Abstract
Cancer cells adapt to their inherently increased oxidative stress through activation of the glutathione (GSH) and thioredoxin (TXN) systems. Inhibition of both of these systems effectively kills cancer cells, but such broad inhibition of antioxidant activity also kills normal cells, which is highly unwanted in a clinical setting. We therefore evaluated targeting of the TXN pathway alone, and more specifically, selective inhibition of the cytosolic selenocysteine-containing enzyme thioredoxin reductase 1 (TXNRD1). TXNRD1 inhibitors were discovered in a large screening effort and displayed increased specificity compared to pan-TXNRD inhibitors such as auranofin that also inhibit the mitochondrial enzyme TXNRD2 as well as additional targets. For our lead compounds, TXNRD1 inhibition correlated with cancer cell cytotoxicity, and inhibitor-triggered conversion of TXNRD1 from an antioxidant to a pro-oxidant enzyme correlated with corresponding increases in cellular production of H2O2. In mice, the most specific TXNRD1 inhibitor, here described as TXNRD1 inhibitor 1 (TRi-1), impaired growth and viability of human tumor xenografts and syngeneic mouse tumors, while having little mitochondrial toxicity and being better tolerated than auranofin. These results display the therapeutic anticancer potential of irreversibly targeting cytosolic TXNRD1 using small molecules and present potent and selective TXNRD1 inhibitors. Given the pronounced upregulation of TXNRD1 in several metastatic malignancies, it seems worthwhile to further explore the potential benefit of specific irreversible TXNRD1 inhibitors for anticancer therapy.
One Sentence Summary
Selective irreversible inhibitors of selenoprotein TXNRD1 yield anticancer efficacy without overt systemic toxicity in mouse models.
Introduction
Excessive oxidative stress due to a distorted metabolism and exaggerated replicative drive is a common feature of cancer cells (1–4). In both normal and cancerous cells, oxidative stress can be compensated by activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-activated glutathione (GSH) and thioredoxin (TXN) systems (5, 6). These systems regulate the cellular amounts of reactive oxygen species (ROS) for control of signaling pathways and growth processes and, together with downstream enzymes, actively scavenge ROS to protect against oxidative cellular damage. In cancer cells, pronounced and prolonged activation of the GSH and TXN systems occurs as a response to their increased oxidative stress phenotype. This response results from constitutive activation of Nrf2 (5), which aids in establishing a non-oncogene addiction to the GSH and TXN systems for cancer cell survival (7, 8). The deleterious result of increased antioxidant activity in cancer cells has also been shown through supplementation with exogenous antioxidants, further promoting cancer growth (9). Earlier studies have shown that concomitant disruption of both the GSH and TXN systems results in an anticancer response (10, 11); however, because normal cells also require either the GSH or the TXN systems for survival (12, 13), it can be difficult to therapeutically target both systems without causing adverse toxicity. Therefore, we wished to address whether sole targeting of the TXN system, and more specifically, irreversible inhibition of only the cytosolic TXN reductase (TXNRD1) can form the basis for anticancer therapy.
The cytosolic flavin adenine dinucleotide oxidoreductase TXNRD1 is a selenoprotein that contributes to a wide range of antioxidant and redox regulatory functions (14, 15). The enzyme is overexpressed in multiple types of cancer (16, 17) and is suggested to serve as a key driver for cancer cell growth and viability (10). Additionally, high expression of TXNRD1 is directly correlated with poor prognosis in head and neck, lung, and breast cancers (18, 19). Suppression of TXNRD1 in cancer cells using siRNA-mediated knockdown of the enzyme effectively impedes xenograft establishment in mice (20), whereas normal adult tissues are able to survive in the absence of TXNRD1 (12, 13). Several attempts have thereby been made to develop TXNRD1 inhibitors for cancer treatment (21–23). Although efficient TXNRD1 inhibitors having anticancer efficacy have been described, such as ethaselen (23), we are not aware of any study describing specific cytosolic TXNRD1 inhibitors that do not target the mitochondrial system. Analyzing in vivo effects of specific inhibitors of cytosolic TXNRD1, not inhibiting mitochondrial TXNRD2, would reveal whether it may be sufficient to target the cytosolic TXN system to yield anticancer efficacy. Specific TXNRD1 inhibition should also trigger less toxicity to normal cells.
It should be noted that in addition to their principal mechanisms of action, several clinically used anticancer drugs including cisplatin, carmustine, melphalan, and chlorambucil also inhibit TXNRD1, suggesting that the inhibition of this enzyme may contribute to their therapeutic efficacy (24–26). There are also several pan-TXNRD inhibitors used in clinical practice, such as auranofin, approved by FDA for use in rheumatoid arthritis, and arsenic trioxide, approved for acute promyelocytic leukemia. These compounds also inhibit mitochondria through the targeting of the mitochondrial isoenzyme TXNRD2 and, furthermore, display multiple TXNRD-independent reactivities (27–31). It remains unclear if cytosolic TXNRD1 inhibition significantly contributes to the clinical efficacy of these drugs, or if such inhibition is merely coincidental. It furthermore remains unclear whether targeting mitochondrial TXNRD2 is a prerequisite for the anticancer properties of pan-TXNRD inhibitors, or alternatively, if this can lead to adverse effects.
As with all mammalian TXNRD isoenzymes, TXNRD1 is sensitive to electrophilic attack due to a highly reactive, surface-exposed selenocysteine (Sec) residue in its active site (32). The selenolate of Sec typically has over three orders of magnitude higher reactivity with electrophiles than the thiolate of cysteine (Cys) (33), providing a nucleophilic “handle” that may be exploited for targeted specificity in the presence of excess thiols (34, 35). In addition, some, but not all inhibitors of TXNRD1 can induce an NADPH oxidase-like gain of function in the protein, producing SecTRAPs (selenium compromised thioredoxin reductase-derived apoptotic proteins). SecTRAPs may further exaggerate oxidative stress beyond a mere loss of native TXNRD1 activity (36–38), providing additional and potentially preferable effects for cancer therapy.
Collectively, the Sec residue in TXNRD1, the ability of the inhibited enzyme to be converted into a prooxidant, and its possible importance in cancer cells versus dispensability in normal cells, potentially make it a prime candidate for cancer therapy. To thoroughly examine this proposal, we evaluated the selective targeting of TXNRD1 using newly discovered inhibitors, and subsequently assessed their anticancer efficacy.
