Keywords: acupuncture, basic fibroblast growth factor, brain cell protection, cerebral hemorrhage, electron microscope, glial cell line-derived neurotrophic factor, immunohistochemistry, Nestin, western blot assay
Abstract
Acupuncture is widely used in the treatment of cerebral hemorrhage, and it improves outcomes in experimental animal models and patients. However, the mechanisms underlying the effectiveness of acupuncture treatment for cerebral hemorrhage are still unclear. In this study, a model of intracerebral hemorrhage was produced by injecting 50 μL autologous blood into the caudate nucleus in Wistar rats. Acupuncture at Baihui (DU20) and Qubin (GB7) acupoints was performed at a depth of 1.0 inch, 12 hours after blood injection, once every 24 hours. The needle was rotated at 200 r/min for 5 minutes, For each 30-minute session, needling at 200 r/min was performed for three sessions, each lasting 5 minutes. For the positive control group, at 6 hours, and 1, 2, 3 and 7 days after induction of hemorrhage, the rats were intraperitoneally injected with 1 mL aniracetam (0.75 mg/mL), three times a day. The Bederson behavioral test was used to assess palsy in the contralateral limbs. Western blot assay was used to examine the expression levels of Nestin and basic fibroblast growth factor in the basal ganglia. Immunohistochemistry was performed to count the number of Nestin- and glial cell line-derived neurotrophic factor-positive cells in the basal ganglia. Acupuncture effectively reduced hemorrhage and brain edema, elevated the expression levels of Nestin and basic fibroblast growth factor in the basal ganglia, and increased the number of Nestin- and glial cell line-derived neurotrophic factor-positive cells in the basal ganglia. Together, these findings suggest that acupuncture promotes functional recovery after cerebral hemorrhage by increasing the expression of neurotrophic factors. The study was approved by the Committee for Experimental Animals of Heilongjiang Medical Laboratory Animal Center (approval No. 2017061001) on June 10, 2017.
Chinese Library Classification No. R454; R245; R741
Introduction
Nestin is a class VI intermediate filament protein that was originally described as a neuronal stem cell marker during central nervous system development. Nestin expression is increased in the adult during pathological conditions, such as the formation of the glial scar after central nervous system injury and during regeneration of injured muscle tissue (Ishiwata et al., 2011). Nestin, together with other structural proteins, participates in cell remodeling. The role of Nestin in cell dynamics, particularly structural organization, appears tightly regulated by phosphorylation, especially its incorporation into heterogeneous intermediate filaments with vimentin or α-internexin (Michalczyk and Ziman, 2005). Nestin is widely used as a marker of central nervous system progenitor cells (Michalczyk and Ziman, 2005).
Neurotrophic factors are proteins with trophic and neuroprotective functions in the central and peripheral nervous systems (Johnson et al., 2011). Neurotrophic factors include nerve growth factor, brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5. In addition, some researchers also consider glial cell line-derived neurotrophic factor (GDNF) a member of the neurotrophic factor family (Masure et al., 1999). GDNF, nerve growth factor and brain-derived neurotrophic factor (BDNF) play important roles in the regulation of neural ontogeny (Allen et al., 2013). These trophic factors participate in neuronal survival, nerve growth, development and differentiation, and help maintain neural function.
Acupuncture, an ancient traditional medical procedure originating in China more than 3000 years ago, has been used clinically and experimentally to treat cerebral hemorrhage (Wang et al., 2011; Zheng et al., 2011; Ko et al., 2013), and has become very popular worldwide as a complementary medical approach. Acupuncture treatment has been used for nervous system diseases, heart disease, neuropathic pains, cancer, asthma, polycystic ovary syndrome and psychological disorders (Lee et al., 2010; Li et al., 2018). Acupuncture is also widely used for the treatment of hemorrhagic stroke because it has been shown to improve outcomes in experimental animals as well as in the clinical setting (Chen, 2015). Aniracetam is a drug shown to protect the brain, improve brain function, enhance memory and learning abilities, and ameliorate memory impairment (Canonico et al., 1991). Therefore, aniracetam was used as a positive control in this study.
In this experiment, stereotactic injection of autologous blood was used to produce a rat model of intracerebral hemorrhage. Then, we investigated the effects of acupuncture treatment by examining changes in the expression of neurotrophic factors, Nestin, GDNF and basic fibroblast growth factor (bFGF) in the brain tissue.
