Abstract
Background
There is scientific evidence that older adults aged 65 and over walk with increased step width variability which has been associated with risk of falling. However, there are presently no threshold levels that define the optimal reference range of step width variability. Thus, the purpose of our study was to estimate the optimal reference range for identifying older adults with normative and excessive step width variability.
Methods
We searched systematically the BMC, Cochrane Library, EBSCO, Frontiers, IEEE, PubMed, Scopus, SpringerLink, Web of Science, Wiley, and PROQUEST databases until September 2018, and included the studies that measured step width variability in both younger and older adults during walking at self-selected speed. Data were pooled in meta-analysis, and standardized mean differences (SMD) with 95% confidence intervals (CI) were calculated. A single-decision threshold method based on the Youden index, and a two-decision threshold method based on the uncertain interval method were used to identify the optimal threshold levels (PROSPERO registration: CRD42018107079).
Results
Ten studies were retrieved (older adults = 304; younger adults = 219). Step width variability was higher in older than in younger adults (SMD = 1.15, 95% CI = 0.60; 1.70; t = 4.72, p = 0.001). The single-decision method set the threshold level for excessive step width variability at 2.14 cm. For the two-decision method, step width variability values above the upper threshold level of 2.50 cm were considered excessive, while step width variability values below the lower threshold level of 1.97 cm were considered within the optimal reference range.
Conclusion
Step width variability is higher in older adults than in younger adults, with step width variability values above the upper threshold level of 2.50 cm to be considered as excessive. This information could potentially impact rehabilitation technology design for devices targeting lateral stability during walking.
Keywords: Walking, Gait, Biomechanics, Rehabilitation, Lateral stability, Biomarkers
Background
Maintaining lateral stability during walking is a considerable challenge to the motor control system of the older adult, for whom age-related declines in sensorimotor functions could result in increased step width variability. Lateral stabilization during walking occurs due to the passive dynamics of the musculoskeletal system and the active control of the central nervous system [1, 2]. It has been also suggested that step width variability reflects the amount of active control that is required for lateral stabilization [3]. Accordingly, it appears that the age-related decrease in sensorimotor precision results in higher step width variability [4]. Based on this theoretical framework, when lateral foot placement becomes more stable the required amount of active control decreases, resulting in a consonant decrease in step width variability [1, 5].
Furthermore, evidence has surfaced to support the link between increased step width variability and risk of falling in older adults [6]. Step width variability was able to predict falls [7–10], and to differentiate older adults who fell from those who did not fall after a slip [11]. It could be suggested, therefore, that an intervention to decrease fall risk in older adults during walking could be effective if it targets to reduce the increased step width variability. However, a critical question for such an intervention to be successful is the amount of such a reduction. This is because the threshold level for identifying older adults with increased step width variability is presently unknown.
Thus, the purpose of this systematic review and meta-analysis was to establish the optimal threshold levels for identifying the boundaries of optimal reference range of step width variability. Such information could potentially impact the development of rehabilitation technology for devices targeting lateral stability to decrease risk of falling in older adults. In addition, it could potentially allow for better diagnostic and prognostic technology for individuals at risk of falling.
Methods
Protocol and registration
This systematic review and meta-analysis was conducted and reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement, and the recommendations from the Cochrane collaboration initiative [12–15]. The review protocol and inclusion criteria were specified in advance based on the PRISMA-P statement and registered on the PROSPERO register of systematic reviews website (registration number: CRD42018107079). The PRISMA checklist is provided as Additional file 1.
Literature review
A computerized systematic literature search, based on the Population Intervention Comparison Outcome (PICO) method was performed in the BMC, Cochrane Library, EBSCO, Frontiers, IEEE, PubMed, Scopus, SpringerLink, Web of Science, Wiley, and PROQUEST databases limited to research articles that have been published until September 2018. A string with Boolean search syntax operators was used to retrieve the titles and abstracts of the articles. The string contained all combinations of keywords and/or wildcards that specified the task, cohort, and outcome, combined with synonyms and terms from the MeSH thesaurus. The given query’s combinations were used to search the databases (see Additional file 2, which shows the search string).
Inclusion criteria
Only peer-reviewed journal articles were included. Articles had to describe studies: (i) with samples whose participants were healthy younger (19–35 years) and older adults (65 years and over) free of overt neurological disorders and significant disabilities who were independently residing in the community; (ii) that measured step width during overground forward walking on a solid surface or on the treadmill at self-selected preferred speed, by using an optical system, pressure mat, instrumented walkway, or force plates; (iii) where all the participants had been measured under identical experimental conditions.
Exclusion criteria
Articles were excluded if: (i) they were abstracts, conference proceedings, pilot studies, reviews with or without meta-analysis, qualitative studies, and technical reports; (ii) participants walked with an assistive device or had progressive neurological conditions, neurological impairments, lower limb disabilities, injuries or diseases that influence gait, or taking medications known to influence gait; (iii) participants walked over obstacles or sideways, backward, and not in a straight line or without a constant walking speed (e.g., accelerated, or decelerated walking speed); (iv) a metronome was used during walking (auditory, visual or any other sensorimotor stimulus or feedback); (v) the described studies used non-representative samples; (vi) they were published in languages other than English.
Study selection
After removal of duplicate items, the titles and abstracts of the articles were screened independently by two review team members and excluded according to the predefined criteria; disagreement between reviewers was settled by consensus. Further, the reference list of each included article was checked and screened with the initial screening criteria to identify additional studies. This step was repeated until there were no further candidates for inclusion.
Assessment of Methodologic quality
Quality has been assessed independently by two reviewers using an adaptation of the Downs and Black quality index checklist [16]. This scale is considered appropriate for assessing both randomized and non-randomized studies and provides an overall study quality score and score profiles for quality reporting, internal validity, power, and external validity [16]. Moreover, the method used by the authors to compute step width variability was evaluated. Only studies that computed step width variability as the standard deviation of the mediolateral distance between left and right foot during forward walking, whose coordinates were defined either on a global or a local reference system, and the anteroposterior axis of the reference system was matched with the direction of walking have been chosen. Disagreement between reviewers was settled by consensus.
