Abstract
Surgical treatment of peripheral nerve injuries is still a major challenge in human clinic. Up to now, none of the well-developed microsurgical treatment options is able to guarantee a complete restoration of nerve function. This restriction is also effective for novel clinically approved artificial nerve guides. In this review, we compare surgical repair techniques primarily for digital nerve injuries reported with relatively high prevalence to be valuable attempts in clinical digital nerve repair and point out their advantages and shortcomings. We furthermore discuss the use of artificial nerve grafts with a focus on chitosan-based nerve guides, for which our own studies contributed to their approval for clinical use. In the second part of this review, very recent future perspectives for the enhancement of tubular (commonly hollow) nerve guides are discussed in terms of their clinical translatability and ability to form three-dimensional constructs that biomimick the natural nerve structure. This includes materials that have already shown their beneficial potential in in vivo studies like fibrous intraluminal guidance structures, hydrogels, growth factors, and approaches of cell transplantation. Additionally, we highlight upcoming future perspectives comprising co-application of stem cell secretome. From our overview, we conclude that already simple attempts are highly effective to increase the regeneration supporting properties of nerve guides in experimental studies. But for bringing nerve repair with bioartificial nerve grafts to the next level, e.g. repair of defects > 3 cm in human patients, more complex intraluminal guidance structures such as innovatively manufactured hydrogels and likely supplementation of stem cells or their secretome for therapeutic purposes may represent promising future perspectives.
Keywords: bioartificial nerve graft, biological nerve graft, cell transplantation, cellular products, luminal structures, peripheral nerve repair
Introduction
Peripheral nerve injuries are revealed in about 2.8% of all trauma surgeries (Boecker et al., 2019). In this respect, they are more likely to appear if the upper extremity of the human body is affected (Kouyoumdjian et al., 2017). In association to peripheral nerve injuries affecting the upper extremity, the common and proper digital nerves, the median nerves, and the ulnar nerves are most frequently injured (Kouyoumdjian et al., 2017). Following a nerve reconstruction, the recovery of fine and gross motor function constitutes the most important success (Fugleholm et al., 2000; Valero-Cabre et al., 2001). But with regard to this, it has to be considered, that, unfortunately, one-third of the patients do not recover significant sensitivity of the affected fingers, leading to numbness and therefore to an impaired function of their whole hand (Neubrech et al., 2016b). The factors determining the success rate of recovery include the severity of the injury, e.g., crush or complete transection, as well as the timespan between the initial injury and the reconstruction (Faroni et al., 2015). Not only the primary injury, but especially unsatisfying outcome of repair approaches are leading to sick leave, a longer rehabilitation period and thereby to further costs for the society (Dahlin and Wiberg, 2017) resulting in serious socioeconomic consequences (Miller et al., 2017).
However, for digital nerve lesions, the recovery times are reported to be considerably short, clinical evaluation of sensory recovery by estimating the 2-Point-Discrimination is an established method and the results can be directly transferred to the functionality of the affected nerve (Boesch et al., 2017). Thus, digital nerve lesions do also represent an ideal subject for clinical research on novel biomimetic peripheral nerve grafts (Lohmeyer et al., 2014).
Over time, several treatment strategies for digital nerve reconstruction have been developed in preclinical models and the most promising approaches were also clinically studied. In order to properly evaluate novel biomimetic peripheral nerve grafts in a preclinical setting, the rat median nerve model has received increasing consideration over the last decade as reviewed by Ronchi et al. (2019). But since the most commonly applied model is injury and repair of the rat sciatic nerve, even more reports present preclinical results from evaluating novel biomimetic peripheral nerve grafts in the latter model (Navarro, 2016; Haastert-Talini, 2017).
This compact review gives a short overview on digital nerve repair approaches currently used in clinical practice. With regard to the application of biomimetic peripheral nerve graft implants, the authors focus on recent reports about clinical use of chitosan-based tubular implants. Since our own work did significantly contribute to the successful translation of this kind of grafts into clinical use, we focus the second part of this paper on recent experimental work using more bendable chitosan-based nerve guides for rat median nerve repair. The paper will close with a critical outlook on promising perspectives to develop nerve implants also for the use in nerves of larger lengths and diameters and long gap injuries (> 3 cm in human patients) in the future, e.g., simple or more complex structuring of the lumen of otherwise hollow tubular implants (Figure 1).
Figure 1.
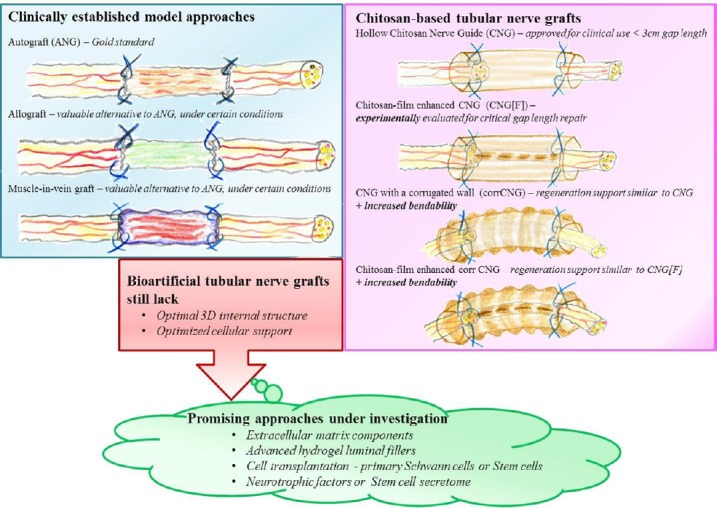
Strategies for peripheral nerve repair.
Model approaches for peripheral nerve repair in the clinics (left/blue box) are commonly based on transplantation of nerve tissue (autograft or decellularized allograft) or vein transplants filled with muscle fibers. So far, optimal three-dimensional structure and cellular support for successful regeneration is only realized in nerve tissue grafts. Different clinically approved bioartificial nerve grafts exist. With regard to bioartificial nerve graft, this review focuses on recent modifications chitosan-based nerve grafts (pink box) and gives an outlook (green balloon) on promising approaches currently under investigation for further modification of tubular nerve grafts.
The following data bases were used for comprehensive literature research: PubMed, Google Scholar, using several combinations of the following words: “peripheral nerve, regeneration, digital nerve, repair, surgical treatment, muscle-in-vein graft, autologous nerve graft, processed nerve allograft, direct coaptation, tissue-engineering, biomimicking, aligned, hydrogel, extracellular matrix, laminin, collagen, chitosan, guidance structure, transplantation, Schwann cell, mesenchymal stem cells, Schwann cell-like, artificial nerve guide, secretome, neurotrophic factors”. The outputs were analyzed with regard to their year of publication and focus of this review and included when relevant to this article and not older than 4 years, unless no recent review of older publications was found. Reports from experimental in vivo studies were excluded from this review in case they were not comprehensively evaluated with more than one functional read-out.
Clinically Established and Applied Surgical Treatment Strategies for Digital Nerve Repair – Advantages and Shortcomings
The intrinsic capability of peripheral nerve fibers to first degenerate upon injury, and then to regrow and finally reinnervate their target tissue is reliant on an intact basal lamina that needs to be provided as guidance structure by neighboring Schwann cells (Jessen and Mirsky, 2016). Since degeneration and removal of axonal and myelin debris is a prerequisite for getting the regeneration process started, it is obvious that spontaneous recovery can only occur in cases of neurapraxia (severe nerve crush) or neurotmesis (axotomy or nerve transection injury (Belanger et al., 2016)). The neurotmesis condition goes along with destruction of all layers of the nerves’ connective tissue and requires surgical intervention for repair (Belanger et al., 2016). Up to now, several microsurgical treatment strategies are available. The decision on the appropriate technique depends on different limitations such as the length of the gap between the nerve ends or the location of the injury, which is considerably mobile in cases of injured digital nerves. The advantages and shortcomings of the respective currently performed surgical treatment strategies are depicted in Table 1. In the following first part of this review, clinically applicable treatment strategies are introduced and discussed. With regard to the outcome of the respective repair method for digital nerves, the authors report meaningful or successful recovery when restoration of sensory recovery is achieved, measured by 2-Point-Discrimination.
Table 1.
