Abstract
Background:
Adaptation of implantable cardioverter defibrillator (ICD) systems to the needs of pediatric and congenital heart patients is problematic due to constraints of vascular and thoracic anatomy. An improved understanding of the defibrillation energy and postshock pacing requirements in such patients may help direct more tailored ICD therapy. We describe the first prospective evaluation of defibrillation threshold (DFT) and postshock rhythm in this population.
Methods:
We prospectively studied patients ≤60 kg at time of ICD intervention. DFTs were obtained using a binary search protocol with three VF inductions. Postshock pacing was programmed using a stepwise protocol, lowering the rate prior to each VF induction.
Results:
Twenty patients were enrolled: 11 had channelopathy, five congenital heart disease, and four cardiomyopathy. The median age was 16 years, median weight 48 kg. Twelve patients had a transvenous high-voltage coil; eight had pericardial +/− subcutaneous coil(s). Median DFT was 7 J (range 3–31 J); 19/20 patients had DFT ≤15 J and all patients <25 kg had DFT ≤9 J (n = 6). There was no difference in DFT between patients with transvenous versus pericardial +/− subcutaneous coils (median 7 J vs 6 J, P = 0.59). No patient with normal atrioventricular conduction prior to defibrillation required postshock pacing (n = 16). There were no adverse events.
Conclusions:
These data suggest that many pediatric ICD patients have low DFTs and adequate postshock escape rhythm. This may help determine appropriate parameters for future design of pediatric-specific ICDs.
Keywords: pediatrics—electrophysiology, pediatrics—implantable devices, implantable device—defibrillation, implantable devices—pacemaker-bradyarrhythmias
Introduction
Some children with cardiac channelopathy, congenital heart disease, and cardiomyopathy have an increased risk of sudden cardiac death.1–3 The use of implantable cardioverter-defibrillator (ICD) therapy in these children has benefited from recent advances in patient selection, downsized devices, improved implant techniques, and programming strategies.4–9 Nonetheless, the vast majority of pediatric ICD practice and literature has been limited to extrapolation of data gained from experience with ICD therapy in adults. There are unique challenges to ICD implantation in younger children: limited venous capacitance increases the risk of vessel occlusion, and rapid somatic growth and physical activity likely predispose to premature lead failure. In addition, patients with congenital heart disease not infrequently have abnormal systemic venous pathways, intracardiac shunts, right-sided atrioventricular valve disease, and lack of venous access to the ventricle, all of which present additional challenges for standard ICD implantation.10 Because the conventional ICD paradigm generally assumes uncomplicated transvenous access and adult body habitus, there is a growing need to differentiate between that which is necessary, superfluous, and detrimental in an ideal pediatric-specific ICD. This study focused on two questions toward that end: (1) what are the actual defibrillation energy requirements in pediatric patients, and (2) is postshock pacing necessary?
Methods
Study Population
A prospective single-center pilot study was performed from June 2009 through May 2010. Appropriate review and approval of the Institutional Review Board was obtained. Inclusion criteria for study participants included: (1) weight ≤60 kg, (2) new or existing ICD system, and (3) clinically necessary assessment of the defibrillation efficacy of the ICD system. Transvenous systems utilized a high-voltage ICD coil with active-fixation lead attached to the right ventricular endocardial surface, whereas nontransvenous systems depended upon a high-voltage shocking coil placed (“off-label”) within the pericardial, subcutaneous, or pleural space. To be included in the postshock pacing portion of the study, adequate sinus and atrioventricular (AV) node function had to be present at baseline. Exclusion criteria included (1) tenuous hemodynamic status felt to warrant abbreviation of the defibrillation efficacy testing or (2) inability to induce fibrillation during defibrillation threshold testing (DFT). Informed consent was obtained from all subjects/legal guardians, and when appropriate, assent was also obtained from minors.
Binary Search Protocol for Defibrillation Threshold Testing
Precise measurement of the DFT was performed using a modified binary search protocol (Fig. 1). This protocol specified three distinct inductions of ventricular fibrillation (VF) for all subjects, with a 3–5-minute observation/waiting period between inductions. The initial shock energy was programmed at 9 J for all patients, with internal rescue shocks at 31 J followed by device-specific maximum deliverable energy. The outcome of the initial induction determined the programmed energies for both the initial and internal rescue shocks for the second induction, and likewise for the third induction. All shocks were biphasic and delivered at manufacturers’ default tilt, polarity, and duration, and all final programmed shock vectors included an active can. External defibrillation pads were in place for delivery of external rescue shock should the internal shocks fail. Monte Carlo analysis demonstrated that the study algorithm would predict the E70% (shock energy associated with 70% defibrillation success), with high concordance in conditions of low, average, or high true DFT.
