Abstract
The high morbidity and mortality rate of ischemic stroke in humans has led to the development of numerous animal models that replicate human stroke to further understand the underlying pathophysiology and to explore potential therapeutic interventions. Although promising therapeutics have been identified using these animal models, with most undergoing significant testing in rodent models, the vast majority of these interventions have failed in human clinical trials. This failure of preclinical translation highlights the critical need for better therapeutic assessment in more clinically relevant ischemic stroke animal models. Large animal models such as non-human primates, sheep, pigs, and dogs are likely more predictive of human responses and outcomes due to brain anatomy and physiology that are more similar to humans-potentially making large animal testing a key step in the stroke therapy translational pipeline. The objective of this review is to highlight key characteristics that potentially make these gyrencephalic, large animal ischemic stroke models more predictive by comparing pathophysiological responses, tissue-level changes, and model limitations.
Keywords: brain ischemia, clinical translation, gyrencephalic, large animal model, magnetic resonance imaging, stroke
Introduction
Resulting in approximately 142,000 deaths a year, stroke ranks 5th among all causes of death in the United States (Benjamin et al., 2019). Although the age-adjusted mortality rates for stroke decreased between 1990 and 2015, the absolute number of people who have strokes annually have increased worldwide (Benjamin et al., 2019). Given the high morbidity and mortality of stroke, animal models have been developed over the last four decades to replicate various aspects of human stroke to further understand underlying pathophysiological responses and explore potential treatments. Ischemic stroke caused by a blockage in the brain vasculature leading to brain ischemia is the most common type of stroke, accounting for 85% of all clinical cases (Mackay et al., 2004).
Due to ischemic stroke prevalence, a number of rodent ischemic stroke models (e.g., permanent middle cerebral artery occlusion and thromboembolic models) have been developed. Although current rodent models offer many advantages including low cost, well-characterized physiological responses, and the ability to investigate genetic manipulations and co-morbidities (e.g., diabetes, hypertension), the number of failed human clinical trials suggests additional testing in translational ischemic stroke models, more representative of the human condition, are needed for the assessment of novel therapies (Perel et al., 2007; Hossmann, 2009; Sicard and Fisher, 2009). Stroke Therapy Academic Industry Roundtable meetings of leading stroke experts have recommended that after sufficient evidence of therapeutic efficacy has been collected in rodent models, large animal models of stroke should be used to increase clinical predictive value (Fisher and Stroke Therapy Academic Industry, 2003; Fisher et al., 2007, 2009). The use of an intermediate species may enhance successful translation given similarities in neuroanatomical structures and clinical hallmarks relative to humans (Figure 1).
Figure 1.
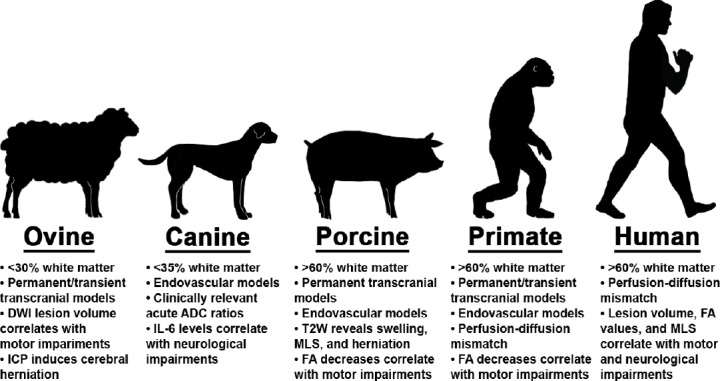
Major large animal models utilized to evaluate human ischemic stroke pathophysiology.
Inherent white matter composition, stroke type, surgical occlusion methods, and magnetic resonance assessments provide a platform to investigate human ischemic stroke pathophysiology and consequent functional outcomes. Collectively, these clinically relevant modalities enable researchers to characterize unique tissue-level and functional (e.g., sensorimotor) changes across acute and chronic time points. These tools possess indisputable value in identifying therapeutic targets and testing of novel treatments prior to clinical trials. ADC: Apparent diffusion coefficient; FA: fractional anisotropy; ICP: intracranial pressure; IL-6: interleukin 6; MLS: midline shif; T2W: T2 weighted.
In order to advance our current understanding of human stroke pathophysiology and to develop novel therapies and devices, numerous pre-clinical animal models are widely employed. In this review, we will compare relevant brain anatomical and physiological characteristics between humans, non-human primates (NHPs), sheep, pigs, and dogs as well as consequential neurologic and motor function deficits post-stroke. Clinically relevant magnetic resonance-based outcomes will also be evaluated in these large animal models to assess tissue-level changes across acute and chronic time points (Figure 2). Finally, clinical translatability and experimental practicality will also be considered.
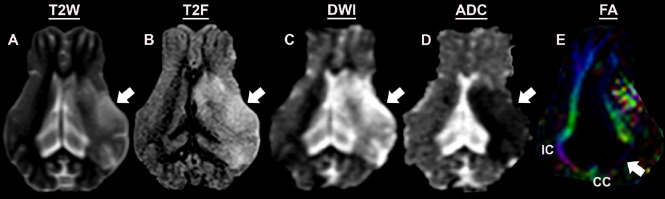
Figure 2.
Magnetic resonance assessment of a preclinical pig ischemic stroke model 1-day post-middle cerebral artery occlusion.
Pathophysiological changes including increased hemispheric swelling, midline shift, and hyperintense lesion formation are observed in T2 weighted (T2W) (A) and T2 fluid-attenuated inversion recovery (T2F) (B) sequences. DWI (C) sequences showed hyperintense lesions indicative of territorial edematous injury, while corresponding hypointense lesions on ADC maps (D) confirmed restricted diffusion and cytotoxic edema. FA maps (E) revealed increased loss of WM integrity in the internal capsule (IC) and the corpus callosum (CC). ADC: Apparent diffusion coefficient; SWI: diffusion weighted imaging; FA: fractional anisotropy; WM: white matter.
Search Strategy and Selection Criteria
The articles in this review were retrieved using the following electronic databases: PubMed, MEDLINE, and Google Scholar. The search was limited to articles published between July 7, 1953 and July 25, 2019. Search terms were identified in the title, abstract, and key words using the following search terms: stroke, ischemic infarct, stroke patients, middle cerebral artery occlusion, large animal model, non-human primates, sheep/ovine, pig/porcine/swine, dog/canine, magnetic resonance imaging, motor function, neurological score.
Large Animal Ischemic Stroke Models
Non-human primate ischemic stroke models
The use of gyrencephalic NHP species for translational stroke research is an attractive alternative due to their remarkable anatomical similarity to the human brain. NHPs have comparable complex cortical organization with deep white matter (WM) tracts, white (> 60%) and gray matter composition, and cerebral vasculature that closely resembles humans (Figure 1) (Cook and Tymianski, 2011). Furthermore, NHPs possess relatively thick cortices and numerous, high-velocity neurons, thus allotting for superior cognitive and behavioral processing capacities during neurobehavioral testing (Cook and Tymianski, 2012; Roth and Dicke, 2012). These favorable anatomical similarities have led to the development of several NHP ischemic stroke models.
Historically, the primary NHP stroke model utilized to investigate cerebral ischemia was the baboon permanent middle cerebral artery occlusion (MCAO) model (Spetzler et al., 1983; Nehls et al., 1986). This model resulted in key stroke hallmarks such as ischemia, classical cytotoxic edema and lesioning of the brain. However, this model produces marked cerebral edema requiring prolonged intensive care of the animal and is associated with a high risk of mortality making it a challenging model to study. In addition, this permanent occlusion model did not allow for reperfusion, which is common in human patients, limiting its potential to assess key aspects of ischemic stroke like reperfusion injury. As a result, transient NHP models were developed in order to gain a better understanding of secondary reperfusion injury and successive microvasculature failure. Del Zoppo et al. (1986) describes a balloon transorbital reperfusion baboon model with increasing cortical lesion volumes observed between 10 days (3.2 ± 1.5 cm3) and 14 days (3.9 ± 1.9 cm3) post-stroke with functional deficits including contralateral hemiparesis, facial paresis, and mydriasis of variable degrees due to internal capsule, putamen, and caudate nucleus involvement. In addition, microvascular clip reperfusion baboon models exhibit lesion volumes (30% of the ipsilateral hemisphere) and progressive temporal evolution similar to humans as assessed by MRI diffusion weighted imaging (DWI) and T2 Weighted (T2W) structural sequences (Huang et al., 2000; D’Ambrosio et al., 2004; Giffard et al., 2005). Functional deficits including hemiparesis of the contralateral arm and leg were thought to arise from the loss of the tissue integrity in the striato-capsular area with internal capsule and basal ganglia involvement. In the context of human ischemic stroke, these results possess important implications for researchers given that many human studies confirm this delayed evolution of ischemic areas with mean lesion volumes significantly (P < 0.0001) higher at 7 days compared to 1-day post-stroke in patients (Pantano et al., 1999; Saver, 2006). Moreover, most stroke patients also exhibit hemiparesis with correlative asymmetries in arm, leg, trunk, and face movements due to internal capsule, putamen, and basal ganglia involvement in the complex communication and feedback loops between the areas of the cerebral cortex and the brainstem (Ng et al., 2007; Patterson et al., 2008). Restoring functional deficits in stroke patients is critical for improvements in patient quality of life and is an important measure of a treatment’s therapeutic potential in animal models (Sofuwa et al., 2005; Liao et al., 2008; Veerbeek et al., 2011).
