Abstract
Mannheimia haemolytica serotype A2 is the principal cause of pneumonic mannheimiosis in ovine and caprine livestock; this disease is a consequence of immune suppression caused by stress and associated viruses and is responsible for significant economic losses in farm production worldwide. Gram-negative bacteria such as M. haemolytica produce outer membrane (OM)-derived spherical structures named outer membrane vesicles (OMVs) that contain leukotoxin and other biologically active virulence factors. In the present study, the relationship between M. haemolytica A2 and bovine lactoferrin (BLf) was studied. BLf is an 80 kDa glycoprotein that possesses bacteriostatic and bactericidal properties and is part of the mammalian innate immune system. Apo-BLf (iron-free) showed a bactericidal effect against M. haemolytica A2, with an observed minimal inhibitory concentration (MIC) of 16 µM. Sublethal doses (2–8 µM) of apo-BLf increased the release of OMVs, which were quantified by flow cytometry. Apo-BLf modified the normal structure of the OM and OMVs, as observed through transmission electron microscopy. Apo-BLf also induced lipopolysaccharide (LPS) release from bacteria, disrupting OM permeability and functionality, as measured by silver staining and SDS and polymyxin B cell permeability assays. Western blot results showed that apo-BLf increased the secretion of leukotoxin in M. haemolytica A2 culture supernatants, possibly through its iron-chelating activity. In contrast, holo-BLf (with iron) did not have this effect, possibly due to differences in the tertiary structure between these proteins. In summary, apo-BLf affected the levels of several M. haemolytica virulence factors and could be evaluated for use in animals as an adjuvant in the treatment of ovine mannheimiosis.
Introduction
Mannheimia haemolytica is a normal inhabitant of the nasal cavity and tonsil crypts of healthy ruminants. However, when animals suffer from shipping stress and/or an acute infection by Mycoplasma bovis (in cattle) or viruses (e.g., parainfluenza-3, adenovirus, and respiratory syncytial virus), they become immunocompromised. This condition leads to the rapid proliferation of M. haemolytica, which subsequently reaches the lungs and infects the alveolar epithelium. M. haemolytica serotype A2 causes acute pneumonic mannheimiosis in lambs, sheep and goats. Several studies have demonstrated the importance of mannheimiosis as a cause of mortality in these livestock species, as well as the negative effects on weight gain and a low efficiency in feed conversion in chronically affected sheep [1, 2]. In the USA, the occurrence of this disease in cattle (bovine respiratory disease, BRD) caused by serotype A1 results in productivity losses of 23.60 USD per sick calf and is a major cause of economic losses to farms [3]. The BRD complex accounts for approximately 70 to 80% of the morbidity and 40 to 50% mortality of cattle [4]. However, corresponding data with respect to serotype A2 is currently unavailable.
Mannheimia haemolytica A2 produces several virulence factors that together lead to acute fibrinous pleuropneumonia in sheep. The most important of these virulence factors is the leukotoxin (Lkt), a member of the RTX family of toxins from Gram-negative bacteria that is primarily secreted during the bacterial logarithmic growth phase. Lkt is a 104-kDa, thermolabile soluble protein that is toxic to ruminant macrophages, leukocytes and erythrocytes. Interestingly, the N-terminal region of Lkt has been shown to interact through nonspecific (electrostatic) contacts and through a specific protein receptor (β-integrin) of the target cells to mediate pore formation and lysis [5–10].
Gram-negative bacteria typically produce outer membrane (OM)-derived spherical structures named outer-membrane vesicles (OMVs) harboring biologically active proteins and other virulence factors that have various functions. The release of OMVs increases when bacteria are subjected to stress conditions, such as the addition of gentamicin [11, 12]. OMVs are 50–250 nm in diameter, and as they originate from the OM, they possess LPS, phospholipids and OM proteins (OMPs). In addition, OMVs harbor periplasmic components, such as enzymes and DNA fragments. The results of previous studies suggest that the OMV-mediated transport of virulence factors has major advantages compared with their conventional transport, since the molecules are packed into a structure that protects them (i.e., the membrane forming the vesicle) [13]. Our group has demonstrated that M. haemolytica A2 contains several immunogenic OMPs inside the OMVs, among which three proteins of 45, 54 and 60 kDa have been shown to react with the sera of sick sheep. In addition, the presence of Lkt, LPS, and a 23-kbp DNA fragment [11]. Cysteine- and metallo-proteases have been detected in OMVs based on zymography assays [14].
In recent years, the testing of M. haemolytica A2 isolates from animals with mannheimiosis has revealed increasing proportions of antimicrobial resistance [15–17]. In addition, due to the lack of a vaccine with 100% efficacy, new strategies to reduce the presence of this disease in farms has emerged. Lactoferrin (Lf) is a glycoprotein that belongs to the mammalian innate immune system possessing bactericidal and bacteriostatic effects and exhibiting immune regulatory functions. Lf is an 80 kDa protein with high homology among mammalian species [18] that is produced by glandular epithelial cells and secreted to the mucosae and by neutrophils at infection sites in all mammals. Lf is a non-haem iron-binding protein that is a member of the transferrin family, which includes serum transferrin and other proteins. It is now accepted that apo-Lf plays a direct antimicrobial role in secretions and epithelial surfaces by limiting the proliferation and adhesion of microbes and by frequently killing them. The bacteriostatic effect of apo-Lf has been attributed to its ability to capture Fe3+ ions and limit their use by pathogenic bacteria, as this ion is an essential factor for their growth and the expression of virulence factors [19]. The bactericidal effect of apo-Lf is primarily attributable to interactions with LPS, porins, and other OM proteins in Gram-negative bacteria [20–22].
Therefore, in this study we evaluated the effect of apo-BLf on the production of OMVs released by M. haemolytica A2, as well as on the secretion and cytotoxicity of free and OMV-harbored Lkt. The results of this study shows the potential for the use of apo-BLf in the prevention and treatment of ovine mannheimiosis.
Materials and methods
Lactoferrin, bacterial strain and OMV purification
Bovine apo-Lf with a purity of 97% was purchased from NutriScience Innovations, LLC, CT, USA. Apo-BLf was saturated with iron to obtain holo-BLf according to the method described by Xiao and Kisaalita [23]; iron in holo-BLf was 93% and it was quantified by an enzymatic automated method (MicroTech Laboratories, Mexico). The strain of M. haemolytica A2 was obtained from the pneumonic lungs of a sheep that died from mannheimiosis, and the capsular serotype was determined by indirect haemagglutination using a reference anti-serum [11]. Bacteria were grown on blood-agar plates for 24 h at 37 °C, and then an inoculum was cultured in brain heart infusion (BHI) broth for 24 h. Bacteria were harvested by centrifugation (9000 × g for 15 min). Culture supernatants (CS) were filtered through a 0.45 pore-size membrane (Millipore, Ireland) to remove residual cells. Finally, OMVs were recovered by ultracentrifugation (150 000 × g for 3 h at 4 °C) as previously described [11]. To verify that whole bacteria were not present in the OMV samples, thirty fields of OMVs were observed under an electron microscope. In addition, cultures from OMV suspensions were made in blood agar to confirm the absence of bacteria.
Effect of apo-BLf and holo-BLf on M. haemolytica A2 growth
To explore the microbicidal effect of apo-BLf (iron-free) and holo-BLf (iron-saturated) in M. haemolytica A2, each BLf was added to 5 mL of BHI medium (total concentration of BLf: 2, 8, 16 or 20 μM). The assay was started with 106 CFU, and the cells were incubated at 37 °C with agitation (180 rpm) for 3, 6 and 9 h. Cell growth was monitored by measuring the OD595 (Coleman Jr. II spectrophotometer, IL, USA), with each sample simultaneously plated on BHI-agar and incubated for 24 h for subsequent CFU enumeration. The experiment was performed five times, each in triplicate.