Figure 1.
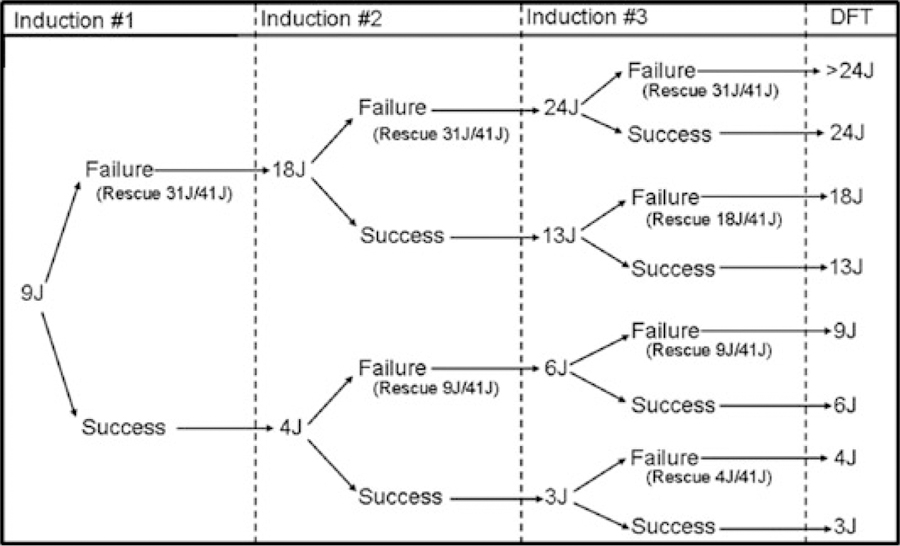
Modified binary search study protocol. All shock energies are displayed in joules (J); internal rescue shock energies are displayed in parentheses.
Postshock Pacing Protocol
To address the second study question, postshock intrinsic rhythm was assessed in all subjects who met the additional inclusion criteria of adequate sinus node function and intact AV node conduction. Prior to each of the three VF inductions performed as part of the binary search protocol, postshock pacing was reprogrammed using a predetermined, stepwise protocol that progressively decreased the lower rate limit (Fig. 2). For the purpose of this protocol, postshock pacing was considered necessary if (1) ≥7 ventricular-paced beats or (2) asystole >4 seconds was observed in the first 20 seconds after defibrillation, or if the systolic blood pressure demonstrated a >10% decrease from preinduction baseline at follow-up measurement 1 minute postdefibrillation. Rescue ventricular pacing via the programmer was available for all subjects.
Figure 2.
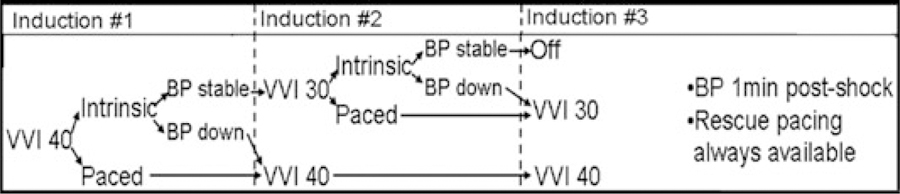
Postshock pacing protocol.
Statistical Analysis
Continuous variables were described using medians and interquartile ranges, with comparisons between groups performed using the nonparametric Mann-Whitney test. If ICD system configuration was revised during the study procedure, the final measured DFT was used in the analysis.
Results
Patient Characteristics
There were 26 ICD procedures in patients weighing ≤60 kg during the study period. Subject recruitment is detailed in Figure 3, with 20 subjects undergoing DFT testing using the binary search protocol and 16 participating in the postshock pacing portion. Baseline demographic data are outlined in Table I. Of the 11 patients with underlying channelopathy, seven had long QT syndrome, two catecholaminergic polymorphic ventricular tachycardia, and two idiopathic ventricular tachycardia/fibrillation. Three patients had hypertrophic cardiomyopathy, and one patient had left ventricular noncompaction cardiomyopathy. System configuration was relatively evenly divided amongst transvenous single coil (n = 7), transvenous dual coil (n = 5), and nontransvenous (n = 8) systems. All but one transvenous system included a left pectoral active can, and nontransvenous systems utilized both right (n = 5) and left (n = 3) subrectus muscle active cans located in the upper abdomen.
