Keywords: caspase, cell apoptosis, cobalt chloride, Lycium barbarum polysaccharides, mitochondrial membrane potential, oxidative stress injury, reactive oxygen species, retinal ganglion cells
Abstract
The accumulation of excessive reactive oxygen species can exacerbate any injury of retinal tissue because free radicals can trigger lipid peroxidation, protein damage and DNA fragmentation. Increased oxidative stress is associated with the common pathological process of many eye diseases, such as glaucoma, diabetic retinopathy and ischemic optic neuropathy. Many studies have demonstrated that Lycium barbarum polysaccharides (LBP) protects against oxidative injury in numerous cells and tissues. For the model of hypoxia we used cultured retinal ganglion cells and induced hypoxia by incubating with 200 µM cobalt chloride (CoCl2) for 24 hours. To investigate the protective effect of LBP and its mechanism of action against oxidative stress injury, the retinal tissue was pretreated with 0.5 mg/mL LBP for 24 hours. The results of flow cytometric analysis showed LBP could effectively reduce the CoCl2-induced retinal ganglion cell apoptosis, inhibited the generation of reactive oxygen species and the reduction of mitochondrial membrane potential. These findings suggested that LBP could protect retinal ganglion cells from CoCl2-induced apoptosis by reducing mitochondrial membrane potential and reactive oxygen species.
Chinese Library Classification No. R453.9; R774.1; Q539
Introduction
Increased oxidative stress is associated with the common pathological process of many eye diseases, such as glaucoma, diabetic retinopathy and ischemic optic neuropathy (Gaydar et al., 2011; Tarr et al., 2013; Tanito et al., 2016). Glaucoma is considered a degenerative and progressive optic neuropathy that causes damage to the optic nerve and retinal ganglion cells (RGCs) (Zmijewski and Slominski, 2011; Agilan et al., 2016; Sharf, 2018; Adocnetto et al., 2019). A study confirmed that RGC apoptosis and optic nerve axon degeneration caused by ischemia, oxidative stress and inflammatory response are important causes of the occurrence and development of glaucoma (Chen and Zhao, 2017). The accumulation of excessive reactive oxygen species (ROS) can exacerbate the injury of retinal tissue because free radicals can cause lipid peroxidation, protein damage and DNA fragmentation (Forman, 2016).
Lycium barbarum polysaccharides (LBP), extracted from Lycium barbarum fruit, is thought to be the main component responsible for its biological activities (Amagase et al., 2009). Based on the antioxidant activity of LBP, many studies have demonstrated that LBP has protective effect against oxidative injury in various cells and tissues (Yang et al., 2017; Li et al., 2018; Niu et al., 2018; Huang et al., 2019; Yu et al., 2019). In our previous studies, we confirmed its protective effect on retinal pigment epithelial cells (Liu et al., 2015). Cobalt can cause oxidative stress by rupturing the outer cell membrane and disturbing mitochondrial respiration. Cobalt chloride acts as an hypoxia mimicking agent and is commonly used for the induction of neurodegeneration in different models (Grasselli et al., 2005; Caltana et al., 2009; del Olmo-Aguado et al., 2013; Zimmerman et al., 2018; Cheng et al., 2019).
In the present study, we assessed the ability of LBP to protect RGC-5 cells from cobalt chloride (CoCl2) damage in an in vitro oxidative stress model (Chavez and LaManna, 2002; Chowdhury et al., 2008; Ohtomo et al., 2008) and analyzed its effects on cell damage and apoptosis and their mechanism in vitro.
Materials and Methods
Cell culture
The cell line RGC-5 was provided by Jinan University, Guangzhou, China. The rat RGC-5 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen) 1 g/L glucose, 1% antibiotic solution (100 U/mL penicillin and 100 μg/mL streptomycin; Invitrogen) in the presence of 95% air and 5% CO2 at 37°C. When grown to 80–85% confluence, the cells were collected for different assays.