Materials and Methods
Animals
The animal studies were approved by the Committee for Experimental Animals of Heilongjiang Medical Laboratory Animal Center, China (approval No. 2017061001) on June 10, 2017, and were performed according to the experimental animal management regulations of Heilongjiang Province of China. Precautions were taken to minimize the number of animals used and their suffering in each experiment.
Healthy specific-pathogen-free male Wistar rats (n = 160), 50 days of age and weighing 350 ± 20 g, were supplied by Weitong Lihua Animal Co., Ltd., Beijing, China (certification No. SCXK (Jing) 2007-0001). All rats were housed at 22 ± 2°C, with a humidity of 50 ± 5% and noise < 60 dB. The rats were randomly divided into control (n = 10), model (n = 50), acupuncture (n = 50) and aniracetam (n = 50) groups.
Establishment of a cerebral hemorrhage model
In accordance with a previous method (Rosenberg et al., 1990), rats were intraperitoneally anesthetized with 10% chloral hydrate (350 mg/kg) (Royalton, Dalian, Liaoning Province, China), and fixed in the prone position to a stereotaxic apparatus (STW-1, China Chengdu Instrument Factory, Chengdu, China). Rats were positioned with the anterior and posterior fontanelles in the same horizontal plane (determined by the upper incisor tooth hook plane being 2.4 mm lower than the plane between the ears). The scalp was shaved and sterilized, and a median incision, approximately 1 cm long, was made. The periosteum was stripped using a bone stripper, and the anterior fontanelle and coronal suture were exposed. A circular hole (1.0 mm diameter) was made using a dental drill (3.5 mm right of and 0.2 mm posterior to the bregma) until the dural surface was reached. The tail was disinfected with alcohol, and a 3-cm piece was cut from the caudal end. A microinjector, containing blood from the tail artery, was inserted approximately 6 mm into the hole. A 50-µL volume of the non-heparinized blood was infused into the caudate putamen at 25 µL/min, and the needle was maintained in place for 5 minutes. Gauze was used to bandage the tail wound. Finally, the skull wound was sealed with zinc phosphate cement (Shanghai Shangchi, Shanghai, China), and the scalp was sutured.
Bederson’s score (Bederson et al., 1986) was used to assess neurological function. Briefly, rats were lifted by their tails until they were 10 cm higher than the desktop. The paw of a normal rat was straight. Rats with a score of 1–3 were selected, and the intracranial hematoma was confirmed at the end of the experiment, indicating successful modeling of cerebral hemorrhage.
Acupuncture treatment
In the acupuncture group, rats received acupuncture 12 hours after induction of hematoma, and once every 24 hours thereafter. For the treatment, rats were fixed on the acupuncture board. Baihui (DU20) (specific location: head between the middle ears) and Qubin (GB7) (specific location: leading edge of the ear root) acupoints were needled on the affected side using a 1.3 inch stainless steel needle (Huatuo, Suzhou, Jiangsu Province, China) (Figure 1). The needling depth was 1.0 inch, and the needle was maintained in place for 30 minutes. During this time, the needle was twisted-rotated three times each for 5 minutes, at a speed of 200 cycles per minute.
Figure 1.
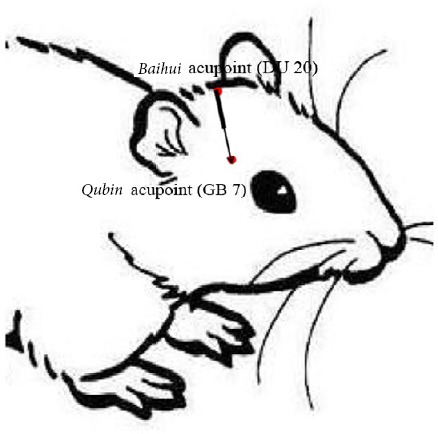
Baihui (DU20)-penetrating-Qubin (GB7) acupuncture treatment method.
Reprinted with permission from Zhang et al. (2018).
Drug administration
The rats were intragastrically administered 1 mL aniracetam (concentration: 0.75 mg/mL) (certification No. H20030229, Shanxi Yabao Pharmaceutical Group Co., Ltd., Shanxi Province, China) three times a day (at 6:00,14:00 and 22:00) starting at 6 hours, and 1, 2, 3 and 7 days after hematoma induction.
Neurological function evaluation
Five motor functional tests were performed at 6 hours, and 1, 2, 3 and 7 days after hematoma induction. The Bederson behavioral test was used to measure palsy of the contralateral limbs, and the score ranged from 0 (no palsy) to 3 points (circling to the contralateral side).