Figure 3.
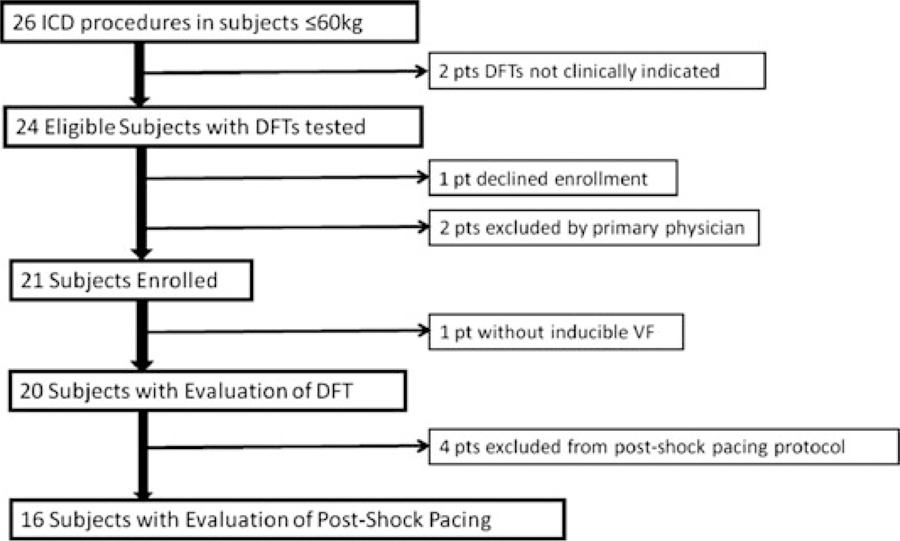
Subject recruitment flow diagram. A total of six patients were not enrolled in the binary search protocol. Two did not have clinical indication for DFT assessment secondary to previously acceptable DFT, no lead revision, and (1) hypertrophic cardiomyopathy with tenuous hemodynamic status or (2) unpalliated complete atrioventricular canal with a risk-benefit ratio that weighed against repeating DFT assessment. Two patients were excluded by the primary physician for the following reasons: One with catecholaminergic polymorphic ventricular tachycardia felt to be at high-risk for protocolized DFT assessment due to perceived risk of electrical storm, and one with extenuating social circumstances. One patient with long QT syndrome had no inducible ventricular fibrillation.
Table I.
Baseline Demographics of Study Cohort
| n = 20 | ||
|---|---|---|
| Cardiac diagnosis | Channelopathy | 11 |
| Congenital heart disease | 5 | |
| Cardiomyopathy | 4 | |
| Age (years)* | 16 (8–23) | |
| Weight (kg)* | 48 (22–57) | |
| ICD system configuration | Transvenous | 12 |
| Nontransvenous | 8 | |
| Location of high-voltage coil | Transvenous single coil | 7 |
| Transvenous dual coil | 5 | |
| Pericardial | 6 | |
| Subcutaneous | 1 | |
| Pleural | 1 | |
| Indication for defibrillation threshold testing | New implant | 7 |
| Lead revision | 5 | |
| Generator change | 5 | |
| Surveillance | 3 | |
| Antiarrhythmic medications at testing† | Beta-blocker | 13 |
| Calcium-channel blocker | 3 | |
| Amiodarone | 2 | |
| Other | 3 |
Age and weight are medians (interquartile range).
Five patients were on >1 antiarrhythmic agent; other agents included sotalol (1), mexiletine (1), and labetolol (1).
Defibrillation Thresholds
Measured DFTs in the study cohort are shown in Figure 4. The median DFT was 7 J (interquartile range 4–9 J). The DFT was ≤15 J in 19/20 subjects, and all six subjects with a weight <25 kg had a DFT ≤9 J. There was no significant difference observed in DFT between transvenous and nontransvenous ICD systems (median 7 J vs 6 J) or single and dual coil ICD leads (median 7 J in both groups). Of note, clinical care required that three subjects received more than the specified three VF inductions with a single configuration: two because of recurrent secondary terminations, and one at the discretion of the implanting EP. Conversely, one subject who had secondary termination received only two sustained inductions at the discretion of the implanting EP, and one subject who required intraoperative system revision received only two inductions in testing of the revised configuration. Eleven subjects required 1-J deviations from protocol for programmed shock energy secondary to manufacturer-specific constraints on available shock energies.
