Abstract
Spinal cord injury (SCI) population with injury below T10 or injury to the cauda equina region is characterized by denervated muscles, extensive muscle atrophy, infiltration of intramuscular fat and formation of fibrous tissue. These morphological changes may put individuals with SCI at higher risk for developing other diseases such as various cardiovascular diseases, diabetes, obesity and osteoporosis. Currently, there is no available rehabilitation intervention to rescue the muscles or restore muscle size in SCI individuals with lower motor neuron denervation. We, hereby, performed a review of the available evidence that supports the use of electrical stimulation in restoration of denervated muscle following SCI. Long pulse width stimulation (LPWS) technique is an upcoming method of stimulating denervated muscles. Our primary objective is to explore the best stimulation paradigms (stimulation parameters, stimulation technique and stimulation wave) to achieve restoration of the denervated muscle. Stimulation parameters, such as the pulse duration, need to be 100–1000 times longer than in innervated muscles to achieve desirable excitability and contraction. The use of electrical stimulation in animal and human models induces muscle hypertrophy. Findings in animal models indicate that electrical stimulation, with a combination of exercise and pharmacological interventions, have proven to be effective in improving various aspects like relative muscle weight, muscle cross sectional area, number of myelinated regenerated fibers, and restoring some level of muscle function. Human studies have shown similar outcomes, identifying the use of LPWS as an effective strategy in increasing muscle cross sectional area, the size of muscle fibers, and improving muscle function. Therefore, displaying promise is an effective future stimulation intervention. In summary, LPWS is a novel stimulation technique for denervated muscles in humans with SCI. Successful studies on LPWS of denervated muscles will help in translating this stimulation technique to the clinical level as a rehabilitation intervention after SCI.
Keywords: denervation, DXA, electrical stimulation, LMN injury, LPWS, MRI, spinal cord injury, stimulation parameters
Introduction
Prevalence of spinal cord injury (SCI) has been estimated to be 250,000–400,000 with an estimated 14% growth since 1988 (Devivo et al., 2002; Strauss et al., 2006). An immediate loss of voluntary movement and contractile force is one of the most crippling effects of SCI which often results in remarkable muscle atrophy within the first few months post injury (Kern et al., 2010). This is accompanied by increased intramuscular fat. This is followed by dramatic changes in body composition characterized by decreasing percentage lean mass and increasing fat mass as well as subsequent reduction in basal metabolic rate and thus leads to increasing prevalence of obesity among the SCI population (Gorgey and Gater, 2007; Gorgey et al., 2014). This increase in ectopic adiposity is likely to be associated with reduction of muscle mass and is linked to several cardio-metabolic disorders and other health-related comorbidities, such as reduced aerobic fitness, glucose intolerance, insulin resistance, and decrement in mitochondrial function (Gorgey et al., 2014; Sumrell et al., 2018). These secondary health related consequences may lead to several disparities which challenge the productivity, quality of life, and well-being of those with SCI, but may be reversible with appropriate exercise or pharmaceutical interventions (Gorgey et al., 2019). This is primarily of interest because advances in neuroscience research may offer the development of novel rehabilitative intervention for functional restoration after SCI (McDonald and Sadowsky, 2002).
Individuals with SCI may suffer an upper motor neuron (UMN) lesion, lower motor neuron (LMN) lesion, or a combination of both. Clinically, it is necessary to determine whether an individual has an UMN or LMN lesion (Kirshblum et al., 2011). The type of lesion whether UMN or LMN, cannot be determined solely based on the neurological level of the injury (Doherty et al., 2002). Within the spinal column, damage to the motor unit can involve motor neuron cell bodies in the spinal segment of the cord or axonal damage at the spinal root level. Following UMN lesion, the inhibitory pathways get disrupted and can be followed by loss of voluntary motor controls. Clinically, a UMN lesion above the lumbar spinal cord segments may lead to spastic paralysis and exaggerated deep tendon reflexes in the paralyzed limbs (Doherty et al., 2002). In contrast, the effects of LMN damage can be associated with severe muscle atrophy, hypotonia, fibrillations in the muscles, and flaccid paralysis in the lower limbs (Doherty et al., 2002).
Anecdotal evidence from our laboratory suggests that about 20–25% of the SCI population experiences LMN denervation. Due to the lack of innervation, the denervated muscles cannot be activated using the standard commercially available electrical stimulator units. Currently, there is no available rehabilitation intervention for SCI individuals with LMN denervation.
The primary objectives of the current mini-review are 1) to review the consequences of denervation in SCI persons with LMN injury and 2) to explore the available stimulation paradigms of electrical stimulation from animal and human models with LMN injury. Results from this review will facilitate a greater understanding of the methodology necessary to determine the impact of long-term exercise on muscle hypertrophy, as well as metabolic and local cellular adaptations following LMN injury after SCI.
Search Strategy and Selection Criteria
We searched PubMed, Web of Knowledge, MEDLINE, and Google Scholar for studies that reported the effects electrical stimulation in denervated muscles after spinal cord injury (SCI). We used the following search strategy (SCI* OR LMN injury* OR Denervation* OR Cauda Equina Injury) AND (electrical stim* OR Functional Electrical Stimulation* OR LPWS* OR stimulation therapy). No restrictions by publication period were used. In addition, we manually scanned the references list of retrieved articles for relevant studies.
Lower Motor Neuron Injury and Denervation
LMN injury is commonly accompanied by peripheral nerve injury to the sensory, motor, autonomic, and terminal branches of the peripheral nervous system. Based on the severity of the injury, and whether there is continuity of the nerve preserved or not, peripheral nerve injury is classified into three major types (Seddon, 1968); 1) Neurapraxia, 2) Axonotmesis and 3) Neurotmesis. A simplified illustration of the different types of peripheral nerve injury based on Seddon’s classification is shown in Figure 1.
Figure 1.
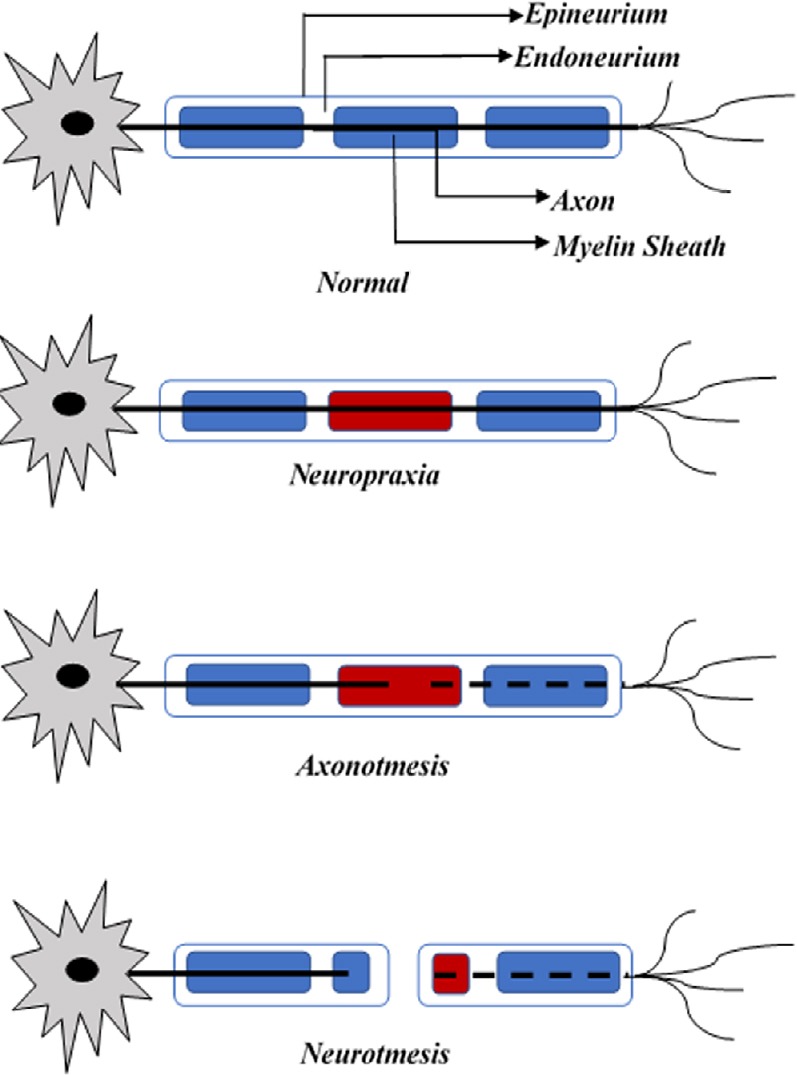
Seddon’s classification of peripheral nerve injury.
