Abstract
A series of aryloxyacetic acid derivatives were designed and synthesized as 4-hydoxyphenylpyruvate dioxygenase (HPPD) inhibitors. Preliminary bioassay results reveal that these derivatives are promising Arabidopsis thaliana HPPD (AtHPPD) inhibitors, in particular compounds I12 (Ki = 0.011 µM) and I23 (Ki = 0.012 µM), which exhibit similar activities to that of mesotrione, a commercial HPPD herbicide (Ki = 0.013 µM). Furthermore, the newly synthesized compounds show significant greenhouse herbicidal activities against tested weeds at dosages of 150 g ai/ha. In particular, II4 exhibited high herbicidal activity for pre-emergence treatment that was slightly better than that of mesotrione. In addition, compound II4 was safe for weed control in maize fields at a rate of 150 g ai/ha, and was identified as the most potent candidate for a novel HPPD inhibitor herbicide. The compounds described herein may provide useful guidance for the design of new HPPD inhibiting herbicides and their modification.
Keywords: aryloxyacetic acid, herbicidal activity, 4-hydroxyphenylpyruvate dioxygenase, modification, synthesis
Introduction
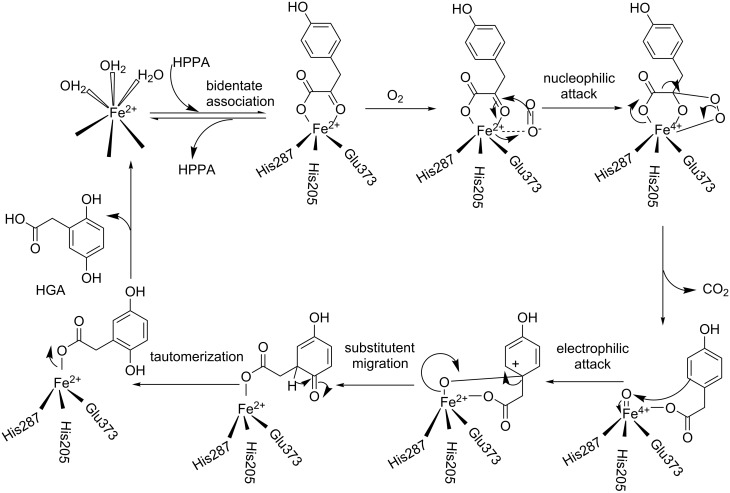
4-Hydroxyphenylpyruvate dioxygenase (EC 1.13.11.27, HPPD), which belongs to the family of non-heme FeII-containing enzymes, is a vital enzyme for tyrosine catabolism. This enzyme is found in microbes, mammals, and plants, and has different functions in different organisms [1]. In the catalytic process of HPPD, 4-hydroxyphenylpyruvic acid (HPPA) and FeII form a chelate complex, from which the HPPA substrate is converted into homogentisic acid (HGA). The generally accepted catalytic mechanism for this process is shown in Scheme 1 [2–6]. The HPPD amino acid sequence homologies in plants and mammals are significantly different [7–8], and this difference affects the binding stability between an inhibitor and HPPD, leading to inhibitor activities that differ among various species and genera and providing a theoretical basis for the design of inhibitors that are highly selective and safe [2].
Scheme 1.
The commonly recognized HPPD catalytic reaction mechanism.
In plants, HPPD inhibitors competitively restrain HPPA from chelating to FeII. The production of plastoquinone is inhibited and phytoene is accumulated when the transformation of HPPA to HGA is interfered with an HPPD inhibitor [9–10]; consequently, plants become severely damage when exposed to sunlight, ultimately resulting in bleaching symptoms followed by necrosis and death [11–12]. Therefore, HPPD inhibitors play important roles in the herbicide industry. In addition, HPPD inhibiting herbicides are advantageous because of their low toxicities, high efficiencies, broad-spectrum weed control, and safety toward crops and the environment [13–15]. However, the abuse of HPPD inhibitors has led to increased weed resistance and crop damage. Furthermore, the long-term applications of a single herbicide result in the resistance of the weed to the agent [14]. Therefore, exploring effective HPPD-inhibiting compounds for the control of resistant weeds is an emergent and important objective [2].
A considerable number of HPPD inhibiting herbicides have recently been commercialized and applied in the agrochemical industry. These herbicides are mainly divided into three categories: triketones, pyrazoles, and isoxazoles [9,15–16]. Figure 1 shows some HPPD-inhibiting herbicides, namely mesotrione, tefuryltrione, isoxaflutole, topramezone, and pyrasulfotole. Among them, mesotrione is a highly successful representative triketone HPPD herbicide. Figure 1 reveals that the HPPD inhibiting herbicides mostly contain 1,3-dicarbonyl or analogous structures [11,15]. Arabidopsis thaliana HPPD (AtHPPD) and its inhibitors have been reported to interact in two ways: 1) through 1,3-dicarbonyl bidentate chelation with the active center metal, and 2) through favorable sandwich π–π stacking interactions between aromatic rings and the Phe360, Phe403 residues of the active site. Thus, 1,3-dicarbonyl and aromatic moieties are indispensable pharmacophores for potent HPPD-inhibiting compounds that interact with surrounding residues in AtHPPD [16–19].
Figure 1.
Chemical structures of the commercial HPPD inhibitors.
2,4-Dichlorophenoxyacetic acid (2,4-D), which acts as a plant growth hormone, was synthesized in 1941. It is a selective pre- and post-emergence herbicide that has applied to several crops [17]. 2,4-D interferes with the hormone balance of the plant, which interrupts nucleic-acid and protein metabolism, and is especially effective in broadleaf weeds, such as Amaranthus retroflexus and Alfalfa. The application of 2,4-D causes excessive growth that ultimately results in plant death. Consequently, 2,4-D has become one of the world’s major herbicides because low dosage is used and less investment costs are required.
