Abstract
Aim:
Common indoor pollutants, as fine particulate matter (PM2.5), can damage people's health and cause skin allergies. However, it remains unknown which common pollutants can lead to allergy, such as, in children atopic dermatitis, and what is the key molecule. This study aimed to investigate the thymic stromal lymphopoietin (TSLP) produced from keratinocytes after environmental pollutant stimulation.
Methods:
PAM212 cells were treated by several pollutants, including PM2.5, formaldehyde, m-xylene, and 1,2,4-trimethylbenzene, and tried to analyze their relationships. The mRNA expression level of TSLP was determined by qPCR. The protein level of TSLP was detected by ELISA analysis.
Results:
The mRNA expression of TSLP was significantly up-regulated when PAM212 cells were stimulated by PM2.5 at 25 μg/ml for 12 h. Meanwhile, the protein level of TSLP in culture supernatant was increased. However, TSLP protein production was not detected in culture supernatant treated with formaldehyde, m-xylene, and 1, 2, 4-trimethylbenzene.
Conclusion:
PM2.5 promotes the expression of TSLP and may aggravate allergic response using this pathway.
KEY WORDS: Atopic dermatitis, keratinocyte cell, PM2.5, thymic stromal lymphopoietin
Introduction
In recent years, more and more patients are suffering from atopic dermatitis. The prevalence of 3–6-year-old children with atopic dermatitis reached 8.3% in 2010.[1] Many studies have focused on the molecular mechanism of the development of atopic dermatitis. Thymic stromal lymphopoietin (TSLP), an IL-7 like cytokine produced by dendritic cells, is recognized as a regulator in the process of allergy.[2] Mou et al. demonstrated that the mRNA of TSLP and its protein expression were highly increased in allergic rhinitis patients. Furthermore, serum TSLP level was increased in children with atopic dermatitis.[3,4] It has been reported that xylene may induce TSLP resulting in an exacerbation of allergy.[5] These findings indicated that production of TSLP could be induced by environmental pollutants. Obviously, effects of air pollutant on children immune system are still developing.[6] Exposure of children to formaldehyde and fine particular matter (PM2.5) is associated with decreasing lung function in atopic boys.[7,8] Indoor PM2.5 is significantly associated with asthma and allergic diseases.[9]
The primary aim of this study was to investigate whether there was a relationship between air pollutants (PM2.5, formaldehyde, xylene, and 1,2,4-trimethylbenzene) and TSLP expression in vitro. We hypothesized the PM2.5 exposure might induce TSLP expression in the keratinocyte cell; the effect of different titers of PM2.5, and other pollutants on TSLP expression in the PAM212 cell were examined to test the hypothesis. We also examined the effects of 12-O-Tetradecanoylphorbol 13-acetate (TPA) which were demonstrated that those could increase TSLP expression.
Materials and Methods
Materials
The murine keratinocyte cell line PAM212, which was derived from BALB/c mouse skin, was kindly bestowed by Dr. Yuspa (National Institutes of Health, NCI, USA).
Main reagents: RPMI-1640 culture medium (Gibco, 11875093), FBS (Gibco, 12483020), 0.25% trypsin-EDTA (Gibco, 25200114), m-xylene (sigma, 95670), 1,2,4-trimethylbenzene (sigma, 45996), formaldehyde solution (sigma, 252549), TPA (sigma, P1585), PM2.5 (bestowed by the Department of Environmental Health, School of Public Health, Fudan University), mouse TSLP ELISA kit (R&D, MTLP00), reverse transcription kit PrimeScriptTM (Takara, RR037A), and GoTap qPCR master mix (promega, A6002).
Methods
Cell culture
PAM212 cells were cultured in RPMI-1640 medium supplemented with 10% FBS. The passage was made when cells were 90% confluent. After the supernatant was discarded, cells were washed twice with Dulbecco's phosphate-buffered saline (DPBS) and then digested with trypsin-EDTA (0.25%) for 3–5 min. The same amount of medium was added to terminate the digestion, then the cells were centrifuged at 200 g for 5 min, re-suspended and inoculated at 1:3–5.