Data extraction
The data extracted by two reviewers were: age, sample size, exposure, preferred walking speed, step width variability (standard deviation of step width), step width calculation, and the instrumentation used for measuring step width. Measurements that reported stride width or base of support during walking were considered synonyms to step width since all measured mediolateral distance between feet. Variability in these measures was treated as equivalent to step width variability. The term lateral variability was treated as equivalent to step width variability, as well. When required, underlying numerical data were extracted through scaling of graphical representation. To reduce any error in this procedure, numerical data were extracted 10 times and mean values were computed and recorded as the measure of step width variability. When needed, the standard deviation of the step width variability was obtained from the parameters of the statistical analysis (p-value, t-value, standard error).
Quantitative synthesis
Data were analyzed using standard meta-analytic modeling in R statistical 3.6.1 software with the meta, metafor, and dmetar packages [17–20]. Considerable heterogeneity was expected between the studies. Therefore, a meta-analysis of the mean difference in step width variability between older and younger adults’ groups was conducted using a random effects model, and Cohen’s method for pooling standardized mean differences (SMD) [21, 22]. Because of the small number of pooled studies and the expected heterogeneity the standard error estimates were adjusted using the Hartung-Knapp-Sidik-Jonkman correction [23–25] Confidence intervals for the SMD reported in each study and the overall SMD estimate were presented using forest plots.
Heterogeneity between studies was assessed using Cochran’s Q statistic [26]. Moreover, the τ2, H, and the Higgins’I2 measures for statistical heterogeneity were also incorporated as a cross-check [27, 28]. We performed influence and graphical display of study heterogeneity (GOSH) diagnostics, including leave-one-out analysis [29–32]. GOSH is a novel all-subsets combinatorial meta-analysis approach that calculates the effects sizes of 210–1 subgroup to explore heterogeneity. Publication biases were evaluated visually with a contour-enhanced Funnel plot and formally checked by Begg’s and Egger’s tests [33–35].
Identification of the optimal threshold levels of step width variability
The upper threshold level of step width variability set the bound of excessive step width variability. To identify the threshold level, a binary logistic regression analysis was conducted with aging (0 = younger adults; 1 = older adults) as the dependent variable, and the younger and older adults’ group averages of step width variability values across studies as the predictor variable. Therefore, the regression analysis was performed on group averages. It was assumed that the step width variability values in healthy younger adults set the optimal reference range. The Hosmer-Lemeshow chi-square test was used to measure model’s goodness of fit [36]. Non-significant chi-square indicates a failure to reject the null hypothesis implying that the model’s estimates fit the data at an acceptable level. The receiver-operating characteristic (ROC) curve was used to evaluate the discrimination ability of the binary logistic model by calculating the area under the ROC curve (AUC). The cutoff value to identify the excessive step width variability was estimated from the ROC analysis using Youden’s index.
We expected that there would be a degree of overlap between the step width variability values of the younger and older adults’ groups. Thus, a novel trichotomization method was used also to provide two threshold levels that define an interval of uncertainty around Youden index. Around Youden index the step width variability values are inter-mixed and have a near equal probability of indicating ‘reference’ or ‘excessive’ providing little or no information whether an individual is a younger or older adult [37]. The cutoff points of the uncertain interval method were chosen at specificity (Sp) = sensitivity (Se) = 0.50.
Results
Study selection
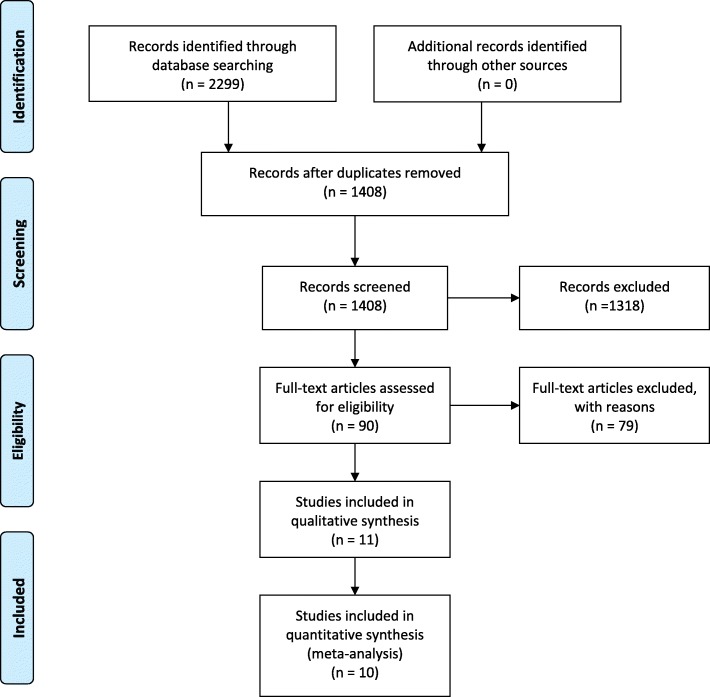
Figure 1 displays a flowchart summarizing the results of the literature search. In summary, from the 1408 unduplicated studies identified, 1318 of them were excluded during the title and abstract screening, and from the remaining 90 full-text reviewed articles, 79 of them were excluded after full-text screening because step width variability values were not stated, subjects did not walk at preferred speed, the age of the older adults group was not over 65 years, did not include both older and younger groups or because the pace of the preferred speed was maintained by an auditory metronome. The remaining 11 articles were included in the qualitative synthesis [38–48] (see Additional file 3, for detailed flow diagram). Of the 11 eligible studies, two studies recruited the same younger adults’ population [39, 40]. In one study the same younger and older adults’ populations contributed twice; in a repeated single and in a continuous overground walking [46]. The continuous protocol has been chosen because it involves walking without interruptions [46].
Fig. 1.
Study selection flowchart
Study subjects
Table 1 reports the characteristics of the 11 studies included in the systematic review. All studies used slightly different cut points to distinguish younger from old. Of the 11 studies, two studies [46, 47] recruited only female subjects; one study [45] only reported the total (male and female) subjects. Data were extracted from 323 older adults with mean age 74.41 ± 6.29 years old, and 239 younger adults with mean age 25.3 ± 4.6 years old. Eight studies assessed health of the younger or older adults’ population by medical history and/or physical, psychiatric or neurological examinations [38–41, 43, 44, 46, 48]. One assessed only the health of the older population [45], and one study detailed specific exclusion criteria [47]. One study did not specify any physical or other examinations for assessing subjects’ health [42].
Table 1.