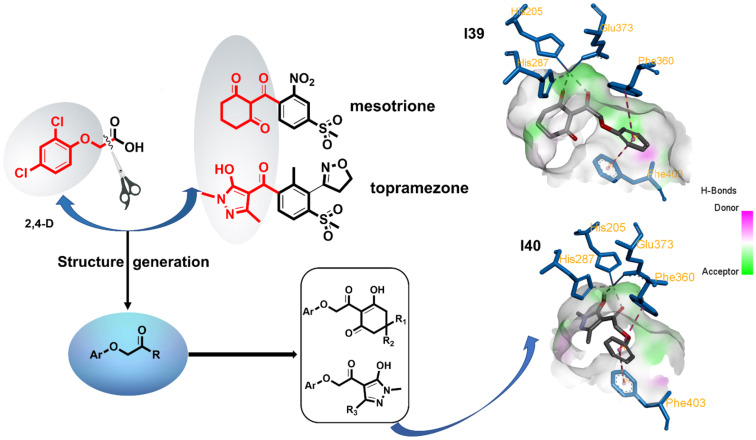
Many researches in HPPD inhibitors have revealed that modifying of aromatic moieties is an effective way of producing new HPPD inhibiting herbicides [20–23]. However, little effort has been directed toward modifying pyrazole derivatives and the carbon−carbon bond between 1,3-dicarbonyl and aroyl moieties. Previously, a series of 2-(aryloxyacetyl)cyclohexane-1,3-diones was synthesized by Wang et al. [24]. We have been interested in inserting a carbon−oxygen bond between the triketone and aroyl moieties of HPPD inhibitors. Initially, molecular docking studies were performed on two representative compounds, namely I39 and I40 [25], in order to explore their binding modes. The result revealed the presence of two main interactions, the sandwich π−π interaction and the bidentate interaction, which are similar to those of commercial mesotrione. Inspired by the above revelations, we synthesized a group of new HPPD inhibitors that contain pyrazole and triketone moieties to study their bioactivities; the design strategy is shown in Figure 2. By combining the two bioactive structures, namely the aromatic moieties of 2,4-D and the 1,3-dicarbonyl unit, we designed and synthesized a series of novel aryloxyacetic acid derivatives. In this context, these derivatives were subjected to HPPD inhibition, herbicidal activity, crop safety and structure–activity relationship (SAR) studies. As expected, many of the title compounds displayed promising inhibitory activity against Arabidopsis thaliana HPPD (AtHPPD) in vitro and excellent herbicidal activities at a rate of 150 g ai/ha.
Figure 2.
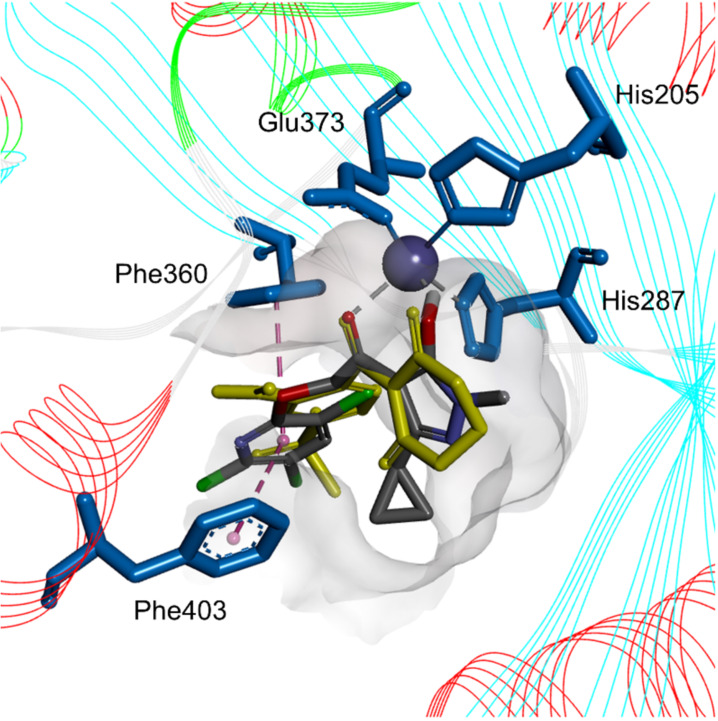
The design strategy of aryloxyacetic acid derivatives as HPPD inhibitors and simulate the binding modes of compound I39 and I40 in a target enzyme (AtHPPD). The key residues in the active site are shown in blue sticks, the FeII is shown as a dark blue sphere, and compound I39 and I40 is shown in gray sticks.
Results and Discussion
Chemistry
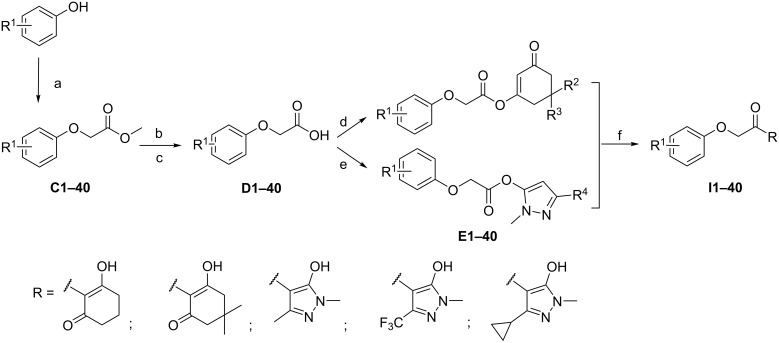
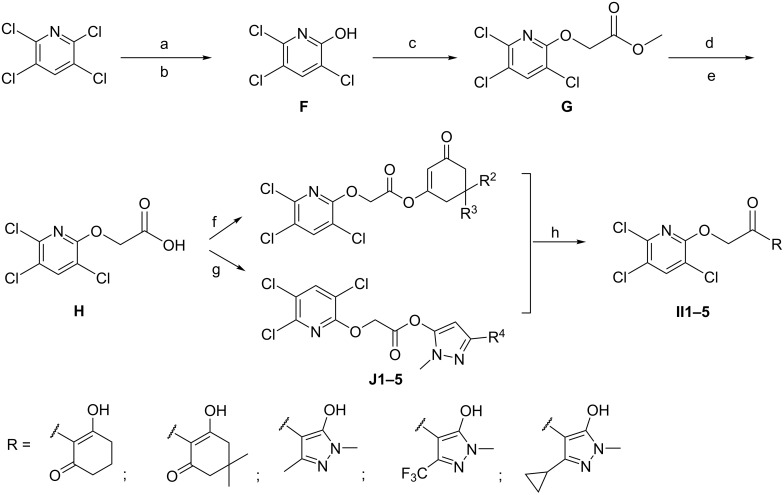
Title compounds were classified into three series (I, II and III). The preparation of the title compounds is shown in Scheme 2, Scheme 3 and Scheme 4. The synthesis of compounds I and III was depicted in Scheme 2 and Scheme 3. The commercially available starting materials reacted with methyl chloroacetate in CH3CN and anhydrous potassium carbonate (K2CO3) as the base, and the corresponding products C and K were prepared. The products were hydrolyzed using K2CO3 as a base to yield the product D and L [26–30]. In the presence of 3-(ethyliminomethylideneamino)-N,N-dimethylpropane-1-amine, hydrochloride (EDCI), the aromatic oxyacetic acid reacted with substituted 1,3-cyclohexanediones or substituted 1,3-dimethyl-1H-pyrazole-5-ol, using DMAP as the catalyst. Subsequently, the key enol ester E and M were respectively obtained. Finally, Fries-type rearrangements were performed in anhydrous DCM at room temperature to afford the title compounds I and III [31].