Displays three types of injury along the axon of a neuron as compared to a healthy nerve.
Neurapraxia is known to be the mildest form of peripheral nerve injury and is characterized by temporary interruption in the conduction of the impulses however, there is no loss of axonal continuity. Concussive or shock-like injury to the fiber by either a compressive, tensile, or chemotoxic insult can also result in a biomechanical lesion. The other two types of peripheral nerve injury are axonotmesis and neurotmesis. These types of injury usually occur due to a more severe crush or a contusion injury and can be characterized by loss of relative continuity of the neuron (Carp, 2015). Neurotmesis, is the most severe kind of injury involving peripheral nerves, based on the level of trauma and the impact of the damage. In this case, severe compression, or tension injury occurs affecting the nerve and axonal continuity. Along with axonal injury, the encapsulating connective tissue is damaged resulting in complete loss of motor, sensory and autonomic functions. In addition, electromyography examinations performed 2–3 weeks after the injury shows fibrillations and denervation potentials in the skeletal muscles distal to the injury site. Partial functions may be retained in some cases if there is a dual dermatomal or myotomal innervation present through an undamaged peripheral nerve fiber (Carp, 2015).
The cellular changes that occur in the nerve stumps located distal to the lesion site are collectively described as Wallerian degeneration (Rotshenker, 2011). This is a process that follows after axonotmetic and neurotmetic nerve injuries (Kaye, 1991). Following loss of axonal continuity, axonal degeneration occurs as a result of separation from the neuron’s nucleus.
The process of degeneration usually begins 24 to 36 hours after the injury (Coleman and Freeman, 2010). The rate of Wallerian degeneration depends on various factors like species, axonal diameter and the length of the distal segment (Gilliatt and Hjorth, 1972; Lubińska, 1977; Beirowski et al., 2005).
The first response following axonal injury is acute axonal degeneration, characterized by the separation of the proximal and distal parts of the axon within the first 30 minutes of injury (Kerschensteiner et al., 2005). The axolemma then swells up in the form of a bead; which takes about 24 hours. The axolemma then undergoes degradation followed by granular disintegration of the axonal cytoskeleton and the inner organelles.
Response following Denervation
The severity of SCI is described by the International Standards for Neurological Classification of Spinal Cord Injury. Individuals with denervation after SCI could have traumatic complete or incomplete SCI with injury level T10 or below; also known as conus and cauda equina lesion. The lesion results in motor deficit below the level of injury. Figure 2 shows the conus medullaris and the cauda equina region.
Figure 2.
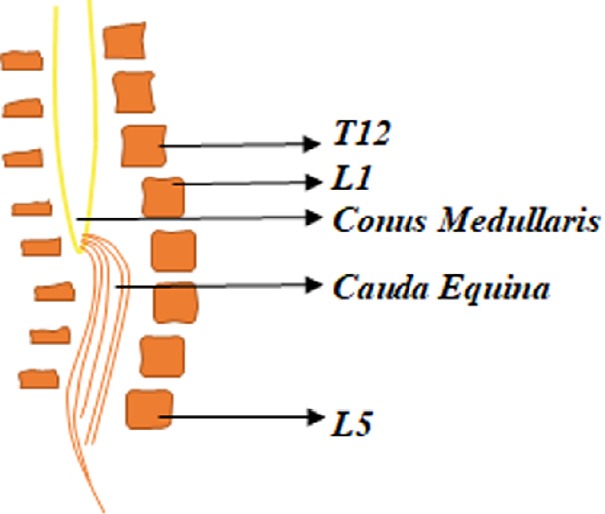
Briefly summarizes the anatomy of the conus medullaris and the cauda equina.
The Conus Medullaris forms the end of the spinal cord near the lumbar vertebral sections L1 and L2. The cauda equina is comprised of a bundle of lumbar and sacral nerve roots and connects to the conus medullaris.
Individuals with SCI and LMN denervation exhibit dramatic musculoskeletal and dermal changes below the level of injury. Denervation leads to a great loss in muscle mass and preserving skeletal muscle integrity is vital for the maintenance of cellular and whole-body metabolism (Pedersen and Febbraio, 2008, 2012; Kanzleiter et al., 2014). These individuals are at an increased risk for several chronic diseases such as type II diabetes, dyslipidemia, cardiovascular disease and osteoporosis.
Skin quality following denervation
Skin is the largest organ in the body and acts as a protection for the internal organs against physical, chemical and biological insults (Albertin et al., 2018). Skin quality is associated with the integrity of the skin, including perfusion of the deep tissue and sufficient blood flow in the vascular system. Loss of blood supply to the soft tissue, immobility, and pressure from internal bony prominence can typically lead to pressure sores (Dolbow et al., 2013). These physiological changes are why persons with SCI are known to be at the highest risk for developing pressure ulcers (Kruger et al., 2013). Most decubitus ulcers or pressure injuries occur in the sacral area in persons with spinal denervation. In this region, the ischial tuberosities as well as the coccyx are particularly exposed. Anatomically, there is no muscle mass superficial to protect these bony structures. Muscle atrophy also decreases the presence of soft tissue and vascularity. This results in the loss of the desired cushioning effect, heightening the risk of pressure ulcers (Liu et al., 2006). Pressure ulcers can have consequences like limited mobility, decreased quality of life, and they can hinder everyday activities (Chen et al., 2005). Pressure ulcers, when left untreated, can increase in severity and become extremely difficult to restore leading to serious medical conditions and possibly even life-threatening problems (Garber and Rintala, 2003).
Electrical stimulation has been widely used as a treatment or prevention method for pressure ulcers in the past few decades (Houghton et al., 2010; Curtis et al., 2011; Franek, et al., 2012). It has been demonstrated that electrical stimulation therapy stimulates wound healing, by promoting the activation and proliferation of major key factors such as fibroblast, growth factors, and epithelial cells (Bourguignon and Bourguignon, 1987; Sheridan et al., 1996; Houghton et al., 2010).
Bone degradation following denervation
Denervation also leads to continuous bone resorption and osteoporosis. Previous studies have concluded that bone osteoporosis occurs as a result of lack of muscle activity (Gillespie, 1954; Gargiulo et al., 2011). This may be attributed to the micro and macro structural changes due to the loss of muscle contraction and weight bearing (Zamarioli et al., 2014). Figure 3 shows the DXA scan of the pelvic region including hip bone and femoral neck in a person with T7 American Spinal Injury Association Impairment Scale grade C following 6.5 years with LMN SCI. Notice the anatomical changes that occur in the femoral head and hip joint following injury. Based on the anatomical region, the bone mineral density can diminish by about 50% at the distal epiphyses of the femur and by 60% at the proximal epiphyses of the tibia post 3–4 years after SCI (Eser et al., 2004). The risk of fracture in an individual with SCI is twice as high the risk of fracture in an able-bodied individual (Eser et al., 2004). About 50% of individuals with complete SCI experience a post-injury bone fracture at some point (Morse et al., 2009). Complications such as increased pain, spasticity, pressure ulcers, risk of non-union fracture, and limb amputation are common post fracture (Morse et al., 2009).
Figure 3.
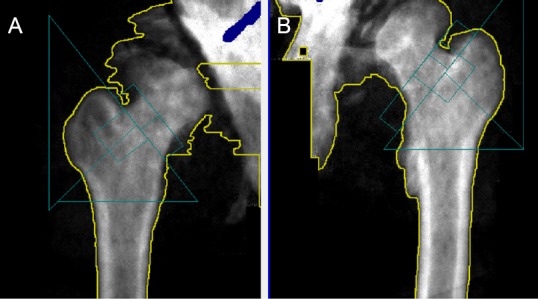
Dual-energy X-ray absorptiometry scan of the right (A) and left (B) hip joints of a denervated individual with T7 level of injury.
Femoral head and bone density deterioration post 6.5 years of injury.