Specimen collection
The control group was only examined at one time point, 6 hours. Rats in the control group did not receive any treatment, and were directly decapitated for sample collection. In the model and aniracetam groups, samples were collected at 6 hours, and 1, 2, 3 and 7 days after hematoma induction. In the acupuncture group, specimens were collected at 6 hours, and 1, 2, 3 and 7 days after acupuncture.
Rats were intraperitoneally anesthetized with an overdose of chloral hydrate at the various time points after surgery. The chest was opened rapidly, and the heart was exposed. Catheterization from the left ventricle into the aortic root was performed. A small incision was made in the right atrial appendage for an exit hole. The ventricle was perfused with approximately 200 mL each of 4°C saline and 4% paraformaldehyde/0.1 M phosphate buffer. Samples were dehydrated, paraffin embedded, and then sliced into 6-µm-thick sections for hematoxylin-eosin staining and immunohistochemistry. Some rats were directly decapitated after anesthesia overdose, and basal ganglia tissue was immediately stored in liquid nitrogen at −80°C for western blot assay.
Hematoxylin-eosin staining
The basal ganglia/hematoma tissue was fixed in 10% (v/v) formalin, embedded in paraffin, and sliced into 2-mm-thick sections. The sections were heated at 65°C for 30 minutes, dewaxed in xylene for 20 minutes, immersed in alcohol for 10 minutes, rehydrated, and stained with hematoxylin for 3–5 minutes. After washing, the sections were immersed in 1% (v/v) hydrogen peroxide for 10–30 seconds, washed in water, treated with ammonia water for 10–30 seconds, and immersed in water for 5–10 minutes. Subsequently, the sections were stained with eosin for 20 minutes, washed with water, dehydrated with alcohol for 10 minutes, permeabilized in xylene for 10 minutes, mounted with neutral resin, and observed using a light microscope (Olympus Medical Systems, Beijing, China).
Western blot assay
Hematoma specimens were extracted using the RIPA lysis buffer protein BCA kit (Beyotime, Shanghai, China) to determine total protein concentration. Total protein loaded from each sample was 50 µg/well. Samples were electrophoresed and transferred to membranes. The membrane was blocked with 5% skim milk in phosphate/Tween buffer at 4°C overnight. Samples were incubated with primary antibody (mouse anti-rat Nestin monoclonal antibody, 1:200, Beyotime, Shanghai, China; mouse anti-rat basic fibroblast growth factor monoclonal antibody, 1:200, Beyotime) for 16–24 hours at 4°C. Membranes were then washed with Tris-buffered saline/Tween 20, and incubated in secondary antibody (rabbit anti-mouse IgG; 1:1000; Beyotime) for 1 hour at room temperature. Optical density values for specific bands were measured using ImageJ software (NIH, Bethesda, MD, USA). A gel imaging analysis system (ProteinSimple, Santa Clara, CA, USA) was used to determine the gray values of each sample band and that of the corresponding beta-actin band. The relative expression level of the target protein was calculated as the optical density of the target band/the optical density of the beta-actin band.
Immunohistochemistry
Nestin and GDNF expression in the area of the hematoma was determined by immunohistochemistry. After paraffin embedding and dehydration, sections were placed in 3% fresh H2O2 solution at 25°C for 10 minutes to block endogenous peroxidase, and then washed in 0.01 M phosphate-buffered saline, 3 × 2 minutes. Sections were immersed in 0.01 M citrate buffer (pH 6) and heated in the microwave for 2 × 5 minutes, with a 10 minute interval. After cooling to 25°C, sections were incubated with primary antibody (mouse anti-rat Nestin monoclonal antibody, 1:60, Santa Cruz Biotechnology, Santa Cruz, CA, USA; rabbit anti-GDNF polyclonal antibody, 1:80, Golden Bridge Biotechnology Co., Ltd., Beijing, China) overnight at 4°C, and washed with 0.01 M phosphate-buffered saline, 3 × 2 minutes. Sections were then incubated with secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG; 1:100; Golden Bridge Biotechnology Co., Ltd.) at 37°C for 30 minutes, washed with 0.01 M phosphate-buffered saline, 3 × 2 minutes, visualized with 3,3′-diaminobenzidine (Golden Bridge Biotechnology Co., Ltd.), counterstained with hematoxylin, mounted with neutral gum, and observed under an optical microscope (Olympus, Tokyo, Japan). Data were analyzed using Motic Med 6.0 pathological image analysis system (Motic Med Industrial Group Co., Ltd., Xiamen, China). Each section was randomly observed and counted at 400× magnification. Total positive cell number was calculated.
