Abstract
Background:
The hypocretin/orexin system is involved in regulating arousal, and much recent work demonstrates that decreasing hypocretin receptor-1 (HCRTr1) activity using antagonists decreases appetitive behavior, including stimulant drug self-administration and reinstatement.
Methods:
The present study determined the effects of hypocretin-1 and HCRTr1 antagonists on responding reinforced by intravenous (i.v.) cocaine self-administration (0.0125 – 0.05 mg/kg/infusion) in 5 female rhesus monkeys. Responding was examined using 3 schedules of reinforcement: 1) a Fixed interval 1 min, Fixed ratio 10 Chain schedule [FI 1-min (FR10:S)], 2) a Progressive Ratio (PR) schedule, and 3) a cocaine vs. candy.
Results:
Choice schedule: the HCRTr1 antagonist SB-334867 (8–24 mg/kg, i.m.) decreased cocaine taking under the Chain schedule and PR schedule in all 5 monkeys and in 4 of the 5 monkeys under the Choice schedule. d-Amphetamine (0.06 – 0.25 mg/kg, i.m.), tested as a control manipulation, decreased cocaine taking in all 5 monkeys under the Chain schedule. The peptide hypocretin-1 (0.072 mg/kg, i.v.) increased cocaine taking in the monkeys with low rates of cocaine taking under the Chain (3/4) and Choice (4/5) schedules. Reinstatement of extinguished cocaine responding following response-independent delivery of a large dose of cocaine (0.3 mg/kg) was attenuated in 3 of the 5 monkeys by the HCRTr1 antagonist SB-334867.
Conclusions:
These data expand upon work accomplished in predominantly male rodents suggesting that the hypocretin system modulates the response to appetitive stimuli. A better understanding of this system offers promise as a novel approach in medication development for appetitive disorders.
Keywords: Hypocretin, Orexin, Cocaine, Amphetamine, Self-administration, Non-human primate, Rhesus monkey, Female
1. Introduction
The neuropeptides hypocretin-1 and −2 or orexin-1 and orexin-2 (de Lecea et al., 1998; Sakurai et al., 1998) derive from the lateral hypothalamus and project throughout the brain (Koob, 2008). Hypocretins interact with the noradrenergic, cholinergic, serotonergic, and dopaminergic systems as well as with the hypothalamic-pituitary-adrenal (HPA) axis. In concert, these actions have been shown to modulate sleep-wake regulation, energy homeostasis, motivational activation, and stress responsivity (Carter et al., 2009; Giardino and de Lecea, 2014; Sutcliffe and de Lecea, 2002). Given its role in modulating arousal and appetitive behaviors, the hypocretin system offers promise as a novel approach in medication development for appetitive disorders.
Data obtained in laboratory rodents demonstrates a role for hypocretin in the development of sensitization, conditioned-place preference (CPP), self-administration, and reinstatement to cocaine and amphetamine. For example, an HCRTr1 antagonist blocked the development of sensitization to cocaine (Borgland et al., 2006) and amphetamine (Winrow et al., 2010) in rats, and sensitization to amphetamine was associated with increased c-FOS activation in many hypocretin-containing neurons in the rat brain (McPherson et al., 2007). With respect to CPP, Harris et al. (2005) trained a place preference for locations that had been paired with cocaine, morphine, or food in rats and measured c-FOS activation in hypocretin neurons in the lateral hypothalamus. In each case, the acquisition of a CPP was associated with increased c-FOS activation of hypocretin neurons in the lateral hypothalamus, and there was a positive correlation between c-FOS activation and magnitude of the CPP. HCRTr1 antagonism also blocked the development of CPP for an environment paired with cocaine (Gentile et al., 2018).
Few studies have examined the effects of the peptide hypocretin-1 on cocaine self-administration (Boutrel et al., 2005; España et al., 2011). Although there are slight differences across these 2 studies, intracerebroventricular (i.c.v.) infusion of hypocretin-1 did not alter responding that was reinforced under a Fixed Ratio 1 (FR1) schedule of reinforcement in both studies, but infusion of hypocretin-1 into the ventral tegmental area slightly increased responding for cocaine that was reinforced under a progressive ratio (PR) schedule in both studies.
SB-334867 [SB; (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl-urea hydrochloride); Smart et al., 2001] has been the most commonly used HCRTr1 antagonist in studies assessing the role of hypocretin in cocaine reinforcement. The administration of a HCRTr1 antagonist reduced i.v. cocaine self-administration by rats most commonly only when responding was maintained under more complex or effortful schedules of reinforcement. When cocaine was delivered after every response (FR1), HCRTr1 antagonism had minimal effects on cocaine self-administration (España et al., 2010; Smith et al., 2009; Zhou et al., 2012; but see Hutcheson et al., 2011 for an exception). When cocaine was delivered under a larger FR schedule or a PR schedule of reinforcement, HCRTr1 antagonism consistently decreased cocaine self-administration (Borgland et al., 2009; Brodnik et al., 2015; Gentile et al., 2018; Hollander et al., 2012; España et al., 2010; Levy et al., 2017). Assuming that rats who respond with greater effort under more challenging reinforcement schedules are more motivated for cocaine, and this increased motivation is a model for human cocaine abuse, these findings suggest that the hypocretin system may be involved in the transition from lighter to heavier cocaine use. For example, Schmeichel et al. (2017) examined the effects of HCRTr1 antagonism on responding for cocaine in rats who had short-access (ShA) or long-access (LgA) to cocaine during daily sessions. Under the LgA condition in this model, rats accelerated their cocaine use over days, while rats under the ShA condition did not. It is hypothesized that the increase in cocaine intake under the LgA condition is a model for escalating drug use by humans. When cocaine was delivered under an FR1 schedule, HCRTr1 antagonism decreased cocaine self-administration under the LgA condition without affecting responding under the ShA condition. The lack of effect under the ShA condition parallels the earlier studies using a FR1 schedule. Of note, when cocaine was delivered under a PR schedule, HCRTr1 antagonism decreased cocaine self-administration under both the LgA and ShA conditions; this effect under an effortful schedule parallels the above PR studies. In summary, HCRTr1 antagonism significantly affects cocaine-taking behavior, but the effects in rats appear to be modulated by schedule of access to cocaine both effort-wise and duration-wise.
The hypocretin system also plays a major role in modulating reinstatement of cocaine seeking behavior in rats with a history of self-administering i.v. cocaine. The central administration of hypocretin-1 reinstated cocaine-seeking behavior in rats (Boutrel et al., 2005; Wang et al., 2009). In contrast, administration of an HCRTr1 antagonist attenuated cue-induced reinstatement of responding for cocaine in rats (Bentzley and Aston-Jones, 2015; Mahler et al., 2013; Smith et al., 2009, 2010) but had no effect on cocaine-elicited reinstatement (Mahler et al., 2013). Based on the differential effect of HCRTr1 antagonism on cue-induced vs. cocaine-induced reinstatement, Bentzley and Aston-Jones (2015) have suggested that one way that hypocretin systems affect cocaine taking is by modulating the motivational effects of cues paired with cocaine.
The hypocretin system also affects drug seeking, drug taking, and perhaps transition to heavy use of other drugs in addition to cocaine (e.g., Baimel et al., 2015; Corrigall, 2009; Richards et al., 2008; Smith and Aston-Jones, 2012). There is clearly significant data based on rodent studies supporting an important role for hypocretinergic systems in modulating both the motivation to consume cocaine and the possible transition from controlled to uncontrolled patterns of cocaine use. In contrast, there are no data in human and non-human primates on this topic. The first purpose of this series of studies was to investigate the effects of pretreatment with HCRTr1 antagonists and the peptide hypocretin-1 on i.v. cocaine self-administration by experimentally naïve rhesus monkeys. In addition, the effects of pretreatment with an HCRTr1 antagonist on cocaine-induced reinstatement were examined. Although sex differences have been reported in the hypocretin system in a few studies (e.g., Cason and Aston-Jones, 2013; 2014; Grafe et al., 2017; Zhou et al., 2012), the vast majority of laboratory animal studies have been conducted in males. Therefore, the second purpose of this series of studies was to investigate the effects of these hypocretin manipulations in female monkeys.
2. Methods
2.1. Animals
Five adult female rhesus monkeys (Macaca mulatta) initially weighing between 4.9 and 7.7 kg were fitted with a chronic indwelling catheter in the femoral vein (Access Technologies, Skokie, IL) that terminated in a subcutaneous vascular access port (VAP) (Wojnicki et al., 1994; Cooper et al., 2013). Monkeys were housed in customized, squeeze-capable, rack-mounted, non-human primate cages (Hazleton Systems, Inc., Aberdeen, MD) in the AAALAC-approved animal care facility of The New York State Psychiatric Institute. Each monkey had access to 2 identically-sized chambers (61.5 cm wide x 66.5 cm deep x 88 cm high) connected by a 40 cm × 40 cm opening. Water was freely available from spouts located on the back wall of both chambers. Fluorescent room lights were controlled by an automatic timer and were illuminated from 0700 to 1900. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the New York State Psychiatric Institute Animal Care and Use Committee. In addition to operant responding during sessions, other forms of environmental enrichment included access to a variety of other tactile stimuli (e.g., toys, mirrors, and other objects to manipulate), visual access to the other monkeys, music, and television.