CoCl2-induced oxidative damage model
Cell counting kit-8 (CCK-8; Transgen, Beijing, China) was used in to determine the cell viability of RGCs. The RGC-5 cells were seeded into 96-well plates with six replicates for each group. When grown to 80–85% confluence, cells were treated with different concentrations of CoCl2 (St. Louis, MO, USA) (0, 50, 100, 200, 400, and 800 µM, respectively). After 24 hours of incubation, the cells were incubated with 10 μL of CCK-8 solution for 2 hours at 37°C and measured by Microplate reader (BioTek, Winooski, VT, USA) at 450 nm optical density (Liu et al., 2015).
LBP intervention
LBPs are a group of water-soluble polysaccharides, including rhamnose, fructose, arabinose, galactose, and galacturonic acid, with a molecular weight of 10–2300 kDa. LBPs were provided and identified by Professor Kwok-fai So. The preparation for LBP was the same as reported previously (Liu et al., 2015). To determine the optimal and safe concentration of LBP, RGC-5 cells were seeded into 96-well plates with six replicates for each group. When grown to 80–85% confluence, cells were treated with different concentrations of LBP (0, 0.01, 0.05, 0.1, 0.5, 1.5 or 10 mg/mL, respectively) for 24 hours. Then the cells were exposed to 200 µM CoCl2 and incubated for 24 hours. After that, the cells were incubated with 10 μL of CCK-8 solution for 2 hours at 37°C and their optical density was measured at 450 nm.
Cell apoptosis analysis
To quantify apoptosis cells, Annexin V and propidium iodide staining were used. Briefly, RGC-5 cells were grown on a six-well plate at 2 × 105 cells per plate and incubated with or without 0.5 mg/mL LBP for 24 hours before 200 µM CoCl2 treatment. Thereafter, cells were harvested and stained with Annexin V-Fluorescein and propidine iodide in a binding buffer for 20 minutes. The fluorescence was measured by flow cytometry (BD Biosciences, Becton, NJ, USA). The percentage of early apoptosis (cells in Q3 area) and total apoptosis cell was calculated by flow cytometry.
Detection of intracellular ROS
To investigate the intracellular ROS generation in RGC-5 cells, RGC-5 cells were treated with CoCl2 (200 μM) for 24 hours, the experimental group was preincubated with LBP (0.5 mg/mL) for 24 hours. Cells were resuspended with 200 μL diluted 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (10 μM) and incubated for 20 minutes. After washing, cells were harvested and the average fluorescence intensity of dichlorofluorescein (DCF) was measured by flow cytometry (BD Biosciences). The excitation wavelength was set to 488 nm and the emission wavelength was set to 525 nm.
Measurement of mitochondria transmembrane potential
In this assay, cells were incubated with 0.5 mg/mL LBP for 24 hours, then exposed to 200 μM CoCl2 for 24 hours (except the control without CoCl2). Cells were incubated with 200 μL 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) (BD Biosciences Pharmingen, San Diego, CA, USA) for 15 minutes at 37°C away from the light, then rapidly washed twice with buffer solution. The fluorescence intensity of JC-1 monomer and JC-1 polymer was measured by flow cytometry. JC-1 is an ideal fluorescent probe widely used for mitochondrial membrane potential (MMP) detection. When MMP is higher, JC-1 forms aggregates in the mitochondrial matrix and emits red fluorescence; while when MMP is lower, JC-1 exists as a monomer and produces a green fluorescence. Therefore, the change of fluorescence color can be used to reflect the change of MMP, and the decline of MMP is a marker event in the early stages of apoptosis, so the conversion of JC-1 from red fluorescence to green fluorescence can also be used as an indicator for detection of early apoptosis (Chinopoulos et al., 1999).
Statistical analysis
Data were analyzed using the SPSS 19.0 (IBM, Armonk, NY, USA). Data were presented as the mean ± SD. For comparison of the different groups, statistical comparisons were performed by one-way analysis of variance and Student-Newman-Keuls test. Differences were considered significant at P < 0.05.