Statistical analysis
The data were analyzed using SPSS 17.0 (SPSS, Chicago, IL, USA). Measurement data are expressed as the mean ± SD. Comparisons among multiple groups were performed using one-way analysis of variance, based on the least significant difference. A value of P < 0.05 was considered statistically significant.
Results
Acupuncture increases the expression of neurotrophic factors around the hematoma in rats with cerebral hemorrhage
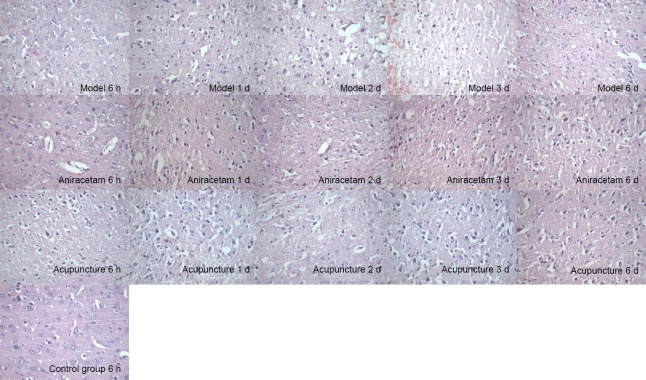
Hemorrhagic foci and brain edema were obvious in the model group. The types of pathological changes in the focal brain tissue in the acupuncture and aniracetam groups were similar to those in the model group; however, the degree of brain tissue damage was less in the acupuncture and aniracetam groups compared with the model group (Figure 2).
Figure 2.
Effect of acupuncture on brain histomorphology in rats with cerebral hemorrhage, assessed by hematoxylin-eosin staining.
In the control group, brain tissue was intact, and neurons and glial cells appeared normal (arrow). In the model group, brain tissue showed hemorrhagic foci at 6 hours and edema at 1 and 2 days, and vacuoles of different sizes were visible (arrows). Numbers of dema and vacuoles were the greatest at 3 days (arrows). The nuclei of the neurons around the focus were condensed and stained (arrows), and the edema around the 7-day hematoma tissue was less (arrows). In the acupuncture group, the brain tissue showed hemorrhagic foci at 1 day, but the cellular morphology appeared more normal (arrows). At 3 days, brain tissue around the hematoma appeared improved, the degree of edema was less (arrows), and the number of inflammatory cells was reduced (arrows). Brain edema had resolved at 7 days. Original magnification: 400×.
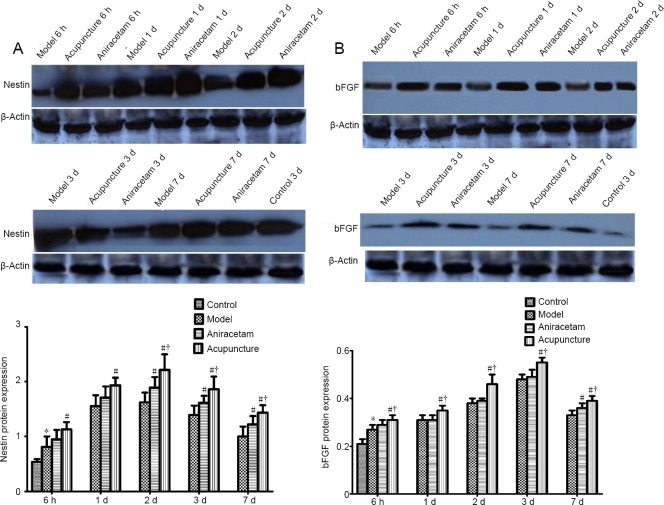
Acupuncture increases Nestin expression in the basal ganglia of rats with cerebral hemorrhage
Compared with the control group, Nestin protein expression was higher in the model group at the various time points after cerebral hemorrhage induction. Compared with the model group, Nestin protein expression was higher in the aniracetam group at 2, 3 and 7 days (P < 0.05). Compared with the model group, Nestin protein expression was higher in the acupuncture group at each time point (P < 0.05). Notably, compared with the aniracetam group, Nestin protein expression was higher in the acupuncture group at 2, 3 and 7 days (P < 0.05; Figure 3A).
Figure 3.