Results
High-throughput screen to identify TXNRD1 inhibitors
A previously validated assay (39) was used to screen 392,548 substances for inhibition of TXNRD1 (PubChem Bioassay ID:588453, https://pubchem.ncbi.nlm.nih.gov/bioassay/588453). Assessing chemical tractability and drug-like nature of 4,037 hit compounds meeting preset cut-off requirements including complete titration curves with high inhibitory efficiencies and few activities in other Bioassays, we identified 53 structurally diverse TXNRD1 inhibitors suitable for further analyses. The compounds were first validated in a competitive assay against TXN, the principal natural substrate of TXNRD1. Concomitant glutathione disulfide reductase (GSR) inhibition was used as an exclusion criterion because GSR supports the GSH system and is structurally and functionally similar to TXNRD1, although GSR lacks a Sec-containing active site (32, 40). These experiments helped to identify more specific TXNRD1 inhibitors rather than general inhibitors of flavoenzyme disulfide reductases. Effective and selective TXNRD1 inhibitors were subsequently assessed for cytotoxicity in cancer cell line cultures, yielding two top candidate TXNRD1 inhibitors, here named TRi-1 and TRi-2 (fig. S1).
Structure-activity relationship of TXNRD1 inhibition
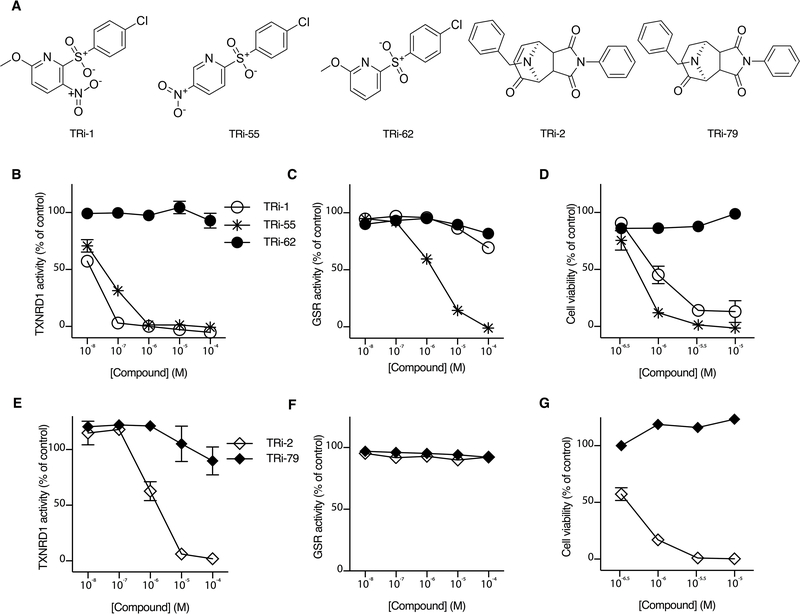
The TRi-1 and TRi-2 compounds represent structurally discrete molecular scaffolds (Fig. 1A). Analyses of TRi-1 and TRi-2 analogs offered insights into their functional activity profiles (tables S1,S2). Removal of the nitro group in TRi-1, exemplified with TRi-62 (Fig. 1A), rendered the compound inactive with regards to TXNRD1 inhibition (Fig. 1B). Placement of the nitro group at the para position of the nitropyridine ring and removal of the methoxy group (TRi-55, Fig. 1A) resulted in a loss of TXNRD1 over GSR specificity (Fig. 1C). The lack of TXNRD1 inhibition with TRi-62 also resulted in a loss of cytotoxicity towards human FaDu head and neck cancer cells, the human cancer cell and tumor model initially chosen for this study (Fig. 1D). For Tri-2, saturation of a single double bond (Fig. 1A; TRi-79) led to a loss of TXNRD1 inhibition (Fig. 1E), whereas neither TRi-2 nor TRi-79 inhibited GSR (Fig. 1F). Again, the capacity of inhibiting TXNRD1 (Fig. 1E) correlated with cancer cell cytotoxicity (Fig. 1G). These findings prompted us to further examine the properties and anticancer potential of TRi-1 and TRi-2. We also wished to compare their effects with auranofin, a potent pan-TXNRD inhibitor (30, 41, 42) that is FDA-approved for treatment of rheumatoid arthritis and also evaluated in trials for cancer therapy (see: www.clinicaltrials.gov trial numbers NCT01747798, NCT01419691 and NCT01737502).
Fig. 1. Correlation of TXNRD1 inhibition with cytotoxicity.
A, Structures of inhibitors (Tri-1 and TRi-2), inactive analogs (TRi-62 and TRi-79), and a non-specific analog also inhibiting GSR (TRi-55). B-G, Comparative inhibitory activities of TRi compounds and their analogs in enzymatic and cell culture assays. B, TRi-1 and its analogs tested for inhibition of TXNRD1 or (C) GSR inhibition. D, TRi-1 with analogs tested for cytotoxicity towards FaDu cells after 72 hours of incubation. E, TRi-2 and its inactive analog TRi-79 tested for inhibition of TXNRD1 and (F) GSR inhibition. G, Cytotoxicity towards FaDu cells after 72 hours of incubation with TRi-2 or TRi-79. Results from recombinant enzyme and cell culture experiments are shown as averages of triplicates ± SEM.
Mechanistic effects on redox components
The electrophilic propensity of TXNRD1 inhibitors prompted examination of the ability of Tri-1, TRi-2, and auranofin to interact with additional components of the GSH and TXN systems. We also examined the effects of the compounds on downstream targets of TXNRD1. Pre-incubation with GSH before exposure to NADPH-reduced TXNRD1 prevented TXNRD1 inhibitory activity for all three compounds, demonstrating their inherent reactivity with nucleophilic moieties such as the thiol of GSH (Fig. 2A). Co-incubating increasing concentrations of GSH with TXNRD1 in the presence of NADPH prevented TRi-2 from inhibiting the enzyme, whereas TRi-1 and auranofin were still effective TXNRD1 inhibitors in the presence of excess GSH (Fig. 2B). When the compounds were incubated in the presence of TXNRD1, NADPH, TXN1, and GSH, only TRi-1 sustained TXNRD1 inhibition (Fig. 2C). The preferential inhibition of TXNRD1 in the presence of GSH may help to explain how TRi-1, Tri-2, and auranofin can display efficacy in cells in spite of their high inherent chemical reactivity and rather rapid metabolism in liver microsomes (table S3). A complex reactivity of auranofin with GSH was demonstrated previously, with detection of at least six different reaction products (43). Here we performed similar analyses of the products of TRi-1 or TRi-2 upon reaction with GSH, finding that TRi-1 only forms one major adduct with GSH, whereas TRi-2 conjugation with GSH results in two separate products (fig. S2).
Fig. 2. Specificity of TXNRD1 targeted inhibition.
A-C, TXNRD1 activity in the presence of TXN1 and GSH examined in a TXN1-coupled insulin reduction assay. A, 10 μM TRi-1, TRi-2, or auranofin were pre-incubated with GSH before incubation with TXNRD1, NADPH, TXN1, and insulin. B, Incubation of compounds in the presence of TXNRD1, NADPH, and GSH before addition of TXN1 and insulin. C, Incubation of compounds in the presence of TXNRD1, NADPH, TXN1, and GSH simultaneously, before addition of insulin. D, E, Comparisons in inhibition of human TXNRD1 versus human TXNRD2 using DTNB as substrate with either (D) TRi-1 or (E) auranofin. F, Compound inhibitory activity toward GPX1. G, Inhibition of cellular TXNRD activity in FaDu cells after 3 hours of compound exposure. Activity was determined using the insulin-endpoint assay. H, Cellular GSH concentrations after exposure of FaDu cells to compounds for 6 hours at 10×IC50. Results from recombinant enzyme and cell culture experiments are shown as averages of triplicates ± SEM. I-L, Western blot analyses of FaDu cells treated with compounds for 6 hours, examining (I) JNK phosphorylation, (J) total JNK, (K) p38 phosphorylation, and (L) total p38.
