Abstract

Glycoside hydrolase family 31 (GH31) enzymes show both highly conserved folds and catalytic residues. Yet different members of GH31 show very different substrate specificities, and it is not obvious how these specificities arise from the protein sequences. The fungal α-xylosidase, AxlA, was originally isolated from a commercial enzyme mixture secreted by Aspergillus niger and was reported to have potential as a catalytic component in biomass deconstruction in the biofuel industry. We report here the crystal structure of AxlA in complex with its catalytic product, a hydrolyzed xyloglucan oligosaccharide. On the basis of our new structure, we provide the structural basis for AxlA’s role in xyloglucan utilization and, more importantly, a new procedure to predict and differentiate C5 vs C6 sugar specific activities based on protein sequences of the functionally diverse GH31 family enzymes.
Keywords: α-Xylosidase, Crystal structure, Aspergillus niger, Biomass deconstruction, GH31, Specificity
Short abstract
This work provides the structural and bioinformatics bases for α-xylosidase’s role in xyloglucan utilization and substrate specificity determinants in GH31 family glycosidases.
Introduction
The past two decades have witnessed a boost in lignocellulose-based bioenergy research led by US DOE Bioenergy Research Centers, which proposes to take advantage of the use of both renewable waste biomass (such as corn stover and paper waste) and energy crops grown on marginal lands (such as switchgrass).1−3 These missions are aimed at cost-effective production of renewable bioenergy as a sustainable solution to the world’s unmet energy needs rather than sole reliance on fossil fuels. These “green” efforts owe thanks to the rising awareness of the worldwide environmental impact of fossil fuels as well as economic concerns about energy security. In addition to the bioengineering of both plant feedstock and microbial workhorses, enzyme discovery for biomass deconstruction also remains a major research focus.3,4 Enzyme based biomass deconstruction represents a “green” approach to the alternative harsh chemical pretreatment of biomass as an initial step before conversion into renewable fuels, such as bioethanol and biodiesel, and other value-added products.4−6
In the current study, we present a crystal structure of a recombinant Aspergillus niger α-xylosidase AxlA in complex with its catalytic product, a hydrolyzed xyloglucan oligosaccharide. AxlA was originally isolated from a commercial enzyme mixture secreted by Aspergillus niger.7 AxlA can effectively release terminal xylose from xyloglucan, a major plant hemicellulose.7,8 It belongs to the GH31 family of glycoside hydrolases and catalyzes the hydrolysis of an α1,6-linked xyloside via a two-step retaining mechanism (classification according to CAZy database, www.cazy.org).9 Optimized ratios in the combinations of AxlA and several other glycosidases have led to significant improvements in yield and efficiency for the total deconstruction of xyloglucan to C6 and C5 fermentable sugars.8
Either native or engineered microbial GH31 family enzymes have also proved useful for oligosaccharide synthesis due to their transglycosylation activity.12,13 Some newly characterized GH31 enzymes were shown to act on uncommon substrates like sulfoquinovosyl diacylglyceride sulfolipids as for E. coli YihQ14 or produce cyclic oligosaccharides such as cycloalternan by Listeria monocytogenes Lmo2446.15 The human genome also encodes multiple GH31 enzymes with varied physiological localizations and functions, such as lysosomal α-glucosidase A (Uniprot ID: P10253), α-glucosidase II (Q14697), intestinal maltase-glucoamylase (Q43451), intestinal sucrose-isomaltase (P14410), myogenesis-regulating glycosidase (Q6NSJ0), and neutral α-glucosidase C (Q8TET4) according to the CAZy database.9
The α-xylosidase activity of AxlA confers great potential to green chemistry: it not only facilitates biomass deconstruction for renewable biofuel production7,8 but also specifically releases d-xylose, a precursor for various bioactive compounds useful to humans.10,11 For example, xylose derived xylitol is used as a food additive for dental protection and as a low-calorie sweetener.10 C-xyloside (as opposed to O-glycoside) series skincare products developed by L’Oreal have shown protective properties for human skin.11 In this work, we elucidate the structural determinants of α-xylosidase activity versus the activities of other GH31 family α-glucosidases. The mechanistic insights gained from the structure–function relationship could inspire design and make use of the multifaceted GH31 family enzymes in effective and ecofriendly bioconversion applications.
Experimental Procedures
Materials and Reagents
Xyloglucan oligosaccharide, XFG heptasaccharide with purity >80%, was purchased from Elicityl (Crolles, France). XFG (see Abbreviations for nomenclature details) was chosen for subsequent crystal soaking experiments for the following reasons: First, it represents the complex sugar components of natural xyloglucan to help reveal different sugar binding sites in the XFG bound enzyme structure. Second, its relatively low degree of polymerization (DP7) may allow successful diffusion into the enzyme active site in the crystalline state to form a complex. d-xylose was purchased from Sigma (U.S.A.). Polyethylene glycol 20000 as a 30% (w/v) stock solution as well as crystallization screens Index HT, PEGRx HT, Crystal Screen HT, and SaltRx HT were obtained from Hampton Research (Aliso Viejo, CA, U.S.A.). Morpheus and MIDAS screens were from Molecular Dimensions (Altamonte Springs, FL, U.S.A.). All other chemicals and reagents used for crystal growth were purchased from Sigma (U.S.A.) or Fisher Scientific (U.S.A.) and were used without further purification.