Non-invasive rehabilitative techniques to restore bone strength and density post-SCI are becoming increasingly popular. Several studies have demonstrated that electrical stimulation for exercise, cycling, and standing, for muscle strengthening, induces an increase in bone mass (Chen et al., 2005; Shields and Dudley-Javoroski, 2006; Lauer et al., 2011; Zamarioli et al., 2013). Previous work by Johnston et al. (2016) has suggested that 6 months of functional electrical stimulation (FES) to the paralyzed muscles results in remarkable improvement in trabecular bone parameters after SCI. However, this supportive evidence is still lacking following LMN injury after SCI. There is also inconclusive evidence to support that improvement in muscle quality may lead to direct improvement to bone health following SCI.
Muscle atrophy following denervation
There are several biochemical, morphological and physiological changes that occur in the muscle fiber following denervation with muscle atrophy being the most prominent. Within the first few months post-injury, there is rapid onset of skeletal muscle atrophy and decrease in fat free mass (Kocina, 1997; Castro et al., 1999; Spungen et al., 2000; Buchholz and Bugaresti, 2005; Md and Clasey, 2006; Gorgey and Dudley, 2007, 2011; Gorgey et al., 2011). The dramatic muscle atrophy after SCI begins within a few weeks of injury and continues at least until the end of the first year (Castro et al., 1999; Gorgey and Dudley, 2007). Skeletal muscle cross sectional area (CSA) could be as low as 50% compared to healthy able-bodied controls (Castro et al., 1999). Muscle atrophy is also associated with a massive adipose tissue infiltration of intramuscular fat (Gorgey and Dudley, 2007). The extensive muscle atrophy has been attributed to a number of factors including reduction in the level of physical activity, unloading, disuse, and reduction in anabolic hormone secretion as well as an increased release of proinflammatory cytokines (Castro et al., 1999; Md and Clasey, 2006; Sumrell et al., 2018; Abilmona et al., 2019). This is further accompanied by an increase in fat mass (Kocina, 1997; Spungen et al., 2000; Buchholz and Bugaresti, 2005; Md and Clasey, 2006), waist circumference (Buchholz and Bugaresti, 2005) and visceral adiposity (Castro et al., 1999; Gorgey and Gater, 2011).
The rate of muscle atrophy varies among different species, different individuals of the same species, different muscle groups in the same individual, and sometimes even within the fibers in the same muscle group (Eberstein and Eberstein, 1996). In rats, two weeks after denervation, the hind-limb weight is reduced by 50%. Whereas in humans, within the same time frame, changes may be negligible, taking about 2–3 months of denervation for the muscle fibers to reduce by 50% in diameter (Adams, 1975; Ohira, 1989). Furthermore, in rats, the extensor digitorum longus muscle atrophies at a slower rate as compared to the soleus muscle (al-Amood et al., 1991; Wroblewski et al., 1989).
Following denervation, the muscles proceed to terminal atrophy; this process can be divided into various stages. The duration of these stages is different in different species. For example, each stage could be several months in laboratory animals like rats; but it might take years to progress through similar stage in case of humans (Carlson, 2014). This progressive decline of the muscles post-denervation can be mainly divided into three main stages (Tower, 1935; Lapalombella et al., 2008). The first stage begins immediately after the nerve injury and is mainly characterized by immediate loss of function resulting in expedited muscle fiber atrophy and rapid weight loss. In rats, this phase lasts for two months. The denervated muscles have restorative capacity equal to that of a normal control muscle, although by the end of the first phase, the muscle loses 90% of its original mass. During the second stage, there is an extreme muscle atrophy, along with the break-down of sarcomere and muscle fiber organization. In rats, from 2–7 months, there is a striking fall in the restorative ability of the muscles during this stage. The third stage, also known as the terminal stage, is when the tissue architecture undergoes fibrosis; this phase starts post 7 months in rats and is characterized by severely diminished muscle fibers accompanied by an excessive increase of adipocytes that is referred to as fat infiltration (Gulati, 1990; Carlson et al., 1996, 2002). Mödlin et al. (2005) found that in human subjects the rate of muscle degeneration after denervation is slower. The quadriceps muscles of an individual denervated for 0.7 years consists of small, multiangular myofibers with a mean diameter of 18.6 µm. After 4 years quadriceps consist of severely atrophic myofibers with mean diameter of 9.0 µm. It is also during this period that the muscle fibers begin to be substituted by adipocytes and collagen. Post 8.7 years of denervation the muscles enter the long-term stage defined by minimal muscular structure consisting of multiangular, atrophied fibers measuring 7.9 µm (Mödlin et al., 2005). As severe atrophy develops post SCI, the muscle tissue undergoes both fibrosis as well as fat infiltration, hence producing denervated degenerated muscle (Carraro et al., 2015). Using sciatic nerve injury as a common denervation model, there were elevations of ubiquitin ligase genes, MAFbx and MURF1 over the course of 2 weeks. Despite the initial increase in insulin-like growth factor-1 in the first 3 days, with increase in fibrillation, there were noted declines in insulin-like growth factor-1 which was correlated with the extent of atrophy (Zeman et al., 2009). In Figure 4, note the dramatic muscle atrophy in the right thigh following SCI in a person with partial denervation as measured by magnetic resonance imaging. Contractile tissue is completely diminished, and muscle fibers were replaced with infiltrated fat and connective tissues. Failure to restore muscle size may lead to serious health-related complications including obesity, higher risk of type II diabetes and cardiovascular diseases (Castro et al., 1999; Buchholz and Bugaresti, 2005; Md and Clasey, 2006; Gorgey and Dudley, 2007; Gorgey et al., 2011; Gorgey and Gater, 2011). Therefore, providing electrical stimulation paradigm may facilitate restoration of denervated muscle following SCI. Surface neuromuscular electrical stimulation (NMES) has been recommended as an effective rehabilitation approach that facilitates restoration of muscle bulk after UMN lesion SCI (Ryan et al., 2013).
Figure 4.
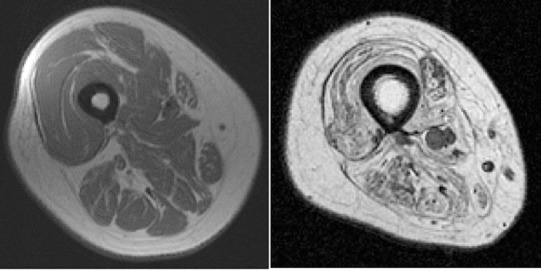
A comparison between two patients with SCI.
The right thigh shows dramatic muscle atrophy in an individual with a T12 complete denervated SCI as compared to an innervated individual with SCI (left). SCI: Spinal cord injury.
Excitability of denervated muscles
The excitability of a tissue is determined mainly by two parameters, stimulation amplitude and pulse duration. The relationship between these two parameters is called the strength-duration curve. Rheobase and Chronaxie are two points on this curve that can be used to accurately describe the excitability of the tissue. Rheobase is defined as the minimum amplitude of the stimulus that is required to trigger a response at an infinitely long pulse duration. Whereas, chronaxie can be defined as the minimum pulse duration required to elicit a response when the amplitude is twice the rheobasic strength (Ashley et al., 2005).
One of the many changes that occur in the skeletal muscles after denervation is decrease in the excitability of the muscle. Chronaxie, the tissue-excitability constant is especially high in case of denervated muscles. Based on the chronaxie, the optimum pulse duration used to stimulate the muscles is determined (Geddes, 2004). It has to be about 100–1000 times longer for denervated muscle to respond to stimulation as compared to innervated muscle (Kern et al., 1999). Denervated muscles respond to longer pulse duration compared to those that are adequate to elicit a response for innervated muscles (Ashley et al., 2005).
Previous studies on the strength-duration curve by Ashley et al. (2005) have successfully found the effect of denervation on parameters like rheobase and chronaxie. Six New Zealand rabbits were denervated and their TA muscle was implanted with stimulator. Rheobase was determined with a constant pulse duration of 100 ms and the amplitude used to determine the Chronaxie was twice the Rheobase. Chronaxie increased 3 times over the course of 2 weeks of denervation (Ashley et al., 2005).