Study summary for the step width variability during walking in young and older adults
| Author / Year | Sample size | Exposure | Preferred walking speed protocol | Step width calculation | Step width variability / analyzed steps (avg.) |
|---|---|---|---|---|---|
| Almarwani et al. (2016a) [39] |
OA, n = 111 (82 f), age = 77.25 ± 6.0 yr., ht. = 163.4 ± 9.5 cm, wt. = 77.4 ± 15.7 kg; YA, n = 40 (30 f), age = 26.60 ± 6.0 yr., ht. = 168.4 ± 8.3 cm, wt. = 66.4 ± 12.4 kg |
Walking on a 4 m walkway in 3-speed conditions |
Participants were instructed to walk at a pace that represented their usual walking speed. OA, PWS = 1.07 ± 0.26 m/s; YA, PWS = 1.29 ± 0.19 m/s. |
The distance between the outermost borders of two consecutive footprints (GaitMat II). |
OA: 3.70 ± 1.80 cm; YA: 2.40 ± 0.60 cm. OA: 23 steps YA: 38 steps |
| Almarwani et al. (2016b) [40] |
OA, n = 46 (35 f), age = 78.09 ± 6.2 yr., ht. = nr cm, mass = nr kg; YA, n = 40 (30 f), age = 26.6 ± 6.0 yr., ht. = nr cm, mass = nr kg; |
Walking on an 8 m walkway at preferred speed |
Authors did not describe how the preferred walking speed was determined. OA, PWS = 0.95 ± 0.28 m/s; YA, PWS = 1.29 ± 0.19 m/s. |
The distance between the outermost borders of two consecutive footprints (GaitMat II). |
OA: 3.00 ± 1.41 cm; YA: 2.50 ± 1.41 cm OA: 23 steps YA: 38 steps |
| Decker et al. (2016) [41] |
OA, n = 19 (9 f), age = 69.26 ± 1.11 yr., ht. = 171 ± 2 cm, mass = 77.45 ± 2.78 kg; YA, n = 20 (12 f), age = 24.45 ± 0.87 yr., ht. = 173 ± 2 cm, mass = 70.41 ± 2.63 kg |
Three-minutes treadmill walking at 4 attentional demands conditions at a preferred speed |
Participants started walking at a slow speed while the treadmill was slowly accelerated by 0.1 km/h until the participants reported their PWS. Then the speed was increased by 1.5 km/h and was slowly decreased by 0.1 km/h until the participants reported their PWS. This procedure was repeated until a less than 0.4 km/h difference was achieved. OA, PWS = 0.77 ± 0.04 m/s; YA, PWS = 1.06 ± 0.03 m/s. |
Mediolateral distance between foot midpoints calculated over the consecutive instants when the left (or right) swing limb’s knee passed in front of the right (or left) stance limb’s knee |
OA: 1.70 ± 0.17 cm; YA: 1.92 ± 0.08 cm OA: ≥ 256 steps YA: ≥ 256 steps |
| Ihlen et al. (2012) [42] |
OA, n = 10 (4 f), age = 75.4 ± 4.6 yr., ht. = 170.9 ± 11.8 cm, mass = 76 ± 13.1 kg; YA, n = 10 (4 f), age = 25.7 ± 4.7 yr., ht. = 177.6 ± 8.3 cm, mass = 74.5 ± 9.5 kg |
Ten-minutes treadmill walking in 3-speed conditions |
Authors did not describe how the preferred walking speed was determined. OA, PWS = 1.17 ± 0.10 m/s; YA, PWS = 1.11 ± 0.15 m/s. |
Step width was defined as the mediolateral distance between heel markers at the time of heel strike |
OA: 2.55 ± 0.35 cm; YA: 1.91 ± 0.30 cm OA: nr steps YA: nr steps |
| Kang et al. (2008) [43] |
OA, n = 18 (6 f), age = 72.1 ± 6.0 yr., ht. =170 ± 10.4 cm, mass = 73.2 ± 12.3 kg; YA, n = 17 (5 f), age = 23.6 ± 2.6 yr., ht. = 173 ± 9.4 cm, mass = 71.1 ± 9.86 kg |
Five-minutes treadmill walking in 5-speed conditions |
Participants reported the limits of their preferred speed while the treadmill was slowly accelerated, then decelerated three times. These upper and lower limits were averaged to determine their preferred walking speed. OA, PWS = 1.29 ± 0.15 m/s; YA, PWS = 1.30 ± 0.10 m/s. |
Step width was defined as the distance between the heel and the contralateral heel at each heel contact in the mediolateral direction |
OA: 2.14 ± 0.54 cm; YA: 2.01 ± 0.56 cm OA: nr steps YA: nr steps |
| Lovden et al. (2008) [38] |
OA, n = 32 (16 f), age = 73.6 ± 2.9 yr., ht. = 169.4 ± 10 cm, mass = 74.3 ± 11.5 kg; YA, n = 32 (16 f), age = 25.0 ± 2.9 yr., ht. = 177.6 ± 9.8 cm, mass = 71.6 ± 13.1 kg |
Twenty-secs treadmill walking in 4 conditions of working memory load at a preferred speed |
Participants gradually increased speed to determine preferred walking speed. After walking at their self-selected speed for 3 min were asked again if they felt comfortable with their choice. OA, PWS = 0.87 ± 0.13 m/s; YA, PWS = 1.04 ± 0.11 m/s. |
The step width was measured as the perpendicular distance between the line of progression and the heel location of the contralateral foot. |
OA: 2.19 ± 0.11 cm; YA: 1.97 ± 0.12 cm OA: 18 steps YA: 18 steps |
| Marigold et al. (2008) [44] |
OA, n = 10 (5 f), age = 74.1 ± 7.2 yr., ht. = nr cm, mass = nr kg; YA, n = 10 (5 f), age = 26.1 ± 5.2 yr., ht. = nr cm, mass = nr kg |
walking on a multi-surface terrain in 4 different terrain configurations for YA and in 3 different conditions for OA, respectively, at a preferred speed |
Authors did not describe how the preferred walking speed was determined. OA, PWS = 1.20 ± 0.12 m/s; YA, PWS = 1.32 ± 0.16 m/s. |
The mediolateral distance between ankle markers |
OA: 4.09 ± 0.70 cm; YA: 2.96 ± 1.29 cm OA: nr steps YA: nr steps |
| Owings et al. (2004a) [45] |
OA, n = 12 (nr), age = 73.4 ± 2.3 yr., ht. = 172 ± 13 cm, mass = 76.3 ± 15.5 kg; YA, n = 18 (nr), age = 27.7 ± 3.3 yr., ht. = 168 ± 11 cm, mass = 35.9 ± 10.2 kg |
Ten-minutes treadmill walking for OA and 15-min for YA at a preferred speed |
Authors did not describe how the preferred walking speed was determined. OA, PWS = 0.97 ± 0.17 m/s; YA, PWS = 1.06 ± 0.28 m/s. |
Step width was determined as the mediolateral distance between the sequential left and right heel-strikes |
OA: 2.50 ± 0.70 cm; YA: 2.10 ± 0.50 cm OA: nr steps YA: nr steps |
| Paterson et al. (2009) [46] |
OA, n = 32 (32 f), age = 67.4 ± 6.3 yr., ht. = 162 ± 7 cm, mass = 65.1 ± 13.2 kg; YA, n = 22 (22 f), age = 21.2 ± 2.5 yr., ht. = 166 ± 8 cm, mass = 62.6 ± 9.8 kg |