Cloning, Expression, and Purification of AxlA
AxlA (Uniprot ID: G3XMN9) was expressed and purified according to previous procedures.7,8 Briefly, a cDNA corresponding to Aspni5|43342 (Department of Energy Joint Genome Institute numbering) was synthesized by GeneArt (Invitrogen, U.S.A.) with the addition of restriction sites for PmlI (5′ end) and XbaI (3′ end) and cloned into pPICZB (Invitrogen, U.S.A.). Transformed Pichia pastoris strain X-33 (Invitrogen, U.S.A.) was grown at 30 °C for 4 days. AxlA expression was induced by adding 0.5% v/v methanol and 1% Casamino acids (Difco Laboratories, U.S.A.) every 24 h starting at time zero, the latter of which enhanced yield and stability of AxlA. Secretion was driven by the native signal peptide of AxlA. The secreted AxlA was then concentrated, desalted, and purified by cation exchange HPLC chromatography (TSK-Gel SP-5PW, Tosoh Bioscience, U.S.A.).
Bioinformatics Analysis
The SignalP 4.0 server16 was used to predict the potential N-terminal native signal peptide MYFSSFLALGALVQAAAA of AxlA to be cleaved upon secretion by P. pastoris. The cleaved protein contains an N-terminal Thr residue and total of 718 residues with a molecular weight of around 80.8 kDa (not considering glycosylation). NetNGlyc 1.0 Server17 was used to predict nine potential N-glycosylation sites on Asn residues at positions 6, 261, 314, 358, 369, 453, 637, 658, and 683 (here, residues were numbered for the mature protein without the signal peptide). Protein interfaces and quaternary assemblies in the structures were analyzed by PDBePISA18 and EPPIC.19 Protein sequence alignment and phylogenetic analysis were performed by NCBI COBALT server20 using default parameter settings.
Crystallization, Diffraction Data Collection, and Structure Determination
Because proteins expressed in P. pastoris can possess excessive N-glycosylation with high-mannose saccharides,7,21 we first treated AxlA with various glycosidases prior to crystallization trials. These included Endo H (500000 units/ml), peptide N-glycosidase F (500000 units/ml), and α1–2,3 mannosidase (32000 units/ml), all from New England Biolabs, Ipswich, MA, U.S.A. with manufacture listed specific activity in parentheses. Deglycosylation reactions contained 10 volumes of AxlA, 1 volume of glycosidases, and 1 volume of 10X reaction buffer provided by the manufacturer. The reactions were incubated at room temperature (22 ± 2 °C) for 24–48 h. Initial crystallization trials were set up by a Mosquito Crystal nanoliter dispenser robot (TTP Labtech, Melbourn, UK) on MRC 2 Well crystallization plates (Hampton Research; Aliso Viejo, CA, U.S.A.) using sitting drop vapor diffusion method testing against all 6 commercial high-throughput screens listed in the Materials and Reagents section each with 96 conditions. Briefly, 200 nl of reservoir solution was laid on top of 200 nL of AxlA stock solution (15 mg mL–1 supplemented with 10 mM d-xylose) and the mixture was equilibrated against the reservoir solution at 20 °C. Neither the native nor the treated AxlA crystallized under these conditions. However, Endo H pretreatment led to precipitation in the crystallization experiments and in the protein stock solution indicating decreased solubility due to potential deglycosylation of AxlA.29 Subsequent optimization of the Endo H pretreatment at various pH and temperature values was performed for crystallization trials. In particular, Endo H pretreatment at 50 mM in Tris pH 7 and 4 °C overnight did not show precipitation of protein stock solution and led to initial crystal hit in the PEGRx HT screen at the H9 condition (5% v/v 2-propanol, 0.1 M citric acid pH 3.5, 6% w/v polyethylene glycol 20000). The crystal appeared as near cubic shape with the longest dimension of ∼70 μm after growing for 2 days. Further optimization of crystallization conditions was performed at varied pH and precipitant concentrations. Prior to harvesting, a subset of crystals was soaked with xyloglucan oligosaccharide XFG for 1–5 min. Crystals were either directly flash-frozen in liquid nitrogen or cryoprotected by transferring into MiTeGen’s LV CryoOil (MiTeGen, Ithaca, NY) before being frozen.