Cell toxicity testing
PAM212 cells in the logarithmic phase were digested with trypsin. After centrifugation and supernatant discard, fresh medium was added to re-suspend cells. The number of cells was counted and concentration adjusted. Cells were inoculated to 96-well plates at 2.4 × 104 cells/well and 100 μl/well, and then put into an incubator (37°C, 5% CO2) overnight. On the next day, fresh medium without blood serum was used for substitution and formaldehyde, TPA, m-xylene, 1,2,4-trimethylbenzene, PM2.5 of various concentrations were added to incubate for another 24 or 48 h.
After the incubation, 10 μl CCK-8 solution was added to each well followed by agitation. After the additional incubation of 3 h at 37°C, 10 ml 0.1M HCl was added to each well to terminate the reaction. Microplate reader was used to detect absorbance value at 450 nm, and cell viability was calculated.
Detecting the expression of mRNA for TSLP in PAM212 cells
The mRNA of cells was extracted and reverse transcriptase using a PrimeScriptTM RT reagent kit (Perfect Real Time) according to the manufacturer's instructions. The sequences of the primer used and polymerase chain reaction (PCR) conditions for the amplification of TSLP cDNA were (forward) 5’-GCTAAGTTCGAGCAAATCGAGG-3’ and (reverse) 5’-GCCAGGGATAGGATTGAGAGTA-3’, 38 cycles of denaturation at 95°C for 5 min, annealing at 95°C for 10 s, and extension at 60°C for 30 s. The murine actin gene was used as an internal standard. The sequences of primer used for the amplification of actin cDNA were (forward) 5’-AGAGGGAAATCGTGCGTGAC-3’ and (reverse) 5’-CAATAGTGATGACCTGGCCGT-3’. PCR was performed for 38 cycles of denaturation at 95°C for 5 min, annealing at 95°C for 10 s, and extension at 60°C for 30 s.
Determination of TSLP protein in supernatant of cells
PAM212 cell stimulated by PM2.5 and TPA were chosen to test the level of TSLP protein in supernatant. One group of PAM212 stimulated by drugs was arranged for culture for another 24 h after replacement of solution following the first 24-h treatment, whereas the other group was, after stimulation, cultivated non-stop for 48 h followed by supernatant collection. Mouse TSLP ELISA kit was used to test the level of TSLP in supernatant.
Statistical analysis
The statistical significance of results was analyzed with chi-square test for group comparisons.
Results
Cell toxicity
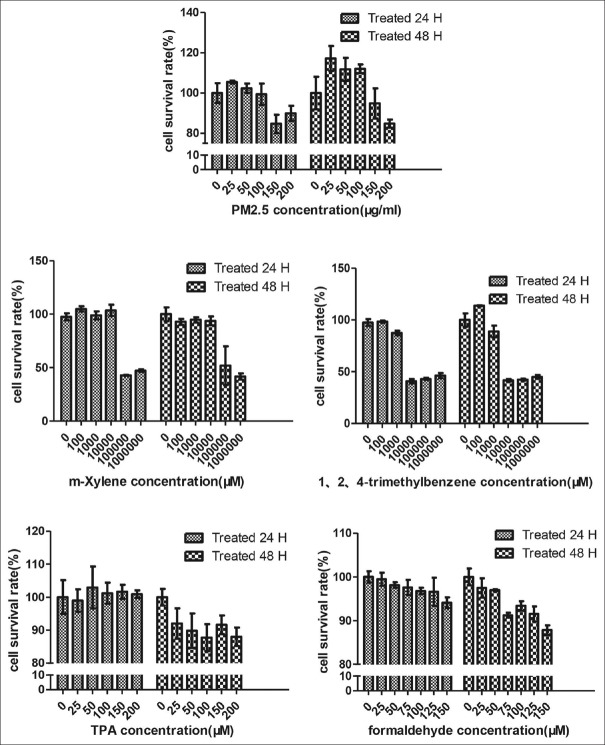
Cell toxicity analysis showed that cell viability was significantly decreased when exposed to PM2.5 at 150 μg/ml (24 h), formaldehyde at 75 μM (48 h), m-xylene at 100000 μM (24 h), and 1,2,4-trimethylbenzene at 10000 μM (24 h) [Figure 1, P ≤ 0.05].
Figure 1.
