Abstract
Gamma oscillations that occur within the entorhinal cortex-hippocampal circuitry play important roles in the formation and retrieval of memory in healthy brains. Recent studies report that gamma oscillations are impaired in the entorhinal-hippocampal circuit of Alzheimer’s disease (AD) patients and AD animal models. Here we review the latest advancements in studies of entorhinal-hippocampal gamma oscillations in healthy memory and dementia. This review is especially salient for readers in Alzheimer’s research field not familiar with in vivo electrophysiology. Recent studies have begun to show a causal link between gamma oscillations and AD pathology, suggesting that gamma oscillations may even offer a plausible future therapeutic target.
1. Gamma oscillations in entorhinal cortex-hippocampus circuits of healthy subjects
The hippocampus and the entorhinal cortex (EC) play critical roles in the formation of declarative memory (Gomez-Isla et al., 1996; Scoville and Milner, 1957; Van Hoesen et al., 1991). The EC interfaces the hippocampus with a number of cortical regions, and most of the cortical input to the hippocampus comes from the EC (Cajal, 1911; Witter and Amaral, 2004). The medial part of EC (medial entorhinal cortex, MEC) processes information about spatial memory (Steffenach et al., 2005), and contains spatially modulated cells including grid cells, head direction cells and border cells, which provide spatial information to place cells in the hippocampal CA1 (Moser et al., 2014). In contrast, the lateral entorhinal cortex (LEC) handles information for updating environmental cues, such as odors (Igarashi et al., 2014; Leitner et al., 2016; Li et al., 2017) or objects (Deshmukh and Knierim, 2011; Tsao et al., 2013). The information encoded within the EC is sent to the hippocampal CA1 through the temporoammonic (direct) pathway, and to the CA3 and dentate gyrus via performant (indirect) pathway, providing information for memory to these regions (Witter et al., 2000).
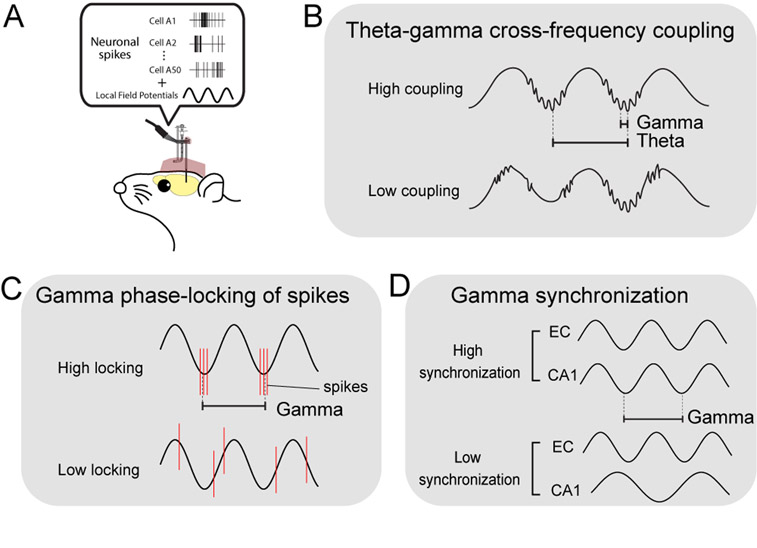
Multi-channel (>32 channel) electrophysiological recording technique is a powerful method for investigating in vivo neuronal activities in the entorhinal-hippocampal circuit of behaving animals (Fig. 1A). The recorded data are normally filtered out as spike activity and slower local field potential (LFP) activity. The most prominent activities observed in the LFP recordings are theta oscillations and gamma oscillations (30–100 Hz). Theta oscillations are 4–12 Hz oscillatory activity generated mainly by the input from the medial septal nucleus. Gamma oscillations in contrast, are local activity derived from the transmembrane current of a population of periodically synchronized neurons (Buzsaki et al., 2012; Einevoll et al., 2013). In healthy rodents and humans, the hippocampus and EC exhibit prominent gamma oscillations that emerge at specific phases of theta oscillations (a phenomenon called “theta-gamma cross-frequency coupling”; Fig 1B) (Bragin et al., 1995; Buzsaki et al., 1983; Canolty et al., 2006; Chrobak and Buzsaki, 1998; Colgin et al., 2009; Mormann et al., 2005; Soltesz and Deschenes, 1993). Because oscillatory membrane current of neurons also contributes to spike generation, spike timing normally coincides with specific phases of gamma oscillations (“gamma phase locking” of spikes; Fig 1C). Computational models suggest that cross-frequency coupling offers an effective mechanism for inter-regional information transfer, because slower theta oscillations can spread to wider areas and synchronize local gamma generators in individual regions (Lisman, 2005; Lisman and Idiart, 1995). In the entorhinal-hippocampal circuit, theta oscillations are thought to coordinate the activity of gamma generators within the entorhinal cortex and hippocampus, whereas these gamma generators coordinate spike timing of neuronal populations in each region. Because of the coordination of multiple gamma oscillators by a common theta rhythm, gamma oscillations in the entorhinal cortex and hippocampus often show synchronization (here we refer as “gamma synchronization”; also referred to as “gamma coupling”; Fig 1D). Theta-gamma cross-frequency coupling, gamma phase-locking of spikes and gamma synchronization are indexes commonly used for evaluating the strength of the neuronal activity coordination by gamma oscillations.