Scheme 2.
Synthetic route of the title compounds I. Reagents and conditions: (a) methyl chloroacetate, K2CO3, CH3CN, 65 °C; (b) K2CO3, H2O, 65 °C; (c) aqueous HCl solution (10%), rt; (d) substituted 1,3-cyclohexanediones, EDCI, DMAP, DCM, rt; (e) substituted 1,3-dimethyl-1H-pyrazol-5-ol, EDCI, DMAP, DCM, rt; (f) Et3N, acetone cyanohydrin, DCM, rt.
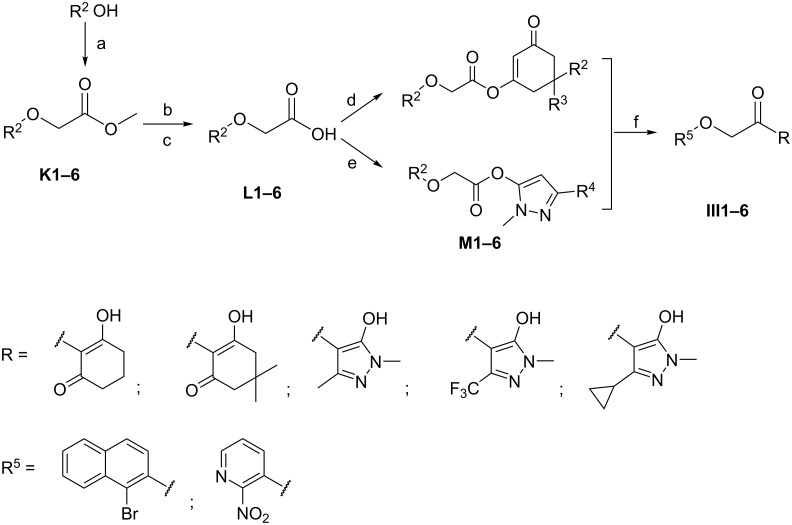
Scheme 3.
Synthetic route of the title compound III. Reagents and conditions: (a) methyl chloroacetate, K2CO3, CH3CN, 65 °C; (b) K2CO3, H2O, 65 °C; (c) aqueous HCl solution (10%), rt; (d) substituted 1,3-cyclohexanediones, EDCI, DMAP, DCM, rt; (e) substituted 1,3-dimethyl-1H-pyrazol-5-ol, EDCI, DMAP, DCM, rt; (f) Et3N, acetone cyanohydrin, DCM, rt.
Scheme 4.
Synthetic route of the title compounds II. Reagents and conditions: (a) NaOH, TBAB, H2O, 100 °C; (b) concentrated HCl solution, rt; (c) methyl chloroacetate, K2CO3, CH3CN, 65 °C; (d) K2CO3, H2O, 65 °C; (e) aqueous HCl solution (10%), rt; (f) substituted 1,3-cyclohexanediones, EDCI, DMAP, DCM, rt; (g) substituted 1,3-dimethyl-1H-pyrazol-5-ol, EDCI, DMAP, DCM, rt; (h) Et3N, acetone cyanohydrin, DCM, rt.
As shown in Scheme 4, the title compounds II were obtained by a five-step synthetic route using the commercially available 2,3,5,6-tetrachloropyridine as the starting material. In the presence of TBAB, the starting material was hydrolyzed using NaOH in water at 100 °C. The resulting solution was cooled and hydrolyzed with HCl solution that yielded compound F. Subsequent preparations for compounds G, H, J and II were respectively the same as for compounds C, D, E and I.
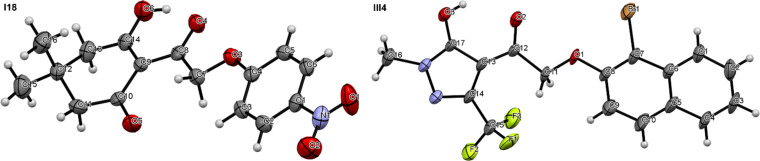
All intermediates were synthesized and characterized as detailed in Supporting Information File 1. The structures of all prepared compounds were confirmed by 1H and 13C NMR spectroscopy, and HRMS. Furthermore, the structures of compounds I18 and III4 were verified by X-ray diffractometry (Figure 3). Crystallographic data for crystalline I18 and III4 have been deposited with the Cambridge Crystallographic Data Centre (CCDC 1959130, CCDC 1959152).
Figure 3.
Crystal structures of I18 and III4.