The best diffracting crystal showed diffraction to 2.7 Å resolution and was collected at the Argonne National Laboratory on the LS-CAT 21-ID-G beamline from a single crystal (grown at the modified condition 2% v/v 2-propanol, 0.1 M citric acid pH 3.5, 3% w/v polyethylene glycol 20000, soaked with xyloglucan derived oligosaccharide XFG for 2 min, cryoprotected with oil) using a X-ray wavelength of 0.97856 Å. The data were indexed, integrated, and scaled using XDS.22 The initial phase problem was solved by molecular replacement using AutoMR (Phaser)23 using Cellvibrio japonicus GH31 α-glucosidase as the search model (PDB code 4B9Y).12 Autobuild programs of the PHENIX suite24 were used to partially complete the polypeptide coordinates without including ligands or water. Two copies of the protein were found in the asymmetric unit displaying an overall noncrystallographic pseudo-C2 symmetry. The phases were improved, and the structure was completed with alternating rounds of manual model building with COOT25 and refinement with PHENIX using default noncrystallographic symmetry restraints. Water molecules were added and updated during refinement. For relatively poor electron density regions, the loops were built based on traceable albeit weak backbone electron density and by relying on favorable side-chain rotamers and favorable geometry statistics after real-space refinement in COOT.25 N-Glycosylation GlcNAc and mannose residues as well as solvent molecules were also built in based on clear residual electron density in the 2mFo–dFc omit maps. Prior to final rounds of refinement, enzyme ligands were built into the structure based on clear difference density, revealing the hydrolysis product of an XFG molecule at each active site of the dimer and also one putative xyloglucan oligosaccharide fragment per asymmetric unit sandwiched between two aromatic residues mediating a crystallographic contact.
The final structures were refined to the same resolution limit as in data collection with favorable Rcryst and Rfree values (Table 1). Model quality was assessed using MolProbity.26 All structures in the figures were rendered using PyMOL.27 All the information on data collection and refinement statistics is summarized in Table 1, which includes the CC1/2 value28 as the resolution cutoff criteria. The final coordinate and structure factors were deposited in the Protein Data Bank (PDB) under the assess code 6DRU and held pending the completion of the CASP13 competition.35
Table 1. Statistics for X-ray Data Collection and Structural Refinement.
| Statistic | AxlA in complex with hydrolyzed XFG |
|---|---|
| Protein Data Bank code | 6DRU |
| Spacegroup | P32 2 1 |
| Cell dimensions | |
| a, b, c (Å) | 146.1, 146.1, 220.1 |
| α, β, γ (deg) | 90.0, 90.0, 120.0 |
| Wavelength (Å) | 0.97856 |
| Resolution of data collection (Å) | 47.83–2.7 (2.797–2.7) |
| No. of unique reflections | 75130 (7400) |
| Completeness % | 99.81 (98.97) |
| Redundancy | 22.1 (19.4) |
| Rsyma | 0.418 (5.55) |
| I/σb | 9.39 (0.62) |
| CC1/2c | 0.995 (0.315) |
| CC*c | 0.999 (0.692) |
| Resolution range in refinement (Å) | 47.83–2.7 (2.80–2.7) |
| No. of unique reflections (work/test) | 75006/1990 |
| Rworkd | 17.99 (36.37) |
| Rfreee | 21.18 (36.83) |
| Mean coordinate errorf (Å) | 0.47 |
| Rmsd bond length (Å) | 0.003 |
| Rmsd bond angles (deg) | 0.54 |
| Average B value (Å2) (overall/protein/waters/ligand) | 67.0/65.6/62.4/88.5 |
| No. of non-hydrogen atoms | 12553 |
| No. of protein atoms | 11442 |
| No. of waters | 293 |
| No. of ligand atoms | 818 |
| Ramachandran Statisticsg (%) | 95.9, 4.1, 0 |
Values in parentheses are for the highest resolution shell.
Rsym = ∑hkl ∑i |Ii(hkl) – ⟨I(hkl)⟩|/ ∑hkl ∑iIi(hkl), where Ii(hkl) is the intensity of an individual measurement of the symmetry related reflection and ⟨I(hkl)⟩ is the mean intensity of the symmetry related reflections.
I/σ is defined as the ratio of averaged value of the intensity to its standard deviation.
CC1/2 = percentage of correlation between intensities from random half-data sets. CC1/2 above 0.1 is considered significant.33 CC* = [2CC1/2/(1+ CC1/2)]1/2. CC* estimates the value of CCtrue. CC* (or CC1/2) is a robust, statistically informative quantity useful for defining the high-resolution cutoff of diffraction data to improve model quality.33
Rwork = ∑hkl ||Fobs| – |Fcalc||/ ∑hkl |Fobs|, where Fobs and Fcalc are the observed and calculated structure-factor amplitudes for the reflections being refined against.
Rfree was calculated as Rcryst using randomly selected small fractions (∼2.65%) of the unique reflections that were omitted from the structure refinement.
Mean coordinate error was calculated based on maximum likelihood.