Figure 1.
A, Schematic diagram showing recordings of spike and local field potentials from behaving rodents. B, Theta-gamma cross-frequency coupling. C, Gamma phase locking of spikes. Red line shows spiking activity of neurons. D, Gamma synchronization.
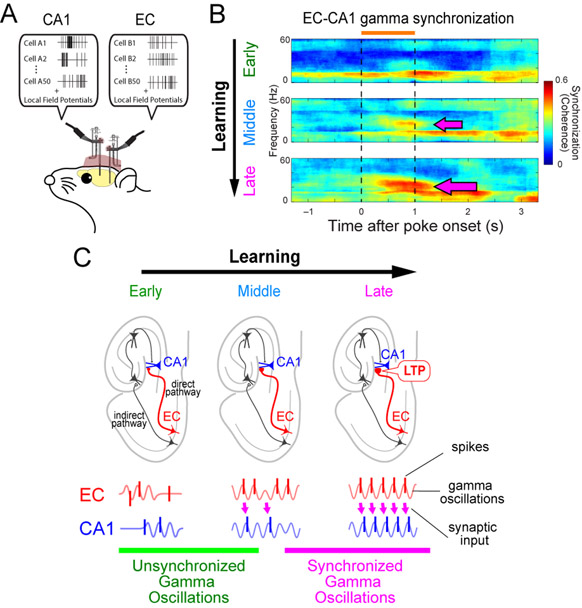
Several studies have demonstrated that strengthening of theta-gamma cross-frequency coupling or gamma synchronization parallels with increased memory formation. The strength of theta-gamma cross-frequency coupling in the hippocampus is correlated with the task load of a working memory in human (Axmacher et al., 2010). In rats, the degree of theta-gamma coupling in the hippocampus is correlated with the amount of task demands (Tort et al., 2009). In the rat EC, strengthening of theta-gamma cross-frequency coupling also occurs during olfactory association learning. In our previous study, we recorded neural activities in the LEC and hippocampal CA1 while rats learned an odor-place association task and asked whether oscillatory coupling in the LEC and CA1 developed during task learning (Igarashi et al., 2014) (Fig. 2A). Prior investigation reports that the acquisition of odor-place association requires an intact hippocampus (Day et al., 2003), which takes ~3 weeks. We found that LFP activity in both LEC and CA1 exhibited strong oscillations in the 20 – 40 Hz band (slow gamma band) during sampling of the odor cues. Recording during the task learning showed that cross-frequency coupling between the theta oscillations and the 20 – 40 Hz band became strengthened gradually over the learning in both the LEC and CA1. The theta-gamma coupling paralleled with gradual strengthening of gamma synchronization between the CA1 and LEC (Fig. 2B). Furthermore, spiking activity of neurons in both the LEC and hippocampus developed phase-locking to slow gamma oscillations as learning progressed. These results suggest that the coordination of EC-hippocampus circuit is supported by synchronized gamma oscillations between the LEC and CA1, together with the cross-frequency coupling in the individual areas. These studies strongly suggest that the coordination of neuronal activities by gamma oscillations in the EC-hippocampus circuit plays crucial roles in memory function in healthy animal’s brains. Since gamma oscillations are in the high-frequency burst range that generates synaptic long-term potentiation (LTP), we speculate that gamma oscillations enhance LTP in the entorhinal → hippocampal synapse during the learning process (Fig. 2C) (Igarashi, 2015).