HPPD inhibition
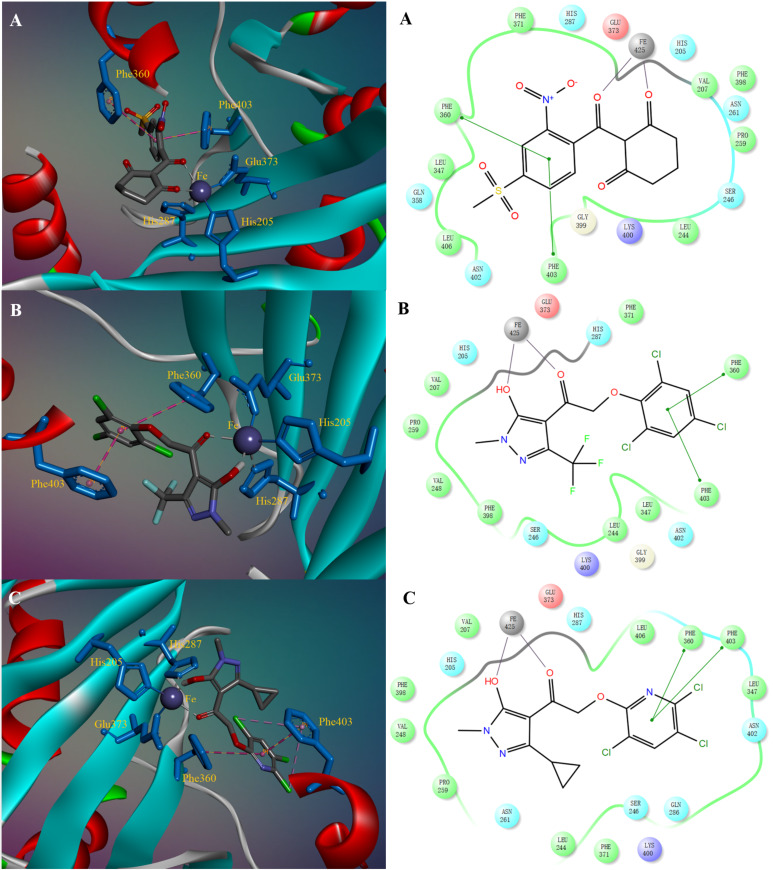
The title compounds displayed promising AtHPPD inhibitory activities, with Table 1 and Table 2 revealing that compounds I12 (Ki = 0.011 µM) and I23 (Ki = 0.012 µM) exhibit similar inhibitor potencies to that of mesotrione (Ki = 0.013 µM). Docking studies using the CDOCKER module within Discovery Studio 4.0 revealed the bioactive binding site positions of potential inhibitors within the targets active site. We modeled the interactions of I12 and II4 (C) with AtHPPD (PDB ID: 1TFZ). The structure of AtHPPD was taken from the PDB data bank. All molecular modeling studies were carried out as previously reported [10,19,32–34]. The results show that two main interactions exist between I12 and the AtHPPD active site (Figure 4), as was observed for mesotrione; the 1,3-dicarbonyl unit is chelated to the iron ion, and the aromatic ring moiety formed π–π interactions with Phe403 and Phe360.
Table 1.
Chemical structures of title compound I and their biological activity against AtHPPD.
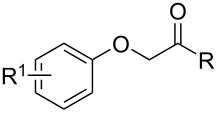 I1–40 | |||
| compound | R1 | R |
AtHPPD inhibition Ki (μM) |
| I1 | H |  |
1.5 ± 0.031 |
| I2 | H |  |
1.3 ± 0.017 |
| I3 | 2-chloro | 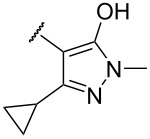 |
0.36 ± 0.012 |
| I4 | 2-chloro | 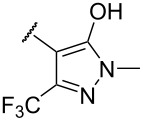 |
0.59 ± 0.043 |
| I5 | 4-chloro | 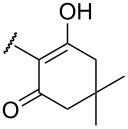 |
1.0 ± 0.036 |
| I6 | 4-chloro | 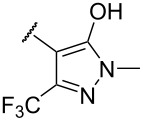 |
0.93 ± 0.032 |
| I7 | 2,4-dichloro | 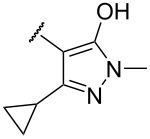 |
0.36 ± 0.012 |
| I8 | 2,4-dichloro | 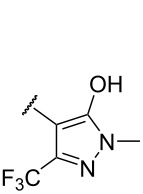 |
0.22 ± 0.023 |
| I9 | 2,4,6-trichloro | 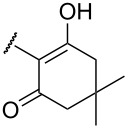 |
0.31 ± 0.048 |
| I10 | 2,4,6-trichloro | 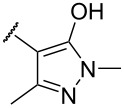 |
0.24 ± 0.003 |
| I11 | 2,4,6-trichloro | 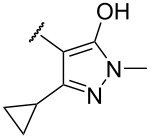 |
0.081 ± 0.001 |
| I12 | 2,4,6-trichloro | 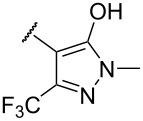 |
0.011 ± 0.012 |
| I13 | 2-nitro | 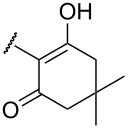 |
0.45 ± 0.033 |
| I14 | 2-nitro | 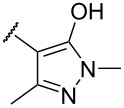 |
0.21 ± 0.042 |
| I15 | 2-nitro | 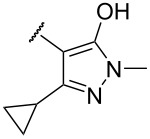 |
0.27 ± 0.004 |
| I16 | 2-nitro | 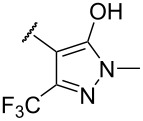 |
0.44 ± 0.013 |
| I17 | 4-nitro | 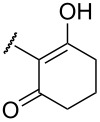 |
0.23 ± 0.004 |
| I18 | 4-nitro | 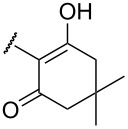 |
0.93 ± 0.006 |
| I19 | 4-nitro | 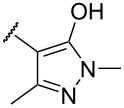 |
0.63 ± 0.002 |
| I20 | 4-nitro | 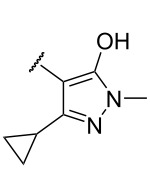 |
0.50 ± 0.003 |
| I21 | 4-nitro | 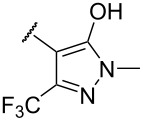 |
1.5 ± 0.041 |
| I22 | 2-chloro-4-nitro | 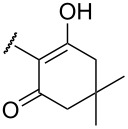 |
1.02 ± 0.009 |
| I23 | 2-chloro-4-nitro | 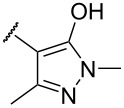 |
0.012 ± 0.009 |
| I24 | 2-chloro-4-nitro | 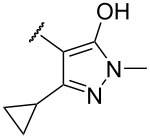 |
0.26 ± 0.012 |
| I25 | 2-chloro-4-nitro | 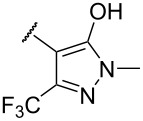 |
0.21 ± 0.043 |
| I26 | 2-(2,4-dichlorophenoxy)-4-chloro | 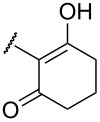 |
1.9 ± 0.001 |
| I27 | 2-(2,4-dichlorophenoxy)-4-chloro | 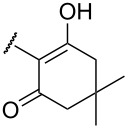 |
2.2 ± 0.041 |
| I28 | 2-(2,4-dichlorophenoxy)-4-chloro | 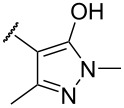 |
0.032 ± 0.002 |
| I29 | 2-(2,4-dichlorophenoxy)-4-chloro | 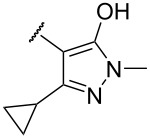 |
1.3 ± 0.022 |
| I30 | 2,4-dimethyl | 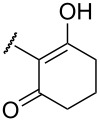 |
2.2 ± 0.034 |
| I31 | 2,4-dimethyl | 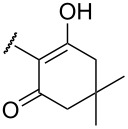 |
3.1 ± 0.34 |