Results
Effect of CoCl2 on cell viability in RGC-5 cells
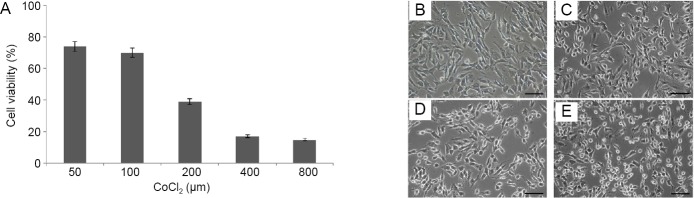
We assessed the effect of CoCl2 on RGC-5 cell viability using the CCK-8 assay. Cell viability was reduced with increasing concentrations of CoCl2, when incubated for 24 hours (Figure 1). There was an approximate 50% decrease in cell viability using 200 μM CoCl2. We chose this concentration for all subsequent treatments in this study.
Figure 1.
Cobalt chloride (CoCl2) reduces the survival of RGC-5 cells in a concentration-dependent manner.
(A) Cell viability of RGC-5 cells was detected by Cell counting kit-8. Data were presented as the mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. (B–E) As the concentration of CoCl2 increased, the cells shriveled, their boundaries blurred, the junctions between cells were loosened and obvious cell fragmentation could be seen. (B–E) Control (0 μM CoCl2), 100, 200, 400 μM CoCl2 group, respectively. Scale bars: 200 μm.
The optimal concentration of LBP for apoptosis induction
Cells were treated with seven different concentrations of LBP for 24 hours and then incubated with 200 μM CoCl2 for 24 hours. The viability of cells was assessed by CCK-8. As shown in Figure 2, the cells treated with more than 0.1 mg/mL LBP improved their viability and 0.5 mg/mL of LBP was determined to give the optimal protection against induced apoptosis.
Figure 2.
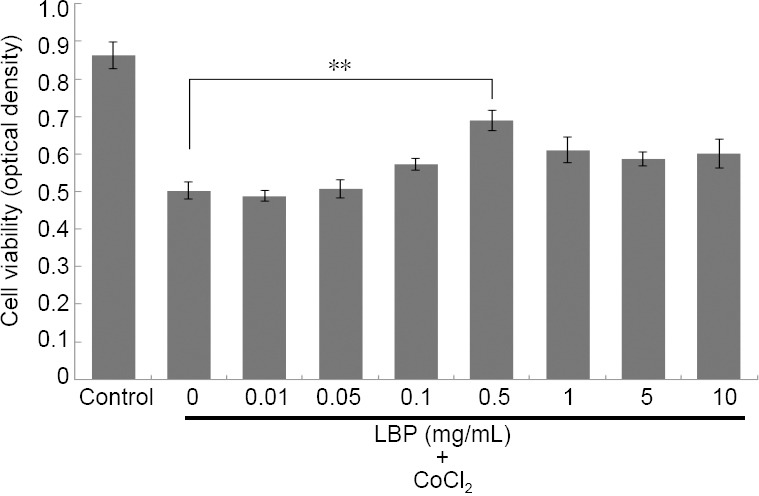
Lycium barbarum polysaccharides (LBP) increases the viability of cobalt chloride (CoCl2) treated RGC-5 cells.
Cells were treated with different concentrations of LBP for 24 hours and then incubated with 200 μM of CoCl2 for 24 hours. Data were presented as the mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. **P < 0.01.
Protective effect of LBP on CoCl2-induced cell apoptosis
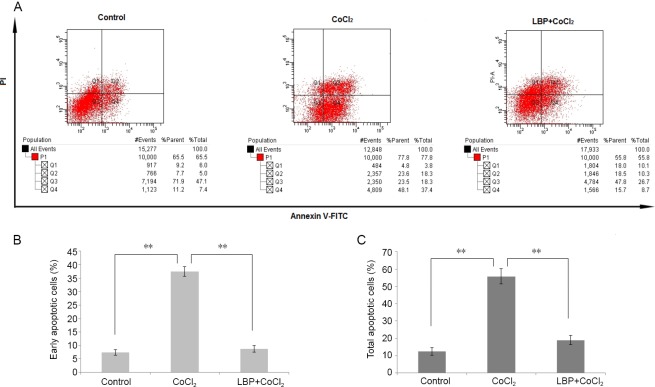
We also evaluated the effect of LBP on CoCl2 induced cell apoptosis using flow cytometry (Figure 3A). As shown in Figure 3C, CoCl2 increased the number of apoptotic cells from 12.45 ± 0.18% to 55.72 ± 4.39% (P < 0.01). In contrast, the LBP group significantly decreased the CoCl2 induced apoptotic proportion (P < 0.01). The early apoptotic rate of RGC-5 pretreated with LBP for 24 hours was significantly lower than that in the CoCl2 group (Figure 3B).