Figure 2.
A, Simultaneous recordings of spike and local field potentials from the EC and CA1 of behaving rodents. B, Synchronization of gamma oscillations in the EC and CA1 increases during memory task learning (arrows). Modified from Igarashi et al. (2014). C, Synchronization of gamma oscillations emerges between the EC and CA1 during learning and may induce LTP in EC→CA1 synapses in the direct pathway. Modified from Igarashi (2015).
2. Impairment of gamma oscillations in Alzheimer’s disease
Accumulating evidence suggests that the entorhinal-hippocampal circuit is severely affected during the progression of Alzheimer’s disease (AD). AD is the leading cause of age-related dementia and involves the accumulation of oligomeric and fibrillar species of amyloid-β (Αβ) and tau proteins that together lead to synaptic impairment, network disturbances, and cognitive dysfunction. Several transgenic mouse models of AD have been developed and used to examine the impact of AD pathology on entorhinal-hippocampal circuitry. Most of these models utilize overexpression of mutant forms of amyloid precursor protein (APP), the holoprotein from which Αβ is generated. In some models these APP mutations are further coupled with mutations in presenilin 1 (PSEN1), a protease that cleaves APP and influences the length and toxicity of Αβ fragments. To model tau pathology yet other transgenic models have been developed that express human tau with mutations that cause a related neurodegenerative disease, frontotemporal dementia. Together these varying transgenic mice have been used to mimic the pathology that occurs in the entorhinal cortex and hippocampus of AD patients including the accumulation of Αβ plaques and tau-laden neurofibrillary tangles within these regions (Gomez-Isla et al., 1996; Hyman et al., 1984; Van Hoesen et al., 1991).
Using these transgenic models, recent studies have shown that the hippocampus exhibits impairments in both neuronal spike activity and LFP oscillatory activity. For example, Cacucci and colleagues (2008) showed that place cells have a larger place field and encode less spatial information in pathological (16-month-old) Tg2576 AD mice. As a result, the AD mice exhibited impaired spatial memory in a T-maze task that correlated closely with reduced spatial information. Furthermore, in Tg2576 mice crossed with PSEN1dE9 mice, which dramatically accelerates the onset of plaque pathology, increase in the number of task-relevant place cells observed in wild-type mouse were reduced at 6-monhts of age (Cayzac et al (2015)). Interestingly, place cell field size was unchanged in ~11-month-old inducible APP transgenic mice with abundant plaque pathology, but the cells exhibited an impairment in the normal shrinkage of place field size which occurred in control mice with repetitive daily training (Zhao et al., 2014). The prior studies all utilized APP transgenic mice at ages at which plaque pathology was present. Recently, similar reductions in place cell spatial information have been reported in the 3xTg-AD mouse model, which also develops tau pathologies, although at ages prior to plaque formation (Mably et al., 2017). Taken together, these studies suggest that impairments in spatial representation by place cells may underlie the observed spatial memory loss in multiple AD mouse models.
More recently, hippocampal gamma oscillations in AD models has gained increasing attention. For example, Goutagny and colleagues (2013) found that theta-gamma cross-frequency coupling is impaired in isolated hippocampus preparation from 1-month old TgCRND8 transgenic mice, an age prior to plaque formation. Similarly, Iaccarino and colleagues (2016) showed that hippocampal gamma oscillations during sharp-wave ripples were impaired in young (3-month old) 5XFAD transgenic AD mice prior to the onset of extracellular Αβ plaques. This notion is also consistent with a recent paper by Verret et al. (2012), in which the authors found that the impairment of gamma oscillations in the parietal cortex of the J20 AD mouse model can be rescued by enhancing the activity of interneurons via overexpressing interneuron-specific voltage-gated sodium channel subunit Nav1.1. Together, these results indicate that considerable impairments in gamma oscillations exists in the hippocampus of several AD mouse models, and this impairment can even occur prior to Αβ plaque pathology. However, mechanisms leading to the impairments in gamma oscillations before the Αβ plaque pathology are so far unclear, and future works will be required.