Effect of acupuncture on Nestin and bFGF protein expression in the basal ganglia of rats with cerebral hemorrhage (western blot assay).
The Y-axis shows the relative optical density values, and the X-axis shows each time point. Measurement data are expressed as the mean ± SD. Comparisons among multiple groups were performed using one-way analysis of variance followed by the least significant difference test. *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. aniracetam group. BFGF: Basic fibroblast growth factor.
Acupuncture increases bFGF expression in the basal ganglia of rats with cerebral hemorrhage
Compared with the control group, bFGF protein expression was higher in the model group at the various time points after cerebral hemorrhage induction. Compared with the model group, bFGF protein expression was higher in the aniracetam group at 7 days (P < 0.05). Compared with the model group, bFGF protein expression was higher in the acupuncture group at each time point (P < 0.05). Interestingly, compared with the aniracetam group, Nestin protein expression was higher in the acupuncture group at each time point (P < 0.05; Figure 3B).
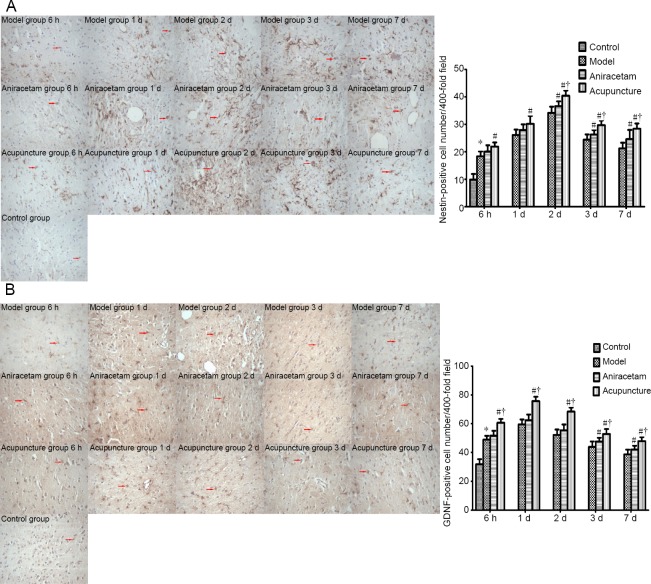
Acupuncture increases Nestin-positive cell number in the basal ganglia of rats with cerebral hemorrhage
The number of Nestin-positive cells was higher in the model group than in the control group at the various time points after cerebral hemorrhage induction. Nestin-positive cells were more numerous in the acupuncture group than in the model group at the various time points (P < 0.05). Notably, compared with the aniracetam group, Nestin-positive cells were more numerous in the acupuncture group at 2, 3 and 7 days (P < 0.05; Figure 4A).
Figure 4.
Effect of acupuncture on Nestin- (A) and GDNF- (B) positive cell number in the basal ganglia of rats with cerebral hemorrhage.
Nestin and GDNF immunoreactivity in the basal ganglia of rats with cerebral hemorrhage given acupuncture treatment (immunohistochemical staining, original magnification: 400×). Arrows show Nestin and GDNF immunoreactivity. Quantitation of Nestin- and GDNF-positive cell number in these animals at each time point. Data are expressed as the mean ± SD. Comparisons among multiple groups were performed using one-way analysis of variance followed by the least significant difference test. *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. aniracetam group. GDNF: Glial cell line-derived neurotrophic factor.
Acupuncture increases GDNF-positive cell number in the basal ganglia of rats with cerebral hemorrhage
The number of GDNF-positive cells was higher in the model group than in the control group at the various time points after cerebral hemorrhage induction. Interestingly, GDNF-positive cells were more numerous in the acupuncture group than in the model or aniracetam group at the various time points (P < 0.05; Figure 4B).
Discussion
Intracerebral hemorrhage is one of the most devastating types of stroke. Patients with intracerebral hemorrhage have poor prognosis, and 80% of those that survive beyond the acute phase suffer prolonged neurological deficits and brain atrophy (Naval et al., 2008). Currently, the management of intracerebral hemorrhage is generally supportive as there are no specific treatments that improve the outcome of intracerebral hemorrhage (Yang et al., 2008). It is therefore important to alleviate the neurological functional impairment and improve the quality of life of the patients.