| I32 | 2,4-dmethyl | 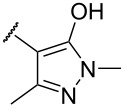 |
2.0 ± 0.009 |
| I33 | 2,4-dimethyl | 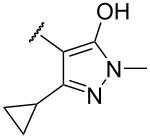 |
2.8 ± 0.045 |
| I34 | 2,4-dimethyl | 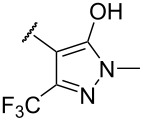 |
2.8 ± 0.67 |
| I35 | 4-methyl-5-methoxy | 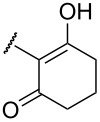 |
3.3 ± 0.14 |
| I36 | 4-methyl-5-methoxy | 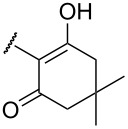 |
3.4 ± 0.21 |
| I37 | 4-methyl-5-methoxy | 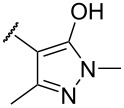 |
2.7 ± 0.53 |
| I38 | 4-methyl-5-methoxy | 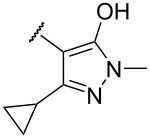 |
2.9 ± 0.038 |
| I39 | H | 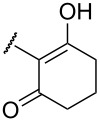 |
1.3 ± 0.056 |
| I40 | H | 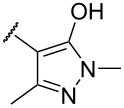 |
1.2 ± 0.031 |
| mesotrione | 0.013 ± 0.001 | ||
Table 2.
Chemical structures of title compound II, III and their biological activity against AtHPPD.
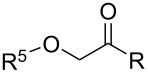 II1–5, III1–6 | |||
| compound | R5 | R |
AtHPPD inhibition Ki (μM) |
| II1 | 2,3,5-trichloro-6-pyridyl | 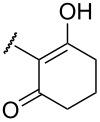 |
0.093 ± 0.007 |
| II2 | 2,3,5-trichloro-6-pyridyl | 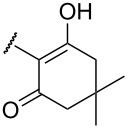 |
0.097 ± 0.010 |
| II3 | 2,3,5-trichloro-6-pyridyl | 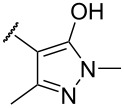 |
0.021 ± 0.004 |
| II4 | 2,3,5-trichloro-6-pyridyl | 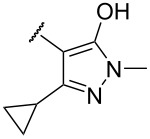 |
0.023 ± 0.006 |
| II5 | 2,3,5-trichloro-6-pyridyl | 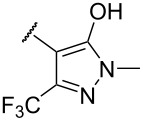 |
0.12 ± 0.003 |
| III1 | 2-bromo-2-naphthyl | 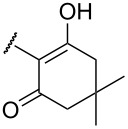 |
2.50 ± 0.011 |
| III2 | 2-bromo-2-naphthyl | 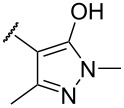 |
2.2 ± 0.090 |
| III3 | 2-bromo-2-naphthyl | 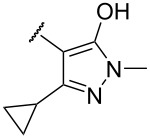 |
2.2 ± 0.13 |
| III4 | 2-bromo-2-naphthyl | 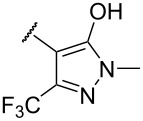 |
2.0 ± 0.012 |
| III5 | 2-nitro-3-pyridyl | 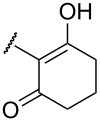 |
0.23 ± 0.011 |
| III6 | 2-nitro-3-pyridyl | 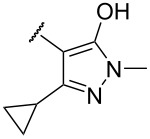 |
0.21 ± 0.042 |
| mesotrione | 0.013 ± 0.001 | ||
Figure 4.
Simulated binding mode of mesotrione (A), compound I12 (B) and compound II4 (C) with AtHPPD. The key residues in the active site are shown in blue sticks, and FeII is shown as a dark blue sphere. mesotrione, compound I12, and II4 are shown in gray sticks.
Electron-withdrawing and electron-donating groups were introduced onto the benzene ring of I1, which significantly influenced the HPPD inhibition activity. We found that electron-withdrawing groups improve the activity; for example, I3 (Ki = 0.36 µM) and I4 (Ki = 0.59 µM) were more potent than I1 (Ki = 1.5 µM) and I2 (Ki = 1.3 µM). In addition, the position of the electron-withdrawing group played an essential role in determining the HPPD inhibitory activity. In most cases, compounds with a chlorine atom at the 2-position (I4) were more active than those with the chlorine at the 4-position (I6), clearly an electron-withdrawing group at the 2-position provides enhanced activity compared to the 4-position. In addition, electron-donating groups were found to be detrimental to HPPD inhibition activity (I1 > I33, I38). We observed that methyl groups at the 5-position of the 1,3-cyclohexane ring were unfavorable to activity (I17 > I18, I26 > I27, II1 > II2), and that the introduction a nitro group led to more potent activity compared to that generated by a chlorine atom (except for I6 and I21), such that I15 > I3, I18 > I5, and I24 > I7 in terms of activity. Generally speaking, compounds with a pyrazole ring exhibited better HPPD inhibitory activities than those with cyclohexanedione rings (I28, I29 > I26, I27; II3, II4 > II1, II2).