Cell activity analysis after several pollutants treatment
TSLP mRNA induced by pollutants
qPCR results showed that when stimulated by PM2.5 at 25 μg/ml for 12 h, the mRNA expression level of TSLP was significantly higher, and slightly decreased following increasing the concentration of PM2.5. When formaldehyde was at the concentration of 50 μM, the level of TSLP mRNA was as low as control group, much lower than PM2.5. After stimulating with m-xylene and 1,2,4-trimethylbenzene for 24 h, lower levels of TSLP mRNA were detected than with formaldehyde [Figure 2, P < 0.05, and Table 1].
Figure 2.
Induction of mRNA of TSLP by pollutants. Various concentrations of pollutant solvents were applied on PAM212 cells. The level of mRNA in treated PAM212 cells was determined by RT-qPCR. Data are shown as the relative quantity of mRNA induced by pollutants vs. control group
Table 1.
Induction of mRNA of TSLP by pollutants
| Pollutant | Concentration | TSLP mRNA expression | |
|---|---|---|---|
| 12 h | 24 h | ||
| Control | 0 | 0.63±0.23 | 0.63±0.23 |
| Formaldehyde | 50 μM | 1.29±0.22 | 0.14±0.03 |
| PM2.5 | 25 μg/ml | 3.72±1.29 | 4.18±1.05* |
| 50 μg/ml | 2.85±1.28 | 3.45±0.62* | |
| 100 μg/ml | 6.12±2.99* | 8.70±0.43* | |
| TPA | 0.02 μM | 22.21±13.52* | 10.64±0.68* |
| 0.05 μM | 20.00±13.47* | 8.40±0.37* | |
| 0.1 μM | 18.36±17.44* | 3.32±0.37* | |
| m-Xylene | 100 μM | 0.31±0.07 | 0.52±0.26 |
| 1000 μM | 0.32±0.24 | 0.44±0.33 | |
| 10000 μM | 0.54±0.23 | 0.34±0.02 | |
| 1, 2, 4-trimethylbenzene | 100 μM | 0.36±0.07 | 0.34±0.11 |
| 500 μM | 0.74±1.16 | 0.25±0.20 | |
| 1000 μM | 0.92±0.35 | 0.61±0.05 | |
Varous concentration of pollutant solvents were applied on PAM212 cells. The level of mRNA in treated PAM212 cells was determined by RT-qPCR. Data are shown as the relative quantity of mRNA induced by pollutants vs control group. Statistical significance: *P<0.05
TSLP production induced by PM2.5
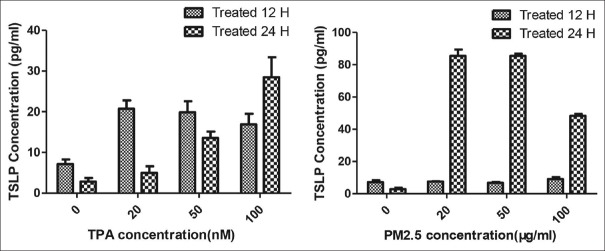
After different dose stimulation with PM2.5 and TPA, the concentration of TSLP production in cell supernatant was significantly higher, and the production of TSLP showed dose dependency [Figure 3, P ≤ 0.05, and Table 2]. Meanwhile, formaldehyde, m-xylene, and 1,2,4-trimethylbenzene groups were not found to produce TSLP in cell supernatant.
Figure 3.
PM2.5 (25 μg/ml, 50 μg/ml, and 100 μg/ml) was treated on the PAM212 cell for 24 h and 48 h. Data are shown as the mean ± SEM. TPA (20 nM, 50 nM, and 100 nM) was treated on the cells for 24 h and 48 h
Table 2.