Figure 3.
Lycium barbarum polysaccharides (LBP) inhibits apoptosis of RGC-5 cells induced by cobalt chloride (CoCl2).
Cells were treated with LBP (0.5 mg/mL) for 24 hours and then incubated with 200 μM of CoCl2 for 24 hours. Apoptosis in RGC-5 cells treated with LBP was analyzed by flow cytometry. The cells were stained with enhanced green fluorescent protein-conjugated Annexin V and propidium iodide. The enhanced green fluorescent protein and PI fluorescence was measured using flow cytometer with Fluorescein-A and propidium iodide-A filter. (A) Representative dot plots of Annexin V/PI staining. The lower left quadrant contains the vital (double negative) population. (B) Quantitative result of early apoptotic cells. (C) Quantitative result of total apoptotic cells. Data were presented as the mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. **P < 0.01.
LBP pretreatment prevents CoCl2-induced oxidative stress
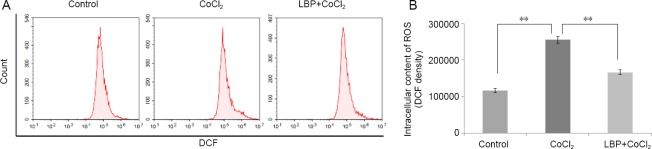
We investigated whether the protective effects of LBP were related to its ability to inhibit CoCl2-induced oxidative stress in vitro. The degree of oxidative stress generated by CoCl2 was determined by measuring the intracellular content of ROS. As shown in Figure 4, CoCl2 increased intracellular ROS production 2-fold over that of the control, which was in turn lessened by LBP pretreatment (P < 0.01).
Figure 4.
Inhibition by Lycium barbarum polysaccharides (LBP) on the increase of reactive oxygen species (ROS) in RGC-5 cells induced by cobalt chloride.
Cells were treated with LBP (0.5 mg/mL) for 24 hours and then incubated with 200 μM of CoCl2 for 24 hours. (A) Flow cytometry to detect dichlorofluorescein (DCF) fluorescence intensity; (B) DCF fluorescence intensity chart. Data were presented as the mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. **P < 0.01.
CoCl2 and LBP alter the mitochondrial transmembrane potential of RGC-5 cells
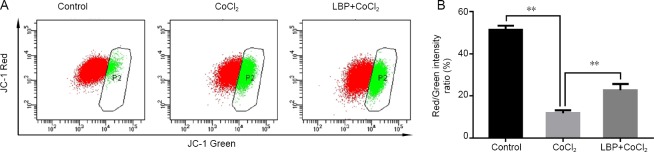
Mitochondrial transmembrane potential, as measured by the ratio of JC-1 red/green fluorescence intensity, in the CoCl2 injury group was significantly lower than that in the control group (Figure 5). The ratio of JC-1 red/green fluorescence intensity in LBP pretreated group was twice as high as the CoCl2 group, which meant that LBP can inhibit the decrease of MMP induced by CoCl2 in RGC-5 cells, thereby reducing the early apoptosis of cells (P < 0.01).
Figure 5.
Inhibitory effect of Lycium barbarum polysaccharides (LBP) on mitochondrial membrane potential reduction induced by cobalt chloride (CoCl2) in RGC-5 cells.
Cells were treated with LBP (0.5 mg/mL) for 24 hours and then incubated with 200 μM of CoCl2 for 24 hours. Their mitochondrial membrane potential was evaluated by JC-1 red/green fluorescence intensity. Data were presented as the mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. **P < 0.01.
