The World Health Organization has predicted that neurodegenerative diseases affecting the motor function will become the second most prevalent cause of death in the next 20 years. New therapies can result from three main sources: synthetic compounds, natural products, and existing drugs.
Parkinson’s disease (PD) is a common neurodegenerative disease affecting 1–3% of the adult population over 50 years of age worldwide. It is initially characterized by the death of dopaminergic neurons in the substantia nigra pars compact and later by the widespread loss of nondopaminergic neurons, including those in the cortex. Inflammation is the main underlying cause in most, if not all, neurodegenerative diseases, playing a protective role in their initial acute phases, but a pernicious one in their later chronic phases. Increasing evidence has disclosed that microglia-mediated neuroinflammation is crucial for PD progression (Hirsch and Hunot, 2009). Another neuropathological hallmark of PD is the presence of Lewy bodies, which are primarily composed of α-synuclein (α-Syn) aggregates. In recent years, important studies on the role of α-Syn in PD have been conducted. The α-Syn aggregation in the central nervous system is a pathological process of fundamental importance in the development and progression of PD. Aggregates of α-Syn, in oligomeric and fibril forms, are thought to be capable of causing neurodegeneration either by directly damaging neurons or by activating microglia to produce neuroinflammatory mediators, which are neurotoxic (Hirsch and Hunot, 2009). Due to the consequent neuronal damage, an aggregation and release process of endogenous α-Syn occurs, triggering microglial activation and leading to neuroinflammation (Sanchez-Guajardo et al., 2015). In this way, α-Syn aggregates generate a vicious circle of neuroinflammation and neuronal death in PD. The interaction between these two players, α-Syn aggregates and microglial cells, is thus believed to be strongly implicated in the inflammatory process that accompanies PD progression. However, the molecular mechanisms that underlie α-Syn-induced microglia activation are not well understood.
The α-Syn aggregates act as agonists of some membrane receptors, such as Toll-like receptors (TLRs) and P2X purinoceptor 7 (P2X7), on microglial cells (Dos-Santos-Pereira et al., 2018), representing an interesting physiological substance to mimic the neuroinflammatory response in vitro and in vivo in a PD context. These aggregates are an alternative to the chemical substances commonly used to model PD, such as rotenone, paraquat, or 1-methyl-4-phenyl pyridinium. Therefore, therapeutic strategies are actively pursued to decrease, or better yet, prevent α-Syn-induced microglial cell activation and thus neuroinflammation in PD (Sanchez-Guajardo et al., 2015).
Rifampicin (Rif) as a candidate for drug repurposing in PD: The complex technology used in drug finding, the long-term assessment procedures, the difficulties in blood-brain barrier permeability, among other factors, make the process of drug discovery for treating neurodegenerative diseases unsuccessful. Repurposing drugs is currently an expeditious approach to detect drug candidates for PD. In this sense, great expectations have been generated regarding the role of some antibiotics as neuroprotective agents. Experimental evidence currently shows that many features of antibiotics, including anti-inflammatory, anti-aggregating, and antioxidant properties, can be beneficial to the treatment of neurological disorders (Socias et al., 2018). Rif is a macrocyclic bactericidal antibiotic, widely used in the treatment of infectious diseases, especially against those caused by Mycobacterium, such as tuberculosis and leprosy. After oral administration, this antibiotic is rapidly absorbed and distributed throughout all tissues and humors, as it has the ability to pass into the cerebrospinal fluid in significant amounts and act against infectious agents in both cellular and extracellular locations.
Besides its well-known antibacterial activity, Rif was the first antibiotic reported to play a significant protective role in neurodegenerative diseases. It was shown that leprosy patients under chronic treatment with Rif displayed a significantly decreased prevalence of dementia (McGeer et al., 1992). Since that paper was published, a growing number of studies have also revealed that Rif provides therapeutic benefits in chronic neurodegenerative disorders. However, the structural or molecular characteristic responsible for conferring this therapeutic effect remains unknown. In this scenario, we have focused our attention on the structural and chemical features of Rif to elucidate a possible structure-neuroprotective function relationship. Indeed, when Rif is immersed in an aqueous solution, the resulting oxidation processes lead to the formation of different species, influenced mainly by pH (Li et al., 2004). The most abundant and probably the most interesting of these species is Rif quinone (RifQ), which differs from Rif in that a central group corresponding to a dihydro quinone, such as p-benzoquinone, is found in a higher oxidation state.