10 m continuous laps of a walking circuit at a preferred speed |
Authors did not describe how the preferred walking speed was determined. OA, PWS = nr; YA, PWS = nr. |
The midline midpoint of the current footprint to the midline midpoint of the previous footprint on the opposite foot (GaitRite). |
OA: 2.50 ± 0.83 cm; YA: 1.90 ± 0.83 cm OA: nr steps YA: nr steps |
| Thies et al. (2005) [47] |
OA, n = 12 (12 f), age = 70.2 ± 4.1 yr., ht. = nr cm, mass = nr kg; YA, n = 12 (12 f), age = 22.2 ± 3.0 yr., ht. = nr cm, mass = nr kg, |
Walking on a 10 m walkway in 4 task conditions at a preferred speed |
Subjects were asked throughout the experiment to walk at a comfortable speed as if they were going to mail a letter. OA, PWS = 1.15 ± 0.06 m/s; YA, PWS = 1.08 ± 0.06 m/s. |
Mediolateral distance between the left and right foot ankle (tibiotalar joint) markers during double support |
OA: 2.99 ± 0.20 cm; YA: 2.50 ± 0.17 cm OA: 55 steps YA: 63 steps |
| Woledge et al. (2005) [48] |
OA, n = 21 (8 f), age = 72.7 ± 1.21 yr., ht. = 166 ± 2 cm, mass = 68.3 ± 2.6 kg; YA, n = 17 (11 f), age = 27.3 ± 1.5 yr., ht. = 171 ± 2 cm, mass = 64.3 ± 2.9 kg |
Walking on 8 m walkway at a preferred speed |
Authors did not describe how the preferred walking speed was determined. OA, PWS = 1.12 ± 0.06 m/s; YA, PWS = 1.19 ± 0.03 m/s. |
The lateral difference between successive footfall positions (medial malleoli) |
OA: 2.32 ± 0.28 cm; YA: 1.73 ± 0.37 cm OA: 34 steps YA: 34 steps |
Note. OA older adults; YA younger adults; PWS preferred walking speed; f females; nr not reported; n number; yr years; m/s meters per second; cm centimeters; avg average; ht height
Instrumentation used for measuring step width
Six studies measured step width during overground walking [39, 40, 44, 46–48] and five studies during walking on a treadmill [38, 41–43, 45]. Of the six overground walking studies, five studies [39, 40, 44, 47, 48] used repeated single walking protocols and one study [46] both repeated single and continuous walking protocols. Data collection during overground walking was done by using either an instrumented walkway [39, 40, 46] or a motion capture system [44, 47, 48]. During treadmill walking data were collected using motion capture systems [38, 41–43] or force plates (i.e., center of pressure - COP) [45]. In addition, Table 2 reports the quality assessment performed on the selected studies used in the meta-analysis.
Table 2.
Methodologic assessment of study design quality using an adaptation of the quality index [16]. Numbering refers to the quality index item
| ITEM | STUDY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Almarwani et al. (2016a) [39] | Almarwani et al. (2016b) [40] | Decker et al. (2016) [41] | Ihlen et al. (2012) [42] | Kang et al. (2008) [43] | Lovden et al. (2008) [38] | Marigold et al. (2008) [44] | Owings et al. (2004a) [45] | Paterson et al. (2009) [46] | Thies et al. (2005) [47] | Woledge et al. (2005) [48] | |
| 1. Is the hypothesis/aim/objective of the study clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods section? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Are the characteristics of the participants included in the study clearly described? | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 5. Are the distributions of the principal confounders in each group of subjects to be compared clearly described? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 6. Are the main findings of the study clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10. Have actual probability values been reported (e.g. 0.035 rather than < 0.05) for the main outcomes except where the probability value is less than 0.001? | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 11. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 12. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13. Were the staff, place and facilities where the study was set representative of a laboratory environment? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 16. If any of the results of the study were based on “data dredging”, was this made clear? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20. Were the main outcome measures used accurate (valid and reliable)? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 21. Were the subjects recruited from the same population? | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| 22. Were study subjects recruited over a defined period of time? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25. Was there adequate adjustment for confounding in the analysis from which the main findings were drawn? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 27. Did the study have a power analysis? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TOTAL | 14 | 14 | 12 | 10 | 13 | 13 | 14 | 11 | 11 | 14 | 13 |
Assessment of publication Bias and heterogeneity sensitivity analysis
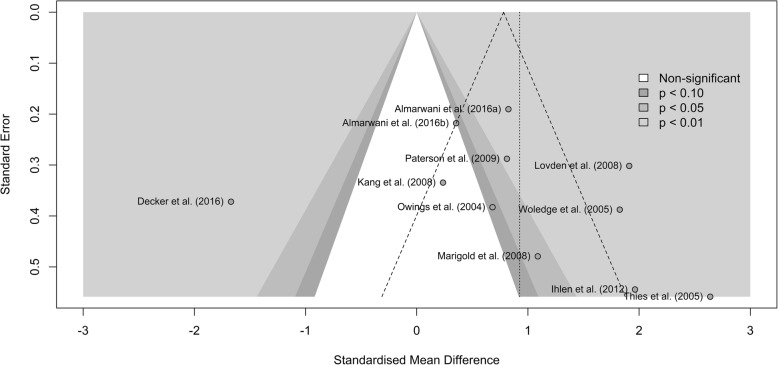
Visual inspection of the contour-enhanced funnel plot (Fig. 2) indicated the presence of publication bias. Neither Begg’s rank correlation test (z = 1.79, p-value = 0.07) nor Egger’s regression test (t = 1.99, df = 8, p-value = 0.08) returned statistically significant results. Diagnostics plots identified the study of Decker et al., [41] as a potential outlier (see Additional file 4). Thus, we omitted the study of Decker et al., [41] from the meta-analysis. Therefore, for the meta-analysis, data were extracted from the 10 remaining studies.