Herbicidal activity
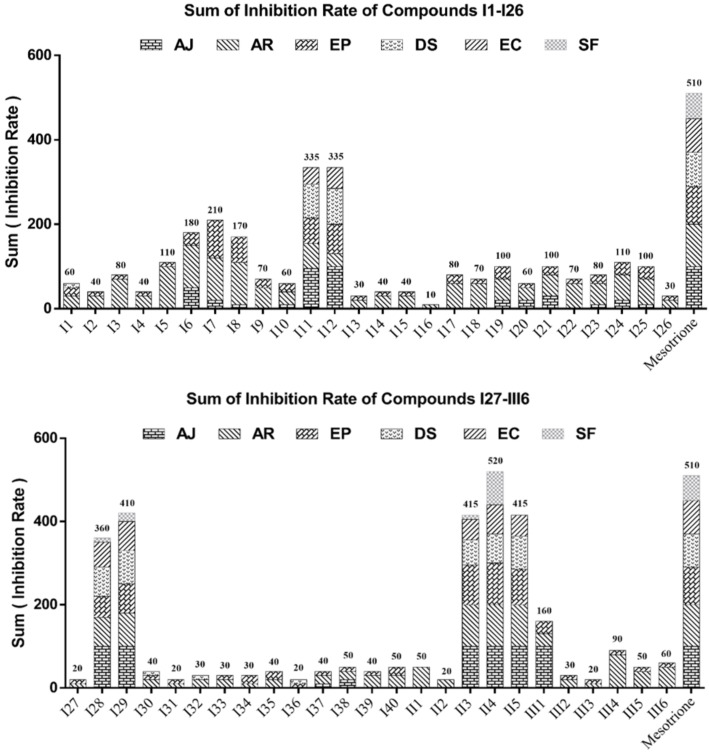
The post-emergence herbicidal activities of the title compounds are summarized in Figure 5. In our work, these weeds, E. crus-galli (EC), S. faberii (SF), D. sanguinalis (DS), A. retroflexus (AR), E. prostrata (EP), and A. juncea (AJ), were selected for evaluating the post-emergence herbicidal activities of the title compounds.
Figure 5.
Sum of inhibition rate of title compounds at 150 g ai/ha. (Abbreviations: AJ, Abutilon juncea; AR, Amaranthus retroflexus; EP, Eclipta prostrata; DS, Digitaria sanguinalis; EC, Echinochloa crus-galli; SF, Setaria faberii.)
Some of the synthesized compounds exhibited better control efficiencies for the test weed; among them, compounds I28, I29, II3 and II4 showed broad-spectrum herbicidal activities, with II4 even showing a slightly higher herbicidal activity than mesotrione at a rate of 150 g ai/ha. When the structure of compound II4 was superimposed onto that of mesotrione, the positive control drug, we observed that it perfectly fits into the active pocket, as shown in Figure 6.
Figure 6.
Simulated folding mode of mesotrione (yellow sticks) and compound II4 (gray sticks) with AtHPPD. The key residues in the active site are shown in blue sticks, and FeII is shown as a dark blue sphere.
In this work, two categories of HPPD inhibitors were synthesized, including triketone and pyrazole derivatives. Compared with the triketone derivatives, the pyrazole-containing derivatives were generally more herbicidally potent. For instance, pyrazole-containing compounds I11 and I12 displayed enhanced activities relative to compound I9, which contains a cyclohexanedione ring. We also observed that the introduction of methyl groups at the 5-position of the 1,3-cyclohexane ring was detrimental to herbicidal activity (I17 > I18, I26 > I27, II1 > II2). Compounds with electron-withdrawing groups on the aromatic ring were found to displayed higher herbicidal activities than those with electron-donating groups (i.e., I7 > I33, I8 > I34), which is consistent with the observed AtHPPD inhibitory activity, and I28 and I29 had significantly superior herbicidal activities. Thus, the introduction of large groups on the benzene ring appears to be beneficial to the activity and deserves further structural optimization.
The herbicidal activities of compounds containing other aromatic rings, compound II and III bearing a pyridine ring and a naphthalene ring, were examined. The results show that the chloro-substituted pyridine exhibited superior herbicidal activity, which provides a theoretical basis for the further development of highly effective HPPD herbicides. Some compounds with significant AtHPPD inhibitory activities were found not to exhibit promising herbicidal activities. For example, compound I12, with the best AtHPPD inhibition activity (Ki = 0.011 µM) exhibited poorer than expected herbicidal activity, which is possibly related to its stability and metabolism in the plant [2].
Crop safety
Crop safety is one of the main considerations during herbicide discovery. Compound II3, II4, and II5, which exhibited excellent herbicidal activities, were chosen for further crop safety studies and to evaluate whether or not they have the potential to be developed as herbicides (Table 3). Commercial mesotrione was selected as the positive control HPPD herbicide. We found that wheat and maize showed high tolerance to compound II3 at a dosage of 150 g ai/ha, however, its herbicidal activity could not compete with that of mesotrione. In addition, maize displayed tolerance to compound II4, indicating that II4 had the potential to be developed as a postemergence herbicide for weed control in maize fields.
Table 3.
Postemergence crop safety of compounds II3, II4 and II5 (150 g ai/ha).
| compound | dosage(g ai/ha) | % injury | |||||
| rice | wheat | maize | cotton | soybean | canola | ||
| II3 | 150 | 40 | 10 | 10 | 60 | 50 | 90 |
| II4 | 150 | 30 | 50 | 10 | 60 | 30 | 100 |
| II5 | 150 | 50 | 50 | 30 | 90 | 70 | 100 |
| mesotrione | 150 | 50 | 40 | 10 | 80 | 50 | 100 |
Conclusion
A series of aryloxyacetic acid derivates was synthesized as novel HPPD inhibitors. The bioassay studies revealed that some of the title compounds, such as compound I12 (Ki = 0.011 µM), I23 (Ki = 0.012 µM), showed similar AtHPPD inhibitor potencies to that of mesotrione (Ki = 0.013 µM). Moreover, several newly synthesized compounds displayed strong, broad spectrum weed control when dosed at 150 g ai/ha. Most importantly, compound II4, with good HPPD inhibition activity (Ki = 0.023 µM), exhibited a slightly higher herbicidal activity than mesotrione. In addition, II4 was found to be safe for use on maize. These results suggest that compound II4 is a promising HPPD inhibiting herbicide candidate deserving of further optimization studies.