The anti-neuroinflammatory capacity of RifQ: We have recently reported the results of a study on Rif and RifQ (Acuña et al., 2019). The main goals of our study were to describe some of the mechanisms through which α-Syn fibrils induce in vitro microglial inflammation leading to neuron death and to evaluate the ability of RifQ to mitigate this process in comparison with Rif. We initially observed that the fibril state was the most inflammogenic α-Syn species in primary murine microglial cultures. Then, we described the cell receptors through which α-Syn fibrils interact and the signaling pathway leading to the production of pro-inflammatory substances. We noted that the crucial metabolites released as a result of this microglial response induce the death of primary murine cortical neurons. Finally, we demonstrated that a microglial pretreatment with RifQ is able to significantly reduce the inflammogenic response and the consequent neuronal damage. Our results suggest that after the interaction of α-Syn fibrils with TLR2 and P2X7 membrane receptors, the PI3K/AKT pathway is activated. As a consequence, we detected tumor necrosis factor-alpha and interleukin-6 release as well as reactive oxygen species generation. The pretreatment of microglial cultures with RifQ prevents the production of α-Syn fibril-induced pro-inflammatory factors, thus increasing the neuron survival. Notably, the anti-inflammatory properties of the RifQ pretreatment were not exclusive to α-Syn fibril induction, since we also observed this anti-inflammatory ability when microglia were challenged with TLR2 or P2X7 agonists, Pam3CSK4 and bz-ATP, respectively. Our results confirm that inflammatory microglia release neurotoxic metabolites and that a compound like RifQ could prevent the neuronal death by a suppressive effect on microglial-inflammation (Figure 1A). In a previous study, we had evaluated the pro-inflammatory potential of amyloid fibrils of α-Syn by using glutamate as an inflammatory marker (Dos-Santos-Pereira et al., 2018). We found that α-Syn fibrils had a strong stimulatory effect on glutamate release through a sequence of signaling events and that this stimulatory effect was suppressed by the dopamine neurotransmitter. Altogether, these results show the inflammatory mechanism involved in PD and the way in which some molecules may be useful in preventing or slowing the progression of the neurodegenerative diseases, such as PD, which exhibit altered glial function.
Figure 1.
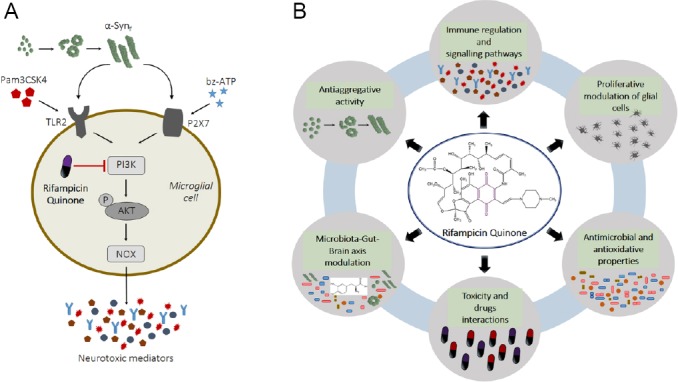
RifQ and Parkinson’s disease.
(A) A model showing how RifQ affects the inflammatory reaction of microglial cells. α-Syn fibrils bind microglial TLR2 and P2X7 receptors, which results in phosphorylation of AKT by PI3K. This induces reactive oxygen species production by activation of the reduced NOX and release of cytokines. These substances are potentially harmful for neuronal cells. RifQ has a suppressive effect on inflammatory mediators release due, in part, to inhibition of PI3K dependent signaling events. The inhibitory effects that RifQ exerts on α-Syn fibrils activated microglia could provide indirect protection to neuronal cells. AKT: Protein kinase B; NOX: nicotinamide adenine dinucleotide phosphate oxidase enzyme; P2X7: P2X purinoceptor 7; PI3K: phosphatidylinositol-3-kinase; RifQ: rifampicin quinone; α-Syn: α-synuclein; TLR2: Toll-like receptor 2. (B) Biological assays to determine the potential of rifampicin quinone in therapies for Parkinson’s disease. The research path towards efficient use of rifampicin quinone involves a multimodal analysis. Such analysis should comprise immune modulation, anti-aggregative, antioxidant, and antimicrobial features, followed by a set of tests including in vivo toxicity, drug interactions, and effects on microbiota-gut-brain axis signaling to identify whether there could be positive effects against Parkinson’s disease.