Following complete denervation, paralyzed muscles in the lower limb may demonstrate large amounts of fibrillation potentials (Katirji, 2007). The fibrillation activity is an indication of significant LMN pathology with likely damage at the segmental or at the radicular level where the peripheral axons are damaged leaving the muscles denervated (Purves et al., 2004). In this situation, the muscle fiber membrane becomes electrically unstable and the fiber starts to fibrillate at about 21 days after the injury (Katirji, 2007). Fibrillation continues until the fiber is either re-innervated or it degenerates to the point where it becomes electrically silent. However, most persons with SCI lesions are discomplete, showing signs of residual neurophysiological brain control below the level of lesion (Sherwood et al., 1992). These individuals with T11–12 complete SCI may show no signs of abnormal fibrillation activity in the lower limb muscles, or only a very limited amount of abnormal spontaneous activity and could actually have some reflexively activated or otherwise limited spontaneous motor unit activity. In these individuals, the peripheral axons and motor neurons at the segmental level are most likely relatively intact with the pathology being primarily suprasegmental. Using electromyography techniques, muscle activity can be determined before and after long pulse electrical stimulation treatment to evaluate the effects on denervated muscles.
Neuromuscular Electrical Stimulation
The application of Electrical current to neuromuscular junctions and its surrounding muscle fibers to trigger muscle contraction is referred to as NMES (Bax et al., 2005). This form of electrical stimulation has been widely used in conjunction with other rehabilitation techniques in individuals with SCI. It is used to evoke muscle hypertrophy, relax muscular spasms, increase range of motion, prevent muscle atrophy, and increase peripheral blood flow (Pichon et al., 1995; Maffiuletti et al., 2000). NMES has been used to train atrophied muscles in rehabilitative settings as well as athletic or preventive strength training (Pichon et al., 1995; Maffiuletti et al., 2000; McDonough and Kitchen, 2002). Pichon et al. (1995) found that by using electrical stimulation strength training on the latissimus dorsi of athletes, performance was increased by 30% over the course of 3 weeks. Ryan et al. (2013) noted an increase in muscle mass of 30% post sixteen weeks of home-based electrical stimulation resistance training. Gorgey et al. (2019) have recently used electrical stimulation with resistance training to evoke muscle hypertrophy and increase basal metabolic rate in persons with SCI. Several other studies have used electrical stimulation during cycling or exercise to improve muscle health, bone health and metabolic profile in the SCI population (Johnston et al., 2016; Gorgey et al., 2019). Several reports have highlighted the significance of using NMES-resistance training to provoke 35–40% hypertrophy in individuals with chronic SCI (Mahoney et al., 2005).
Unfortunately, individuals with SCI who present with LMN injury as evidenced by muscle denervation cannot benefit from standard NMES. Following LMN injury, the affected skeletal muscle experiences extensive loss of contractile material as well as undergoes fibrosis and fat infiltration (Boncompagni, 2012; Carraro et al., 2015). Unpublished data from previous studies suggests that approximately 20–25% of the SCI population experience injury to their conus medullaris which may lead to LMN denervation. Denervated muscles do not respond to typical stimulation, therefore we aim to explore the benefits of LPWS as a means to stimulate these muscles.
Electrical Stimulation as a Treatment for Denervated Muscles
According to the current European regulations for the stimulators, the maximum output energy for the standard NMES is 300 mJ per impulse. Most commercially available stimulators have an amplitude that does not exceed 200 mA because of an increased risk of skin irritation and burns, especially in individuals with SCI. However, this is not sufficient to trigger usable contractions in denervated muscles via surface electrodes, unless these muscles remain partially innervated (Mayr et al., 2002). Unpublished data suggests that 25% of the SCI population cannot benefit from the standard NMES protocol because of LMN denervation.
Denervated muscles have lost their peripheral nerve supply. This results in a decrease in the amount of tension which can be generated and an increase in the time required for contraction. In individuals with denervated muscles, various parameters need to be modified as compared to the standard NMES in order to achieve desirable muscle contraction and minimize muscle atrophy. As the amplitude or pulse duration increase, the nerve fibers nearest the electrodes are depolarized to activate the target muscle. All available NMES applications are based on direct excitation of neural structures and indirect activation of the target muscle. Due to the denervation, the cellular membrane of each target muscle fiber must be depolarized to elicit muscular contractions (Boncompagni, 2012). The average pulse duration used for individuals with LMN denervation is 100–200 ms. The intensity of the current should be sufficient to elicit strong contractions and the pulse duration should be greater than or equal to the chronaxie of denervated muscles. This pulse has extremely long duration and is expressed in milliseconds (ms) as compared to the standard NMES in microseconds (µs).
The European project, Research and Innovation Staff Exchange has developed a novel technique, LPWS, which is capable of training and restoring muscle size following denervation in persons with SCI (Kern et al., 2009; Boncompagni, 2012; Carraro et al., 2015). Biphasic rectangular direct current (DC) impulses with duration between 30–150 ms and a frequency in between 2–22 Hz may have to be applied with an amplitude higher than that of standard NMES (200 mA) to maximize the electrical charges and to exceed the chronaxie of the denervated muscles. This imposes direct activation of the myofibers independent of LMN denervation. Continuous stimulation can increase the excitability of the denervated muscles, and the pulse duration can be gradually regulated down to 30–50 ms. The LPWS has the capacity to penetrate deeply and activate muscle fibers. For this much current, large size rubber electrodes of 12 × 15 cm with salt free gel covered with wet spongy pads should be used to reduce chances of electrical burn of the exposed skin (Figure 5).
Figure 5.

Placement of the electrodes in a SCI patients with LMN.
A rubber-based electrode is covered in a salt free gel (A) before being placed in a wet, conductive sponge (B) which prevents surface tissue damage. The covered electrode is then secured with elastic straps in position above the knee extensor groups (C). LMN: Lower motor neuron; SCI: spinal cord injury.
LPWS has been proven to restore functional use of muscles post denervation. An extensive amount of research has been done to explore the benefits of FES in denervated muscles in animal models. One study on 42 male rats with denervated tibialis anterior muscle showed positive effects on the muscle weight and a significant increase in bone strength in the denervation with electrical stimulation group (Tamaki et al., 2017). Morphological findings in another study, post electrical stimulation on cranial tibialis muscle in rats showed that the experimentally denervated and treated group had no significant difference when compared with initial control group (Bueno et al., 2017). Another study involving adult horses with transected laryngeal nerve showed improvement in inspiratory loads following 8 weeks of stimulation (Cheetham et al., 2015).
Human studies are beginning to emerge, that support the findings of previous animal studies showing that LPWS can potentially be established as the rehabilitation technique for muscle restoration post denervation. Previous study on FES, on individuals with varied levels of denervation demonstrated regain of active standing up of 7 patients over a 2-year training period (Kern et al., 1999). The effects of home-based FES using LPWS (120–150 ms) at an intensity of 250 mA for 5 days/week has been studied for two years in 25 SCI persons with complete LMN denervation (Kern et al., 1999, 2002; Carraro et al., 2002; Hofer et al., 2002). The trial showed that knee extensor CSA increased by 24% following the first year and an additional 7% in the second year, respectively, with no changes in the CSA of the hamstring muscles (Kern et al., 2010).
Tables 1 and 2 provide a brief literature review of electrical stimulation of denervated muscles in animal models and humans, respectively. The parameters and the specific techniques used are highlighted in order to better understand the effects of electrical stimulation on denervated muscles.
Table 1.
