Abstract
Spinal cord injury is linked to the interruption of neural pathways, which results in irreversible neural dysfunction. Neural repair and neuroregeneration are critical goals and issues for rehabilitation in spinal cord injury, which require neural stem cell repair and multimodal neuromodulation techniques involving personalized rehabilitation strategies. Besides the involvement of endogenous stem cells in neurogenesis and neural repair, exogenous neural stem cell transplantation is an emerging effective method for repairing and replacing damaged tissues in central nervous system diseases. However, to ensure that endogenous or exogenous neural stem cells truly participate in neural repair following spinal cord injury, appropriate interventional measures (e.g., neuromodulation) should be adopted. Neuromodulation techniques, such as noninvasive magnetic stimulation and electrical stimulation, have been safely applied in many neuropsychiatric diseases. There is increasing evidence to suggest that neuromagnetic/electrical modulation promotes neuroregeneration and neural repair by affecting signaling in the nervous system; namely, by exciting, inhibiting, or regulating neuronal and neural network activities to improve motor function and motor learning following spinal cord injury. Several studies have indicated that fine motor skill rehabilitation training makes use of residual nerve fibers for collateral growth, encourages the formation of new synaptic connections to promote neural plasticity, and improves motor function recovery in patients with spinal cord injury. With the development of biomaterial technology and biomechanical engineering, several emerging treatments have been developed, such as robots, brain-computer interfaces, and nanomaterials. These treatments have the potential to help millions of patients suffering from motor dysfunction caused by spinal cord injury. However, large-scale clinical trials need to be conducted to validate their efficacy. This review evaluated the efficacy of neural stem cells and magnetic or electrical stimulation combined with rehabilitation training and intelligent therapies for spinal cord injury according to existing evidence, to build up a multimodal treatment strategy of spinal cord injury to enhance nerve repair and regeneration.
Keywords: brain-computer interface technology, multimodal rehabilitation, nerve regeneration, neural circuit reconstruction, neural regeneration, neuromodulation, rehabilitation training, spinal cord injury, stem cells, transcranial direct current stimulation, transcranial magnetic stimulation
Introduction
Spinal cord injury (SCI) is a severe neural trauma that, depending on the damaged segment and severity (Tanabe et al., 2019), is classified into complete and incomplete SCI. Approximately 42% of patients with SCI have complete dysfunction without any movement or sensation below the site of injury. Interestingly, only 14.3% of all SCIs are believed to be anatomically complete injuries, while the remainder of SCIs are considered as an incomplete functional deficiency with a few spared connections that could be established under proper interventions (Kakulas, 2004).
Neurogenesis and neuroregeneration are two of the most important scientific directions in neuroscience of the 21st century. In the past two decades, research on neurogenesis and neuroregeneration has significantly improved our understanding of the neural repair of central nervous system (CNS) injuries (Wagner et al., 2018; Rezaei Haddad et al., 2019; Staudt et al., 2019). However, the efficiency of functional restoration and reconstruction remains a worldwide issue, and could involve relationships between the microenvironment (Lukovic et al., 2015), exogenous neural stimulation (Yang et al., 2015a), and exogenous/endogenous cell resources (Bellenchi et al., 2013). Neural circuits could act as a bridge between neuronal activations and functions. A neural circuit is a fibrous connection between specific neurons in functional areas of the cortex, and motor and sensory neurons in the spinal cord. Neural circuit reconstruction is a long-term process and a primary goal of nerve repair and regeneration (Priori et al., 2014). Neural circuit reconstruction also aims to achieve functional reconstruction after nerve damage (Angeli et al., 2014).
In the field of neuroregeneration, nerve repair of SCI is still considered to be a major challenge. SCI is a severe and complicated clinical condition that mostly results from accidents during work, recreational activities, or in motor vehicles (Armour et al., 2016), and can be classified into primary and secondary injuries. A primary injury results in varying degrees of neuronal and glial cell necrosis, apoptosis, axonal rupture, demyelination, glial scar formation, impaired neurotransmission, and inflammation (Ahuja et al., 2017). This seriously affects spinal cord function in the early stage of SCI. A secondary injury coexists with the regenerative process of the myelin sheath, vasculature, endogenous neural progenitor/stem cells (Ramadan et al., 2018), and axons (Oyinbo, 2011). To date, SCI is considered extremely difficult to treat. Therapeutic options include surgical decompression, anti-inflammatory drugs, hyperbaric oxygen therapy, and rehabilitation interventions. Despite the intensive rehabilitation programs carried out in hospitals worldwide, SCI is still associated with a high disability rate. The main issue is how to induce activations or reorganization in the remaining neural network and axonal regeneration, which occur within the weak innate regeneration ability of neurons (Fagoe et al., 2014), the cellular microenvironment with immune cell infiltration, microglial activation, and inhibitory effect of myelin debris to the injured CNS (Lang et al., 2015).
The nerve microenvironment is composed of neurons, axons, dendrites, glial cells, myelin, the cell matrix, and neurotransmitters, and is regulated by various nutrient factors and secreted cytokines. The microenvironment supplies neural stem cells (NSCs) with the fundamental substances required for nerve growth; therefore, it is important to optimize the environment in which neurogenesis takes place. Usually, the CNS lesion area of acute SCI is an inflammatory and inhibitory microenvironment that contains inhibitors for axon regeneration and repair, such as myelin and myelin-associated glycoprotein, reactive astrocytes and microglia, and infiltrated macrophages, which form glial scars at the damaged site (Rodriguez et al., 2014). This inhibits axon regeneration and limits the capacity of activated NSCs to differentiate into neurons and establish nascent neural circuits (Lukovic et al., 2015; Ramadan et al., 2018). In addition, the water content of the area of the injured spinal cord increases with the increasing level of Ca2+ and decreasing level of Mg2+ (Kim et al., 2017). Given these conditions, neurogenesis and neural regeneration are limited and additional resources are needed to facilitate functional recovery in acute or chronic SCI. It remains unclear how to adjust the microenvironment such that it contributes to nerve growth and function and maintains basic substances for microcirculation and metabolism. Many previous attempts have been made to eliminate external factors to enhance axon regeneration; however, no satisfactory treatments have yet been developed. Consequently, internal factors are receiving more attention. It has been reported that several cell-derived molecules, such as the mammalian target of rapamycin, Krüppel-like factors, RhoA, cAMP, and the phosphatase and tensin homolog, play essential roles in determining the potential for neuronal growth (Wilems et al., 2015; Nguyen et al., 2018).
After SCI, the upper motor neurons lose feedback of the afferent signal, and the output signal is lost or terminated at the proximal end of the injured level; this results in an interruption of the neural pathway that controls movement. Also, signal transduction, axonal growth, and myelination are disrupted; this inhibits the recovery of spinal cord function (Sonksen and Hillier, 2010), which is largely determined by neural circuit reconstruction. The goal of neural circuit reconstruction in SCI is to achieve recanalization of the neural pathway after disruption of the upper and lower nerve fiber conduction bundles, as well as functional remodeling of autonomous and involuntary movements, shallow sensation, proprioception, and autonomic nerves.
Traditionally, spinal nerve cells are considered to be highly differentiated cells that do not have the ability to undergo mitosis. However, modern theories have proposed that, after SCI, ependymal cells in the central canal can be reactivated to produce cell types for repair (Bauchet et al., 2013), and spinal progenitor cells can proliferate and generate motor and interneurons. Both proliferation and an influx of ependymal cells are observed following SCI (Chara et al., 2014). Moreover, the axon-side expenditure bud regenerates and extends to the corresponding target cells by forming synaptic connections, which fully or partially restores target cell innervation. In particular, synaptic regeneration and axonal regrowth play a crucial role in the effective recovery of SCI (De Miguel et al., 2012). Considering this, neural circuit reconstruction does not only represent the growth of new nerves, but also stimulates or mobilizes potential nerve fibers to function or to strengthen the existing neural network.
In recent years, a number of translational research studies in areas such as stem cell transplantation and brain-computer interfaces have aimed to restore sensorimotor function following SCI (Shaked et al., 2010; Salisbury et al., 2016). However, these scientific explorations are still in progress (Doulames and Plant, 2016; Wagner et al., 2018). This review focuses on the relatively new field of multimodal neuromodulation techniques that combine personalized rehabilitation strategies as a novel means to enhance neural circuit reconstruction, thus achieving neuroregeneration and functional motor recovery in SCI. This review also provides a discussion on the effect of endogenous and exogenous NSCs on neurogenesis in SCI. It is of vital importance to provide a new framework for exploring the diverse and integrated rehabilitation for functional recovery after SCI.
Search Strategy
We conducted a PubMed search for patients with SCI/animal models of SCI, neurogenesis/neuroregeneration, and neuromodulation strategies and their outcomes. General information on neuromodulation for SCI was searched using the term “SCI” in combination with the desired term (e.g., “magnetic stimulation”). To explore the existing rehabilitative paradigms in more depth, the term (e.g., “voluntary wheel training”) was combined with the term “SCI”. Publications on neurogenesis and neuroregeneration were sourced by combining these two terms with the term “SCI”. The relevance of identified publications was verified by checking the presence of search terms in the title, abstract, and key words. We focused on general rather than recent findings wherever possible.
Neuromodulation Induces Neuroregeneration and Neural Repair in SCI
Due to the development of medical biology and medical engineering technology, neuromodulation has been the fastest-growing discipline in the field of medical sciences over the past couple of decades. Neuromodulation has the potential to induce enormous and subversive changes in the treatment of several nervous system disorders (Staudt et al., 2019), but it can also affect any organ and tissue in the body. Moreover, the development of neuromodulation technology has had a profound and lasting impact on functional neurosurgery. One example is the replacement of ablative techniques, such as stereotactic radiofrequency ablation, with deep brain stimulation (Bilge et al., 2018; Jakobs et al., 2019; Rezaei Haddad et al., 2019).
At the neuroscience level, the broad definition of neuromodulation is the treatment pattern that achieves therapeutic effects by altering the function or state of the nervous system, either electrically or chemically (De Ridder et al., 2017). Specifically, it is the method of generating therapeutic effects on influencing signal transmission in the nervous system, including excitation, inhibition, and regulation of neuronal and neural network activities (Blackmore et al., 2019) by implanted or non-implantable devices that use electrical or chemical methods.