Fig. 2.
Contour-enhanced Funnel plot of standardized mean differences. Standardized mean differences in the white area are statistically non-significant (p > 0.1). The dashed angled lines represent the bounds within which 95% of studies should fall if there is no statistical heterogeneity. The dashed vertical line represents the estimate for the overall effect from the random-effect model
Step width variability in younger adults vs older adults
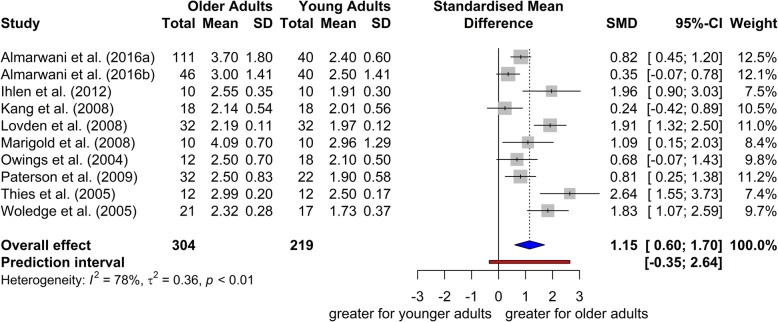
The study of Decker et al., [41] was omitted from the meta-analysis as an influential outlier, and the meta-analysis was conducted with the 10 remaining studies (304 older adults with mean age 74.74 ± 6.34 years old, and 219 younger adults with mean age 25.4 ± 4.8 years old). The meta-analysis revealed a significant overall effect size (SMD = 1.15, 95% CI = 0.60; 1.70; t = 4.72, p = 0.001), indicating that step width variability was higher in older adults than in younger adults (Fig. 3). The between studies heterogeneity was moderate (τ2 = 0.36, H = 2.14 [1.58; 2.89]; I2 = 78% [60%; 80%]; Q = 41.14, p-value < 0.001). Two of the studies recruited only female participants [46, 47]. Subgroup meta-analysis using a mixed-effects model (random-effects model within subgroups, fixed-effects model between subgroups) [17] was conducted to test whether gender modified the meta-analytic results. The test for subgroup differences indicated that there is no statistically significant subgroup effect (p = 0.52) (analysis not presented). Additionally, a subgroup meta-analysis was conducted to test if heterogeneity varies according to walking environment (‘overground walking’ vs. ‘treadmill walking’). The test for subgroup differences indicated that there is no statistically significant subgroup effect (p = 0.94).
Fig. 3.
Forest plot of standardized mean difference (SMD) and 95% confidence intervals (CI) for the step width variability between older and younger adults. The difference found for the step width variability between the younger and older adults was statistically significant (p = 0.001) indicating that step width variability was higher in older adults than in younger adults. SD: standard deviation, SMD: standardized mean difference, CI: confidence interval
Discrimination of step width variability for predicting age group
The binary logistic regression showed a good association (z = − 1.928, p = 0.057) and a good accuracy (ACC) (correct classification of older and younger adults’ group) for the step width variability to predict subjects’ age group (younger adults vs. older adults) (ACC = 0.70; no information rate (NIR) = 0.50; p-value [ACC > NIR] = 0.06; Hosmer-Lemeshow: χ2 = 7.58, p = 0.37). Using the ROC curve approach, the Youden’s index value was chosen as the cutoff value, which corresponds to step width variability value of 2.14 cm. The accuracy of the cutoff value based on the dichotomization approach (Youden’s index) was ACCdi = 0.80 of the older and younger adults’ groups. The sensitivity (older adults’ groups with excessive step width variability) was Sedi = 1.00, and the specificity (younger adults’ groups with healthy step width variability) was Spdi = 0.60. The positive predictive value (probability to belong to the older adults’ groups when step width variability is excessive) was PPVdi = 0.71, and the negative predictive value (probability to belong to the younger adults’ groups when step width variability is healthy) was NPVdi = 1.00.
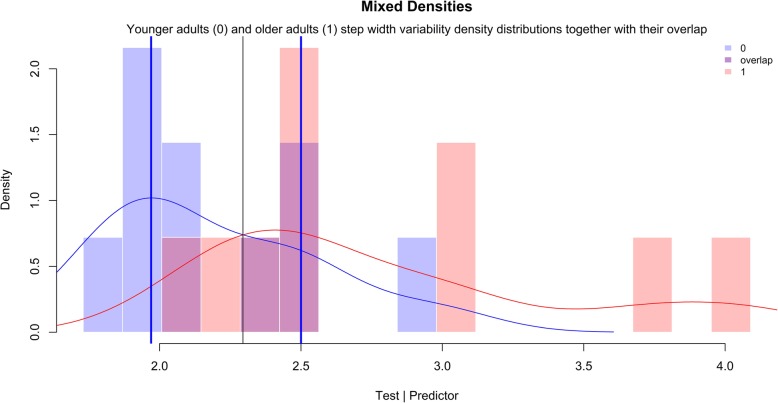
Using the approach of Landsheer, [37] the optimal reference range was separated from the excessive step width variability by an uncertainty interval. The lower and upper threshold levels of the uncertainty interval were Lo = 1.97 cm and Hi = 2.50 cm, respectively. Eleven observations were considered as uncertain (Table 3). The uncertain step width variability of the younger adults was compared with that of the older adults. The t-test did not reveal statistically difference between the two groups (t = − 0.13, p = 0.89). The trichotomization approach improved accuracy (ACCtr = 0.88) (Table 3). Moreover, the sensitivity (Setr = 1.00), specificity (Sptr = 0.75), positive predicted value (PPVtr = 0.83), and negative predicted value (NPVtr = 1.00) were improved. Within the interval it is impossible to decide about the absence or not of excessive step width variability (CCRun = 0.55; Seun = 0.60; Sp = 0.50; PPVun = 0.50; NPVun = 0.60). The trichotomization approach removed inter-mixed step width variability values providing more information for the classification. Therefore, step width variability values above the threshold level of Hi = 2.50 cm were considered excessive, while step width variability values below the threshold level of Lo = 1.97 cm were considered within the optimal reference range (Fig. 4).