Determination of the number of M. haemolytica A2 OMVs by flow cytometry
To quantify the number of OMVs, the method reported by Hernandez et al. [24] for detecting vesicles in peripheral blood from breast cancer patients was adapted for use with bacterial cell OMVs. Twenty microliters of PBS (filtered with a nitrocellulose membrane of 0.22 μm in diameter) was added to a Trucount tube (BD Biosciences, CA, USA) to hydrate the tube pearls. Then, OMVs (50 μL) were added, after which the mixture was vortexed for 30 s and then incubated for 15 min at room temperature (RT). Subsequently, PBS (450 μL) was added, after which the tube was vortexed for 30 s and then incubated for 15 min. Finally, the samples were analyzed on a flow cytometer (BD LSRFortessa, NJ, USA), and the size and granularity of the OMVs were determined. To determine the number of OMVs present in each sample, the following formula was used to analyze different populations in the dot plot: (population pearls events/population vesicles events) × (pearls per tube/sample volume). The results were analyzed using Attune® Cytometric Software 2.1.0, CA, USA. Each experiment was performed three times, each in triplicate.
Electron microscopy of whole bacteria and OMVs
Samples of bacteria grown in different sublethal concentrations of apo-BLf (2, 4, 6 and 8 µM) and in the same concentrations of holo-Lf were obtained. In addition, OMVs obtained from all cultures were placed on carbon- and Formvar-coated copper grids (Electron Microscopy Sciences, PA, USA), negatively stained via successive incubations with 2% uranyl acetate for 1 s, 30 s and 2 min, removing the uranyl after each step, and then observed under a transmission electron microscope (JEOL, JEM 2000 EX, PA, USA).
Determination of outer membrane permeability
To determine whether the function of the bacterial OM was damaged after treatment with apo- or holo-BLf, an MIC assay was performed. This assay was carried out in 96-well plates with bacteria grown in BHI medium supplemented with different sublethal concentrations of apo- or the same concentrations of holo-BLf (1, 2, 4, 6 and 8 μM) for 2 h without agitation. Subsequently, SDS (3, 6, 9 and 15 µg/µL) or polymyxin B (3, 6, 9 and 12 μg/mL) was added and incubated for 6 h at 37 °C. Finally, the OD595 values and the MICs were measured (a minor MIC corresponded to a major damage of the OM) [25]. Each experiment was performed three times, each in triplicate.
Determination of LPS in culture supernatants
Culture supernatants proteins and LPS were separated by 12% SDS-PAGE and then stained for LPS using a modified specific silver staining method [26]. Briefly, proteins were fixed in the gel overnight with a solution containing 25% isopropanol and 7% acetic acid (setting solution). Subsequently, the solution was decanted, and the gel was oxidized for 5 min in a solution containing 150 mL of deionized water, 1.05 g of periodic acid and 4 mL of setting solution. The gel was then washed eight times (20 min each) in deionized water and then stained for 10 min with a solution containing 28 mL of 0.1 N NaOH, 1.25 mL of 29.4% NH4OH and 5 mL of 20% AgNO3. Subsequently, the gel was washed four times (10 min each) in deionized water and then incubated at RT for 3–5 min in a solution containing 50 mg of citric acid in 0.5 mL of 37% formaldehyde. To preserve the gel, it was submerged for 60 min in 7% acetic acid. Subsequently, the stained gel was scanned and analyzed by densitometry using ImageJ Software (Fiji 1.51w, NIH, NY, USA).
Detection of Lkt from M. haemolytica A2 in culture supernatants and OMVs by Western blot
To assess the presence of Lkt in the CS, M. haemolytica A2 cells (5 × 106 CFU) were cultured in BHI supplemented with apo- or holo-BLf (2, 4, 6 or 8 µM, final concentration). Subsequently, the cultures were incubated for 4 h at 37 °C with agitation. Next, the cultures were centrifuged (6400 × g), the bacteria were discarded, and the CS was filtered through a cellulose membrane (0.22 µm diameter, Millipore). Samples from each CS were precipitated with ethanol, dried, and diluted in PBS. Protein samples (10 µL) were resolved by 8% SDS-PAGE for 2 h. Subsequently, the proteins were transferred to a nitrocellulose membrane (BioRad, Germany) at 400 mA for 1 h, after which the membrane was blocked with 0.1% TBS (Tris-buffered saline)-Tween plus 5% skim milk for 2 h and then washed in 0.1% TBS-Tween three times for 10 min each. The membrane was then incubated with a rabbit anti-Lkt Ab (Biorbyt, SF, USA) in 0.1% TBS-Tween (1:5000), washed with 0.1% TBS-Tween, incubated with a secondary peroxidase-coupled anti-rabbit Ab (1:5000) and detected by chemiluminescence.
To determine the presence of Lkt in OMVs, the same culturing procedure was performed with apo- or holo-BLf, but the bacterial cultures were incubated for 24 h. The OMVs were obtained as previously mentioned. Each experiment was performed three times, each in triplicate. Importantly, the results were analyzed by densitometry using ImageJ (Fiji) and normalized to the amount of Lkt secreted by 1 × 106 bacteria for CS and Lkt harbored by 1000 vesicles for the OMV samples.
Interaction of Lkt with ovine macrophages
Enrichment of ovine peripheral blood mononuclear cells (PBMCs) and their differentiation into macrophages
To obtain sheep monocyte-derived macrophages, we used a method reported for human monocytes, as we did not identify a specific method for sheep in the literature. We optimized the centrifugation step for the Lymphoprep (Axis-Shield, Norway) gradient at 1000 × g for 15 min (instead of 800×g for 20 min), because the density of sheep blood is different from that of human blood. Ovine cells were subjected to a second purification step by a magnetic separation system using human MiniMac CD14 (Miltenyi Biotec, CA, USA), following the manufacturer’s instructions. The obtained monocytes were incubated in 96-well plates in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Gibco, NY, USA), 1% antibiotics and an anti-mycotic solution (Caisson Labs, UT, USA) in a CO2 incubator (Thermo Scientific Forma I, OH, USA) at 37 °C for 24 h to promote cell adhesion to the wells. Subsequently, the medium was changed, and 100 ng of recombinant human granulocyte macrophage-colony stimulating factor (GM-CSF) (PeproTech, Inc, NJ, USA) was added to 1 × 105 cells. After 5 days, fresh GM-CSF was added every other day to maintain stimulated cell differentiation.
Assay of macrophage viability
Lkt secreted into the CS or OMVs released from M. haemolytica A2 cultured with different concentrations of apo- or holo-BLf was purified and added to cells and incubated for 1 h. In the other group, the same concentration of apo- and holo-BLf (2, 4, 6 and 8 µM) used to grow bacteria was added to RPMI medium supplemented with purified Lkt (only in the purified Lkt assay). Lkt and OMVs from bacteria grown in medium without BLf, human macrophages (Lkt assay) and cells without Lkt or OMVs were used as controls. Due to the results obtained in the pure Lkt assay, only the lowest and highest concentrations of BLf were used for the OMV experiment. After the incubation period, the medium was changed to fresh medium without phenol red and 10% FBS plus 10 µL of MTT in a final volume of 110 μL. Then, the cells were incubated for 3 h, after which DMSO was added and the absorbance of the samples was read in a spectrophotometer at 570 nm.
Statistical analysis
Statistical comparisons among groups were performed using one-way analysis of variance (ANOVA), followed by Tukey’s posttest. Significance for all analyses was established with P < 0.05. All statistical analyses were performed using GraphPad Prism 6.01 (GraphPad, CA, USA).