All monkeys were experimentally naïve and were trained in getting in and out of primate restraint chairs for experimental sessions. Based on daily assessment of menstrual cycle status, only 2 of the 5 female monkeys exhibited normal menstrual cycles during the period of data collection; the other 3 cycled intermittently. During the initial study phase, monkeys earned their daily food ration during operant sessions on weekdays and received a ration of approximately 7–9 chow each weekend day (High protein monkey diet #5047, 3.37 Kcal/g; LabDiets®, PMI Feeds, Inc., St. Louis, MO). Food-maintained responding was only assessed during the initial phase of the study. Upon completion of the first tests with an HCRTr1 antagonist, operant sessions for food ceased, and all food was given as chow. Monkeys were not food-restricted, and all gained weight over the course of the study.
2.2. Experimental set-up
For self-administration sessions, monkeys were taken from their home cage and placed into primate chairs for cocaine self-administration sessions that began at 9:00 A.M. A custom-designed right-angle Huber needle infusion set was used to connect the VAP to drug and saline infusion pumps (Multi-Phaser, Model NE-1000, New Era Pump Systems, Inc., Farmingdale, NY). The response panel was mounted on the wall in front of each primate chair. Session lights were evenly spaced around the outside edges of each panel. Each panel had 2 Lindsley levers (Gerbrands, Arlington, MA) with stimulus lights above each lever, one for drug and one for candy (for the choice schedule) mounted at the bottom. A food hopper, a pair of lights over the hopper, and a pellet-dispenser (BRS-LVE model PDC-005, Beltsville, MD) were mounted on the outside of the panel. The catheters had a volume of about 0.4 ml that was always filled with saline. Intravenous cocaine reinforcement (0, 0.0125, 0.0250, 0.050, 0.100, and 0.300 mg/kg/infusion) was accomplished by flushing the 0.4 ml of saline through the catheter and then immediately delivering 0.4 ml of cocaine solution followed by a 0.4 ml saline flush to leave 0.4 ml of saline in the catheter space; thus, each infusion consisted of 0.8 ml of fluid delivered over a 20-sec period. A pair of green lights, located at monkey eye level above the lever, flashed for 20 s (1 s on/1 s off) during the delivery of cocaine. Candy reinforcement consisted of 5 plain chocolate M and Ms® (Mars, Hackettstown, NJ; about 4.5 kcal per piece). Red lights above the food hopper were illuminated during candy delivery. All schedule contingencies were programmed using Pascal on a Macintosh (Cupertino, CA) computer.
2.3. Schedules of reinforcement
2.3.1. Chain FI: FR10 schedule
Monkeys were initially trained to respond for food pellets with the response increasing to 10 (FR10) as responding stabilized. Once responding was stable, monkeys were fitted with a chronic indwelling VAP for i.v. drug administration; cocaine was substituted for food pellets, and responding was then shaped under a chain schedule of reinforcement. Monkeys responded under a 2-chain, second-order operant schedule of reinforcement that differentiated responding reinforced by only the stimuli paired with primary reinforcement (conditioned reinforcer) from responding reinforced with the primary reinforcer and reinforcer-related stimuli. Monkeys were initially trained with 10 trials per session, and then the number of trials increased to 15 once behavior stabilized. The first chain of the schedule was a 1- min interval (FI1′), and the second chain of the schedule was a fixed-ratio that required 10 responses (FR10). Monkeys needed to complete the response requirement of the initial FI schedule in order to gain access to the second FR of chain. Completion of 10 responses during the initial 1-min interval was reinforced by only the stimuli paired with primary reinforcement (every 10 responses completed during the 1′-interval was reinforced by drug-related stimuli (FR10:S; Kelleher, 1966). Completion of a single FR10 after the interval ended resulted in reinforcer and paired-stimuli delivery [FI1′ (FR10:S)]. If an FR10 was not completed within 2 min after the expiry of the interval component, then no primary reinforcement occurred. After a 30-second time-out interval, during which no lever lights were illuminated, the next trial started. The FI was signaled by illumination of a single white light above the operant lever. If the response requirement was met, i.e., 10 responses were made after 1 min had elapsed, the FR was signaled by illumination of 2 white lights above the lever. During this initial part of the study, monkeys had a morning session with cocaine available (9:00 AM) and then 2 h later a second session with food pellets available (up to 80) to determine if cocaine self-administration or HCRTr1 antagonist administration affected food intake. Because the initial evaluation of the effects of a HCRTr1 antagonist on responding during the food session were minimal, the food session was then removed from the experimental day.
2.3.2. Progressive ratio schedule
During the PR sessions, the response requirement for a single cocaine infusion increased by 40% after each reinforcer delivery. Because of baseline differences in the reinforcing efficacy of cocaine, 3 monkeys started with an initial ratio of 50 (50, 70, 98, 137, 192…), and 2 started with an initial ratio of 25 (25, 35, 49, 69, 96…). There was a 30-second time-out interval after each reinforcer delivery during which no stimulus lights were illuminated. Each session continued until responding ceased and no reinforcers were received for a minimum of 30 min; the last completed ratio was the breakpoint.
2.3.3. Candy vs. cocaine choice schedule
Responding on the left manipulandum resulted in cocaine delivery, while responding on the right manipulandum resulted in candy delivery. Completion of a single FR resulted in the delivery of cocaine or candy and the presentation of the stimuli paired with reinforcement. Because of baseline differences in the reinforcing efficacy of cocaine, 3 monkeys were tested using a FR50 schedule, and 2 were tested with a FR25 schedule for cocaine; a FR10 schedule for candy delivery was used for all monkeys. Choice sessions consisted of 15 discrete choice trials with a 1-min interval between choice opportunities. Choices were signaled by the illumination of the lights over the cocaine lever and over the candy lever. Making a single response on either lever turned off the light over the non-selected lever and indicated the choice of that option. Once the choice was made, the ratio on that lever had to be completed for delivery of the reinforcer. Completion of the FR requirement resulted in the delivery of cocaine or candy and the presentation of the stimuli paired with reinforcement. There was a 5-min limited hold in effect for each choice trial. If an animal failed to complete the FR on the selected lever in 5 min, the choice opportunity terminated, and after a 1-min time out both lever lights again illuminated to signal another choice trial.
2.4. Reinstatement of cocaine-maintained responding
In order to test the effects of an HCRTr1 antagonist on cocaine-induced reinstatement, reinstatement sessions were accomplished when responding was reinforced with a low dose of cocaine (0.0125 mg/kg/infusion) and the largest self-administered dose of cocaine (0.05 mg/kg/infusion) using an FR10 schedule of reinforcement with 15 maximal doses. Extinction sessions closely matched cocaine-reinforced sessions. After being placed in the chairs, the VAP was punctured with a needle as if the Huber needle was being placed in the port. During the session, the infusion pumps ran and the lights paired with drug delivery flashed as usual once the response requirement for cocaine was met even though no drug was given. Once responding decreased to less than 3 completed response requirements per session, a 2-day test sequence began. The monkeys were given a “free” response-independent large dose of cocaine [0.3 mg/kg; in one monkey a smaller cocaine dose (0.1 mg/kg) was used because the larger dose reliably abolished all responding] for 2 consecutive days. 24 mg/kg of the HCRTr1 antagonist SB or placebo was also given 45 min before the 2 sessions, and SB vs. placebo order was balanced among the 5 monkeys. All monkeys also experienced a second type of extinction session during which they did not receive a needle stick into the VAP and there were no stimulus cues (pump sounds, flashing stimulus cues) paired with completion of the response requirement; the 30-second timeout after completion of the response requirement was the only stimulus change associated with responding. Under these conditions, most or all of the monkeys stopped responding entirely in a matter of days. Because monkeys made so few responses under these conditions, the first extinction procedure described above (VAP puncture, flashing stimulus cues) was used for assessing the effects of the HCRTr1 antagonist SB on cocaine-induced reinstatement.
2.5. Procedure
As listed in Table 1, 8 experimental conditions were examined in the presented order. Five monkeys were tested in each condition, and monkeys were generally tested with the same cocaine doses as a group. Due to a limited supply of the HCRTr1 antagonist RTIOX-276 (Condition 8 in Table 1), only 4 monkeys were tested; the largest monkey was not tested. Drug effects on responding reinforced by cocaine were examined by administering a test dose of drug before the session at most twice per week with placebo administered intermittently. Testing occurred in the early morning (9:00 AM) when daily circulating levels of hypocretin were expected to be low, as hypocretin levels increase across the day in monkeys (Zeitzer et al., 2003). Complete cocaine dose-response functions were determined under each reinforcement schedule but not presented here. Different cocaine doses were tested across the schedules in order to provide a range of cocaine self-administration within each schedule.
Table 1.