Neuromodulation includes invasive neuromodulation and noninvasive neuromodulation. Invasive neurological regulation includes brain deep stimulation, spinal electrical stimulation, dorsal root ganglion stimulation, and drug pumping. The stimulation technology has gradually replaced invasive surgery, and has the advantages of being minimally invasive, reversible, and controllable (Shah and Padalia, 2019); this is the case for, for example, drug pumps, which use intramuscular drug release technology to inject into the nervous system, providing a precise targeted treatment for therapeutic purposes.
Noninvasive neuromodulation includes peripheral nerve stimulation, vagus nerve stimulation, transcranial magnetic stimulation, transcranial electrical stimulation, transcranial focused ultrasound stimulation, and optogenetic stimulation (Blackmore et al., 2019). Noninvasive magnetic and electrical stimulation technology has been safely applied in clinical practice and has achieved therapeutic effects in many neuropsychiatric diseases (Concerto et al., 2015; Lanza et al., 2018; Wessel and Hummel, 2018). In the early stage of disease, the application of magnetic neuromodulation reduces the imbalance of calcium and magnesium ions that is seen after SCI, thereby reducing secondary spinal edema and inhibiting blood flow loss (Tekieh et al., 2016). In the recovery of an injury, magnetic neuromodulation can also regulate neurotrophic factors such as nerve growth factor and brain-derived neurotrophic factor (BDNF), as well as nerve growth-related genes (e.g., c-fos), glial cells, and cell withering, thus creating a microenvironment that alleviates secondary damage and has a beneficial effect on nerve repair and regeneration (Yao et al., 2008; Luo et al., 2017a).
Neuromodulation is a broad term that refers to multiple components of plasticity, which can act on diverse regulatory mechanisms and produce different behavioral results. The mechanisms underlying the efficacy of electromagnetic stimulation techniques have been demonstrated to involve neural plasticity or neural circuit reconstruction, activation of neurons and nerve conduction, regulation of the nervous system microenvironment, and gene regulation (Feng et al., 2014; Cantone et al., 2017). The selection of different stimulation targets, such as cortical, subcortical, and spinal targets, depends on the initial pathological process and downstream effect on neural synapses, pathways, and circuits. Noninvasive strategies to study the mechanisms underlying the neurogenesis of stem cells will aid the development of future studies and therapies to improve the overall use of NSCs in the treatment of SCI.
Although a growing number of studies have highlighted the potential of magnetic/electrical neuromodulation for both the healthy and the sick (Coffman et al., 2014), the underlying mechanisms of neural circuit reconstruction following magnetic/electrical neuromodulation remain unknown. However, magnetic and electrical neuromodulation has been found to serve as useful neuromodulation techniques to avoid neuronal dysfunction following SCI, as they can help to train spinal interneuron networks by enhancing afferent inputs (Dietz, 2010; Hubli et al., 2011). Furthermore, according to metaplasticity theory, the morphology and function of a synapse can change over time in certain circumstances (e.g. nerve damage), whereby synapse connection strength can change. Therefore, to make the most of the feature or phenomenon of synapses, multiple neuromodulation techniques could increase the chances of establishing connections of remaining nerve fibers to affect functional outcomes. It should be noted that longer interventions durations are required to achieve long-lasting effects and to detect possible mechanisms of the therapeutic effect at the spinal cord level.
The variability in the efficacy of neuromodulation could be attributed to genetic features. In particular, the BDNF Val66Met polymorphism, a substitution of valine-to-methionine at codon66 in the normal population, has been reported to enhance spinal plasticity and is associated with motor neuronal connections, multiple neuronal phenotypes, and motor cortex plasticity (Cardenas-Morales et al., 2014; Morin-Moncet et al., 2018). During transcutaneous spinal direct current stimulation for at least 15 minutes, a remarkable leftward shift of the H-reflex recruitment curve was induced by the anode after offset in Val/Val individuals, but not in Met allele carriers (Jean-Charles et al., 2012). These findings illustrate that the BDNF Val66Met genotype may significantly enhance spinal and synaptic plasticity in those recovering from SCI, and may lead to differences in the natural response to injury or disease of the spinal cord (Jean-Charles and Maxwell, 2013).
Transcranial Magnetic Stimulation and Neural Circuit-Magnetic Stimulation: Safety and Effects on SCI
Magnetic stimulation as an exogenous stimulus is a noninvasive diagnostic and therapeutic technique that can cause excitability in certain electrically conducting tissues (Wagner and Valero-Cabre, 2007). More than three decades ago, transcranial magnetic stimulation (TMS) was the first noninvasive brain stimulation technique to activate the cortex (Barker et al., 1985). TMS has the ability to safely affect brain metabolism and nerve power (Du et al., 2018), based on the principle of using a time-varying magnetic field that acts on the cerebral cortex to produce an induced current that either changes the action potential of cortical neurons or modifies tissue excitability. Among the TMS-activated areas of the cortex, the motor cortex is the most frequently examined because it can cause muscle contraction. Recorded using electromyography, motor evoked potential is a well-recorded response and the simplest biomarker of individual motor excitability. In 1998, Kolosova et al. demonstrated in animal experiments that local magnetic stimulation can increase the regeneration rate of axons and promote the recovery of nerve function after injury. Its advantages of no pain, no damage, safety, reliability, and easiness to operate, mean that magnetic stimulation has been increasingly applied in clinics. With the rapid development of neuromodulation, the influence of magnetic stimulation technology on nerve regeneration has attracted increasing attention.
The effects of magnetic stimulation on cells are not caused by a single factor, but are associated with the stimulation parameters (e.g., magnetic field type, intensity, frequency, location, amplitude, and duration) and the functional state of the stimulated cells. A single-pulse current has been found to activate neuronal action potentials, and a sufficiently strong TMS pulse over the motor cortex can induce muscle contraction and motor evoked potentials (Kremer et al., 2016). Moreover, neuronal excitability, function, and behavior can be altered by repeated pulses of TMS (rTMS) under the right conditions (Klomjai et al., 2015), and the effects can continue for hours after stimulation. Different rTMS and paired associative stimulation protocols in stimulation frequency, pattern, and location (Fernandez et al., 2018; Ross et al., 2018) can enhance or suppress neural activity beyond the stimulation duration. rTMS affects synaptic plasticity according to the principle of long-term potentiation and long-term depression, which involve modification of activity of the N-methyl-D-aspartic acid receptor systems (Zhang et al., 2015; Shang et al., 2016). rTMS also induces promotion (> 5 Hz) or inhibition (~1 Hz or < 5 Hz) of cortical regions according to the frequency of stimulation (De Pisapia et al., 2018; Yang et al., 2018). In the study of Ljubisavljevic et al. (2015), the expression of 52 genes related to inflammation, neural protection, neural transmission, angiogenesis, injury repair, structural reconstruction, and neuronal plasticity increased significantly after two weeks of intermittent theta-burst stimulation, but not after 1 or 5 Hz rTMS and continuous theta-burst stimulation. This indicates that stimulation frequency and/or stimulation pattern influence the stimulation effect. The duration of this modulation also varies, and the enhancement of cortical plasticity can be achieved with longer durations. The TMS-induced change in cortical excitability is key in such studies, because such changes are characteristic of SCI and normalization of excitability reflects a better outcome.
TMS produces an induced current in the cerebral cortex and acts on the upper motor neurons. The artificially induced action potentials are transmitted via the descending conduction beam and promote axoplasmic transport through the accumulation effect of repeated stimulation, thereby promoting metabolism and growth, and stimulating neural plasticity to take effect on neuroregeneration (Yang et al., 2015a). One study found that exposure to very low frequency electromagnetic fields could enhance synaptic transmission by increasing the amplitude of post-tetanic potentiation, a short-term plasticity; the very low frequency electromagnetic fields could enhance the expression of voltage-gated calcium channels in presynaptic nerve terminals, mainly P/Q subtypes, and promote an increase in calcium influx, vesicle swallowing, and synaptic plasticity (Sun et al., 2016). Acceleration of endocytosis can increase synaptic strength and further regulate neuronal development, axonal branching, and refinement. Enhancement of post-tetanic potentiation can also lead to enhanced connections between neurons, which can further support neural circuits (Vyleta et al., 2016). These findings reveal an important regulatory role of magnetic fields in synaptic transmission and CNS plasticity.
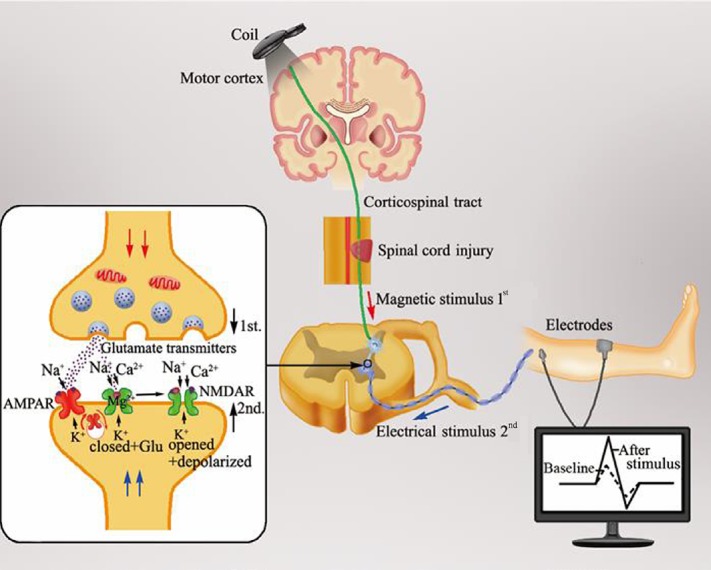
When rTMS applied to two brain regions or the paired-pulse of TMS to the sensory cortex is combined with an appropriately timed peripheral sensory stimulus (paired associative stimulation) (Catarina et al., 2013), it induces spike-timing dependent plasticity, an assessment of neuroplasticity (Feldman, 2012). Taylor and Martin (2009) were the first to demonstrate spike-timing dependent plasticity-like changes of synaptic strength following correlational pre- and post-synaptic spiking in the spinal cord in control subjects (Figure 1). The authors showed that the size of cervicomedullary motor evoked potentials increased when TMS over M1 region triggered repeated corticospinal activity controlling the biceps brachii, which started 1–2 milliseconds earlier than antidromic motoneuronal activation, induced by maximal electrical stimulation on peripheral nerve. Nevertheless, when presynaptic activation lagged behind postsynaptic depolarization, the cervicomedullary motor evoked potential amplitude was decreased. These results demonstrated that the presence of cervicomedullary motor evoked potentials measures spinal cord plasticity, such as corticospinal transmission and excitability of spinal motoneurons (Barthelemy et al., 2015). It is conceivable that other CNS structures, such as spinal cord circuits, can also be modulated by repetitive stimulation. In recent years, several studies have found that TMS and local magnetic stimulation of the damaged spinal cord can induce functional reorganization of neural circuits and promote the remodeling of nervous system by stimulating the central pattern generator (Diaz-Rios et al., 2017). The central pattern generator can help strengthen effective connections between the area and other structures or even regulate the function of the stimulated area to establish functional networks, which are conducive to motor function recovery (Stein, 2018). Furthermore, an electrical noninvasive electromagnetic field over intact vertebrae can effectively stimulate spinal circuits. Particularly, neurite outgrowth from spinal neurons and dorsal root ganglions can be promoted by a pulsed electromagnetic field in vitro (Sisken et al., 1984; Macias et al., 2000), with growth and regeneration of nerve tissue in vivo at frequencies < 100 Hz and field strengths < 5 mT.