Macaque reperfusion models generated by transorbital and pterional craniotomy approaches have been characterized with both methods of cerebrovascular access while producing representative ischemic lesions. In addition to modeling the structural damage of stroke, these models mirror functional deficits including reduced strength and skilled movement of upper limbs with the loss or disruption of motor and sensory cortices, similar to the human condition (Nakayama et al., 1994; Cirstea and Levin, 2000; Nudo et al., 2001). Chin et al. (2010) reported transient decreases in MRI WM fractional anisotropy (FA) values, suggesting loss of integrity of the ipsilateral motor pathways at the dorsal region of the internal capsule at 7 days post-stroke. This correlated with deficits in motor function including paralysis and weakness in upper and lower limbs as well as incoordination. However, motor function recovered to baseline at 6 weeks post-stroke. This gradual motor recovery observed in macaques has also been reported in chronic stroke patients where gradual motor recovery is also observed and correlates with WM FA value changes (Pierpaoli et al., 2001; Biesbroek et al., 2017). West et al. (2009) characterized varying durations of microvascular clip occlusion and consequent mean lesion volumes at 45 minutes (1.983 ± 2.51 cm3), 60 minutes (2.381 ± 6.54 cm3), and 90 minutes (4.707 ± 12.7 cm3) as determined by T2W imaging in macaques (West et al., 2009). Consequent ischemia encompassed portions of the frontal and parietal lobes, insular motor cortex, and cingulate cortex with the severity of hemiparesis in the extremities and face consistent with clinical stroke patients diagnosed with damaged neuronal motor cortex circuitries (Traversa et al., 2000; West et al., 2009). In a similar study by Murphy et al. (2008) a 90-minute occlusion produced infarcts extending from the caudate nucleus and putamen (basal ganglia), external capsule, and adjacent subcortical WM in macaques. Multiple human clinical trials and large cohort studies have characterized these frontotemporal abnormalities with most involving both basal ganglia and WM compartments (Saver, 2006). Final patient volumes in clinical trials range from 19 to 138 cm3 (3.33–24.17% of the ipsilateral hemisphere), thus suggesting 45 minutes (19.0 ± 0.98% of the ipsilateral hemisphere) and 60 minutes (22.1 ± 4.6% of clip application are more clinically relevant macaque models of ischemic stroke as compared to 90 minutes (44.4 ± 4.0% of temporary occlusion) (Saver, 2006).
Endovascular occlusion using an autologous blood clot, coil, or surgical suture has also been widely developed in macaques to induce focal ischemia. Advantages of these methods include 1) they are minimally invasive and avoid the need for enucleation and its associated loss of vision and impacts on neurobehavioral assessments, 2) they are capable of achieving reperfusion, and 3) they directly affect the intracranial vessels, thus avoiding surgically induced damage to the endocranium and intracranial environment. Autologous blood clot models have induced ischemic lesions (28.3 ± 12.4% of the ipsilateral hemisphere) affecting the caudate, globus pallidus, putamen, internal capsule, claustrum, and insular cortex (Hillet al., 1955; Kito et al., 2001). Clinical signs compatible with selective occlusion of these middle cerebral artery (MCA) territories in humans were also observed in macaques exhibiting contralateral facial sensation, pinna and pain reflexes, and severe paralysis of contralateral hands and legs. Furthermore, significant correlations between macaque lesion volumes and total neurologic deficit scores were observed, with similar correlations commonly seen between acute ischemic stroke patient lesion volumes and the National Institutes of Health Stroke Scale neurological scores (Kito et al., 2001; Furlanis et al., 2018).
The ischemic penumbra is classically defined as potentially salvageable hypoperfused tissue and is the difference between the perfusion weighted imaging (PWI) lesion volume and the DWI lesion volume in stroked tissue (Astrup et al., 1981; Kakuda et al., 2008). This PWI/DWI mismatch is a key characteristic in both NHP and human stroke pathology and has prompted alterations in therapeutic targets and treatment windows (Hossmann, 1994; Wey et al., 2011). Alternative penumbra imaging concepts more technologically available have also been recently developed using diffusion tensor imaging (DTI) and associated FA values. For example, in a recent study by Neal et al. (2015), they demonstrated that patients with acute ischemic stroke (< 6 hours) exhibited decreased FA values in regions of ischemic core, yet increased FA values in hypoperfused penumbra tissues. This trend suggests DTI and FA related changes in humans could be used to differentiate penumbra and ischemic core in addition to currently used PWI/DWI comparisons. Comparatively, NHP coil endovascular occlusion revealed a PWI/DTI mismatch with PWI lesion volumes being larger than DTI abnormalities at 1 hour post-stroke with the difference being penumbra (Guo et al., 2011). A similar NHP suture model also identified penumbra evolution as detected by DWI and corresponding apparent diffusion coefficient (ADC) maps followed by T2W sequences (Rodriguez-Mercado et al., 2012). Liu et al. (2007) provided further evidence that the evolution of stroke in macaques is closer to what has been observed in humans than in rodent models by comparing ADC and FA maps with T2W and T2FLAIR sequences to establish the temporal profile of diffusion changes and to determine endpoint lesion volumes in permanent and transient MCAO models. In human studies, the mean time of pseudonormalized ADC (i.e., return to an apparently normal ADC value) was ~10–14 days, while in rodent studies a pseudonormalized ADC was often found much earlier at ~1–4 days post-stroke, likely due to inherent differences in cerebrovascular collateralization and cytoarchitecture (Eastwood et al., 2003; Munoz et al., 2004; Liu et al., 2007). Comparatively, NHP ADC pseudonormalized at ~8 days in lissencephalic marmosets and ~10 days in gyrencephalic macaques, providing further support for the use of gyrencephalic NHP species that more adequately replicate human penumbra evolution post-stroke (Liu et al., 2007; Bihelet al., 2010). Wey et al. (2011a, b) implemented arterial spin labeling to characterize the spatial-temporal characteristics associated with perfusion-diffusion mismatch and provided evidence that reperfusion salvaged damaged penumbra tissue in a transient baboon model. In humans, this perfusion-diffusion mismatch is often detected for up to 12 hours post-stroke with the frequency of detection decreasing over time (Darby et al., 1999; Shen et al., 2003; Zhang et al., 2015b). Comparatively, this mismatch volume has shown to be detectable for up to 6 hours in both baboons and macaques, whereas it is only detectable for up to 3 hours post-stroke in rodent models (e.g., intraluminal reperfusion models). The similarities in the manifestation, evolution and detection of the salvageable penumbra region between humans and primates provides researchers with valuable information on ischemic stroke pathophysiological changes and the ability to better assess drugs targeting the salvageable penumbra tissue (Astrup et al., 1981; Warach et al., 1996).
Non-human primate ischemic stroke model considerations
Despite the translational potential of primate models, there are a number of important practical and scientific disadvantages that have limited the use of these models. Baboons exhibit a network of arteries that communicate between the bilateral anterior cerebral arteries (ACAs) rather than the single vessel found in humans, which may influence Circle of Willis collateralization (Kapoor et al., 2003). Additionally, some NHP species, sheep, and pigs demonstrate complete anterior communicating artery (CoA) hypoblastia, which may result in poor outcomes due to decreased cerebral blood flow (Combs et al., 1990; Sorby-Adams et al., 2018). Baboon MCAO models are associated with high premature mortality rates and prolonged intensive care (Nehls et al., 1986; Huang et al., 2000; D’Ambrosio et al., 2004). Some permanent and transient occlusion methods in baboons and macaques require enucleation for transorbital access to the MCA and the ACA, thus limiting neurobehavioral assessments due to binocular vision loss (Nehls et al., 1986; Tagaya et al., 1997; Mack et al., 2003; D’Ambrosio et al., 2004). Cynomolgus and rhesus macaque endovascular induction methods require substantial technological and surgical skill and may produce unreliable anterior circulation stroke patterns (Kito et al., 2001; Kuge et al., 2001; de Crespignyet al., 2005; Wu et al., 2016). Reperfusion in autologous blood clot models is difficult to control, while microcatheter embolization methods utilizing metal coils or guide wires prohibits the use of MRI prior to reperfusion. The extensive limitations of cost, housing facilities, veterinary care, and ethical challenges associated with NHP models warrant further investigation of alternative large animal species for modeling ischemic stroke.