Results
Apo- but not holo-BLf has bactericidal activity toward M. haemolytica A2
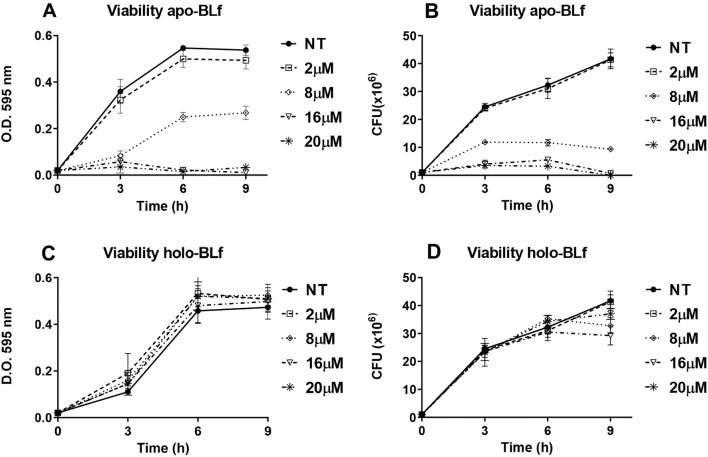
First, apo-BLf was assessed for bactericidal activity toward M. haemolytica A2 using different apo-BLf concentrations and incubation times. Figure 1A shows the bacterial growth results in the presence of apo-BLf, as determined by measuring the OD595 values of cultures, while Figure 1B shows the viable count under the same culture conditions. Using both approaches, we observed that M. haemolytica was unable to grow at 16 µM apo-BLf. Apo-BLf may promote M. haemolytica cell death, in addition to sequestering iron, since the number of viable bacteria was drastically reduced after the third hour. This killing effect was observed after the first hour of incubation with apo-BLf (data not shown). The iron-saturated BLf form (holo-BLf) had no effect on M. haemolytica growth (Figures 1C and D). Interestingly, in more than 20 experiments, we observed a slight stimulation (approximately 10%) in M. haemolytica growth at high holo-BLf concentrations (above 10 µM), despite M. haemolytica being unable to utilize holo-BLf as a sole iron source [20].
Figure 1.
Apo-BLf is bactericidal towardM. haemolyticaA2, while holo-BLf does not affect the growth of this bacterium. A, C OD595 values for the apo-BLf- and holo-BLf-treated cells, respectively. B, D Enumeration of viable bacteria for the same treatments. Bacteria were grown in BHI supplemented with different concentrations of apo- or holo-BLf.
Apo-BLf increases the number of OMVs released
As is the case for all Gram-negative bacteria, M. haemolytica typically produces OMVs. To assess the effect of apo-BLf on the production of OMVs, M. haemolytica was grown in medium supplemented with different concentrations of the glycoprotein, and the number of OMVs was quantified by flow cytometry. Since a concentration of 16 µM apo-BLf resulted in a strong decrease in the number of bacteria, only sublethal concentrations (2, 4, 6 and 8 µM apo-BLf) were used in this experiment. As gentamicin treatment increases the release of OMVs from M. haemolytica [11, 27], the addition of this aminoglycoside was used as a positive control. Figure 2 shows that apo-BLf increased the release of OMVs in a concentration-dependent manner. To determine whether the increase in OMV release caused by apo-BLf was due to iron chelation, we performed a similar experiment with holo-BLf (with the same concentrations used for apo-BLf) and an iron-chelating compound (2′2-dipyridyl) at different concentrations (0.10, 0.15, 0.20, 0.25 and 0.30 mM). Figure 2 shows that the number of OMVs released was not altered when holo-BLf or 2′2-dipyridyl was used at any concentration. The results of this assay suggest that apo-BLf induces the release of OMVs in an iron chelation-independent manner.
Figure 2.
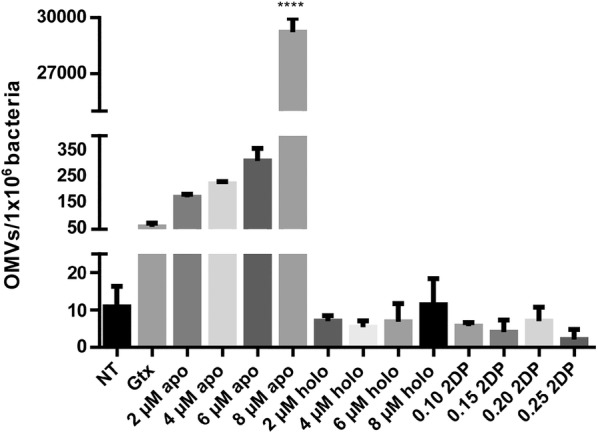
Apo-BLf increases the release of OMVs fromM. haemolyticaA2. Holo-BLf and 2′2-dipyridyl do not affect the number of OMVs. Bacteria were grown in BHI supplemented with 25 µg/mL gentamicin (positive control) or different concentrations of 2′2-dipyridyl, apo- or holo-BLf, after which the outer membrane vesicles (OMVs) were purified and quantified by flow cytometry. NT, no treatment; Gtx, gentamicin; apo, apo-BLf; holo, holo-BLf; 2DP, 2′2-dipyridyl. ****P < 0.0001.
Apo- but not holo-BLf affects the structure of both the M. haemolytica A2 OM and OMVs
We subsequently investigated whether the increase in the release of OMVs from M. haemolytica A2 in the presence of apo-BLf could be due to apo-BLf binding to the OM, resulting in a morphological change. To this end, we analyzed the structure of the OM and OMVs produced by M. haemolytica A2 by negative electron microscopy. Figure 3 shows images of bacteria grown in medium supplemented with different sublethal concentrations of apo-BLf (left side) and corresponding samples of their purified OMVs (right side). In all of the groups treated with apo-BLf, the bacterial OM was affected and exhibited protrusions along the OM that presented discontinuity in some zones and generally occurred at high apo-BLf concentrations. In addition, the OMVs exhibited morphological changes, had membranes that could not be clearly observed in approximately 80% of the OMV population, and had electron-dense contents with minimal electron density (Figure 3, right side).
Figure 3.
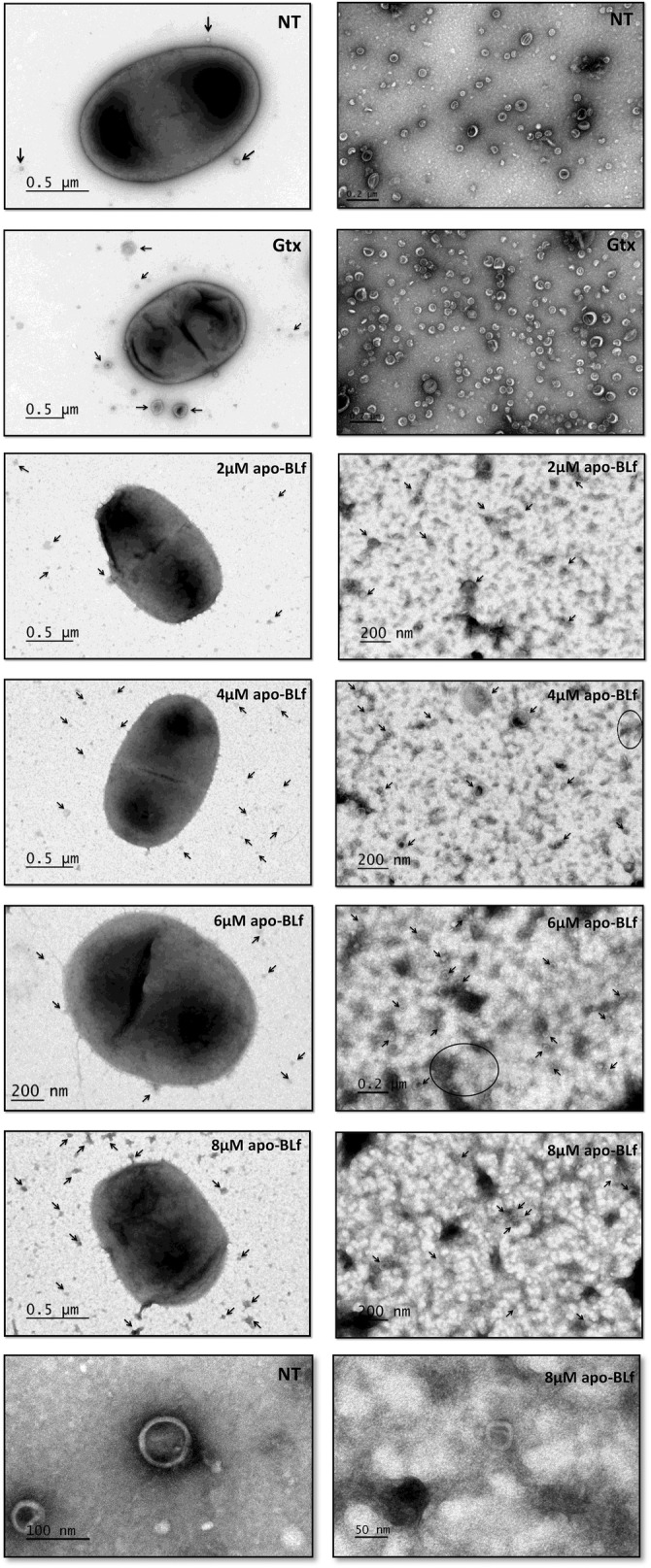
Apo-BLf affects the OM and OMV structure ofM. haemolyticaA2. Bacteria (left side) and purified OMVs (right side); bacteria were grown in BHI supplemented with different concentrations of apo-BLf (2, 4, 6 and 8 µM). Arrows, OMVs; circles and ovals, set of OMVs. Electron transmission microscopy, negative staining.