In addition to the effects of Ab, another major AD risk gene product, apolipoprotein E4 (ApoE4), has also been implicated in gamma oscillation dysfunction. In the first study, Gillespie and colleagues (2016) recorded gamma oscillations within the hippocampus from mice expressing humanized ApoE4. They assessed a specific form of gamma oscillations that occur during short bursts of high-frequency (150–250 Hz) oscillation events (sharp-wave ripples) previously reported by Carr et al (2012), and demonstrated that the gamma power during sharp-wave ripple events was significantly reduced in apoE4 mouse. Interestingly, gamma oscillations were rescued when apoE4 was removed specifically from GABAergic interneurons by crossing floxed apoE4 mice with a Dlx-cre driver line. This result paralleled with the rescue of memory impairment in the same crossed animals in the group’s previous study (Knoferle et al., 2014). Together, these results indicate that dysfunction of GABAergic interneurons plays a key role in the impairment of gamma oscillations in ApoE4 AD model mice.
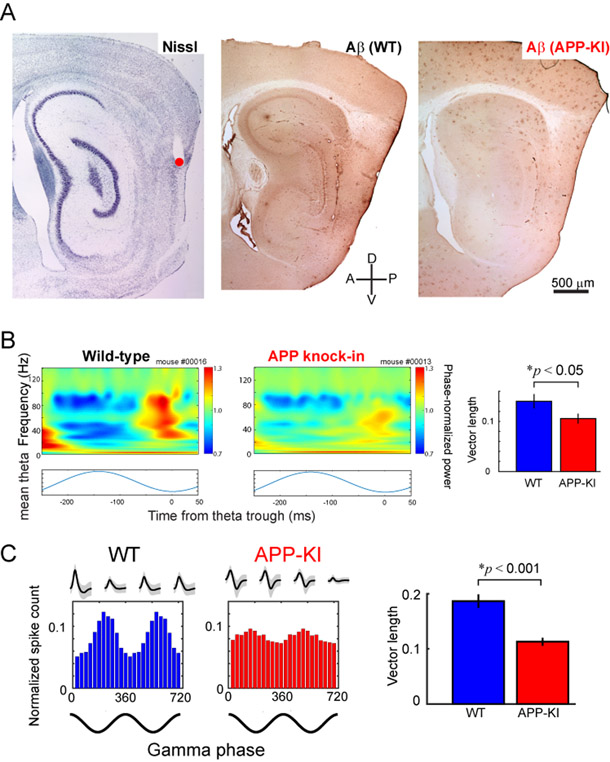
Do similar neuronal activity changes occur within the EC? Compared to the hippocampus, studies of EC physiology in AD models are far more limited. Focusing on models with tau pathology, Fu and colleagues (2017) demonstrated that spike activity of grid cells within the MEC was reduced in 30-month-old transgenic mice that express mutant human tau protein in the MEC. An in vitro slice study by Booth and colleagues (2016) reported that the power of kainate-induced gamma oscillations is reduced in rTg4510 mice that overexpress mutant human tau. Similar reductions have been reported in a model of Αβ pathology whereby a reduction of gamma oscillation power in the lateral entorhinal cortex of ~5-month-old APP/PSEN1 transgenic mouse, but smaller reduction within the MEC, was observed (Klein and colleagues (2016)). To reconcile these results, we recently performed an in vivo recording of both spike and LFP activity from an APP knock-in mouse model (Fig. 3A) (Nakazono et al., 2017). The knock-in model expresses mutated human APP gene in the endogenous locus, and is therefore expected to mimic human AD pathology more precisely, because it avoids the confound of APP overexpression that is found in most other transgenic models of AD. It also avoids the issue of presenilin mutations, which themselves are associated with disruption of many cellular functions, including calcium homeostasis. The APP knock-in model also avoids the potential drift of transgene copy number and potential problems with overexpression of non-Aβ APP fragments (Saito et al., 2014). We selected an age after the appearance of plaques, but prior to memory impairments, in order to model the “preclinical” stage of AD (Bateman et al., 2012; Sasaguri et al., 2017). Using this model, we showed that theta-gamma cross-frequency coupling was reduced in the MEC of 5-month-old APP knock-in mice (Fig. 3B). Furthermore, the phase-locking of spiking activity of the layer II/III principal neurons to the gamma oscillations was significantly reduced (Fig. 3C). These data indicate that the neural circuit activities organized by gamma oscillations were disrupted in the MEC of APP knock-in mice. Together with the studies discussed above, these findings suggest that gamma oscillations are impaired within the EC of AD mice. However, due to the use of varying AD models across these studies, it remains unknown whether the EC gamma impairment starts prior to or after hippocampal impairments. Thus, future studies are needed to understand the time course of gamma impairments within the EC and hippocampus. Additionally, given that these mouse models show impairments in gamma oscillations prior to plaque pathologies, or shortly thereafter, the relevance to human AD needs to be delineated. AD patients develop plaque pathologies many years prior to clinical symptoms (Bateman et al., 2012; Sasaguri et al., 2017), showing a delay between plaques and cognitive impairments; for the most part the various mouse models of AD show cognitive impairments prior to, or shortly after, the appearance of the plaques, and thus it may be that the human brain is better at compensating for impaired gamma oscillations than mice, or that the human brain responds to early AD pathologies differently to that of rodent models.