Table 3.
Confusion matrix for the uncertain interval method
| Classified | Actual | |
|---|---|---|
| Younger adults | Older adults | |
| Younger adults (SWV < Lo) | 3 | 0 |
| Uncertain Interval (Lo ≤ SWV ≤ Hi) | 6 | 5 |
| Older adults (SWV > Hi) | 1 | 5 |
Notes: SWV step width variability; Lo 1.97 cm; Hi 2.50 cm
Fig. 4.
Visual inspection of the uncertain interval method. The densities of older adults and younger adults step width variability distributions together with their overlap are presented. Youden index occurs at the intersection of both density distributions, where the overlap is higher (0 = younger adults; 1 = older adults). The blue vertical lines are the optimal threshold levels
Discussion
Implications for clinical practice
In this systematic review and meta-analysis we sought to define the optimal threshold levels for identifying the boundaries of optimal reference range of step width variability in older adults. As such, we provided evidence of optimal threshold levels of step width variability with an uncertainty interval. For our purposes, the step width variability values in healthy younger adults set the optimal reference range.
Lateral foot placement has been shown to be the dominant mechanism that ensures lateral stability during walking [49, 50]. Simple locomotion models suggest that lateral stability is controlled through active adjustments of lateral foot placement which is determined from integrative sensory feedback with each step [1, 3, 51, 52]. Based on this approach, step width variability serves as an indicator of the required active control, [3] and as a quantifier of the degree to which sensory inputs contributes to the active control [53, 54]. As such, step width variability increases when active control is subjected to noisy inputs [5]. Age-related decrease in sensorimotor precision [55–57] can be treated as a reduced signal-to-noise ratio [5, 58, 59]. It is likely that an imprecise active control in older adults causes increments in step width variability [5, 60] and increases the risk of falling [52]. Indirect evidence comes from many studies demonstrating an increase in step width variability with aging [45, 48, 61, 62] but not between populations of older adults with different balance control abilities [63]. The clinical utility for identifying the older adults with excessive step width variability relies on previous research work that has related increased step width variability in older adults with increased risk of falling [11, 64]. Mechanically, since foot placement is the dominant mechanism that ensures lateral stability during walking, [2, 49, 50] the alignment of the step width variability to the optimal reference values would reflect an improvement in the lateral stability. While age-related, non-pathological decline in walking performance occurs in everybody, older adults who walk with excessively increased step width variability are at a higher risk of falling [11]. Therefore, it is plausible that older adults at decreased risk of falling walk within the optimal reference range of step width variability, while older adults at high risk of falling walk with excessive, non-optimal reference range values. The comparison with reference values could be set realistic goals for interventions targeted to improve lateral stability during walking. The proposed reference values can be used to express older adults’ step width variability as a percentage of what individuals with precise active control can achieve.
Gait training has become an essential component of fall prevention interventions and is recommended in current fall prevention guidelines [65]. The extent to which a gait training intervention has the potential to ameliorate the common age-related deterioration in gait performance of older adults and reduce risk of falling is dependent on the specific population being examined. It can be argued that gait training requires a measure of gait performance that can be used both to profile older adults for screening practices, and as a sensitive indicator for monitoring an individual’s performance. Our results suggest that gait training would be more effective in decreasing risk of falling in older adults if it targets to align excessive step width variability to the optimal reference range values. For example, Wang et al., [66] proposed a 12-week exercise intervention able to decrease gait variability in older adults. However, it is impossible to know whether postintervention gait variability fell within normal values in the absence of an optimal reference range. Step width variability could be used to identify older adults at higher risk of falling, and as a biomarker to be targeted for gait training intervention. Step width variability is a straightforward measurement due to its simplicity, it is noninvasive, easy to perform, and inexpensive. Such information could be implemented in the development of rehabilitation technology for devices targeting lateral stability to decrease risk of falling in older adults.
Recently, it has been shown that a change in attentional demands causes a consonant decrease or increase in step width variability in older adults during treadmill walking (a U-shaped relation) [41]. In this study, step width variability in the most cognitively demanding condition did not exceed that of the control walking condition (i.e., without any attentional demands). This was interpreted as a protective mechanism of the central nervous system to counteract the increased risk of falling that is related with excessive step width variability. However, in the absence of an optimal reference range, we do not know who walk within, near or below to the boundary of excessive step width variability. This meta-analysis contributes to fill this essential gap of knowledge. As the effectiveness of any intervention is related to the specific population being examined, using optimal threshold levels for step width variability can allow the selection of older adults with excessive (or normal) step width variability in the absence of attentional demands other than that of the walking activity itself.
Computation of step width variability
High and low step width variability values (low, < 7–8%; moderate, 8–27%; high, > 27–30%), expressed as the coefficient of variation of the step width, has been related retrospectively with falls and with low levels of physical activity in older adults who did not walk slowly (i.e., gait speed ≥1 m/s) [8, 67]. The coefficient of variation of step width variability has been questioned as being an appropriate parameter to express step width variability during walking. Helbostad and Moe-Nilssen, [68] showed that the coefficient of variation of the step width demonstrates ‘a spurious relation to gait speed’ because the mean step width value is non-linearly associated with walking speed (a U-shaped relation). On the other hand, the standard deviation of step width demonstrates no relation with walking speed indicating that the within-subject standard deviation of step width could be a more suitable parameter to express step width variability [43, 68]. In addition, the coefficient of variation depends on the foot markers used to calculate the step width. Woledge et al., [48] defined step width as the mediolateral distance between the left and right medial malleoli during double support, while Owings and Grabiner, [45] defined it as the mediolateral distance between the sequential left and right heel markers. In other words, if we had collected data on the same subject during walking, the use of different foot markers to calculate step width would have resulted in different coefficient of variations, while the standard deviations of the step width would have been the same (assuming that the foot is a rigid segment and there is no rotation). This is supported by a recent literature review that showed that the coefficient of variation of step width exhibited large differences between studies [69]. Finally, the coefficient of variation is applicable only to ratio data, and the step width is considered interval data as it is not bounded by a meaningful zero point [46, 70]. Therefore, in this systematic review and meta-analysis we included only studies that reported the step width variability as the standard deviation of the step width.