Functional electrical stimulation (FES) of denervated muscles in animal models
| Reference | Purpose | Subjects | Protocol/Parameters used | Results/Conclusion |
|---|---|---|---|---|
| Tamaki et al., 2017 | To test the effects of Electrical Stimulation on bone strength and muscle atrophy | Forty-two males, 7-wk-old Fischer 344 rats with denervated Tibialis Anterior (TA) muscle were used |
Three groups with n = 14 each; randomly grouped into Control (Cont), Denervation (Dn) without E stim and Denervation with Electrical stimulation (ES). Dn + ES group stimulation parameters: intensity 16 mA, frequency 10 Hz, 250 μs pulse duration for 30 min per day for 1 wk. ES consisted of 2 s stimulation and 6 s of rest. |
Body weight did not significantly differ but the relative muscle weight was significantly higher in in the Dn + ES group compared to the Dn group (P < 0.05); 1.3 ± 0.1 in Dn and 1.6 ± 0.2 in Dn + ES group. Bone strength is determined by parameters like max load, stiffness and elastic modulus; there was a significant increase in Dn + ES group compared to the Dn group. |
| Bueno et al., 2017 | To investigate the effects of electrical stimulation through Russian Currents on cranial tibial muscle of experimentally denervated rats. | Thirty-six young male Wistar rats aged 80 d | Four groups: Initial control group euthanized at 80 d (ICG), Final control group (FCG) euthanized at 125 d, Experimental Denervation group (EDG) denervated at 80 d, no treatment for 45 d and Experimental Denervated Treated Group (EDTG) denervated at 80, treated for 45 d, three weekly sessions, two application cycles each session. Cycle duration: 10 min, cycle frequency was 2500 Hz in periods of 0.4 ms; 3/1 intervals, 9 s of stimulation and 27 s rest period with modulation percent at 50%. First cycle stimulates red fibers at 30 Hz, second one for white fibers at 100 Hz. |
Histological observations: the EDTG group has the characteristics of both FCG and EDG groups, most of the muscle fibers observed peripheral nuclei like the FCG, central nuclei were also observed rarely. Morphometric data: significant difference between EDTG (2402 ± 94 µm2) compared to EDG (755 ± 227 µm2); No significant difference when comparing ICG (2441 ± 193 µm2) to EDTG. |
| Cheetham et al., 2015 | To assess the effects of direct intramuscular stimulation with long pulses at low activation frequency; on muscle size and function in denervated laryngeal muscles | Ten adult horses (age range 5–7 yr) with transected recurrent laryngeal nerve | Quadripolar intramuscular electrodes were instrumented in the left posterior cricoarytenoid (PCA) muscle. After 12 wk of denervation, the left PCA was stimulated every day for 8 wk at 50 Hz, 2 ms pulse duration, 2 s ON and 2 s OFF 10 V for 48 min to produce 72,000 impulse/d and daily frequency equivalent 0.83 Hz, biphasic pulses used; 7 animals stimulated (FES + group) and 3 animals implanted but not stimulated, served as control (FES- group). |
Quantitative assessment using strength-duration curve to determine the effects of denervation and stimulation, muscle contraction, PCA volume and laryngeal functions were measured 12-wk post denervation and post 8-wk of FES. The PCA function under increasing inspiratory loads drops down severely post denervation, post FES in the FES + group significantly improved at high levels of inspiratory loads (90 and 100 HR max). |
| Monaco et al., 2015 | To investigate the effects of combination of testosterone and electrical stimulation on functional recovery post laryngeal nerve crush | One hundred and forty-eight adult male Sprague-Dawley rats with a crush injury in the left RLN were used | Animals were divided into four experimental groups 1) no treatment (CTL) 2) ES only 3) TP only and 4) ES + TP. These animals were also subdivided into timepoints of 1, 2, 3 and 4 wk. The ES voltage ranged from 200 to 300 mV with the current from 0.1 to 0.2mA at 20 Hz frequency for 30 min. |
ES and TP had similar recovery. Testosterone did not significantly shorten the treatment time. The Vocal Fold Mobility (VFM) scores determine the recovery. At no time points were the VFM scores from any of the treated group significantly different from any other treatment groups. At 2 wk the VFM score of ES and ES + TP group was significantly higher than the control group, only TP group had similar effects but was not statistically significant. By wk 3 and 4, the VFM scores were reaching max in all the groups. |
| Fujita et al., 2011 | To study the effects of electrical stimulation on muscle atrophy | Twenty-eight adult male Wistar rats were used. Hindlimb unloading was applied to induce muscle atrophy. | The animals were randomly divided into four groups control (Cont); hindlimb unloading (HU); hindlimb unloading plus electrical stimulation (ES) and hindlimb unloading plus combination of electrical stimulation with forceful isometric contraction (ES + IC) | The mean values of wet weight of the Tibialis Anterior muscle in HU was 353 ± 8 mg; 405 ± 5 mg in the ES group and 434 ± 3 mg in the ES + IC group. This was a stastically significant difference as compared to the HU group. The effect of combination of ES + IC was better than just ES. |
| Asensio-Pinilla et al., 2009 | To compare the effects of the combination of electrical stimulation and exercise as compared to their individual effects | Five groups of adult rats with sciatic nerve denervation | One group received acute E stimulation (3 V, 0.1 ms at 20 Hz), immediately after denervation (ESa). All the other groups received treatment for 4 wk. The second group received chronic E stimulation (ESc); the third group receives Treadmill Exercise + E stimulation (ES + TR); the fourth group receives only Exercise (TR); the fifth group does not receive treatment and serves as the control (C). |
The results demonstrate that the final level of reinnervation of the tibialis anterior (TA) muscle was significantly higher in three groups; ESa group by 11 ± 2%, TR group by 9.4 ± 1% and ES + TR by 10.4 ± 2% compared to the control group C with 6 ± 1%. But it was found to be higher in the ES + TR group at earlier timepoint of 1 wk. There was an increase in the number of myelinated regenerated fibers in groups ESa, TR and ES + TR. The increase in ES + TR group (5124 ± 404) compared to the control group (2403 ± 196) was significant. |
| Sharma et al., 2009 | To investigate the combined therapeutic effects of Electrical stimulation and gonadal steroids on peripheral nerve regeneration | Ninety-eight adult male Sprague-Dawley rats with its right facial nerve crushed near its exit from the stylomastoid foramen | A total of 8 groups; 1) Untreated 2) Treated with E stimulation only 3) Treated with Testosterone Propionate (TP) only 4) E stimulation + TP 5) Dihydrotestosterone (DHT) only 6) E stimulation + DHT 7) Estradiol (E2) only 8) E stimulation + E2; Stimulation was conducted with Supramaximal pulses with 20 Hz frequency; 1-d post axotomy for 30 min every day until sacrifice (4 and 7 d). | The group 4 with E stimulation + TP showed the highest rate of regeneration with the facial nerve outgrowth distance being 11.8 ± 0.7 mm at 4 dpo as compared to untreated animals at 7.3 ± 1.0 mm; and 25.0 ± 0.7 mm at 7 dpo compared to untreated at 19.0 ± 1.6 mm. Increase in regeneration rate (E stimulation) and delay in sprout formation (TP) has beneficial effects. DHT and E2 did not prove to be as effective as TP. |
| Ashley et al., 2008 | To evaluate the effects of stimulation parameters on the extent of regeneration | Twenty-six male New Zealand white rabbits were used | Rectangular bipolar constant-current pulses used. A total of 5 different patterns with different stimulation parameters were used. Varying pulse duration (20, 20, 20, 10 & 10 ms); frequency (20, 20, 20, 40 & 40 Hz); daily stimulation minutes (1 × 60, 1 × 60, 2 × 30, 1 × 300 and 1 × 20 min); no: of impulses per day (24,000, 48,000, 24,000, 480,000 & 24,000) and the duration of stimulation (6, 6, 6, 6 & 10 wk) were used in patterns 1 to 5 respectively. |
The group with Pattern 1 proved to me more effective with maximum CSA 62.5 ± 8.8 and fibre number 18,200 ± 885 as compared to denervated group with CSA 36.0 ± 3.1 and fibre number 15,500 ± 1120. |
| Vivo et al., 2008 | To investigate whether electrical stimulation performed immediately after the injury may boost axonal regeneration | Thirty Sprague-Dawley female rats; subjected to complete transection of the sciatic nerve and repaired immediately by epineural suture | Two groups with n = 15 each. The first group received electric stimulation using a Grass S44 stimulation for 1 h immediately after the injury. Parameters used were continuous 20 Hz square pulses of 3 V, 0.1 ms. The second group n = 15 was the control group. | Nerve regeneration, as indicated by higher number of axons distal to the lesion post 1 and 2 mon seen in the E stimulation group. Test performed 1-wk post injury showed denervation of the muscles. Proximal Tibialis Anterior (TA) muscles showed signs of reinnervation 3 wk post injury with indications of small amplitude M waves; final reinnervation values were 28% in the control group and 33% in the E stimulation group. |
| Demiryurek and Babül, 2004 | Effects of vitamin E treatment and electrical stimulation on progression of atrophy in denervated rat gastrocnemius muscle studied | Thirty Wistar albino rats (both sexes) were used | Five groups (n = 6): Protocol 1, only incision; protocol 2, denervation group; protocol 3, E stimulation + denervation; protocol 4, vitamin E + denervation; protocol 5: vitamin E + E stimulation + denervation. Vitamin E: 30 mg/kg per day for 7 d; E stimulation: bipolar, 1 ms, 3–10 mA strong pulses-10 min for 7 d. |
Malondialdehyde levels found significantly increased in denervated gastrocnemius muscle (62.6 ± 4.9 nmol/g) compared to the control group (49.7 ± 2 nmol/g). There was no significant difference between E stim or vitamin E treatment. The best results were in vitamin E + E stimulation + denervated muscle group lowest MDA levels (25.8 ± 1.3 nmol/g). |
Table 2.