Figure 3. Impaired gamma oscillations in the MEC of APP knock-in mice.
A, Recorded position (left, red) and Aβ deposition in MEC (middle, WT; right, APP-KI). B, Theta-gamma cross-frequency coupling was diminished in the APP-KI mice as revealed by the vector length of theta-gamma phase-locking analysis (right). C, Gamma phase locking of layer II/III principal cells were impaired in the MEC of APP-KI mice, as revealed by the vector length of spike-gamma phase-locking analysis (right). Modified from Nakazono et al (2017).
3. Can gamma reactivation rescue cognitive deficit in AD?
The studies above clearly demonstrate that impairments of gamma oscillation activity occur in the entorhinal-hippocampal circuit of AD mouse models and are closely related to cognitive impairments. While similar studies of human AD subjects are far more limited, it is reasonable to question whether approaches can be developed to safely and effectively reactivate gamma oscillations in the entorhinal-hippocampal circuit in mouse models. If such approaches can be developed they could perhaps eventually be tested in AD subjects.
Several attempts to reactivate gamma oscillations have already been performed in the animal studies. In the Iaccarino study mentioned above, the authors further crossed 5XFAD mouse with parvalbumin (PV)-Cre mouse line to optogenetically stimulate PV interneurons in the hippocampus. By stimulating this subpopulation of interneurons at 40 Hz frequency, they generated gamma oscillations in the hippocampus. Theoretical studies suggest that gamma oscillations are generated by a loop circuit composed of inhibitory cells (Buzsaki and Wang, 2012). Indeed, experimental studies showed that optogenetic stimulation of PV-expressing interneurons generated gamma oscillations (Cardin et al., 2009; Sohal et al., 2009). Surprisingly, 40 Hz optogenetic stimulation in 5XFAD×PV-Cre mouse reduced Αβ peptide levels and induced changes in microglia morphology followed by increased Aβ uptake in these glial cells in the hippocampus. They further performed 40 Hz flickering visual stimulation to the animals, and showed that AD pathology became reduced in the visual cortex. In another study, Roy and colleagues (2016) showed that optogentic stimulation of EC perforant path at high-gamma (100 Hz) frequency increased spine density of cells in the dentate gyrus before the plaque formation (7-months-old) APP/PSEN1 mouse, and rescued memory impairments. In this study, authors stimulated only the terminals of EC cells that were activated in the previous exposure to the experimental box (“engram cells”). This activation strategy may contribute to the generation of gamma oscillations at the focal site within the dentate gyrus that influence the memory specifically needed for the task. In another recent study, Xia and colleagues (2017) performed electrical stimulation of EC cells at 130 Hz in the TgCRND8 mouse model. Although this stimulation frequency is above the range of biological fast gamma oscillations (60–100 Hz), they found that stimulating the EC during the pre-plaque period blocked subsequent Αβ accumulation and reduced cognitive deficit. Altogether, these results strongly indicate that function of gamma oscillations in recruiting neuronal and glial responses to attenuate not only AD-associated cognitive impairments but also to reduce AD pathology.