Ovine ischemic stroke models
Sheep are a highly promising surrogate for modeling human stroke due to inherent anatomical similarities including gyrencephalic cerebral structure with dense WM tracts (Bataille et al., 2007). A strong fibrous dura mater and tentorium cerebelli also play a significant role in modeling human ischemic stroke by confining post-stroke increases in intracranial pressure (ICP) to the supratentorial compartment (Klintworth, 1968; Gabrielian et al., 2011). Comparatively, rodents have a weak vestigial connective tissue membrane that enables distribution of ICP into other compartments. Increases in ICP in humans and large gyrencephalic animals post-stroke are therefore more significant and more common than in rodents and often leads to loss of consciousness, cerebral herniation, and premature death. Sheep cerebrovasculature also facilitates the development of sheep stroke models as the intradural internal carotid artery (ICA) supplies blood to a majority of the supratentorial structures and, like humans, the terminal intradural ICA bifurcates to the ACA and MCA (Ashwini, 2008). Despite these similarities, sheep possess a reticulated arterial anastomosis between the maxillary and internal carotids known as the rete mirabile (Daniel 1953; Hoffmann et al., 2014). This capillary network at the branch of the common carotid artery renders endovascular models of cerebral ischemia virtually impossible due to the minute diameter of arterial vessels, thus transcranial stroke induction approaches are typically required.
Permanent occlusion in sheep via frontotemporal craniectomy boasts a number of advantages including preservation of post-operative binocular vision, unlike NHP transorbital models, as well as lesion reproducibility due to the length of the ICA which aids in proximal MCA accessibility (Kapoor et al., 2003). Boltze et al. (2008) reported DWI-based lesion volumes could be titrated, with complete MCAO lesion volumes being significantly greater than 2-branch and 1-branch-MCAO lesion volumes (16.3 ± 5.2 vs. 8.7 ± 3.9 vs. 5.6 ± 3.6 cm3, respectively) at 1-day post-stroke. These significant differences in lesion volumes were maintained between subgroups up to 42 days post-stroke. Lesion volume was found to be correlated with functional outcomes based on a novel sheep-specific neurobehavioral score system with important metrics including unconsciousness, ataxia, fetlock flexion weakness, delayed hemistanding, circling behaviors, and impaired hopping reactions. Comparatively, complete proximal MCA occlusion in humans resulted in similar motor and somatosensory deficits including ataxia and hemiparesis of contralateral upper and lower extremities as well as loss of consciousness due to significant cerebral edema and swelling (Battey et al., 2014). Furthermore, T2W sequences in sheep revealed significantly higher atrophy ratios for the complete MCAO group compared to the 2-branch-MCAO group, whereas 1-branch-MCAO resulted in significantly less atrophy ratios compared with 2-branch-MCAO 42 days post-stroke (Boltze et al., 2008). Although this model maintains clinically relevant tissue and functional-level deficits at acute and chronic time points, it also exhibits unrealistic survivability rates (100%) post-stroke. Researchers believe these survival rates are due to reduced ICP at the craniectomy site and is therefore a notable limitation of the model.
To address the translational limitations of the permanent sheep occlusion model, Wells et al. later developed a transient sheep occlusion model and performed a head to head comparison to more accurately assess intracranial dynamics and reperfusion mechanism differences between these models (Wells et al., 2012, 2015). As expected, lesion volumes were greater in permanent occlusion sheep compared to transient occlusion sheep (27.4–28.8% vs. 7.9–14.6% of the ipsilateral hemisphere, respectively) with ischemia affecting both cortical and subcortical structures in both models. DWI deficits were also greater in permanent occlusion sheep compared to transient occlusion sheep (25.4 ± 6.8% vs. 10.7 ± 3.9%, respectively) with restricted diffusion reported throughout the entire right MCA territory and basal ganglia in permanent occlusion sheep and throughout the right caudate head and genu of the internal capsule in transient occlusion sheep. T2W sequences revealed similar model differences in edema volume (25.0 ± 4.9% vs. 5.4 ± 4.1% of the ipsilateral hemisphere) resulting in increasing midline shift (MLS) (3.3 ± 0.6 mm vs. 1.0 ± 0.8 mm). Sorby-Adams et al. (2019) recently reported significantly elevated ICP levels in excess of 20 mmHg at 5–6 days post-transient MCAO in sheep. These findings are comparable to human patients in which cerebral edema and ICP peak around 3–5 days following initial ischemic insult (Hewitt, 2012). Clinically, ICP readings > 20 mmHg warrant treatment intervention as persistently elevated ICP following MCA infarction is commonly associated with rapid neurological deterioration (Treadwell and Thanvi, 2010; Lavinio and Menon, 2011; Battey et al., 2014). Death is common in the 78% of patients that experience herniation and consequent brainstem compression due to ICP (Hacke et al., 1996; Wang et al., 2011). The sequela of herniation and comparative mortality rates observed in the transient sheep model further supports the use of this clinically relevant large animal model in order to study ICP related pathology and decompressive therapies.
Ovine ischemic stroke model considerations
Arguably the most notable disadvantage of sheep models is the rete mirabile. This dense network of small diameter arteries renders endovascular methods of ischemic induction unfeasible, thus necessitating rather complex transcranial surgical approaches to induce MCAO. Transcranial approaches disturb endocranium dynamics and may lead to hematomas or hemorrhagic transformation as seen in approximately ~12% patients with MCA infarctions that undergo decompressive craniectomies (Kenning et al., 2012; Lee et al., 2012). Furthermore, surgical craniectomy permits the loss of cerebrospinal fluid (CSF) upon dural excision and minimizes the pathological development of elevated ICP following MCA occlusion (Boltze et al., 2008). Common assessments of patient injury severity and prognosis in clinical settings are often based on neurological symptoms including pupillary dilation, anisocoria, consciousness, and paralysis which are caused by excessive ICP (Chen et al., 2011). Without representative increases in ICP, craniectomy sheep occlusion models display notable alterations in stroke pathophysiology, thus decreasing this model’s translational potential. Furthermore, with < 30% WM, sensorimotor impairments commonly associated with WM injuries in stroke patients may not be fully replicated in sheep models (Figure 1) (Sahin, 2001; Nitzsche et al., 2015; Wang et al., 2016). Perhaps similarities in other critical brain characteristics overcome this limitation, yet this remains to be determined.
Porcine ischemic stroke models
Pigs possess notable translational advantages due to inherent neuroanatomical similarities including gyrification, large intracranial vessel diameter, and a high white-to-gray matter ratio (Gralla et al., 2006; Kobayashi et al., 2012). Proportionally comparable cerebral volumes between humans and pigs (1273.6 cm3 for men and 1131.1 cm3 for women vs. 111.09 cm3 for males and 103.15 cm3 for females) allows for a more direct assessment of therapeutic dosing in a preclinical model (Allen et al., 2002; Conrad et al., 2012). In terms of cytoarchitecture, human and pig brains are composed of > 60% WM (Figure 1) (Tanakaet al., 2008; Nakamura et al., 2009). These attributes are critically important as WM and gray matter demonstrate different metabolic needs due to neuroanatomical differences. Specifically, neuron-rich gray matter requires 2.5 times more ATP and consequently 3–5 times more vascularization than WM (Borowsky and Collins, 1989; Nonaka et al., 2003; Peters et al., 2004). The increased vasculaturization of gray matter permits some protection following ischemic events, however the limited collateralization of WM leaves these tissues particularly susceptible to ischemic insult and is a critical factor to consider when modeling human ischemic stroke. Experimental pig stroke models have also been characterized with human MRI modalities and may help provide critical insight into the refinement of acute stroke detection (Figure 2). Lastly, high purchase and housing costs as well as ethical challenges associated with other large animal models are less pronounced in pigs making them an attractive alternative (Kobayashi et al., 2012).
Similar to sheep, the rete mirabile network in pigs makes endovascular methods of MCA occlusion challenging (Stroke Therapy Academic Industry, 1999; Sakoh et al., 2000b; Watanabe et al., 2007; Ashwini, 2008). Watanabe et al. (2007) developed a permanent pig MCAO model in which the proximal MCA was occluded via permanent electrocautery utilizing a transorbital approach. Although transorbital induction has been well characterized in NHP models as an efficient method of inducing focal cortical infarction, DWI analysis in pigs revealed varying lesion volumes in relation to the magnitude of residual flow following MCAO (Sakoh et al., 2000a; Watanabe et al., 2001, 2007). Subsequent loss of binocular vision and limited neurobehavioral and gait assessments were additional limitations (Sakoh et al., 2000b). Comparatively, frontotemporal MCAO approaches avoid the need for enucleation and intraorbital decompression making it a favorable alternative over transorbital approaches (Imai et al., 2006; Platt et al., 2014; Baker et al., 2017; Webb et al., 2018). This model provides increased visibility of the MCA and its associated branches, thus resulting in repeatable ischemic injury with consistent lesion volumes. Platt et al. (2014) showed pigs, like humans, exhibit a standard evolution of ischemic injury with cytotoxic edema primarily observed as hypointense ADC abnormalities followed by delayed vasogenic edema (Ho et al., 2012). Additionally, as pig cerebrums are approximately 7.5 times smaller than humans, the West Laboratory has recently confirmed pig DWI lesion volumes (9.91 ± 3.14 cm3; unpublished data) closely replicate patient DWI lesion volume thresholds of 72 cm3, which frequently correspond to major cerebral artery occlusions and poor neurological outcome (Sanak et al., 2006; Gonzalez, 2012).