Experimental
The experimental details and analytical data for intermediates C to M and title compounds were given in Supporting Information File 1. The chemical structures of all title compounds were confirmed by 1H and 13C NMR spectroscopic analyses and HRMS spectrometric analyses.
X-ray diffraction
Single crystals of compounds I18 and III4 were cultivated for structure validation. Compound I18 was recrystallized from a mixture of DCM/methanol to afford a colorless transparent crystal. It crystallized in the monoclinic space group: P–1 (2), cell: a = 6.425(4) Å, b = 9.854(6) Å, c = 13.205(8) Å, α = 93.974(7)°, β = 102.211(7)°, γ = 107.567(7)°, temperature: 298 K. Compound III4 was recrystallized from a mixture of DCM/methanol to afford a colorless transparent crystal. It crystallized in the monoclinic space group: P–1 (2), cell: a = 5.191(5) Å, b = 12.133(12) Å, c = 13.576(14) Å, α = 80.141(13)°, β = 81.978(12)°, γ = 79.496(12)°, temperature: 296 K. X-ray crystal structure of compound I18 and III4 are shown in Figure 3.
Crystallographic data for crystal compounds I18 and III4 were deposited with the Cambridge Crystallographic Data Centre as supplementary publications with the deposition numbers CCDC 1959130 and CCDC 1959152, respectively. The data can be obtained free of charge from http://www.ccdc.cam.ac.uk/.
Docking study
The docking study was conducted with the method reported previously [10,19,32–34]. Crystal structures of Arabidopsis thaliana HPPD (PDB ID: 1TFZ) with the native ligand, named DAS869 were downloaded from the Protein Data Bank. The docking was carried out using Discovery Studio 4.0. During the docking process, all water molecules were removed. The ligand and protein were prepared with the Dock Ligands tool before docking. By using Define and Edit Binding Site tool to identify the active site. Then the center of the native ligand was deleted. Utilizing the CDOCKER, the prepared ligand was docked into the protein receptor binding site. After the docking calculations were performed, the best binding modes were determined by docking scores and also compared with the simulated binding mode of mesotrione with AtHPPD.
Enzyme inhibition study
AtHPPD was prepared and purified according to the reported methods in the literature [19–20,35–37]. The inhibition constant (Ki) was obtained and shown in Table 1 and Table 2.
Herbicidal activities
The post-emergence herbicidal activities of the title compounds were evaluated against monocotyledon weeds (E. crus-galli, S. faberii, and D. sanguinalis) and broadleaf weeds (A. retroflexus, E. prostrata, and A. juncea) in the greenhouse experiments. The commercial HPPD herbicide mesotrione was regarded as a control. All tested compounds were dissolved in DMF as 100 g/L emulsified concentrates, containing 1% Tween-80 as emulsifier. Then the solvent was diluted with distilled water. Flowerpots with an inner diameter of 7.5 cm were filled with complex nutrient soil to three-fourths of their height. The above six weed targets were respectively grown in the pots and covered with soil to a thickness of 0.2 cm and grown in the greenhouse. When the weeds grew to about the three-leaf stage, they were treated by the title compounds at the rate of 150 g ai/ha. After 18 days of treatment with inhibitors, the herbicidal activities were surveyed and evaluated with two duplicates per experiment [19]. (Figure 5)
Crop selectivity
The representative crops, rice, wheat, maize, cotton, soybean, and canola were selected to test the crop safety of compound II3, II4, and II5. The six crops were separately planted in flowerpots (12 cm diameter) containing the composite nutrient soil and grown at room temperature. When the crops had reached the four-leaf stage, the safety experiments were conducted at the rate of 150 g ai/ha. After 15 days, the final results of crop safety were evaluated with two duplicates per experiment (Table 3).
Supporting Information
Additional experimental and analytical data, and NMR spectra of synthesized compounds.
Acknowledgments
The author thanks Prof. Da-Yong Zhang and Hao Huang for experimental guidance, Zhejiang University Chemical Industry Research Institute for biological activity assay, and National Natural science Foundation of China (30973607, 81172934), "Double First-Class" New Drug Development Project of China Pharmaceutical University (CPU2018PZQ15) for financial supports
Contributor Information
Man-Man Wang, Email: mmwang17@163.com.
Da-Yong Zhang, Email: cpuzdy@163.com.
References
- 1.Yang C, Pflugrath J W, Camper D L, Foster M L, Pernich D J, Walsh T A. Biochemistry. 2004;43:10414–10423. doi: 10.1021/bi049323o. [DOI] [PubMed] [Google Scholar]
- 2.He B, Wang D, Yang W, Chen Q, Yang G. Chin J Org Chem. 2017;37(11):2895. doi: 10.6023/cjoc201705031. [DOI] [Google Scholar]
- 3.Riggins C W, Peng Y, Stewart C N, Jr, Tranel P J. Pest Manage Sci. 2010;66(10):1042–1052. doi: 10.1002/ps.2006. [DOI] [PubMed] [Google Scholar]
- 4.Wójcik A, Broclawik E, Siegbahn P E M, Lundberg M, Moran G, Borowski T. J Am Chem Soc. 2014;136(41):14472–14485. doi: 10.1021/ja506378u. [DOI] [PubMed] [Google Scholar]
- 5.Lee D L, Knudsen C G, Michaely W J, Chin H-L, Nguyen N H, Carter C G, Cromartie T H, Lake B H, Shribbs J M, Fraser T. Pestic Sci. 1998;54(4):377–384. doi: 10.1002/(sici)1096-9063(199812)54:4<377::aid-ps827>3.0.co;2-0. [DOI] [Google Scholar]
- 6.Diebold A R, Brown-Marshall C D, Neidig M L, Brownlee J M, Moran G R, Solomon E I. J Am Chem Soc. 2011;133:18148–18160. doi: 10.1021/ja202549q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall M G, Wilks M F, Provan W M, Eksborg S, Lumholtz B. Br J Clin Pharmacol. 2001;52(2):169–177. doi: 10.1046/j.0306-5251.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis R W, Botham J W. Crit Rev Toxicol. 2013;43:185–199. doi: 10.3109/10408444.2013.764279. [DOI] [PubMed] [Google Scholar]
- 9.Hawkes T R, Langford M P, Viner R, Blain R E, Callaghan F M, Mackay E A, Hogg B V, Singh S, Dale R P. Pestic Biochem Physiol. 2019;156:9–28. doi: 10.1016/j.pestbp.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Li H-B, Li L, Li J-X, Han T-F, He J-L, Zhu Y-Q. Pest Manage Sci. 2018;74:579–589. doi: 10.1002/ps.4739. [DOI] [PubMed] [Google Scholar]
- 11.Neidig M L, Decker A, Choroba O W, Huang F, Kavana M, Moran G R, Spencer J B, Solomon E I. Proc Natl Acad Sci U S A. 2006;103(35):12966–12973. doi: 10.1073/pnas.0605067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borowski T, Bassan A, Siegbahn P E M. Biochemistry. 2004;43(38):12331–12342. doi: 10.1021/bi049503y. [DOI] [PubMed] [Google Scholar]
- 13.Ahrens H, Lange G, Müller T, Rosinger C, Willms L, van Almsick A. Angew Chem, Int Ed. 2013;52(36):9388–9398. doi: 10.1002/anie.201302365. [DOI] [PubMed] [Google Scholar]
- 14.Heap I. International survey of herbicide resistant weeds. Available from: http://www.weedscience.com.