4. Conclusion and future challenges
The frequency used in the Xia study discussed above was motivated by two previous clinical trials, in which 130 Hz deep brain stimulation (DBS) was given to AD patients (Laxton et al., 2010; Lozano et al., 2016). DBS is a neurosurgical procedure to treat neurological disorders characterized by the deterioration of neuronal function at a particular brain region. It operates through sending electrical impulses from the implanted electrodes with the aim of improving neural circuit deficits. DBS has become a successful therapy option for several different neurologic disorders, including Parkinson’s disease. In a phase I clinical trial to apply DBS for the therapy for AD patients, Laxton and colleagues (2010) performed DBS (130 Hz) targeting the fornix/hypothalamus in AD patients to determine its efficacy in treating AD patients. The study demonstrated marked decrease in cognitive decline without serious adverse effects from the subjects receiving the treatment. Unfortunately, the subsequent phase II trial study of this approach showed little therapeutic benefit (Lozano et al., 2016). However, these initial clinical studies tested only a 130 Hz stimulation frequency which is widely used in DBS studies, and the effect of the 60–100Hz gamma-frequency stimulation has not yet been examined. Considering the effect of gamma stimulations in AD animal models, lower frequency stimulation may provide more favorable effects in DBS trials. Thus, the optimization of stimulation conditions using animal studies, and applying the optimal stimulus protocol in the clinical trials could still lead to an effective therapy. Recently, a method for non-invasive electrical stimulation was developed using interference of multiple high-frequency electric fields (Grossman et al., 2017). If this approach can be harnessed to non-invasively produce gamma stimulations in the entorhinal-hippocampal circuit, it could offer another promising therapeutic for AD in a near future.
Acknowledgments
We thank members of the Igarashi lab for their valuable comments and discussions. The work was supported by PRESTO Grant from Japan Science and Technology Agency (JPMJPR1681), Fay-Frank Seed Grant from Brain Research Foundation (BRFSG-2017-04), Alzheimer’s Association Research Grant (AARG-17-532932) and Whitehall Foundation Research Grant (2017-08-01) to KMI, and NIH R01-AG056303, RF1-AG055524 and P50-AG016573 to MBJ.
Reference
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, and Fell J (2010). Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences of the United States of America 107, 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CA, Ridler T, Murray TK, Ward MA, de Groot E, Goodfellow M, Phillips KG, Randall AD, and Brown JT (2016). Electrical and Network Neuronal Properties Are Preferentially Disrupted in Dorsal, But Not Ventral, Medial Entorhinal Cortex in a Mouse Model of Tauopathy. J Neurosci 36, 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, and Buzsaki G (1995). Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, and Koch C (2012). The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, and Vanderwolf CH (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain research 287, 139–171. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, and Wang XJ (2012). Mechanisms of gamma oscillations. Annu Rev Neurosci 35, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacucci F, Yi M, Wills TJ, Chapman P, and O’Keefe J (2008). Place cell firing correlates with memory deficits and amyloid plaque burden in Tg2576 Alzheimer mouse model. Proceedings of the National Academy of Sciences of the United States of America 105, 7863–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal S.R.y. (1911). Histologie du système nerveux de l’homme et des vertébrés. Vol. II (Paris: A. Maloine; ). [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, and Knight RT (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, and Moore CI (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, and Frank LM (2012). Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75, 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayzac S, Mons N, Ginguay A, Allinquant B, Jeantet Y, and Cho YH (2015). Altered hippocampal information coding and network synchrony in APP-PS1 mice. Neurobiol Aging 36, 3200–3213. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, and Buzsaki G (1998). Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci 18, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, and Moser EI (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, and Morris RG (2003). Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424, 205–209. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, and Knierim JJ (2011). Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, and Panzeri S (2013). Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 14, 770–785. [DOI] [PubMed] [Google Scholar]