Further considering the specificity of TRi-1, we compared auranofin with TRi-1 in inhibition of TXNRD1 over TXNRD2. This showed that TRi-1 displayed about 5- to 10-fold higher specificity for TXNRD1 compared to inhibition of TXNRD2 (Fig. 2D and fig. S3A), whereas auranofin was confirmed as a pan-TXNRD inhibitor (Fig. 2E and fig. S3B). The compounds were also tested for their ability to inhibit glutathione peroxidase 1 (GPX1). GPX1 uses reduced GSH for the conversion of H2O2 into water and, like TXNRD1, is a Sec-containing enzyme (44). When preincubated with GPX1 in the absence of GSH, high concentrations of auranofin yielded moderate inhibition of the enzyme, as did TRi-1, but to a lesser extent (Fig. 2F). However, these compounds were over 1000-fold less efficient in inhibiting GPX1 than in inhibiting TXNRD1 (Figs. 2D–F).
TRi-1 and TRi-2 inhibited cellular TXNRD activity with equal or greater potency compared to that of auranofin (Fig. 2G and fig. S4). TRi-1 treatment also had no effect on cellular GSH concentrations, whereas doses of TRi-2 and auranofin at 10 times the IC50 for the respective cell line lowered GSH (Fig. 2H). Both TRi-1 and TRi-2 efficiently activated JNK and p38 phosphorylation (Figs. 2I–L), which is an expected downstream effect of increased signaling through apoptosis signaling kinase (ASK1) that is inhibited by reduced TXN1 (45). Auranofin, however, did not activate JNK and p38 phosphorylation as robustly under the same conditions (Fig 2I–L). Collectively, the differential reactivity profiles with recombinant enzymes, GSH, and cellular redox pathway effects present TRi-1 as the most selective inhibitor of cytosolic TXNRD1.
Conversion of TXNRD1 from an antioxidant to a prooxidant enzyme
The mechanisms of TXNRD1 inhibition were investigated in greater detail. TRi-1, TRi-2, and auranofin were all found to irreversibly inhibit TXNRD1 in an NADPH-dependent manner (Fig. 3A). Irreversible inhibition occurs with several inhibitors of TXNRD1, typically via covalent modification of its Sec residue (46). NADPH-reduced TXNRD1 is less thermostable than the oxidized enzyme, but its stability was increased upon incubation with TRi-1, though not with TRi-2 or auranofin (fig. S5). This observation reinforces our proposal that these compounds have different reaction mechanisms toward TXNRD1, as was also seen with their different reactivities with GSH.
Fig. 3. SecTRAP formation, cellular H2O2 production, and mitochondrial function.
A, TXNRD1 activity with DTNB as a model substrate analyzed before (black bars) and after desalting (white bars) upon incubation with compounds in the presence or absence of NADPH. B, SecTRAP activity as indicated by juglone reduction measured after desalting upon pre-incubations of NADPH-reduced TXNRD1 with enzyme inhibitors at concentrations completely inhibiting native TXNRD1 activity. C,D, H2O2 production in cultured FaDu cells treated with TXNRD1 inhibitors, measured with an Amplex red assay in a (C) concentration-dependent or (D) time-dependent manner. E,F, Mitochondrial respiration in HCT116 cells using a Seahorse assay, examining ATP coupled basal respiration and maximal respiratory capacity determined after (E) 30-minute or (F) 5-hour exposure times. Results are shown as the averages of three experiments performed in triplicate (n=3), and differences were compared to vehicle using an unpaired t-test (*p<0.05, **p<0.01,***p<0.001, ****p<0.0001).
After the normal enzymatic activities of TXNRD1 had been inhibited with TRi-1 or auranofin, it still maintained substantial NADPH-dependent redox cycling with juglone (Fig. 3B), indicating formation of pro-oxidant SecTRAPs (36, 37). TRi-1 or auranofin consequently increased cellular H2O2 production, whereas TRi-2 did not have SecTRAPs forming characteristics and did not further accelerate cellular H2O2 production (Fig. 3C,D). This differential H2O2 production occurred despite TRi-1 and TRi-2 inhibiting cellular TXNRD1 activity to an equal or greater extent as auranofin (Fig. 2G and fig. S4), connecting the increased cellular H2O2 production to a gain of function through formation of SecTRAPs and not only the loss of cellular TXNRD activity. The observed exaggerated cellular generation of H2O2 could, however, not be ruled out as an off-target effect on mitochondrial function, especially because auranofin is known to target mitochondria (47, 48). We confirmed that auranofin severely impairs mitochondrial function in cultured cells, causing deteriorated ATP-coupled respiration and impaired maximal respiratory capacity after both short and long exposure times (Fig. 3E,F). TRi-2 also decreased basal respiration rates and maximal respiratory capacity, though to a lesser degree than auranofin, whereas TRi-1 lacked effects on basal respiration and had little effect on maximal respiratory capacity (Fig. 3E,F). These results confirm that TRi-1 triggers increased cellular production of H2O2 through transformation of inhibited cytosolic TXNRD1 into prooxidant SecTRAPs, whereas the cellular effects of auranofin are not solely driven through cytosolic TXNRD1 inhibition.
TXNRD1 inhibitor cytotoxicity toward cancer cells
Because of the diverse mechanistic effects of the three compounds, we assessed their potencies in cytotoxicity toward cancer cells. TRi-1 was tested for its cytotoxicity profile against the NCI-60 cell panel and compared to TRi-2 and auranofin, which had been screened previously [https://dtp.cancer.gov, NSC351105 (TRi-2) and NSC321521 (auranofin)]. TRi-1, TRi-2, and auranofin all displayed potency against every cell line tested, with an average growth inhibition to 50% (GI50) of 6.31 μM, 4.14 μM, and 0.76 μM, respectively (table S4). It has been shown that cytotoxicity correlations between compounds over the NCI-60 cell panel can indicate similar mechanisms of action (49). When comparing TRi-1, TRi-2, and auranofin, we found that TRi-1 and auranofin display the least similarity in cytotoxicity profiles (fig. S6). This is in agreement with the mechanistic evidence from the biochemical and cellular studies above, which differentiate the compounds from each other and identify TRi-1 as the most specific inhibitor of cytosolic TXNRD1.
