high parenchymal T cell infiltration. CD4 T cell depletion was confirmed by flow cytometry using an anti-CD4 clone (RM4-5) (Supplementary figure 2).
Behavioral testing and Infarct analysis:
Behavioral testing and analysis were performed by a blinded investigator on day 7 following MCAo. Details of procedures including infarct analysis are provided in supplementary methods.
Cytokine Measurement
Blood was obtained from anesthetized mice via cardiac puncture and collected into heparinized tubes. Blood was spun at 500 RCF for 10 minutes at 4°C, and the plasma supernatant was removed. Plasma was stored frozen (−80°C) until assay. Plasma levels were assessed per manufacturer’s instructions (Bio-Plex Pro Mouse Cytokine 23-Plex Immunoassay BioRad, Hercules, CA and Mouse IP-10 Platinum ELISA, eBioscience Inc., San Diego, CA).
Outcome Measures
For the human group and animal cohort one, the primary objective was to determine the impact of age and stroke on levels of pro-inflammatory IP-10. For animal cohort two, the primary objective was to examine the impact of CD4+ T cell depletion on post-stroke recovery, including infarct size, behavioral outcomes, and circulating IP-10 levels. We also examined the impact of depleting CD4 cells on other inflammatory cytokines and chemokines, utilizing a 23-multiplex.
Statistics
Data are expressed as mean ±SD except for the NDS where the interquartile range is provided (Mann-Whitney). Two-sample t-test or Wilcoxon rank-sum test was used to compare variables between different groups. Two-way ANOVA or a general linear model was used depending on whether the number of mice in each category was equal, followed by post-hoc tests adjusted by Tukey method. P<0.05 was considered statistically significant. All statistical analyses were performed in GraphPad Prism 7.
3. Results
IP-10 levels increase in age and ischemic stroke
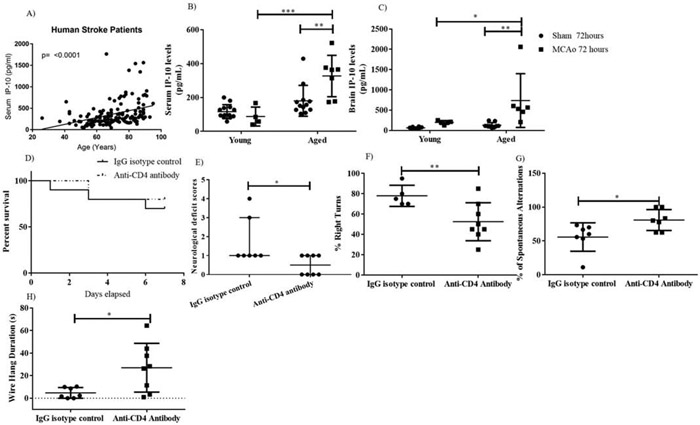
Circulating IP-10 levels increased with age in stroke patients (p<0.0001, Fig 1A) IP-10 levels were significantly higher in aged stroke mice compared to young stroke mice (p=0.0003, Fig 1B). Additionally, there was a significant main effect of stroke: Both brain (p=0.0048) and serum (p= 0.0032) IP-10 levels were significantly higher in aged stroke mice as compared to aged sham (p= 0.0032). Lastly, aged stroke mice had significantly higher brain IP-10 levels than young stroke mice at day 3, with no differences in sham mice, suggesting that age may be an important factor in propagating inflammation but only during brain injury (p=0.0352, Fig 1C). Peripheral and central IP-10 levels did not significantly differ by age in sham mice (p=0.2216, Fig 1B, p=0.9726, Fig 1C).
Figure 1: Depletion of CD4 T cells resulted in improved functional outcomes at day 7 post stroke.
A) Circulating IP-10 levels after stroke were significantly associated with increasing patient age. B) Serum IP-10 were higher in aged stroke mice at 72 hours. C) Aged stroke mice had higher brain IP-10 levels than young stroke mice at 72 hours. D) No difference in survival between the treatment groups. E) NDS, F) Percent of right turns was reduced. G) Percent spontaneous alterations were higher. H) Motor strength was increased in the CD4 T cell depleted mice after stroke.*p<0.05, **p<0.01, ***p<0.001.