Strength and limitations of the analysis
We provided scientific evidence to use step width variability as an age determinant of gait control. All participants in the studies identified were considered healthy younger and older adults free of overt neurological disorders and significant disabilities who were independently residing in the community. However, subclinical gait deficiencies that occur with aging could result in increased step width variability. In our study we identified boundaries of optimal reference range of step width variability, and we provide the highest level of evidence that step width variability in older adults is higher than that of younger adults. However, our study cannot claim that this optimal reference range of step width variability can discriminate fallers and non-fallers in older adults. Future research should investigate this question and endeavor to investigate whether fall risk among older adults could be reduced by decreasing the excessive step width variability. This would set step width variability as a robust and sensitive marker to be targeted for intervention to ameliorate age-related deterioration in lateral stability during walking.
However, our results should be considered in lieu of certain limitations. A key requirement for maximizing the likelihood to detect a true difference between younger and older adults is to perform a power analysis beforehand. Any lack of accuracy or reliability of the step width variability measurement can reduce the likelihood of detecting a true difference (study power). For example, it has been suggested that for treadmill walking, an accurate measure of step width variability can be achieved with at least 400 steps (i.e., about 10 min treadmill walking) [71, 72]. Of the seven included studies that used treadmill, only three had a 10 min walking protocol. Similarly, it has been showed that reliability (minimum detectable change) of step width variability during overground walking improves with an increase in sample size (i.e., number of steps) [39, 73]. Thus, longer evaluations of step width variability during overground and treadmill walking are necessary to obtain accurate and reliable measurement of step width variability. Nevertheless, this may impose an unnecessary burden for older adults due to physical limitations and it can introduce confounding factors like fatigue [74]. Another drawback is the difficulty to measure step width variability on overground walking over long straight distances. To overcome this drawback repeated trials of consecutive steps can be measured during overground walking. Repeated short duration measurements of continuous overground walking protocols are preferred because are more reliable than repeated single walking protocols [46]. Of the six overground studies, five studies used repeated single walking protocols and only one study continuous walking protocol (Table 2). Regardless of such limitations, we still support that a meta-analysis is the best level of evidence providing the least-biased estimate. An additional limitation of our meta-analysis is that the probabilistic approach we used to estimate threshold levels of step width variability, was based on group data rather than on individual data. Further research to explore any loss in information in our meta-analytic approach is necessary.
Conclusions
In summary, older adults walk with higher step width variability than younger adults. Older adults who walk with step width variability values above the upper threshold level of 2.50 cm, could be characterized as having excessive step width variability. This information could potentially impact rehabilitation technology design for devices targeting lateral stability during walking.
Supplementary information
Additional file 3. Detailed flow diagram of literature search and selection of studies.
Additional file 4. Heterogeneity analyses.
Acknowledgments
The authors would like to thank Dr. Studenski, Professor Emeritus at the University of Pittsburgh, for the constructive feedback of the manuscript.
Abbreviations
- ACC
Accuracy
- AUC
Area under curve
- CCR
Cutoff correctly classified
- GOSH
Graphical display of study heterogeneity
- Hi
High
- Lo
Low
- NIR
No information rate
- NPV
Negative predictive value
- PICO
Population Intervention Comparison Outcome
- PPV
Positive predictive value
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- ROC
Receiver-operating characteristic
- Se
Sensitivity
- SMD
Standardized mean differences
- Sp
Specificity
- SWV
Step width variability
Authors’ contributions
AS: Study concept and design, acquisition of data, data analysis, interpretation of data, drafting first version of manuscript. EM: Acquisition of data, data analysis, revision of manuscript for intellectual content. HS: Design, revision of manuscript for intellectual content. KS: Design, revision of manuscript for intellectual content. NS: Study concept and design, interpretation of data, critical revision of manuscript for intellectual content. All authors approved the definitive version of the manuscript.
Funding
This work was supported by the National Institutes of Health [COBRE-NIH/NIGMS P20GM109090, NIH/NICHD R15HD08682] and the University of Nebraska Collaboration Initiative. The sponsors were not involved in the design of this study, and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its additional file).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12984-020-00671-9.
References
- 1.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Bruijn SM, van Dieën JH. Control of human gait stability through foot placement. J R Soc Interface. 2018;15:20170816. doi: 10.1098/rsif.2017.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/S0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 4.Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther. 2010;90:157–174. doi: 10.2522/ptj.20090125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- 6.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol Ser A Biol Med Sci. 2007;62:983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Pai Y-C. Can stability really predict an impending slip-related fall among older adults? J Biomech. 2014;47:3876–3881. doi: 10.1016/j.jbiomech.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PRISMA-P Group. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package For The Guide “Doing Meta-Analysis in R” [Internet]. 2019 [cited 2019 Nov 2]. Available from: http://dmetar.protectlab.org.
- 18.R Core Team. R: a language and environment for statistical computing., [internet]. Vienna, Austria.: R Foundation for statistical Computing; 2018. Available from: http://www.r-project.org.
- 19.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Cham: Springer International Publishing; 2015. [Google Scholar]
- 20.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft [Internet]. 2010 [cited 2019 Nov 4];36. Available from: http://www.jstatsoft.org/v36/i03/.
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Jacob. Statistical Power Analysis for the Behavioral Sciences. 1977. The Concepts of Power Analysis; pp. 1–17. [Google Scholar]
- 23.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 24.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidik K, Jonkman JN. A comparison of heterogeneity variance estimators in combining results of studies. Stat Med. 2007;26:1964–1981. doi: 10.1002/sim.2688. [DOI] [PubMed] [Google Scholar]
- 26.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 27.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 30.Harrer M, Cuijpers P, Furukawa T, Ebert DD. Doing Meta-Analysis in R: A Hand-on Guide. [Internet]. Available from: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/.