Advantages and shortcomings of surgical digital nerve repair approaches currently used in clinical practice with relatively high prevalence
| Treatment strategy | Advantages | Shortcomings |
|---|---|---|
| Direct coaptation | • Method of choice (Dahlin and Wiberg, 2017) • Match of axon sizes, numbers, distributions (Houschyar et al., 2016) • No additional material required (Dahlin and Wiberg, 2017) |
• Only when tension-free (Houschyar et al., 2016; Dahlin and Wiberg, 2017) • Not applicable with very proximal injuries (Moore et al., 2015) • Congruent alignment of nerve fibers (Dahlin and Wiberg, 2017) • Less promising outcome for mixed nerves (Nadi and Midha, 2018) |
| Autologous nerve graft | • Up to 5 cm gap (Siemers and Houschyar, 2017; Wieringa et al., 2018) • Good functional results (Wieringa et al., 2018; Houshyar et al., 2019) • Providing original nerve structure (Houshyar et al., 2019) • Protection against scar tissue formation (Houshyar et al., 2019) • Reduced rejection rate, non-immunogenic (Houshyar et al., 2019) |
• Not off-the-shelf • Donor site morbidity (Muheremu and Ao, 2015; Belanger et al., 2016; Houshyar et al., 2019) • Limited donor tissue availability (Muheremu and Ao, 2015; Wieringa et al., 2018) • Functional recovery not guaranteed (Muheremu and Ao, 2015; Houshyar et al., 2019) • Potential for neuroma formation and persistent pain (Muheremu and Ao, 2015; Belanger et al., 2016; Siemers and Houschyar, 2017) • Polysurgery (Siemers and Houschyar, 2017; Wieringa et al., 2018; Houshyar et al., 2019) • Time consuming (Dahlin and Wiberg, 2017; Siemers and Houschyar, 2017) • Mismatches of axon sizes, numbers, distributions (Li et al., 2017; Siemers and Houschyar, 2017) • Risk of infection (Siemers and Houschyar, 2017) |
| Autologous muscle-in-vein graft | • Up to 6 cm gap (Sabongi et al., 2015) • Good functional results (Jones et al., 2016; Wieringa et al., 2018) • Abundant amount of donor tissue (Sabongi et al., 2015; Stößel et al., 2018) • Minor donor site morbidity (Sabongi et al., 2015; Jones et al., 2016) • Non-immunogenic (Sabongi et al., 2015) • Cost saving (Sabongi et al., 2015) • Permeable (Sabongi et al., 2015) • Good blood supply (Sabongi et al., 2015) • Providing elements of original nerve structure (Sabongi et al., 2015) |
• Not off-the-shelf • No reports for successful repair of nerves with larger diameters • Not valuable for delayed repair in experimental models (Stößel et al., 2018) |
| Processed nerve allograft | • Off-the-shelf product (López-Cebral et al., 2017; Siemers and Houschyar, 2017) • Good functional results for noncritical gap repair (Siemers and Houschyar, 2017; Wieringa et al., 2018) • No donor site morbidity (Jones et al., 2016) • Good biomimicking of nerve structure (Wieringa et al., 2018) • Non-immunogenicity of newer products (Belanger et al., 2016) |
• Disease transmission (He et al., 2015; Siemers and Houschyar, 2017) • Elaborate protocols (Jones et al., 2016; Siemers and Houschyar, 2017) |
| Artificial nerve graft | • No donor site morbidity (Muheremu and Ao, 2015; Belanger et al., 2016) • Off-the-shelf product (Muheremu and Ao, 2015; Jones et al., 2016) • Chemiotropism (Muheremu and Ao, 2015) • Ease of handling (Dahlin and Wiberg, 2017) • Enrichment with luminal fillers possible (Muheremu and Ao, 2015; Houshyar et al., 2019) • Control of properties (Siemers and Houschyar, 2017) |
• Approved for use ≤ 3 cm gap (Belanger et al., 2016; Houshyar et al., 2019) • Variable functional outcomes (Muheremu and Ao, 2015; Belanger et al., 2016) • Material stiffness (Muheremu and Ao, 2015; Belanger et al., 2016) • Single cases of nerve guide extrusion reported (Duncan et al., 2015; Means et al., 2016; Costa Serrao de Araujo et al., 2017) • Inappropriate degradation (Muheremu and Ao, 2015; Houshyar et al., 2019) |
Direct end-to-end suture
For reconstruction of digital nerve injuries, direct coaptation of the proximal and the distal nerve ends by end-to-end suture is presently preferred (Dunlop et al., 2019). If applicable, the primary and immediate end-to-end suture should always be the method of choice. This treatment strategy is, however, only indicated when the gap length guarantees a tension-free connection of the proximal and the distal nerve ends after their debridement (Dahlin and Wiberg, 2017). In this preferred condition, no additional graft material (Dahlin and Wiberg, 2017) as well as mismatch of axon sizes, numbers and distributions within the coapted nerve ends need to be considered (Houschyar et al., 2016). Nevertheless, surgeons need to perform as accurately as possible to guarantee congruent alignment of the nerve fibers (Dahlin and Wiberg, 2017). Otherwise, outcomes for mixed nerves are less promising (Nadi and Midha, 2018). Tension at the nerve coaptation sites has been reported to negatively affect the clinical outcome of nerve regeneration (Neubrech et al., 2016b). Large nerve gaps are consequences of tissue retraction or loss upon transection and a proper debridement of the nerve ends may additionally contribute to elongation of the nerve gaps. The presence of concomitant injuries due to complex trauma, like injuries of bones, tendons or muscles, may also display contraindications for primary nerve repair (Assmus, 2017). Primary nerve repair is attractive in cases of distal, isolated, single nerve injuries. Whenever the nerve injury is found very proximal, a sole primary end-to-end suture is less favorable due to long recovery times (Moore et al., 2015). When the nerve gap is exceeding an extent that prohibits tension-free coaptation, usually nerve grafting or nerve repair by biological or biomimetic implants is performed (Moore et al., 2015; Dahlin and Wiberg, 2017). It is noteworthy, however, that some surgeons prefer to perform an end-to-end repair with subsequent temporal fixation of finger flexion in order to avoid tension at the coaptation sites, rather than using a nerve graft. This can be led back to the time factor for reconstruction, which is shorter when directly suturing the nerve ends instead of bridging the distance between them by an extra implant (Bertleff et al., 2005).
Autologous nerve graft
The gold standard treatment strategy for bridging a peripheral nerve gap is the application of autologous nerve grafts (ANGs) and until today, this graft type is repetitively reported to have the highest probability to result in at least partial functional recovery even when used for long gap repair (Means et al., 2016; Siemers and Houschyar, 2017). Besides, ANGs provide the original nerve structure, protect against scar tissue formation and lead to minor rejection rates (Houshyar et al., 2019). The sensory nerves commonly harvested as donor tissue are the sural nerve, the posterior interosseous nerve, and the medial antebrachial cutaneous nerve (Panagopoulos et al., 2017). Although ANGs represent the gold standard, this repair method does not guarantee complete functional recovery in all patients (Neubrech et al., 2016b). In addition to that, the use of ANGs carries several other downsides such as donor-site morbidity and a limited availability of donor tissue for repair of extended injuries, e.g., of nerve plexus injuries (Faroni et al., 2015; Siemers and Houschyar, 2017). In any case, a more time consuming polysurgery needs to be performed for autologous nerve grafting (Dahlin and Wiberg, 2017; Siemers and Houschyar, 2017; Wieringa et al., 2018), which leads to a higher risk of infection (Siemers and Houschyar, 2017). When autologous donor nerves are used, mismatches of axon sizes, numbers and distributions need to be considered (Li et al., 2017). Also possible neuroma formation at the proximal end of the donor nerve may lead to persisting pain (Neubrech et al., 2016a).
Autologous muscle-in-vein graft
To avoid the downsides of autologous nerve grafting, another alternative to bridge a nerve gap is the application of vein conduits (Sabongi et al., 2015; Siemers and Houschyar, 2017). Either veins from the ipsilateral dorsum of the hand or the palmar forearm can be taken to reconstruct digital nerve lesions (Paprottka et al., 2013). Thus, this method still requires harvest of donor tissue. Besides, a potential collapse of the vein walls could lead to scar formation and impairment of nerve regeneration (Lohmeyer and Machens, 2009). Collapsing of the vein can be prevented by inserting skeletal muscle into the vein conduit. These muscle-in-vein conduits reveal less donor-site morbidity, when compared to ANGs (Siemionow et al., 2010; Manoli et al., 2014). It has been reported that muscle-in-vein conduits could additionally be used to provide a growth permissive collagen and laminin axis to the regenerating axons (Siemionow et al., 2010). Therefore, muscle-in-vein conduits are considered to display valuable implants not only for the reconstruction of sensory but also for mixed nerves in a clinical setting (Battiston et al., 2005; Marcoccio and Vigasio, 2010; Tos et al., 2012; Manoli et al., 2014). While application of either muscle or vein conduits alone have been reported to allow for regeneration of up to 1–2 cm (Brunelli et al., 1993; Battiston et al., 2005; Siemionow et al., 2010), the combination of both, in form of muscle-in-vein-grafts, is considered to be applicable for nerve gaps of up to 6 cm in length (Battiston et al., 2000; Marcoccio and Vigasio, 2010; Manoli et al., 2014).