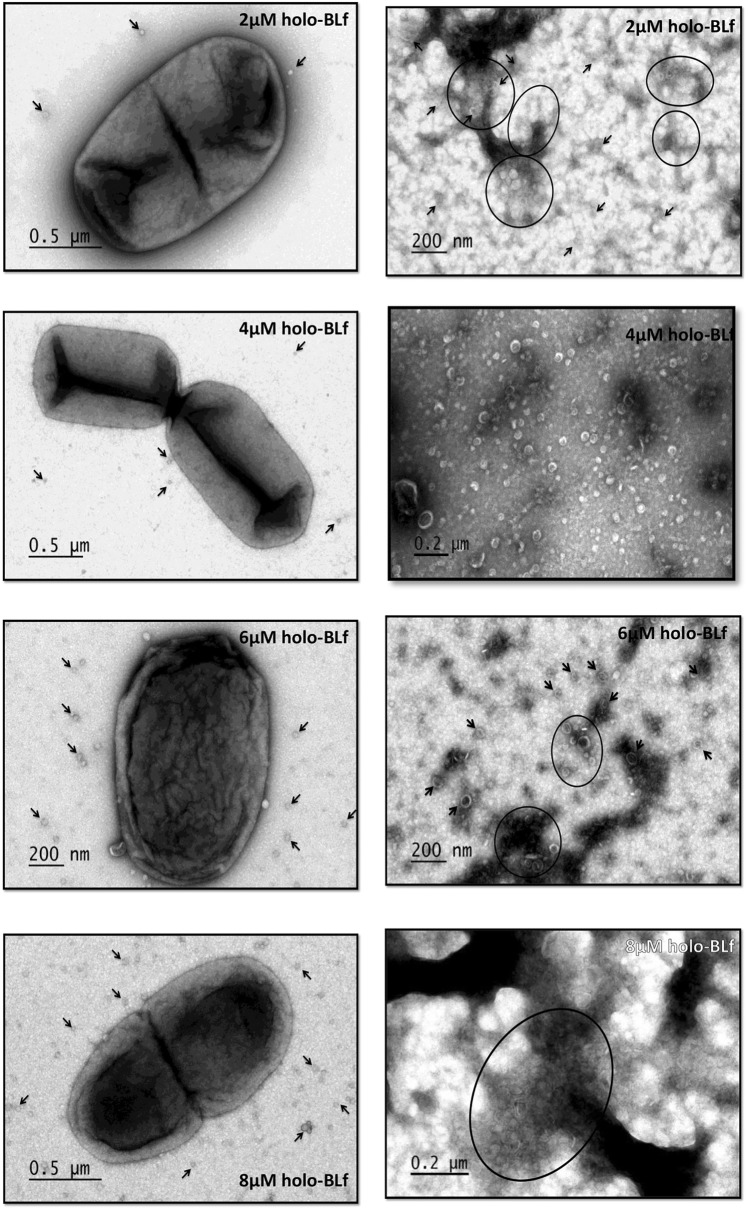
Since the M. haemolytica A1 OMPs that bind to apo-BLf also bind holo-BLf [20], we assessed whether holo-BLf has the same effect as apo-BLf on the OM and OMV structure of M. haemolytica A2. Structural damage of the bacterial cells was not observed with holo-BLf (Figure 4, left side). However, a few number of bacteria with the abovementioned morphological characteristics detected when apo-BLf was added, were also observed (approximately 10% of the total bacteria, data not shown). In addition, the morphology of OMVs purified from M. haemolytica A2 treated with holo-BLf was similar to that of the control without BLf, but a decrease in the size of the OMVs was observed.
Figure 4.
Holo-BLf does not affect the OM and OMV structure ofM. haemolyticaA2. Bacteria (left side) and purified OMVs (right side) were grown in BHI supplemented with different concentrations of holo-BLf (2, 4, 6 and 8 µM). Arrows, OMVs; circles and ovals, a set of OMVs. Electron transmission microscopy, negative staining.
Apo-BLf affects the permeability of the M. haemolytica A2 OM
To determine whether apo-BLf destabilizes and damages the OM of M. haemolytica A2, thereby altering its permeability, bacterial membrane integrity assays were performed. Figure 5 shows the MIC results for SDS (Figure 5A) and polymyxin B (Figure 5B) treatments of M. haemolytica A2 previously grown for 2 h in BHI supplemented with apo-BLf. Bacteria grown in medium supplemented with apo-BLf showed a lower MIC toward SDS and polymyxin B than that observed for the control group, indicating that their OM was more susceptible to the entry of these compounds. This same assay was performed with holo-BLf, and a decrease in the MIC was observed, although to a much lesser degree. These results demonstrate that apo-BLf affects the permeability and continuity of the M. haemolytica A2 OM, which likely allows for the increased release of OMVs.
Figure 5.
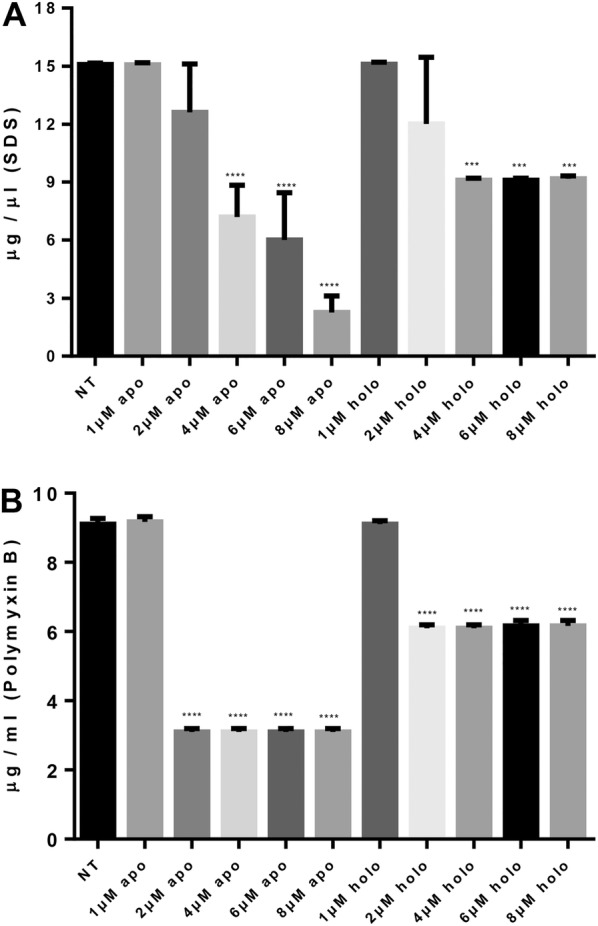
Apo-BLf affects the outer membrane permeability ofM. haemolyticaA2. Apo-BLf diminishes the MIC for SDS (A) and polymyxin B (B). NT, No treatment with BLf (only BHI); apo, BHI plus apo-BLf; holo, BHI plus holo-BLf. The MICs of the SDS and polymyxin B controls without BLf were 15 and 9 µg/mL, respectively. ***P < 0.001, ****P < 0.0001.