Ramachandran statistics indicate the percentage of residues in the most favored, additionally allowed, and outlier regions of the Ramachandran diagram as defined by MolProbity.26
Results and Discussion
The overall structure of the Aspergillus niger Ax1A is similar to those of the other GH31 family hydrolases, a core (β/α)8-barrel with additional components, including two β-sandwich domains and some residual glycosylation. Additional details regarding the mechanism of action and the specificity of this enzyme are revealed by the presence of a hydrolyzed oligosaccharide in the active site of the enzyme.
Oligomeric State of AxlA
AxlA comprises two chains in the asymmetric unit of the unit cell of the crystal with pseudo-C2 noncrystallographic symmetry (Figure 1A). This 2-fold interaction was predicted to be stable by the PDBePISA server18 based on calculations of interface area of ∼2358 Å2 per dimer (Figure 1B) and a positive dissociation free energy of 21.1 kcal/mol.
Figure 1.
Tetrameric structure of AxlA. (A) Two half-tetramers are related by crystal symmetry. Each monomeric subunit (A, B, A′, B′) is color-coded individually. Steric surface is rendered transparently, and secondary structures are shown as ribbon cartoons, the experimentally observed post-translational N-glycans (white) and bioinformatically predicted glycosylation site Asn residues (yellow, with residue numbers labeled for subunit B) are shown as spheres. The active site ligands (hydrolyzed xyloglucan oligosaccharides) are shown as black spheres. (B, C, and D) Tetrameric oligomerization interfaces between different subunits are represented by interfacial residues within 4.5 Å shown as spheres for the AB, AA′ and BB′ interfaces.
EPPIC19 is a biological assembly analysis program recommended by PDB to distinguish biological interfaces from artificial crystal contacts. It enumerates all possible symmetric assemblies with a prediction of the most likely assembly based on probabilistic scores from pairwise evolutionary scoring (sequence entropy signals).19 The EPPIC server19 predicted AxlA to be a homotetramer with 0.99 probability based on four additional interfaces between crystallographic symmetry-related dimers of either ∼1255 (Figure 1C) or ∼1082 Å2 (Figure 1D) forming a total interfacial area of ∼9390 Å2 in the tetrameric assembly. Thus, we conclude that the physiological state of Ax1A is most likely a tetramer based on both bioinformatic analysis and physical analysis of favorable interfacial interactions.
Post-Translational modifications of AxlA
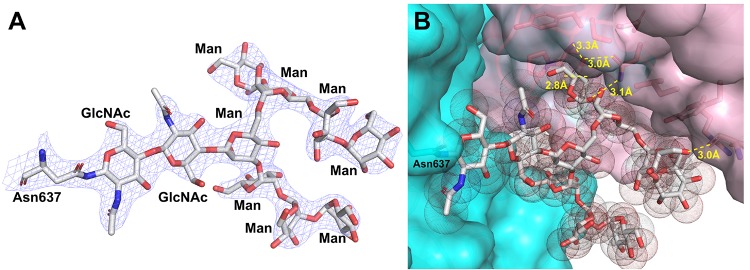
Seven out of the nine N-glycosylation sites of AxlA as predicted by the NetNGlyc 1.0 Server17 displayed glycosylation in the structure (Figure 1A). These include asparagine amino acids at positions 6, 261, 314, 358, 453, 637, and 683 but not 369 and 658 (residue numbers are based on the secreted form of AxlA with the N-terminal signal peptide sequence removed). The N-glycans on Asn358 and 637 both retained a chitobiose core and high mannose type substitutions, possibly protected from Endo H due to limited solvent exposure. At residues 6, 314, 453, and 683, only one GlcNAc is present, indicating a result of successful deglycosylation by Endo H treatment prior to setup of crystallization. Asn261 shows high mannose glycan in one chain but not the other, which possesses only a GlcNAc. The N-linked glycan on Asn637 contains a complex branched structure of GlcNAcβ1-4GlcNAcβ1-4[Manα1-3(Manα1-2Manα1-6)Manα1-6]Manα1-3Manα1-2Manα1-2Man (Figure 2A). We have previously reported a similar effect of Endo H treatment on a glycosylated GH29 family α-fucosidase secreted by the same Pichia eukaryotic expression system.29 Asn369 is not glycosylated and is relatively more buried with its side-chain amide group forming favorable interactions with two nearby main-chain amide groups. This is consistent with a warning by the NetNGlyc 1.0 server17 on the accuracy of the prediction of Asn369 glycosylation because of a preceding Pro residue in the sequence. There is very little electron density in our experimental maps to convincingly suggest N-glycosylation on Asn658 despite bioinformatics predictions.
Figure 2.
Representative N-glycan structure as seen on Asn637 of AxlA. (A) Unbiased 2mFo-dFc omit electron density map contoured at the 1.5 σ level (blue meshes) around the sugars and Asn637 (sticks, carbon in white, oxygen in red, and nitrogen in blue). (B) The shape complementarity and polar interactions between the N-glycan and the interfacial pocket between adjacent subunits of the dimer in the same asymmetric unit. The two subunits surfaces are in cyan and pink, respectively. The N-glycan are shown as both sticks and dotted vander Waals spheres by PyMOL.27 The hydrogen-bonding interactions (yellow dashes) and corresponding distances (in Å unit) between the N-glycan on one subunit and the protein residues on the adjacent subunit are labeled.