Figure 1.
Change of synaptic plasticity in the spinal cord following a neuromodulation stimulation mode called spike-timing dependent plasticity.
When electrical impulses by transcranial magnetic stimulation over the M1 region passes through the corticospinal tract to the presynaptic membrane of the downstream neuron in the spinal cord anterior horn controlling the targeted muscle, the glutamate released from the presynaptic membrane binds to the postsynaptic membrane of the motor neuron. After a delay of 1–2 ms, the electrical signal transmitted to the postsynaptic membrane generated from the electrical stimulus on the peripheral nerve forms an inverse synchronous stimulus at the anterior horn of the spinal cord. This inverse stimulus depolarizes the postsynaptic membrane and the Mg2+ block is removed with the Ca2+ influx through the N-methyl-D-aspartic acid receptor. As a result, the change of intracellular Ca2+ concentration resulting from the Ca2+ influx reduces the excitability threshold of the postsynaptic membrane and enhances the induction possibility of long-term potentiation effects, with an increased motor evoked potential amplitude, as revealed by electromyography. Hence, spike-timing dependent plasticity with a synchronous stimulus in the anterior horn efficiently enhances synaptic plasticity in the spinal cord.
Motor functional recovery from SCI relies heavily on the involvement of multiple descending motor pathways, one of which is the corticospinal tract. In a recent study, Christiansen and Perez (2018) demonstrated that using a targeted TMS protocol based on the principle of spike-timing dependent plasticity could induce plasticity of residual corticospinal projections and spontaneously increase motor output in patients with chronic incomplete SCI, thus enhancing motor function. According to animal experiments, magnetic stimulation after SCI can protect spinal nerve tissue and promote the regeneration of nerve fibers to achieve nerve reinnervation of the damaged limbs. With the application of 10 Hz rTMS for 8 weeks, the movements of SCI rats with damage to the T10–11 segments were significantly improved, but this was not observed in rats with T4–5 injury; this improvement was associated with the density of serotonergic fibers in the caudal segments of the injured spinal cord (Anne-Lise et al., 2004). The authors suggested that the benefit of rTMS in low thoracic lesions could be derived from activating the central pattern generator, probably via descending serotonin pathways. Moreover, this effect could be a result of increased expression of Nestin in the damaged local spinal cord induced by magnetic stimulation therapy (Cullen and Young, 2016). rTMS can activate neurons in different horizontal levels, which not only causes biological effects, but also affects the local and functionally related distant cortical functions to realize the regional reconstruction of cortical functions. More nerve impulses from the brain travel down the spinal cord to motor neurons and thus residual nerve fiber connections can be maximally used; this not only contributes to nerve regeneration after SCI, but also enhances brain plasticity (Yamanaka et al., 2013). That study found that TMS can rapidly increase excitability in the M1 area of the cerebral cortex, which may contribute to motor learning and rehabilitation; TMS also enhanced cognitive ability by enhancing connections within functional networks, and promoting learning, memory, picture naming, analogy, reasoning, and decision making. Therefore, TMS has been widely used clinically for the rehabilitation of neurocognitive functions (Luber and Lisanby, 2014).
Neural circuit reconstruction depends not only on local nerve regeneration, but also on effective stimulation of remaining nerve fibers in the damaged area to maximize their use. According to this theoretical framework, nerve circuit stimulation should not be limited to cortical stimulation; therefore, a single transcranial cortical target is not enough, and more multi-target, multimodal progressive repetitive intervention is needed for sensorimotor circuit reconstruction. Based on this principle, our team has been developing a neural circuit-magnetic stimulation protocol in patients with incomplete SCI facing the bottleneck period of rehabilitation. We have already witnessed some active movement restoration in the lower extremities. Although our stimulation pattern is a nonsynchronous stimulation of the cerebral cortex and nerve roots, the M1 region is stimulated by intermittent theta-burst TMS stimulation to achieve downward conduction of the corticospinal tract. In this case, the lateral corticospinal tract controls the motor neurons of distal muscles of the limb to control voluntary movements. In addition, the corticospinal anterior bundle supports the trunk and the muscles at the proximal end of the limbs, and underlies posture maintenance and gross movement. After activation by the intermittent theta-burst stimulation of the motor cortex, the root stimulation is given during the effective time of cortex activation. The sensory signal can be transmitted to the thalamus via the spinal thalamus bundle which, when stimulated, induces a subjective anesthesia sensation in patients. Simultaneously, the signal can be transmitted along the nerve root to the muscle at the distal end of the limb, which causes uncontrolled target muscle activity; when asked to perform active movements, patients perform better than before. Neural circuit-magnetic stimulation has started to be used in patients with SCI assessed by Grade C or D in a functional assessment of spinal cord injury by the American Spinal Injury Association (ASIA). Combining magnetic stimulation technology with rehabilitation tasks, the establishment of task-oriented functional magnetic stimulation rehabilitation has shown promising results. However, more accurate stimulation using neurophysiological assessment and neuroimaging techniques is required.
Noninvasive electrical stimulation regulates neuronal activities to improve motor function
A few years after first application of TMS, transcranial direct current stimulation applied to the scalp started to be used, three decades ago. Transcranial direct current stimulation develops a constant electrical field between a cathode and anode electrode to prolong stimulus timing, thus making a greater impact on neuronal function and activating spinal neuronal circuitries, which is regarded as another noninvasive technique to effectively modulate brain and spinal cord excitability (Nitsche and Paulus, 2001; Nitsche et al., 2003; Ahmed, 2011, 2013; Alberto et al., 2015). Although transcranial direct current stimulation cannot produce enough current to induce neuronal action potentials (Purpura and McMurtry, 1965), it can modulate ongoing neuronal activity (Fujiwara et al., 2011) and prolong focal shifts in cortical activity. The placement of the electrode over the targeted region determines whether the activity elicited will be excitatory or inhibitory. For example, when placing the anodal electrode over the motor cortex, there is an excitation shift below the electrode, and cathode electrode induces an inhibition change in the cortex. Polarity from the two electrodes exerts an enormous impact on the transcranial direct current stimulation; anodic stimulation generally contributes to cortical excitability of the underlying tissue (Rawji et al., 2018), and cathodal stimulation increases the circular threshold of neurons (Murray and Knikou, 2019). Transcutaneous spinal direct current stimulation generates a constant low-intensity direct current that ranges from 1.5–2.5 mA over the spinal cord, through a pair of sponge electrodes (one over the spinal cord and the other over the right arm for reference), the effects of which can last from minutes to hours (Lenoir et al., 2018; Powell et al., 2018; Aplin and Fridman, 2019).
A previous study found that direct current stimulation acting on the whole spinal cord of mice can influence GABAergic and glutamatergic systems, and cathodal stimulation on mice is related to GABA and glycine receptors (Ahmed, 2011). Furthermore, anodal spinal direct current stimulation reduced, while cathodal stimulus increased the glutamate analogue aspartate; this indicates that glutamate has an influence on the outcome of transcutaneous spinal direct current stimulation (Ahmed and Wieraszko, 2012). Hubli et al. (2013) found that anodal transcutaneous spinal direct current stimulation could regulate the spinal neuronal circuit excitability responsible for locomotion in complete SCI. Additionally, research has revealed that somatosensory, motor, and nociceptive spinal neuronal circuits in the human spinal cord can be affected by transcutaneous spinal direct current stimulation (Cogiamanian et al., 2012; Priori et al., 2014). Likewise, some studies have also found that epidural electrical stimulation can facilitate spinal sensorimotor networks to resume a strong and harmonious motor activity in paralyzed individuals who are functionally disrupted from brain to spinal cord due to SCI (van den Brand et al., 2012; Angeli et al., 2014). Gill et al. (2018) described a clinical case of complete sensorimotor paralysis of the lower limbs; epidural electrical stimulation restored the ability to stand and control step-like activity when lying on the side and when placed upright in a body-weight support system. Moreover, transcutaneous electrical spinal cord stimulation can modulate excitability at various levels of the spinal neuraxis, ranging from the cervical to the coccygeal cord levels, to facilitate motor function (Hofstoetter et al., 2013, 2014, 2015; Gerasimenko et al., 2015a, b, 2016; Minassian et al., 2016). Rath et al. (2018) demonstrated that using transcutaneous electrical spinal cord stimulation over the lumbosacral enlargement improved postural control of upright sitting more than 2 years following the onset of complete or partial paralysis.
Combined rehabilitation training accelerates functional recovery
After the nerve conduction bundle or circuit has been activated by neuromodulation, an intensive rehabilitation training is required for the following reasons: (1) follow-up cognitive motor rehabilitation training can actively interact with neuromodulation and maintain neural excitability; (2) a combination of neural regulation and training helps to consolidate the initial activation state and continues the treatment training mode to achieve circuit reconstruction. Although clinical and experimental studies have shown that rehabilitative training can accelerate motor function recovery following brain injury and SCI, much remains to be discovered, including the optimal timing and intensity, as well as the neuronal mechanisms underlying motor improvements (Cote et al., 2017).
After SCI, the terminal membrane structure of the damaged axon actually has increased synaptic efficiency, axonal sprouting, and restarting of the latent pathway, such as vigorous metabolism and active growth. This suggests that the spinal cord has a certain degree of plasticity, which is the basis of neural circuit reconstruction. This injury-induced plasticity is called spontaneous plasticity. In addition, spinal plasticity can also be initiated by special forms of training called training task-dependent plasticity, which is induced by reactivation of the central pattern generator (Dunlop, 2008) and reorganization of spinal neuron circuits. However, in contrast to the peripheral nervous system, axonal regeneration and regrowth through the injured areas of the spinal cord are hardly seen spontaneously in certain conditions because of limited plasticity, which is a characteristic of the reconstruction of spared connections and broken axons (Loy and Bareyre, 2019). Thus, it is important to determine how rehabilitation triggers increased sprouting and plasticity of axonal connections to regulate and enhance connections between the brain and spinal cord.