Discussion
The main constituents of wolfberries include LBP, scopoletin, and 2-O-β-D-glucopyranosyl-L-ascorbic acid (Chen et al., 2014). The polysaccharide LBP, the main effective ingredient of Lycium barbarum, isolated from the aqueous extracts of Lycium barbarum, consists of six monosaccharides; glucose, arabinose, galactose, mannose, rhamnose, and xylose (Cheng et al., 2015). Published studies have associated LBP intake with a number of therapeutic effects, including antiaging (Deng et al., 2003), metabolic effects (Luo et al., 2004), neuroprotective effects in neurodegeneration (Ho et al., 2007) and neurotoxicity (Ho et al., 2009), including ocular neuroprotective effects (Chiu et al., 2009). The neuroprotective effects of LBP are some of the most widely studied fields regarding LBP’s therapeutic effects. Yu and coworkers (Yu et al., 2018) used an in vitro model to study the effects of LBP in oxygen glucose deprived/reperfused primary hip-pocampal mice neuron cells and found that LBP significantly increased cell viability, reduced lactate dehydrogenase levels and reduced ROS in a dose-dependent manner. Others demonstrated LBP’s ability to protect retinal neurons and could be used to prevent or slow down the progression of diseases such as diabetic retinopathy, glaucoma, and retinopathy of prematurity (Yang et al., 2017). The latest LBP chemical component analysis demon-strated that all glycopeptides in LBP act to eliminate lipid peroxidation (Varoni et al., 2017; Chen et al., 2018; Schoppet et al., 2018). Thus, it was speculated that as an antioxidant, LBP may decrease cell injury induced by oxygen free radicals and protect cell development and differentiation.
Oxidative stress damage is a major cause of nerve damage, and the retina is very sensitive to oxidative stress (Chen and Zhao, 2017; Zhao et al., 2019). When damage occurs, such as ischemia-reperfusion or glaucoma, ROS will exceed the maximum capacity of the endogenous antioxidant system and result in its accumulation and destructive action on macromolecules, cells and tissues. (Osborne et al., 2004; Tezel, 2006; Kupsco and Schlenk, 2015). Mitochondria are the main subcellular structures for oxidative respiration and the most important source of endogenous ROS production. Hypoxia can inhibit the mitochondrial inner membrane electron transport chain, reduce the MMP and cause an increase in mitochondrial membrane permeability, leading to the release of pro-apoptotic factors and ROS into the cytoplasm (Marques et al., 2017). At the same time, ROS can cause an increase of mitochondrial membrane permeability, so ROS and mitochondrial membrane permeability affect each other. In our experiments, CoCl2 caused a significant decrease of MMP in RGC-5 cells. After pretreating with LBP, cells significantly increased the level of MMP. The level of intracellular ROS was significantly inhibited when LBP was administered beforehand.
In summary, we constructed an CoCl2-injured RGC-5 cell model to simulate the oxidative stress damage of RGCs in glaucoma and found LBP can prevent RGC-5 cells apoptosis induced by CoCl2. Regulation of MMP and lowering ROS levels may be the underlying mechanism by which LBP protects RGC-5 cells from oxidative injury. This study used hypoxia induced in cultured retinal cells as the model. Further research in vitro might use isolated retinas. In later stages of research, the therapeutic effect should be studied in an animal model to provide the basis of a new treatment for glaucoma patients in the clinic.