Processed nerve allograft
Another promising approach towards successful sensory recovery is the use of processed nerve allografts. This type of graft is commercially available as Avance® (AxoGen, Inc., Alachua, FL, USA). It has been described that these decellularized and predegenerated human nerve tissues obtain the nerve continuity on the one hand, as well as provide a supporting microenvironment on the other hand (López-Cebral et al., 2017). The number of applications of this kind of graft is influenced by some debate about a minor risk for disease transmission (He et al., 2015) between the graft donor and the recipient. Production protocols, however, are strictly followed and the outcomes after different processing techniques, e.g., physical and chemical protocols, are well studied in the rat animal model (Lovati et al., 2018) and have shown to be save. By using the RANGER database 50 digital nerve injuries larger than 25 mm defect size in 28 patients were surveyed. Levels of meaningful sensory recovery were achieved in 86% of the patients and can be compared to results after using nerve autografts while no donor site morbidity was caused (Rinker et al., 2017).
Artificial nerve graft
The clinically established biological nerve grafts that have been described above are expected to provide an optimal milieu for nerve regeneration. This includes maintenance of axotomised neurons and their axonal regrowth into a pro-regenerative tissue matrix. After re-growing axons have crossed the nerve gap and reached denervated targets, functional recovery is established in cases with sufficient outcome (Faroni et al., 2015). Appropriate substrates need to be provided at the lesion site. Reactive repair Schwann cells as well as the secretion of trophic and tropic factors contribute to the formation of the optimal milieu (Jessen and Mirsky, 2016). Extracellular matrix components as well as repair Schwann cells guarantee the formation of guidance structures (bands of Büngner) for the re-growing axons (Jessen and Mirsky, 2016).
As substitutes or replacements for biological grafts, artificial nerve grafts have been developed and up to now, a broad range of them has been experimentally studied as reviewed in detail by Tian et al. (2015) and some were also approved for clinical use in humans (Kornfeld et al., 2019). The use of artificial nerve guides allows an adequate refreshment of the nerve stumps even if this increases additionally the gap length between the separated nerve ends (Moore et al., 2015). Ideally, bioartificial nerve conduits are degradable, prevent neuroma formation and inhibit the ingrowth of fibrous tissue (Boecker et al., 2019). Transparent conduits even secure the control of the position of the nerve endings and the presence of blood cloths, which are known to hinder the growth of nerve fibers (Wang et al., 2017), can be visibly excluded. Up to now, a variety of US Food and Drug Administration-approved artificial nerve guides are commercially available. Among them collagen, poly(DL-lactide-ε-caprolactone), and chitosan are the most frequently used ones. Different materials provide differentially controllable properties (Siemers and Houschyar, 2017) and support for the regeneration process chemiotropism (Muheremu and Ao, 2015). Nevertheless, these artificial nerve guides have only been proven to be applicable in humans for bridging gap lengths of up to 3 cm, and their use for larger gaps will most likely fail to support recovery of sensory function (Kornfeld et al., 2019).
Therefore, until today, the clinical use of bioartificial nerve grafts is still less frequent than the use of biological conduits (Siemers and Houschyar, 2017). Developmental research is continuing to be very active in this field and specific focus is given to luminal fillers enriching hollow tubular implants.
Chitosan-based bioartificial nerve grafts
Our own collaborative work on the development of an improved bioartificial nerve graft did comprehensively study chitosan-based nerve guides in the last years (Haastert-Talini et al. 2013). Chitosan is a hydrolysation derivative from chitin and with its inherent bioactivity, it supports the survival and orientation of Schwann cells (Yuan et al., 2004), promotes the survival and differentiation of neuronal cells (Freier et al., 2005; Simoes et al., 2011), as well as could prevent painful neuroma formation (Marcol et al., 2011). In comprehensive preclinical analyses, our collaboration partners and ourselves have proven that hollow chitosan nerve guides support axonal and functional regeneration of acutely injured and repaired rat sciatic nerves (gap length 10–15 mm) (Gonzalez-Perez et al., 2015; Meyer et al., 2016a; Shapira et al., 2016). We have further studied the outcome of delayed nerve repair, a condition that is also clinically relevant, and could again demonstrate the regeneration-supporting properties of chitosan-based nerve guides (Stenberg et al., 2017). Interestingly, 45-days delayed repair of critical gap length (15 mm) rat sciatic nerve defects with muscle-in-vein grafts was less supportive for functional motor recovery as determined during 150 days of observation than application of chitosan-based nerve guides (Stößel et al., 2018).
Our study results contributed to the approval of chitosan nerve guides for clinical use for reconstruction of nerve gaps up to 2.6 cm (Reaxon® Nerve Guides (Boecker et al., 2019)). The first clinical study using pieces of chitosan-based nerve guides in human patients was published by Neubrech et al. (2018). Seventy-four patients with sensory nerve injuries in the hand were subjected to either classical end-to-end repair or end-to-end repair combined with application of ring-like structures derived from Reaxon® Nerve Guides in order to cover the sutures from outside. The additional application of a chitosan-protection ring surrounding the sutures is reported to significantly increase recovery of tactile gnosis and sensitivity when compared to the commonly applied unprotected end-to-end suture. Since only a short circular segment of the Reaxon® Nerve Guide was installed around the end-to-end suture (Neubrech et al., 2018), no signs of decreased finger mobility in patients with digital nerve repair were reported.
For bridging a nerve gap in digital nerves, it needs to be considered that nerve guides need to provide an increased bendability to follow joint movements and to provide preserved collapse stability during the same. In this context, especially the material stiffness plays a crucial role (Muheremu and Ao, 2015; Belanger et al., 2016). In order to address these needs, innovative chitosan nerve guides with a corrugated wall structure (corrCNGs) were developed. We demonstrated that in the 15 mm rat sciatic nerve gap repair model, these nerve guides revealed comparable results to the classic hollow chitosan nerve guides with regard to the functional outcome, and, at the same time, corrCNGs demonstrated preserved compression resistance and significantly increased flexibility (Stößel et al., 2018).
We have also shown previously that longitudinal introduction of chitosan-films into otherwise hollow nerve guides further increases the regeneration supporting properties of these two-chambered chitosan-film enhanced chitosan nerve guides (CNG[F]s) in comparison to classic hollow chitosan nerve guides (CNGs) in the 15 mm rat sciatic nerve model (Meyer et al., 2016a). Perforations within the films did allow for formation of potentially capillary guiding tissue bridges between the two nerve strands that regenerated along both sides of the film (Stenberg and Stößel et al., 2017). We have hypothesized that increased vascularization along with chitosan-driven increased availability of pro-regenerative macrophages inside the implants was responsible for better functional recovery after implantation of CNG[F]s (Stenberg and Stößel et al., 2017).
In order to address the question, if digital nerve repair could receive profit from using more bendable chitosan nerve guides (corrCNGs) in gap repair, we recently compared the performance of classic hollow CNGs, two-chambered CNG[F]s, and two-chambered corrCNG[F]s to that of ANGs in the enhanced rat median nerve model (Stößel et al., 2017; Dietzmeyer et al., 2019). The model is based on the classic rodent median nerve model as reviewed in Ronchi et al. (2019) and we have shown before that our combination of functional evaluation of reinnervation of thecal muscle motor endplates, recovery of skilled forelimb reaching, and recovery of reflex-based grasping allows precise determination of the onset, progress, and completeness of motor recovery (Stößel et al., 2017). In our latest study, 10 mm rat median nerve gaps were repaired by the above listed implants (CNGs, CNG[F]s, corrCNG[F]s, ANGs) and we demonstrated that animals receiving corrCNG[F]s or ANGs displayed comparable recovery of thenar muscle reinnervation, skilled forelimb reaching, and electrodiagnostic recordings (Dietzmeyer et al., 2019). We therefore conclude, that corrCNG[F]s represent a good alternative for bridging gaps of small nerves in a mobile extremity region. The insertion of the unstructured chitosan-film was certainly the simplest way to modify the properties of the graft by introducing a guiding structure for invading cells and regrowing axons inside the hollow nerve guide.
Up to now, there is one engineered nerve guidance channel with a more complex intraluminal guidance structure on the market (Bozkurt et al., 2017). Neuromaix® is a collagen-based tube, which is filled with an inner sponge-like structure also made out of collagen. In experimental studies, this device proved to support the structural as well as functional regeneration process in the 2 cm rat sciatic nerve injury and repair model (van Neerven et al., 2017). A clinical first-in-human study from 2017 also revealed the safety of the reconstruction of sural nerve gaps with the Neuromaix® device (Bozkurt et al., 2017). While this is already a progressive development for the field, the embodiment of physical cues as well as extracellular matrix components as luminal fillers for peripheral nerve guides has been proposed as promising approach towards the development of an ideal nerve bridge (Muheremu and Ao, 2015; Sarker et al., 2018b; Wieringa et al., 2018). The following paragraphs will focus on some attempts that have recently been evaluated and revealed promising results towards reaching increased similarity of nerve guide luminal structures with the original nerve structural cues and properties.