Functional electrical stimulation (FES) of denervated muscles in humans
| Reference | Purpose | Subjects | Protocol/Parameters used | Results/Conclusion |
|---|---|---|---|---|
| Albertin et al., 2018 | To investigate how the skin was affected by electrical stimulation | Three human subjects with complete conus and cauda equina lesion. | The FES protocol, for first few months- 4 s stimulation duration with impulses of 150 ms and 2 s stimulation pause with impulse pause of 500 ms. The parameters are modified with the progress. The home-based FES (hb-FES) conducted for 2 yr. | Skin biopsies from both left and right thighs before and after about 2 yr of FES were analyzed. Four out of six biopsies found statistically significant increase in the epidermis. On an average, there was a 28% increase in the epidermis biopsies post the hb-FES. |
| Gargiulo et al., 2011 | Using finite element analysis to determine structural changes in bone, muscle and tendon during FES treatment | Two males with conus cauda syndrome | Parameters as used in the European Project RISE (Kern et al., 2009): 0–4 mon 2–6 mon 4–12 mon 8–24 mon |
Muscle restoration in the FES group was visible through segmentation and Finite Element Analysis (increasing from 42% to 58%); decrease in fat from 8% to 2% was also observed. |
| Kern et al., 2010 | To determine the effectiveness of home-based FES training | Twenty-five patients with complete conus/cauda equina lesions | At the beginning of the treatment, biphasic stimulation impulses of very long duration (120– 150 ms, 60–75 ms per phase at high intensity (up to 80 V and 250 mA). Parameters modified every 12 wk for 2 yr (depending on patient’s improvement). |
Increase in muscle cross sectional area of the quadriceps muscle by an average of 35%. An increase in the size of muscle fiber was observed; especially in vastus lateralis by an average of 75% from 16.6 ± 14.3 to 29.1 ± 23.3 µm; recovery of tetanic contractility of muscles observed. |
| Kern et al., 1999 | To discuss the effects of FES after denervation | Patients ranged from 1–30 yr of denervation; the degeneration rate varied widely | Performed using non-invasive surface electrodes with gel or wet sponge conducting layer; Parameters were very specific for each individual; Pulse duration was varied more than 150 ms in individuals with severe degeneration and regulated down to 40 ms with the training progress. | The study proved that the restoration and functional use of denervated muscle is possible using FES therapy. Seven patients could regain active standing up, although it took up to 2 yr of training for the first standing up; other patients achieved different levels of progress in their muscles. |
Simulation parameters used with lower motor neuron
Currents
There are two types of electrical stimulation current that have been commonly used to activate the paralyzed muscles: either DC or alternating current (AC). In DC, the flow of electrons is in one direction and is considered constant, while the flow using AC could be either unidirectional (monophasic) or bidirectional (biphasic); symmetrical or asymmetrical. AC can be represented as a sine wave to visualize the flow of electrons. AC is usually delivered at high frequencies which reduces the effect of skin impedance, resulting in more current being delivered to the motor units of the target area. With continuous DC, the current intensity needs to reach the threshold for the motor unit for muscle contraction to occur. The ability of DC to cause chemical changes is one of the biggest differences between AC and DC (Howe and Trevor, 2002; McDonough and Kitchen, 2002).
Pulse
A pulse could be discrete, or it could be a series of pulses or a pulse train. A sudden step of short duration of voltage or current to form a steady value is called a pulse (Howe and Trevor, 2002). The pulse can have different shapes like square, rectangular and triangular pulse. They could be delivered at different durations depending on the requirement of the training.
Pulse duration
The pulse duration, also known as the pulse width is the time between the onset and offset of the stimulus. It is expressed in µs or ms. The pulse duration required to generate a contraction in denervated muscle is usually 100–1000 times greater than that required to elicit the same response for the innervated muscles (Kern et al., 1999).
It is worth noting that the outcome of the training is primarily determined by the initial condition of the denervated muscles. Those in the chronic stage, 2 years or more after denervation, may take up to 12 weeks to illicit a twitch response and even longer to create a tetanic contraction. Denervated muscles following 5 years of SCI, especially in older individuals (> 40 years of age) may require extensive training and longer duration of training to elicit reasonable tension. Muscle restoration within 5 years of denervation is more desirable. Post 5 years of denervation, there is severe loss of muscle and fat substitution advances so far that it is highly challenging at this point to attempt restoration (Kern et al., 2002).
Initially, the LPWS with limited frequency is likely to elicit twitches of the denervated muscles in the acute and subacute phase after SCI. After 3 months, the pulse duration can be shortened by 30–50 ms with the intention to gradually increase the frequency of the pulses to 15–25 Hz. This may enhance tension generating capacity in the denervated knee extensor muscles and would allow the stimulated muscles to gradually produce tetanic contraction. It is recommended that the frequency of the pulses should not exceed 30 Hz to reduce the possibility of developing muscle fatigue (Gorgey et al., 2009). Direct muscle stimulation with the described parameters requires attention due to a risk of developing electrical burns as a result of high current density developed under the electrodes. Applications are commonly performed in prone position or in standing position in a standing frame or on a tilting table. It is recommended to stimulate the muscles for 30–60 minutes every day (Boncompagni et al., 2007; Gargiulo et al., 2011). In our laboratory, we have been successful in stimulating the denervated knee extensor in a seated position. The emphasis is to move the leg fully against gravity prior to load the muscles using standard ankle weights.
Summary and Conclusion
Denervation after SCI is a serious consequence that leads to extensive muscle atrophy. Skeletal muscle integrity is vital for the maintenance of cellular and whole-body metabolism. It is important to continue developing exercise interventions that benefit these individuals and decrease the risk for chronic diseases. LPWS is an emerging rehabilitation intervention technique which is used to stimulate denervated muscles. Parameters like pulse duration, frequency, and amplitude are altered in order to achieve muscle contractions. The excitability of denervated muscles is extremely low and hence, the pulse duration needs to be exponentially long to achieve desirable contraction. Muscle atrophy can be mitigated, and hypertrophy can be achieved in denervated muscles via LPWS and appropriate loading. The application may be feasible in clinical settings and may help reducing the associated healthcare costs. Future studies using LPWS are likely to maximize the rehabilitation potential for clinical care of LMN in persons with SCI.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Abilmona SM, Sumrell RM, Gill RS, Adler RA, Gorgey AS. Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord. 2019;57:229–239. doi: 10.1038/s41393-018-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams R. In a study in pathology. New York: Harper & Row; 1975. Diseases in muscle; pp. 112–130. [Google Scholar]
- 3.al-Amood WS, Lewis DM, Schmalbruch H. Effects of chronic electrical stimulation on contractile properties of long‐term denervated rat skeletal muscle. J Physiol. 1991;441:243–256. doi: 10.1113/jphysiol.1991.sp018749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertin G, Hofer C, Zampieri S, Vogelauer M, Löfler S, Ravara B, Guidolin D, Fede C, Incendi D, Porzionato A, De Caro R, Baba A, Marcante A, Piccione F, Gargiulo P, Pond A, Carraro U, Kern H. In complete SCI patients, long-term functional electrical stimulation of permanent denervated muscles increases epidermis thickness. Neurol Res. 2018;40:277–282. doi: 10.1080/01616412.2018.1436877. [DOI] [PubMed] [Google Scholar]