Depletion of CD4 T cells resulted in improved functional outcomes
CD4 depletion in aged male and female stroke mice led to significant improvement in behavioral recovery although no difference in mortality was observed (Fig 1D, 1E, 1F, 1G, 1H and supplementary Fig 3). Specifically, CD4 T cell depletion in aged males led to significant improvement in the NDS (lower scores on day 3, p=0.033, Fig 1E), sensorimotor function (reduced percentage of right turns, p= 0.0184, Fig 1F), short-term memory (increase percentage spontaneous alterations, p= 0.0253, Fig 1G), and strength (longer grip, p=0.0196, Fig 1H). Similarly CD4 T cell depletion in aged females resulted in improvement in NDS (p=0.075, supplementary Fig 3A) sensorimotor function (reduced percentage of right turns, p= 0.0344, supplementary Fig 3B) and strength (longer grip, p=0.0161, supplementary Fig 3C). Importantly, for both males and females, this occurred in the absence of significant changes in percentage infarct area (males: hemispheric percentage infarct area isotype control vs antibody group was 44.52±5.76 vs. 48.23±4.19, respectively; p>0.05 in males; females: hemispheric percentage infarct area isotype control vs antibody group was 31.20 ± 4.55 vs 28.92 ±5.37).
CD4 T cell depletion reduced circulating pro-inflammatory cytokines
CD4 depletion reduced multiple inflammatory cytokines and chemokines. There was a significant decline in circulating IFN-γ (p= 0.0403, sup. Fig 1A), IP-10 (p= 0.0265, sup. Fig. 1B), CCL2 (p=0.0385, sup. Fig 1C), and CXCL1 (p=0.0329, sup. Fig 1D) in aged stroke mice treated with anti-CD4 vs. the IgG control group. There were no differences in circulating IL-α, IL-12, IL-β and TNF-α.
4. Discussion
We have previously shown that the aged brain is more permissive to T cell infiltration under naive conditions and following ischemic stroke (Ritzel et al., 2016). However, no studies have investigated the effects of T cell depletion after stroke in aged mice. This is the first study to show that CD4 T cell depletion reduces plasma cytokine levels and improves functional outcome in aged male and female mice, independent of infarct size. The anti-CD4 antibody was administered at peak inflammation (72 and 96 hours post-stroke) and after the stroke was histologically complete, therefore it is not surprising that there was no difference in infarct volume. Prior studies have demonstrated functional improvement following stroke in the absence of histological changes (Harris et al., 2016) and this work suggests that inflammatory factors contribute to functional outcome.
Both serum and brain concentrations of IP-10, which is largely activated by IFN-γ, were higher after stroke in mice, with significantly higher levels in aged stroke mice compared to young stroke mice. Our study supports the current literature showing that aging is linked to higher levels of baseline inflammation, which is exacerbated in the setting of stressors such as ischemic stroke. Depletion of CD4 in aged animals led to reduced IP-10 levels and improved functional recovery following ischemic stroke, confirming that T cells are heavily involved in age-related inflammatory responses. Improvement in the Y maze test in this study suggests that IP-10also plays a role in cognitive outcomes after ischemic stroke.
CD4 depletion also has non-specific downstream effects distinct from its role in the CD4-IFN-y-IP-10 feed-forward pathway. CD4 T cell depletion led to significant reduction in a range of inflammatory factors including IFN-y, CXCL10, and CCL2 at day 7 after stroke, some of which have been found to improve recovery following stroke (Benson et al., 1991). Together, these findings suggest that a reduction in inflammation, mediated by IP-10and CD4 T cells, contributed to improved functional outcomes. Although this study demonstrates compelling evidence for the benefit of depleting CD4 T cells after stroke in aged mice, there are several limitations to this study. Firstly, we showed beneficial effects of an antiCD4 antibody therapy in acute stroke recovery; efficacy of this treatment should be tested in more chronic cohorts. Secondly, CD4 cells are highly dynamic and polarize into various effector and regulatory phenotypes, which demonstrate both protective and detrimental functions after injury. We did not explore the effects of anti CD4 antibody on different T helper cells including Th1, Th2, Th17 and Tregs. Thirdly, T cells promote regeneration post stroke. Effects of CD4 depletion on regenerative processes post stroke warrants further investigation.