If a spinal cord lesion occurs on one side, reticulospinal tract projections of the contralateral brainstem could cross the midline to the injured area or contact double midline crossing intrinsic interneurons of the spinal cord to form a detour circuit that reconnects the brainstem motor center caudal to the lesion (Zörner et al., 2014; Filli et al., 2014). However, with rehabilitation and/or neuromodulation, the reticulospinal tract is more likely to form a cortico-reticulospinal detour circuit to relay neural conduction, which induces compensatory plasticity following SCI (Asboth et al., 2018).
Some studies have shown that spontaneous compensatory sprouting of the corticospinal tract fibers following injury could either be regulated by forced use of the denervated limb to cause the well-preserved fibers to play a compensatory role (Weidner et al., 2001; Maier et al., 2008) or by the establishment of connections between injured axons and new partners (Lang et al., 2013). Ten days after injury, when the corticospinal tract fibers join the middle of the ventral gray matter rostral to the injured cervical spinal cord segment, the connecting pathway between the corticospinal tract and neurons of the long descending intrinsic tract of the spinal cord is initially formed; this process can be accelerated and enhanced by rehabilitation with irregular running wheels, as reported in a recent study by Loy et al. (2018). Within the first 3 to 4 weeks post injury, fiber growth and established connections with long descending propriospinal neurons are formed and subsequently refined. Moreover, if animals are constantly trained in irregularly-spaced running wheels for remodeling of the corticospinal tract, the connections between corticospinal tract fibers and long descending propriospinal neurons and the number of propriospinal neurons are observed to be further increased over time. Similarly, a reinforced density and length of fibers around the lesion can also result from treadmill training and wheel running because of integral remodeling and local sprouting effects (Hayashibe et al., 2015). However, one study found that combined treatment with an electrochemical prosthesis in the lumbar spinal cord and treadmill rehabilitation restored stepping ability, even via all severed direct descending connections with the brain; this was achieved by the formation of a corticospinal tract detour circuit with inherent spinal interneurons and neuronal re-wiring (Brand et al., 2012).
Voluntary wheel training on a flat surface wheel has been found to promote outgrowth of serotonergic tract fibers in incomplete SCI (Engesser-Cesar et al., 2007). Likewise, an irregular running wheel training paradigm was more recently found to increase connections between serotonergic fibers and cholinergic motoneurons in the lumbar spinal cord following incomplete SCI (Loy et al., 2018). Complete loss of central serotonergic inputs to spinal motor neurons was found to lead to paralysis after injury. Moreover, post-injury serotonin application and endogenous serotonin level restoration has been found to facilitate the reconstruction of acute spinal networks, thus enhancing motor function (Gackiere and Vinay, 2014). Strikingly, it has been demonstrated that motor abilities can improve quickly with a combination of rehabilitation and serotonin agonists, and the rehabilitation effect persists for a longer time than serotonin treatment alone (Ghosh and Pearse, 2014).
Research studies have further indicated that exercise training may participate in the regulation of CNS regeneration and up-regulation of nerve growth factor and receptor expression, and may enhance the intrinsic regenerative capacity and induce a beneficial effect on the regeneration microenvironment (Kim et al., 2016). One study found that active rehabilitation training can increase the protein and mRNA expression of various neurotrophic factors and their receptors, such as BDNF, nerve growth factor, and growth-associated protein-43 in SCI rats (Keefe et al., 2017).
Rehabilitation is the process of retraining; task repetition facilitates plasticity, leading to reinforcement of one task and inhibition of others (Mawase et al., 2017). A recent study reported that after 43 weeks of multimodal rehabilitation, including dynamic task-specific training and epidural electrical stimulation, humans with paralysis were able to step bilaterally on treadmill without trainer assistance or a body-weight support system, and could step on the ground with trainer assistance at the hips and using a front-wheeled walker to maintain balance (Gill et al., 2018). This reveals that spinal networks can be modified several years following SCI and can be generated from powerful spinal motor outputs such that patients can walk and stand independently. It has been suggested that multimodal rehabilitation promotes supraspinal-spinal connections to maintain functional reorganization. Concerning neurorehabilitation, functional neuroimaging has shown that brain reactivation can be induced by mental imagery of sports, its observation, or passive training (Butler et al., 2011). Furthermore, electrophysiological study has confirmed the efficacy of traditional training. Thomas et al. (2005) investigated the effects of forced treadmill training in 10 patients with incomplete SCI. The authors found that the TMS-evoked motor evoked potential amplitude increased and was correlated with the degree of locomotor recovery. The optimal stage of SCI for rehabilitation remains a controversial topic.
Modern intelligent modulation technology promotes rehabilitation development
With the accumulation of clinical evidence and the need for more precise modulation, neuromodulation technology is facing many bottlenecks that require breakthroughs in theory and strong methodological foundations. Such issues include the optimal intervention time and the intensity of rehabilitation required to reinforce the neuromodulation effect. The combination of functional engineering and regenerative medicine is also indispensable to promote functional rehabilitation. With the advancement in our social and technological understanding, the treatment and rehabilitation techniques for post-traumatic motor system dysfunction have rapidly progressed. The use of tissue engineering and regenerative medicine for damage repair has become the focus of much attention, for example.
Robotic devices are now used in neurorehabilitation training. These have been shown to hold great promise, and can significantly reduce the load on therapists during ambulation training (Hussain, 2014). Robotic devices and other replacements may increase plasticity, particularly in sensory function, even many years after injury (Stevenson et al., 2015; Duret and Mazzoleni, 2017). However, patients with robotic-assisted Lokomat walking showed significantly higher quadriceps-hamstring activity in the swing-phase and reduced ankle flexor-extensor activity compared with treadmill ambulation, which suggests that forced robotic gait training may alter the natural patterns of muscle activation (Aurich-Schuler et al., 2017). A study by Rodgers et al. (2019) also supports this point; compared with conventional care, robot-assisted training and enhanced upper arm therapy did not significantly improve upper arm function after stroke, and the incidence of moderate to severe side effects was higher. The results of that study indicate that robotic-assisted training should not be used in clinical practice.
With the development of polymer materials technology and its integration into the biomedical field, micro-nano biomaterials are increasingly used in biotherapy. Protecting signal molecules, maintaining their stability, and transporting them to target sites could hold potential for wound healing in the CNS, similarly the micro-nanoparticle systems in complete SCI (Santos et al., 2012). After injection of neurotrophin-3-coupled chitosan biomaterial into a completely severed rat’s thoracic spinal cord with a 5-mm gap, NSCs were attracted to the injured area by the biomaterial’s slow releasing neurotrophin-3, differentiated into neurons, and established functional neural networks (Yang et al., 2015b). The interconnecting ascending and descending axons and the nascent networks in the transection could contribute to sensorimotor behavioral recovery. Therefore, the biomaterial enhanced spinal cord regeneration by eliciting robust activation of endogenous NSCs, enhancing vascularization, and suppressing inflammatory immune responses. Moreover, the novel material helped to optimize the microenvironment for endogenous NSCs, such as CD133+ ependymal cells and their downstream lineage cells, transform these into new-born neurons, and reconstruct a new neural synaptic network (Duan et al., 2015). This study indicates that enhancing endogenous neurogenesis could represent an advanced strategy for treating SCI.
One study reported that a specially designed electrical stimulation technique allowed patients with SCI to stand up (Wagner et al., 2018). A brain-computer interface technology developed in the United States has also been found to aid lower limb movements in patients with SCI (Salisbury et al., 2016). Brain-computer interfaces can be self-regulated by electroencephalographic activity of the scalp, by an electrode inserted into the brain surface within M1 or subcortical structures (Sburlea and Muller-Putz, 2018), or by the blood oxygen level-dependent signal from real-time functional magnetic resonance imaging (Paret et al., 2019). Using an adapted information processing algorithm, an external device that provides suitable somesthetic, visual, and auditory feedback could be controlled by the conversion from electrophysiological or hemodynamic input into an output. Therefore, brain-computer interfaces make it possible to restore communication to a certain extent in patients with severe paralysis (Pandarinath et al., 2017). Furthermore, one recent study introduced a new deep neural network that decodes the framework for brain-computer interface systems; this achieves discrete movements with high precision, minimal daily setup, short response times, and functional variety (Lecun et al., 2015; Schwemmer et al., 2018). Combined with an updated procedure without the need for human supervision, the network could sustain the above four performance characteristics for more than one year without the need for specific daily retraining (Bacher et al., 2015; Beata et al., 2015; Jarosiewicz et al., 2016). It could also enhance function with minimal retraining using transfer learning techniques, which means applying mature knowledge of one domain to other scenarios. Moreover, with functional electrical stimulation, the participant can use the decoder in real-time to reactivate their paralyzed forearm, manipulating three objects from the grasp and release test accurately. Thus, it can be concluded that the clinical translation of brain-computer interface technology is very likely to advance in the realm of deep neural network decoders.
Various novel methods, including three-dimensional printing, exoskeletons, and robotics, have been developed with the aims of functional restoration after SCI. In the field of stem cell transplantation, it is critical to find reliable methods to promote sensory and motor behavioral recovery, as well as for the formation of functional neural networks. Notably, considering the inconsistencies and uncertainties of novel techniques, many clinical trials need to be carried out.
Endogenous and Transplanted Stem Cells are Involved in Neuroregenesis and Neural Repair in SCI
In general, NSCs are in a relatively “quiescent” state that can be activated when stimulated by certain factors (e.g. vascular endothelial growth factor) or injury. After activation, NSCs can re-enter the cell proliferation cycle and differentiate into neurons, astrocytes, and oligodendrocytes to self-renew and provide a sufficiently large number of tissue cells (Yu and Jin, 2014).
Neurogenesis is a process involving the proliferation of NSCs, a balanced or unbalanced division into directed progenitor cells, gradual migration to functional areas, plasticity changes, and establishment of synaptic connections with other neurons to produce neurological integrity. In brief, neurogenesis is regarded as the maturation of new neurons into adulthood (Dey et al., 2019). However, a recent paper from Sorrells et al. (2018) questioned the presence of human hippocampal neurogenesis during adulthood. The authors proposed that neurogenesis is extremely rare or even nonexistent in the dentate gyrus in adults; indeed, the number of young neurons developing in the hippocampus decreases sharply after the first few years of life. With the advancement and development of neuronal detection and tissue processing approaches, Moreno-Jimenez et al. (2019) identified many immature neurons in the dentate gyrus in healthy persons without neurological diseases aged up to their 90s, with various degrees of maturation.