Figure 4.
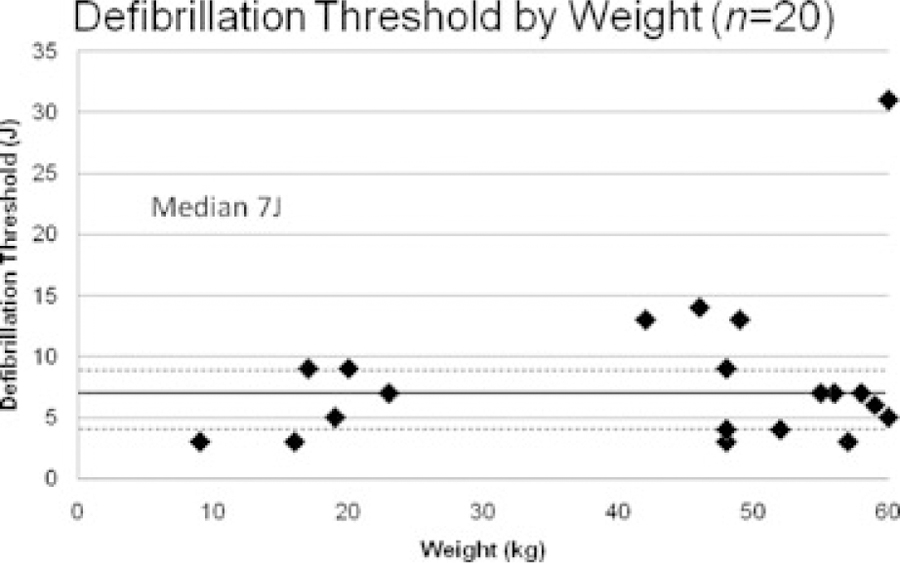
Defibrillation thresholds as measured by the binary search protocol. The solid horizontal line at 7 J marks the median DFT, with the dashed horizontal lines at 4 J and 9 J representing the 25th and 75th quartiles, respectively.
Two subjects required revision of the original ICD system configuration secondary to elevated initial DFT measurement. Both subjects had nontransvenous ICD systems. The first was a 7-year-old, 19-kg boy with long QT syndrome whose initial shock vector between a left lateral subcutaneous high-voltage coil and an active right upper quadrant subrectus generator yielded a DFT of 21 J. With addition of a right anterior subcutaneous coil to the vector, the repeat DFT assessment was 5 J. The other subject was a 20-year-old, 52-kg young woman with an unbalanced complete atrioventricular canal defect status postpalliation to a Fontan circulation. The initial system was tested with the shock vector between an inferior/leftward pericardial coil and a second rightward pericardial coil, with DFT of 35 J. Reprogramming the shock vector to include the left upper abdominal generator decreased the repeat DFT measurement to 4 J.
In those subjects with previous clinical DFT assessment and no interval revision of the shocking vector configuration, the DFT measurements obtained using the binary search protocol in this study were compared to that individual patient’s prior measured DFT (median time interval between DFTs = 56 months, range 14–73 months). Five subjects had a previous true DFT recorded, and although two subjects displayed a potentially clinically significant increase in DFT, this did not qualitatively track with somatic growth (Fig. 5). Another seven subjects had undergone previous DFT assessment using a less rigorous “lowest energy tested” strategy; the current DFT utilizing this study’s binary search protocol was, not surprisingly, lower in six of seven of these patients.
Figure 5.