This is the first study to demonstrate the importance of this inflammatory pathway in aged animals, with supporting data from a clinical stroke population. Moreover, this study showed benefits of CD4 depletion well after the window for standard of care therapies including thrombolysis and mechanical thrombectomy. Thus, therapies aimed to target this inflammatory pathway may benefit a larger population of stroke patients due to the more easily achievable timeframe of administration.
Supplementary Material
Supplement figure 1: CD4 depletion led to reduced serum levels of inflammatory cytokines after stroke in aged mice. IFN-y (A), IP-10(B), CCL2 (C), CXC11 (D) No difference was seen in the serum levels of IL-α (E), TNF-α (F), IL-12 (G), and IL-β (H) between groups. *p<0.05.
Supplement figure 2: (A) Representative flow plots showing CD4+T cells in the blood and spleen of aged mice. Reduction in circulating (B) and splenic (C) CD4+ T cells after anti-CD4 antibody treatment. **p<0.01.
Supplement figure 3: Depletion of CD4 T cells in aged females resulted in improved functional outcomes at day 7 post stroke. A) NDS, B) Percent of right turns was reduced. C) Motor strength was increased in the CD4 T cell depleted mice after stroke.*p<0.05.
Highlights:
Aging alters the immunological response to stroke
Aged animals and humans have poorer outcomes after stroke
The CD4-IFNy-IP-10 pathway propagates the post-stroke inflammatory cascade
CD4 depletion during peak inflammation resulted in improved functional outcomes in both male and female aged mice
Therapies targeting this inflammatory pathway should be explored
Acknowledgements:
This work was supported by 16POST27490032 AHA post-doctoral fellowship (AC) and R01 5R01NS094543 (LDM) and F31 NS083244 (RMR)
Footnotes
Conflict of Interest
The authors declare no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benson CA, Spear J, Hines D, Pottage JC Jr., Kessler HA, & Trenholme GM 1991. Combined APACHE II score and serum lactate dehydrogenase as predictors of in-hospital mortality caused by first episode Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. Am Rev Respir Dis, 144, 319–323. [DOI] [PubMed] [Google Scholar]
- Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, & Zhao H 2012. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke, 43, 1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NM, Ritzel R, Mancini NS, Jiang Y, Yi X, Manickam DS, Banks WA, Kabanov AV, McCullough LD, & Verma R 2016. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav, 150-151, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, & McCullough LD 2011. Functional recovery in aging mice after experimental stroke. Brain Behav Immun, 25, 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochimaru H, Usui T, Yaguchi T, Nagahama Y, Hasegawa G, Usui Y, Shimmura S, Tsubota K, Amano S, Kawakami Y, & Ishida S 2008. Suppression of alkali burn-induced corneal neovascularization by dendritic cell vaccination targeting VEGF receptor 2. Invest Ophthalmol Vis Sci, 49, 2172–2177. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, Jellison ER, & McCullough LD 2016. Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J Immunol, 196, 3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, & Pennypacker KR 2014. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol, 9, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, & Pennypacker KR 2012. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol, 7, 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, & McCullough LD 2018. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol, 84, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, & Stenzel-Poore MP 2002. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res, 932, 110–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement figure 1: CD4 depletion led to reduced serum levels of inflammatory cytokines after stroke in aged mice. IFN-y (A), IP-10(B), CCL2 (C), CXC11 (D) No difference was seen in the serum levels of IL-α (E), TNF-α (F), IL-12 (G), and IL-β (H) between groups. *p<0.05.
Supplement figure 2: (A) Representative flow plots showing CD4+T cells in the blood and spleen of aged mice. Reduction in circulating (B) and splenic (C) CD4+ T cells after anti-CD4 antibody treatment. **p<0.01.
Supplement figure 3: Depletion of CD4 T cells in aged females resulted in improved functional outcomes at day 7 post stroke. A) NDS, B) Percent of right turns was reduced. C) Motor strength was increased in the CD4 T cell depleted mice after stroke.*p<0.05.