The involvement of endogenous stem cells in neural repair and neural network reconstruction is a classic scientific theory (Christie and Turnley, 2013). Endogenous stem cells play a key role in neurogenesis and differentiate to form nascent neural circuits that are integrated into damaged neural networks (Bellenchi et al., 2013). Furthermore, endogenous NSCs are mainly situated the subventricular zone of the lateral ventricle wall and the subgranular layer of the dentate gyrus in the hippocampus of adult mammals (including humans) (Hayashi et al., 2018). Later, NSCs are also found in the ependymal zone around the central canal of the spinal cord (Christian et al., 2014; Luo et al., 2015; Liu and Chen, 2019). Luo et al. (2015) used single-cell transcriptome and network analyses to identify a subpopulation of resting NSCs in non-neurogenic regions of the brain. This indicates that there are dormant NSCs that exist throughout the CNS, which are located on the ventricular surface (e.g., the central canal of the spinal cord). After exposure to factors such VEGF, these cells can be mitotically activated and can differentiate into neurons or glial cells to participate in neural repair (Luo et al., 2015). The discovery of the prevalence of NSCs has challenged the traditional notion that spinal cord cells cannot regenerate. Additionally, it has been reported that most activation sources of NSCs for proliferation and differentiation were ependymal cells (Weiss et al., 1996). By supplying endogenous cells with a stable microenvironment, specialized stem cell “niches” are thought to modulate their proliferation and regulate downstream differentiation (Walker et al., 2010).
SCI causes damage to multiple specialized cell types. The focus of ongoing studies has thus been to repair and regenerate the entire neurovascular unit. Besides using endogenous NSCs to induce neurogenesis in damaged areas, exogenous NSC transplantation is an emerging effective method for repairing and replacing damaged tissues, to reconstruct part of the neural circuits and functioning in CNS diseases (Doulames and Plant, 2016). However, the inflammatory microenvironment following acute SCI of the adult CNS where endogenous NSCs gather may affect stem cell survival, self-renewal, migration, and neuronal differentiation (Cheng et al., 2017). The inflammation that determines either pro- or anti-neurogenic properties is probably based on activation of macrophages, microglia, and/or astrocytes (Russo et al., 2011). For instance, releasing inflammatory factors, including tumor necrosis factor-α, interleukin-6, interleukin-1β, reactive oxygen species, and nitric oxide, and microgliosis reaction could cause harm to the NSC niche, resulting in neuronal dysfunction and degeneration, and a reduction in neuronal proliferation and differentiation (Horgusluoglu et al., 2017). Conversely, certain factors and conditions, such as antigen-specific autoimmune T cells and their cytokines, create a more beneficial microenvironment for neurogenesis of the grafted stem cells (Shaked et al., 2010). Additionally, it has been reported that NSC transplantation triggering the inflammatory response to disease or injury rather than cell replacement itself may provide protection for the CNS against inflammation (Gincberg et al., 2012), mostly depending on up/down regulation of specific inflammatory factors. Moreover, the neuroprotective capability of endogenous NSCs could also be developed by secreting neurotrophic factors at the site of damaged tissue to promote neuronal differentiation (Chen et al., 2014). Therefore, it is important to identify the optimal transplantation timing after injury with consideration to the association between neuroinflammation and neurogenesis, which may directly affect the efficacy of the therapy (Aguzzi et al., 2013).
Exogenous stem cells currently used for transplantation come from a variety of sources, such as embryos, bone marrow, adipose tissue, and umbilical cord blood (Liao et al., 2019). These types of stem cells can provide nutritive support and nerve growth substrates by differentiating into mature neurons, enhancing regeneration of damaged neurons, and promoting nerve axon growth across the gelatinous barrier. Co-transplantation therapy is another aspect of stem cell research that offers promising effects on neuronal differentiation and survival. The transplantation of astrocytes with NSCs has been found to result in a higher ratio of survival and proliferation compared with transplanting NSCs alone (Luo et al., 2017b).
In states of disease or injury, the migration and differentiation patterns of stem cells change to participate in nerve regeneration at the injury site (Zheng et al., 2013). Indeed, NSC proliferation and activation have been observed within 24 hours after SCI; furthermore, activated ependymal cells differentiated into astrocytes and oligodendrocytes, but only a small proportion differentiated into neurons. Similarly, NSCs transplanted into the lesion area mostly differentiate into astrocytes, which can only achieve limited functional recovery. It follows that nerve regeneration cannot be fully exerted as a result of glial scars or other undesirable outcomes (Barnabe-Heider et al., 2010). Moreover, due to the difficulties in availability and mobilization of NSCs, the therapeutic effects of endogenous stem cells are also seriously restricted within the CNS.
Concerning the future direction of this field, a tracking method to follow stem cells on their paths to neurogenesis would provide clinicians and researchers with visualized knowledge on the progress of stem cells, including the exact location of mobilization and proliferation (Wang et al., 2017). Monitoring the acceptance, growth, migration, differentiation, and survival of injected cells is vital to better analyze and understand the process that occurs after stem cell transplantation. Although many animal model studies have been conducted in recent years, the progression from basic research to clinical trials is still a challenge. Moreover, the most effective source of NSCs and creating a microenvironment that can neutralize inhibitory effects of neuroregeneration following nerve injury have yet to be identified, and the scarcity of exogenous stem cells and ethical issues are also potential barriers to the clinical application of NSCs. The use of animal models can prepare medical communities for future clinical trials and the application of stem cells as a primary option for SCI treatment.
Future work should investigate whether endogenous and exogenous NSCs truly participate in the repair of SCI and differentiate into the required cell types. Finding ways to modulate and induce neurogenesis in SCI is currently the focus and challenge of nerve regeneration (Yu et al., 2016). In recent years, promotion of axonal guidance, blocking of factors inhibiting regeneration (e.g., glial scars) (Okubo et al., 2018), supplementing growth factors (Li et al., 2019), and even the modulation of immune responses (Wenker et al., 2016) have been considered to facilitate graft efficacy. Interestingly, researchers have found that physical factors such as magnetic, electric, and ultrasound can alter the activation of stem cells for neural repair and neural regeneration in the CNS (Cui et al., 2017; Blackmore et al., 2019). Abbasnia et al. (2015) found that after a 2-week application of rTMS, there was a remarkable increase in NSC proliferation in the intact brain of adult mice, at both low (1 Hz) and high (30 Hz) frequencies. Similar findings have been reported in vitro, whereby rTMS treatments applied for 1 week with both 1 and 30 Hz also promoted NSC proliferation and neuronal differentiation (Abbasnia et al., 2015). Moreover, the very low frequency electromagnetic field could activate the excitability of neural progenitor cells, which is the molecular basis for electrical activity of neural cells (He et al., 2013). In addition, T-type calcium channels can be regulated by extremely low frequency magnetic fields, which provides an important theoretical basis for the activation of neurons and the formation of neural circuits (Cui et al., 2014). However, there have been no reports on the ability of rTMS to mobilize endogenous NSCs in the spinal cord after SCI, and there is great potential to study and explore this subject.
Prospects and Challenges
Surgical technology is developing rapidly; in comparison, surgical rehabilitation, especially orthopedic rehabilitation, is lagging behind. With the development of global economy and the improvement of people’s living standards, society’s demand for rehabilitation medicine is growing, and the concept of “surgical-rehabilitation integration”, i.e., integrating rehabilitation into the entire surgical procedure, including before and after surgery, has emerged. However, whether acute or early rehabilitation intervention can maximally promote rehabilitation after spinal surgery is still in the early stages of exploration.
At present, there have been very few clinical reports on the efficacy of magnetic stimulation technology for SCI, and the methodology relies on the “transcranial” stimulation technique. However, traditional TMS has the following main clinical problems: (1) it is unclear whether it effectively activates the conduction of the corticospinal tract and establishes the big “perception-cortex-motion” circuit; (2) it is unclear whether activation of the motor function of spinal anterior horn (small circuit) is involved in TMS. Therefore, more precise neuromodulation models are needed.
The following issues remain to be explored in the development of SCI treatment by neuromodulation technology: (1) the mechanism of TMS pulsed magnetic field on nerve conduction; (2) the influence of a TMS pulse magnetic field on synaptic plasticity of the anterior horn in the injured spinal cord; (3) the technical path of neuromodulation combined with microenvironment regulation to promote neural circuit reconstruction.
Although there have been promising advances in the diagnosis and therapy of CNS disorders in the field of neurogenesis, supporting evidence for wide-range clinical applications in neurological disorders is still in its infancy. Therefore, an increasing number of case studies and clinical trials are emerging. In medical research, we have been striving to accurately locate the target using imaging technology, and accurately evaluate the efficacy through multidimensional techniques. With the rapid development of neurobiological technology, the reconstruction of neural circuits can be effectively observed and the trajectory of nerve conduction beam can be tracked by diffusion tensor imaging, and the growth of nerve fibers can also be dynamically monitored. There is great potential for the study of nervous system and brain function. Functional magnetic resonance imaging provides an in-depth study of functional recovery and neurological functional reorganization after disease treatment by monitoring the corresponding cortex in the brain; this work could help to fine-tune clinical diagnosis, treatment, and prognosis of SCI.
A better understanding of the principles underlying the above-mentioned therapies will allow us to better combine them to guide neural plastic potential, and thus modulate the dynamics of solid networks and enhance functional recovery following SCI. However, at present, the existing neuromodulation parameters are adjusted based on artificial settings. In the future, if an active interaction system integrating assessment and intervention can be developed, artificial intelligence methods such as deep learning could be introduced into current neuromodulation facilities to achieve a more precise and effective rehabilitation system. In addition, the most important criterion for evaluating the technique of neuromodulation is long-term efficacy; that is, whether and how the effect of neuromodulation therapy can be maintained for a long time, for which neural circuit reconstruction is a crucial indicator. Therefore, the approriate time for starting neuromodulation treatment to match with sensation and movement is a vital issue for future research and clinical practice, and may open a window for SCI patients.
Additional file: Open peer review reports 1 (92.5KB, pdf) and 2 (88.8KB, pdf) ..
Acknowledgments
The authors are grateful to Ying Shen from Jiangsu Province Hospital, China for her theoretical guidance of the figure.