We suggest that RifQ could be a candidate molecule for the treatment of PD either by interfering with inflammatory processes or by preventing the accumulation of pro-inflammatory microglial cell activators, like α-Syn aggregates. In addition, our results suggest that RifQ could be behind some of the properties attributed to Rif and that the p-benzoquinone group of the molecule could be specifically associated with some chemical and biological effects, as previously observed by Konrad and Stenberg (1988). Regarding aggregation protein inhibition, it was previously reported that Rif plays an anti-aggregating role not only in the human α-Syn, stabilizing its monomeric form, but also in other proteins involved in neurodegenerative diseases, like amyloid-β and tau, thus indicating its broad spectrum (Li et al., 2004; Socias et al., 2018). It was also shown that Rif and p-benzoquinone served as effective inhibitors against in vitro amyloid fibrillogenesis of lysozyme, suggesting that these molecules may be used in a rational design of effective therapeutics for amyloidogenic diseases (Lieu et al., 2007). In this sense, RifQ is like a combination of Rif and p-benzoquinone in the same molecule. However, as opposed to Rif, not many trials have been conducted with RifQ over time. The only study using oxidized species of Rif in processes related to neurodegenerative diseases was conducted by Li et al. (2004), employing an aged preparation of Rif, where a combination of rusty species was present. This formulation was reported to have more potent anti-aggregating effects than Rif (Li et al., 2004). Furthermore, other studies have also reported that oxidized derivatives of certain molecules would be more effective in inhibiting protein aggregation. Quinone formation has previously been reported as the potential mechanism of inhibition of α-Syn aggregation by the phenolic compound baicalein (Zhu et al., 2004). Also, oxidized derivatives of benzoic acid are more effective in inhibiting α-Synfibrillation and could be attributed to the combined properties of their three -OH groups and quinone-forming structures (Ardah et al., 2014). The presence of such oxidized products probably increases the stability of the compound binding to the α-Syn fibrils, accounting for the increased inhibitory potency of these compounds in a protein-aggregation process.
As regards the background on inflammation processes, several studies have also reported the positive effects of Rif, supporting its potential clinical applications to prevent the neuroinflammation (Socias et al., 2018). In addition, experiments carried out with quinone molecules have been reported to have effects on both the prevention and stimulation of inflammation. However, these effects are not fully understood yet. It is necessary to determine whether the presence of the structural motifs mentioned above, which have anti-aggregative implications, is also important in drugs that can be used as immunomodulators against neuroinflammatory processes. In this regard, Rif was tested as a potential immunosuppressive agent in rats, and the immunosuppressive effect ascribed to Rif was found to be, in fact, due to RifQ (Konrad and Stenberg, 1988).
Reflections and perspectives: Despite the great number of reports on the beneficial effects of Rif in PD pathology, analyses using pure RifQ have not been performed to date. Our data are promising adequately to justify further experiments to confirm the potential of RifQ as an antiparkinsonian drug. We suggest that RifQ should be carefully examined to know this molecule in-depth before this antibiotic is administered in chronic therapy.
As we previously noticed, a noxious circle in which protein aggregation induces neuroinflammation and neuronal death and vice versa is playing in PD. Keeping this in mind, therapeutic strategies should be focused in blocking all the possible factors leading to neurodegeneration. In this line, since α-Syn aggregation is one of the most important events of that detrimental circle, RifQ should be block the amyloid pathway to be adequate for using in the early stage of PD. Unfortunately, the difficulty for early diagnosis may interfere with the success of therapy and the pertinent starting points for treatment may have passed. Nevertheless, RifQ could be also beneficial at later stages as long as it could mitigate the injurious microglia reaction induced by α-Syn aggregates (particularly oxidative stress and inflammation) and diminish the neuronal death. In vivo anti-oxidative, anti-proliferative and anti-inflammatory properties may contribute to combat degeneration and neuronal death. In this scenario, it is now crucial to investigate these parameters in animal models when the process of neurodegeneration is already engaged.