In side-by-side comparisons of cytotoxic potency using a selection of human cancer cell lines, TRi-2 and auranofin were on average more potent than TRi-1 (Fig. 4A–C). Colony-forming assays with human FaDu cells also showed that auranofin was more cytotoxic than TRi-1 (fig. S7). We saw no apparent resistance to any of the three compounds upon altered p53 status or BCL-2 overexpression, as assessed using subclones of human colon carcinoma HCT116 cells (Fig. 4A–C). The broad-range efficacy over the NCI-60 cell panel (table S4) and minor effects of p53- and BCL-2 status suggest that cancer cell genotype has little influence on the efficacy of these compounds. Such genotype-independent cytotoxicity should be expected if the mechanisms of action are based upon exaggerated oxidative stress (4, 50), and therefore target an important mechanism of non-oncogene addiction (7, 8). Depleting cells of GSH using buthionine sulphoximine (BSO), an irreversible inhibitor of γ-glutamylcysteine synthetase, enhanced the cytotoxicity of the compounds (fig. S8), which further supports the notion that the mechanisms of cytotoxicity of TRi-1, TRi-2, and auranofin involve sensitization of the cancer cells to oxidative stress.
Fig. 4. TRi-1, TRi-2, and auranofin cancer cell cytotoxicity and potency against non-cancerous cell lines.
A-C, Viability of selected human carcinoma cell lines was determined after 72 hours of incubation with (A) TRi-1, (B) TRi-2, or (C) auranofin to determine dose-dependent cytotoxicity profiles. D-F, Comparison of cytotoxicities towards TXNRD1-overexpressing human A549 lung carcinoma cells or MEFs with (Txnrd1fl/fl) and without (Txnrd1−/−) the gene encoding for TXNRD1 expression using (D) TRi-1, (E) TRi-2, or (F) auranofin. G-I, Viability of normal human primary fibroblasts, primary keratinocytes, and colon epithelial CCD841 cells after 72 hours of incubation with (G) TRi-1, (H) TRi-2, or (I) auranofin. The averages of the cancer cell viability data for the eight cancer cell lines shown in (A-F) were pooled and plotted here together with the normal cells for reference.
Comparing cytotoxic effects of TRi-1, TRi-2, and auranofin between non-cancerous mouse embryonic fibroblast (MEF) cells and human A549 lung adenocarcinoma cells, which express excessively high amounts of TXNRD1 (51), we found that TRi-2 and auranofin were similarly cytotoxic towards these two cell types, whereas TRi-1 was preferentially toxic towards the A549 cells (Fig. 4D–E). MEFs with the Txnrd1 gene deleted (52) displayed additional resistance to Tri-1 treatment, whereas TRi-2 and auranofin sensitivity was minimally affected (Fig. 4D–E). In further examinations of the toxicity of the compounds to non-cancerous cells, both TRi-1 and TRi-2 showed reduced potency against primary human fibroblasts, primary keratinocytes, and CCD841 colon epithelial cells, whereas auranofin only displayed lower potency against the CDD841 cells when compared to the previously tested cancer cell lines (Fig. 4G–I). These observations strengthen the idea that TRi-1 mainly exerts its cytotoxic mechanism of action through cytosolic TXNRD1 inhibition, and that cancer cells display increased sensitivity to such inhibition.
Anticancer efficacy and minimal toxicity in mice
We next examined in vivo activities of TRi-1 and TRi-2 using dose escalation studies in mice. Very good tolerance was observed up to the highest administrable dose in terms of solubility, yielding 10 mg/kg TRi-1 or 15 mg/kg TRi-2 given intravenously (i.v.), without any overt signs of toxicity over a 72-hour observation period. SCID mice bearing established human FaDu cell xenografts were subsequently treated twice a day i.v. with 10 mg/kg TRi-1, 15 mg/kg TRi-2, or 10 mg/kg auranofin, as a repeated high-dose toxicity study with tumor-bearing mice. This resulted in decreased tumor growth compared to vehicle controls within four days (Fig. 5A) with no signs of overt toxicity or changes in mouse weight relative to vehicle control (fig. S9).
Fig. 5. Rapid anticancer effects of TXNRD1 inhibitors in FaDu xenograft-bearing mice.
A, Human FaDu cell xenograft growth in SCID mice, treated twice daily for four days starting 13 days after inoculation (“day 0”) with 10 mg/kg TRi-1 (n=6), 15 mg/kg TRi-2 (n=5), 10 mg/kg auranofin (n=5), or vehicle (n=3). The graph displays percentage increase in tumor growth compared to day 0 (average ± S.E.M.). Groups were compared with a repeated measures ANOVA and Tukey’s multiple comparisons post-test (*p<0.05, **p<0.01). B, Representative PET image of [18F]-FDG uptake in a FaDu cell xenograft tumor (T), heart (H), and brain (Br), with elimination of radiotracer through kidneys (K) and bladder (Bl). C, Side-by-side [18F]-FDG uptake in individual tumors on Day 0 and Day 3, treated with 5 mg/kg TRi-1 (n=8) or vehicle (n=9) once daily. D, Relative [18F]-FDG uptake in the viable parts of the tumor masses shown in (C) analyzed using a paired t-test. E, Representative three-plane image of a TRi-1 treated low uptake tumor core with sagittal (S), coronal (C), and trans axial (T) projection. F, Caspase 3 staining in excised FaDu cell xenografts from mice treated with 5 mg/kg TRi-1 once daily, i.p., from day 0 to day 3 (n=4) or day 4 (n=4). 5 image fields per xenograft were quantitated using Image J analysis software and grouped according to treatment duration.
Positron emission tomography (PET), using [2-18F]-2-fluoro-2-deoxy-D-glucose ([18F]-FDG) to reflect viability through glucose uptake, was next performed to assess TRi-1 antitumor efficacy with a clinically relevant diagnostic method (53) (Fig. 5B). Here, each mouse served as its own baseline control over the course of treatment (Fig. 5C). The [18F]-FDG uptake in viable parts of the tumors markedly decreased in the TRi-1-treated group after only four days of treatment, whereas xenografts in the vehicle-treated group instead displayed an increase in [18F]-FDG uptake during the same period (Fig. 5D). In the individual PET images, many of the tumors in the TRi-1-treated mice also displayed a very low radiotracer uptake in their central regions (Fig. 5C,E). It should be noted that these lower-uptake tumor cores were not included in the [18F]-FDG uptake quantitation (Fig. 5D), avoiding exaggeration of the effects of TRi-1. The lower [18F]-FDG uptake in the tumors of the TRi-1 treated group could represent a combination of metabolic effects and increased cell death, which was subsequently supported by increased staining for activated caspase-3 between days three and four, as evaluated in sections of the excised xenograft tumors (Fig. 5F).
We next evaluated the anticancer efficacy of TRi-1 and auranofin in a longer-duration experiment using PyMT-MMTV mice that spontaneously develop malignant breast cancer tumors (54). With a three-week low-frequency dosing regimen of i.p. treatment twice a week, TRi-1 (5 mg/kg) and auranofin (10 mg/kg) both impaired tumor growth in this model (Fig. 6A–C). A waterfall plot analysis demonstrates that the largest tumors all belonged to the vehicle group (Fig. 6D), and the tumor volumes of both the TRi-1 and auranofin treatment groups were significantly smaller compared to the vehicle controls (p<0.05), but were not significantly different from each other (Fig. 6E). All mice in the TRi-1 treated group survived during the time of observation, whereas two mice in the vehicle group and two mice in the auranofin group had to be sacrificed due to poor health.