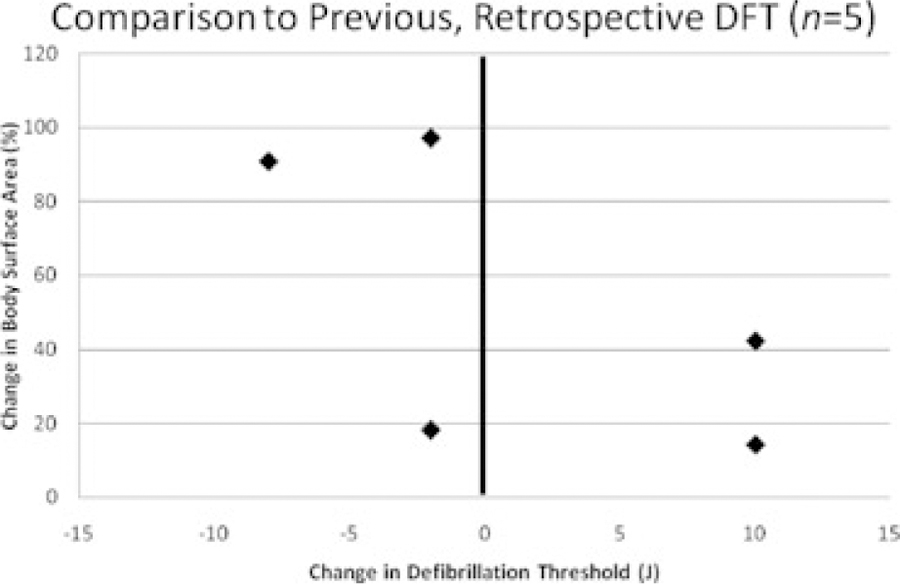
Change in defibrillation threshold (study DFT minus previous DFT) plotted against somatic growth (change in body surface area).
Postshock Rhythm
Sixteen subjects of the 20 subjects underwent evaluation of postshock intrinsic rhythm. Of the four subjects excluded from this assessment, two had complete AV block, one had second-degree AV block, and one was excluded at the discretion of the implanting electrophysiologist. Fifty-six VF inductions were performed in the 16 subjects, with postshock rhythm evaluable following 49 events. No patient required >7 paced beats, developed asystole >4 seconds, or experienced >10% decrease in systolic blood pressure. Of note, 2/49 events had data collection truncated to ~10 seconds postevent secondary to interference of wireless telemetry. The postshock rhythm was nonevaluable following 7/56 events secondary to inadvertent failure to reprogram prior to two inductions, interference of wireless telemetry following three conversions, and other unavailable data on two occasions.
There were no adverse events related to the study protocol.
Discussion
ICD therapy has been used in children and young adults for primary and secondary prevention of sudden cardiac death.4–9 There is a growing body of pediatric ICD literature addressing technical implant considerations, programming strategies, and clinical outcomes. Unfortunately, ICD therapy remains suboptimal in pediatric and congenital heart disease patients as compared to the adult cohorts for whom ICD therapy was primarily intended.5,6,11,12 This study was designed with the goal of starting to identify which elements of the conventional ICD system are truly necessary in pediatric-specific ICD therapy, while conversely recognizing those features which may be superfluous or even detrimental. In other words, this represents an attempt to step away from describing “how” adult ICD therapy can be applied to the pediatric population and instead asks “what” high-risk pediatric patients need from ICDs.
Defibrillation Energy Requirements in Young ICD Recipients
Although there has been much progress over the past two decades in reducing ICD generator size, the requirement for relatively large-volume cans limits pocket site selection and increases the risk for pocket breakdown in younger pediatric patients. Many factors have driven the default to manufacture only ICDs capable of delivering ≥31 J, but the defibrillation energy requirements in the pediatric ICD population remain incompletely defined. The two largest reports of pediatric ICD therapy provide a global report of implant experience and clinical outcomes without specific report of systematic DFTs.4,5 The adult DFT experience has well demonstrated that the testing strategy matters,13 but the pediatric ICD series that have specifically reported DFTs have not typically specified the method used to assess defibrillation efficacy.11,14–19 The notable exception is the retrospective report by Stefanelli et al.,20 which found a median DFT of 10 J in 27 subjects by use of the step-down method. The current prospective trial used a binary search algorithm for measurement of defibrillation efficacy in a pediatric and congenital heart disease cohort. The median DFT was 7 J in 20 subjects, suggesting that it may be possible to identify a group for whom lower-energy devices may be a sufficient “bridge” therapy until somatic growth accommodates standard therapy.