Novel Approaches to Increase the Performance of Tubular Nerve Implant by Adding Luminal Fillers
The predominant aim of current research is to not only provide axonal guidance structures but also to mimic the physiological environment as far as possible during peripheral nerve regeneration. Therefore, researchers in the field of artificial nerve guide design focus on different approaches for further accelerating functional regeneration after nerve gap repair with bioartificial grafts. A brief overview of the different approaches including their advantages, shortcomings and potential for their translation into the clinic (translatability) is depicted in Table 2. The following section of this compact review deals with attempts to fill the nerve guide devices’ remaining lumen with supporting physical cues and molecules such as natural components of the extracellular matrix through optimized hydrogels and/or cell and secretome supplementation. Although there are many existing reports dealing elaborately with the innovative concepts for possible luminal fillers, in our review we focus on a few approaches that were already comprehensively studied for their outcome in vivo. Our selection was supported by the abundance of reports from comprehensive outcome measurements, like electrodiagnostic evaluation of motor function, and, where applicable (e.g., in the critical gap length sciatic nerve model), recovery of mechanosensitivity, and unbiased evaluation of axonal regeneration by standard nerve morphometry.
Table 2.
Advantages and shortcomings and rating of the translatability of recently researched approaches for enhancing nerve guidance channels with biomicking luminal fillers
| Biomimicking approach | Advantages | Shortcomings | Translatability |
|---|---|---|---|
| Natural ECM components | • Representing neurotropic factors (Gonzalez-Perez et al., 2017; Wieringa et al., 2018) • Biomimicking (Du et al., 2017; Wieringa et al., 2018) • Hydrophilic (Sarker et al., 2018b) • Low stiffness (Hsu et al., 2019) • Non-toxic degradation products (Sarker et al., 2018b) • Non-immunogenic (Sarker et al., 2018b; Wieringa et al., 2018) • Enrichment with supplementary cues (Gonzalez-Perez et al., 2018) |
• Lack of structural guidance (Sarker et al., 2018a) • Impairment by high concentrations (Gonzalez-Perez et al., 2017; Wieringa et al., 2018) • Instability (Sarker et al., 2018b) • Production costs (Sarker et al., 2018b) |
√ (chemical modification) |
| Advanced Hydrogels | • Representing neurotropic factors (Gonzalez-Perez et al., 2018) • Biomimicking (Wieringa et al., 2018; Hsu et al., 2019) • Hydration (Sarker et al., 2018b; Wieringa et al., 2018) • Quality control (Carballo-Molina and Velasco, 2015) • Stability (Sarker et al., 2018b) • Structural guidance (Muheremu and Ao, 2015; Hsu et al., 2019) • Low stiffness (Wieringa et al., 2018; Hsu et al., 2019) • Enrichment with supplementary cues (Gonzalez-Perez et al., 2018; Wieringa et al., 2018; Hsu et al., 2019) |
• Lack of cell binding peptides (Sarker et al., 2018b; Wieringa et al., 2018) • Impairment by high concentrations (Gonzalez-Perez et al., 2017; Wieringa et al., 2018) • Uncontrolled degradation (Wieringa et al., 2018) • Hydrophobic properties (Sarker et al., 2018b) |
√ |
| Linear guidance structures | • Structural guidance (Wieringa et al., 2018; Houshyar et al., 2019) • Off-the-shelf product (Bozkurt et al., 2017) • Cell attachment (Houshyar et al., 2019) • Diverse sources (Wieringa et al., 2018) |
• Production costs • Combination with cells possibly needs supportive milieu (Meyer et al., 2016a) |
√ (material of approved conduits) |
| Cell transplantation | • Release of neurotropic and neurotrophic factors (Muheremu and Ao, 2015; Belanger et al., 2016; Sarker et al., 2018b) • Biomimicking (Muheremu and Ao, 2015; Gonzalez-Perez et al., 2018) • Differentiation of stem cells (Muheremu and Ao, 2015; Jones et al., 2016; Sullivan et al., 2016) • Gradient derived guidance (Hsu et al., 2019) • Genetic modification (Sarker et al., 2018b) • Remyelination (Jones et al., 2016; Sullivan et al., 2016) |
• Donor site morbidity for the use of primary Schwann cells (Jones et al., 2016; Gonzalez-Perez et al., 2018) • Difficult to harvest primary Schwann cells (Houshyar et al., 2019) • Cultivation/storage costs (Jones et al., 2016; Gonzalez-Perez et al., 2018) • Ethical concerns (Jones et al., 2016) • Limited viability (Jones et al., 2016) • Limited availability (Jones et al., 2016) • Immunogenicity (Sarker et al., 2018b) • Arrangement within conduit may be difficult / crucial • Clinical trials only for central nervous system (Houshyar et al., 2019) • Cell-type specific potentials for differentiation and / or proliferation (Jones et al., 2016; Sarker et al., 2018b) |
? |
| Neurotrophic factors | • Biomimicking (Belanger et al., 2016; Sarker et al., 2018b) • Gradient derived guidance (Hsu et al., 2019) • Promote cell survival (Sarker et al., 2018b) • Induce cell proliferation, differentiation (Ching et al., 2018) |
• Short bioactivity/Half-life time (Belanger et al., 2016; Li et al., 2017; Sarker et al., 2018b) • Instability (Li et al., 2017) • Production costs (Sarker et al., 2018b) • Unpredictable release, leakage (Houshyar et al., 2019) ·• Limited availability of clinical data for peripheral nerve • Inconsistent data on appropriate dosage (Belanger et al., 2016) |
√ (protection during scaffold manufacturing) |
ECM: Extracellular matrix; √: probable; ?: under debate.
Extracellular matrix components as three-dimensional luminal fillers for advanced peripheral nerve guides
The extracellular matrix (ECM) is as a three-dimensional network arranged in the intercellular space of all tissues. With regard to the peripheral nerve, the ECM is found in the basal lamina of Schwann cells as well as in the endoneurium. Playing an important role in cell migration, proliferation, differentiation, structural support, and intercellular communication, different ECM components were used by researchers in the field of peripheral nerve repair.
Natural extracellular matrix components
First attempts in using molecules of the ECM as luminal fillers have been made several decades ago. Glycoproteins of the ECM, such as collagen and laminin, have been used in various experimental studies and shown to effectively support peripheral nerve regeneration as reviewed elsewhere (Fairbairn et al., 2015; Dalamagkas et al., 2016; Lackington et al., 2017; Boni et al., 2018; Wieringa et al., 2018). In many of the earlier experimental studies on ECM components as luminal fillers, silicone tubes have been used as nerve guidance channels. And, although silicone has once been the most frequently used material for bridging peripheral nerve gaps in experimental animal models, the materials’ stiffness and non-degradability may lead to compression of the regenerated nerve tissue as well as to a fibrous foreign body reaction due to a permanent fibrotic encapsulation (Pinho et al., 2016). Consequently, the use of silicone tubes does not provide potential for clinical translation.
Luminal fillers should therefore be introduced in nerve guide materials of clinical relevance which are mainly collagen, chitosan, Poly(DL-lactide-ε-caprolactone), and human nerve allografts as already demonstrated (Kornfeld et al., 2019). Natural ECM components (Table 2) do not only represent neurite guiding cues via cell binding motifs (Gonzalez-Perez et al., 2017; Wieringa et al., 2018), they can also be applied safely due to their non-immunogenicity and non-toxic degradation products (Sarker et al., 2018b). Also newer studies focus on the use of for example collagen as natural ECM component as reviewed by Jahromi et al. (2019). In a 35 mm dog sciatic nerve model nerve growth factor loaded longitudinally oriented collagen conduits have been shown to allow functional as well as morphological nerve regeneration within a 9 months follow-up period (Yao et al., 2018). Here, the mechanical characteristics of the classic ECM component have already been enhanced. However, without modifying and adapting their mechanical characteristics, their use goes along with several disadvantages. On the one hand, their isotropic character lacks structural guidance which results in a higher axonal dispersion, lowering the probability that regenerating axons reach the distal target tissues. Secondly, their original mechanical properties lead to an instability that would prevent an additional seeding with additional cues (Sarker et al., 2018a). Therefore ECM components are nowadays used in form of hydrogels which allow the adaptation of mechanical properties where appropriate, and qualify them for their combination with e.g., neurotrophic factors or living cells.