Footnotes
Conflicts of interest: The authors have no actual or potential conflicts of interest.
Financial support: This work was supported by the Major International (Regional) Joint Research Project of the National Natural Science Foundation of China, No. 81820108013 (to LMC); the General Research Project of the National Natural Science Foundation of China, No. 81772453 (to DSX); the National Key Research and Development Program of China, No. 2016YFA0100800 (to LMC). The funding sources had no role in paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Wenhui Hu, Temple University, USA; Valerio Chiurchiù, European Center for Brain Research (CERC), Italy.
Funding: This work was supported by the Major International (Regional) Joint Research Project of the National Natural Science Foundation of China, No. 81820108013 (to LMC); the General Research Project of the National Natural Science Foundation of China, No. 81772453 (to DSX); the National Key Research and Development Program of China, No. 2016YFA0100800 (to LMC).
P-Reviewers: Hu W, Chiurchiù V; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Cason N, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Abbasnia K, Ghanbari A, Abedian M, Ghanbari A, Sharififar S, Azari H. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 2015;48:104–113. doi: 10.5115/acb.2015.48.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J Appl Physiol. 2011;110:1414–1424. doi: 10.1152/japplphysiol.01390.2010. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed Z. Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements. J Neurosci. 2013;33:14949–14957. doi: 10.1523/JNEUROSCI.2793-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed Z, Wieraszko A. Trans-spinal direct current enhances corticospinal output and stimulation-evoked release of glutamate analog D-2 3-(3)H-aspartic acid. J Appl Physiol (1985) 2012;112:1576–1592. doi: 10.1152/japplphysiol.00967.2011. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 7.Alberto P, Matteo C, Marta P, Maurizio V, Roberta F. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol. 2015;592:3345–3369. doi: 10.1113/jphysiol.2013.270280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anne-Lise P, Yves N, Felix S, Sylvie M, Charline R, Géraldine W, Delphine B, Gary B, Rachelle F, Jean S. Repetitive transcranial magnetic stimulation improves open field locomotor recovery after low but not high thoracic spinal cord compression-injury in adult rats. J Neurosci Res. 2004;75:253–261. doi: 10.1002/jnr.10852. [DOI] [PubMed] [Google Scholar]
- 10.Aplin FP, Fridman GY. Implantable direct current neural modulation: theory feasibility and efficacy. Front Neurosci. 2019;13:379. doi: 10.3389/fnins.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armour BS, Courtney-Long EA, Fox MH, Fredine H, Cahill A. Prevalence and causes of paralysis-united states 2013. Am J Public Health. 2016;106:1855–1857. doi: 10.2105/AJPH.2016.303270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pages S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 13.Aurich-Schuler T, Grob F, van Hedel HJA, Labruyere R. Can Lokomat therapy with children and adolescents be improved? An adaptive clinical pilot trial comparing Guidance force, Path control, and FreeD. J Neuroeng Rehabil. 2017:14–76. doi: 10.1186/s12984-017-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacher D, Jarosiewicz B, Masse NY, Stavisky SD, Simeral JD, Newell K, Oakley EM, Cash SS, Friehs G, Hochberg LR. Neural point-and-click communication by a person with incomplete locked-in syndrome. Neurorehabil Neural Repair. 2015;29:462–471. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Barthelemy D, Willerslev-Olsen M, Lundell H, Biering-Sorensen F, Nielsen JB. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog Brain Res. 2015;218:79–101. doi: 10.1016/bs.pbr.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Bauchet L, Lonjon N, Vachiery-Lahaye F, Boularan A, Privat A, Hugnot JP. Isolation and culture of precursor cells from the adult human spinal cord. Methods Mol Biol. 2013;1059:87–93. doi: 10.1007/978-1-62703-574-3_8. [DOI] [PubMed] [Google Scholar]
- 19.Beata J, Sarma AA, Daniel B, Masse NY, Simeral JD, Brittany S, Oakley EM, Christine B, Chethan P, Vikash G. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med. 2015:7–313ra179. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, di Porzio U. Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem. 2013;124:159–167. doi: 10.1111/jnc.12084. [DOI] [PubMed] [Google Scholar]
- 21.Bilge MT, Gosai AK, Widge AS. Deep brain stimulation in psychiatry: mechanisms, models, and next-generation therapies. Psychiatr Clin North Am. 2018;41:373–383. doi: 10.1016/j.psc.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO. Ultrasound neuromodulation: a review of results mechanisms and safety. Ultrasound Med Biol. 2019;45:1509–1536. doi: 10.1016/j.ultrasmedbio.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand RVD, Heutschi J, Barraud Q, Digiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 24.Butler AJ, James TW, James KH. Enhanced multisensory integration and motor reactivation after active motor learning of audiovisual associations. J Cogn Neurosci. 2011;23:3515–3528. doi: 10.1162/jocn_a_00015. [DOI] [PubMed] [Google Scholar]
- 25.Cantone M, Bramanti A, Lanza G, Pennisi M, Bramanti P, Pennisi G, Bella R. Cortical plasticity in depression: a neurochemical perspective from transcranial magnetic stimulation. ASN Neuro. 2017 doi: 10.1177/1759091417711512. doi:10177/175909141771151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardenas-Morales L, Gron G, Sim EJ, Stingl JC, Kammer T. Neural activation in humans during a simple motor task differs between BDNF polymorphisms. PLoS One. 2014:9–e96722. doi: 10.1371/journal.pone.0096722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catarina F, Faranak F, Alvaro PL. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Front Neurosci. 2013;7:42. doi: 10.3389/fnins.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chara O, Tanaka EM, Brusch L. Mathematical modeling of regenerative processes. Curr Top Dev Biol. 2014;108:283–317. doi: 10.1016/B978-0-12-391498-9.00011-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Qiu R, Li L, He D, Lv H, Wu X, Gu N. The role of exogenous neural stem cells transplantation in cerebral ischemic stroke. J Biomed Nanotechnol. 2014;10:3219–3230. doi: 10.1166/jbn.2014.2018. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Z, Bosco DB, Sun L, Chen X, Xu Y, Tai W, Didier R, Li J, Fan J, He X, Ren Y. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. Cell Transplant. 2017;26:469–482. doi: 10.3727/096368916X693473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christiansen L, Perez MA. Targeted-plasticity in the corticospinal tract after human spinal cord injury. Neurotherapeutics. 2018;15:618–627. doi: 10.1007/s13311-018-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci. 2013;6:70. doi: 10.3389/fncel.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffman BA, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage. 2014;85:895–908. doi: 10.1016/j.neuroimage.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 35.Cogiamanian F, Ardolino G, Vergari M, Ferrucci R, Ciocca M, Scelzo E, Barbieri S. Transcutaneous spinal direct current stimulation. Front Psychiatry. 2012;3 doi: 10.3389/fpsyt.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, Aguglia E. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19:252–258. doi: 10.3109/13651501.2015.1084329. [DOI] [PubMed] [Google Scholar]
- 37.Cote MP, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J Neurotraum. 2017;34:1841–1857. doi: 10.1089/neu.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui M, Ge H, Zhao H, Zou Y, Chen Y, Feng H. Electromagnetic fields for the regulation of neural stem cells. Stem Cells Int 2017. 2017 doi: 10.1155/2017/9898439. 9898439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y, Liu X, Yang T, Mei YA, Hu C. Exposure to extremely low-frequency electromagnetic fields inhibits T-type calcium channels via AA/LTE4 signaling pathway. Cell Calcium. 2014;55:48–58. doi: 10.1016/j.ceca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Cullen CL, Young KM. How does transcranial magnetic stimulation influence glial cells in the central nervous system? Front Neural Circuits. 2016;10:26. doi: 10.3389/fncir.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 42.De Pisapia N, Barchiesi G, Jovicich J, Cattaneo L. The role of medial prefrontal cortex in processing emotional self-referential information: a combined TMS/fMRI study. Brain Imaging Behav. 2018;13:603–614. doi: 10.1007/s11682-018-9867-3. [DOI] [PubMed] [Google Scholar]
- 43.De Ridder D, Perera S, Vanneste S. State of the art: novel applications for cortical stimulation. Neuromodulation. 2017;20:206–214. doi: 10.1111/ner.12593. [DOI] [PubMed] [Google Scholar]
- 44.Dey J, Alam MT, Chandra S, Gandhi S, Tripathi PP. Recalibrating the existence of new neurons in adult brain. ACS Chem Neurosci. 2019;10:2091–2093. doi: 10.1021/acschemneuro.9b00196. [DOI] [PubMed] [Google Scholar]
- 45.Diaz-Rios M, Guertin PA, Rivera-Oliver M. Neuromodulation of spinal locomotor networks in rodents. Curr Pharm Des. 2017;23:1741–1752. doi: 10.2174/1381612823666170124111729. [DOI] [PubMed] [Google Scholar]
- 46.Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol. 2010;6:167–174. doi: 10.1038/nrneurol.2009.227. [DOI] [PubMed] [Google Scholar]
- 47.Doulames VM, Plant GW. Induced pluripotent stem cell therapies for cervical spinal cord injury. Int J Mol Sci. 2016;17:530. doi: 10.3390/ijms17040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du X, Rowland LM, Summerfelt A, Wijtenburg A, Chiappelli J, Wisner K, Kochunov P, Choa FS, Hong LE. TMS evoked N100 reflects local GABA and glutamate balance. Brain Stimul. 2018;11:1071–1079. doi: 10.1016/j.brs.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan H, Ge W, Zhang A, Xi Y, Chen Z, Luo D, Cheng Y, Fan KS, Horvath S, Sofroniew MV, Cheng L, Yang Z, Sun YE, Li X. Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:13360–13365. doi: 10.1073/pnas.1510176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci. 2008;31:410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Duret C, Mazzoleni S. Upper limb robotics applied to neurorehabilitation: an overview of clinical practice. NeuroRehabilitation. 2017;41:5–15. doi: 10.3233/NRE-171452. [DOI] [PubMed] [Google Scholar]
- 52.Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- 53.Fagoe ND, van Heest J, Verhaagen J. Spinal cord injury and the neuron-intrinsic regeneration-associated gene program. Neuromol Med. 2014;16:799–813. doi: 10.1007/s12017-014-8329-3. [DOI] [PubMed] [Google Scholar]
- 54.Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng G, Xiaohua H, Jinghui Z, Xiuxiu Z, Jicheng L, Hong C, Xiaolin H. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS One. 2014;91:e109267. doi: 10.1371/journal.pone.0109267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG. The impact of stimulation intensity and coil type on reliability and tolerability of cerebellar brain inhibition (CBI) via dual-coil TMS. Cerebellum. 2018;17:540–549. doi: 10.1007/s12311-018-0942-5. [DOI] [PubMed] [Google Scholar]