Fig. 6. Anticancer effects in PyMT-MMTV syngeneic and MDA-MB-231 xenograft-bearing mice.
A-C, Average mammary gland tumor volumes over time in individual PyMT-MMTV mice treated i.p. twice a week with (A) vehicle (n=7), (B) 5 mg/kg TRi-1 (n=6), or (C) 10 mg/kg auranofin (n=8). Mammary tumor volume was measured at a minimum of four nodes per mouse at regular intervals for 20–22 days and upon sacrifice. D, Waterfall plot of averaged tumor volumes from the PyMT-MMTV mice surviving 20–22 days. E, Grouped averaged mammary gland tumor volumes of mice surviving 20–22 days compared using an ordinary one-way ANOVA with Tukey’s multiple comparisons post-test. F-H, Tumor volumes of MDA-MB-231 xenografts in athymic mice treated daily for five days followed by two day of no treatment, then treatment three times per week for two weeks with (E) vehicle, (F) 10 mg/kg TRi-1 i.v., or (G) 10 mg/kg TRi-1 i.p.. I, Waterfall plot of the final MDA-MB-231 xenograft tumor volumes after treatment for 22 days. J, Tumor volumes were compared to vehicle using a two-way ANOVA with Tukey’s multiple comparison post-test (***p<0.001, ****p<0.0001, n=12 in each group).
Attempting to further increase the anticancer efficacy of auranofin, some PyMT-MMTV mice were treated in combination with BSO. However, this combination was lethal to the mice after the first round of administration and was therefore discontinued (table S5. This lethality likely reflects the dependence of normal cells upon GSH in the absence of TXNRD activity, further confirming the notion that at least one of the two cellular antioxidant pathways needs to be functional for survival of normal cells and tissues (12, 13).
Finally, we analyzed the comparative efficacy of TRi-1 administered i.v, versus i.p. injection. For this we used a third tumor model with xenografts of the human breast cancer cell line MDA-MB-231 inoculated orthotopically into the mammary fat pads of athymic mice (Fig. 6F–H). Again, no signs of overt systemic toxicity of TRi-1 were observed and mouse weights did not significantly differ between the groups (fig. S10). A waterfall plot analysis of the final tumor volumes illustrates that the smallest tumors were found in the two TRi-1 treatment groups, with one tumor in the TRi-1 i.p. group completely regressing during the experiment (Fig 6I). Tumor growth in both of the TRi-1 treatment groups was significantly decreased compared to the vehicle controls (p<0.0001), but did not significantly differ from each other (Fig. 6J).
Discussion
The results described here demonstrate that drug-mediated, irreversible inhibition of cytosolic TXNRD1 can yield anticancer efficacy without overt systemic toxicity. This was exemplified with a specific inhibitor of TXNRD1, TRi-1. Cytosolic TXNRD1 inhibition with TRi-1 provoked cytotoxicity towards cultured cancer cells essentially independent of genotype, likely because TXNRD1 supports a non-oncogene addiction of cancer cells by protecting against their inherently increased oxidative stress. The anticancer efficacy of TRi-1 in mouse tumor models is consistent with the idea that TXNRD1 is important for cancer cell growth in vivo, and the lack of overt systemic toxicity suggests that the enzyme can be dispensable for overall viability and function of normal adult non-cancerous tissues.
Distinguishing between TXNRD1 inhibition and inhibition of other components of the GSH and TXN pathways were crucial steps in our compound selection process. Had the counter screen for omitting dual GSR and TXNRD1 inhibitors not been performed, several compounds with broad, non-selective inhibition of flavoprotein pyridine nucleotide oxidoreductases would have been included. The comparisons in mechanisms of action and cellular effects revealed rather promiscuous activity of auranofin. Our results show that in addition to auranofin having TXNRD1 inhibitory activity, the gold compound cross-reacts with multiple components of both the GSH and TXN pathways and, importantly, disrupts cellular function independent of its inhibition of TXNRD1. Recent claims have stated that simultaneous targeting of both the TXN and GSH systems might yield synergistic effects in cancer treatment (10). However, as reasoned above and accentuated through the non-specific activity of auranofin, care must be taken to avoid the risk of excessive toxicity to healthy cells and tissues. This caution should be emphasized, because we found that mice died after receiving a combination of BSO (to inhibit GSH synthesis) and auranofin. Lower doses of BSO and auranofin have been combined for partial inhibition of the TXN and GSH pathways, and such dual treatment indeed results in inhibited cancer cell growth (55), but we suggest that effective inhibition of only cytosolic TXNRD1 can be a superior alternative therapeutic principle that maintains anticancer efficacy with lower risks for toxic side effects.
In certain cases of TXNRD1 inhibition, the enzyme may be converted from an antioxidant into prooxidant SecTRAPs, as seen with TRi-1. SecTRAPs were previously studied using recombinant enzyme and cellular model systems (37, 38), whereas our study correlated SecTRAP formation with increased production of cellular H2O2. Our results suggest that oxidative stress can be exaggerated in cancer cells through the combination of inhibiting cellular antioxidant activities and actively increasing H2O2 production through a single drug target. Furthermore, because formation of SecTRAPs in cells seemed to be triggered by TRi-1 without strong effects on mitochondrial respiration, these results also support the idea that selective inhibition of cytosolic TXNRD1 is sufficient to yield anticancer efficacy. Auranofin has been previously shown to exert pronounced effects in the mitochondria (29, 47), which is in line with our results. The efficacy of TRi-1 suggests that such mitochondrial targeting is not necessarily required for anticancer efficacy, and is not a necessary consequence of inhibiting cytosolic TXNRD1. A side-by-side comparison of the effects of TRi-1 with those of auranofin is given in table S6. Considering the results of our studies and the fact that a range of clinically used anticancer compounds have TXNRD1 inhibitory activity as part of their mechanisms of action, we suggest that specific targeting of cytosolic TXNRD1 is a pertinent anticancer therapeutic principle that should be further evaluated.
Materials and Methods
Study design
The aim of this study was to search for small molecule inhibitors of TXNRD1 and examine whether or not such inhibitors could serve as anticancer drug candidates. We used a reverse chemical genetics approach, examining 392,548 substances. Subsequently, the drug-like nature and specificity of inhibitory compounds was assessed as an additional selection criterion. A specificity profile of TXNRD1 inhibitors was determined through the analysis of cross reactivity with other redox-active and ROS-regulating cellular components. The most potent and specific TXNRD1 inhibitors discovered in the high-throughput screen were then examined for their potential efficacy in cell culture and animal models and benchmarked relative to auranofin. All animals in these studies were randomized into control or treatment groups. Animal caretakers involved in treatment of animals, animal observations and tumor measurements by caliper were not involved in summarizing or analyzing the results. The number of replicates differed between experimental sets and are stated in the figure legends. No power analyses were performed before commencement of any of the studies. Statistical analyses of animal treatment results were performed under the guidance of an experienced expert in statistics from Charles River Laboratories.