- Fu H, Rodriguez GA, Herman M, Emrani S, Nahmani E, Barrett G, Figueroa HY, Goldberg E, Hussaini SA, and Duff KE (2017). Tau Pathology Induces Excitatory Neuron Loss, Grid Cell Dysfunction, and Spatial Memory Deficits Reminiscent of Early Alzheimer’s Disease. Neuron 93, 533–541 e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie AK, Jones EA, Lin YH, Karlsson MP, Kay K, Yoon SY, Tong LM, Nova P, Carr JS, Frank LM, et al. (2016). Apolipoprotein E4 Causes Age-Dependent Disruption of Slow Gamma Oscillations during Hippocampal Sharp-Wave Ripples. Neuron 90, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW Jr., Morris JC, Growdon JH, and Hyman BT (1996). Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16, 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot JG, Quirion R, Krantic S, and Williams S (2013). Alterations in hippocampal network oscillations and theta-gamma coupling arise before Abeta overproduction in a mouse model of Alzheimer’s disease. Eur J Neurosci 37, 1896–1902. [DOI] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, Cassara AM, Neufeld E, Kuster N, Tsai LH, et al. (2017). Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169, 1029–1041 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, and Barnes CL (1984). Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225, 1168–1170. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, et al. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM (2015). Plasticity in oscillatory coupling between hippocampus and cortex. Current opinion in neurobiology 35, 163–168. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, and Moser EI (2014). Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 510, 143–147. [DOI] [PubMed] [Google Scholar]
- Klein AS, Donoso JR, Kempter R, Schmitz D, and Beed P (2016). Early Cortical Changes in Gamma Oscillations in Alzheimer’s Disease. Front Syst Neurosci 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoferle J, Yoon SY, Walker D, Leung L, Gillespie AK, Tong LM, Bien-Ly N, and Huang Y (2014). Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. J Neurosci 34, 14069–14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, et al. (2010). A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68, 521–534.d [DOI] [PubMed] [Google Scholar]
- Leitner FC, Melzer S, Lutcke H, Pinna R, Seeburg PH, Helmchen F, and Monyer H (2016). Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nature neuroscience 19, 935–944. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu J, Liu Y, Zhu J, Liu N, Zeng W, Huang N, Rasch MJ, Jiang H, Gu X, et al. (2017). A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nature neuroscience 20, 559–570. [DOI] [PubMed] [Google Scholar]
- Lisman J (2005). The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15, 913–922. [DOI] [PubMed] [Google Scholar]
- Lisman JE, and Idiart MA (1995). Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science 267, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos JM, Munro C, Oh E, Drake KE, Lyman CH, Rosenberg PB, Anderson WS, et al. (2016). A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J Alzheimers Dis 54, 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably AJ, Gereke BJ, Jones DT, and Colgin LL (2017). Impairments in spatial representations and rhythmic coordination of place cells in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 27, 378–392. [DOI] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, and Fernandez G (2005). Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15, 890–900. [DOI] [PubMed] [Google Scholar]
- Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T, and Moser MB (2014). Grid cells and cortical representation. Nat Rev Neurosci 15, 466–481. [DOI] [PubMed] [Google Scholar]
- Nakazono T, Lam TN, Patel AY, Kitazawa M, Saito T, Saido TC, and Igarashi KM (2017). Impaired in vivo gamma oscillations in the medial entorhinal cortex of knock-in Alzheimer model. Front Syst Neurosci 11, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, and Tonegawa S (2016). Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, and Saido TC (2014). Single App knock-in mouse models of Alzheimer’s disease. Nature neuroscience 17, 661–663. [DOI] [PubMed] [Google Scholar]
- Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, Hardy J, Vassar R, Winblad B, and Saido TC (2017). APP mouse models for Alzheimer’s disease preclinical studies. EMBO J 36, 2473–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, and Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, and Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, and Deschenes M (1993). Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol 70, 97–116. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, and Moser EI (2005). Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron 45, 301–313. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, and Eichenbaum H (2009). Theta-gamma coupling increases during the learning of item-context associations. Proceedings of the National Academy of Sciences of the United States of America 106, 20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser MB, and Moser EI (2013). Traces of experience in the lateral entorhinal cortex. Current biology : CB 23, 399–405. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT, and Damasio AR (1991). Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1, 1–8. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, and Amaral DG (2004). Hippocampal Formation In The Rat Nervous System 3rd edn (ed Paxinos G), Paxinos G, ed. (Amsterdam, The Netherlands: Elsevier; ). [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, and Van Haeften T (2000). Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci 911, 1–24. [DOI] [PubMed] [Google Scholar]
- Xia F, Yiu A, Stone SS, Oh S, Lozano AM, Josselyn SA, and Frankland PW (2017). Entorhinal Cortical Deep-Brain Stimulation Rescues Memory Deficits In Both Young And Old Mice Genetically Engineered To Model Alzheimer’s Disease. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Fowler SW, Chiang AC, Ji D, and Jankowsky JL (2014). Impairments in experience-dependent scaling and stability of hippocampal place fields limit spatial learning in a mouse model of Alzheimer’s disease. Hippocampus 24, 963–978. [DOI] [PMC free article] [PubMed] [Google Scholar]