- 57.Filli L, Engmann AK, Zorner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34:13399–13410. doi: 10.1523/JNEUROSCI.0701-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujiwara T, Tsuji T, Honaga K, Hase K, Ushiba J, Liu M. Transcranial direct current stimulation modulates the spinal plasticity induced with patterned electrical stimulation. Clin Neurophysiol. 2011;122:1834–1837. doi: 10.1016/j.clinph.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Gackiere F, Vinay L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Front Neural Circ. 2014;8:102. doi: 10.3389/fncir.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie EV. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015a;58:225–231. doi: 10.1016/j.rehab.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol. 2015b;113:834–842. doi: 10.1152/jn.00609.2014. [DOI] [PubMed] [Google Scholar]
- 62.Gerasimenko Y, Gad P, Sayenko D, McKinney Z, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Shigueva T, Tomilovskaya E, Kozlovskaya I, Edgerton VR. Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion. J Neurophysiol. 2016;116:98–105. doi: 10.1152/jn.00146.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh M, Pearse DD. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circ. 2014;8:151. doi: 10.3389/fncir.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, Beck LA, Sayenko DG, Van Straaten MG, Drubach DI, Veith DD, Thoreson AR, Lopez C, Gerasimenko YP, Edgerton VR, Lee KH, Zhao KD. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24:1677–1682. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- 65.Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI. Neural stem cells: therapeutic potential for neurodegenerative diseases. Br Med Bull. 2012;104:7–19. doi: 10.1093/bmb/lds024. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi Y, Jinnou H, Sawamoto K, Hitoshi S. Adult neurogenesis and its role in brain injury and psychiatric diseases. J Neurochem. 2018;147:584–594. doi: 10.1111/jnc.14557. [DOI] [PubMed] [Google Scholar]
- 67.Hayashibe M, Homma T, Fujimoto K, Oi T, Yagi N, Kashihara M, Nishikawa N, Ishizumi Y, Abe S, Hashimoto H. Locomotor improvement of spinal cord-injured rats through treadmill training by forced plantar placement of hind paws. Spinal Cord. 2015;54:521–529. doi: 10.1038/sc.2015.186. [DOI] [PubMed] [Google Scholar]
- 68.He YL, Liu DD, Fang YJ, Zhan XQ, Yao JJ, Mei YA. Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in rat cerebellar granule cells through increase of AA/PGE2 and EP receptor-mediated cAMP/PKA pathway. PLoS One. 2013;8:e54376. doi: 10.1371/journal.pone.0054376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37:202–211. doi: 10.1179/2045772313Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofstoetter US, Hofer C, Kern H, Danner SM, Mayr W, Dimitrijevic MR, Minassian K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed Tech (Berl) 2013 doi: 10.1515/bmt-2013-4014. doi: 101515/bmt-2013-4014. [DOI] [PubMed] [Google Scholar]
- 71.Hofstoetter US, Krenn M, Danner SM, Hofer C, Kern H, McKay WB, Mayr W, Minassian K. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39:E176–186. doi: 10.1111/aor.12615. [DOI] [PubMed] [Google Scholar]
- 72.Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174:93–112. doi: 10.1002/ajmg.b.32429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubli M, Bolliger M, Dietz V. Neuronal dysfunction in chronic spinal cord injury. Spinal Cord. 2011;49:582–587. doi: 10.1038/sc.2010.147. [DOI] [PubMed] [Google Scholar]
- 74.Hubli M, Dietz V, Schrafl-Altermatt M, Bolliger M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin Neurophysiol. 2013;124:1187–1195. doi: 10.1016/j.clinph.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 75.Hussain S. State-of-the-art robotic gait rehabilitation orthoses: design and control aspects. NeuroRehabilitation. 2014;35:701–709. doi: 10.3233/NRE-141174. [DOI] [PubMed] [Google Scholar]
- 76.Jakobs M, Fomenko A, Lozano AM, Kiening KL. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation-a systematic review on established indications and outlook on future developments. EMBO Mol Med. 2019;11:e9575. doi: 10.15252/emmm.201809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarosiewicz B, Sarma AA, Saab J, Franco B, Cash SS, Eskandar EN, Hochberg LR. Retrospectively supervised click decoder calibration for self-calibrating point-and-click brain-computer interfaces. J Physiol Paris. 2016;110:382–391. doi: 10.1016/j.jphysparis.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jean-Charles L, Chris H, Anne B, Arrigo RT, Maxwell B. Modulation of soleus H reflex by spinal DC stimulation in humans. J Neurophysiol. 2012;108:906–914. doi: 10.1152/jn.10898.2011. [DOI] [PubMed] [Google Scholar]
- 79.Jean-Charles L, Maxwell B. BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol. 2013;110:109–116. doi: 10.1152/jn.00116.2013. [DOI] [PubMed] [Google Scholar]
- 80.Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 81.Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF BDNF and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18:E548. doi: 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HJ, Lim CS, Lee HW, Lee HS, Um YJ, Kumar H, Han I, Kim HM. A ratiometric two-photon probe for Ca(2+) in live tissues and its application to spinal cord injury model. Biomaterials. 2017;141:251–259. doi: 10.1016/j.biomaterials.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Kim SH, Cho SR, Lee JY, Kim JH, Baek A, Jung HS. The modulation of neurotrophin and epigenetic regulators: implication for astrocyte proliferation and neuronal cell apoptosis after spinal cord injury. Ann Rehabil Med. 2016;40:559–567. doi: 10.5535/arm.2016.40.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann Phys Rehabil Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Kolosova LI, Akoev GN, Ryabchikova OV, Avelev VD. Effect of low-intensity millimeter-range electromagnetic irradiation on the recovery of function in lesioned sciatic nerves in rats. Neurosci Behav Physiol. 1998;28:26–30. doi: 10.1007/BF02461908. [DOI] [PubMed] [Google Scholar]
- 86.Kremer KL, Smith AE, Sandeman L, Inglis JM, Ridding MC, Koblar SA. Transcranial magnetic stimulation of human adult stem cells in the mammalian brain. Front Neural Circ. 2016;10:17. doi: 10.3389/fncir.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang BT, Cregg JM, Depaul MA, Tran AP, Kui X, Dyck SM, Madalena KM, Brown BP, Yi-Lan W, Shuxin L. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lang C, Bradley PM, Jacobi A, Kerschensteiner M, Bareyre FM. STAT3 promotes corticospinal remodelling and functional recovery after spinal cord injury. EMBO Rep. 2013;14:931–937. doi: 10.1038/embor.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lanza G, Cantone M, Arico D, Lanuzza B, Cosentino FII, Paci D, Papotto M, Pennisi M, Bella R, Pennisi G, Paulus W, Ferri R. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory-motor network in patients with restless legs syndrome. Ther Adv Neurol Disord. 2018 doi: 10.1177/1756286418759973. doi: 101177/1756286418759973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015:521–436. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 91.Lenoir C, Jankovski A, Mouraux A. Anodal Transcutaneous Spinal Direct Current Stimulation (tsDCS) Selectively inhibits the synaptic efficacy of nociceptive transmission at spinal cord level. Neuroscience. 2018;393:150–163. doi: 10.1016/j.neuroscience.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li WY, Zhu GY, Yue WJ, Sun GD, Zhu XF, Wang Y. KLF7 overexpression in bone marrow stromal stem cells graft transplantation promotes sciatic nerve regeneration. J Neural Eng. 2019;16:056011. doi: 10.1088/1741-2552/ab3188. [DOI] [PubMed] [Google Scholar]
- 93.Liao LY, Lau BW, Sánchez-Vidaña DI, Gao Q. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen Res. 2019;14:1129–1137. doi: 10.4103/1673-5374.251188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu S, Chen Z. Employing endogenous NSCs to promote recovery of spinal cord injury. Stem Cells Int 2019. 2019:1958631. doi: 10.1155/2019/1958631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ljubisavljevic MR, Javid A, Oommen J, Parekh K, Nagelkerke N, Shehab S, Adrian TE. The effects of different repetitive transcranial magnetic stimulation (rTMS) protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injury. PLoS One. 2015;10:e0139892. doi: 10.1371/journal.pone.0139892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loy K, Bareyre FM. Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen Res. 2019;14:405–412. doi: 10.4103/1673-5374.245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loy K, Schmalz A, Hoche T, Jacobi A, Kreutzfeldt M, Merkler D, Bareyre FM. Enhanced voluntary exercise improves functional recovery following spinal cord injury by impacting the local neuroglial injury response and supporting the rewiring of supraspinal circuits. J Neurotrauma. 2018;35:2904–2915. doi: 10.1089/neu.2017.5544. [DOI] [PubMed] [Google Scholar]
- 98.Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2014;85:961–970. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys? Stem Cells. 2015;33:1036–1041. doi: 10.1002/stem.1959. [DOI] [PubMed] [Google Scholar]
- 100.Luo J, Zheng H, Zhang L, Zhang Q, Li L, Pei Z, Hu X. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017a;18:E455. doi: 10.3390/ijms18020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo L, Guo K, Fan W, Lu Y, Chen L, Wang Y, Shao Y, Wu G, Xu J, Lü L. Niche astrocytes promote the survival proliferation and neuronal differentiation of co-transplanted neural stem cells following ischemic stroke in rats. Exp Ther Med. 2017b;13:645–650. doi: 10.3892/etm.2016.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, Shi Z, Zhang K, Li C, Cui Y, Lin H, Luo D, Wang J, Lin C, Dai Z, Zhu H, Zhang J, Liu J, Liu H, deVellis J, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161:1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Macias MY, Battocletti JH, Sutton CH, Pintar FA, Maiman DJ. Directed and enhanced neurite growth with pulsed magnetic field stimulation. Bioelectromagnetics. 2000;21:272–286. [PubMed] [Google Scholar]
- 104.Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mawase F, Uehara S, Bastian AJ, Celnik P. Motor learning enhances use-dependent plasticity. J Neurosci. 2017;37:2673–2685. doi: 10.1523/JNEUROSCI.3303-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, Tansey KE. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair. 2016;30:233–243. doi: 10.1177/1545968315591706. [DOI] [PubMed] [Google Scholar]