It should be noticed that the partially deglycosylated recombinant AxlA in the crystal structure has an average molecular weight per monomer (∼85.4 kDa considering both protein and N-glycan contents) very similar to that reported for its native form from A. niger (∼85 kDa).7 This suggests that the crystallographically observed N-glycan modifications and tetrameric assembly of AxlA may also be preserved in the native form. However, in the absence of deglycosylation treatment, recombinant AxlA heterologously expressed in P. pastoris was reported to have a much higher average molecular weight per monomer (∼110 kDa) than the native form from A. niger despite their very similar kinetic properties in catalyzing the hydrolysis of small α-xyloside substrates.7 This suggests a much higher extent of glycosylation of AxlA expressed in P. pastoris than its native host A. niger.
While the function of glycans are not known, several scenarios are possible such as the enhancment of solubility or stability of the protein. A support of solubility function comes from the observation of decreased solubility of the AxlA protein upon deglycosylation treatment as described in the Experimental Procedures section. One argument in support of stability function is that the Asn637 linked glycan locates at the dimer interface and possibly stabilizes dimerization via hydrogen-bonding and van der Waals interactions because of its proximity and its shape complementarity between the glycan and the dimer crevice (Figure 2B). This observation suggests that glycan modifications can be a useful strategy for protein design and engineering.21
Structure Determinants of Substrate Specificity
We observed a reaction product at the active sites of both AxlA subunits in the crystal asymmetric unit from the hydrolysis of an added xyloglucan oligosaccharide substrate XFG (Figure 3A–C). In particular, the α1–6 glycosyl bond between the terminal xylose at −1 site and glucose at +1 site is cleaved to yield a free d-xylose. We were able to trace the experimental electron density for all the sugar residues of the XFG except for the +2 site branched fucose (Fuc) residue (Figure 3A–C) possibly due to disorder of the fucose in exposure to the bulk solvent. AxlA forms multiple polar and van der Waals interactions with the reaction product. All the hydroxyl groups of −1 site xylose and nearly all for the +1 site glucose interact with one or more hydrogen-bonding partners from the protein active site (Figure 3A). The protein–ligand interactions at +2 site, + 3 site and +2 branch sites appear to be dominated by van der Waals interactions and shape complementarity. The current product bound complex supports the specific role of AxlA in xyloglucan biomass deconstruction7,8 and provides a structural basis for further optimization of its enzyme activity in the renewable biofuel industry.
Figure 3.
Reaction product complex structure of AxlA and proposed catalytic mechanism. (A) The hydrolyzed XFG heptasaccharide catalytic product is shown as sticks (carbon in white) with the corresponding difference omit map contoured at 3.5 σ. The active site residue side chains within 4 Å of the hydrolyzed oligosaccharide are shown as sticks (carbon in cyan) with corresponding 2mFo-dFc map contoured at 2 σ. The hydrogen-bonding interactions between the ligand and active site residues are indicated as black dashes. The conserved nucleophile D395 and general acid D487 aspartate residues and catalytic labile C1 of xylose at −1 site are also labeled. The branched xyloglucan oligosaccharide binding site is connected to a surface pocket of the adjacent subunit (pink), although with no apparent direct interactions with the ligand. (B) shown the same way as part A but in the other active site of the dimer in the asymmetric unit. (C) chemical structures of XFG and d-xylose (atom number labeled). XFG is named according to an existing nomenclature for xyloglucan-derived oligosaccharide (see Abbreviations).34 (D) Proposed two-step double displacement catalytic mechanism of AxlA leads to conformational retention at the catalytic labile C1 position between the substrate and product.