Order of Testing Experimental Conditions.
| Hypocretin Manipulation | Cocaine Dose/Infusion | |
|---|---|---|
| Chain Schedule - [FI1’ (FR10:S)] | ||
| 1a) | HCRTr1 antagonist SB-334867 (24 mg/kg) | 0.05 mg/kg |
| 1b) | HCRTr1 antagonist SB-334867 (24 mg/kg) | 0.0125 mg/kg |
| 2) | d-Amphetamine (0.12, 0.25, 0.50 mg/kg) | 0.05 mg/kg |
| 3a) | Hypocretin-1 (0.072 mg/kg) | 0.025 mg/kg |
| 3b) | Hypocretin-1 (0.072 mg/kg) | 0.018 mg/kg |
| Cocaine-induced Reinstatement | ||
| 4a) | HCRTr1 antagonist SB-334867 (24 mg/kg) | 0.0125 mg/kg |
| 4b) | HCRTr1 antagonist SB-334867 (24 mg/kg) | 0.05 mg/kg |
| Cocaine vs. Candy Choice | ||
| 5) | Hypocretin-1 (0.072 mg/kg) | 0.0188 mg/kg |
| 6a) | HCRTr1 antagonist SB-334867 (8, 24 mg/kg) | 0.0188 mg/kg |
| 6a) | HCRTr1 antagonist SB-334867 (8, 24 mg/kg) | 0.025 mg/kg |
| Progressive Ratio Schedule | ||
| 7a) | HCRTr1 antagonist SB-334867 (4, 8, 24 mg/kg) | 0.05 mg/kg |
| 7b) | HCRTr1 antagonist SB-334867 (4, 8, 24 mg/kg) | 0.025 mg/kg |
| 8) | HCRTr1 antagonist RTIOX-276 (4, 8, 16 mg/kg) | 0.025 mg/kg |
HCRTr1 = hypocretin receptor-1.
2.6. Drugs
Cocaine hydrochloride (provided by The National Institute on Drug Abuse) was dissolved into USP sterile saline for injection and filtered using a 0.22 μm millipore filter. The HCRTr1 antagonist SB-334867 [8, 16, 24 mg/kg; ((1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl-urea hydrochloride)); produced by Dr. Fei Liu] was dissolved in dimethyl sulfoxide (DMSO; Alfa Aesor, Ward Hill, MA) at a concentration of 35 mg/ml and injected intramuscularly (i.m.) 45 min prior to the session (Hollander et al., 2012). The hypocretin agonist, hypocretin-1 (Orexin-A; 0.072 mg/kg; PolyPeptide Group, San Diego, CA; courtesy of NIDA) was dissolved in sterile saline at a concentration of 0.6 mg/ml and injected intravenously (i.v.) 10 or 15 min prior to the session (Deadwyler et al., 2007). The limited availability of the agonist peptide prevented testing additional doses. D- Amphetamine (0.06 – 0.5 mg/kg; Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline at a concentration of 10 mg/ml and injected i.m. 15 min prior to the session. The HCRTr1 antagonist RTIOX-276 (4–16 mg/kg; Research Triangle Institute; Research Triangle Park, NC) was dissolved in DMSO at a concentration of 40 mg/ml and injected i.m. 20 min prior to the session (Levy et al., 2017). HCRTr1 antagonist pretreatment times were initially selected based on onset of action in rodent studies and then adjusted based on behavioral effects in the monkeys. Each pretreatment drug dose was tested once. The limited solubility of the HCRTr1 antagonists prevented testing of larger doses.
3. Results
3.1. Effects of HCRTr1 antagonism on cocaine self-administration
3.1.1. Chain FI: FR10 schedule
Under the Chain schedule of cocaine delivery, only 4 of the 5 monkeys self-administered the 0.0125 mg/kg/infusion cocaine dose (Ginger did not). Increasing the dose of cocaine from 0.0125 to 0.05 mg/kg/infusion increased the number of cocaine deliveries in 4 of the 5 monkeys (Fig. 1); 3 of the 5 monkeys self-administered nearly the maximal number of available doses when 0.05 mg/kg/infusion cocaine was available. One monkey (Gina) decreased the number of cocaine deliveries when the higher dose of cocaine was available. Two monkeys (Deena and Gina, shown in open symbols on all figures) self-administered less cocaine than the other 3 monkeys under all 3 schedules. Administration of the HCRTr1 antagonist SB decreased responding for 0.0125 mg/kg/infusion cocaine in 3 of 4 monkeys and decreased responding for 0.05 mg/kg/infusion cocaine in 4 of 5 monkeys. Of note, the decreases in cocaine self-administration following SB administration occurred consistently in the 4 monkeys who were on the ascending limb of the cocaine dose-response function. Responding for food pellets 2 h after the cocaine session was not affected by the HCRTr1 antagonist SB (data not shown).
Fig. 1.
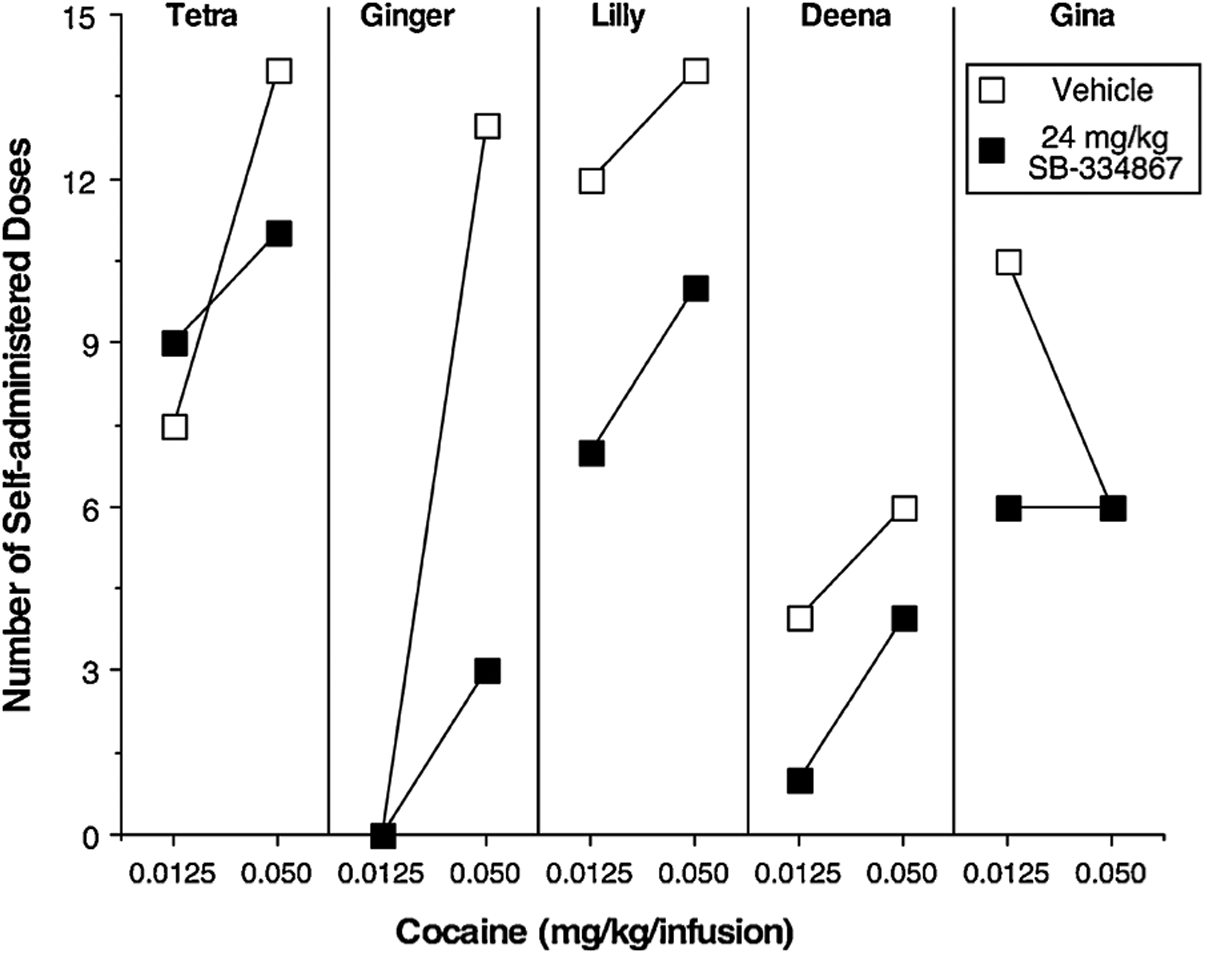
Number of self-administered cocaine doses as a function of cocaine dose and HCRTr1 antagonist SB-334867 dose in 5 female rhesus monkeys. Responding was maintained under a Fixed interval 1 min; Fixed ratio 10 chain schedule [FI 1-min (FR10:S)] with a maximum of 15 drug infusions.
D-Amphetamine, tested as a positive control, produced dose-dependent decreases in responding maintained by 0.05 mg/kg/infusion cocaine in 4 of the 5 monkeys (Fig. 2: top panel) and produced dose-dependent decreases in responding for food pellets 2 h after the cocaine session in 3 of the 5 monkeys (Fig. 2: bottom panel). The magnitude of effects of the HCRTr1 antagonist SB and d-amphetamine on cocaine-maintained responding was similar.
Fig. 2.