- 5.Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Ashley Z, Sutherland H, Lanmuller H, Unger E, Li F, Mayr W, Kern H, Jarvis JC, Salmons S. Determination of the chronaxie and rheobase of denervated limb muscles in conscious rabbits. Artif Organs. 2005;29:212–215. doi: 10.1111/j.1525-1594.2005.29037.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashley Z, Sutherland H, Russold MF, Lanmüller H, Mayr W, Jarvis JC, Salmons S. Therapeutic stimulation of denervated muscles: The influence of pattern. Muscle Nerve. 2008;38:875–886. doi: 10.1002/mus.21020. [DOI] [PubMed] [Google Scholar]
- 8.Bax L, Staes F, Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomised controlled trials. Sports Med. 2005;35:191–212. doi: 10.2165/00007256-200535030-00002. [DOI] [PubMed] [Google Scholar]
- 9.Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6 doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boncompagni S. Severe muscle atrophy due to spinal cord injury can be reversed in complete absence of peripheral nerves. Eur J Transl Myol. 2012;22:161–200. [Google Scholar]
- 11.Boncompagni S, Kern H, Rossini K, Hofer C, Mayr W, Carraro U, Protasi F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci U S A. 2007;104:19339–19344. doi: 10.1073/pnas.0709061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourguignon G, Bourguignon L. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J. 1987;1:398–402. doi: 10.1096/fasebj.1.5.3678699. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz A, Bugaresti J. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- 14.Bueno CRS, Pereira M, Favaretto IA, Junior, Bortoluci CHF, Santos TCPD, Dias DV, Daré LR, Rosa GM Junior Electrical stimulation attenuates morphological alterations and prevents atrophy of the denervated cranial tibial muscle. Einstein (São Paulo) 2017;15:71–76. doi: 10.1590/S1679-45082017AO3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson BM. The biology of long-term denervated skeletal muscle. Eur J Transl Myol. 2014;24:3293–3293. doi: 10.4081/ejtm.2014.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson BM, Billington L, Faulkner J. Studies on the regenerative recovery of long-term denervated muscle in rats. Restor Neurol Neurosci. 1996;10:77–84. doi: 10.3233/RNN-1996-10203. [DOI] [PubMed] [Google Scholar]
- 17.Carlson BM, Borisov AB, Dedkov EI, Dow D, Kostrominova TY. The biology and restorative capacity of long-term denervated skeletal muscle. Basic Appl Myol. 2002;12:249–256. [Google Scholar]
- 18.Carp SJ. Philadelphia: F.A Davis Company; 2015. Peripheral nerve injury: an anatomical and phisiological approach for physical therapy intervention. [Google Scholar]
- 19.Carraro U, Boncompagni S, Gobbo V, Rossini K, Zampieri S, Mosole S, Ravara, Nori A, Stramare R, Ambrosio F, Piccione F, Masiero S, Vindigni V, Gargiulo P, Protasi F, Kern H, Pond A, Marcante A. Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J Transl Myol. 2015;25:77–92. doi: 10.4081/ejtm.2015.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carraro U, Rossini K, Zanin M, Rizzi C, Mayr W, Kern H. Induced myogenesis in long-term permanent denervation: perspective role in functional electrical stimulation of denervated legs in humans. Basic Appl Myol. 2002;12:53–63. [Google Scholar]
- 21.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 22.Cheetham J, Perkins JD, Jarvis JC, Cercone M, Maw M, Hermanson JW, Mitchell LM, Piercy RJ, Ducharme NG. Effects of functional electrical stimulation on denervated laryngeal muscle in a large animal model. Artif Organs. 2015;39:876–885. doi: 10.1111/aor.12624. [DOI] [PubMed] [Google Scholar]
- 23.Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJ. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27:1337–1341. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Devivo MJ, Jackson AB. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil. 2005;86:1208–1213. doi: 10.1016/j.apmr.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Coleman MP, Freeman MR. Wallerian degeneration, WldS, and Nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis CA, Chong SL, Kornelsen I, Uwiera RR, Seres P, Mushahwar VK. The effects of intermittent electrical stimulation on the prevention of deep tissue injury: varying loads and stimulation paradigms. Artif Organs. 2011;35:226–236. doi: 10.1111/j.1525-1594.2011.01212.x. [DOI] [PubMed] [Google Scholar]
- 27.Demiryürek S, Babül A. Effects of vitamin E and electrical stimulation on the denervated rat gastrocnemius muscle malondialdehyde and glutathione levels. Int J Neurosci. 2004;114:45–54. doi: 10.1080/00207450490249374. [DOI] [PubMed] [Google Scholar]
- 28.DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- 29.Doherty JG, Burns AS, O’Ferrall DM, Ditunno JF. Prevalence of upper motor neuron vs lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J Spinal Cord Med. 2002;25:289–292. doi: 10.1080/10790268.2002.11753630. [DOI] [PubMed] [Google Scholar]
- 30.Dolbow DR, Gorgey AS, Dolbow JD, Gater DR. Seat pressure changes after eight weeks of functional electrical stimulation cycling: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19:222–228. doi: 10.1310/sci1903-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberstein A, Eberstein S. Electrical stimulation of denervated muscle: is it worthwhiles? Med Sci Sports Exerc. 1996;28:1463–1469. doi: 10.1097/00005768-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Franek A, Kostur R, Polak A, Taradaj J, Szlachta Z, Blaszczak E, Dolibog P, Dolibog P, Koczy B, Kucio C. Using high-voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized controlled clinical study. Ostomy Wound Manage. 2012;58:30–44. [PubMed] [Google Scholar]
- 34.Fujita N, Murakami S, Arakawa T, Miki A, Fujino H. The combined effect of electrical stimulation and resistance isometric contraction on muscle atrophy in rat tibialis anterior muscle. Bosn J Basic Med Sci. 2011;11:74–79. doi: 10.17305/bjbms.2011.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garber S, Rintala D. Pressure ulcers in veterans with spinal cord injury: a retrospective study. J Rehabil Res Dev. 2003;40:433–441. doi: 10.1682/jrrd.2003.09.0433. [DOI] [PubMed] [Google Scholar]
- 36.Gargiulo P, Reynisson PJ, Helgason B, Kern H, Mayr W, Ingvarsson P, Helgason T, Carraro U. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res. 2011;33:750–758. doi: 10.1179/1743132811Y.0000000007. [DOI] [PubMed] [Google Scholar]
- 37.Geddes L. Accuracy limitations of chronaxie values. IEEE Trans Biomed Eng. 2004;51:176–181. doi: 10.1109/TBME.2003.820340. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie J. The nature of the bone changes assosication with nerve iujuries and disuse. J Bone Joint Surg Br. 1954;3:464–473. [Google Scholar]
- 39.Gilliatt R, Hjorth R. Nerve conduction during Wallerian degeneration in the baboon. J Neurol Neurosurg Psychiatry. 1972;35:335–341. doi: 10.1136/jnnp.35.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab. 2011;36:107–114. doi: 10.1139/H10-091. [DOI] [PubMed] [Google Scholar]
- 41.Gorgey AS, Khalil RE, Gill R, Gater DR, Lavis TD, Cardozo CP, Adler RA. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma. 2019;36:2631–2645. doi: 10.1089/neu.2018.6136. [DOI] [PubMed] [Google Scholar]
- 42.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 43.Gorgey AS, Gater DR., Jr Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12:1–7. doi: 10.1310/sci1204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorgey AS, Black CD, Elder CP, Dudley GA. Effects of electrical stimulation parameters on fatigue in skeletal muscle. J Orthop Sports Phys Ther. 2009;39:684–692. doi: 10.2519/jospt.2009.3045. [DOI] [PubMed] [Google Scholar]
- 45.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile – Part I. J Spinal Cord Med. 2014;37:693–702. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorgey AS, Mather KJ, Poarch HJ, Gater DR. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imagings. J Spinal Cord Med. 2011;34:99–109. doi: 10.1179/107902610X12911165975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulati AK. Restoration of denervated skeletal muscle transplants after reinnervation in rats. Restor Neurol Neurosci. 1990;2:23–29. doi: 10.3233/RNN-1990-2103. [DOI] [PubMed] [Google Scholar]
- 48.Hofer C, Mayr W, Stöhr H, Unger E, Kern H. A stimulator for functional activation of denervated muscles. Artif Organs. 2002;26:276–279. doi: 10.1046/j.1525-1594.2002.06951.x. [DOI] [PubMed] [Google Scholar]
- 49.Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, Hayes KC, Woodbury MG. Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil. 2010;91:669–678. doi: 10.1016/j.apmr.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 50.Howe T, Trevor M. Low-frequency currents - an introduction. In: Kitchen S, Bazin S, Bellis E, editors. Electrotherapy: evidence based practice. London: Churchill Livingstone; 2002. pp. 233–240. [Google Scholar]