Acupuncture therapy, which is a major therapeutic approach in Chinese traditional healing systems, has also been used in stroke therapy for many years. Acupuncture dilates blood vessels to improve local blood circulation, facilitates metabolism and promotes the restoration of injured tissues. Studies show that acupuncture improves neurological function in patients with cerebral hemorrhage (Li et al., 2019). The therapy comprises a short course of treatments, and is characterized by rapid effectiveness and few side effects (Zheng et al., 2011).
In this study, we used aniracetam as a positive control. Aniracetam is a new generation γ-lactam developed by Hoffmann-La Roche (Basel, Switzerland) that improves brain function. It is suitable for treating neuronal dysfunction and memory impairment caused by cerebrovascular disease after brain damage, and has a good effect in vascular dementia. A pharmacodynamic study showed that the drug reduces cerebral ischemia and alleviates hypoxia-induced brain damage and memory impairment (Elston et al., 2014). Therefore, in this study, we chose this drug for the positive control group.
Acupuncture effectively improves the symptoms of neurological deficits after cerebral hemorrhage. In the early 1990s, we began to use scalp acupuncture for acute cerebral hemorrhage in the clinic. We observed the effects of “Baihui-Qubin” acupuncture in approximately 100 patients and showed good clinical efficacy (Shouzhuang and Chao, 2006). Recently, a meta-analysis showed that scalp acupuncture therapy ameliorates neurological deficits in acute hypertensive intracerebral hemorrhage patients (Zheng et al., 2011).
Rats in the control group displayed normal activities. Rats in the model group at each time point exhibited varying degrees of contralateral hemiplegia. Wrist, elbow and shoulder flexion appeared on the contralateral side of the brain lesion. Resistance was reduced when pushing to the paralyzed side. Neurological function at each time point was markedly improved in the acupuncture and aniracetam groups. However, there were significant differences between the aniracetam and acupuncture groups at 2, 3 and 7 days. Functional improvement was better in the acupuncture group than in the aniracetam group.
Under the light microscope, pyknosis, chromatin condensation or karyolysis, swollen astrocytes and mitochondria, and varying degrees of synaptic swelling were visible in the model group. These pathological changes were most obvious at 3 days after induction of intracerebral hemorrhage. Astrocyte foot processes were visible and the capillary basement membrane had separated. Separation and swelling of endothelial cells were obvious. Necrosis and degeneration of some neurons and glial cells was noticeable at 7 days. Compared with the model group, neuronal swelling and cell and nuclear membrane damage was reduced in the acupuncture group. At 7 days, distinct nucleoli, evenly distributed chromatin, a well-defined synaptic structure, rich Golgi complex, and normal-looking rough endoplasmic reticulum and mitochondria were visible. Therefore, acupuncture markedly reduced cerebral edema and protected neurons in the brain.
Nestin is a 1621-amino acid class VI intermediate filament protein that was originally described as a neuronal stem cell/progenitor cell marker during central nervous system development (Lendahl et al., 1990), and is involved in the multi-directional differentiation of neuroepithelial stem cells. Nestin is therefore also known as neuroepithelial stem cell protein. In the mammalian central nervous system, Nestin protein expression reflects the birth of somatic cells before and after differentiation or proliferation. Nestin can also be expressed in the brain and spinal cord of adult animals (Clarke et al., 1994; Brook et al., 1999). GDNF is a distant member of the transforming growth factor β superfamily that was originally isolated from the rat B49 glial cell line (Lin et al., 1993). GDNF is expressed throughout the central nervous system during development, and is mainly expressed and detected in neurons in the adult brain (Barroso-Chinea et al., 2005). The cellular localization of bFGF changes during development, suggesting that it is a dynamic protein with multiple roles. Indeed, bFGF participates in wound healing, tissue repair, survival, proliferation and differentiation of immature neural cells. bFGF can promote neuronal survival and neurite growth, induce neural stem cells to differentiate and mature, and it plays an important role in protecting neurons from degeneration, necrosis and apoptosis caused by various factors (Bhindi et al., 2005; Jin-qiao et al., 2009). bFGF expression is low in normal brain tissue, but increases after ischemic damage. bFGF improves blood supply, inhibits ischemia and hypoxia, reduces brain edema, and promotes the proliferation of fibroblasts and neural cells. Studies have shown that bFGF can maintain and enhance the growth of various neurons in vitro and in vivo, and can promote the repair and regeneration of injured neurons (Li and Stephenson, 2002; Stachowiak et al., 2003). The addition of bFGF to cultured fetal rat hippocampal neurons prolongs their survival. In the current study, Nestin, GDNF and bFGF expression increased to different degrees at the various time points in the model and aniracetam groups, but no significant difference was found between these two groups. In comparison, acupuncture markedly increased the expression levels of Nestin, GDNF, bFGF and BDNF, and exerted a neuroprotective effect in the rat model of cerebral hemorrhage.