Acknowledgments
The authors expressed their thanks to Professor Kwok-fai So and Da-Xiang Lu (Jinan University, Guangzhou, China) for providing LBP and cell line RGC-5.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Financial support: This work was supported by grants from Project of Administration of Traditional Chinese Medicine of Guangdong Province of China, No. 20161071 (to LL), and Medical Scientific Research Foundation of Guangdong Province of China, No. A2019098 (to LL). All authors declare that the financial supports did not affect the paper’s views and statistical analysis of the objective results of the research data and their reports.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by grants from Project of Administration of Traditional Chinese Medicine of Guangdong Province of China, No. 20161071 (to LL), and Medical Scientific Research Foundation of Guangdong Province of China, No. A2019098 (to LL).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Adornetto A, Russo R, Parisi V. Neuroinflammation as a target for glaucoma therapy. Neural Regen Res. 2019;14:391–394. doi: 10.4103/1673-5374.245465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agilan B, Rajendra Prasad N, Kanimozhi G, Karthikeyan R, Ganesan M, Mohana S, Velmurugan D, Ananthakrishnan D. Caffeic acid inhibits chronic UVB-induced cellular proliferation through JAK-STAT3 signaling in mouse skin. Photochem Photobiol. 2016;92:467–474. doi: 10.1111/php.12588. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Caltana L, Merelli A, Lazarowski A, Brusco A. Neuronal and glial alterations due to focal cortical hypoxia induced by direct cobalt chloride (CoCl2) brain injection. Neurotox Res. 2009;15:348–358. doi: 10.1007/s12640-009-9038-9. [DOI] [PubMed] [Google Scholar]
- 5.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Li W, Qi D, Wang D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis oxidative stress and inflammation in pulmonary endothelial cells. Free Radic Res. 2018;52:480–490. doi: 10.1080/10715762.2018.1447105. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, Qin J, Tian M, Jin G, Zhang X. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9:e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhao Y. Diagnostic performance of isolated-check visual evoked potential versus retinal ganglion cell-inner plexiform layer analysis in early primary open-angle glaucoma. BMC Ophthalmol. 2017;17:77. doi: 10.1186/s12886-017-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, Sun T, Zhang X, Zhao RJ, Gu L, Cao C, Zhou SF. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2015;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Z, Yao W, Zheng J, Ding W, Wang Y, Zhang T, Zhu L, Zhou F. A derivative of betulinic acid protects human retinal pigment epithelial (RPE) cells from cobalt chloride-induced acute hypoxic stress. Exp Eye Res. 2019;180:92–101. doi: 10.1016/j.exer.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiu K, Chan HC, Yeung SC, Yuen WH, Zee SY, Chang RC, So KF. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J Ocul Biol Dis Infor. 2009;2:47–56. doi: 10.1007/s12177-009-9023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury R, Hardy A, Schofield CJ. The human oxygen sensing machinery and its manipulation. Chem Soc Rev. 2008;37:1308–1319. doi: 10.1039/b701676j. [DOI] [PubMed] [Google Scholar]
- 14.del Olmo-Aguado S, Núñez-Álvarez C, Ji D, Manso AG, Osborne NN. RTP801 immunoreactivity in retinal ganglion cells and its down-regulation in cultured cells protect them from light and cobalt chloride. Brain Res Bull. 2013;98:132–144. doi: 10.1016/j.brainresbull.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Deng HB, Cui DP, Jiang JM, Feng YC, Cai NS, Li DD. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on nonenzyme glycation in D-galactose induced mouse aging model. Biomed Environ Sci. 2003;16:267–275. [PubMed] [Google Scholar]
- 16.Forman HJ. Redox signaling: An evolution from free radicals to aging. Free Radic Biol Med. 2016;97:398–407. doi: 10.1016/j.freeradbiomed.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaydar V, Ezrachi D, Dratviman-Storobinsky O, Hofstetter S, Avraham-Lubin BC, Goldenberg-Cohen N. Reduction of apoptosis in ischemic retinas of two mouse models using hyperbaric oxygen treatment. Invest Ophthalmol Vis Sci. 2011;52:7514–7522. doi: 10.1167/iovs.11-7574. [DOI] [PubMed] [Google Scholar]
- 18.Grasselli F, Basini G, Bussolati S, Bianco F. Cobalt chloride a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod Fertil Dev. 2005;17:715–720. doi: 10.1071/rd05059. [DOI] [PubMed] [Google Scholar]