T2W sequences in the pig MCAO model revealed acute hemispheric swelling (126.8 ± 3.4% change from contralateral hemisphere), pronounced MLS, and cerebellar herniation with functional deficits in gait and behavior performance as well as premature death (Webb et al., 2018). T2W visualized hemispheric swelling and MLS in patients is also considered a robust predictor of cerebral herniations and patient death as rapid neurological deterioration often leads to 60–80% 30-day patient mortality rates (Ropper, 1986; Hacke et al., 1996; Berrouschot et al., 1998; Walberer et al., 2007; Treadwell and Thanvi, 2010). Hemispheric swelling often instigates herniation leading to abnormal protrusion into adjacent neural structures or through rigid intracranial barriers (i.e., the foreman magnum) (Klintworth 1968; Kotwica et al., 1991; Gabrielian et al., 2013). The tentorium cerebelli in humans and large animal species is relatively strong, which limits and alters the displacement of the brain post-stroke. Comparatively, rodents possess a weak tentorium cerebelli that permit the displacement of the brain. This results in inconsistencies between rodent and human swelling responses post-stroke. DTI sequences have revealed permanent pig MCAO also replicates patient reductions in WM integrity of the internal capsule and corpus callosum at acute and chronic time points (Baker et al., 2017; Webb et al., 2018). These reductions in WM integrity were coupled with deteriorations in pig spatiotemporal and relative gait pressure measurements including velocity, cadence, swing percent of cycle, stride length, cycle time, and mean pressure. Likewise, these deficits correlate with contralateral deteriorations in patient motor function, as studies utilizing Functional Ambulatory Categories found patients with internal capsule lesions exhibited persistent (> 6 months) motor deficits and required aids for balance and support during ambulation (Baltan et al., 2008; Srikanth et al., 2009; Ahmad et al., 2015; Lee et al., 2017). Understanding how brain ischemia leads to WM changes and consequent motor deficits, preferably in models with comparable WM content (> 60%), is a research priority that will help advance strategies for WM repair and regeneration.
Consequential damage to the dura and disrupted ICP evolution in the pig craniectomy models has led to the development of an endovascular pig model. Cui et al. (2013) established an endovascular model of focal ischemic stroke in miniature pigs in which sodium alginate microspheres, a biodegradable material, were injected through the femoral artery to embolize the rete mirabile as confirmed by angiographic and DWI analysis. Signal abnormalities on T2W sequences revealed lesion volumes encompassing approximately 30% of the ipsilateral hemisphere with notable ischemia in the temporal and parietal lobes and the basal ganglia. Infarction in these regions prompted mild hemiplegia and associated ambulation impairments in balance and coordination as early as 12 hours post-stroke. Interestingly, stroke patients with confined basal ganglia and internal capsule injury exhibit persistently impaired balance and ambulation performance as well (Miyai et al., 1997). These observed motor memory deficits are likely due to persistent dysfunction in the cortico-basal ganglia-thalamo-cortical loop, an intricate neural circuit system responsible for facilitating voluntary movements while simultaneously inhibiting competing or interfering movements (Boyd et al., 2009; Simonyan, 2019).
Porcine ischemic stroke model considerations
Like the sheep, the rete mirabile necessitates transcranial approaches that damage the cranium and dura and result in uncharacteristic intracranial dynamics and cerebrovascular pathophysiological changes post-stroke (Imai et al., 2006). Although an endovascular occlusion method has been described, minimal sample size, short observation windows, and occlusion of the anatomical anastomosis the rete mirabile (a more distal vasculature structure), limits the clinical translatability of this model. Furthermore, the posterior CoA in the Circle of Willis in both pigs and sheep is comparable to the diameter of the anterior cerebral artery whereas in humans the posterior CoA is approximately half the diameter of the anterior cerebral artery. (Ashwini, 2008; Deepthi, 2016). These variations in vessel diameter are an important consideration when modeling ischemic stroke as equal blood volume flows through both divisions of internal carotid artery in animals whereas in humans the blood flow through the posterior CoA is reduced, thus impacting collateral flow within the Circle of Willis. Pig plasminogen also displays high resistance to tPA activation due to unique plasminogen-streptokinase interactions (Yakovlev et al., 1995; Flight et al., 2006). As a result, investigation of combination therapies in conjunction with tPA are hindered, even if a clot model were available.
Canine ischemic stroke models
Similar to previously discussed large animal models, dogs demonstrate conserved gyrencephalic structure, well-established WM tracts, and large cerebrovascular diameter (Traystman, 2003). Additionally, unlike sheep and pigs, dogs lack a rete mirabile. This anatomical similarity to humans favors endovascular approaches of stroke induction and is one notable translational advantage of dog models (Gralla et al., 2006; Howells et al., 2010). Permittable endovascular access avoids the need for enucleation or invasive transcranial procedures to access the MCA thus preserving dura, cerebral spinal fluid volumes, and post-stroke ICP. The use of dogs also minimizes economic, housing, and handling difficulties commonly associated with NHP use, however ethical concerns remain a notable consideration (Hillock, 2006).
First described by Hill et al. (1955) several studies have since reported on various endovascular methods for inducing MCAO in dog models (Shaibaniet al., 2006; Rink et al., 2008; Christoforidis et al., 2011). Permanent MCA, achieved via ICA injection of synthetic emboli, induced lesion volumes 32.13 ± 11.98% in the basal ganglia, left ventral cortex, left ventrolateral cortex, and left cortex of the cerebrum with swelling, MLS, and mass effect observed via T1W sequences (Kang et al., 2007). Kang et al. (2009) later reported a mean ADC ratio (0.77 ± 0.08) that closely replicated human ADC ratios (0.5–0.8) at acute post-stroke time points (van Everdingen et al., 1998; Lee et al., 2005). Accumulation of cytotoxic and vasogenic edema were well-preserved within this model, thus presenting a significant advantage over photothrombotic rodent models (Carmichael, 2005; Kang et al., 2007, 2009; Macrae, 2011). Interestingly, dog CSF concentration of interleukin-6 (IL-6) positively correlated with the severity of neurological deterioration and death. This correlation has also been observed in several human stroke studies in which elevated CSF levels of IL-6 were found to positively correlate with ischemic lesion volumes and poor functional outcome (Tarkowski et al., 1995, 1997). As CSF IL-6, unlike serum IL-6, maintains significant predicative value in both dog and human stroke, further examination of this potential biomarker may help identify at-risk patients prior to onset of severe neurological symptoms and death.
Endovascular occlusion can also be achieved via coil placement in dogs through the vertebral artery (VA) in order to control for the variable intricacies of the ICA as previously mentioned (Atchaneeyasakul et al., 2016). Coil models offer a number of translational advantages as angiographic guidance in dogs is significantly superior when compared to rodent models where the relative size of the cranial arteries limits procedural evaluation and confirmation of occlusion (Flecknell et al., 2009). This method utilizes comprehensive imaging techniques to precisely position the coil encased within a microcatheter through the proximal M1 until appropriate permanent or transient occlusion of the MCA is achieved. Although the use of imaging guidance requires a complex understanding of dog cerebrovasculature, it enables specificity in coil placement that cannot be achieved by emboli injection via ICA. This yields definite and reproducible dog ischemic lesions of approximately 9.81–20.58 cm3 (30.9 ± 2.1% and 31.2 ± 4.3%, as reviewed by two independent observers) as determined by T2 structural sequences 1-day post-stroke (Rink et al., 2008, 2011). DTI fiber tract projections from the region of the internal capsule to the corona radiata were dramatically reorganized with impaired connectivity in stroke dogs (Rink et al., 2011). These corticospinal and corticobulbar tracts that descend through the corona radiata and posterior limbs of the internal capsule mainly originate in the primary motor cortex in humans and play an important role in recovery of motor deficits (Higano et al., 2001; Kunimatsu et al., 2003). These anatomical similarities may provide important insights into the WM responses to hypoxia as well as necessary therapeutic measures to induce recovery of these WM structures.