Apo-BLf can remove LPS from the OM of M. haemolytica A2
It has been reported that the LPS embedded in the OM of Gram-negative bacteria is a Lf-binding molecule [21, 28–30], which neutralizes LPS and promotes its release while simultaneously destabilizing and permeabilizing the bacterial OM [31–34]. Thus, silver staining specific for LPS was performed for the CS from M. haemolytica A2 grown in BHI supplemented with different concentrations of apo-BLf. Figure 6A shows that when apo-BLf was added (at a minimum concentration of 2 µM), an LPS band was detected in the CS, and this LPS release occurred in an apo-BLf concentration-dependent manner. The densitometry graph of the LPS band is shown in Figure 6C. Next, the same experiment was performed using holo-BLf, and the results are shown in Figure 6B (silver staining) and C (densitometry). It can be clearly observed that small amounts of LPS were present in the CS when holo-BLf was used at all concentrations tested. This result is similar to that observed for the OM integrity, where apo-BLf affected the permeability of the OM to a greater extent than holo-BLf.
Figure 6.
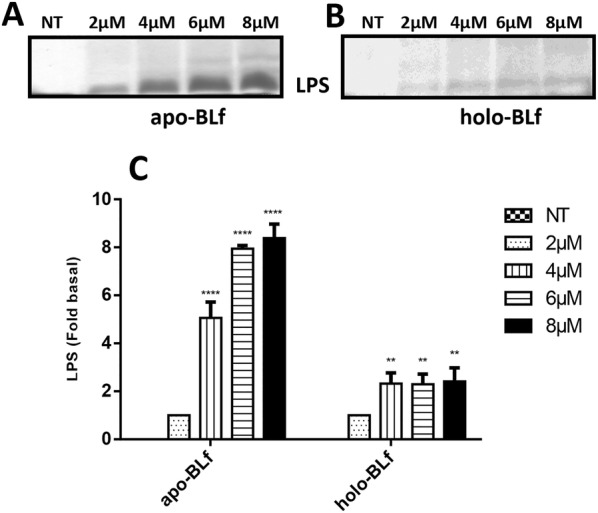
Apo-BLf induces the release of LPS from the outer membrane ofM. haemolyticaA2. Silver staining of LPS in SDS-PAGE gels from samples of culture supernatants of M. haemolytica A2 grown for 4 h in BHI supplemented with apo-BLf (A) or holo-BLf (B). C Graph of the LPS release data (densitometry). **P < 0.01, ****P < 0.0001.
Apo-BLf increases the secretion of Lkt into the CS of M. haemolytica A2 as a result of iron chelation
We evaluated the secretion of Lkt into the CS by M. haemolytica A2 grown under different treatments. Figure 7A shows the Western blot results for the CS samples of bacteria grown in medium supplemented with apo-BLf, where the increase in Lkt secretion was dependent on its concentration such that the greatest effect was observed at 8 µM apo-BLf. Additionally, to evaluate whether the increase in Lkt secretion was due to the iron-chelating ability of apo-BLf, we performed this assay with holo-BLf (Figure 7B) and 2′2-dipyridyl (Additional file 1). In contrast to the results observed using apo-BLf, the samples treated with holo-BLf did not show an increase in Lkt secretion. However, for the 2′2-dipyridyl-treated samples, we observed an increase in Lkt secretion, which was more evident at concentrations of 0.20 and 0.25 mM, similar to apo-BLf. Thus, we suggest that the observed increase in Lkt secretion into the CS could be partially due to the iron-chelation activity of apo-BLf.
Figure 7.
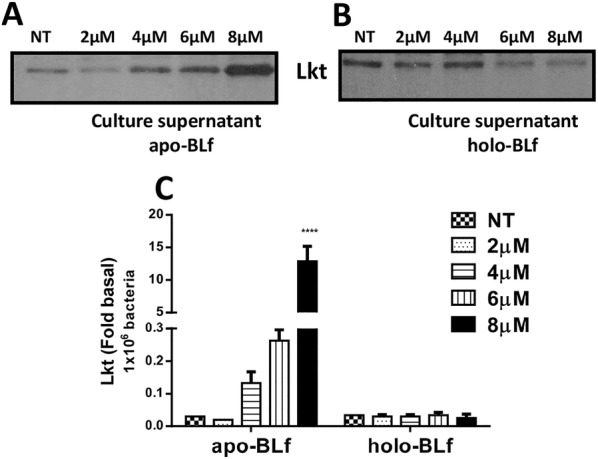
Apo-BLf increases the secretion of leukotoxin (Lkt) into culture supernatants (CS) ofM. haemolyticaA2. A, B Western blotting of Lkt secreted into CS from bacteria grown in BHI supplemented with apo- or holo-BLf, respectively. C Graph of Lkt secreted into CS (densitometry, adjusted to Lkt release by 1 × 106 bacteria). ****P < 0.0001.
The Lkt content in OMVs was also measured and showed different results compared to those observed for the CS samples. Although an increase in Lkt was observed in the samples with high concentrations of apo-BLf, when these values were adjusted for the number of vesicles released, an increase in the Lkt contents of OMVs was not observed (Figures 8A and C). The same increase in Lkt was observed in the holo-BLf-treated samples (Figures 8B and C), but unlike the apo-BLf-treated samples, this increase was maintained when adjusted for the number of vesicles released, suggesting that the holo-BLf treatment increased the secretion of Lkt contained in OMVs.
Figure 8.
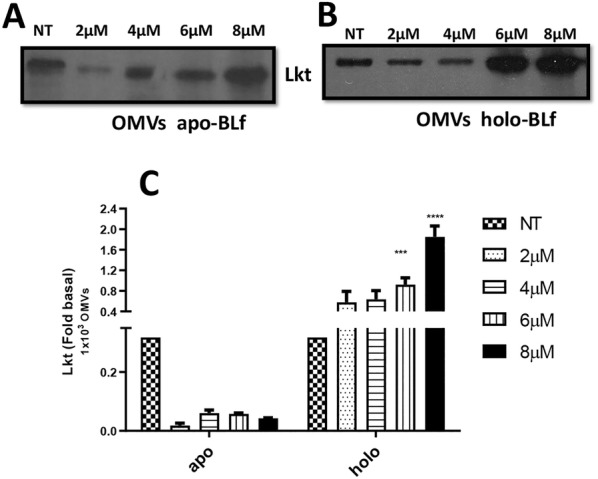
Holo-BLf increases the leukotoxin (Lkt) concentration in OMVs released byM. haemolyticaA2. A, B Western blotting of Lkt contained within OMVs of bacteria grown in BHI supplemented with apo- or holo-BLf, respectively. C Graph of Lkt contained within OMVs (densitometry, adjusted to Lkt release from 1000 OMVs). ***P < 0.001, ****P < 0.0001.
Lkt from the CS and OMVs of M. haemolytica A2 grown in medium supplemented with apo- and holo-BLf are cytotoxic toward ovine macrophages
An MTT assay was performed on ovine macrophages by treating cells with purified Lkt secreted by M. haemolytica A2 grown in the presence of apo- or holo-BLf (bacteria grown in BHI were used as a control), after which the Lkt cytotoxicity was measured. Lkt had a toxic effect toward ovine macrophages in all groups analyzed, and the same result was obtained when apo- or holo-BLf was added to the cell medium (Figure 9A). Human macrophages were used as a control group, where the Lkt of M. haemolytica had no toxic effect. Therefore, we concluded that the Lkt released from cells grown in medium supplemented with apo- or holo-BLf is active and presents classic cytotoxic activity. With respect to OMVs (Figure 9B), the Lkt from bacteria grown with apo-BLf and holo-BLf was also cytotoxic. These results indicate that CS and OMV Lkt is biologically functional.
Figure 9.