Induction by PM2.5 and TPA of TSLP production in the cell supernatant
| TSLP Concentration (pg/ml) | ||
|---|---|---|
| 24 h | 48 h | |
| Control | 7.12±1.15 | 2.85±0.86 |
| TPA | ||
| 20 μg/ml | 20.76±2.01* | 4.99±1.58 |
| 50 μg/ml | 19.84±2.73* | 13.53±1.58* |
| 100 μg/ml | 16.89±2.59* | 28.49±4.89* |
| PM2.5 | ||
| 25 nM | 7.53±0.29 | 85.37±3.89* |
| 50 nM | 6.82±0.43 | 85.37±1.30* |
| 100 nM | 9.16±1.15 | 48.23±1.15* |
TPA (20 nM, 50 nM, 100 nM) was treated on the cells for 24 h and 48 h. Data are shown as the mean±SEM. PM2.5 (25 μg/ml, 50 μg/ml, 100 μg/ml) was treated on the PAM212 cell for 24 h and 48 h. Data are shown as the mean±SEM. *P<0.01 versus control group
Discussion
TSLP is a cytokine induced by Th2-type allergic inflammation. Exposure to air pollutants has a strong relationship with allergic diseases[10,11,12], but it remains a debate how the pollutants affect the allergic immune system. In the present study, PM2.5 and formaldehyde significantly up-regulated mRNA expression of TSLP in vitro. However, only PM2.5 promoted TSLP production. Interestingly, m-xylene, and 1,2,4-trimetlybenzene did not result in the expression of TSLP, which were observed that those solvents could induce TSLP in vivo in previous study.[5]
In a recent study, aryl hydrocarbon was reported to have a positive relationship to the patients with atopic dermatitis. Aryl hydrocarbon is the main component of PM2.5, which can regulate the expression of gene encoding inflammation in the mice through binding to AhR (aryl hydrocarbon receptor).[13] Our data indicated that PM2.5 promoted TSLP production in vitro, partly by binding to a specific protein, such as AhR.
12-O-Tetradecanoylphorbol 13-acetate (TPA) induced production of TSLP in the mouse keratinocyte cell was observed by Segawa in 2014,[14] which was also detected in our study. Meanwhile, formaldehyde, m-xylene, and 1,2,4-trimetlybenzene did not stimulate TSLP expression after stimulating for 48 h, which was reported to be triggers in allergic inflammation.[5] Exposure to cigarette smoke extract was reported to induce TSLP production and promote allergic response and inflammation.[15] Therefore, considering those chemical solvents often detected in the indoor environment, the inhalation of these chemical solvents may induce TSLP production after a long-time exposure.
In conclusion, the present study revealed that PM2.5, one of the common indoor pollutants could promote the production of TSLP and aggravated allergic response in vitro.
Financial support and sponsorship
The study was supported by a grant from the National Science Foundation of China (No. 81301361).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Xu F, Yan S, Li F, Cai M, Chai W, Wu M, et al. Prevalence of childhood atopic dermatitis: An urban and rural community-based study in Shanghai, China. Plos One. 2012;7:e36174. doi: 10.1371/journal.pone.0036174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–60. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 4.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008;181:4311–9. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 5.Satou N, Ishihara K, Hiratsuka M, Tanaka H, Endo Y, Saito S, et al. Induction of thymic stromal lymphopoietin production by xylene and exacerbation of picryl chloride-induced allergic inflammation in mice. Int Arch Allergy Immunol. 2012;157:194–201. doi: 10.1159/000327545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–373e5. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environ Health Perspect. 2002;110(Suppl 4):573–89. doi: 10.1289/ehp.02110s4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu F, Yan S, Wu M, Li F, Xu X, Song W, et al. Ambient ozone pollution as a risk factor for skin disorders. Br J Dermatol. 2011;165:224–5. doi: 10.1111/j.1365-2133.2011.10349.x. [DOI] [PubMed] [Google Scholar]
- 9.Park HK, Cheng KC, Tetteh AO, Hildemann LM, Nadeau KC. Effectiveness of air purifier on health outcomes and indoor particles in homes of children with allergic diseases in Fresno, California: A pilot study. J Asthma. 2017;54:341–6. doi: 10.1080/02770903.2016.1218011. [DOI] [PubMed] [Google Scholar]
- 10.Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Miller RL, Flak E, et al. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155:275–81. doi: 10.1159/000320376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song S, Lee K, Lee YM, Lee JH, Lee SI, Yu SD, et al. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ Res. 2011;111:394–9. doi: 10.1016/j.envres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–8e1. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18:64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 14.Hirasawa N, Ohsawa Y, Ishihara K, Seyama T, Hong J, Ohuchi K. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: establishment of a modified allergic dermatitis model in mouse ear lobes by application of 12-O-tetradecanoyl phorbol 13-acetate: Putative involvement of thymic stromal lymphopoietin and roles of histamine. J Pharmacol Sci. 2009;110:245–50. doi: 10.1254/jphs.09r03fm. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Miyata M, Hobe T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–14. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]