The conserved nucleophile Asp395 (D395) and general acid Asp487 (D487) residues are located at the opposite sides of the catalytic labile C1 position of xylose. The averaged distances between the carboxylic oxygen atoms of D395 and D487 are 6.38 and 6.55 Å for each active site of the dimer, respectively. These values are consistent with a retaining catalytic mechanism involving double displacement steps (Figure 3D), as seen in other GH31 enzymes.9,30,31
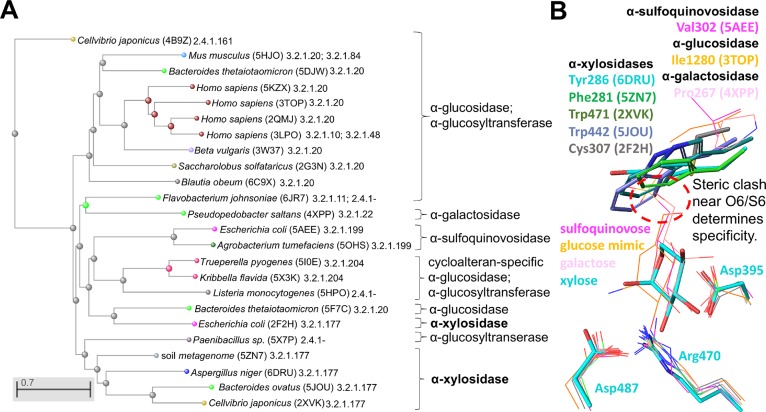
GH31 family α-glycosidases comprise a variety of enzyme activities with highly conserved catalytic residues conferring catalytic activity in the structurally conserved core (β/α)8-barrel domain. In addition, the sequence identity ranges from 21%–33% between AxlA and other GH31 members (α-xylosidases, a majority of α-glucosidases, α-quinovosidases, and α-galactosidases, etc.) with no clear identity cutoff value to specify a particular type of substrate specificity. Thus, the overall protein structure and pairwise sequence identity are relatively poor predictors of α-xylosidase activity. However, we differentiated α-xylosidases from other GH31 enzymes based on sequence phylogenetic analysis of all the structurally characterized GH31 enzymes in the CAZy9 databases (Figure 4A). The only exception is an E. coli α-xylosidase (PDB code 2F2H) that appears to be clustered with the α-glucosidases, α-glucosyltransferase, and α-quinovosidase GH31 family members.
Figure 4.
A procedure to determine substrate specificities of GH31 family glycosidases. (A) Protein sequence based phylogenetic analysis allows clustering of specific carbohydrate hydrolytic activities among structurally characterized and functionally annotated GH31 family members in CAZy database.9 This can serve as a basis for the prediction of functionally unknown GH31 family sequences. (B) The simplified view of active site structures aligned based on highly conserved residues (nucleophile Asp395, general acid Asp487 and Arg470 in AxlA). The carbon atoms of aligned structures are individually color coded. Tyr286 of AxlA and spatially equivalent residues in homologues are shown as sticks with their corresponding PDB codes and substrate specificities indicated. The steric clash between bulky aromatic residues at Tyr286 equiv positions of α-xylosidases (shown as thicker sticks) and the O6/S6 containing substrates from the aligned α-glucosidase, α-glucosyltransferase, and α-quinovosidases (shown as thinner sticks in magenta, orange, and pink) is indicated by a red dashed circle.
We further identify a key molecular determinant of substrate specificity at the −1 site corresponding to Tyr286 of AxlA (PDB code 6DRU) despite the poor sequence conservation at this position among GH31 family enzymes. Almost all the structurally available GH31 α-xylosidases possess a bulky aromatic residue at the spatially equivalent position to Tyr286 again except for the E. coli α-xylosidase with Cys307 (PDB code 2F2H) compared to relatively smaller residues at this site in α-glucosidase, α-quinovosidase, and α-galactosidase (Figure 4B). These bulky aromatic side chains necessarily form steric clash with C6 hydroxyl groups of glucose, galactose and the C6 sulfo group of sulfoquinovose but not xylose. This is because xylose is a “C5 sugar” which lacks C6 and hence the corresponding O6/S6 groups that are present in the “C6 sugars”. In addition, the exact spatial orientation and proximity of the side chain to −1 site sugar also appear to play a role in the discrimination of substrates. For example, the relatively large sulfur atom of Cys307 is located closer to the O6 equiv position at the −1 site to cause potential steric clash, although perhaps to a lesser extent. Consistent with this molecular basis, the E. coli α-xylosidase (PDB code 2F2H) was reported to show minimal but not completely abolished α-glucosidase activity compared to its much higher α-xylosidase activity.31 Interestingly, among the limited number of structurally characterized GH31 enzymes, both Bacteroides ovatus and Cellvibrio japonicus GH31 α-xylosidases (PDB codes 5JOU and 2XVK) were also reported to act on xyloglucan derived substrates.32,33 The functional diversity of the GH31 enzymes suggests evolutionary robustness of their conserved fold to be tailored for different carbohydrate chemistry to gain specific fitness advantages.
Conclusions
In summary, we determined the structure of a GH31 family α-xylosidase AxlA from an industrially relevant fungus, Aspergillus niger, in a form with its bound catalytic product. Both the active site shape complementarity and polar interactions with the ligand support the role of AxlA in xyloglucan utilization with application potential in the renewable biofuel industry. We identified useful functional predictors on the basis of phylogenetic and active site residue analyses, and also suggested that the overall protein fold and pairwise sequence identity are poor functional predictors of substrate specificity of GH31 family enzymes.
Acknowledgments
We thank the staff at the LS-CAT beamline for help and advice on data collection. Use of the Advanced Photon Source was supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-+06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corp. and Michigan Technology Tri-Corridor under Grant 085P1000817. We acknowledge U.S. Department of Energy, DOE Great Lakes Bioenergy Research Center, DOE Office of Science, for Grant No. BER DE-FC02-07ER64494 (to GNP and JDW) and National Institutes of Health for Grant No. U01 GM098248 (to GNP).