- 107.Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 108.Morin-Moncet O, Latulipe-Loiselle A, Therrien-Blanchet JM, Theoret H. BDNF Val66Met polymorphism is associated with altered activity-dependent modulation of short-interval intracortical inhibition in bilateral M1. PLoS One. 2018;13:e0197505. doi: 10.1371/journal.pone.0197505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murray LM, Knikou M. Repeated cathodal transspinal pulse and direct current stimulation modulate cortical and corticospinal excitability differently in healthy humans. Exp Brain Res. 2019;237:1841–1852. doi: 10.1007/s00221-019-05559-2. [DOI] [PubMed] [Google Scholar]
- 110.Nguyen LK, Kholodenko BN, von Kriegsheim A. Rac1 and RhoA: networks loops and bistability. Small GTPases. 2018;9:316–321. doi: 10.1080/21541248.2016.1224399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 112.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 113.Okubo T, Nagoshi N, Kohyama J, Tsuji O, Shinozaki M, Shibata S, Kase Y, Matsumoto M, Nakamura M, Okano H. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC- derived transplants for chronic spinal cord injury. Stem Cell Rep. 2018;11:1416–1432. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 115.Pandarinath C, Nuyujukian P, Blabe CH, Sorice BL, Saab J, Willett FR, Hochberg LR, Shenoy KV, Henderson JM. High performance communication by people with paralysis using an intracortical brain-computer interface. ELife. 2017;6:e18554. doi: 10.7554/eLife.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paret C, Goldway N, Zich C, Keynan JN, Hendler T, Linden D, Kadosh KC. Current progress in real-time functional magnetic resonance-based neurofeedback: methodological challenges and achievements. Neuroimage. 2019;9:116107. doi: 10.1016/j.neuroimage.2019.116107. [DOI] [PubMed] [Google Scholar]
- 117.Powell ES, Carrico C, Salyers E, Westgate PM, Sawaki L. The effect of transcutaneous spinal direct current stimulation on corticospinal excitability in chronic incomplete spinal cord injury. NeuroRehabilitation. 2018;43:125–134. doi: 10.3233/NRE-172369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Priori A, Ciocca M, Parazzini M, Vergari M, Ferrucci R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol. 2014;592:3345–3369. doi: 10.1113/jphysiol.2013.270280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- 120.Ramadan WS, Abdel-Hamid GA, Al-Karim S, Zakar N, Elassouli MZ. Neuroectodermal stem cells: A remyelinating potential in acute compressed spinal cord injury in rat model. J Biosci. 2018;43:897–909. [PubMed] [Google Scholar]
- 121.Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, Gerasimenko YP, Sayenko DG. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35:2540–2553. doi: 10.1089/neu.2017.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rawji V, Ciocca M, Zacharia A, Soares D, Truong D, Bikson M, Rothwell J, Bestmann S. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018;11:289–298. doi: 10.1016/j.brs.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rezaei Haddad A, Lythe V, Green AL. Deep brain stimulation for recovery of consciousness in minimally conscious patients after traumatic brain injury: a systematic review. Neuromodulation. 2019;22:373–379. doi: 10.1111/ner.12944. [DOI] [PubMed] [Google Scholar]
- 124.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, Aird L, Alvarado N, Andole S, Cohen DL, Dawson J, Fernandez-Garcia C, Finch T, Ford GA, Francis R, Hogg S, Hughes N, Price CI, Ternent L, Turner DL, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394:51–62. doi: 10.1016/S0140-6736(19)31055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci. 2014;34:10285–10297. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ross JM, Iversen JR, Balasubramaniam R. The role of posterior parietal cortex in beat-based timing perception: a continuous theta burst stimulation study. J Cogn Neurosci. 2018;30:634–643. doi: 10.1162/jocn_a_01237. [DOI] [PubMed] [Google Scholar]
- 127.Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salisbury DB, Parsons TD, Monden KR, Trost Z, Driver SJ. Brain-computer interface for individuals after spinal cord injury. Rehabil Psychol. 2016;61:435–441. doi: 10.1037/rep0000099. [DOI] [PubMed] [Google Scholar]
- 129.Santos T, Maia J, Agasse F, Xapelli S, Ferreira L, Bernardino L. Nanomedicine boosts neurogenesis: new strategies for brain repair. Integr Biol (Camb) 2012;4:973–981. doi: 10.1039/c2ib20129a. [DOI] [PubMed] [Google Scholar]
- 130.Sburlea AI, Muller-Putz GR. Exploring representations of human grasping in neural muscle and kinematic signals. Sci Rep. 2018;8:16669. doi: 10.1038/s41598-018-35018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schwemmer MA, Skomrock ND, Sederberg PB, Ting JE, Sharma G, Bockbrader MA, Friedenberg DA. Meeting brain–computer interface user performance expectations using a deep neural network decoding framework. Nat Med. 2018;24:1669–1676. doi: 10.1038/s41591-018-0171-y. [DOI] [PubMed] [Google Scholar]
- 132.Shah N, Padalia D. StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC; 2019. Intrathecal Delivery System. [Google Scholar]
- 133.Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, Hart RP, Schwartz M. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2010;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- 134.Shang Y, Wang X, Shang X, Zhang H, Liu Z, Yin T, Zhang T. Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol Learn Mem. 2016;134(Pt B):369–378. doi: 10.1016/j.nlm.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 135.Sisken BF, Fowler I, Barr EJ, Kryscio RJ. The threshold quantity of nerve required to induce limb regeneration in the chick embryo. J Neurosci Res. 1984;12:623–632. doi: 10.1002/jnr.490120411. [DOI] [PubMed] [Google Scholar]
- 136.Sonksen P, Hillier S. A patient’s journey: spinal cord injury. BMJ. 2010;340:922–924. doi: 10.1136/bmj.b5204. [DOI] [PubMed] [Google Scholar]
- 137.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Staudt MD, Herring EZ, Gao K, Miller JP, Sweet JA. Evolution in the treatment of psychiatric disorders: from psychosurgery to psychopharmacology to neuromodulation. Front Neurosci. 2019;13:108. doi: 10.3389/fnins.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stein PSG. Central pattern generators in the turtle spinal cord: selection among the forms of motor behaviors. J Neurophysiol. 2018;119:422–440. doi: 10.1152/jn.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stevenson AJ, Mrachacz-Kersting N, van Asseldonk E, Turner DL, Spaich EG. Spinal plasticity in robot-mediated therapy for the lower limbs. J Neuroeng Rehabil. 2015;12:81. doi: 10.1186/s12984-015-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun ZC, Ge JL, Guo B, Guo J, Hao M, Wu YC, Lin YA, La T, Yao PT, Mei YA, Feng Y, Xue L. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci Rep. 2016;6:21774. doi: 10.1038/srep21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tanabe N, Kuboyama T, Tohda C. Matrine promotes neural circuit remodeling to regulate motor function in a mouse model of chronic spinal cord injury. Neural Regen Res. 2019;14:1961–1967. doi: 10.4103/1673-5374.259625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci. 2009;29:11708–11716. doi: 10.1523/JNEUROSCI.2217-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tekieh T, Sasanpour P, Rafii-Tabar H. Effects of electromagnetic field exposure on conduction and concentration of voltage gated calcium channels: a Brownian dynamics study. Brain Res. 2016;1646:560–569. doi: 10.1016/j.brainres.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 145.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 146.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 147.Vyleta NP, Borges-Merjane C, Jonas P. Plasticity-dependent, full detonation at hippocampal mossy fiber-CA3 pyramidal neuron synapses. Elife. 2016;5:e17977. doi: 10.7554/eLife.17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seáñez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 149.Wagner T, Valero-Cabre AL, Pascual-Leone A. Noninvasive human brain stimulation. Ann Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 150.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2010;217:169–180. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 151.Wang M, Ong LS, Dauwels J, Asada HH. Automated tracking and quantification of angiogenic vessel formation in 3D microfluidic devices. PLoS One. 2017;12:e0186465. doi: 10.1371/journal.pone.0186465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wenker SD, Leal MC, Farias MI, Zeng X, Pitossi FJ. Cell therapy for Parkinson’s disease: Functional role of the host immune response on survival and differentiation of dopaminergic neuroblasts. Brain Res. 2016;1638:15–29. doi: 10.1016/j.brainres.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 155.Wessel MJ, Hummel FC. Non-invasive cerebellar stimulation: a promising approach for stroke recovery? Cerebellum. 2018;17:359–371. doi: 10.1007/s12311-017-0906-1. [DOI] [PubMed] [Google Scholar]
- 156.Wilems TS, Pardieck J, Iyer N, Sakiyama-Elbert SE. Combination therapy of stem cell derived neural progenitors and drug delivery of anti-inhibitory molecules for spinal cord injury. Acta Biomater. 2015;28:23–32. doi: 10.1016/j.actbio.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamanaka K, Kadota H, Nozaki D. Long-latency TMS-evoked potentials during motor execution and inhibition. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang CC, Vollm B, Khalifa N. The effects of rTMS on impulsivity in normal adults: a systematic review and meta-analysis. Neuropsychol Rev. 2018;28:377–392. doi: 10.1007/s11065-018-9376-6. [DOI] [PubMed] [Google Scholar]
- 159.Yang HY, Liu Y, Xie JC, Liu NN, Tian X. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav Brain Res. 2015a;281:149–155. doi: 10.1016/j.bbr.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 160.Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, Sun YE, Li X. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015b;112:13354–13359. doi: 10.1073/pnas.1510194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yao C, Mi Y, Hu X, Li C, Sun C, Tang J, Wu X. Experiment and mechanism research of SKOV3 cancer cell apoptosis induced by nanosecond pulsed electric field. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1044–1047. doi: 10.1109/IEMBS.2008.4649338. [DOI] [PubMed] [Google Scholar]
- 162.Yu JH, Seo JH, Lee JY, Lee MY, Cho SR. Induction of neurorestoration from endogenous stem cells. Cell Transplant. 2016;25:863–882. doi: 10.3727/096368916X690511. [DOI] [PubMed] [Google Scholar]
- 163.Yu W, Jin S. Production of neural stem cells from human pluripotent stem cells. J Biotechnol. 2014;188:122–129. doi: 10.1016/j.jbiotec.2014.07.453. [DOI] [PubMed] [Google Scholar]
- 164.Zhang N, Xing M, Wang Y, Tao H, Cheng Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015;311:284–291. doi: 10.1016/j.neuroscience.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 165.Zheng W, ZhuGe Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma. 2013;30:1872–1880. doi: 10.1089/neu.2010.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zörner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Röthlisberger M2, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014;137:1716. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.