- 51.Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Leiby BE, Sendecki J, Singh H, Modlesky CM. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2016;97:1413–1422. doi: 10.1016/j.apmr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450:1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 53.Katirji B. Electromyography in clinical practice: a case study approach. USA: Mosby; 2007. [Google Scholar]
- 54.Kaye AH. Essential Neurosurgery. London: Churchill Livingstone; 1991. Classification of Nerve Injuries; pp. 333–334. [Google Scholar]
- 55.Kern H, Carraro U, Adami N, Biral D, Hofer C, Forstner C, Mödlin M, Vogelauer M, Pond A, Boncompagni S, Paolini C, Mayr W, Protasi F, Zampieri S. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair. 2010;24:709–721. doi: 10.1177/1545968310366129. [DOI] [PubMed] [Google Scholar]
- 56.Kern H, Hofer C, Mayr W, Carraro U, Vogelauer M. European Project RISE: Partners, protocols, demography. Eur J Transl Myol. 2009;19:211–216. [Google Scholar]
- 57.Kern H, Hofer C, Mödlin M, Forstner C, Raschka-Högler D, Mayr W, Stöhr H. Denervated muscles in humans: limitations and problems of currently used functional electrical stimulation training protocols. Artif Organs. 2002;26:216–218. doi: 10.1046/j.1525-1594.2002.06933.x. [DOI] [PubMed] [Google Scholar]
- 58.Kern H, Hofer C, Strohhofer M, Mayr W, Richter W, Stöhr H. Standing up with denervated muscles in humans using functional electrical stimulation. Artif Organs. 1999;23:447–452. doi: 10.1046/j.1525-1594.1999.06376.x. [DOI] [PubMed] [Google Scholar]
- 59.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinalcord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 60.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocina P. Body composition of spinal cord injured adultss. Sports Med. 1997;23:48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- 62.Kruger EA, Pires M, Ngann Y, Sterling M, Rubayi S. Comprehensive management of pressure ulcers in spinal cord injury: Current concepts and future trends. J Spinal Cord Med. 2013;36:572–585. doi: 10.1179/2045772313Y.0000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lapalombella R, Kern H, Adami N, Biral D, Zampieri S, Scordari A, di Tullio S, Marini M. Persistence of regenerative myogenesis in spite of down-regulation of activity-dependent genes in long-term denervated rat muscle. Neurol Res. 2008;30:197–206. doi: 10.1179/174313208X281091. [DOI] [PubMed] [Google Scholar]
- 64.Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE. Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal Cord. 2011;49:917–923. doi: 10.1038/sc.2011.19. [DOI] [PubMed] [Google Scholar]
- 65.Liu LQ, Nicholson GP, Knight SL, Chelvarajah R, Gall A, Middleton FR, Ferguson-Pell MW, Craggs MD. Pressure changes under the ischial tuberosities of seated individuals during sacral nerve root stimulation. J Rehabil Res Dev. 2006;43:209–218. doi: 10.1682/jrrd.2005.04.0078. [DOI] [PubMed] [Google Scholar]
- 66.Lubińska L. Early course of wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977;130:47–63. doi: 10.1016/0006-8993(77)90841-1. [DOI] [PubMed] [Google Scholar]
- 67.Maffiuletti NA, Cometti G, Amiridis IG, Martin A, Pousson M, Chatard JC. The effects of electromyostimulation training and basketball practice on muscle strength and jumping ability. Int J Sports Med. 2000;21:437–443. doi: 10.1055/s-2000-3837. [DOI] [PubMed] [Google Scholar]
- 68.Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D, Jr, Dudley GA. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:1502–1504. doi: 10.1016/j.apmr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 69.Mayr W, Hofer C, Bijak M, Rafolt D, Unger E, Reichel M, Sauermann S, Lanmueller H, Kern H. Functional electrical stimulation (FES) of denervated muscles: existing and pospective technological solutions. Basic Appl Myol. 2002;12:287–290. [Google Scholar]
- 70.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 71.McDonough S, Kitchen S. Neuromuscular and muscular electrical stimulation. In: Kitchen S, Bazin S, Bellis E, editors. Electrotherapy: evidence based practice. London: Churchill Livingstone; 2002. p. 241.p. 258. [Google Scholar]
- 72.Md D, Clasey J. Body composition assessment in spinal cord injury clinical trials. Top Spinal Cord Inj Rehabil. 2006;11:36–49. [Google Scholar]
- 73.Mödlin M, Forstner C, Hofer C, Mayr W, Richter W, Carraro U, Protasi F, Kern H. Electrical stimulation of denervated muscles: first results of a clinical study. Artif Organs. 2005;29:203–206. doi: 10.1111/j.1525-1594.2005.29035.x. [DOI] [PubMed] [Google Scholar]
- 74.Monaco GN, Brown TJ, Burgette RC, Fargo KN, Akst LM, Jones KJ, Foecking EM. Electrical stimulation and testosterone enhance recovery from recurrent laryngeal nerve crush. Restor Neurol Neurosci. 2015;33:571–578. doi: 10.3233/RNN-130334. [DOI] [PubMed] [Google Scholar]
- 75.Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20:385–392. doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohira Y. Effects of denervation and deafferentation on mass and enzyme activity in rat skeletal muscles. Jpn J Physiol. 1989;39:21–31. doi: 10.2170/jjphysiol.39.21. [DOI] [PubMed] [Google Scholar]
- 77.Pedersen BK1, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 78.Pedersen BK, Febbraio MA. Muscles exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 79.Pichon F, Chatard JC, Martin A, Cometti G. Electrical stimulation and swimming performance. Med Sci Sports Exerc. 1995;27:1671–1676. [PubMed] [Google Scholar]
- 80.Purves D, Augustine G, Fitzpatrick D, Katz L, LaMantia AS, McNamara J, Williams S. Neuroscience. 3rd. Sunderland, MA: Sinauer Associates, Inc; 2004. The lower motor neuron circuits and motor control; pp. 371–392. [Google Scholar]
- 81.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109–109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil. 2013;94:2166–2173. doi: 10.1016/j.apmr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 83.Seddon H. Advances in nerve repair. Triangle. 1968;8:252–259. [PubMed] [Google Scholar]
- 84.Sharma N, Coughlin L, Porter RG, Tanzer L, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci. 2009;27:633–644. doi: 10.3233/RNN-2009-0489. [DOI] [PubMed] [Google Scholar]
- 85.Sheridan DM, Isseroff RR, Nuccitelli R. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J Invest Dermatol. 1996;106:642–646. doi: 10.1111/1523-1747.ep12345456. [DOI] [PubMed] [Google Scholar]
- 86.Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci. 1992;110:90–98. doi: 10.1016/0022-510x(92)90014-c. [DOI] [PubMed] [Google Scholar]
- 87.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol (1985) 2000;88:1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- 89.Strauss DJ, Devivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 90.Sumrell RM, Nightingale TE, McCauley LS, Gorgey AS. Anthropometric cutoffs and associations with visceral adiposity and metabolic biomarkers after spinal cord injury. PLoS One. 2018:13–e0203049. doi: 10.1371/journal.pone.0203049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tamaki H, Yotani K, Ogita F, Hayao K, Nakagawa K, Sugawara K, Kirimoto H, Onishi H, Kasuga N, Yamamoto N. Electrical stimulation of denervated rat skeletal muscle ameliorates bone fragility and muscle loss in early-stage disuse musculoskeletal atrophy. Calcif Tissue Int. 2017;100:420–430. doi: 10.1007/s00223-017-0250-y. [DOI] [PubMed] [Google Scholar]
- 92.Tower S. Atrophy and degeneration in skeletal muscle. Am J Anat. 1935;56:1–43. [Google Scholar]
- 93.Vivó M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol. 2008;211:180–193. doi: 10.1016/j.expneurol.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 94.Wroblewski R, Edström L, Jakobsson F. Effect of short time denervation on intracellular elemental content and fibre atrophy pattern of slow and fast twitch rat muscle. J Submicrosc Cytol Pathol. 1989;21:685–690. [PubMed] [Google Scholar]
- 95.Zamarioli A, Battaglino RA, Morse LR, Sudhakar S, Maranho DA, Okubo R, Volpon JB, Shimano AC. Standing frame and electrical stimulation therapies partially preserve bone strength in a rodent model of acute spinal cord injury. Am J Phys Med Rehabil. 2013;92:402–410. doi: 10.1097/PHM.0b013e318287697c. [DOI] [PubMed] [Google Scholar]
- 96.Zamarioli A, Maranho DA, Butezloff MM, Moura PA, Volpon JB, Shimano AC. Anatomic changes in the macroscopic morphology and microarchitecture of denervated long bone tissue after spinal cord injury in rats. Biomed Res Int 2014. 2014:853159. doi: 10.1155/2014/853159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch. 2009;458:525–535. doi: 10.1007/s00424-009-0643-5. [DOI] [PubMed] [Google Scholar]