Postshock Intrinsic Rhythm in Young ICD Recipients
For those pediatric patients who are not candidates for transvenous ICD systems, an epicardial pacing lead has been the sole option for pace-sense function. In regard to sensing, there has been recent development of an alternative approach to recognition of tachyarrhythmias using only minimally invasive subcutaneous leads.21 From a pacing perspective, many pediatric patients at high risk for sudden death do not have a concomitant indication for antibradycardia or biventricular pacing.5 The role of antitachycardia pacing in the pediatric population continues to evolve, but clinical practice has demonstrated that this therapy offers minimal benefit for some pediatric subcohorts such as children with a primary channelopathy. In subjects who at baseline had no primary indication for antibradycardia pacing, this study specifically examined the postshock intrinsic rhythm by temporarily reprogramming postshock pacing. None of the 16 evaluated subjects demonstrated clinically significant bradycardia or pauses following defibrillation. If such pilot findings are confirmed in broader pediatric cohorts and there is increased availability of technology allowing identification of tachyarrhythmias without dependence on near-field endo- or epicardial sensing, there may be increased confidence in deferring placement of an epicardial pacing lead for selected patients without transvenous ventricular access.
Special Considerations in Young ICD Recipients
Although anecdotal, the observed influence of shocking vector on defibrillation efficacy for two subjects in this cohort reinforces the need for improved processes to allow preprocedural assessment of multiple potential shock vectors in subjects requiring nonconventional (e.g., wholly nontransvenous) ICD configurations. Such predictive modeling is currently in development,22 but has not yet progressed to be readily available for implanting physicians to apply to specific clinical patients. Also worth noting are the changes in defibrillation energy requirements over time for those subjects in this cohort who had previous rigorously measured DFTs. We observed that two of five such subjects had a 10-J increase in serial DFTs. Although there remains an active debate in the electrophysiology community regarding the utility of DFTs, these very small numbers are in concordance with data from both pediatric and adult series finding a clinically significant increase in 15% of patients undergoing routine surveillance DFTs.23,24 A priori identification of these patients remains an elusive goal that is prerequisite for the advancement of defibrillation therapy.
Study Limitations
There are inherent limitations that influence the interpretation and applicability of the findings from this study. The small sample size is of particular importance given the pronounced heterogeneity of the patient population and the configuration of the ICD systems. Of note, patients with primary channelopathy were relatively overrepresented in our cohort as compared to larger registry data5 (55% vs 31%). Both patients with congenital heart defects and very young patients were under-represented in this single-center pilot study. The physiology of defibrillation in adolescents with long QT syndrome may be as different from infants with palliated single ventricle congenital heart disease as adults with ischemic cardiomyopathy, and it may be no more appropriate to indiscriminately combine the first two “pediatric” cohorts as it would be to merge the latter two groups. Furthermore, defibrillation is by nature probabilistic and not deterministic, and any generalizations (particularly those arising from anecdotal observations) need be tempered by acknowledgement of this reality. Any proposed modification of clinical ICD therapy in children must be constrained by the potentially extraordinarily high cost of failed therapy in an outlying patient. In addition, it should be recognized that measurement of the DFT is simply one approach to measuring defibrillation efficacy; this study was not designed as an analysis of the DFT measurement in a pediatric cohort.
Conclusion
These data suggest that many pediatric ICD patients have low DFTs and hemodynamically adequate postshock intrinsic rhythm. These findings may help determine appropriate parameters for future design of pediatric-specific ICDs.
Acknowledgments:
The authors wish to acknowledge the contributions of Doug Lang, PhD (Boston Scientific Corporation), who contributed technical expertise for performance of Monte Carlo analysis.
Financial support: Supported in part by the John R. Grey IV Cardiology Fellowship Fund at Children’s Hospital, Department of Cardiology, Children’s Hospital Boston.
Footnotes
None of the authors have any relevant financial relationships to disclose.