Glossary
Abbreviations
- AxlA
α-xylosidase of Aspergillus niger
- PDB
Protein Data Bank
- P. pastoris
Pichia pastoris
- GH
glycoside hydrolase
- Xyl
d-xylose
- Glc
d-glucose
- Gal
d-galactose
- Fuc
l-fucose
- GlcNAc
N-acetyl-d-glucosamine
- Man
d-mannose. XFG is short for a xyloglucan oligosaccharide comprising a backbone of β1,4-linked d-glucoses modified by additional branched sugar units, where X is for Xylα1-6Glc, L is for Galβ1-2Xylα1-6Glc, F is for Fucα1-2Galβ1-2Xylα1-6Glc, and G is for Glc, according to an existing nomenclature.34
The authors declare no competing financial interest.
Dedication
$ In memory of pioneer Dr. Jonathan Walton, deceased Oct 18, 2018.
References
- Gilna P.; Lynd L. R.; Mohnen D.; Davis M. F.; Davison B. H. Progress in understanding and overcoming biomass recalcitrance: a BioEnergy Science Center (BESC) perspective. Biotechnol. Biofuels 2017, 10, 285. 10.1186/s13068-017-0971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. DOE . Lignocellulosic Biomass for Advanced Biofuels and Bioproducts: Workshop Report, DOE/SC-0170. U.S. Department of Energy Office of Science. 2015, https://genomicscience.energy.gov/biofuels/lignocellulose/.
- Dale B. E.; Holtzapple M. The Need for Biofuels. Chem. Eng. Prog. 2015, 111 (3), 36–40. [Google Scholar]; (SBE Special Section: Lignocellulosic Biofuels, 32-page supplement)
- Himmel M. E.; Ding S. Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Meng X.; Sun Q.; Kosa M.; Huang F.; Pu Y.; Ragauskas A. J. Physicochemical Structural Changes of Poplar and Switchgrass during Biomass Pretreatment and Enzymatic Hydrolysis. ACS Sustainable Chem. Eng. 2016, 4, 4563–4572. 10.1021/acssuschemeng.6b00603. [DOI] [Google Scholar]
- Zabed H.; Sahu J. N.; Suely A.; Boyce A. N.; Faruq G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renewable Sustainable Energy Rev. 2017, 71, 475–501. 10.1016/j.rser.2016.12.076. [DOI] [Google Scholar]
- Scott-Craig J. S.; Borrusch M. S.; Banerjee G.; Harvey C. M.; Walton J. D. Biochemical and molecular characterization of secreted α-Xylosidase from Aspergillus niger. J. Biol. Chem. 2011, 286, 42848–42854. 10.1074/jbc.M111.307397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour D.; Borrusch M. S.; Banerjee G.; Walton J. D. Enhancement of fermentable sugar yields by α-xylosidase supplementation of commercial cellulases. Biotechnol. Biofuels 2013, 6, 58. 10.1186/1754-6834-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard V.; Ramulu G.; Drula E.; Coutinho P. M.; Henrissat B. The Carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L. V.; Goli J. K.; Gentela J.; Koti S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. 10.1016/j.biortech.2016.04.092. [DOI] [PubMed] [Google Scholar]
- Deloche C.; Minondo A. M.; Bernard B. A.; Bernerd F.; Salas F.; Garnier J.; Tancrede E. Effect of C-xyloside on morphogenesis of the dermal epidermal junction in aged female skin. An ultrastuctural pilot study. Eur. J. Dermatol. 2011, 21, 191–196. 10.1684/ejd.2010.1225. [DOI] [PubMed] [Google Scholar]
- Larsbrink J.; Izumi A.; Hemsworth G. R.; Davies G. J.; Brumer H. Structural enzymology of Cellvibrio japonicus Agd31B protein reveals α-transglucosylase activity in glycoside hydrolase family 31. J. Biol. Chem. 2012, 287 (43288), 43299. 10.1074/jbc.M112.416511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K.; Davis B. G. Creation of an α-mannosynthase from a broad glycosidase scaffold. Angew. Chem., Int. Ed. 2012, 51, 7449–7453. 10.1002/anie.201201081. [DOI] [PubMed] [Google Scholar]
- Speciale G.; Jin Y.; Davies G. J.; Williams S. J.; Goddard-Borger E. D. YihQ is a sulfoquinovosidase that cleaves sulfoquinovosyl diacylglyceride sulfolipids. Nat. Chem. Biol. 2016, 12, 215–217. 10.1038/nchembio.2023. [DOI] [PubMed] [Google Scholar]
- Light S. H.; Cahoon L. A.; Halavaty A. S.; Freitag N. E.; Anderson W. F. Structure to function of an α-glucan metabolic pathway that promotes Listeria monocytogenes pathogenesis. Nat. Microbiol. 2017, 2, 16202. 10.1038/nmicrobiol.2016.202. [DOI] [PubMed] [Google Scholar]
- Petersen T. N.; Brunak S.; von Heijne G.; Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Gupta R.; Jung E.; Brunak S.. Prediction of N-glycosylation sites in human proteins. 2004http://www.cbs.dtu.dk/services/NetNGlyc/.