The distal injection of autologous clots through the ICA accurately mimics human ischemia and is amenable to studies investigating the efficacy of novel thrombolytic therapies or thrombectomy devices. Despite clinical applicability, this model induces inconsistent lesion distribution as the precise site of occlusion cannot be controlled due to individual vascular variability, particularly of the ICA (Liu et al., 2012). Variations in emboli size have also contributed to variations in lesion volumes. Liu et al. (2012) reported clots 1.7 mm in diameter resulted in small, variable lacunar infarcts of 0.148 ± 0.133 cm3 at 6 hours (DWI), 0.150 ± 0.154 cm3 at 24 hours (T2W), and 0.095 ± 0.115 cm3 at 7 days post-stroke (T2W) affecting the internal capsule and caudate. Zu et al. (2013) reported clots 1.4–1.7 mm in diameter resulted in mean infarct volumes of 4.17 ± 0.06 cm3 at 24 hours (T2W) and 3.27 ± 0.062 cm3 at 7 days post-stroke (T2W) affecting the basal ganglia and cortex. Van der Bom et al. (2012) described larger clots of 2.33 mm in diameter that induced extremely variable infarct volumes 4 hours post-stroke (0.12 to 12.53 cm3, ADC). These discrepancies were suggested to be due to convoluted ICA and perfusion differentials from the extensive leptomeningeal collaterals and have contributed to heterogeneous strokes in dogs (Symon, 1960; Harris et al., 2009; Christoforidis et al., 2011).
In addition to MCAO models, vertebral artery occlusion (VAO) and basilar artery occlusion (BAO) models have also been developed. In VAO models, high vertebral artery blood pressure often prevents emboli from anchoring to the vessel wall, thus limiting experimental efficacy. To address this, a recent study utilized a preinstalled self-expanding thrombus filter in the delivery catheter to allow for successful implantation of emboli (Zhang et al., 2015a). Although further imaging and clinical outcome studies of this canine model are still needed, this model may be useful to evaluate novel endovascular therapy for acute VAO in humans. The BAO model comparatively is commonly used to test recanalization by intravenous versus intraarterial thrombolysis. Qureshi et al. (2004) reported significant lesion formation following autologous clot injection into the proximal portion of the basilar artery with intraarterial thrombolysis affording a recanalization rate similar to that of intravenous thrombolysis, but with a lower rate of intra-cerebral hemorrhage. The most relevant disadvantages of BAO models are the exaggerated neurological deficits, prolonged intensive care, and premature mortality which limit longitudinal assessments of therapeutic efficacy, functional outcomes, and tissue recovery post-stroke.
Canine ischemic stroke model considerations
An appreciable concern in dog models is the low extent of WM (35%), similar to that observed in sheep (< 30%) (Figure 1) (Krafft et al., 2012). Limited WM in these models may hinder advancements in understanding WM pathophysiology and mechanisms involved in ischemic injury (Ahmad et al., 2015). Conversely, NHPs and pigs display comparable WM to humans (> 60%) that results in similar prognosis and motor function decline commonly associated with human WM hyperintensities post-stroke (Debette and Markus, 2010). Occlusion of the MCA is primarily achieved via ICA access, yet this procedure requires great surgical skill due to the intracavernous connection between the torturous ICAs as well as the small diameter of microcatheter administration systems (Nanda and Getty, 1975; Rink et al., 2008). A number of dogs exhibit extensive leptomeningeal anastomoses branching from the posterior, middle, and anterior cerebral artery, potentiating variation in lesion volume due to differentials in collateralization (Symon, 1960). Variation in vascular diameter, organization and physiological responses such as vasospasms between dog breeds may also limit reproducibility and experimental outcomes especially as mongrel dogs are commonly used (Rink et al., 2008, 2011). In the endovascular coil model, Rink et al. (2011) reported the coil positioned to occlude the proximal MCA, ACA, and distal ICA resulted in premature mortality of ~38% of the dogs within 1-day post-stroke. These experimental challenges in combination with pronounced ethical complications, limit the widespread use of canine species.
Conclusion
The stroke field agrees that a therapeutic breakthrough will most likely require a multifaceted approach including innovations in clinical trial design, consideration of systemic biomarkers, and optimization of therapeutic dosing. Large animal ischemic stroke models are a key part of this approach providing more predictive models for identifying potential treatment targets and testing novel therapeutics. While rodent models provide invaluable insight into the initial characterization and screening of potential therapeutic interventions, the significant failures of novel pharmacological agents in clinical trials may be partly due to the use of animal models that do not fully reflect stroke pathophysiology as it occurs in humans. As highlighted throughout this review, NHP, ovine, porcine, and canine ischemic stroke models possess significant advantages including comparative brain anatomy, similar tissue-level and functional responses, and compatibility with clinically relevant MRI techniques. To further enhance the predictive power of these large animal models, advances need to be made to develop thromboembolic clot models that will more closely mimic patient vascular occlusion mechanisms, enable the study of reperfusion injury and evaluate the effects of novel combined therapies with thrombolytic agents. These large animal models must be enlisted as an important step in the translational framework in order to successfully bridge the gap between preclinical basic studies and effective therapies in human patients.
Acknowledgments
The authors would like to thank Dr. Kylee Duberstein, Dr. Holly Kinder, and Kelly Scheulin for their valuable editorial critiques of this review.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke, No. R01NS093314.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke, No. R01NS093314.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Ahmad AS, Satriotomo I, Fazal J, Nadeau SE, Doré S. Considerations for the optimization of induced white matter injury preclinical models. Front Neurol. 2015:6–172. doi: 10.3389/fneur.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- 3.Ashwini C A, Shubba R, Jayanthi KS. Comparative anatomy of the circle of Willis in man, cow, sheep, goat, and pig. Neuroanatomy. 2008;7:54–85. [Google Scholar]
- 4.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 5.Atchaneeyasakul K, Guada L, Ramdas K, Watanabe M, Bhattacharya P, Raval AP, Yavagal DR. Large animal canine endovascular ischemic stroke models: A review. Brain Res Bull. 2016;127:134–140. doi: 10.1016/j.brainresbull.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Baker EW, Platt SR, Lau VW, Grace HE, Holmes SP, Wang L, Duberstein KJ, Howerth EW, Kinder HA, Stice SL, Hess DC, Mao H, West FD. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci Rep. 2017:7–10075. doi: 10.1038/s41598-017-10406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bataille B, Wager M, Lapierre F, Goujon JM, Buffenoir K, Rigoard P. The significance of the rete mirabile in Vesalius’s work: an example of the dangers of inductive inference in medicine. Neurosurgery. 2007;60:761–768. doi: 10.1227/01.NEU.0000255391.92785.ED. [DOI] [PubMed] [Google Scholar]
- 9.Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, Davis SM, Donnan GA, Sheth KN, Kimberly WT. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–3648. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 11.Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–623. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 12.Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci (Lond) 2017;131:715–728. doi: 10.1042/CS20160452. [DOI] [PubMed] [Google Scholar]
- 13.Bihel E, Pro-Sistiaga P, Letourneur A, Toutain J, Saulnier R, Insausti R, Bernaudin M, Roussel S, Touzani O. Permanent or transient chronic ischemic stroke in the non-human primate: behavioral, neuroimaging, histological, and immunohistochemical investigations. J Cereb Blood Flow Metab. 2010;30:273–285. doi: 10.1038/jcbfm.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boltze J, Förschler A, Nitzsche B, Waldmin D, Hoffmann A, Boltze CM, Dreyer AY, Goldammer A, Reischauer A, Härtig W, Geiger KD, Barthel H, Emmrich F, Gille U. Permanent middle cerebral artery occlusion in sheep: a novel large animal model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1951–1964. doi: 10.1038/jcbfm.2008.89. [DOI] [PubMed] [Google Scholar]