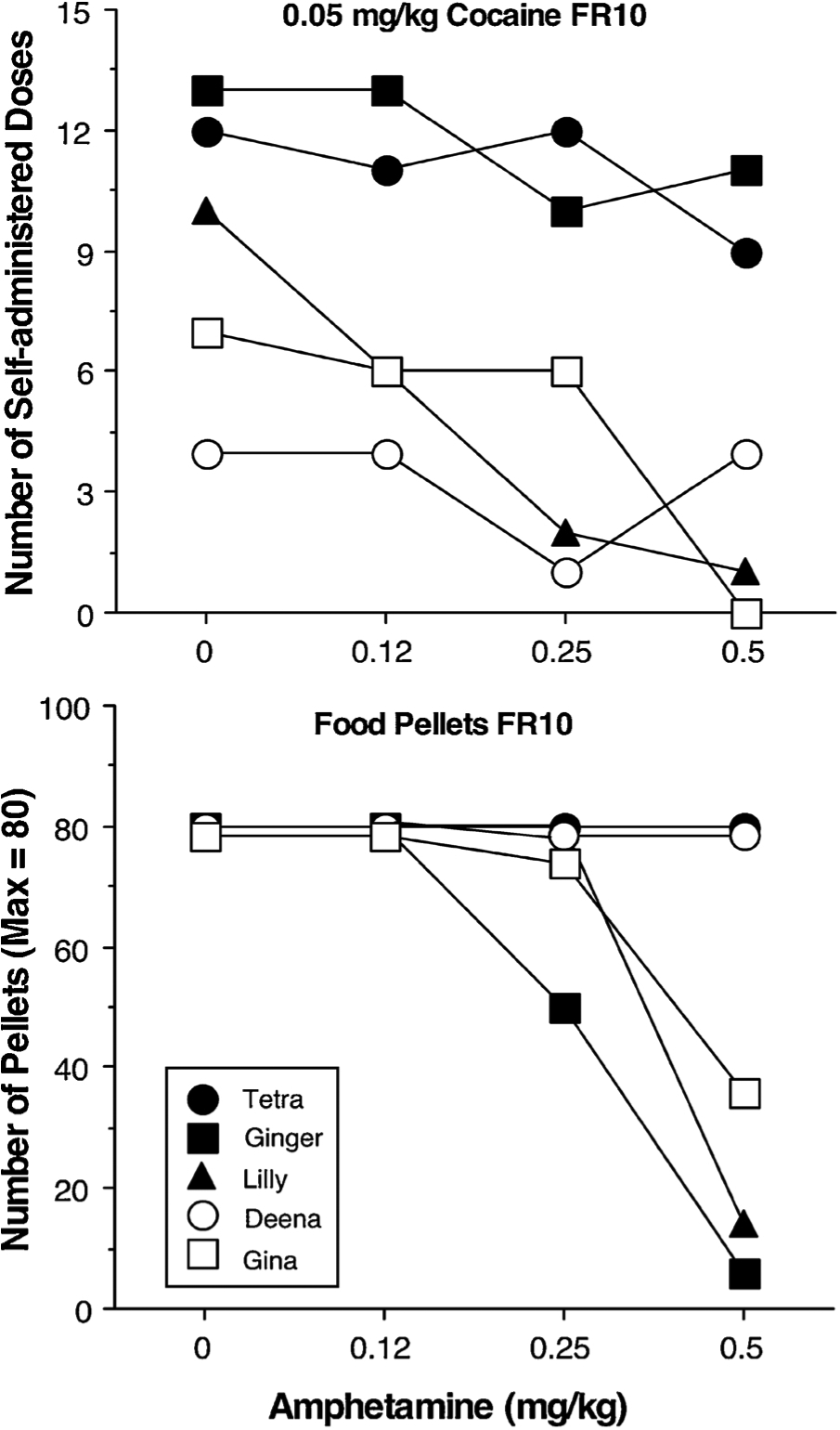
Top Panel: Number of self-administered cocaine doses as a function of d-amphetamine dose in 5 female rhesus monkeys. Responding was maintained under a Fixed interval 1 min; Fixed ratio 10 chain schedule [FI 1-min (FR10:S)] with a maximum of 15 drug infusions.
Bottom Panel: Number of food pellets earned as a function of d-amphetamine dose in 5 female rhesus monkeys. Responding was maintained under a Fixed interval 1 min; Fixed ratio 10 chain schedule [FI 1-min (FR10:S)] with a maximum of 80 food pellets (8 trials for 10-pellets each). The food pellet session occurred 2 h after completion of the cocaine session.
3.1.2. Progressive ratio schedule
Under the PR schedule, increasing the cocaine dose from 0.025 mg/kg/infusion to 0.05 mg/kg/infusion increased the PR breakpoint from a range of 200 to 450 for the lower dose to a range of 400 to 600 for the higher dose in 3 of the monkeys (Fig. 3; 0 mg SB). PR breakpoints slightly decreased for Deena and Gina with the higher dose of cocaine. The effects of the HCRTr1 antagonist SB was first examined with the lower cocaine dose. Because the PR breakpoints for this dose of cocaine were increased in 3 monkeys after 8 mg/kg SB administration, the effects of 4 mg/kg SB were also examined at the lower dose of cocaine. The 4 mg/kg SB dose had minimal effects, and the 24 mg/kg SB dose only decreased the PR breakpoint for the 0.025 mg/kg cocaine dose in 2 of the 5 monkeys. When SB was tested in combination with the 0.05 mg/kg/infusion cocaine dose, the 8 mg/kg dose of SB only increased the breakpoint in 1 monkey, and the 24 mg/kg SB decreased the PR breakpoint only in the 3 monkeys with the greater breakpoints. As observed with the chain schedule, decreases in cocaine self-administration following SB administration occurred consistently in the 3 monkeys who were on the ascending limb of the cocaine dose-response function.
Fig. 3.
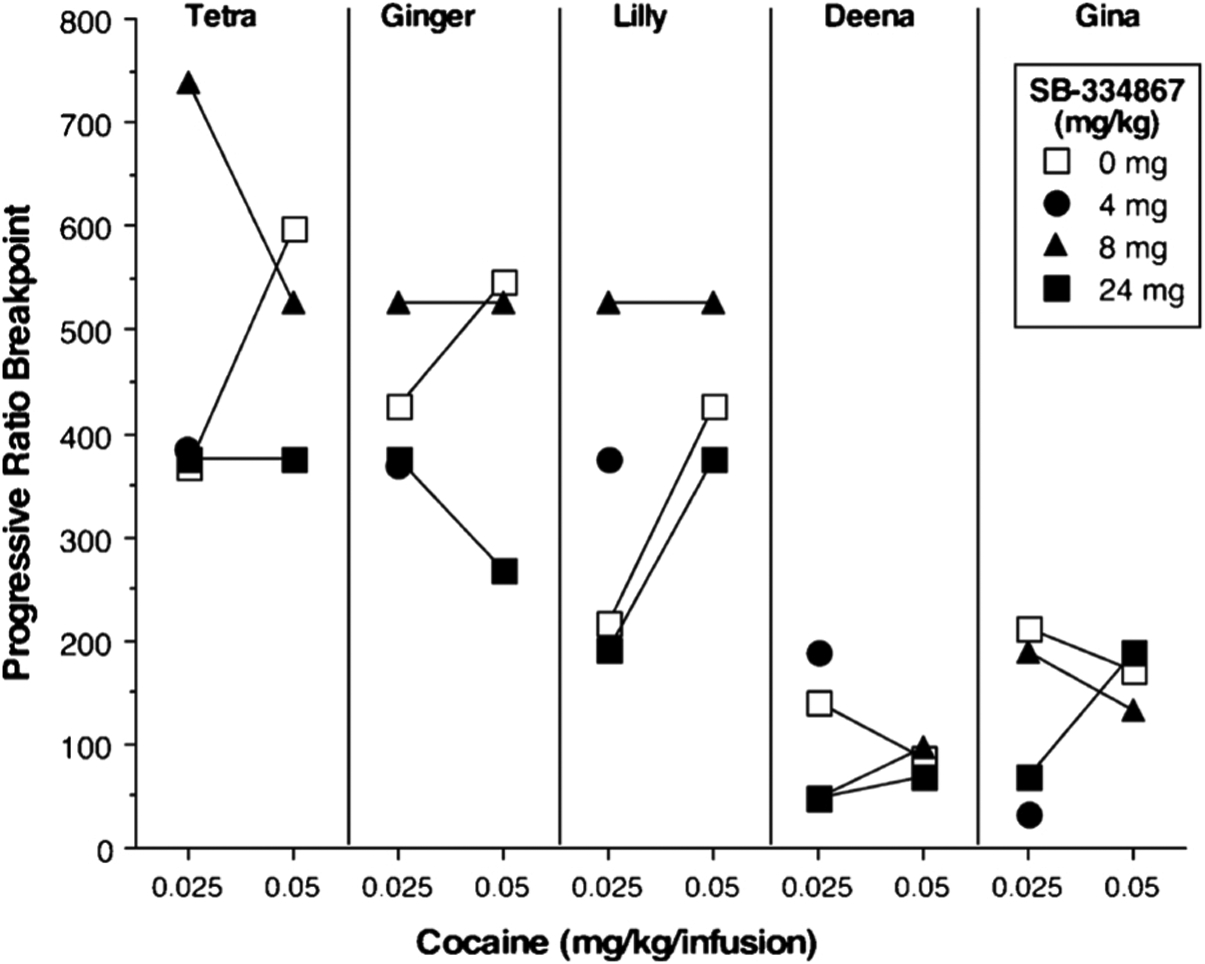
Progressive ratio breakpoint for cocaine as a function of cocaine dose and HCRTr1 antagonist SB-334867 dose in 5 female rhesus monkeys.