Although RifQ could represent a potential option to treat neuroinflammation, as explained above, its anti-bacterial activity might also be a potential problem for long-term therapy. An alternative to avoid this inconvenience is to test whether RifQ could exert neuroprotective action at sub-antibiotic doses in vivo without disturbing microbiome communities. Another option would involve developing RifQ chemical analogs without anti-bacterial action but retaining their neuroprotective properties, or with the same anti-bacterial action but having an enhanced neuroprotective effect for long-term administration at lower doses. Another interesting perspective in understanding the antibiotic features of RifQ is the antimicrobial activity against bacteria from microbiota involved in the brain-gut axis, particularly the bacterial strains that have been found to play a leading role in PD. A recently described pathway for gut bacterial levodopa metabolism was found to reduce its bioavailability and to probably decrease its efficacy in PD patients (Maini Rekdal et al., 2019). This pathway involves the decarboxylation of levodopa to dopamine by Enterococcus faecalis and, subsequently, dehydroxylation of dopamine by Eggerthella lenta to form m-Tyramine (Maini Rekdal et al., 2019). Does RifQ have antimicrobial activity against these strains? Can RifQ block or diminish the activity of the microbial enzymes involved in L-dopa metabolism in the human gut at sub-antibiotic concentrations? Deciphering these connections may be an important research avenue to know drug interactions to eventually improve levodopa efficacy and the success in the treatment of PD.
Against this background, it remains to be determined whether any effects of Rif would be mainly caused by the oxidized species RifQ. Therefore, multiple comparisons between RifQ and Rif would help elucidate the structure-function relationship of these molecules. RifQ, like any other antibiotic, may exert selective pressure against microorganisms normally found in the human microbiota, and therefore, affecting the physiologic processes that they are involved by perturbing the symbiotic interaction microbia/human. For that, in vitro differences between RifQ and Rif of their antimicrobial properties including spectral action, resistant bacteria selection and minimal inhibitory concentrations against human gut bacteria could also be relevant points to be compared in order to elucidate the linkage between chemical structure and biological functions. By determining these relationships, we would expand the knowledge required to establish the chemical components that should be present to repurpose or design drugs to combat neurodegenerative diseases. An overview of experiments and future perspectives is summarized in (Figure 1B).
This work was supported by Grants PIP 0906CO from CONICET, PICT 3776 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and CIUNSa 2530/0 from Universidad Nacional de Salta and PIUNT D641/1 (all to LA).
Additional file: Open peer review report 1 (80.5KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jing Wang, Boston Children’s Hospital, USA.
P-Reviewer: Wang J; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Acuña L, Hamadat S, Corbalán NS, González-Lizárraga F, Dos-Santos-Pereira M, Rocca J, Díaz JS, Del-Bel E, Papy-García D, Chehín RN, Michel PP, Raisman-Vozari R. Rifampicin and its derivative rifampicin quinone reduce microglial inflammatory responses and neurodegeneration induced in vitro by α-synuclein fibrillary aggregates. Cells. 2019 doi: 10.3390/cells8080776. doi: 103390/cells8080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardah MT, Paleologou KE, Lv G, Abul Khair SB, Kazim AS, Minhas ST, Al-Tel TH, Al-Hayani AA, Haque ME, Eliezer D, El-Agnaf OM. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front Aging Neurosci. 2014;6:197. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dos-Santos-Pereira M, Acuña L, Hamadat S, Rocca J, González-Lizárraga F, Chehín R, Sepulveda-Diaz J, Del-Bel E, Raisman-Vozari R, Michel PP. Microglial glutamate release evoked by α-synuclein aggregates is prevented by dopamine. Glia. 2018;66:2353–2365. doi: 10.1002/glia.23472. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 5.Konrad P, Stenberg P. Rifampicin quinone is an immunosuppressant, but not rifampicin itself. Clin Immunol Immunopathol. 1988;46:162–166. doi: 10.1016/0090-1229(88)90017-7. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Lieu VH, Wu JW, Wang SS, Wu CH. Inhibition of amyloid fibrillization of hen egg-white lysozymes by rifampicin and p-benzoquinone. Biotechnol Prog. 2007;23:698–706. doi: 10.1021/bp060353n. [DOI] [PubMed] [Google Scholar]
- 8.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019 doi: 10.1126/science.aau6323. doi: 101126/scienceaau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGeer PL, Harada N, Kimura H, McGeer EG, Schulzer M. Prevalence of dementia amongst elderly Japanese with leprosy: apparent effect of chronic drug therapy. Dementia. 1992;3:146–149. [Google Scholar]
- 10.Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. The relation between α-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience. 2015;302:47–58. doi: 10.1016/j.neuroscience.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Socias SB, González-Lizárraga F, Avila CL, Vera C, Acuña L, Sepulveda-Diaz JE, Del-Bel E, Raisman-Vozari R, Chehin RN. Exploiting the therapeutic potential of ready-to-use drugs: Repurposing antibiotics against amyloid aggregation in neurodegenerative diseases. Prog Neurobiol. 2018;162:17–36. doi: 10.1016/j.pneurobio.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