- 15.Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- 16.Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol Learn Mem. 2009;92:35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int. 2011:2–82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin Y, Sato Y, Mase M, Kato T, Herculano B, Sekino M, Ohsaki H, Ageyama N, Ono F, Terao K, Yoshikawa Y, Hisatsune T. Transient decrease in cerebral motor pathway fractional anisotropy after focal ischemic stroke in monkey. Neurosci Res. 2010;66:406–411. doi: 10.1016/j.neures.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Christoforidis GA, Rink C, Kontzialis MS, Mohammad Y, Koch RM, Abduljalil AM, Bergdall VK, Roy S, Khanna S, Slivka AP, Knopp MV, Sen CK. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol. 2011;46:34–40. doi: 10.1097/RLI.0b013e3181f0cbc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 22.Combs DJ, Dempsey RJ, Kumar S, Donaldson D. Focal cerebral infarction in cats in the presence of hyperglycemia and increased insulin. Metab Brain Dis. 1990;5:169–178. doi: 10.1007/BF00997070. [DOI] [PubMed] [Google Scholar]
- 23.Conrad MS, Dilger RN, Johnson RW. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci. 2012;34:291–298. doi: 10.1159/000339311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- 25.Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9:371–379. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui Y, Tian Y, Tang Y, Jia L, Wu A, Peng P, Yang J, Du H, Wang X, Wu L. Application of sodium alginate microspheres in ischemic stroke modeling in miniature pigs. Neural Regen Res. 2013;8:1473–1480. doi: 10.3969/j.issn.1673-5374.2013.16.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel PM, Dawes JDK, Prichard MML. Studies of the carotid rete and its associated arteries. Philos Trans R Soc Lond B Biol Sci. 1953 doi: https:// doi.org/10.1098/rstb.1953.0003. [Google Scholar]
- 28.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, Li T, Tress BM, Davis SM. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 29.de Crespigny AJ, D’Arceuil HE, Maynard KI, He J, McAuliffe D, Norbash A, Sehgal PK, Hamberg L, Hunter G, Budzik RF, Putman CM, Gonzalez RG. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J Stroke Cerebrovasc Dis. 2005;14:80–87. doi: 10.1016/j.jstrokecerebrovasdis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. :341–c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deepthi SSD, Pramod Kumar D, Saradadevi SS, Subhadradevi V. Comparative study of formation of Circle of Willis in human and sheep brain. J Anat Soc. 2016;65:S16–19. [Google Scholar]
- 32.Del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- 33.Eastwood JD, Engelter ST, MacFall JF, Delong DM, Provenzale JM. Quantitative assessment of the time course of infarct signal intensity on diffusion-weighted images. AJNR Am J Neuroradiol. 2003;24:680–687. [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH STAIR Group. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher M, Hanley DF, Howard G, Jauch EC, Warach S STAIR Group. Recommendations from the STAIR V meeting on acute stroke trials technology and outcomes. Stroke. 2007;38:245–248. doi: 10.1161/01.STR.0000255951.37434.aa. [DOI] [PubMed] [Google Scholar]
- 36.Fisher M Stroke Therapy Academic Industry Roundtable. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- 37.Flecknell P. Laboratory animal anaesthesia. London: Elsevier/Academic Press; 2009. [Google Scholar]
- 38.Flight SM, Masci PP, Lavin MF, Gaffney PJ. Resistance of porcine blood clots to lysis relates to poor activation of porcine plasminogen by tissue plasminogen activator. Blood Coagul Fibrinolysis. 2006;17:417–420. doi: 10.1097/01.mbc.0000233374.79593.57. [DOI] [PubMed] [Google Scholar]
- 39.Furlanis G, Ajčević M, Stragapede L, Lugnan C, Ridolfi M, Caruso P, Naccarato M, Ukmar M, Manganotti P. Ischemic volume and neurological deficit: correlation of computed tomography perfusion with the National Institutes of Health Stroke Scale score in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27:2200–2207. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Gabrielian L, Helps SC, Thornton E, Turner RJ, Leonard AV, Vink R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir Suppl. 2013;118:201–204. doi: 10.1007/978-3-7091-1434-6_37. [DOI] [PubMed] [Google Scholar]
- 41.Gabrielian L, Willshire LW, Helps SC, van den Heuvel C, Mathias J, Vink R. Intracranial pressure changes following traumatic brain injury in rats: lack of significant change in the absence of mass lesions or hypoxia. J Neurotrauma. 2011;28:2103–2111. doi: 10.1089/neu.2011.1785. [DOI] [PubMed] [Google Scholar]
- 42.Giffard C, Young AR, Mézenge F, Derlon JM, Baron JC. Histopathological effects of delayed reperfusion after middle cerebral artery occlusion in the anesthetized baboon. Brain Res Bull. 2005;67:335–340. doi: 10.1016/j.brainresbull.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez RG. Clinical MRI of acute ischemic stroke. J Magn Reson Imaging. 2012;36:259–271. doi: 10.1002/jmri.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gralla J, Schroth G, Remonda L, Fleischmann A, Fandino J, Slotboom J, Brekenfeld C. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2006;27:1357–1361. [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J, Zheng HB, Duan JC, He L, Chen N, Gong QY, Tang HH, Li HX, Wang L, Cheng JQ. Diffusion tensor MRI for the assessment of cerebral ischemia/reperfusion injury in the penumbra of non-human primate stroke model. Neurol Res. 2011;33:108–112. doi: 10.1179/016164110X12761752770177. [DOI] [PubMed] [Google Scholar]
- 46.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 47.Harris AD, Kosior RK, Chen HS, Andersen LB, Frayne R. Evolution of hyperacute stroke over 6 hours using serial MR perfusion and diffusion maps. J Magn Reson Imaging. 2009;29:1262–1270. doi: 10.1002/jmri.21763. [DOI] [PubMed] [Google Scholar]
- 48.Hewitt AEC. Brain oedema, intracranial pressure and cerebral blood flow. Surgery. 2012;30:102–106. [Google Scholar]
- 49.Higano S, Zhong J, Shrier DA, Shibata DK, Takase Y, Wang H, Numaguchi Y. Diffusion anisotropy of the internal capsule and the corona radiata in association with stroke and tumors as measured by diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2001;22:456–463. [PMC free article] [PubMed] [Google Scholar]
- 50.Hill NC, Millikan CH, Wakim KG, Sayre GP. Studies in cerebrovascular disease VII.Experimental production of cerebral infarction by intracarotid injection of homologous blood clot; preliminary report. Proc Staff Meet Mayo Clin. 1955;30:625–633. [PubMed] [Google Scholar]
- 51.Hillock SM, Stefanacci JD, Fondacaro JV. Vascular encephalopathies in dogs: incidence, risk factors, pathophysiology, and clinical signs. Compend Contin Educ Vet. 2006;28:196–207. [Google Scholar]
- 52.Ho ML, Rojas R, Eisenberg RL. Cerebral edema. AJR Am J Roentgenol. 2012;199:W258–273. doi: 10.2214/AJR.11.8081. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann A, Stoffel MH, Nitzsche B, Lobsien D, Seeger J, Schneider H, Boltze J. The ovine cerebral venous system: comparative anatomy, visualization, and implications for translational research. PLoS One. 2014:9–e92990. doi: 10.1371/journal.pone.0092990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 55.Hossmann KA. Pathophysiological basis of translational stroke research. Folia Neuropathol. 2009;47:213–227. [PubMed] [Google Scholar]
- 56.Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Mocco J, Choudhri TF, Poisik A, Popilskis SJ, Emerson R, DelaPaz RL, Khandji AG, Pinsky DJ, Connolly ES., Jr A modified transorbital baboon model of reperfused stroke. Stroke. 2000;31:3054–3063. doi: 10.1161/01.str.31.12.3054. [DOI] [PubMed] [Google Scholar]
- 58.Imai H, Konno K, Nakamura M, Shimizu T, Kubota C, Seki K, Honda F, Tomizawa S, Tanaka Y, Hata H, Saito N. A new model of focal cerebral ischemia in the miniature pig. J Neurosurg. 2006;104:123–132. doi: 10.3171/ped.2006.104.2.123. [DOI] [PubMed] [Google Scholar]
- 59.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, Moseley ME, Marks MP, Albers GW DEFUSE Investigators. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28:887–891. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang BT, Jang DP, Gu SH, Lee JH, Jung DI, Lim CY, Kim HJ, Kim YB, Kim HJ, Woo EJ, Cho ZH, Park HM. MRI features in a canine model of ischemic stroke: correlation between lesion volume and neurobehavioral status during the subacute stage. Comp Med. 2009;59:459–464. [PMC free article] [PubMed] [Google Scholar]
- 61.Kang BT, Lee JH, Jung DI, Park C, Gu SH, Jeon HW, Jang DP, Lim CY, Quan FS, Kim YB, Cho ZH, Woo EJ, Park HM. Canine model of ischemic stroke with permanent middle cerebral artery occlusion: clinical and histopathological findings. J Vet Sci. 2007;8:369–376. doi: 10.4142/jvs.2007.8.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kapoor K, Kak VK, Singh B. Morphology and comparative anatomy of circulus arteriosus cerebri in mammals. Anat Histol Embryol. 2003;32:347–355. doi: 10.1111/j.1439-0264.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- 63.Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW. Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy. J Neurosurg. 2012;116:1289–1298. doi: 10.3171/2012.2.JNS111772. [DOI] [PubMed] [Google Scholar]