After completion of the study with the HCRTr1 antagonist SB, the effects of another HCRTr1 antagonist, RTIOX-276, on cocaine PR breakpoint was examined in 4 monkeys (due to a limited drug supply the heaviest animal was not tested). Similar to the pattern that was observed with SB-334867, RTIOX-276 (Fig. 4) produced dose-dependent decreases in the PR breakpoint for 0.025 mg/kg/infusion cocaine dose in the 2 monkeys with the greater breakpoints (425–475) without affecting the breakpoint in the 2 monkeys with the lesser breakpoints (125–150).
Fig. 4.
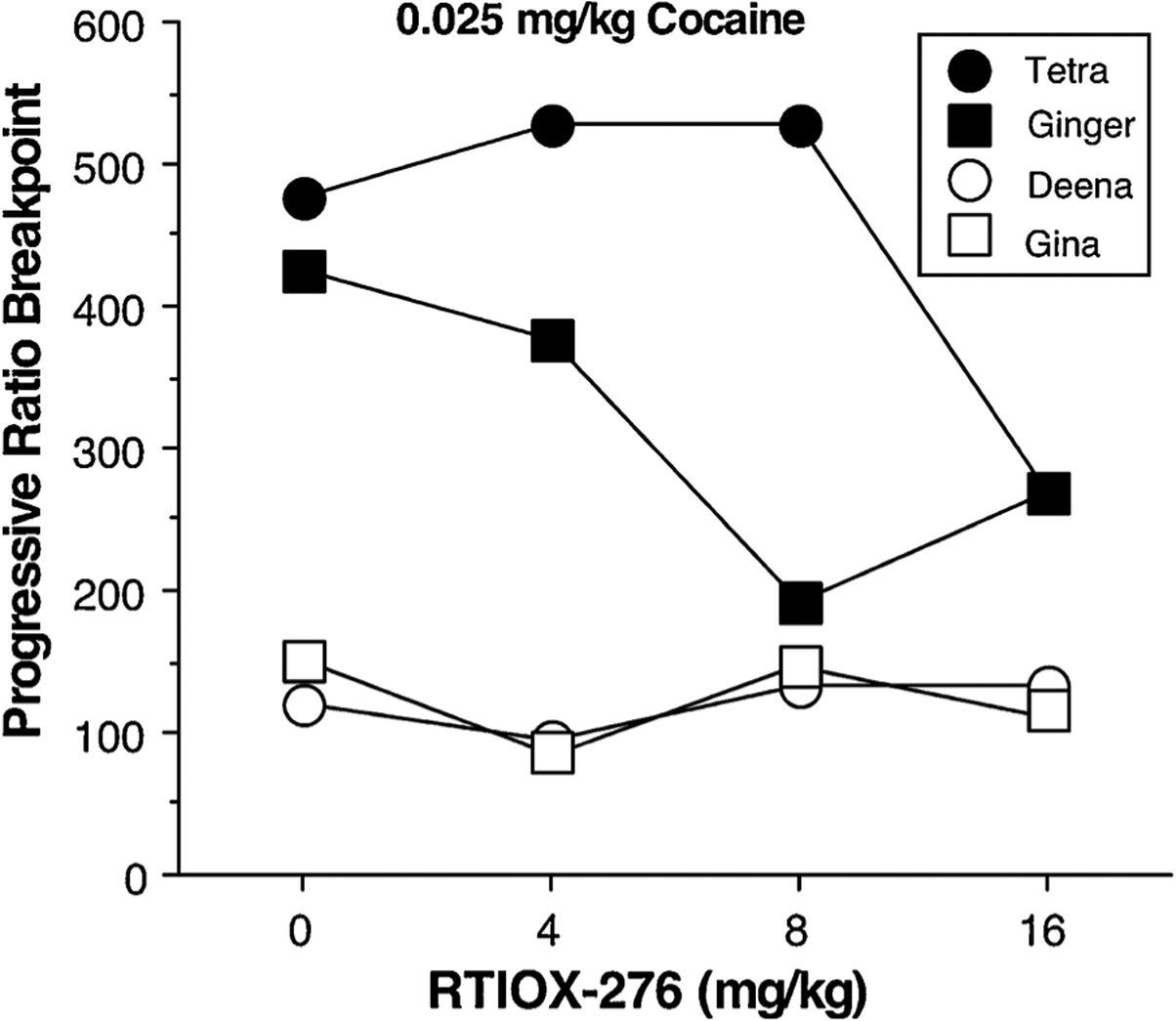
Progressive ratio breakpoint for 0.025 mg/kg/infusion cocaine as a function of HCRTr1 antagonist RTIOX-276 dose in 4 female rhesus monkeys.
3.1.3. Candy vs. cocaine choice schedule
Prior to testing the effects of the HCRTr1 antagonist SB on choice behavior, a number of cocaine and candy options were tested in order to find options that would generate intermediate levels of cocaine choice. The effects of 8 mg/kg and 24 mg/kg of SB were examined under 3 cocaine dose conditions (Fig. 5): 0 mg/kg/infusion cocaine vs 5 candies; 0.0188 mg/kg/infusion cocaine vs 5 candies; 0.025 mg/kg/infusion cocaine vs 5 candies. The number of cocaine choices increased in all 5 monkeys when cocaine was available (Fig. 5; top panel), while the number of candy choices decreased in all 5 monkeys when cocaine was available (Fig. 5; bottom panel). Maximal cocaine choice occurred at the smaller cocaine dose in 3 monkeys and at the larger cocaine dose in 2 monkeys. [Of note, Gina self-administered more cocaine under the choice schedule than she did under the chain FR10 schedule or the PR schedule.] Administration of the HCRTr1 antagonist SB had modest effects on cocaine choices: choices for 0.0188 mg/kg/infusion cocaine decreased in 2 of 4 monkeys (Ginger chose no cocaine under this condition), and choices for 0.025 mg/kg/infusion cocaine decreased in 2 of 5 monkeys.
Fig. 5.
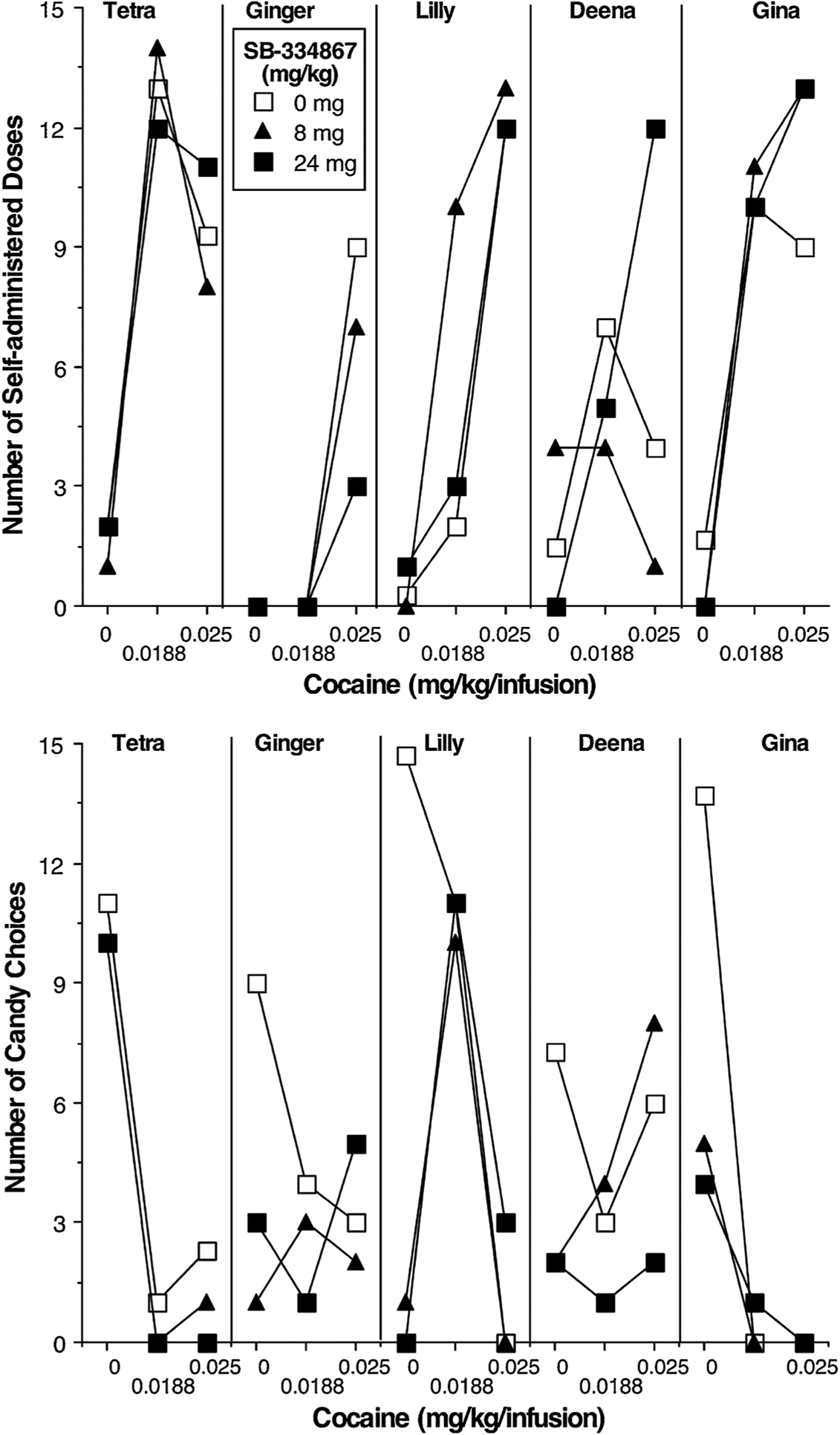
Number of cocaine dose choices (top panel) and candy choices (bottom panel) as a function of cocaine dose and HCRTr1 antagonist SB-334867 dose in 5 female rhesus monkeys. There were 15 discrete choice trials with cocaine as one option and 5 M&M® candies as the other option.