High-throughput screen for inhibitors of TXNRD1
The assay used in the high-throughput screen was described previously in a validation study using the LOPAC1280 library, identifying protoporphyrin IX as an inhibitor of TXNRD1 (39). For further details, see Supplementary Materials and Methods.
Recombinant enzyme assays
TXNRD1 and GSR recombinant enzyme assays were performed in a reaction buffer containing 50 mM Tris pH 7.5 with 2 mM EDTA and 0.1 mg/mL bovine serum albumin. Assays were performed in triplicate using 96-well plates, with activity inferred from absorbance changes using a Spectra Max plate reader. All compounds were dissolved in DMSO. Recombinant rat TXNRD1 (21 U/mg) was used in all follow-up screens except for the comparison of TXNRD1 versus TXNRD2, where human recombinant enzymes were used. TXNRD1-based assays were adapted from 1 mL cuvette protocols (56). Results of recombinant enzyme assays were normalized to DMSO and to no enzyme controls. For details of all assay conditions, see Supplementary Materials and Methods.
Cell culture
Cell cultures were maintained at 37 °C in 5% CO2 in medium containing 100 μg/mL penicillin/streptomycin, 2 mM L-glutamine, and 10% fetal bovine serum (FBS) (GE Healthcare, A15–102). Experiments were performed in triplicate in medium containing 10% FBS and 25 nM sodium selenite. All compounds were diluted in DMSO, 0.01% final concentration. For choice of media and further details, see Supplementary Materials and Methods.
Single dose and repeated dose in vivo toxicity
Fox Chase male severe combined immunodeficiency (SCID, Charles River, #250) mice were treated once with 0.7–10 mg/kg TRi-1 or 0.5–20 mg/kg TRi-2 via i.v. injections, and mouse health status was observed for up to 72 hours, at the Adlego AB facility in Stockholm, Sweden. For repeated dose toxicity studies with tumor bearing animals, mice were first inoculated with 1×106 FaDu cells in PBS at a pre-shaved region located at the anterior lateral thoracic wall (also performed by Adlego AB). After 13 days of growth, tumors were measured by calipers and treatments were initiated. Mice were injected with 10 mg/kg TRi-1, 15 mg/kg TRi-2, 10 mg/kg auranofin, or vehicle a total of nine times during a five day span via i.v. tail injection. Upon the final day of dosing, injections were performed subcutaneously (s.c.) due to hematomas in several of the mice at the tail injection site. Mouse health status was monitored daily, weight was measured, and tumor volume was recorded from caliper measurements.
Positron emission tomography xenograft studies
Male SCID mice were inoculated with 1×106 FaDu cells. On the day of baseline imaging, after 11 days of tumor growth, food was removed from the cages. Mice were anesthetized using isoflurane (initially 5%, then 1.5% blended with 3:2 air/O2), controlled by an E-Z anesthesia vaporizer delivered through individual Microflex non-rebreathing masks (Euthanex Corporation) and placed on a heating pad at 37 °C on the bed of a MicroPET Focus 120 camera (CTI Concorde Microsystems). [18F]-FDG was obtained as an aliquot from the daily production for the clinical PET at the Karolinska University hospital that had passed all quality requirements for administration in humans, and was administered via i.v. tail vein injection. After baseline PET scanning, mice were treated with 5 mg/kg TRi-1 or vehicle by i.p. injection once daily. On the day three of treatment, mice were again injected with [18F]-FDG and imaged by PET. The PET data were acquired every second from the time of injection until 60 min after injection, in full three-dimensional mode. Images were reconstructed by standard two-dimensional filtered back projection using a ramp filter. Data were processed with MicroPET Manager and evaluated using Inveon Research Workplace (Siemens Medical Solution) software. Data were corrected for dead time, decay, and randoms, and steady state uptake was included for analysis from 30 minutes until the end of data acquisition (57). The minimum tumor diameter for inclusion was 3 mm, with a minimum threshold set at 1.8×105 Bq/mL to minimize partial volume effects (57). Regions of interest (ROIs) were drawn applying the set threshold of 1.8×105 Bq/mL. [18F]-FDG uptake was quantitated as a standard uptake value mean (SUVmean) distribution over the imaged tumor. Regions of treated tumors with radiotracer uptake below 1.8×105 Bq/mL were not included in the analysis. All PET images were created using the same color scheme and intensity range. Day 0 to day 3 image comparisons were set to the same zoom and captured at the most proximal position within the tumor. Mice were euthanized on day three or four by cervical dislocation under continued anesthesia.
PyMT-MMTV tumor low frequency drug dosing studies
PyMT-MMTV mice were genotyped at 6–8 weeks for the polyoma virus middle T antigen for inclusion into the study. Mice confirmed as transgenes were treated twice a week via i.p. injection of 5 mg/kg TRi-1, 10 mg/kg auranofin, or vehicle. Treatments involving 12.5 mg of BSO in saline (50 mg/ml) via i.p. injection were administered to mice alone (n=3) or six hours before treatment with auranofin (n=3). Tumor volume was determined using caliper measurements.
MDA-MB-231 xenograft efficacy studies
These experiments were performed on a service contract by Charles River Laboratories. In short, athymic nude mice were orthotopically inoculated with 5×106 MDA-MB-231 breast cancer cells into the mammary fat pad, and randomized for treatment when tumors reached an average volume of 80–120 mm3 (n=12 in each group). Mice were treated with 10 mg/kg TRi-1 via either i.v. or i.p. injection, or with vehicle i.v., once a day for the first five days, then two days off treatments, and thereafter three times per week for two weeks. Mouse health status was monitored, weight was measured, and tumor volume was assessed using caliper measurements.
Ethical considerations
Studies were performed in accordance with national legislation on laboratory animal protection, and all animal experiments performed by us were approved by the regional ethical vetting board (Regionala Etikprövningsnämnden) in Stockholm, Sweden. The dose escalation study was performed at Adlego AB under approval no. N476/11, and the repeated dose toxicity study with xenografts was performed at Adlego under approval no. N447/12. The PET study was performed under approval no. N416/12, and the PyMT-MMTV mouse study was performed under approval no. N178/13. The MDA-MB-231 xenograft experiments were performed by Charles River Laboratories following the animal policies of the Office of Laboratory Animal Welfare, assurance number #A4358–01.