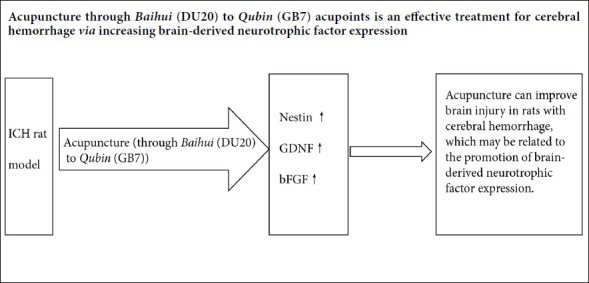
In summary, acupuncture in the treatment of acute cerebral hemorrhage improves neurological function, reduces intracerebral hematoma, and accelerates the resolution of cerebral edema. The mechanism of action likely involves increasing BDNF, Nestin, GDNF and bFGF expression, thereby promoting the protection and repair of neuronal cells. While we only examined a select few neurotrophic factors, our findings suggest that acupuncture has therapeutic potential in the treatment of cerebral hemorrhage. Further study is needed to more fully clarify the mechanisms underlying the effectiveness of acupuncture.
Footnotes
Conflicts of interest: There were no conflicts of interest in this experiment.
Financial support: This study was supported by the National Natural Science Foundation of China, Nos. 81473764, 81273824, 30772840 (to WZ); the Doctoral Fund of Ministry of Education of China, No. 20102327110003 (to WZ); the Natural Science Foundation of Heilongjiang Province of China, No. ZD201204 (to WZ); the Special Fund for Technological Innovation Research of Harbin of China, No. 2012RFXXS062 (to WZ); the Doctoral Innovation Fund of Heilongjiang University of Chinese Medicine of China, No. 2015bs03 (to QXC); the Chunhui Plans Research Cooperation Project of China, No. Z2007-1-15010 (to WZ), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province of China, No. UNPYSCT-2018234 (to QXC). The funding bodies played no role in the study design, collection, analysis and in-terpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Committee for Experimental Animals of Heilongjiang Medical Laboratory Animal Center (approval No. 2017061001) on June 10, 2017 and according to the Experimental Animal Management Regulations of Heilongjiang Province of China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81473764, 81273824, 30772840 (to WZ); the Doctoral Fund of Ministry of Education of China, No. 20102327110003 (to WZ); the Natural Science Foundation of Heilongjiang Province of China, No. ZD201204 (to WZ); the Special Fund for Technological Innovation Research of Harbin of China, No. 2012RFXXS062 (to WZ); the Doctoral Innovation Fund of Heilongjiang University of Chinese Medicine of China, No. 2015bs03 (to QXC); the Chunhui Plans Research Cooperation Project of China, No. Z2007-1-15010 (to WZ), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province of China, No. UNPYSCT-2018234 (to QXC).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Norman C, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- 3.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 4.Bhindi R, Brieger D, Ishii H, Di Girolamo N, Kumar RK, Khachigian LM, Lowe HC. Fibroblast growth factor 2 and the transcription factor Egr-1 localise to endothelial cell microvascular channels in human coronary artery occlusion. Thromb Haemost. 2005;93:172–174. [PubMed] [Google Scholar]
- 5.Brook GA, Pérez-Bouza A, Noth J, Nacimiento W. Astrocytes re-express nestin in deafferented target territories of the adult rat hippocampus. Neuroreport. 1999;10:1007–1011. doi: 10.1097/00001756-199904060-00021. [DOI] [PubMed] [Google Scholar]
- 6.Canonico V, Forgione L, Paoletti C, Casini A, Colonna CV, Bertini M, Acito R, Rengo F. Efficacy and tolerance of aniracetam in elderly patients with primary or secondary mental deterioration. Riv Neurol. 1991;61:92–96. [PubMed] [Google Scholar]