When 0 mg/kg/infusion cocaine was available, monkeys chose 7–15 candy deliveries. The number of candy deliveries decreased to 0 in 4 of the 5 monkeys following administration of the HCRTr1 antagonist SB. When either dose of cocaine was available, monkeys, with the exception of Ginger, chose 0–3 candy deliveries. Candy choice was not affected by administration of the HCRTr1 antagonist SB when cocaine was also available.
3.1.4. Behavioral effects of HCRTr1 antagonists
Experienced monkeys readily climb into their non-human primate chair prior to sessions and quickly return to their home cage after sessions. Animals were observed after HCRTr1 antagonist dosing and after the self-administration session for signs of sedation or somnolence. While there were no signs of sedation, the research technician reported that monkeys were often more “relaxed” and deliberate in climbing in and out of the chair after HCRTr1 antagonist dosing.
3.2. Effects of Hypocretin-1 on cocaine self-administration
The effects of hypocretin-1 on cocaine self-administration were determined under 3 cocaine conditions: 0.0125 mg/kg/infusion cocaine under the Chain schedule, 0.05 mg/kg/infusion cocaine under the Chain schedule (Fig. 6: left panel), and 0.0188 mg/kg/infusion cocaine vs 5 candies under the Choice schedule (Fig. 6: right panel). Administration of 0.072 mg/kg hypocretin-1 increased responding for 0.0125 mg/kg/infusion cocaine in 3 of 4 monkeys (Ginger took no cocaine under this condition) and increased responding for 0.05 mg/kg/infusion cocaine in 1 of the 2 monkeys whose cocaine intake was not already at maximal levels.
Fig. 6.
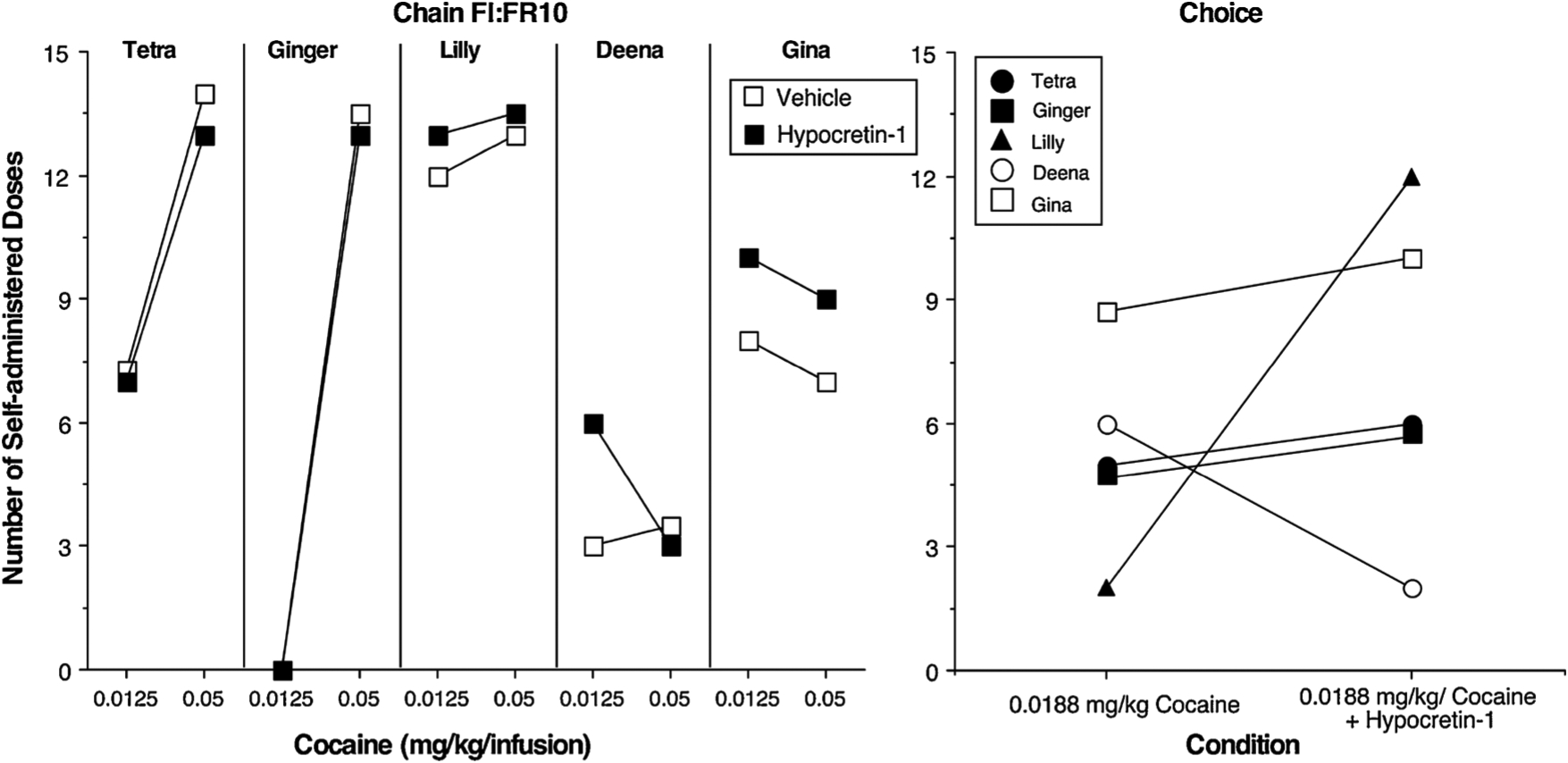
Number of self-administered cocaine doses as a function of cocaine dose and 0.072 mg/kg hypocretin-1 administration in 5 female rhesus monkeys. Left Panel: Chain Schedule. Responding was maintained under a Fixed interval 1 min; Fixed ratio 10 chain schedule [FI 1-min (FR10:S)] with a maximum of 15 drug infusions. Right Panel: Choice Schedule. There were 15 discrete choice trials with cocaine as one option and 5 M&M® candies as the other option.
The 3 monkeys who had maximal levels of cocaine intake when 0.05 mg/kg/infusion was available under the Chain schedule had lower levels of cocaine intake under the 0.0188 mg/kg/infusion vs. candy choice condition. Under the Choice schedule, which generated lower levels of cocaine taking than the Chain schedule, 0.072 mg/kg hypocretin-1 increased cocaine taking in all 3 of these monkeys. Hypocretin-1 also increased cocaine intake in 1 of the other 2 monkeys such that under the choice procedure hypocretin-1 increased cocaine intake in 4 of 5 monkeys. Hypocretin-1 had less consistent effects on candy choice; candy choice was unaffected (+ 1) in 3 monkeys, increased from 2 to 4 candy choices in one monkey, and decreased from 11 to 2 candy choices in the remaining monkey. These data complement the data above showing that under the same choice conditions the HCRTr1 antagonist SB decreased cocaine intake in 3 of 4 monkeys.
3.3. Effects of HCRTr1 antagonism on cocaine-induced reinstatement
When monkeys had access to 0.0125 mg/kg/infusion cocaine under the Chain schedule, they completed the response requirement between 5.7 (Deena) and 13.3 (Lilly) times per session (mean = 9.5 cocaine infusions; data not shown). Within 3 experimental sessions when cocaine was not given, but the stimuli paired with cocaine were delivered, i.e., EXT, the number of completed response requirements dropped to less than 3 for all monkeys (Fig. 7). Giving the monkeys a response-independent dose of 0.3 mg/kg cocaine along with cocaine stimulus cues increased the number of completed response requirements in 3 of the 5 monkeys. For these 3 monkeys, when the HCRTr1 antagonist SB was given in combination with the response-independent dose of cocaine, the number of completed response requirements increased only slightly relative to the EXT condition, e.g., SB attenuated the effects of the response-independent dose of cocaine. When monkeys had access to 0.05 mg/kg/infusion cocaine under the Chain schedule, they completed the response requirement between 3.0 (Deena) and 15 (Tetra) times per session (mean = 10.2 cocaine infusions; data not shown). Within 4 experimental sessions when cocaine was not given, but the stimuli paired with cocaine were delivered, the number of completed response requirements dropped to less than 3 for all monkeys. Giving the monkeys a response-independent dose of 0.3 mg/kg cocaine, along with cocaine stimulus cues, increased the number of completed response requirements in 4 of the 5 monkeys. Administering the HCRTr1 antagonist SB in combination with the response-independent dose of cocaine attenuated the effects of response-independent cocaine in only 2 of these 4 monkeys. The HCRTr1 antagonist SB had smaller effects on cocaine-induced reinstatement when the monkeys had been responding for the larger dose of cocaine prior to extinction.
Fig. 7.