Statistical analyses
For the cellular depletion of GSH using BSO, doses of each compound were compared between BSO and no-BSO treated cells. Differences in mitochondrial respiration were analyzed for each treatment, compared to their respective control. These cellular experiments were performed in triplicate and analyzed using an unpaired t-test. The growth of FaDu cell tumor xenograft studies was normalized to day 0 caliper measurements, and the effects of TRi-1 (n=6) and auranofin (n=5) treatments were compared to vehicle (n=3) using repeated measures ANOVA with Tukey’s multiple comparison post-test. Changes in [18F]-FDG uptake were determined by comparing day 3 to day 0 using individual mouse baseline matched pair analysis, and statistical significance was determined using a paired t-test for vehicle (n=9) and TRi-1 (n=8). In the PyMT-MMTV studies, a minimum of four mammary glands on each mouse were palpated, measured for tumor size using calipers, and then averaged together. Averaged mammary gland volumes were grouped by treatment and compared using an ordinary ANOVA with Tukey’s multiple comparison post-test for TRi-1 (n=6), auranofin (n=6), and vehicle (n=5). MDA-MB-231 xenograft tumor growth was measured using calipers, grouped by treatment, and compared on every measurement day using a two-way ANOVA with Tukey’s multiple comparison post-test (n=12 in each group).
Supplementary Material
Fig. S1. TRi-1, TRi-2, and analog structures with enzymatic inhibition activities.
Fig. S2. Schematic reactivity of TRi-1 and TRi-2 with reduced GSH and their respective metabolite percentages.
Fig. S3. Inhibition of human TXNRD1 and TXNRD2 by (A) TRi-1 or (B) auranofin.
Fig. S4. Cellular TXNRD1 activity from HCT116 cells incubated with compounds for 3 hrs.
Fig. S5. Thermal stability of TXNRD1 oxidized, reduced, and the reduced enzyme incubated with compounds.
Fig. S6. Comparison of cytotoxicity profiles of TXNRD1 inhibitory small molecules within the NCI 60 cancer cell panel.
Fig. S7. FaDu cell colony formation assay.
Fig. S8. FaDu cells treated with and without BSO and increasing concentrations of compound.
Fig. S9. Grouped mouse weights during repeated dose toxicity study.
Fig. S10. Grouped mouse weights during MDA-MB-231 xenograft study.
Table S1. Details of 53 top compounds from the high-throughput thioredoxin reductase inhibitor screen.
Table S2. SMILES of TRi-1, TRi-2, and analogs.
Table S3. Mouse liver microsome stability of TRi-1, TRi-2, and auranofin.
Table S4. Growth inhibition for TRi-1, TRi-2, and auranofin tested with the NCI-60 cancer cell panel.
Table S5. Death of mice treated with the combination of auranofin and BSO.
Table S6. Comparisons in effects between TRi-1 and auranofin.
Acknowledgements
The authors wish to thank Arne Holmgren for providing TXN1; Marcus Conrad for providing MEFs; Thomas Helleday and Lars Bräutigam for providing CCD841 cells; Pádraig D’Arcy for compound formulation support; Jonas Grafström for PET data analysis support; Johanna Henriksnäs, and Urban Höglund for assistance at Adlego Biomedical AB; Ulrika Yngve, Richard Svensson and Kristian Sandberg for assistance at the Drug Development Platform and SciLifeLab, Uppsala; Carolina Trkulja, Isabel Löwstedt, and Kjell Andersson for MDA-MB-231 xenograft study support; the Neuroradiology PET radiochemistry group for the PET radiotracer production; Sam Michael and Richard Jones for automation support; Paul Shinn, Danielle van Leer, Crystal McKnight and Misha Itkin for the assistance with compound management; William Leister, Heather Baker, Elizabeth Fernandez, and Christopher Leclair for analytical chemistry support; Alan Meshaw at Charles River Laboratories for expert advice on statistical analyses of the tumor experiments.
Funding: This study was supported by funding from the Karolinska Institutet, the Swedish Research Council, the Swedish Cancer Society, Radiumhemmets forskningsfonder, Barncancerfonden, the Swedish Foundation for Strategic Research, the Knut and Alice Wallenberg Foundations, the US National Institutes of Health (NIH) Intramural Research Program, NIH grant 5R03MH090846, the intramural research program of the National Center for Advancing Translational Sciences and the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research (U54MH084681), and Oblique Therapeutics AB.
Footnotes
Competing interests: W.C.S., D.K.L., T.S.D., N.P.C, D.J.M, A.J., A.S. and E.S.J.A. are inventors on three patents (GB1514015.5, GB1514018.9, GB1514021.3) submitted by E.S.J.A. and the United States of America as represented by the Secretary Department of Health and Human Services Office of Technology Transfer, for protection of intellectual property regarding the TXNRD1 inhibitors as discovered within these studies. O.O. and W.C.S. are employees and shareholders of a company developing TRi compounds towards clinical applications. All other authors state that they have no competing interests.
Data and materials availability: Data from the high-throughput screen for TXNRD1 inhibitors can be found online at https://pubchem.ncbi.nlm.nih.gov (AID:588453). Materials described in this study are available upon request from E.S.J.A.
References and Notes
- 1.Watson J, Oxidants, antioxidants and the current incurability of metastatic cancers. Open biology 3, 120144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wondrak GT, Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal 11, 3013–3069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorrini C, Harris IS, Mak TW, Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12, 931–947 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Trachootham D, Alexandre J, Huang P, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8, 579–591 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Mitsuishi Y, Motohashi H, Yamamoto M, The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Frontiers in oncology 2, 200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arner ES, Holmgren A, The thioredoxin system in cancer. Semin Cancer Biol 16, 420–426 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Solimini NL, Elledge SJ, Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G, Metabolic targets for cancer therapy. Nat Rev Drug Discov 12, 829–846 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Sayin VI et al. , Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6, 221ra215 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Harris IS et al. , Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer cell, (2015). [DOI] [PubMed] [Google Scholar]
- 11.Mandal PK et al. , System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem 285, 22244–22253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson S, Prigge JR, Talago EA, Arner ES, Schmidt EE, Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat Commun 6, 6479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prigge JR et al. , Hepatocyte DNA replication in growing liver requires either glutathione or a single allele of txnrd1. Free Radic Biol Med 52, 803–810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillig CH, Holmgren A, Thioredoxin and related molecules-from biology to health and disease. Antioxid Redox Signal 9, 25–47 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Arner ES, Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim Biophys Acta 1790, 495–526 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Cadenas C et al. , Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res 12, R44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM, The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res 23, 2425–2433 (2003). [PubMed] [Google Scholar]