- 64.Kito G, Nishimura A, Susumu T, Nagata R, Kuge Y, Yokota C, Minematsu K. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods. 2001;105:45–53. doi: 10.1016/s0165-0270(00)00351-4. [DOI] [PubMed] [Google Scholar]
- 65.Klintworth GK. The comparative anatomy and phylogeny of the tentorium cerebelli. Anat Rec. 1968;160:635–642. doi: 10.1002/ar.1091600312. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi E, Hishikawa S, Teratani T, Lefor AT. The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplant Res. 2012:1–8. doi: 10.1186/2047-1440-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotwica Z, Hårdemark HG, Persson L. Intracranial pressure changes following middle cerebral artery occlusion in rats. Res Exp Med (Berl) 1991;191:99–104. doi: 10.1007/BF02576664. [DOI] [PubMed] [Google Scholar]
- 68.Krafft PR, Bailey EL, Lekic T, Rolland WB, Altay O, Tang J, Wardlaw JM, Zhang JH, Sudlow CL. Etiology of stroke and choice of models. Int J Stroke. 2012;7:398–406. doi: 10.1111/j.1747-4949.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuge Y, Yokota C, Tagaya M, Hasegawa Y, Nishimura A, Kito G, Tamaki N, Hashimoto N, Yamaguchi T, Minematsu K. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J Cereb Blood Flow Metab. 2001;21:202–210. doi: 10.1097/00004647-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Kunimatsu A, Aoki S, Masutani Y, Abe O, Mori H, Ohtomo K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology. 2003;45:532–535. doi: 10.1007/s00234-003-0974-4. [DOI] [PubMed] [Google Scholar]
- 71.Lavinio A, Menon DK. Intracranial pressure: why we monitor it how to monitor it what to do with the number and what’s the future? Curr Opin Anaesthesiol. 2011;24:117–123. doi: 10.1097/ACO.0b013e32834458c5. [DOI] [PubMed] [Google Scholar]
- 72.Lee DH, Kang DW, Ahn JS, Choi CG, Kim SJ, Suh DC. Imaging of the ischemic penumbra in acute stroke. Korean J Radiol. 2005;6:64–74. doi: 10.3348/kjr.2005.6.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KB, Kim JS, Hong BY, Sul B, Song S, Sung WJ, Hwang BY, Lim SH. Brain lesions affecting gait recovery in stroke patients. Brain Behav. 2017:7–e00868. doi: 10.1002/brb3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee MH, Yang JT, Weng HH, Cheng YK, Lin MH, Su CH, Chang CM, Wang TC. Hydrocephalus following decompressive craniectomy for malignant middle cerebral artery infarction. Clin Neurol Neurosurg. 2012;114:555–559. doi: 10.1016/j.clineuro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 75.Liao F, Wang J, He P. Multi-resolution entropy analysis of gait symmetry in neurological degenerative diseases and amyotrophic lateral sclerosis. Med Eng Phys. 2008;30:299–310. doi: 10.1016/j.medengphy.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Hu WX, Zu QQ, Lu SS, Xu XQ, Sun L, Zhou WZ, Shi HB. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. J Neurosci Methods. 2012;209:90–96. doi: 10.1016/j.jneumeth.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, D’Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, Pryor J, de Crespigny AJ. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–145. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- 78.Mack WJ, King RG, Hoh DJ, Coon AL, Ducruet AF, Huang J, Mocco J, Winfree CJ, D’Ambrosio AL, Nair MN, Sciacca RR, Connolly ES., Jr An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol Res. 2003;25:280–284. doi: 10.1179/016164103101201346. [DOI] [PubMed] [Google Scholar]
- 79.Mackay J, Mensah GA, Mendis S, Greenlund K World Health Organization. Geneva: World Health Organization; 2004. The atlas of heart disease and stroke. [Google Scholar]
- 80.Macrae IM. Preclinical stroke research--advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol. 2011;164:1062–1078. doi: 10.1111/j.1476-5381.2011.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyai I, Blau AD, Reding MJ, Volpe BT. Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts. Neurology. 1997;48:95–101. doi: 10.1212/wnl.48.1.95. [DOI] [PubMed] [Google Scholar]
- 82.Muñoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, Cvoro V, Rivers CS, Wardlaw JM. Temporal evolution of water diffusion parameters is different in grey and white matter in human ischaemic stroke. J Neurol Neurosurg Psychiatry. 2004;75:1714–1718. doi: 10.1136/jnnp.2003.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy SJ, Kirsch JR, Zhang W, Grafe MR, West GA, del Zoppo GJ, Traystman RJ, Hum PD. Can gender differences be evaluated in a rhesus macaque (Macaca mulatta) model of focal cerebral ischemia? Comp Med. 2008;58:588–596. [PMC free article] [PubMed] [Google Scholar]
- 84.Nael K, Trouard TP, Lafleur SR, Krupinski EA, Salamon N, Kidwell CS. White matter ischemic changes in hyperacute ischemic stroke: voxel-based analysis using diffusion tensor imaging and MR perfusion. Stroke. 2015;46:413–418. doi: 10.1161/STROKEAHA.114.007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura M, Imai H, Konno K, Kubota C, Seki K, Puentes S, Faried A, Yokoo H, Hata H, Yoshimoto Y, Saito N. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis Laboratory investigation. J Neurosurg Pediatr. 2009;3:488–495. doi: 10.3171/2008.6.PEDS0834. [DOI] [PubMed] [Google Scholar]
- 86.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 87.Nanda BS, Getty R. Arteria intercarotica caudalis and its homologue in the domestic animals. Anat Anz. 1975;137:110–115. [PubMed] [Google Scholar]
- 88.Nehls DG, Cartwright M, Spetzler RF. Experimental primate stroke model. Neurosurgery. 1986;18:388–389. doi: 10.1097/00006123-198603000-00032. [DOI] [PubMed] [Google Scholar]
- 89.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 90.Nitzsche B, Frey S, Collins LD, Seeger J, Lobsien D, Dreyer A, Kirsten H, Stoffel MH, Fonov VS, Boltze J. A stereotaxic population-averaged T1w ovine brain atlas including cerebral morphology and tissue volumes. Front Neuroanat. 2015:9–69. doi: 10.3389/fnana.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. The microvasculature of the cerebral white matter: arteries of the subcortical white matter. J Neuropathol Exp Neurol. 2003;62:154–161. doi: 10.1093/jnen/62.2.154. [DOI] [PubMed] [Google Scholar]
- 92.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 93.Pantano P, Caramia F, Bozzao L, Dieler C, von Kummer R. Delayed increase in infarct volume after cerebral ischemia: correlations with thrombolytic treatment and clinical outcome. Stroke. 1999;30:502–507. doi: 10.1161/01.str.30.3.502. [DOI] [PubMed] [Google Scholar]
- 94.Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, Black SE, McIlroy WE. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 95.Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007:334–197. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28:143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 98.Platt SR, Holmes SP, Howerth EW, Duberstein KJJ, Dove CR, Kinder HA, Wyatt EL, Linville AV, Lau VW, Stice SL, Hill WD, Hess DC, West FD. Development and characterization of a Yucatan miniature biomedical pig permanent middle cerebral artery occlusion stroke model. Exp Transl Stroke Med. 2014:6–5. doi: 10.1186/2040-7378-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qureshi AI, Boulos AS, Hanel RA, Suri MF, Yahia AM, Alberico RA, Hopkins LN. Randomized comparison of intra-arterial and intravenous thrombolysis in a canine model of acute basilar artery thrombosis. Neuroradiology. 2004;46:988–995. doi: 10.1007/s00234-004-1180-8. [DOI] [PubMed] [Google Scholar]
- 100.Rink C, Christoforidis G, Abduljalil A, Kontzialis M, Bergdall V, Roy S, Khanna S, Slivka A, Knopp M, Sen CK. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A. 2008;105:14100–14105. doi: 10.1073/pnas.0806678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rink C, Christoforidis G, Khanna S, Peterson L, Patel Y, Khanna S, Abduljalil A, Irfanoglu O, Machiraju R, Bergdall VK, Sen CK. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab. 2011;31:2218–2230. doi: 10.1038/jcbfm.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Mercado R, Ford GD, Xu Z, Kraiselburd EN, Martinez MI, Eterović VA, Colon E, Rodriguez IV, Portilla P, Ferchmin PA, Gierbolini L, Rodriguez-Carrasquillo M, Powell MD, Pulliam JV, McCraw CO, Gates A, Ford BD. Acute neuronal injury and blood genomic profiles in a nonhuman primate model for ischemic stroke. Comp Med. 2012;62:427–438. [PMC free article] [PubMed] [Google Scholar]
- 103.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 104.Roth G, Dicke U. Evolution of the brain and intelligence in primates. Prog Brain Res. 2012;195:413–430. doi: 10.1016/B978-0-444-53860-4.00020-9. [DOI] [PubMed] [Google Scholar]
- 105.Sahin B, Unal B, Canan S, Bilgic S, Kaplan S, Tumkaya L. Brain volumes of the lamb rat and bird do not show hemispheric asymmetry: a stereological study. Image Anal Stereol. 2001;20:9–13. [Google Scholar]