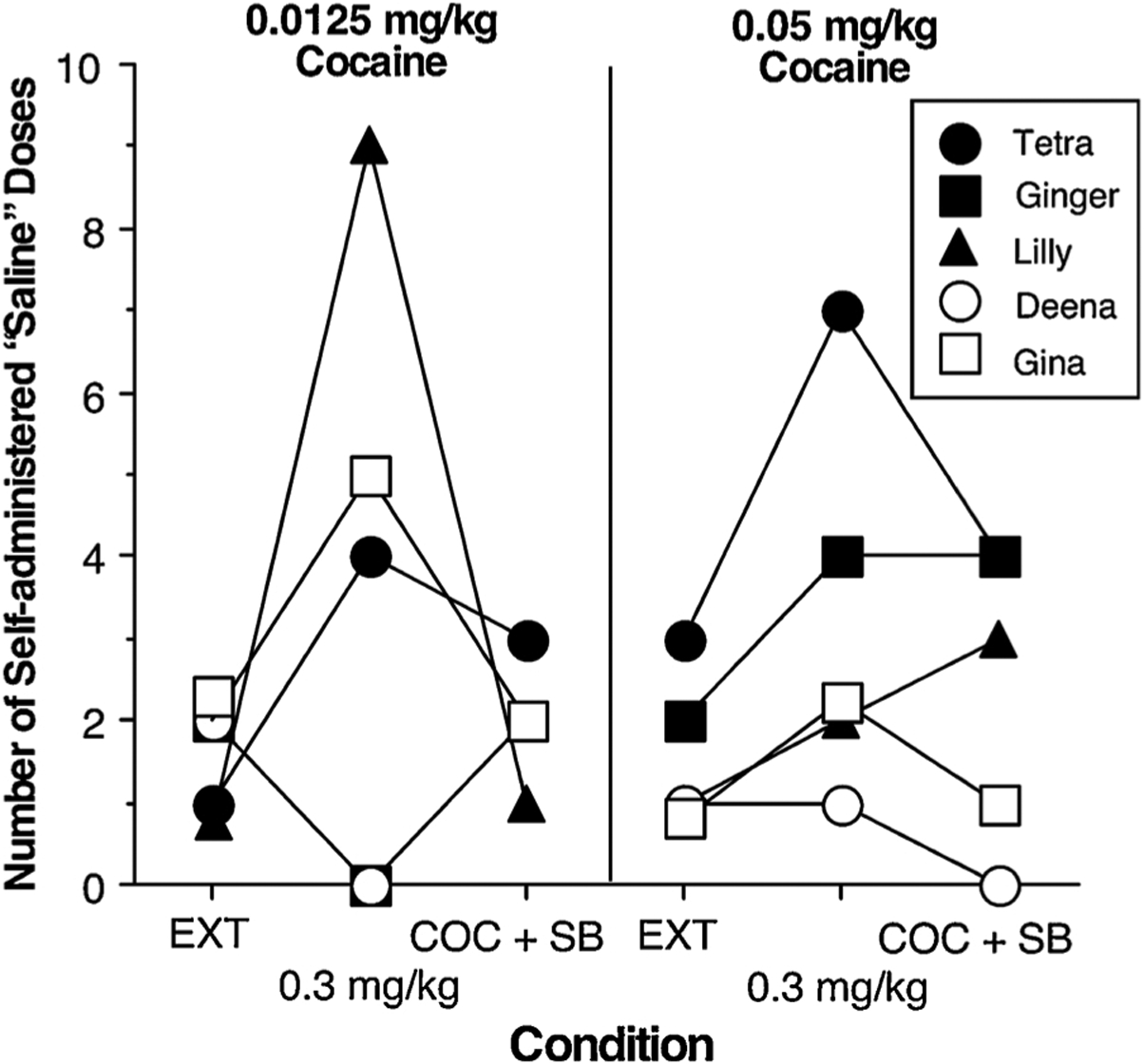
Number of self-administered “saline” doses as a function of cocaine dose that had previously maintained responding and HCRTr1 antagonist SB-334867 administration in 5 female rhesus monkeys. Responding was maintained under a Fixed interval 1 min; Fixed ratio 10 chain schedule [FI 1-min (FR10:S)] with a maximum of 15 drug infusions. Responding was tested under extinction (EXT) conditions when responding resulted in the presentation of the stimuli associated with cocaine but no drug was infused, then after a response-independent administration of a 0.3 mg/kg cocaine infusion prior to the session and after a response-independent administration of a 0.3 mg/kg cocaine infusion and 24 mg/kg SB-334867 administration (COC + SB) prior to the session: order of testing the latter 2 conditions was counterbalanced across monkeys.
4. Discussion
This is the first study demonstrating the impact of the hypocretin system on drug taking by non-human primates. Increasing or decreasing hypocretin activity affects i.v. cocaine self-administration by female rhesus monkeys. The administration of the HCRTr1 antagonist SB decreased self-administration of 1 or more cocaine doses in all 5 monkeys under a Chain FI: FR10 schedule and under a PR schedule and in 4 of 5 monkeys under a cocaine vs. candy choice schedule. The administration of the HCRTr1 antagonist RTIOX-276 decreased self-administration of 1 or more cocaine doses in the 2 monkeys that had the largest PR breakpoints. The effects of HCRTr1 antagonists were greater on the ascending limb of the cocaine dose-response function under the Chain FI: FR10 schedule of reinforcement and when a greater number of responses were required for reinforcement under the PR schedule. The administration of hypocretin-1 increased self-administration of 1 or more cocaine doses in 3 of 5 monkeys under a Chain FI: FR10 schedule and in 4 of 5 monkeys under a cocaine vs. candy choice schedule. Increases were only observed when baseline levels of cocaine self-administration were low. Finally, the administration of the HCRTr1 antagonist SB attenuated reinstatement of extinguished cocaine responding following response-independent delivery of cocaine in 3 of the 5 monkeys under a Chain FI: FR10 schedule.
Data clearly supports a role for the hypocretin system in modulating cocaine self-administration in rodents. A consistent observation, when data are combined across investigators, is that HCRTr1 antagonism decreases cocaine self-administration when self-administration behavior is maintained under schedules of reinforcement that require more than a single response to earn a cocaine delivery, i.e., “effortful” schedules (e.g., Borgland et al., 2009; Brodnik et al., 2015; Gentile et al., 2018; Hollander et al., 2012; España et al., 2010; Levy et al., 2017; Smith et al., 2009). In the present study, responding reinforced by cocaine was examined under 3 different effortful schedules of reinforcement (Chain FI: FR10; PR, choice); under each schedule, administration of a HCRTr1 antagonist decreased cocaine self-administration in the majority or all of the monkeys. Of note, when we tested the HCRTr1 antagonist RTIOX-276 using a PR schedule in 4 monkeys, the antagonist only decreased cocaine self-administration in the 2 monkeys with the greater PR breakpoints (> 425). The size of the decreases was similar to those observed when amphetamine was examined under the Chain FI: FR10 schedule. In most cases, we obtained data using several doses of cocaine and several doses of a HCRTr1 antagonist, and the pattern of antagonist–induced decreases were variable across cocaine doses, schedule of reinforcement, and monkeys. More consistent results may have been observed if it had been possible to administer larger doses of the antagonists. While variable, the effects of HCRTr1 antagonism on cocaine self-administration were robust across 3 schedules of reinforcement and extend the patterns of results observed with rodents to non-human primates.
Much less work has examined the effects of hypocretin peptide administration on cocaine self-administration (Boutrel et al., 2005; España et al., 2011), with increases in cocaine-reinforced responding using a PR schedule but not an FR1 schedule of reinforcement following direct infusion into the ventral tegmental area. We examined the effects of a single dose of i.v. hypocretin-1 on cocaine self-administration under the Chain and Choice schedules. Although the effects were modest, hypocretin-1 increased cocaine self-administration under both schedules in the monkeys who had lower baseline levels of cocaine self-administration. These findings extend the data obtained in rodents with central administration of hypocretin-1 and complement the decreases in cocaine self-administration produced by HCRTr1 antagonism.
Multiple studies in rodents clearly support a role for the hypocretin system in modulating cue-induced reinstatement of cocaine seeking behavior such that HCRTr1 antagonism attenuates cue-induced reinstatement (Bentzley and Aston-Jones, 2015; Mahler et al., 2013; Smith et al., 2009, 2010) but not cocaine-induced reinstatement (Mahler et al., 2013). We had difficulty maintaining stable behavior when neither cues or cocaine were presented during extinction, so we were unable to assess cue-induced reinstatement. Of interest, a previous study in rats reported that the HCRTr1 antagonist SB attenuated cue-induced reinstatement in males but failed to in females (Zhou et al., 2012); thus, this may reflect a sex difference. The non-human primates in the present study were female, so we could not directly address this possibility.