- 18.Bhatia M et al. , The thioredoxin system in breast cancer cell invasion and migration. Redox Biol 8, 68–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leone A, Roca MS, Ciardiello C, Costantini S, Budillon A, Oxidative Stress Gene Expression Profile Correlates with Cancer Patient Poor Prognosis: Identification of Crucial Pathways Might Select Novel Therapeutic Approaches. Oxidative Medicine and Cellular Longevity 2017, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo MH et al. , Targeting thioredoxin reductase 1 reduction in cancer cells inhibits self-sufficient growth and DNA replication. PLoS ONE 2, e1112 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B et al. , Thioredoxin reductase inhibitors: a patent review. Expert Opin Ther Pat, 1–10 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Cai W et al. , Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic Biol Med 52, 257–265 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Wang L et al. , Ethaselen: a potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic Biol Med 52, 898–908 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Chew EH, Holmgren A, Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A 104, 12288–12293 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urig S, Becker K, On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol 16, 452–465 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Witte AB, Anestål K, Jerremalm E, Ehrsson H, Arnér ESJ, Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med 39, 696–703 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Talbot S, Nelson R, Self WT, Arsenic trioxide and auranofin inhibit selenoprotein synthesis: implications for chemotherapy for acute promyelocytic leukaemia. Br J Pharmacol 154, 940–948 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy D et al. , Gold(I)-mediated inhibition of protein tyrosine phosphatases: a detailed in vitro and cellular study. J Med Chem 51, 4790–4795 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Cox AG, Brown KK, Arner ES, Hampton MB, The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharmacol, (2008). [DOI] [PubMed] [Google Scholar]
- 30.Rigobello MP, Folda A, Baldoin MC, Scutari G, Bindoli A, Effect of auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic Res 39, 687–695 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Miller WH Jr., Schipper HM, Lee JS, Singer J, Waxman S, Mechanisms of action of arsenic trioxide. Cancer Res 62, 3893–3903 (2002). [PubMed] [Google Scholar]
- 32.Cheng Q, Sandalova T, Lindqvist Y, Arnér ESJ, Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J Biol Chem 284, 3998–4008 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Huber RE, Criddle RS, Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys 122, 164–173 (1967). [DOI] [PubMed] [Google Scholar]
- 34.Arner ES, Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res 316, 1296–1303 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Johansson L et al. , Exploiting the 21st amino acid - purifying and labeling proteins by selenolate targeting. Nat Methods 1, 61–66 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Fang J, Lu J, Holmgren A, Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 280, 25284–25290 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Anestål K, Prast-Nielsen S, Cenas N, Arnér ESJ, Cell Death by SecTRAPs – Thioredoxin Reductase as a Prooxidant Killer of Cells. PLoS ONE 3, e1846 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anestål K, Arnér ESJ, Rapid Induction of Cell Death by Selenium-compromised Thioredoxin Reductase 1 but Not by the Fully Active Enzyme Containing Selenocysteine. J Biol Chem 278, 15966–15972 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Prast-Nielsen S et al. , Inhibition of thioredoxin reductase 1 by porphyrins and other small molecules identified by a high-throughput screening assay. Free Radic Biol Med 50, 1114–1123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong L, Arnér ESJ, Ljung J, Åslund F, Holmgren A, Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J. Biol. Chem 273, 8581–8591 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Gromer S, Arscott LD, Williams CH, Schirmer RH, Becker K, Human placenta thioredoxin reductase: Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem 273, 20096–20101 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Rackham O et al. , Substrate and inhibitor specificities differ between human cytosolic and mitochondrial thioredoxin reductases: Implications for development of specific inhibitors. Free Radic Biol Med 50, 689–699 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Albert A et al. , Speciation analysis of the antirheumatic agent Auranofin and its thiol adducts by LC/ESI-MS and LC/ICP-MS. J Anal Atom Spectrom 27, 975–981 (2012). [Google Scholar]
- 44.Toppo S, Flohe L, Ursini F, Vanin S, Maiorino M, Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta 1790, 1486–1500 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Tobiume K et al. , ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cebula M, Schmidt EE, Arner ES, TrxR1 as a Potent Regulator of the Nrf2-Keap1 Response System. Antioxid Redox Signal, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rigobello MP, Scutari G, Boscolo R, Bindoli A, Induction of mitochondrial permeability transition by auranofin, a gold(I)-phosphine derivative. Br J Pharmacol 136, 1162–1168 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox AG, Brown KK, Arner ES, Hampton MB, The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharmacol 76, 1097–1109 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Rabow AA, Shoemaker RH, Sausville EA, Covell DG, Mining the National Cancer Institute’s tumor-screening database: identification of compounds with similar cellular activities. J Med Chem 45, 818–840 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Trachootham D et al. , Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10, 241–252 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L, Arner ES, High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic Biol Med 47, 1661–1671 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Peng X et al. , Sec-containing TrxR1 is essential for self-sufficiency of cells by control of glucose-derived H2O2. Cell Death Dis 5, e1235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shields AF, Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol 8, 141–150 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Lin EY et al. , Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. The American journal of pathology 163, 2113–2126 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR, Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res 17, 6206–6217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnér ESJ, Holmgren A, in Current Protocols in Toxicology, Maines M, Costa L, Reed D, Sassa S, Eds. (John Wiley & Sons, Inc., New York, 2000), pp. 7.4.1–7.4.14. [Google Scholar]
- 57.de Langen AJ et al. , Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 53, 701–708 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Rengby O et al. , Assessment of production conditions for efficient use of Escherichia coli in high-yield heterologous recombinant selenoprotein synthesis. Appl Environ Microbiol 70, 5159–5167 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inglese J et al. , Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 103, 11473–11478 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts G, Myatt GJ, Johnson WP, Cross KP, Blower PE Jr., LeadScope: software for exploring large sets of screening data. J Chem Inf Comput Sci 40, 1302–1314 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Cheng Q, Arner ES, Selenocysteine insertion at a predefined UAG codon in a release factor 1 (RF1) depleted Escherichia coli host strain bypasses species barriers in recombinant selenoprotein translation. J Biol Chem, 292, 5476–5487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez Molina D et al. , Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Zhang X et al. , Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun 5, 3295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. TRi-1, TRi-2, and analog structures with enzymatic inhibition activities.
Fig. S2. Schematic reactivity of TRi-1 and TRi-2 with reduced GSH and their respective metabolite percentages.
Fig. S3. Inhibition of human TXNRD1 and TXNRD2 by (A) TRi-1 or (B) auranofin.
Fig. S4. Cellular TXNRD1 activity from HCT116 cells incubated with compounds for 3 hrs.
Fig. S5. Thermal stability of TXNRD1 oxidized, reduced, and the reduced enzyme incubated with compounds.
Fig. S6. Comparison of cytotoxicity profiles of TXNRD1 inhibitory small molecules within the NCI 60 cancer cell panel.
Fig. S7. FaDu cell colony formation assay.
Fig. S8. FaDu cells treated with and without BSO and increasing concentrations of compound.
Fig. S9. Grouped mouse weights during repeated dose toxicity study.
Fig. S10. Grouped mouse weights during MDA-MB-231 xenograft study.
Table S1. Details of 53 top compounds from the high-throughput thioredoxin reductase inhibitor screen.
Table S2. SMILES of TRi-1, TRi-2, and analogs.
Table S3. Mouse liver microsome stability of TRi-1, TRi-2, and auranofin.
Table S4. Growth inhibition for TRi-1, TRi-2, and auranofin tested with the NCI-60 cancer cell panel.
Table S5. Death of mice treated with the combination of auranofin and BSO.
Table S6. Comparisons in effects between TRi-1 and auranofin.