- Krissinel E.; Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Bliven S.; Lafita A.; Parker A.; Capitani G.; Duarte J. M. Automated evaluation of quaternary structures from protein crystals. PLoS Comput. Biol. 2018, 14, e1006104. 10.1371/journal.pcbi.1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos J. S.; Agarwala R. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- Wildt S.; Gerngross T. U. The humanization of N-glycosylation pathways in yeast. Nat. Rev. Microbiol. 2005, 3, 119–128. 10.1038/nrmicro1087. [DOI] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66, 125–132. 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. J.; Grosse-Kunstleve R. W.; Adams P. D.; Winn M. D.; Storoni L. C.; Read R. J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. D.; Afonine P. V.; Bunkoczi G.; Chen V. B.; Davis I. W.; Echols N.; Headd J. J.; Hung L. W.; Kapral G. J.; Grosse-Kunstleve R. W.; McCoy A. J.; Moriarty N. W.; Oeffner R.; Read R. J.; Richardson D. C.; Richardson J. S.; Terwilliger T. C.; Zwart P. H. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66, 213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004, 60, 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Chen V. B.; Arendall W. B.; Headd J. J.; Keedy D. A.; Immormino R. M.; Kapral G. J.; Murray L. W.; Richardson J. S.; Richardson D. C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66, 12–21. 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano W.The PyMOL Molecular Graphics System, v2.0 (Schrödinger, LLC, 2000). https://www.pymol.org.
- Karplus P. A.; Diederichs K. Linking crystallographic model and data quality. Science 2012, 336, 1030–1033. 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H.; Walton J. D.; Brumm P.; Phillips G. N. Jr. Structure and substrate specificity of a eukaryotic fucosidase from Fusarium graminearum. J. Biol. Chem. 2014, 289, 25624–25638. 10.1074/jbc.M114.583286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J. D.; Withers S. G. Mechanisms of enzymatic glycoside hydrolysis. Curr. Opin. Struct. Biol. 1994, 4, 885–892. 10.1016/0959-440X(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Okuyama M.; Kaneko A.; Mori H.; Chiba S.; Kimura A. Structural elements to convert Escherichia coli alpha-xylosidase (YicI) into alpha-glucosidase. FEBS Lett. 2006, 580, 2707–2711. 10.1016/j.febslet.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Hemsworth G. R.; Thompson A. J.; Stepper J.; Sobala L. F.; Coyle T.; Larsbrink J.; Spadiut O.; Goddard-Borger E. D.; Stubbs K. A.; Brumer H.; Davies G. J. Structural dissection of a complex Bacteroides ovatus gene locus conferring xyloglucan metabolism in the human gut. Open Biol. 2016, 6, 160142. 10.1098/rsob.160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsbrink J.; Izumi A.; Ibatullin F. M.; Nakhai A.; Gilbert H. J.; Davies G. J.; Brumer H. Structural and enzymatic characterization of a glycoside hydrolase family 31 α-xylosidase from Cellvibrio japonicus involved in xyloglucan saccharification. Biochem. J. 2011, 436, 567–580. 10.1042/BJ20110299. [DOI] [PubMed] [Google Scholar]
- Fry S. C.; York W. S.; Albersheim P.; Darvill A.; Hayashi T.; Joseleau J. P.; Kato Y.; Lorences E. P.; Maclachlan G. A.; McNeil M.; Mort A. J.; Reid J. S. G.; Seitz H. U.; Selvendran R. R.; Voragen A. G. J.; White A. R. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol. Plant. 1993, 89, 1–3. 10.1111/j.1399-3054.1993.tb01778.x. [DOI] [Google Scholar]
- Lepore R.; Kryshtafovych A.; Alahuhta M.; Veraszto H. A.; Bomble Y. J.; Bufton J. C.; Bullock A. N.; Caba C.; Cao H.; Davies O. R.; Desfosses A.; Dunne M.; Fidelis K.; Goulding C. W.; Gurusaran M.; Gutsche I.; Harding C. J.; Hartmann M. D.; Hayes C. S.; Joachimiak A.; Leiman P. G.; Loppnau P.; Lovering A. L.; Lunin V. V.; Michalska K.; Mir-Sanchis I.; Mitra A.; Moult J.; Phillips Jr G. N.; Pinkas D. M.; Rice P. A.; Tong Y.; Topf M.; Walton J. D.; Schwede T.; et al. Target highlights in CASP13: Experimental target structures through the eyes of their authors. Proteins: Struct., Funct., Genet. 2019, 87, 1037–1057. 10.1002/prot.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]