References
- 1.Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998; 32:245–251. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg I, Moss AJ, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation 2008; 117:2184–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF, Lurie PR, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: Findings from the Pediatric Cardiomyopathy Registry. Circulation 2007; 115:773–781. [DOI] [PubMed] [Google Scholar]
- 4.Silka MJ, Kron J, Dunnigan A, Dick M 2nd. Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. The Pediatric Electrophysiology Society. Circulation 1993; 87:800–807. [DOI] [PubMed] [Google Scholar]
- 5.Berul CI, Van Hare GF, Kertesz NJ, Dubin AM, Cecchin F, Collins KK, Cannon BC, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol 2008; 51:1685–1691. [DOI] [PubMed] [Google Scholar]
- 6.Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol 2004; 15:72–76. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them? Data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation 2010; 122:1272–1282. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007; 298:405–412. [DOI] [PubMed] [Google Scholar]
- 9.Dubin AM, Berul CI, Bevilacqua LM, Collins KK, Etheridge SP, Fenrich AL, Friedman RA, et al. The use of implantable cardioverter-defibrillators in pediatric patients awaiting heart transplantation. J Card Fail 2003; 9:375–379. [DOI] [PubMed] [Google Scholar]
- 10.Berul CI. Defibrillator indications and implantation in young children. Heart Rhythm 2008; 5:1755–1757. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson EA, Batra AS, Knilans TK, Gow RM, Gradaus R, Balaji S, Dubin AM, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol 2006; 17:41–46. [DOI] [PubMed] [Google Scholar]
- 12.Radbill AE, Triedman JK, Berul CI, Fynn-Thompson F, Atallah J, Alexander ME, Walsh EP, et al. System survival of nontransvenous implantable cardioverter-defibrillators compared to transvenous implantable cardioverter-defibrillators in pediatric and congenital heart disease patients. Heart Rhythm 2010; 7:193–198. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow CD, Russo AM, Degroot PJ. The dilemma of ICD implant testing. Pacing Clin Electrophysiol 2007; 30:675–700. [DOI] [PubMed] [Google Scholar]
- 14.Heersche JH, Blom NA, van de Heuvel F, Blank C, Reimer AG, Clur SA, Witsenburg M, et al. Implantable cardioverter defibrillator therapy for prevention of sudden cardiac death in children in the Netherlands. Pacing Clin Electrophysiol 2010; 33:179–185. [DOI] [PubMed] [Google Scholar]
- 15.Botsch MP, Franzbach B, Opgen-Rhein B, Berger F, Will JC. ICD therapy in children and young adults: Low incidence of inappropriate shock delivery. Pacing Clin Electrophysiol 2010; 33:734–741. [DOI] [PubMed] [Google Scholar]
- 16.Goel AK, Berger S, Pelech A, Dhala A. Implantable cardioverter defibrillator therapy in children with long QT syndrome. Pediatr Cardiol 2004; 25:370–378. [DOI] [PubMed] [Google Scholar]
- 17.Tomaske M, Pretre R, Rahn M, Bauersfeld U. Epicardial and pleural lead ICD systems in children and adolescents maintain functionality over 5 years. Europace 2008; 10:1152–1156. [DOI] [PubMed] [Google Scholar]
- 18.Cannon BC, Friedman RA, Fenrich AL, Fraser CD, McKenzie ED, Kertesz NJ. Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. Pacing Clin Electrophysiol 2006; 29:181–187. [DOI] [PubMed] [Google Scholar]
- 19.Hsia TY, Bradley SM, LaPage MJ, Whelan S, Saul JP, Ringewald JM, Reed JH. Novel minimally invasive, intrapericardial implantable cardioverter defibrillator coil system: A useful approach to arrhythmia therapy in children. Ann Thorac Surg 2009; 87:1234–1238. [DOI] [PubMed] [Google Scholar]
- 20.Stefanelli CB, Bradley DJ, Leroy S, Dick M 2nd, Serwer GA, Fischbach PS. Implantable cardioverter defibrillator therapy for life-threatening arrhythmias in young patients. J Interv Card Electrophysiol 2002; 6:235–244. [DOI] [PubMed] [Google Scholar]
- 21.Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med 2010; 363:36–44. [DOI] [PubMed] [Google Scholar]
- 22.Jolley M, Stinstra J, Tate J, Pieper S, Macleod R, Chu L, Wang P, et al. Finite element modeling of subcutaneous implantable defibrillator electrodes in an adult torso. Heart Rhythm 2010; 7:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson EA, Cecchin F, Walsh EP, Berul CI. Utility of routine follow-up defibrillator threshold testing in congenital heart disease and pediatric populations. J Cardiovasc Electrophysiol 2005; 16:69–73. [DOI] [PubMed] [Google Scholar]
- 24.Tokano T, Pelosi F, Flemming M, Horwood L, Souza JJ, Zivin A, Knight BP, et al. Long-term evaluation of the ventricular defibrillation energy requirement. J Cardiovasc Electrophysiol 1998; 9:916–920. [DOI] [PubMed] [Google Scholar]