- 106.Sakoh M, Ostergaard L, Røhl L, Smith DF, Simonsen CZ, Sørensen JC, Poulsen PV, Gyldensted C, Sakaki S, Gjedde A. Relationship between residual cerebral blood flow and oxygen metabolism as predictive of ischemic tissue viability: sequential multitracer positron emission tomography scanning of middle cerebral artery occlusion during the criticalfirst 6 hours after stroke in pigs. J Neurosurg. 2000a;93:647–657. doi: 10.3171/jns.2000.93.4.0647. [DOI] [PubMed] [Google Scholar]
- 107.Sakoh M, Rohl L, Gyldensted C, Gjedde A, Ostergaard L. Cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking after acute stroke in pigs: comparison with [(15)O]H(2)O positron emission tomography. Stroke. 2000b;31:1958–1964. doi: 10.1161/01.str.31.8.1958. [DOI] [PubMed] [Google Scholar]
- 108.Sanák D, Nosál’ V, Horák D, Bártková A, Zelenák K, Herzig R, Bucil J, Skoloudík D, Burval S, Cisariková V, Vlachová I, Köcher M, Zapletalová J, Kurca E, Kanovský P. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 109.Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 110.Shaibani A, Khawar S, Shin W, Cashen TA, Schirf B, Rohany M, Kakodkar S, Carroll TJ. First results in an MR imaging--compatible canine model of acute stroke. AJNR Am J Neuroradiol. 2006;27:1788–1793. [PMC free article] [PubMed] [Google Scholar]
- 111.Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab. 2003;23:1479–1488. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sicard KM, Fisher M. Animal models of focal brain ischemia. Exp Transl Stroke Med. 2009:1–7. doi: 10.1186/2040-7378-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simonyan K. Recent advances in understanding the role of the basal ganglia. F1000Res. 2019 doi: 10.12688/f1000research.16524.1. doi: 1012688/f1000research165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, Jonkers I. Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86:1007–1013. doi: 10.1016/j.apmr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Sorby-Adams AJ, Leonard AV, Elms LE, Marian OC, Hoving JW, Yassi N, Vink R, Thornton E, Turner RJ. Determining the temporal profile of intracranial pressure changes following transient stroke in an ovine model. Front Neurosci. 2019:13–587. doi: 10.3389/fnins.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sorby-Adams AJ, Vink R, Turner RJ. Large animal models of stroke and traumatic brain injury as translational tools. Am J Physiol Regul Integr Comp Physiol. 2018;315:165–190. doi: 10.1152/ajpregu.00163.2017. [DOI] [PubMed] [Google Scholar]
- 117.Spetzler RF, Zabramski JM, Kaufman B, Yeung HN. Acute NMR changes during MCA occlusion: a preliminary study in primates. Stroke. 1983;14:185–191. doi: 10.1161/01.str.14.2.185. [DOI] [PubMed] [Google Scholar]
- 118.Srikanth V, Beare R, Blizzard L, Phan T, Stapleton J, Chen J, Callisaya M, Martin K, Reutens D. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40:175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 119.Stroke Therapy Academic Industry Roundtable STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 120.Symon L. Observations on the leptomeningeal collateral circulation in dogs. J Physiol. 1960;154:1–14. doi: 10.1113/jphysiol.1960.sp006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tagaya M, Liu KF, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka Y, Imai H, Konno K, Miyagishima T, Kubota C, Puentes S, Aoki T, Hata H, Takata K, Yoshimoto Y, Saito N. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke. 2008;39:205–212. doi: 10.1161/STROKEAHA.107.489906. [DOI] [PubMed] [Google Scholar]
- 123.Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S, Tarkowski A. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26:1393–1398. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 124.Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S, Tarkowski A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Traversa R, Cicinelli P, Oliveri M, Giuseppina Palmieri M, Filippi MM, Pasqualetti P, Rossini PM. Neurophysiological follow-up of motor cortical output in stroke patients. Clin Neurophysiol. 2000;111:1695–1703. doi: 10.1016/s1388-2457(00)00373-4. [DOI] [PubMed] [Google Scholar]
- 126.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 127.Treadwell SD, Thanvi B. Malignant middle cerebral artery (MCA) infarction: pathophysiology, diagnosis and management. Postgrad Med J. 2010;86:235–242. doi: 10.1136/pgmj.2009.094292. [DOI] [PubMed] [Google Scholar]
- 128.van der Bom IM, Mehra M, Walvick RP, Chueh JY, Gounis MJ. Quantitative evaluation of C-arm CT cerebral blood volume in a canine model of ischemic stroke. AJNR Am J Neuroradiol. 2012;33:353–358. doi: 10.3174/ajnr.A2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Everdingen KJ, van der Grond J, Kappelle LJ, Ramos LM, Mali WP. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998;29:1783–1790. doi: 10.1161/01.str.29.9.1783. [DOI] [PubMed] [Google Scholar]
- 130.Veerbeek JM, Koolstra M, Ket JC, van Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in thefirst 6 months after stroke: a meta-analysis. Stroke. 2011;42:3311–3315. doi: 10.1161/STROKEAHA.111.623819. [DOI] [PubMed] [Google Scholar]
- 131.Walberer M, Blaes F, Stolz E, Muller C, Schoenburg M, Tschernatsch M, Bachmann G, Gerriets T. Midline-shift corresponds to the amount of brain edema early after hemispheric stroke--an MRI study in rats. J Neurosurg Anesthesiol. 2007;19:105–110. doi: 10.1097/ANA.0b013e31802c7e33. [DOI] [PubMed] [Google Scholar]
- 132.Wang DZ, Nair DS, Talkad AV. Acute decompressive hemicraniectomy to control high intracranial pressure in patients with malignant MCA ischemic strokes. Curr Treat Options Cardiovasc Med. 2011;13:225–232. doi: 10.1007/s11936-011-0121-1. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Prog Neurobiol. 2016;141:45–60. doi: 10.1016/j.pneurobio.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996;16:53–59. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 135.Watanabe H, Andersen F, Simonsen CZ, Evans SM, Gjedde A, Cumming P DaNeX Study Group. MR-based statistical atlas of the Gottingen minipig brain. Neuroimage. 2001;14:1089–1096. doi: 10.1006/nimg.2001.0910. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe H, Sakoh M, Andersen F, Rodell A, Sørensen JC, Østergaard L, Mouridsen K, Cumming P. Statistical mapping of effects of middle cerebral artery occlusion (MCAO) on blood flow and oxygen consumption in porcine brain. J Neurosci Methods. 2007;160:109–115. doi: 10.1016/j.jneumeth.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 137.Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wells AJ, Vink R, Blumbergs PC, Brophy BP, Helps SC, Knox SJ, Turner RJ. A surgical model of permanent and transient middle cerebral artery stroke in the sheep. PLoS One. 2012;7:e42157. doi: 10.1371/journal.pone.0042157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wells AJ, Vink R, Helps SC, Knox SJ, Blumbergs PC, Turner RJ. Elevated intracranial pressure and cerebral edema following permanent MCA occlusion in an ovine model. PLoS One. 2015;10:e0130512. doi: 10.1371/journal.pone.0130512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.West GA, Golshani KJ, Doyle KP, Lessov NS, Hobbs TR, Kohama SG, Pike MM, Kroenke CD, Grafe MR, Spector MD, Tobar ET, Simon RP, Stenzel-Poore MP. A new model of cortical stroke in the rhesus macaque. J Cereb Blood Flow Metab. 2009;29:1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wey HY, Kroma GM, Li J, Leland MM, Jones L, Duong TQ. MRI of perfusion-diffusion mismatch in non-human primate (baboon) stroke: a preliminary report. Open Neuroimag J. 2011a;5:147–152. doi: 10.2174/1874440001105010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wey HY, Wang DJ, Duong TQ. Baseline CBF and BOLD CBF and CMRO2 fMRI of visual and vibrotactile stimulations in baboons. J Cereb Blood Flow Metab. 2011b;31:715–724. doi: 10.1038/jcbfm.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu D, Chen J, Wang B, Zhang M4, Shi J, Ma Y, Zhu Z, Yan F, He X, Li S, Dornbos Iii D, Ding Y, Ji X. Endovascular ischemic stroke models of adult rhesus monkeys: a comparison of two endovascular methods. Sci Rep. 2016;6:31608. doi: 10.1038/srep31608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yakovlev SA, Rublenko MV, Izdepsky VI, Makogonenko EM. Activating effect of the plasminogen activators on plasminogens of different mammalia species. Thromb Res. 1995;79:423–428. doi: 10.1016/0049-3848(95)00131-a. [DOI] [PubMed] [Google Scholar]
- 145.Zhang X, Tong F, Li CX, Yan Y, Kempf D, Nair G, Wang S, Muly EC, Zola S, Howell L. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS One. 2015a;10 doi: 10.1371/journal.pone.0117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y, Jin M, Du B, Lin H, Xu C, Jiang W, Jia J. A novel canine model of acute vertebral arAtery occlusion. PLoS One. 2015b;10 doi: 10.1371/journal.pone.0142251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zu QQ, Liu S, Xu XQ, Lu SS, Sun L, Shi HB. An endovascular canine stroke model: middle cerebral artery occlusion with autologous clots followed by ipsilateral internal carotid artery blockade. Lab Invest. 2013;93:760–767. doi: 10.1038/labinvest.2013.65. [DOI] [PubMed] [Google Scholar]