Optimized extracellular matrix components - hydrogels
Hydrogels are characterized by their high water content, which allows the diffusion and uptake but also the release of soluble molecules. The use of matrices as luminal fillers should thereby enable a balance between the infiltration of nutrients required by potentially transplanted cells and the protection of these cells from endogenous immune cells or antibodies (Jahromi et al., 2019). However, the extent of incorporation and release mechanisms are strongly dependent on the controllable and adaptable mechanical properties of the hydrogel, e.g. pore size and the molecular weight and electrical charge of the hydrogel components (Carballo-Molina and Velasco, 2015).
Despite innovative manufacturing techniques, it is still an unresolved question, how ECM components within gels should be delivered in order to precisely model the intraluminal properties of biological autologous nerve grafts. In our own collaborative work, we have been analyzing a composite hydrogel, consisting of high molecular weight hyaluronic acid and laminin, which to our surprise did not show regeneration permissive properties in the 15 mm rat sciatic nerve injury and repair model, although it had performed very well in previous in vitro studies (Meyer et al., 2016b). A possible explanation for the failure of some kind of hydrogels in their function as regeneration supporting luminal fillers of peripheral nerve implants in vivo could simply be attributed to mechanical hindrance of axonal growth. In this context the concentration of the hydrogel is of outmost importance and lower concentrations may perform better (Dalamagkas et al., 2016; Dodla et al., 2019). As reviewed by Sarker et al. (2018a) this has also been shown when diluted collagen or laminin gels within silicone tubes were compared to more concentrated gels in a 4–6 mm mouse sciatic nerve model. Interestingly, in our animal study the impairment of regeneration was partly resolved by the co-application of low molecular weight fibroblast growth factor-2 overexpressing Schwann cells (Meyer et al., 2016a). We and other researchers stress that luminal fillers should at best mimic the endoneurial tubes as accurately as possible and thereby provide guiding channels for cellular and axonal ingrowth (Sarker et al., 2018b). And indeed, several reports exist on possibilities for aligning ECM components as part of biomimetic nerve engineering strategies.
Optimized hydrogels – alignment and releasing systems
Fibrils of hydrogels may be either aligned by electrical and magnetic fields, by gradients, or by physical and chemical cues to better mimic the endoneurial tubes. This attempt was evaluated by an interesting study, which was carried out in 2017, dealing with an aligned three-dimensional fibrin nanofiber hydrogel (Du et al., 2017). This hydrogel was not only meant to mimic the ECM but also the fibrin cable that is initially formed when peripheral nerve injuries are repaired by means of nerve guidance channels. In the early stage of nerve regeneration, the fibrin cable is the first present loosely aligned matrix that forms along the nerve guidance channels between the proximal and the distal stump. The fibrin formation is crucial for directing the cell invasion and thereby priming axonal regeneration (Dodla et al., 2019). In the study of Du et al. (2017), the aligned three-dimensional fibrin nanofiber hydrogel (AFG) was produced by electrospinning and molecular self-assembly. In vitro analyses showed the ability of the AFG to align Schwann cells parallel to the fibrin nanofibers so that it was afterwards used in vivo in a chitosan nerve guide to bridge 10 mm rat sciatic nerve defects comparing it to hollow chitosan tubes, non-aligned fibrin nanofiber hydrogel (RFG), and ANGs (Du et al., 2017). The AFG group revealed better motor recovery (evaluated by means of CatWalk gait analyses, Sciatic functional index, and electrodiagnostic recordings) when compared to the RFG and hollow tube groups. Furthermore, the bioengineered grafts supported successful axonal regrowth towards the distal target already 6 weeks after surgery as well as a higher nerve fiber density and remyelination in the distal stump 12 weeks after surgery when compared to RFG and empty tube groups (Du et al., 2017). Another advantage, which should be considered when talking about the use of hydrogels for peripheral nerve regeneration, is their consistency. In contrast to other rigid luminal fillers, soft hydrogels with low stiffness (Table 2) would not lead to any restriction of mobility especially with regard to injuries of peripheral nerves in highly mobile areas of the body, such as the digital nerves. Additionally, hydrogels can even be adapted towards mimicking the rigidity of the initial fibrin cable (Du et al., 2017) and the natural ECM.
A second promising example for a regenerative matrix based on collagen type 1 and either containing additional laminin or fibronectin was evaluated by the Navarro group (Gonzalez-Perez et al., 2018). The authors used this composition either as standard hydrogel or further stabilized it by drying-compression and rolling. This luminal filler was placed into hollow chitosan nerve grafts and was surveyed in the 15 mm rat sciatic nerve model.
The group showed that not only adding fibronectin to the collagen type 1-based matrices enhanced peripheral nerve regeneration but also that especially the additional stabilization further increased the outcome by increasing Schwann cell migration and axonal growth. Besides, stabilization and rolling probably leads to slower degradation of the hydrogel components. This may lead to the preservation of their initial characteristic properties and a longer lasting supportive effect when compared to non-stabilized hydrogels.
With the help of orientated ECM components, self-alignment of additionally incorporated regeneration supporting cells can be accomplished, which displays an important approach towards biomimicking of elongated repair Schwann cells playing a key role in natural nerve regeneration. In the presence of endogenous cell-generated tension, cells and also ECM components show a physiological capability to build directed three-dimensional constructs (Georgiou et al., 2013). However, especially when cells are part of these anisotropic three-dimensioral constructs, it has to be taken into account, that again the stability of the cellular anisotropic hydrogels is a crucial factor to make them good candidates for clinical repair. In this context Georgiou et al. designed an aligned collagen matrix containing highly aligned Schwann cells making it stable by plastic compression (Georgiou et al., 2013). This concept was used for aligning fibronectin or laminin matrices with mesenchymal stem cells or Schwann cells within tethered collagen type 1-based gels, which afterwards underwent stabilization and rolling. Combinations with aligned Schwann cells revealed the best regenerative outcome, leading to 100% functional recovery rate compared to combinations with aligned mesenchymal stem cells (90%) or acellular combinations (75%; Gonzalez-Perez et al., 2018).
However, cell transplantation is always accompanied with concerns about their final clinical approval. Cell transplantation would indeed become obsolete if injectable hydrogels themselves would exhibit a suitable permeability for internal cell migration and furthermore boost cell proliferation, distribution, and network forming. Most of the classic injectable nonporous hydrogels are characterized by uncontrolled degradation of their components (Table 2). If not appropriately stabilized, the hydrogel degeneration process will very likely not be consistent with the rate of tissue formation and with that of cell infiltration, proliferation, and neovascularization. Furthermore, by applying an optimized design and crosslinking it to the optimized density, not only degradation will be controlled but also release of incorporated growth factors would become steerable. Very recently, a versatile adaptable hydrogel with spontaneously formed micropores has been developed and described by Hsu et al. (2019). This novel hydrogel consists of differently charged building blocks made of photocrosslinkable gelatin methacrylate and chitosan oligomer-methacrylate and has been shown to improve cell migration and proliferation. Through controlled material degradation and resorption, a nerve growth factor gradient was created within the gel. Functional recovery was evaluated by nerve conduction velocity measurements and calculation of gastrocnemius muscle weight ratios (5 mm rat sciatic nerve model). To confirm the functional results, nerve fiber densities and morphometry of axon diameters and myelin sheaths were evaluated. Recovery of gastrocnemius muscle weight as well as nerve fiber densities and remyelination in the distal stump were comparable to the gold standard, the autograft.
New approaches towards cell transplantation
Although displaying a promising candidate for cell transplantation after peripheral nerve injuries, the use of Schwann cells goes along with several burdens that might limit their clinical use (Sarker et al., 2018b). To avoid an immune response of the receiving patient, the use of autologous Schwann cells would be needed and obviously in the recent years, autologous stem cells have been proposed to be an alternative.