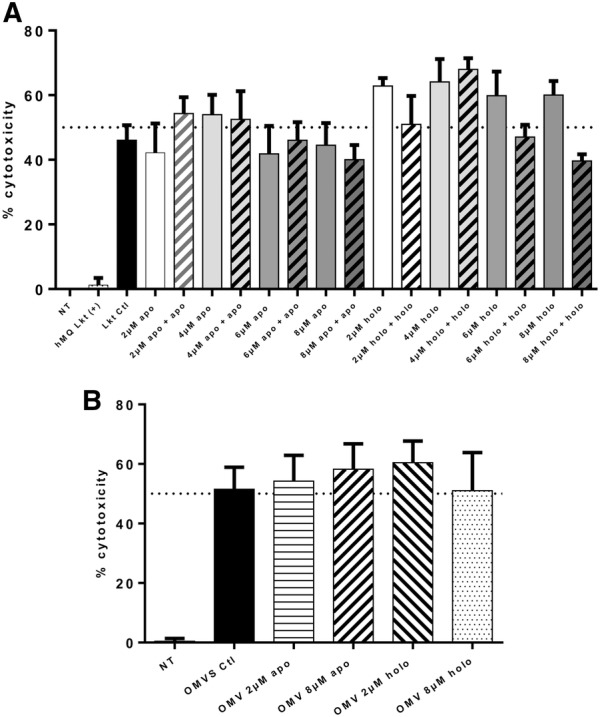
Lkt fromM. haemolyticaA2 grown in medium supplemented with apo- and holo-BLf is active. A Ovine macrophages were treated with purified Lkt secreted by M. haemolytica A2 grown in medium supplemented with apo- and holo-BLf (2, 4, 6 and 8 µM) or purified Lkt plus RPMI with the same concentration of apo- and holo-Lf (2, 4, 6 and 8 apo- plus apo-Lf or 2, 4, 6 and 8 holo- plus holo-Lf). B Ovine macrophages were treated with OMVs released by M. haemolytica A2 grown in medium supplemented with apo- and holo-BLf (2 or 8 µM).
Discussion
The increase in the resistance of M. haemolytica to antibiotics worldwide has created an urgent need for the investigation of novel alternative strategies for the treatment and prevention of ovine mannheimiosis. Lf is a multifunctional glycoprotein that has several activities, the most studied of which is as an antimicrobial compound. In this study, the effect of apo- and holo-BLf on the growth and OMV production of M. haemolytica A2 was evaluated. Apo-BLf showed a bactericidal effect toward M. haemolytica A2, a result that is in agreement with those of other reports, such as for Streptococcus pneumoniae [35], Vibrio cholerae [36], antibiotic resistant strains of Helicobacter pylori [37], Staphylococcus aureus and Escherichia coli [38], Pseudomonas fluorescens in ground beef [39], and Actinobacillus pleuropneumoniae [40]. In contrast, when we performed this assay using BHI supplemented with holo-BLf, we observed that this ferric glycoprotein did not inhibit M. haemolytica A2 growth.
Outer membrane vesicles are spherical structures that are formed from the OM. OMVs enclose a broad range of membrane-associated and periplasmic proteins, a few cytosolic components and DNA fragments, all of which have important biological functions, such as in stress responses, quorum sensing, virulence, and target host cell signaling. Many OMV components may represent an effective long-distance delivery mechanism for a broad range of effectors/virulence factors to host cells [41]. The contents and production of OMVs have been reported to be affected by the growth conditions of bacteria [41–43]. Furthermore, the aminoglycoside antibiotic gentamicin can also perturb the packing order of lipids, thereby destabilizing the bilayer membrane and increasing the release of vesicles by some bacteria, such as Pseudomonas aeruginosa [44].
The effect of mammalian Lf on bacterial OMV release has not been explored. Therefore, we first evaluated the number of OMVs released by M. haemolytica when grown in medium supplemented with different sublethal concentrations of apo- and holo-BLf. An increase in the release of OMVs with apo-BLf but not with holo-BLf was detected. Since apo-BLf is an iron-chelating protein, 2′2-dipyridyl was used as a control for iron starvation conditions. As with holo-BLf, there was no change in the number of OMVs when bacteria were treated with 2′2-dipyridyl. Similar results were reported by Chan et al. [41], who observed that iron-limiting conditions had a minimal effect on the number of OMVs produced from clinical isolates of extraintestinal pathogenic E. coli (ExPEC). Thus, we suggest that the lack of iron in the growth medium does not induce the exacerbated release of OMVs and that the apo-BLf-mediated increase in the release of vesicles is due to another mechanism of action, such as the binding of apo-BLf to OM components, which produces membrane modifications that alters its functions.
Multiple reports have described the damage caused by Lf to the OM of different bacterial species, which has also been observed to be caused by Lf N-terminus-derived peptides named lactoferricins (Lfcins) [31–33, 35]. Thus, we assessed whether the increase in the apo-BLf-mediated increase in OMV production is due to the effect on the structure and function of the OM. We observed severe OM damage for all apo-BLf concentrations assayed and a clear increase in the number of released OMVs with an altered morphology. Similar results were obtained using the Lfcin peptide from BLf by Yamauchi et al. [33], who showed that E. coli O157:H7 exposed to BLfcin (100 µg/mL) showed an altered cell membrane morphology that included the appearance of membrane “blisters”, although these structures were not studied. León-Sicairos et al. [35] reported that Streptococcus pneumoniae (a Gram-positive bacterium) cells treated with BLfcin (40 µM) displayed deformation and thickening of the cell wall as well as thickened septa with irregular features when treated with a BLf chimera [a fusion peptide between Lfcin and lactoferrampin (Lfampin), a peptide containing the 265–284 amino acid region], where atypical bubbling and increased permeability of the inner membrane were observed. In E. coli K12, Lfcin and Lfampin (20 µM each) also lead to OM breakage in such a way that the inner and OM fused and protrusions from the surface were observed. In this case, the authors reported vesicle-like structures of approximately 50 nm in diameter in more than 50% of the cells [45]. Taken together, these results indicate that apo-Lf and its derivate peptides damage bacterial membranes and affect their functionality. Regarding the effects of iron saturation of Lf, the results of electrophoretic and crystallographic studies have indicated that iron chelation of this molecule significantly alters the structural conformation of the protein, as holo-Lf has a more closed structure and is more resistant to high temperature and pH values than apo-Lf [46–48]. Such a conformational change may influence its interaction with bacterial membranes. In our assays with holo-BLf, damage to OM and OMV structures was not observed. Therefore, we suggest that due to this conformational change, holo-BLf protein is unable to affect the bacterial OM, although this iron-loaded protein is able to bind to it.
The OM has an asymmetric lipid bilayer with negatively charged LPS molecules that are primarily localized on the outer leaflet and are stabilized by the presence of divalent cations [49]. Multiple observations have shown that Lf causes LPS release, suggesting that Lf has membrane-permeabilizing activity [31–33, 35]. We assessed the release of LPS from M. haemolytica A2 grown in medium supplemented with apo- and holo-BLf. Apo-BLf-mediated LPS release occurred in a concentration-dependent manner, and holo-BLf also promoted LPS release, although to a much lesser degree than apo-BLf. Additionally, M. haemolytica grown in the presence of apo-BLf displayed diminished MIC value for SDS and polymyxin B. Apo-BLf decreased the functions of the OM, causing this structure to be more susceptible to external compounds. In contrast, holo-BLf permeabilized the OM of M. haemolytica to a lesser degree, a result that was consistent with our previous data and electron microscopy images. We suggest that the increase in OMVs released by M. haemolytica grown in medium supplemented with apo-BLf is in part due to its ability to remove divalent cations, such as Ca2+ and Mg2+ [32, 34], thereby destabilizing LPS, inducing damage to the OM and causing increasing cell permeability, which allows OMVs to be more easily released. This effect was not observed for holo-BLf, perhaps because differences in its tertiary structure does not allow for similar binding to the OM such that it cannot harm bacterial cells.
The ability of bacterial pathogens to adapt to the environment within the host is essential for their virulence [50], and environmental signals have been shown to influence virulence gene expression in several different organisms. Microorganisms have adapted to iron limitation in mammalian hosts by evolving diverse mechanisms for the assimilation of sufficient iron for growth. In some organisms, iron starvation leads to a response through an increase in the expression of RTX toxins and hemolysins, which can lyse host cells and cause them to release their internal stores of iron [51]. We demonstrated that apo-BLf, an iron-chelator, increased the secretion of Lkt into the CS. Moreover, the addition of the iron-chelator 2′2-dipyridyl also increased the secretion of Lkt, indicating that Lkt secretion partially depends on iron. Similar results were reported by Marciel and Highlander [52], who showed that in the presence of 2′2-dipyridyl, the transcription of the M. haemolytica lkt gene promoter was increased more than threefold. These authors performed a detailed study of the transcriptional regulation of this gene through the development of plasmid-borne chloramphenicol acetyltransferase (cat) operon fusions and concluded that the lkt gene is negatively regulated by iron [52]. Balashova et al. [53] also showed that the secretion of leukotoxin by Aggregatibacter actinomycetemcomitans, another member of the Pasteurellaceae family, is increased in low-iron medium. M. haemolytica is capable of reaching the lungs, where the microenvironment is low in iron. Thus, as a mechanism for M. haemolytica to obtain iron for growth, it seems reasonable that iron limitation leads to increased Lkt production, which destroys cells and releases iron. This mechanism has been reported in other bacterial species [51].