- 19.Ho YS, Yu MS, Lai CS, So KF, Yuen WH, Chang RC. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain Res. 2007;1158:123–134. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Ho YS, Yu MS, Yik SY, So KF, Yuen WH, Chang RC. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell Mol Neurobiol. 2009;29:1233–1244. doi: 10.1007/s10571-009-9419-x. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Zhou F, Shen C, Wang H, Xiao Y. LBP reduces theinflammatory injuryof kidney in septic rat and regulates the Keap1-Nrf2ARE signaling pathway1. Acta Cir Bras. 2019;34:e20190010000003. doi: 10.1590/s0102-865020190010000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupsco A, Schlenk D. Oxidative stress unfolded protein response and apoptosis in developmental toxicity. Int Rev Cell Mol Biol. 2015;317:1–66. doi: 10.1016/bs.ircmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ding Z, Yang Y, Mao B, Wang Y, Xu X. Lycium barbarum polysaccharides protect human trophoblast HTR8/SVneo cells from hydrogen peroxideinduced oxidative stress and apoptosis. Mol Med Rep. 2018;18:2581–2588. doi: 10.3892/mmr.2018.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Lao W, Ji QS, Yang ZH, Yu GC, Zhong JX. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int J Ophthalmol. 2015;8:11–16. doi: 10.3980/j.issn.2222-3959.2015.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 26.Marques AP, Rosmaninho-Salgado J, Estrada M, Cortez V, Nobre RJ, Cavadas C. Hypoxia mimetic induces lipid accumulation through mitochondrial dysfunction and stimulates autophagy in murine preadipocyte cell line. Biochim Biophys Acta Gen Subj. 2017;1861:673–682. doi: 10.1016/j.bbagen.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Niu T, Jin L, Niu S, Gong C, Wang H. Lycium barbarum polysaccharides alleviates oxidative damage induced by H2O2 through down-regulating microRNA-194 in PC-12 and SH-SY5Y cells. Cell Physiol Biochem. 2018;50:460–472. doi: 10.1159/000494159. [DOI] [PubMed] [Google Scholar]
- 28.Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou C, Miyata T. Cobalt ameliorates renal injury in an obese hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 29.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Schoppet M, Tailhades J, Kulkarni K, Cryle MJ. Precursor manipulation in glycopeptide antibiotic biosynthesis: are beta-amino acids compatible with the oxidative cyclization cascade? J Org Chem. 2018;83:7206–7214. doi: 10.1021/acs.joc.8b00418. [DOI] [PubMed] [Google Scholar]
- 31.Sharif NA. Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics techniques and tools to help preserve vision. Neural Regen Res. 2018;13:1145–1150. doi: 10.4103/1673-5374.235017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanito M, Kaidzu S, Takai Y, Ohira A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci Rep. 2016;6:25792. doi: 10.1038/srep25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013. 2013 doi: 10.1155/2013/343560. 343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varoni MV, Pasciu V, Gadau SD, Baralla E, Serra E, Palomba D, Demontis MP. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ Sci Pollut Res Int. 2017;24:2946–2955. doi: 10.1007/s11356-016-8050-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Exp Ophthalmol. 2017;45:717–729. doi: 10.1111/ceo.12950. [DOI] [PubMed] [Google Scholar]
- 37.Yu N, Song N, Liu CY, Yang GL. The estrogenlike protective effect of Lycium barbarum polysaccharides in reducing oxidative stress on myocardial cells from ovariectomized rats. Mol Med Rep. 2019;19:2271–2278. doi: 10.3892/mmr.2019.9880. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Wu X, Pu J, Luo P, Ma W, Wang J, Wei J, Wang Y, Fei Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem Biophys Res Commun. 2018;495:1187–1194. doi: 10.1016/j.bbrc.2017.11.165. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Kuang F, You YY, Wu MM, You SW. Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection. Neural Regen Res. 2019;14:2020–2024. doi: 10.4103/1673-5374.259627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman MA, Biggers CD, Li PA. Rapamycin treatment increases hippocampal cell viability in an mTOR-independent manner during exposure to hypoxia mimetic, cobalt chloride. BMC Neurosci. 2018;19:82. doi: 10.1186/s12868-018-0482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zmijewski MA, Slominski AT. Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinol. 2011;3:3–10. doi: 10.4161/derm.3.1.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]