Stem cell transplantation
In contrast to Schwann cells, mesenchymal stem cells can easily be harvested from the bone marrow and different other tissues like mobilized peripheral blood, adipose tissue, the placenta, or the umbilical cord. Moreover, these multipotent cells can not only be differentiated into chondrocytes, osteoblasts adipocytes or neural lineages but also into Schwann cell-like phenotypes (Fairbairn et al., 2015; Faroni et al., 2015; Sullivan et al., 2016; Jiang et al., 2017). There are different types of stem cells which are studied in the field of peripheral nerve regeneration. Embryonic stem cells are indeed able to differentiate into Schwann cell-like phenotypes but their availability is limited and their use goes along with the risk of teratoma formation and immunoreaction. On the other hand, induced pluripotent stem cells, e.g. somatic cells with a stem cell-like phenotype, do not lead to immune rejection but entail the risk of teratoma formation as well. Neural crest stem cells can be harvested minimal invasively, e.g., as hair follicle neural crest stem cells from the skin, and are thereby abundantly available (Jones et al., 2016). One clinical study from Grimoldi et al. (2015) used autologous skin-derived stem cells within a collagen conduit for treatment of one patient with polyinjured motor and sensory nerves of the upper arm with a gap length of 8–10 cm. However, the 3 cm collagen conduits were only placed at the proximal and distal stump and a sural nerve guide was inserted between the stem cell containing collagen conduits. The 3-year-follow-up period revealed a partial but not complete restoration of motor and sensory function. However, the patient was salvaged from upper arm amputation making skin-derived stem cells a good candidate for bridging longer gaps. Adipose-derived stem cells are also harvested minimally invasive by liposuction and have the capability to be differentiated into Schwann cells. But it is known that they on the one hand may de-differentiate back into stem cell and on the other hand may not differentiate to Schwann cells or Schwann-like cells in vivo. For bone marrow-derived mesenchymal stem cells, invasive harvesting of autologous cells as part of bone marrow biopsy is required. Nevertheless, they can be differentiated in Schwann cell-like cells, thereby producing regeneration supportive factors, such as nerve growth factor, brain-derived neurotrophic factor, myelin basic protein, and glial cell line-derived neurotrophic factor. Based on these differences, it has to be taken into account that the clinical potential of stem cells varies depending on their origin (Jones et al., 2016; Sullivan et al., 2016).
Secretome supplementation – a non-immunogenic alternative?
Traditional cell-based therapies, e.g., transplantation of Schwann cells go along with the requirement of an autologous cell origin to avoid immune rejection. As we have demonstrated, the harvesting of donor cells depends on the cell type and must not compulsorily be maximally invasive, especially in terms of stem cells derived from adipose tissue. However, it still remains questionable if these cells have the potential to be ever readily available as comfortable off-the-shelf products. Therefore, newer approaches aim at using rather the secreted extracellular vesicles, the so-called secretome, consisting of relatively non-immunogenic bioactive molecules with paracrine effects on adjacent cells and tissues, e.g., cytokines, chemokines, immunomodulatory molecules, and growth factors that might influence tissue responses to injuries (Konala et al., 2016). As reviewed by Ferreira et al. (2018), the secretome composition of adult stem cells is strongly dependent on the surrounding microenvironment making it possible to precondition these cells with several factors in order to improve their therapeutic capacity depending on the injured tissue. Interestingly, culturing the cells in hypoxic conditions previously (0–10% O2) leads to the rescue of ischemic rat cortical neurons in vitro by the expression of higher levels of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and vascular endothelial growth factor (Kim et al., 2015), a factor also promoting angiogenesis, vasculogenesis, and neuritogenesis in peripheral nerve regeneration (Muratori et al., 2018). Additionally, preconditioning adult stem cells with inflammatory cytokines makes them exhibit the immunomodulatory ability to guide monocyte differentiation towards anti-inflammatory macrophages (Ferreira et al., 2018), which are also known to support peripheral nerve regeneration (Mokarram et al., 2017; Stenberg and Stößel et al., 2017). Taking all these characteristics together, the secretome of stem cells becomes a potentially promising candidate for peripheral nerve regeneration. To the best of our knowledge, there is only little available data about the therapeutic effect of the stem cell secretome in the field of peripheral nerve regeneration up to now. One study underlines that the secretome of Schwann cell-like differentiated adipose stem cells might be a useful therapeutic approach for peripheral nerve injuries as it was able to enhance the neurite outgrowth in vitro (Ching et al., 2018). Sugimura-Wakayama et al. (2015) investigated the effect of human exfoliated deciduous teeth stem cell secretome within silicone nerve guides on peripheral nerve regeneration in vivo in the 10 mm rat sciatic nerve model. Their study revealed successful functional recovery and significantly higher numbers of regenerated axons in the distal nerve stump when compared to simply medium filled silicone tubes suggesting the secretion of various trophic factors that enhance peripheral nerve regeneration (Sugimura-Wakayama et al., 2015). However, the strong dependence of the secretome on its microenvironment goes along with difficulties in homogenizing it for clinical use. Therefore, it is essential to conduct future research that deal with creating preconditions that lead to homogenous secretomes, which would guarantee homogenous regeneration outcomes.
Conclusion
In cases of severe peripheral nerve injuries, when none of the axons as well as the three layers of the connective tissues are preserved, the restoration of a complete functional and axonal regeneration remains a major challenge. Despite progresses in microsurgical treatment strategies, none of the current repair methods guarantees complete recovery. By means of this review, we have given a selective overview on recent research focuses on diverse approaches towards improving intraluminal structure and microenvironment of artificial nerve grafts. The simpliest way is introduction of a central plain guidance structure as reported above for chitosan-film enhanced chitosan nerve guides. More complex attempts will certainly even better biomimick the original nerve structure and components, when designing a three-dimensional biomimicking nerve guide, however, it has to be taken into account that their translatability strongly depends on the material used. Ideally, upcoming new hydrogel manufacturing techniques may potentially overcome the drawbacks of hydrogel-based peripheral nerve regeneration. Innovative hydrogels within permeable nerve guidance channels should have the capability to attract and incorporate endogenous growth factor-producing cells as the translation of exogenous cell transplantation into clinic would have to pass many burdens. Nevertheless, stem cell therapy may become more and more attractive since candidates like skin-derived stem cells can be harvested minimally invasive and show first promising clinical results. In this context, the sole use of regeneration-supportive biomolecules, secreted by stem cells, are also coming more into focus. If stem cells could be preconditioned in a way that these secretomes are consistently homogenous, this strategy would display an optimal progress with regard to the production of non-immunogenic, readily available off-the-shelf products. Future approaches should concentrate on the combination of realistically translatable materials in order to substitute or replace the autologous nerve graft by an accurate three-dimensional artificial nerve guidance channel.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jianxun Ding, Chinese Academy of Sciences, China.
P-Reviewer: Ding J; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Assmus H. Timing and Decision-Making in Peripheral Nerve Trauma. In: Haastert-Talini K, Assmus H, Antoniadis G, editors. Modern Concepts of Peripheral Nerve Repair. Cham, Switzerland: Springer; 2017. pp. 27–40. [Google Scholar]
- 2.Battiston B, Tos P, Cushway TR, Geuna S. Nerve repair by means of vein filled with muscle grafts I Clinical results. Microsurgery. 2000;20:32–36. doi: 10.1002/(sici)1098-2752(2000)20:1<32::aid-micr6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 4.Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C. Recent strategies in tissue engineering for guided peripheral nerve regeneration. Macromol Biosci. 2016;16:472–481. doi: 10.1002/mabi.201500367. [DOI] [PubMed] [Google Scholar]
- 5.Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am. 2005;30:513–518. doi: 10.1016/j.jhsa.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Boecker A, Daeschler SC, Kneser U, Harhaus L. Relevance and recent developments of chitosan in peripheral nerve surgery. Front Cell Neurosci. 2019;13:104. doi: 10.3389/fncel.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesch CE, Medved F, Held M, Bender D, Schaller HE, Fuchsberger T. Analysis of the two-point discrimination test in daily routine practice. Eur J Plast Surg. 2017;40:333–336. [Google Scholar]