- 7.Chen JC. The effects of acupuncture and traditional Chinese medicines on apoptosis of brain tissue in a rat intracerebral hemorrhage model. Physiol Behav. 2015;151:421–425. doi: 10.1016/j.physbeh.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885–1888. doi: 10.1097/00001756-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Elston TW, Pandian A, Smith GD, Holley AJ, Gao N, Lugo JN. Aniracetam does not alter cognitive and affective behavior in adult C57BL/6J mice. PLoS One. 2014;9:e104443. doi: 10.1371/journal.pone.0104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409–418. doi: 10.3748/wjg.v17.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin-qiao S, Bin S, Wen-hao Z, Yi Y. Basic fibroblast growth factor stimulates the proliferation and differentiation of neural stem cells in neonatal rats after ischemic brain injury. Brain Dev. 2009;31:331–340. doi: 10.1016/j.braindev.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Johnson TV, Bull ND, Martin KR. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res. 2011;93:196–203. doi: 10.1016/j.exer.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Ko CN, Lee IW, Cho SY, Lee SH, Park SU, Koh JS, Park JM, Kim GK, Bae HS. Acupuncture for cerebral vasospasm after subarachnoid hemorrhage: a retrospective case-control study. J Altern Complement Med. 2013;19:471–473. doi: 10.1089/acm.2012.0076. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Choi TY, Park JE, Lee SS, Ernst E. Moxibustion for cancer care: a systematic review and meta-analysis. BMC Cancer. 2010;10:130. doi: 10.1186/1471-2407-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 16.Li LX, Zhang MM, Zhang Y, He J. Acupuncture for cerebral palsy: A meta-analysis of randomized controlled trials. Neural Regen Res. 2018;13:1107–1117. doi: 10.4103/1673-5374.233455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Stephenson D. Postischemic administration of basic fibroblast growth factor improves sensorimotor function and reduces infarct size following permanent focal cerebral ischemia in the rat. Exp Neurol. 2002;177:531–537. doi: 10.1006/exnr.2002.7994. [DOI] [PubMed] [Google Scholar]
- 18.Li ZW, Zheng XN, Li P. Time-effect relationship of acupuncture on histopathology, ultrastructure, and neuroethology in the acute phase of cerebral hemorrhage. Neural Regen Res. 2019;14:107–113. doi: 10.4103/1673-5374.243714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 20.Masure S, Geerts H, Cik M, Hoefnagel E, Van Den Kieboom G, Tuytelaars A, Harris S, Lesage AS, Leysen JE, Van Der Helm L, Verhasselt P, Yon J, Gordon RD. Enovin, a member of the glial cell-linederived neurotrophic factor (GDNF) family with growth promoting activity on neuronal cells. Existence and tissue-specific expression of different splice variants. Eur J Biochem. 1999;266:892–902. doi: 10.1046/j.1432-1327.1999.00925.x. [DOI] [PubMed] [Google Scholar]
- 21.Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- 22.Naval NS, Nyquist PA, Carhuapoma JR. Management of spontaneous intracerebral hemorrhage. Neurol Clin. 2008;26:373–384, vii. doi: 10.1016/j.ncl.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 24.Shouzhuang H, Chao L. Acupuncture treatment for 68 cases of functional impairment induced by cerebral hemorrhage at the convalescence stage. J Tradit Chin Med. 2006;26:172–174. [PubMed] [Google Scholar]
- 25.Stachowiak EK, Fang X, Myers J, Dunham S, Stachowiak MK. cAMP-induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1) J Neurochem. 2003;84:1296–1312. doi: 10.1046/j.1471-4159.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Lei HS, Zhao X, Li HH, Dong GR. Effect of penetrative needling of scalp-acupoints on cerebral N-acetyl-aspartate and choline levels in intracerebral hemorrhage rabbits. Zhen Ci Yan Jiu. 2011;36:242–246. [PubMed] [Google Scholar]
- 27.Yang S, Song S, Hua Y, Nakamura T, Keep RF, Xi G. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke. 2008;39:2079–2084. doi: 10.1161/STROKEAHA.107.508911. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Dai XH, Yu XP, Zou W, Teng W, Sun XW, Yu WW, Liu H, Wang H, Sun MJ, Li M. Baihui (DU20)-penetrating-Qubin (GB7) acupuncture inhibits apoptosis in the perihemorrhagic penumbra. Neural Regen Res. 2018;13:1602–1608. doi: 10.4103/1673-5374.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng GQ, Zhao ZM, Wang Y, Gu Y, Li Y, Chen XM, Fu SP, Shen J. Meta-analysis of scalp acupuncture for acute hypertensive intracerebral hemorrhage. J Altern Complement Med. 2011;17:293–299. doi: 10.1089/acm.2010.0156. [DOI] [PubMed] [Google Scholar]