We were only able to examine the effects of HCRTr1 antagonism on cocaine-induced reinstatement. When responding had been reinforced with a low dose of cocaine, administration of a large single dose of cocaine prior to an extinction session increased responding in 3 of 5 monkeys; administration of the HCRTr1 antagonist SB blocked this effect in those 3 monkeys. When responding had been reinforced with a large dose of cocaine, administration of a large single dose of cocaine prior to an extinction session increased responding in 4 of 5 monkeys; administration of the HCRTr1 antagonist SB blocked this effect in only 2 of these 4 monkeys. Thus, HCRTr1 antagonism was effective at decreasing cocaine-induced reinstatement in only about half of the monkeys. This minimal effect is similar to the lack of effect of HCRTr1 antagonism on cocaine-induced reinstatement reported with rodents (Mahler et al., 2013; Zhou et al., 2012). However, the HCRTr1 antagonist SB attenuated cocaine + cue induced reinstatement in male rats but not female rats (Zhou et al., 2012), suggesting that there may be sex differences related to the interaction between the hypocretin system and cocaine reinforcement. At this time, the effect of sex on the relationship between the hypocretin system and drug taking has not been adequately studied. However, there is evidence from other studies that there are sex differences in the hypocretin system. For instance, one study showed that female rats were slower to habituate to restraint stress than male rats, and this difference was associated with greater increases in hypocretin RNA in females than males (Grafe et al., 2017).
Similarly, the hypocretin system modulates food consumption, particularly of palatable food (e.g., Alcaraz-Iborra et al., 2014; Inutsuka et al., 2014; see review by Rodgers et al., 2002). There is also some evidence that these effects are modulated by sex. In one study, Cason and Aston-Jones (2013) assessed the effects of the HCRTr1 antagonist SB on saccharin-reinforced responding and cue-induced reinstatement in males, and in a second study they (Cason and Aston-Jones, 2014) assessed the effects of the HCRTr1 antagonist SB on sucrose-reinforced responding and cue-induced reinstatement in females. The effects of HCRTr1 antagonism were determined when rats were food-restricted or fed ad libitum. In males, the HCRTr1 antagonist SB decreased sweet-food reinforced responding and cue-induced reinstatement under both feeding conditions. In contrast, in females the HCRTr1 antagonist SB decreased sweet-food reinforced responding only when food-restricted but did not alter cue-induced reinstatement under either feeding condition. In the present study, we did not test the effects of hypocretin-1 on candy choice in the absence of cocaine, so it is not entirely surprising that hypocretin-1 did not increase candy choice when monkeys were not food-deprived and there was a highly reinforcing alternative (i.e., cocaine). Different results may have been obtained if we had tested the effects of hypocretin-1 when only candy was available. Although the HCRTr1 antagonist SB decreased candy choice when the alternative was saline, when cocaine was available, the HCRTr1 antagonist SB decreased cocaine choice but did not alter candy choice, perhaps because candy choice was already quite low. Taken together, there is converging evidence to support a role for the hypocretin system in part by modulating the motivational value of stimuli paired with reinforcers (e.g., Ho and Berridge, 2013; Kay et al., 2014; Risco and Mediavilla, 2014) in the maintenance and escalation of dug taking and palatable food-related behavior.
Limitations of this study are related to the relatively small sample size, the inability to assess sex differences since only females were included, and the limited dose range of the hypocretin agents that were tested. In spite of the small sample, behavior of all of the monkeys was affected by the hypocretin manipulations in the same manner under at least one cocaine dose maintenance condition. Furthermore, similar effects of the hypocretin manipulations were observed under 3 different schedules of reinforcement. Other than await novel compounds, there is little to be done about the solubility and dosing issues related to the hypocretin agents. Also, by conducting the hypocretin manipulations in the morning when circulating levels of hypocretin were expected to be low (Zeitzer et al., 2003), we may have limited the ability to observe greater decreases in cocaine self-administration.
Enthusiasm for the therapeutic potential of hypocretin/orexin manipulations should be tempered for multiple reasons (Boutrel et al., 2013). While hypocretin antagonists are being examined for putative use to treat sleep disorders, studies in humans with dual antagonists have reported side effects including excessive somnolence, postural instability, decreased alertness, and visual and motor coordination difficulties (e.g., Bettica et al., 2012; Cruz et al., 2014; Hoever et al., 2012). Fluctuating hypocretin levels may also modulate emotional and social “arousal” (Blouin et al., 2013), limiting their usefulness as a medication.
The results of the present study extend findings previously only reported in rodents to non-human primates about the effects of altering HCRTr1 activity on cocaine self-administration: decreasing HCRTr1 activity decreases cocaine self-administration, while increasing HCRTr1 activity increases cocaine self-administration. These findings strongly support additional research on the effects of hypocretin manipulations on complex motivated behavior in human and non-human primates (e.g.,Giardino and de Lecea, 2014).
Acknowledgements
The assistance of Jean Willi and the New York State Psychiatric Institute’s Division of Comparative Medicine is gratefully acknowledged. We also thank Robert Scalese, Wendy Johnson, and Jon Ehrmann for their expertise in performing the VAP surgeries.
Role of funding source
This research was supported by DA-021319 and KO5 DA 031749 from The National Institute on Drug Abuse.
Footnotes
Conflict of interest
No conflict declared.
References
- Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I, 2014. Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: pharmacological and molecular evidence of orexin involvement. Behav. Brain Res 272, 93–99. [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou L-C, Lawrence AJ, Muschamp JW, Patkar O, Tung L-W, Borgland SL, 2015. Orexin/hypcretin role in reward: implications for opioid and other addictions. Br. J. Pharmacol 172, 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur. J. Neurosci 41, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk D, 2012. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology 37, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KA, Lapierre JL, Siegel JM, 2013. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun 4, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A, 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang S, Bowers MS, Thompson JL, Vittoz N, Floresco NB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, Halfon O, 2013. Hypocretins and the reward function: what have we learned so far? Front. Behav. Neurosci 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Leccea L, 2005. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A 102, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, España RA, 2015. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behav. Brain Res 291, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L, 2009. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J. Neurosci 29, 10939–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G, 2013. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology 228, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G, 2014. Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology 86, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Evans SM, 2013. Effects of menstrual cycle phases on cocaine self-administration in rhesus macaques. Horm. Behav 63, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, 2009. Hypocretin mechanisms in nicotine addiction: evidence and speculation. Psychopharmacology 206, 23–37. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Hay JL, Hoever P, Alessi F, te Beek ET, van Gervan JMA, Dingemanse J, 2014. Pharmacokinetic and pharmacodynamics interactions between almorexant, a dual orexin receptor antagonist, and desipramine. Eur. Neuropsychopharmacol. 24, 1257–1268. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE, 2007. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci 27, 14239–14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Peyron C, Tighe DK, Pol AN, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR, 2010. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur. J. Neurosci 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DC, Jones SR, 2011. Hypocretin 1/Orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology 214, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Barker DJ, Shaw JK, España RA, Muschamp JW, 2018. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict. Biol 23, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L, 2014. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr. Opin. Neurobiol 29, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S, 2017. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol. Psychiatry 81, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G, 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559. [DOI] [PubMed] [Google Scholar]
- Ho C, Berridge KC, 2013. An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever P, Dorffner G, Beneš H, Penzel T, Danker-Hopfe H, Barbanoj MJ, Pillar G, Saletu B, Polo O, Kunz D, Zeitlhofer J, Berg S, Partinen M, Bassetti CL, Högl B, Ebrahim IO, Holsboer-Trachsler E, Bengtsson H, Peker Y, Hemmeter UM, Chiossi E, Hajak G, Dingemanse J, 2012. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin. Pharmacol. Ther 91, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ, 2012. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front. Behav. Neurosci 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C, 2011. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav. Pharmacol 22, 173–181. [DOI] [PubMed] [Google Scholar]
- Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A, 2014. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85, 451–460. [DOI] [PubMed] [Google Scholar]
- Kay K, Parise EM, Lilly N, Williams DL, 2014. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology 231, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, 1966. Conditioned reinforcement in second-order schedules. J. Exp. Anal. Behav 9, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, España RA, 2017. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology 234, 2761–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G, 2013. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 226, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CS, Featherby T, Krstew E, Andrew JL, 2007. Quantification of phosphorylated camp-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: activation of hypothalamic orexin a-containing neurons. J. Pharmacol. Exp. Ther 323, 805–812. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SS, Borgland SL, Bonci A, Bartlett SE, 2008. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long–Evans rats. Psychopharmacology 199, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco S, Mediavilla C, 2014. Orexin-1 receptor antagonist in central nucleus of the amygdala attenuates the acquisition of flavor-taste preference in rats. Pharmacol. Biochem. Behav 126, 7–12. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Ishii Y, Halford JCG, Blundell JE, 2002. Orexins and appetite regulation. Neuropeptides 36, 303–325. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu W, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and g protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF, 2017. Hypocretin neuro-transmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol. Psychiatry 81, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC, 2001. SB-334867-A: the first selective orexin-1 receptor antagonist. Br. J. Pharmacol 132, 1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G, 2012. Orexin/hypocretin-1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur. J. Neurosci 35, 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009. Orexin/hypocretin signaling at the orexin-1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G, 2010. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L, 2002. The hypocretins: setting the arousal threshold. Nat. Rev. Neurosci 3, 339–349. [DOI] [PubMed] [Google Scholar]
- Wang B, You Z, Wise RA, 2009. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol. Psychiatry 65, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ, 2010. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology 58, 185–194. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR, 1994. Use of subcutaneous vascular access ports in rhesus monkeys. Lab. Anim. Sci 44, 491–494. [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E, 2003. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J. Neurosci 23, 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE, 2012. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J. Pharmacol. Exp. Ther 340, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]