- 8.Boni R, Ali A, Shavandi A, Clarkson AN. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci. 2018;25:90. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozkurt A, Claeys KG, Schrading S, Rodler JV, Altinova H, Schulz JB, Weis J, Pallua N, van Neerven SGA. Clinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide Neuromaix. Eur J Med Res. 2017;22:34. doi: 10.1186/s40001-017-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelli GA, Battiston B, Vigasio A, Brunelli G, Marocolo D. Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery. 1993;14:247–251. doi: 10.1002/micr.1920140407. [DOI] [PubMed] [Google Scholar]
- 11.Carballo-Molina OA, Velasco I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Front Cell Neurosci. 2015;9:13. doi: 10.3389/fncel.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching RC, Wiberg M, Kingham PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018;9:266. doi: 10.1186/s13287-018-1017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa Serrao de Araujo G, Couto Neto B, Harley Santos Botelho R, Carpi Malta M. Clinical evaluation after peripheral nerve repair with caprolactone neurotube. Hand (N Y) 2017;12:168–174. doi: 10.1177/1558944716643277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlin LB, Wiberg M. Nerve injuries of the upper extremity and hand. EFORT Open Rev. 2017;2:158–170. doi: 10.1302/2058-5241.2.160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: A biomaterials approach. Mater Sci Eng C Mater Biol Appl. 2016;65:425–432. doi: 10.1016/j.msec.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Dietzmeyer N, Forthmann M, Leonhard J, Helmecke O, Brandenberger C, Freier T, Haastert-Talini K. Two-chambered chitosan nerve guides with increased bendability support recovery of skilled forelimb reaching similar to autologous nerve grafts in the rat 10 mm median nerve injury and repair model. Front Cell Neurosci. 2019;13:149. doi: 10.3389/fncel.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodla MC, Alvarado-Velez M, Mukhatyar VJ, Bellamkonda RV. Chapter 69-Peripheral nerve regeneration. In: Atala A, Lanza R, Mikos AG, Nerem R, editors. Principles of regenerative medicine. 3rd ed. Boston: Academic Press; 2019. pp. 1223–1236. [Google Scholar]
- 18.Du J, Liu J, Yao S, Mao H, Peng J, Sun X, Cao Z, Yang Y, Xiao B, Wang Y, Tang P, Wang X. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309. doi: 10.1016/j.actbio.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Duncan SFM, Kakinoki R, Rizzo M, Kang W. Extrusion of a neurotube: A case report. Ochsner J. 2015;15:191–192. [PMC free article] [PubMed] [Google Scholar]
- 20.Dunlop RLE, Wormald JCR, Jain A. Outcome of surgical repair of adult digital nerve injury: a systematic review. BMJ Open. 2019;9:e025443. doi: 10.1136/bmjopen-2018-025443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J Stem Cells. 2015;7:11–26. doi: 10.4252/wjsc.v7.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26:5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Fugleholm K, Schmalbruch H, Krarup C. Post reinnervation maturation of myelinated nerve fibers in the cat tibial nerve: chronic electrophysiological and morphometric studies. J Peripher Nerv Syst. 2000;5:82–95. doi: 10.1046/j.1529-8027.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 26.Georgiou M, Bunting SC, Davies HA, Loughlin AJ, Golding JP, Phillips JB. Engineered neural tissue for peripheral nerve repair. Biomaterials. 2013;34:7335–7343. doi: 10.1016/j.biomaterials.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Perez F, Cobianchi S, Heimann C, Phillips JB, Udina E, Navarro X. Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery. 2017;80:465–474. doi: 10.1093/neuros/nyw068. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Perez F, Hernandez J, Heimann C, Phillips JB, Udina E, Navarro X. Schwann cells and mesenchymal stem cells in laminin- or fibronectin-aligned matrices and regeneration across a critical size defect of 15 mm in the rat sciatic nerve. J Neurosurg Spine. 2018;28:109–118. doi: 10.3171/2017.5.SPINE161100. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Perez F, Cobianchi S, Geuna S, Barwig C, Freier T, Udina E, Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2015;35:300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- 30.Grimoldi N, Colleoni F, Tiberio F, Vetrano IG, Cappellari A, Costa A, Belicchi M, Razini P, Giordano R, Spagnoli D, Pluderi M, Gatti S, Morbin M, Gaini SM, Rebulla P, Bresolin N, Torrente Y. Stem cell salvage of injured peripheral nerve. Cell Transplant. 2015;24:213–222. doi: 10.3727/096368913X675700. [DOI] [PubMed] [Google Scholar]
- 31.Haastert-Talini K, Haastert-Talini K, Assmus H, Antoniadis G. Modern Concepts of Peripheral Nerve Repair, 1 Edition. Cham, Switzerland: Springer; 2017. Peripheral Nerve Tissue Engineering: An Outlook on Experimental Concepts; pp. 127–138. [Google Scholar]
- 32.He B, Zhu Q, Chai Y, Ding X, Tang J, Gu L, Xiang J, Yang Y, Zhu J, Liu X. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med. 2015;9:286–295. doi: 10.1002/term.1707. [DOI] [PubMed] [Google Scholar]
- 33.Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, Jew OS, Siemers F, van Schoonhoven J. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int 2016. 2016:4175293. doi: 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houshyar S, Bhattacharyya A, Shanks R. Peripheral nerve conduit: materials and structures. ACS Chem Neurosci. 2019;10:3349–3365. doi: 10.1021/acschemneuro.9b00203. [DOI] [PubMed] [Google Scholar]
- 35.Hsu RS, Chen PY, Fang JH, Chen YY, Chang CW, Lu YJ, Hu SH. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Adv Sci (Weinh) 2019;6:1900520. doi: 10.1002/advs.201900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahromi M, Razavi S, Bakhtiari A. The advance on nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J Tissue Eng Regen Med. 2019 doi: 10.1002/term.2945. doi: 101002/term2945. [DOI] [PubMed] [Google Scholar]
- 37.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int J Mol Sci. 2017 doi: 10.3390/ijms18010094. doi: 103390/ijms18010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones S, Eisenberg HM, Jia X. Advances and future applications of augmented peripheral nerve regeneration. Int J Mol Sci. 2016 doi: 10.3390/ijms17091494. doi: 103390/ijms17091494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YS, Noh MY, Cho KA, Kim H, Kwon MS, Kim KS, Kim J, Koh SH, Kim SH. Hypoxia/reoxygenation-preconditioned human bone marrow-derived mesenchymal stromal cells rescue ischemic rat cortical neurons by enhancing trophic factor release. Mol Neurobiol. 2015;52:792–803. doi: 10.1007/s12035-014-8912-5. [DOI] [PubMed] [Google Scholar]
- 41.Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornfeld T, Vogt PM, Radtke C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med Wochenschr. 2019;169:240–251. doi: 10.1007/s10354-018-0675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouyoumdjian JA, Graca CR, Ferreira VFM. Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurol India. 2017;65:551–555. doi: 10.4103/neuroindia.NI_987_16. [DOI] [PubMed] [Google Scholar]
- 44.Lackington WA, Ryan AJ, O’Brien FJ. Advances in nerve guidance conduit-based therapeutics for peripheral nerve repair. ACS Biomater Sci Eng. 2017;3:1221–1235. doi: 10.1021/acsbiomaterials.6b00500. [DOI] [PubMed] [Google Scholar]
- 45.Li G, Xiao Q, Zhang L, Zhao Y, Yang Y. Nerve growth factor loaded heparin/chitosan scaffolds for accelerating peripheral nerve regeneration. Carbohydr Polym. 2017;171:39–49. doi: 10.1016/j.carbpol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Lohmeyer JA, Machens HG. Basics and current approaches to tissue engineering in peripheral nerve reconstruction. Neurosurg Quart. 2009;19:101–109. [Google Scholar]
- 47.Lohmeyer JA, Kern Y, Schmauss D, Paprottka F, Stang F, Siemers F, Mailaender P, Machens HG. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. J Reconstr Microsurg. 2014;30:227–234. doi: 10.1055/s-0033-1358788. [DOI] [PubMed] [Google Scholar]
- 48.López-Cebral R, Silva-Correia J, Reis RL, Silva TH, Oliveira JM. Peripheral nerve injury: Current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater Sci Eng. 2017;3:3098–3122. doi: 10.1021/acsbiomaterials.7b00655. [DOI] [PubMed] [Google Scholar]
- 49.Lovati AB, D’Arrigo D, Odella S, Tos P, Geuna S, Raimondo S. Nerve repair using decellularized nerve grafts in rat models A review of the literature. Front Cell Neurosci. 2018:12–427. doi: 10.3389/fncel.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manoli T, Schulz L, Stahl S, Jaminet P, Schaller HE. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34:608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 51.Marcoccio I, Vigasio A. Muscle-in-vein nerve guide for secondary reconstruction in digital nerve lesions. J Hand Surg Am. 2010;35:1418–1426. doi: 10.1016/j.jhsa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Marcol W, Larysz-Brysz M, Kucharska M, Niekraszewicz A, Slusarczyk W, Kotulska K, Wlaszczuk P, Wlaszczuk A, Jedrzejowska-Szypulka H, Lewin-Kowalik J. Reduction of post-traumatic neuroma and epineural scar formation in rat sciatic nerve by application of microcrystallic chitosan. Microsurgery. 2011;31:642–649. doi: 10.1002/micr.20945. [DOI] [PubMed] [Google Scholar]