- 31.Olkin I, Dahabreh IJ, Trikalinos TA. GOSH – a graphical display of study heterogeneity. Res Synth Methods. 2012;3:214–223. doi: 10.1002/jrsm.1053. [DOI] [PubMed] [Google Scholar]
- 32.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 33.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosmer DW, Lemeshow S. Applied logistic regression. 3. Hoboken: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 37.Landsheer JA. Interval of uncertainty: an alternative approach for the determination of decision thresholds, with an illustrative application for the prediction of prostate cancer. PLoS One. 2016;11:e0166007. doi: 10.1371/journal.pone.0166007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lövdén M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and working-memory load in aging: a dual-process account relating cognitive control to motor control performance. J Gerontol: Ser B. 2008;63:P121–P128. doi: 10.1093/geronb/63.3.P121. [DOI] [PubMed] [Google Scholar]
- 39.Almarwani M, Perera S, VanSwearingen JM, Sparto PJ, Brach JS. The test–retest reliability and minimal detectable change of spatial and temporal gait variability during usual over-ground walking for younger and older adults. Gait Posture. 2016;44:94–99. doi: 10.1016/j.gaitpost.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almarwani M, VanSwearingen JM, Perera S, Sparto PJ, Brach JS. Challenging the motor control of walking: gait variability during slower and faster pace walking conditions in younger and older adults. Arch Gerontol Geriatr. 2016;66:54–61. doi: 10.1016/j.archger.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Decker LM, Cignetti F, Hunt N, Potter JF, Stergiou N, Studenski SA. Effects of aging on the relationship between cognitive demand and step variability during dual-task walking. AGE. 2016;38:363–375. doi: 10.1007/s11357-016-9941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ihlen EAF, Sletvold O, Goihl T, Wik PB, Vereijken B, Helbostad J. Older adults have unstable gait kinematics during weight transfer. J Biomech. 2012;45:1559–1565. doi: 10.1016/j.jbiomech.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait Posture. 2008;27:572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Marigold DS, Patla AE. Age-related changes in gait for multi-surface terrain. Gait Posture. 2008;27:689–696. doi: 10.1016/j.gaitpost.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait Posture. 2004;20:26–29. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 46.Paterson KL, Lythgo ND, Hill KD. Gait variability in younger and older adult women is altered by overground walking protocol. Age Ageing. 2009;38:745–748. doi: 10.1093/ageing/afp159. [DOI] [PubMed] [Google Scholar]
- 47.Thies SB, Richardson JK, Ashton-Miller JA. Effects of surface irregularity and lighting on step variability during gait. Gait Posture. 2005;22:26–31. doi: 10.1016/j.gaitpost.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Woledge RC, Birtles DB, Newham DJ. The variable component of lateral body sway during walking in young and older humans. J Gerontol Ser A Biol Med Sci. 2005;60:1463–1468. doi: 10.1093/gerona/60.11.1463. [DOI] [PubMed] [Google Scholar]
- 49.Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol. 2010;213:2655–2664. doi: 10.1242/jeb.042572. [DOI] [PubMed] [Google Scholar]
- 50.Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 51.Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robotics Res. 1999;18:917–930. doi: 10.1177/02783649922066655. [DOI] [Google Scholar]
- 52.Roos PE, Dingwell JB. Influence of simulated neuromuscular noise on movement variability and fall risk in a 3D dynamic walking model. J Biomech. 2010;43:2929–2935. doi: 10.1016/j.jbiomech.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. 2009;102:1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait Posture. 2012;36:102–107. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brauer SG, Burns YR, Galley P. A prospective study of laboratory and clinical measures of postural stability to predict community-dwelling fallers. J Gerontol A Biol Sci Med Sci. 2000;55:M469–M476. doi: 10.1093/gerona/55.8.M469. [DOI] [PubMed] [Google Scholar]
- 56.Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009;33:271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Paraskevoudi N, Balci F, Vatakis A. “Walking” through the sensory, cognitive, and temporal degradations of healthy aging. Ann N Y Acad Sci. 2018;1426:72–92. doi: 10.1111/nyas.13734. [DOI] [PubMed] [Google Scholar]
- 58.Franz JR, Francis CA, Allen MS, O’Connor SM, Thelen DG. Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Hum Mov Sci. 2015;40:381–392. doi: 10.1016/j.humov.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis CA, Franz JR, O’Connor SM, Thelen DG. Gait variability in healthy old adults is more affected by a visual perturbation than by a cognitive or narrow step placement demand. Gait Posture. 2015;42:380–385. doi: 10.1016/j.gaitpost.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clin Biomech. 2003;18:435–443. doi: 10.1016/S0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 61.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Grabiner P, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82:31–35. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- 63.Moe-Nilssen R, Helbostad JL. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait Posture. 2005;21:164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Hamacher D, Singh NB, Van Dieen JH, Heller MO, Taylor WR. Kinematic measures for assessing gait stability in elderly individuals: a systematic review. J R Soc Interface. 2011;8:1682–1698. doi: 10.1098/rsif.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society Clinical Practice Guideline for Prevention of Falls in Older Persons: AGS/BGS clinical practice guideline for prevention of falls. Journal of the American Geriatrics Society. 2011;59:148–57. [DOI] [PubMed]
- 66.Wang R-Y, Wang Y-L, Cheng F-Y, Chao Y-H, Chen C-L, Yang Y-R. Effects of combined exercise on gait variability in community-dwelling older adults. AGE. 2015;37:40. doi: 10.1007/s11357-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciprandi D, Bertozzi F, Zago M, Ferreira CLP, Boari G, Sforza C, et al. Study of the association between gait variability and physical activity. Eur Rev Aging Phys Act. 2017;14:19. doi: 10.1186/s11556-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helbostad JL, Moe-Nilssen R. The effect of gait speed on lateral balance control during walking in healthy elderly. Gait Posture. 2003;18:27–36. doi: 10.1016/S0966-6362(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 69.Herssens N, Verbecque E, Hallemans A, Vereeck L, Van Rompaey V, Saeys W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture. 2018;64:181–190. doi: 10.1016/j.gaitpost.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 71.Owings TM, Grabiner MD. Measuring step kinematic variability on an instrumented treadmill: how many steps are enough? J Biomech. 2003;36:1215–1218. doi: 10.1016/S0021-9290(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 72.Desmet DM, Sawers A, Grabiner MD. Ensuring accurate estimates of step width variability during treadmill walking requires more than 400 consecutive steps. J Biomech. 2019;91:160–163. doi: 10.1016/j.jbiomech.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagano H, James L, Sparrow WA, Begg RK. Effects of walking-induced fatigue on gait function and tripping risks in older adults. J Neuroeng Rehabil. 2014;11:155. doi: 10.1186/1743-0003-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3. Detailed flow diagram of literature search and selection of studies.
Additional file 4. Heterogeneity analyses.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article (and its additional file).