- 15.Beaudegnies R, Edmunds A J F, Fraser T E M, Hall R G, Hawkes T R, Mitchell G, Schaetzer J, Wendeborn S, Wibley J. Bioorg Med Chem. 2009;17(12):4134–4152. doi: 10.1016/j.bmc.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y Q, Hu F Z, Yang H Z. Huaxue Tongbao. 2004;67:w018/1–w018/7. [Google Scholar]
- 17.Freitas L M, de Assis Valadares L P, Camozzi M G M, de Oliveira P G, Ferreira Machado M R, Lima F C. Hum Exp Toxicol. 2019;38:1178–1182. doi: 10.1177/0960327119860172. [DOI] [PubMed] [Google Scholar]
- 18.Ndikuryayo F, Kang W-M, Wu F-X, Yang W-C, Yang G-F. Eur J Med Chem. 2019;166:22–31. doi: 10.1016/j.ejmech.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y, Zhang S-Q, Liu Y-X, Wang J-Y, Gao S, Zhao L-X, Ye F. Ind Crops Prod. 2019;137:566–575. doi: 10.1016/j.indcrop.2019.05.070. [DOI] [Google Scholar]
- 20.Wang D-W, Lin H-Y, Cao R-J, Ming Z-Z, Chen T, Hao G-F, Yang W-C, Yang G-F. Pest Manage Sci. 2015;71(8):1122–1132. doi: 10.1002/ps.3894. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs P R, Patel K M, Selby T P, Smith B T, Taggi A E, inventors. Herbicidal pyrimidone derivatives. WO2011031658 A1. WO Patent. 2011 Mar 17;
- 22.Lian L, Lu X, Wu J, Zhang H. Chin J Pestic Sci. 2020;22:1–9. [Google Scholar]
- 23.Witschel M. Bioorg Med Chem. 2009;17:4221–4229. doi: 10.1016/j.bmc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang D-W, Lin H-Y, He B, Wu F-X, Chen T, Chen Q, Yang W-C, Yang G-F. J Agric Food Chem. 2016;64(47):8986–8993. doi: 10.1021/acs.jafc.6b04110. [DOI] [PubMed] [Google Scholar]
- 25.Jensen B S. Acta Chem Scand. 1959;13:1668–1670. doi: 10.3891/acta.chem.scand.13-1668. [DOI] [Google Scholar]
- 26.He H-W, Yuan J-L, Peng H, Chen T, Shen P, Wan S-Q, Li Y, Tan H-L, He Y-H, He J-B, et al. J Agric Food Chem. 2011;59(9):4801–4813. doi: 10.1021/jf104247w. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, He H-W, Zuo N, He H-F, Peng H, Tan X-S. J Agric Food Chem. 2012;60(31):7581–7587. doi: 10.1021/jf301829m. [DOI] [PubMed] [Google Scholar]
- 28.He H-W, Peng H, Wang T, Wang C, Yuan J-L, Chen T, He J, Tan X. J Agric Food Chem. 2013;61(10):2479–2488. doi: 10.1021/jf305153h. [DOI] [PubMed] [Google Scholar]
- 29.Verma A K, Rustagi V, Aggarwal T, Singh A P. J Org Chem. 2010;75:7691–7703. doi: 10.1021/jo101526b. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Iwasaki H, Fujikawa Y, Kitahara M, Sakashita M, Sakoda R. Bioorg Med Chem. 2001;9:2727–2743. doi: 10.1016/s0968-0896(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 31.Luan L-b, Song Z-j, Li Z-m, Wang Q-r. Beilstein J Org Chem. 2018;14:1826–1833. doi: 10.3762/bjoc.14.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y-q, Liu P, Si X-K, Zou X-M, Liu B, Song H-B, Yang H-z. J Agric Food Chem. 2006;54(19):7200–7205. doi: 10.1021/jf061573j. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee J M, Johnson-Winters K, Harrison D H T, Moran G R. Biochemistry. 2004;43(21):6370–6377. doi: 10.1021/bi049317s. [DOI] [PubMed] [Google Scholar]
- 34.Schaetzer J, Edmunds A J F, Gaus K, Rendine S, De Mesmaeker A, Rueegg W. Bioorg Med Chem Lett. 2014;24(19):4643–4649. doi: 10.1016/j.bmcl.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt S R, Muller C R, Kress W. Eur J Biochem. 1995;228:425–430. doi: 10.1111/j.1432-1033.1995.00425.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang D-W, Lin H-Y, Cao R-J, Chen T, Wu F-X, Hao G-F, Chen Q, Yang W-C, Yang G-F. J Agric Food Chem. 2015;63:5587–5596. doi: 10.1021/acs.jafc.5b01530. [DOI] [PubMed] [Google Scholar]
- 37.Lei K, Hua X-W, Tao Y-Y, Liu Y, Liu N, Ma Y, Li Y-H, Xu X-H, Kong C-H. Bioorg Med Chem. 2016;24(2):92–103. doi: 10.1016/j.bmc.2015.11.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional experimental and analytical data, and NMR spectra of synthesized compounds.