- 53.Means KR, Jr, Rinker BD, Higgins JP, Payne SH, Jr, Merrell GA, Wilgis EF. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y) 2016 doi: 10.1177/1558944715627233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer C, Stenberg L, Gonzalez-Perez F, Wrobel S, Ronchi G, Udina E, Suganuma S, Geuna S, Navarro X, Dahlin LB, Grothe C, Haastert-Talini K. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials. 2016a;76:33–51. doi: 10.1016/j.biomaterials.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Meyer C, Wrobel S, Raimondo S, Rochkind S, Heimann C, Shahar A, Ziv-Polat O, Geuna S, Grothe C, Haastert-Talini K. Peripheral nerve regeneration through hydrogel-enriched chitosan conduits containing engineered Schwann cells for drug delivery. Cell Transplant. 2016b;25:159–182. doi: 10.3727/096368915X688010. [DOI] [PubMed] [Google Scholar]
- 56.Miller C, Peek AL, Power D, Heneghan NR. Psychological consequences of traumatic upper limb peripheral nerve injury: A systematic review. Hand Therapy. 2017;22:35–45. [Google Scholar]
- 57.Mokarram N, Dymanus K, Srinivasan A, Lyon JG, Tipton J, Chu J, English AW, Bellamkonda RV. Immunoengineering nerve repair. Proc Natl Acad Sci U S A. 2017;114:E5077–5084. doi: 10.1073/pnas.1705757114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore AM, Wagner IJ, Fox IK. Principles of nerve repair in complex wounds of the upper extremity. Semin Plast Surg. 2015;29:40–47. doi: 10.1055/s-0035-1544169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muheremu A, Ao Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. Biomed Res Int 2015. 2015:237507. doi: 10.1155/2015/237507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muratori L, Gnavi S, Fregnan F, Mancardi A, Raimondo S, Perroteau I, Geuna S. Evaluation of vascular endothelial growth factor (VEGF) and its family member expression after peripheral nerve regeneration and denervation. Anat Rec (Hoboken) 2018;301:1646–1656. doi: 10.1002/ar.23842. [DOI] [PubMed] [Google Scholar]
- 61.Nadi M, Midha R. 61 - Management of Peripheral Nerve Injuries. In: Ellenbogen RG, Sekhar LN, Kitchen ND, da Silva HB, editors. Principles of Neurological Surgery. Fourth. Philadelphia: Content Repository Only; 2018. pp. 832–841.e832. [Google Scholar]
- 62.Navarro X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur J Neurosci. 2016;43:271–286. doi: 10.1111/ejn.13033. [DOI] [PubMed] [Google Scholar]
- 63.Neubrech F, Heider S, Harhaus L, Bickert B, Kneser U, Kremer T. Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand without a gap: study protocol for a randomized controlled trial. Trials. 2016a:17–48. doi: 10.1186/s13063-015-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neubrech F, Heider S, Otte M, Hirche C, Kneser U, Kremer T. Nerve tubes for the repair of traumatic sensory nerve lesions of the hand: Review and planning study for a randomised controlled multicentre trial. Handchir Mikrochir Plast Chir. 2016b;48:148–154. doi: 10.1055/s-0042-104505. [DOI] [PubMed] [Google Scholar]
- 65.Neubrech F, Sauerbier M, Moll W, Seegmuller J, Heider S, Harhaus L, Bickert B, Kneser U, Kremer T. Enhancing the outcome of traumatic sensory nerve lesions of the hand by additional use of a chitosan nerve tube in primary nerve repair: A randomized controlled bicentric trial. Plast Reconstr Surg. 2018;142:415–424. doi: 10.1097/PRS.0000000000004574. [DOI] [PubMed] [Google Scholar]
- 66.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40:e141–156. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 67.Paprottka FJ, Wolf P, Harder Y, Kern Y, Paprottka PM, Machens HG, Lohmeyer JA. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plast Surg Int 2013. 2013:704589. doi: 10.1155/2013/704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinho AC, Fonseca AC, Serra AC, Santos JD, Coelho JF. Peripheral nerve regeneration: Current status and new strategies using polymeric materials. Adv Healthc Mater. 2016;5:2732–2744. doi: 10.1002/adhm.201600236. [DOI] [PubMed] [Google Scholar]
- 69.Rinker B, Zoldos J, Weber RV, Ko J, Thayer W, Greenberg J, Leversedge FJ, Safa B, Buncke G. Use of processed nerve allografts to repair nerve injuries greater than 25 mm in the hand. Ann Plast Surg. 2017;78:S292–S295. doi: 10.1097/SAP.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 70.Ronchi G, Morano M, Fregnan F, Pugliese P, Crosio A, Tos P, Geuna S, Haastert-Talini K, Gambarotta G. The median nerve injury model in pre-clinical research - A critical review on benefits and limitations. Front Cell Neurosci. 2019;13:288. doi: 10.3389/fncel.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabongi RG, Fernandes M, Dos Santos JB. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res. 2015;10:529–533. doi: 10.4103/1673-5374.155428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarker M, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol J. 2018a;13:e1700635. doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- 73.Sarker MD, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog Neurobiol. 2018b;171:125–150. doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Shapira Y, Tolmasov M, Nissan M, Reider E, Koren A, Biron T, Bitan Y, Livnat M, Ronchi G, Geuna S, Rochkind S. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery. 2016;36:664–671. doi: 10.1002/micr.22418. [DOI] [PubMed] [Google Scholar]
- 75.Siemers F, Houschyar KS. Alternative Strategies for Nerve Reconstruction. In: Haastert-Talini K, Assmus H, Antoniadis G, editors. Modern Concepts of Peripheral Nerve Repair. 1. Cham, Switzerland: Springer; 2017. pp. 127–138. [Google Scholar]
- 76.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30:574–588. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 77.Simoes MJ, Gartner A, Shirosaki Y, Da Costa RMG, Cortez PP, Gartner F, Santos JD, Lopes MA, Geuna S, Varejao ASP, Mauricio AC. In vitro and in vivo chitosan membranes testing for peripheral nerve reconstruction. Acta Medica Port. 2011;24:43–52. [PubMed] [Google Scholar]
- 78.Stenberg L, Stößel M, Ronchi G, Geuna S, Yin Y, Mommert S, Martensson L, Metzen J, Grothe C, Dahlin LB, Haastert-Talini K. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 2017:18–53. doi: 10.1186/s12868-017-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stößel M, Rehra L, Haastert-Talini K. Reflex-based grasping, skilled forelimb reaching, and electrodiagnostic evaluation for comprehensive analysis of functional recovery – the 7 mm rat median nerve gap repair model revisited. Brain Behav. 2017;7:e00813. doi: 10.1002/brb3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stößel M, Wildhagen VM, Helmecke O, Metzen J, Pfund CB, Freier T, Haastert-Talini K. Comparative evaluation of chitosan nerve guides with regular or increased bendability for acute and delayed peripheral nerve repair: A comprehensive comparison with autologous nerve grafts and muscle-in-vein grafts. Anat Rec (Hoboken) 2018;301:1697–1713. doi: 10.1002/ar.23847. [DOI] [PubMed] [Google Scholar]
- 81.Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, Hibi H. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. 2015;24:2687–2699. doi: 10.1089/scd.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: Stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016 doi: 10.3390/ijms17122101. doi: 103390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds cells and biomolecules. Regen Biomater. 2015;2:31–45. doi: 10.1093/rb/rbu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tos P, Battiston B, Ciclamini D, Geuna S, Artiaco S. Primary repair of crush nerve injuries by means of biological tubulization with muscle-vein-combined grafts. Microsurgery. 2012;32:358–363. doi: 10.1002/micr.21957. [DOI] [PubMed] [Google Scholar]
- 85.Valero-Cabre A, Tsironis K, Skouras E, Perego G, Navarro X, Neiss WF. Superior muscle reinnervation after autologous nerve graft or poly-L-lactide-epsilon-caprolactone (PLC) tube implantation in comparison to silicone tube repair. J Neurosci Res. 2001;63:214–223. doi: 10.1002/1097-4547(20010115)63:2<214::AID-JNR1014>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 86.van Neerven SGA, Haastert-Talini K, Boecker A, Schriever T, Dabhi C, Claeys K, Deumens R, Brook GA, Weis J, Pallua N, Bozkurt A. Two-component collagen nerve guides support axonal regeneration in the rat peripheral nerve injury model. J Tissue Eng Regen Med. 2017;11:3349–3361. doi: 10.1002/term.2248. [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Oh S, Lee HA, Kang J, Jeong KJ, Kang SW, Hwang DY, Lee J. In vivo feasibility test using transparent carbon nanotube-coated polydimethylsiloxane sheet at brain tissue and sciatic nerve. J Biomed Mater Res A. 2017;105:1736–1745. doi: 10.1002/jbm.a.36001. [DOI] [PubMed] [Google Scholar]
- 88.Wieringa PA, Goncalves de Pinho AR, Micera S, van Wezel RJA, Moroni L. Biomimetic architectures for peripheral nerve repair: A review of biofabrication strategies. Adv Healthc Mater. 2018;7:e1701164. doi: 10.1002/adhm.201701164. [DOI] [PubMed] [Google Scholar]
- 89.Yao Y, Cui Y, Zhao Y, Xiao Z, Li X, Han S, Chen B, Fang Y, Wang P, Pan J, Dai J. Efect of longitudinally oriented collagen conduit combined with nerve growth factor on nerve regeneration after dog sciatic nerve injury. J Biomed Mater Res B Appl Biomater. 2018;106:2131–2139. doi: 10.1002/jbm.b.34020. [DOI] [PubMed] [Google Scholar]
- 90.Yuan Y, Zhang P, Yang Y, Wang X, Gu X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]