Other researchers [6, 54] have reported a positive effect of iron on the regulation of Lkt secretion in M. haemolytica. The difference between these observations and our results may be due to apo-BLf being a multifunctional protein with activities other than as a bacteriostatic agent as an iron-chelator, since it has bactericidal activity by being able to bind and damage the bacterial OM. Therefore, the apo-BLf iron-chelating effect may not be the only one involved in the regulation of Lkt secretion.
Interestingly, only holo-BLf increased the level of Lkt present in OMVs. Perhaps this result is due to its ability to promote the production of smaller vesicles than those typically produced by M. haemolytica A2, many of which could not be detected by flow cytometry. Thus, it is necessary to use other methods to accurately evaluate this effect, such as nanoparticle tracking analysis.
Although this study reports the basic interactions between BLf and some M. haemolytica A2 components (OM and LPS), as well as the effect on some virulence factors, such as Lkt and OMVs, our results can be informative for further studies of the use of apo-BLf in the treatment of ovine mannheimiosis. In the future, assays with animals could be performed to determine the appropriate apo-BLf doses for its possible use as an adjuvant in the treatment of ovine mannheimiosis and assess its synergy with antibiotics.
In summary, the results of this study demonstrates that apo-BLf is bactericidal toward M. haemolytica A2. At sublethal concentrations, apo-BLf promotes OMV release, which is dependent on the apo-BLf concentration, induces OM damage and affects its permeability. Apo-BLf increases Lkt secretion via an iron-chelating effect, and Lkt and OMVs released from M. haemolytica grown in medium supplemented with apo- or holo-BLf maintains its toxic effect toward ovine macrophages. Due to these effects, the use of apo-BLf can be recommended for the treatment of ovine mannheimiosis, but the appropriate doses must be determined.
Supplementary information
Additional file 1. 2′2-Dipyridyl increases leukotoxin (Lkt) secretion intoM. haemolyticaA2 culture supernatants (CS). Bacteria were grown in BHI supplemented with 2′2-dipyridyl. A Western blotting of Lkt in the CS of M. haemolytica A2. B Graph of Lkt (densitometry). ****P < 0.0001.
Acknowledgements
First author received a doctorate scholarship from Conacyt, México, No. 447198. We thank Dr. Lourdes Rojas for her advice in the capture of electron microscopy photographs. We also thank to the Unit of Electronic Microscopy and the Unit of Flow Cytometry, Cinvestav, Mexico. MGA thanks to Conacyt, Mexico, for the Grant 2018 CB A1-S-8989.
Abbreviations
- OM
outer membrane
- OMVs
outer membrane vesicles
- Lf
lactoferrin
- apo-BLf
apo-bovine lactoferrin
- holo-BLf
holo-bovine Lactoferrin
- Lkt
leukotoxin
- LPS
lipopolysaccharide
- OMPs
outer membrane proteins
- CS
culture supernatants
- BHI
brain heart infusion
- CFU
colony forming units
- RT
room temperature
- PBS
phosphate-buffered saline
- MIC
minimum inhibitory concentration
- SDS
sodium dodecyl sulfate
- TBS
tris-buffered saline
- PBMC
peripheral blood mononuclear cells
- FBS
fetal bovine serum
- GM-CSF
granulocyte macrophage-colony stimulating factor
- DMSO
dimethyl sulfoxide
- ANOVA
one-way analysis of variance
- 2DP
2′2 dipyridyl
- Lfcins
lactoferricins
- Lfampin
lactoferrampin
- NT
no treatment
- Gtx
gentamicin
Authors’ contributions
CAG carried out the experimental work, participated in the study design and prepared the manuscript. MRL, CGR and GRR participated in the adjustment of methods, analysis and interpretation of data. EDA and EZG participated in the design and helped draft the manuscript. MGA conceived the study and participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Avalos-Gómez, Email: chris8814@hotmail.com.
Magda Reyes-López, Email: magrel2003@yahoo.com.mx.
Gerardo Ramírez-Rico, Email: b.am.element@hotmail.com.
Efrén Díaz-Aparicio, Email: efredia@yahoo.com.
Edgar Zenteno, Email: ezenteno@unam.mx.
Cynthia González-Ruiz, Email: cgrmvz@hotmail.com.
Mireya de la Garza, Email: mireya@cell.cinvestav.mx.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13567-020-00759-z.
References
- 1.Zecchinon L, Fett T, Desmecht D. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet Res. 2005;36:133–156. doi: 10.1051/vetres:2004065. [DOI] [PubMed] [Google Scholar]
- 2.Colín RF, Jaramillo ML, Aguilar F, Trigo JMM. Serotipos de Pasteurella haemolytica en pulmones neumónicos de ovinos de México. Rev Lati Am Microbiol. 1987;29:231–234. [PubMed] [Google Scholar]
- 3.USDA . Feedlot 2011: Part IV: Health and Health Management on US. Feedlots with a capacity of 1000 or more head. Fort Collins: USDA–APHIS–VS–CEAH–NAHMS; 2011. [Google Scholar]
- 4.Hilton WM. BRD in 2014: where have we been, where are we now, and where do we want to go? Anim Health Res Rev. 2014;15:120–122. doi: 10.1017/S1466252314000115. [DOI] [PubMed] [Google Scholar]
- 5.Chang YF, Young R, Post D, Struck DK. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987;55:2348–2354. doi: 10.1128/IAI.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentry MJ, Confer AW, Weinberg ED, Homer JT. Cytotoxin (leukotoxin) production by Pasteurella haemolytica: requirement for an iron-containing compound. Am J Vet Res. 1986;47:1919–1923. [PubMed] [Google Scholar]
- 7.Highlander SK. Molecular genetic analysis of virulence in Mannheimia (pasteurella) haemolytica. Front Biosci. 2001;6:D1128–D1150. doi: 10.2741/highland. [DOI] [PubMed] [Google Scholar]
- 8.Jeyaseelan S, Sreevatsan S, Maheswaran SK. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim Health Res Rev. 2002;3:69–82. doi: 10.1079/AHRR200242. [DOI] [PubMed] [Google Scholar]
- 9.Clinkenbeard KD, Mosier DA, Confer AW. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolytica leukotoxin. Infect Immun. 1989;57:420–425. doi: 10.1128/IAI.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Clinkenbeard KD, Cudd LA, Clarke CR, Clinkenbeard PA. Correlation of Pasteurella haemolytica leukotoxin binding with susceptibility to intoxication of lymphoid cells from various species. Infect Immun. 1999;67:6264–6269. doi: 10.1128/IAI.67.3.1172-1179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González RC, Tenorio GV, Trigo TF, Reyes LM, León SN, Godínez VD, de la Garza M. Characterization of microvesicles of Mannheimia haemolytica serotype A1 (reference strain) and serotype A2 (field isolate) J Anim Vet Adv. 2007;6:1172–1178. [Google Scholar]
- 12.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/JB.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero MA, Hernández CC, Ibarra JA, Castro EG. The outer membrane vesicles: secretion system type zero. Traffic. 2017;18:425–432. doi: 10.1111/tra.12488. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez Rico G, Martínez-Castillo M, González-Ruíz C, Luna CS, de la Garza M. Mannheimia haemolytica A2 secretes different proteases into the culture medium and in outer membrane vesicles. Microb Pathog. 2017;113:276–281. doi: 10.1016/j.micpath.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Esaki H, Asai T, Kojima A, Ishihara K, Morioka A, Tamura Y, Takahashi T. Antimicrobial susceptibility of Mannheimia haemolytica isolates from cattle in Japan from 2001 to 2002. J Vet Med Sci. 2005;67:75–77. doi: 10.1292/jvms.67.75. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz S, Kehrenberg C, Salmon SA, Watts JL. In vitro activities of spectinomycin and comparator agents against Pasteurella multocida and Mannheimia haemolytica from respiratory tract infections of cattle. J Antimicrob Chemother. 2004;53:379–382. doi: 10.1093/jac/dkh059. [DOI] [PubMed] [Google Scholar]
- 17.Shin SJ, Kang SG, Nabin R, Kang ML, Yoo HS. Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet Microbiol. 2005;106:73–77. doi: 10.1016/j.vetmic.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 18.González CSA, Arévalo GS, Rascón CQ. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33:301.e1–301.e8. doi: 10.1016/S0924-8579(09)70071-8. [DOI] [PubMed] [Google Scholar]
- 19.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin. Cell Mol Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samaniego BL, Luna CS, Piña VC, Suarez GF, de la Garza M. Two outer membrane proteins are bovine lactoferrin-binding proteins in Mannheimia haemolytica A1. Vet Res. 2016;47:93. doi: 10.1186/s13567-016-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, de Graaff J, Nuijens JH. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/IAI.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallmann FR, Baveye-Descamps S, Pattus F, Salmon V, Branza N, Spik G, Legrand D. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. J Biol Chem. 1999;274:16107–16114. doi: 10.1074/jbc.274.23.16107. [DOI] [PubMed] [Google Scholar]
- 23.Xiao R, Kisaalita WS. Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Microbiology. 1997;143:2509–2515. doi: 10.1099/00221287-143-7-2509. [DOI] [PubMed] [Google Scholar]
- 24.Galindo HO, Villegas CS, Candanedo F, González VMC, Chavez OS, Jimenez VX, Sierra MM, Salazar EP. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44:208–214. doi: 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, Lass A, Daum G, Reidl J, Feldman MF, Schild S. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai C-M, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- 27.Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/JB.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elass-Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapple DS, Hussain R, Joannou CL, Hancock RE, Odell E, Evans RW, Siligardi G. Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide. Antimicrob Agents Chemother. 2004;48:2190–2198. doi: 10.1128/AAC.48.6.2190-2198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Berkel PHC, Geerts MEJ, Van Veen HA, Kooiman PM, Pieper FR, de Boer HA, Nuijens JH. Glycosylated and unglycosylated human lactoferrins both bind iron and show identical affinities towards human lysozyme and bacterial lipopolysaccharide, but differ in their susceptibilities towards tryptic proteolysis. Biochem J. 1995;312:107–114. doi: 10.1042/bj3120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellison RT, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56:2774–2781. doi: 10.1128/IAI.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison RT, LaForce FM, Giehl TJ, Boose DS, Dunn BE. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+ J Gen Microbiol. 1990;136:1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi K, Tomita M, Giehl TJ, Ellison RT., 3rd Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun. 1993;61:719–728. doi: 10.1128/IAI.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drago SME, de la Garza M, Luna JS, Campos RR. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12:1–9. doi: 10.1016/j.intimp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 35.León-Sicairos N, Angulo-Zamudio UA, Vidal JE, López-Torres CA, Bolscher JG, Nazmi K, Reyes-Cortes R, Reyes-López M, de la Garza M, Canizalez-Román A. Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. Biometals. 2014;27:969–980. doi: 10.1007/s10534-014-9775-y. [DOI] [PubMed] [Google Scholar]
- 36.Acosta SE, Viveros JK, Canizalez RA, Reyes LM, Bolscher JGM, Nazmi K, Flores VH, Alapizco CG, de la Garza M, Martínez GJJ, Velazquez RJ, Leon SN. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front Microbiol. 2018;8:2633. doi: 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciccaglione AF, Di Giulio M, Di Lodovico S, Di Campli E, Cellini L, Marzio L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: an in vitro and in vivo study. J Antimicrob Chemother. 2019;74:1069–1077. doi: 10.1093/jac/dky510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flores VH, Canizalez RA, Reyes LM, Nazmi K, de la Garza M, Zazueta BJ, León SN, Bolscher JG. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals. 2010;23:569–578. doi: 10.1007/s10534-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 39.del Olmo A, Morales P, Nuñez M. Bactericidal activity of lactoferrin and its amidated and pepsin-digested derivatives against Pseudomonas fluorescens in ground beef and meat fractions. J Food Prot. 2009;72:760–765. doi: 10.4315/0362-028X-72.4.760. [DOI] [PubMed] [Google Scholar]
- 40.Luna CS, Aguilar RF, Samaniego BL, Godínez VD, de la Garza M. Effect of bovine apo-lactoferrin on the growth and virulence of Actinobacillus pleuropneumoniae. Biometals. 2014;27:891–903. doi: 10.1007/s10534-014-9752-5. [DOI] [PubMed] [Google Scholar]
- 41.Chan KW, Shone C, Hesp JR. Antibiotics and iron-limiting conditions and their effect on the production and composition of outer membrane vesicles secreted from clinical isolates of extraintestinal pathogenic E. coli. Proteom Clin Appl. 2017;11:1600091. doi: 10.1002/prca.201600091. [DOI] [PubMed] [Google Scholar]
- 42.Bai J, Kim SI, Ryu S, Yoon H. Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infect Immun. 2014;82:4001–4010. doi: 10.1128/IAI.01416-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl Environ Microbiol. 2014;80:3469–3483. doi: 10.1128/AEM.04248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadurugamuwa J, Beveridge TJ. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemother. 1997;40:615–621. doi: 10.1093/jac/40.5.615. [DOI] [PubMed] [Google Scholar]
- 45.van der Kraan MIA, van Marle J, Nazmi K, Groenink J, van’t Hof W, Veerman EC, Bolscher JG, Nieuw Amerongen AV. Ultrastructural effects of antimicrobial peptides from bovine lactoferrin on the membranes of Candida albicans and Escherichia coli. Peptides. 2005;26:1537–1542. doi: 10.1016/j.peptides.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Bezwoda WR, Mansoor N. Lactoferrin from human breast milk and from neutrophil granulocytes. Comparative studies of isolation, quantitation, characterization and iron binding properties. Biomed Chromatogr. 1989;3:121–126. doi: 10.1002/bmc.1130030307. [DOI] [PubMed] [Google Scholar]
- 47.Andersen BF, Baker HM, Morris GE, Rumball SV, Baker EN. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990;344:784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- 48.Sreedhara A, Flengsrud R, Langsrud T, Kaul P, Prakash V, Vegarud GE. Structural characteristic, pH and thermal stabilities of apo and holo forms of caprine and bovine lactoferrins. Biometals. 2010;23:1159–1170. doi: 10.1007/s10534-010-9366-5. [DOI] [PubMed] [Google Scholar]
- 49.Labischinski H, Barnickel G, Bradaczek H, Naumann D, Rietschel ET, Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J Bacteriol. 1985;162:9–20. doi: 10.1128/JB.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/CMR.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mekalanos JJ. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/JB.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marciel AM, Highlander SK. Use of operon fusions in Mannheimia haemolytica to identify environmental and cis-acting regulators of leukotoxin transcription. Infect Immun. 2001;69:6231–6239. doi: 10.1128/IAI.69.10.6231-6239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balashova NV, Diaz R, Balashov SV, Crosby JA, Kachlany SC. Regulation of Aggregatibacter (Actinobacillus) actinomycetemcomitans leukotoxin secretion by iron. J Bacteriol. 2006;188:8658–8661. doi: 10.1128/JB.01253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strathdee CA, Lo RY. Regulation of expression of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:5955–5962. doi: 10.1128/JB.171.11.5955-5962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. 2′2-Dipyridyl increases leukotoxin (Lkt) secretion intoM. haemolyticaA2 culture supernatants (CS). Bacteria were grown in BHI supplemented with 2′2-dipyridyl. A Western blotting of Lkt in the CS of M. haemolytica A2. B Graph of Lkt (densitometry). ****P < 0.0001.